Abstract
Background
We have previously shown that monocyte chemotactic protein-1 (MCP-1) promotes aneurysm healing.
Objective
To determine the temporal cascade and durability of aneurysm healing.
Methods
Murine carotid aneurysms were treated with MCP-1-releasing or poly(lactic-co-glycolic) acid (PLGA)-only coils. Aneurysm healing was assessed by quantitative measurements of intraluminal tissue ingrowth on 5 μm sections by blinded observers.
Results
Aneurysm healing occurred in stages characteristic of normal wound healing. The 1st stage (day 3) was characterized by a spike in neutrophils and T cells. The 2nd stage (week 1) was characterized by an influx of macrophages and CD45+ cells significantly greater with MCP-1 than with PLGA (p<0.05). The third stage (week 2–3) was characterized by proliferation of smooth muscle cells and fibroblasts (greater with MCP-1 than with PLGA, p<0.05). The fourth stage (3–6 months) was characterized by leveling off of smooth muscle cells and fibroblasts. M1 macrophages were greater at week 1, whereas M2 macrophages were greater at weeks 2 and 3 with MCP-1 than with PLGA. Interleukin 6 was present early and increased through week 2 (p<0.05 compared with PLGA) then decreased and leveled off through 6 months. Tumour necrosis factor α was present early and remained constant through 6 months. MCP-1 and PLGA treatment had similar rates of tissue ingrowth at early time points, but MCP-1 had a significantly greater tissue ingrowth at week 3 (p<0.05), which persisted for 6 months.
Conclusions
The sequential cascade is consistent with an inflammatory model of injury, repair, and remodeling.
We have previously shown that local delivery of the cytokine monocyte chemotactic protein-1 (MCP-1), via coils that sustain release MCP-1, promotes inflammatory tissue healing of murine carotid aneurysms via a macrophage inflammatory protein 1α and macrophage inflammatory protein 2-dependent pathway.1 However, the temporal cascade, in which specific cell-type populations and the cytokines that recruit them are seen, is not known. Additionally, since the durability of aneurysm repair is clinically important, the long-term characteristics of MCP-1-aneurysm repair need to be studied. Finally, we believe that macrophages are critical in MCP-1-aneurysm repair but the roles of macrophage subtypes are unknown.2 Here we show that the temporal cascade of cytokines and cell-type populations in MCP-1-mediated aneurysm healing closely resembles an inflammatory model of injury, repair, and remodeling, and that the durability of aneurysm healing extends long term.
METHODS
All animal experimentation was approved by the Institutional Animal Care and Use Committee and in accordance with Animal Research: Reporting in Vivo Experiments guidelines. Murine carotid aneurysms were created in C57BL/6 female mice (6–10 weeks, Charles River, Wilmington, Massachusetts, USA) using a method previously described.3 We have previously shown that these aneurysms lack elastin and histologically resemble human cerebral aneurysms.3 Mice were then randomized to treatment with MCP-1-releasing or poly(lactic-co-glycolic acid) (PLGA)-only coils using a method previously described.1 We have previously demonstrated that the MCP-1-releasing coils release MCP-1 in a sustained manner over 3 weeks.1 Coiled aneurysms were harvested at 3 days, 1 week, 2 weeks, 3 weeks, 3 months, and 6 months after coil treatment (n=10 mice for 3-day, 1-week, and 2-week time points for each coil-type, and n=15 mice for the 3-week time point for each coil-type). The rationale for the chosen time points is based on previously described time points of MCP-1-mediated wound healing in a murine excisional wound healing model (3 days, 1 week, 2 weeks, 3 weeks)4 plus an additional two longer-term time points (3 and 6 months) to assess the long-term durability of MCP-1-mediated aneurysm healing. Aneurysms were split into 5 μm sections (five sections per aneurysm), and stained with hematoxylin and eosin or underwent immunohistochemical staining: hematopoietic cells (anti-CD45, Abcam), macrophages (anti-CD11b, BD Pharmingen), T cells (anti-CD3e, Abcam), smooth muscle cells (anti-smooth muscle cell antibody (anti-SMA), Sigma), fibroblasts (anti-fibroblast-specific protein 1, Abcam), M1 macrophages (anti-inducible nitric oxide synthase (anti-iNOS) antibody, Abcam), M2 macrophages (anti-mannose receptor CD206, Abcam), interleukin (IL)-6 (anti-IL6, Abcam), and tumor necrosis factor (TNF)-α (anti-TNF-α, Abcam). Briefly, except for CD3e and SMA staining, the following antigen heat retrieval methods were used before the primary antibody staining: target retrieval solution (Dako) was used for CD45 and CD11b staining; citrate buffer (pH 6.0) was used for fibroblast-specific protein 1, iNOS, CD206, IL-6, and TNF-α. The primary antibody incubation was performed overnight at 4°C followed by incubation with species-appropriate Alexa Fluor antibodies created in donkey. All sections were mounted with VectaShield with 4′,6-diamidino-2-phenylindole before imaging.
Quantitative tissue healing measurements and cell counting by immunohistochemistry for temporal cascade analysis were performed as follows. Coiled aneurysms were harvested at 3 days, 1 week, 2 weeks, 3 weeks, 3 months, and 6 months after coil treatment. The tissues were split into 5 μm sections and five sections, which were collected every 100 μm (20 sections), from each sample. For tissue ingrowth analysis, sections were stained with hematoxylin and eosin, than microscopic high-resolution images were obtained using a 10× objective lens by blinded observers. Tissue ingrowth was measured by two blinded observers using Image-Pro software (Media-Cybernetics, Silver Spring, Maryland, USA). For the temporal cascade analysis, after the staining, five microscopic high-resolution images for each slide were obtained using a 20× objective lens by blinded observers. None of the images overlapped with other fields and all image files were blindly named. Cells were counted by two blinded observers using Image-Pro software. For IL-6, TNF-α and α-SMA, the fluorescent intensity of their expression was analyzed using ImageJ software.
A two-way analysis of variance model5 was used to compare mean transformed responses between PLGA and MCP-1 animal groups observed at six different time points. Separate groups of animals were observed at each time point. Animal group (PLGA, MCP-1), observation day (3, 7, 14, 21, 91, and 182), and the interaction between animal group and observation day was modeled as fixed effects.
Comparisons of animal groups at each observation day were evaluated for significance using t statistics, with p values for this set of comparisons adjusted to maintain a family-wise type I error rate of 0.05 using the method of Sidak.6 An optimal Box–Cox power transformation7 was applied to the response variable before model fitting to obtain normally distributed errors. The optimal λ coefficient used in the Box–Cox transformation (λ=0.41) was nearly equivalent to applying a square root transformation to the response variable (λ=0.50).
RESULTS
Temporal cascade of cell-types
Figure 1A demonstrates that the first stage (day 3) of MCP-1-mediated aneurysm healing is characterized by an early spike in neutrophil and T-cell infiltration, which occurs also with PLGA-only treatment. The second stage (week 1) is characterized by an influx of macrophages, both M1 and M2 phenotype, and CD45+ cells which are significantly greater with MCP-1 than with PLGA-only (p<0.05 for macrophages, p<0.05 for CD45+ cells). The third stage (week 2–3) is characterized by proliferation of smooth muscle cells and fibroblasts. Fibroblasts are significantly greater at this stage with MCP-1 than with PLGA-only treatment (p<0.05). The fourth stage (3–6 months) is characterized by leveling off of smooth muscle cells and fibroblasts with both MCP-1 and PLGA-only treatment.
Figure 1.
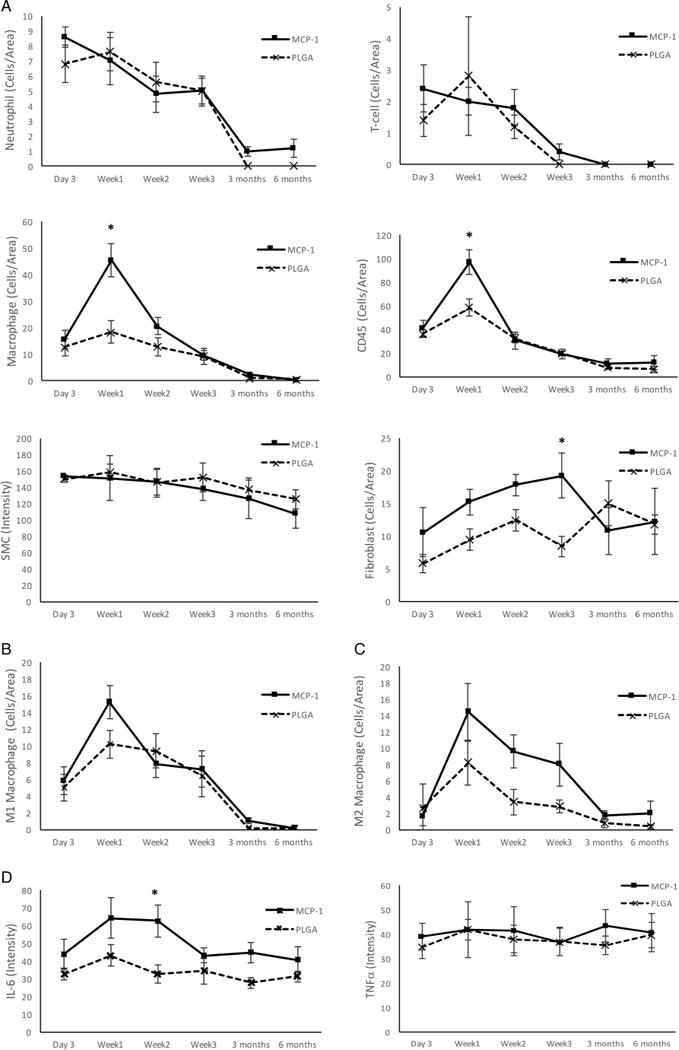
Temporal cascade of monocyte chemotactic protein-1 (MCP-1)-mediated aneurysm healing. (A) Temporal cascade of cell-type populations. (B) Temporal infiltration of M1 proinflammatory macrophages. (C) Temporal infiltration of M2 reparative macrophages. (D) Temporal cascade of inflammatory cytokines. *p<0.05. IL-6, interleukin 6; PLGA, poly(lactic-co-glycolic) acid; SMC, smooth muscle cells.
Macrophage subtypes
There has been great interest in different macrophage subtypes and their different functions. While the paradigm of M1 (proinflammatory) and M2 (anti-inflammatory) macrophages has been postulated, this is not entirely defined.8 Therefore we studied M1 macrophages, which are known to be destructive, and M2 macrophages, which are believed to be reparative.
Figure 1B demonstrates that M1 macrophages infiltrate at stages 1–3 (day 3 to week 3) with a spike at week 1 and then decline thereafter. At week 1, there are more M1 macrophages with MCP-1 than with PLGA-only treatment (p=0.1 underpowered).
Figure 1C demonstrates similarly that M2 macrophages infiltrate at stages 1–3 (day 3 to week 3) with a spike at week 1, and then decline thereafter. However, whereas M1 macrophages are greater at week 1 with MCP-1 compared with PLGA-only, M2 macrophages are greater at week 2 and week 3 with MCP-1 than with PLGA-only treatment (p=0.1 underpowered). This may be evidence that MCP-1-mediated aneurysm healing, as compared with PLGA-only, is characterized by an early M1 ‘destructive’ phase at week 1 followed by a later M2 ‘reparative’ phase at weeks 2 and 3.
Temporal cascade of cytokines
The signaling factors that recruit the different cell-type populations in MCP-1-mediated aneurysm tissue healing are characterized in figure 1D. As others have shown in models of myocardial infarct,9 aortic dissection,10 and tubulointerstitial inflammation,11 IL-6 and TNF-α are downstream mediators of MCP-1. We have also found that IL-6 is a downstream mediator in MCP-1-mediated aneurysm healing.12
In MCP-1-mediated aneurysm healing, IL-6 is present early and increases through week 2 (p<0.05 compared with PLGA-only) then decreases and levels off through 6 months. TNF-α is present early and remains constant through 6 months, similar to PLGA-only treatment.
Long-term durability of aneurysm healing
There was no morbidity or mortality in the animals. Figure 2 demonstrates that MCP-1 and PLGA-only treatment have similar rates of tissue ingrowth at early time points (day 3, week 1, and week 2), but MCP-1 has significantly greater tissue ingrowth at week 3 (p<0.05) compared with PLGA-only, which persists for 6 months (p=0.3 for 3 months, p=0.09 for 6 months, both underpowered). In our mouse model of aneurysm healing, intraluminal aneurysm tissue ingrowth is a surrogate for aneurysm occlusion.
Figure 2.
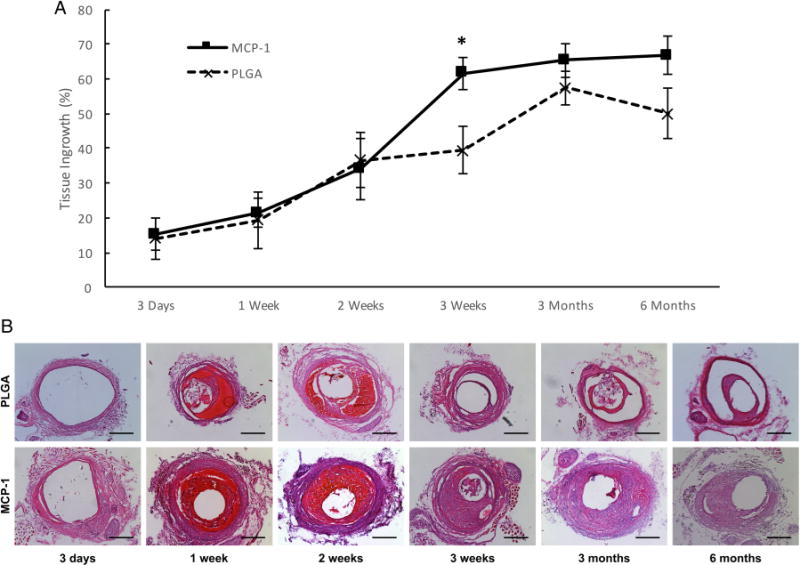
Long-term durability of monocyte chemotactic protein-1 (MCP-1)-mediated aneurysm healing. (A) Tissue ingrowth at time points after MCP-1 or poly(lactic-co-glycolic) acid (PLGA)-only coil treatment, *p<0.05. (B) Representative cross-axial 5 μm sections of mouse aneurysms at the depicted time points after MCP-1 or PLGA-only coil treatment. Scale bar is 200 μm.
DISCUSSION
The goal of brain aneurysm treatment is durable, permanent cure. Our understanding of how a cure can be achieved comes from a histological comparison of cured and incompletely cured human brain aneurysms harvested at surgery or at autopsy. Completely cured human brain aneurysms are characterized by intraluminal inflammatory fibrous scar formation with connective tissue proliferation, fibroblasts, collagen, smooth muscle cells, capillary ingrowth, and macrophages.13
We have previously shown that local delivery of MCP-1 on a MCP-1-releasing coil can create intra-aneurysmal tissue healing inside murine aneurysms characterized by the same findings that are seen in cured human brain aneurysms: connective tissue proliferation, macrophages, fibroblasts, smooth muscle cells, and endothelial cells.1
In this follow-up study, we define the temporal cascade of inflammatory cytokines and cell-type populations, including macrophage subtypes, which produce MCP-1-mediated aneurysm tissue healing.
The sequential cascade we see in this study is consistent with an inflammatory model of injury, repair, and remodeling that has been described in other models, including myocardial repair and remodeling after myocardial infarct.9
In the acute phase, circulating neutrophils are first recruited by chemokines and rapidly infiltrate the area of injury. Neutrophils are then followed in the second stage by proinflammatory monocytes circulating from the bone marrow and recruited to the site of injury to remove extracellular matrix debris by phagocytosis. The third stage is repair and remodeling with proliferation of fibroblasts and smooth muscle cells. The fourth stage is scar formation characterized by collagen and fibrosis.
The chemokines we found in this temporal cascade are consistent with findings in other models of repair and remodeling.9 IL-6, MCP-1, and TNF-α are important and critical mediators of the inflammatory cascade, which appeared in the sequential cascade of MCP-1-mediated aneurysm healing.
This model of aneurysm tissue healing is unlike others in that the scar formation is intraluminal, meaning that there is no native matrix upon which repair and remodeling takes place. While other models aim to reduce or eliminate scar formation, the goal in this model is to create and promote scar. Because it is intraluminal, it is necessary to introduce a scaffold for this cascade to take place, which in our model is the PLGA-coated platinum coil. The local sustained delivery of MCP-1 promotes the inflammatory temporal cascade.
We also investigated the role of macrophage subtypes. It is believed that M1 macrophages are the proinflammatory destructive macrophages and this is consistent in our model with the infiltration of M1 macrophages in the early second stage to create a proinflammatory environment and to remove extracellular debris by phagocytosis. It is believed that M2 macrophages are reparative. In our model, we found that M1 macrophages are greater at week 1 with MCP-1 than with PLGA-only treatment (p=0.1 underpowered), whereas M2 macrophages are greater at week 2 and week 3 with MCP-1 than with PLGA-only treatment (p=0.1 underpowered), demonstrating perhaps that MCP-1-mediated aneurysm healing, as compared with PLGA-only, is characterized by an early M1 ‘destructive’ phase at week 1 followed later by a M2 ‘reparative’ phase at weeks 2 and 3. It is important to note that we chose the antibodies used to demonstrate M1 versus M2 macrophages based on prior studies, but there is controversy as to the most appropriate method for characterizing different macrophage phenotypes. Among multiple models and studies, similar to our model of aneurysm repair, in murine models of remodeling of murine myocardial infarction, CD206+ ‘M2’ macrophages mediate repair, therefore, we used CD206 as a marker for M2 reparative macrophages,14 15 and iNOS has been commonly used as a marker for M1 macrophage phenotype.16 However, no precise method for identifying M1 versus M2 macrophages has been agreed.
We demonstrate the long-term durability of MCP-1-mediated aneurysm healing up to 6 months after treatment, which translates in murine lifespan to decades in human years.17 In the early stages from day 3 to week 2, MCP-1 and PLGA-only have similar rates of tissue ingrowth, and it is not until week 3 that we see a significant difference, where MCP-1 has robust ingrowth. This fits with the possibility that the M1 destructive phase of inflammation occurs early (day 3 to week 1), and that the M2 reparative phase occurs later (week 2–3). It is also expected that the process of MCP-1-mediated aneurysm healing occurs over time and not immediately. The durability of MCP-1-mediated aneurysm healing persists up to 6 months. No statistical difference between MCP-1 and PLGA-only was found at 3 and 6 months because the number of animals was underpowered. Nevertheless, it is reassuring that the tissue ingrowth we demonstrate in our aneurysm repair model is longlasting and demonstrates real fibrous scar.
Our findings are similar to the work of others who have shown that aneurysm healing occurs by cellular-mediated wound healing. The local delivery of mesenchymal stem cells has demonstrated improved aneurysm healing in a rabbit model.18 Controlled release of osteopontin and IL-10,19 vascular endothelial growth factor,20 stromal cell-derived factor 1α (and local delivery of mesenchymal stem cells and endothelial progenitor cells),21 and cyclic peptide SEK-100522 from modified coils have been shown by other groups to accelerate intra-aneurysmal tissue healing in rat models of aneurysms. This work is all consistent with a theme of inflammatory wound healing as a mechanism for aneurysm tissue ingrowth.
MCP-1 has been studied by other groups as a mediator of aneurysm formation rather than aneurysm healing.23,24 This apparent paradox can be explained by the finding of MCP-1 and macrophages within the aneurysm wall in these studies thereby causing inflammation and degeneration of the structural validity of the vessel wall, whereas in our studies, we have delivered MCP-1 locally to the lumen of the aneurysm thereby producing inflammation which creates a scar in the aneurysm sac. The macrophages and MCP-1 in our studies are found in the intraluminal tissue ingrowth.
A limitation of this study is that the sample sizes were not adequately powered to demonstrate a statistically significant difference in cell-specific populations, cytokines, and tissue ingrowth across all time points between MCP-1 coils and PLGA-only coils, although the purpose of this study was not to demonstrate the differences between MCP-1 and PLGA, but rather to characterize the temporal cascade of the MCP-1 inflammatory aneurysm healing pathway. Another limitation is our murine aneurysm model is not intracranial. It is extracranial to accommodate intrasaccular coils. Nevertheless, the pathway of intra-aneurysmal tissue healing should be no different given that the process is intraluminal. Other groups have used extra-cranial carotid animal aneurysm models to study aneurysm healing18–22 and we are unaware of intracranial animal aneurysm models that can accommodate intrasaccular coils. Finally, a limitation of the our murine aneurysm model is that the carotid aneurysms do not rupture. However, our studies are similar to other studies of aneurysm healing and aneurysm devices which use extracranial animal aneurysm models that are not known to rupture, but are rather designed to accommodate intrasaccular devices.18–22,25–30
We are the first to demonstrate the presence of important cytokines, cell populations, and temporal cascade in MCP-1-mediated aneurysm healing, which provides a basis for further knowledge and identification of targets for novel therapies for cerebral aneurysms.
Acknowledgments
We are grateful to Vincent Federico BS, Matthew McCord BS, Antoine Hana, and Robert King for performing blinded cell counting and measurements.
Funding This work was supported by the National Institutes of Health (NIH) grant number R01 NS083673.
Footnotes
Contributors BLH conceived the concept and design, interpreted the data, and drafted the article. HZF, SH, ML, LL acquired the data. KH conceived the design, interpreted the data, and revised the article critically for important intellectual content.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Unpublished data are available for data sharing with the permission of the corresponding author.
References
- 1.Hoh BL, Hosaka K, Downes DP, et al. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1α and macrophage inflammatory protein-2-dependent pathway. Circulation. 2011;124:2243–52. doi: 10.1161/CIRCULATIONAHA.111.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood S, Jayaraman V, Huelsmann EJ, et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE. 2014;9:e91574. doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoh BL, Velat GJ, Wilmer EN, et al. A novel murine elastase saccular aneurysm model for studying bone marrow progenitor-derived cell-mediated processes in aneurysm formation. Neurosurgery. 2010;66:544–50. doi: 10.1227/01.NEU.0000365616.46414.2B. discussion 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low QE, Drugea IA, Duffner LA, et al. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159:457–63. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schabenberger O, Pierce FJ. Contemporary statistical models for the plant and soil sciences. Boca Raton, Florida: CRC Press; 2002. [Google Scholar]
- 6.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–33. [Google Scholar]
- 7.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc B. 1964;26:211–46. [Google Scholar]
- 8.Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tieu BC, Lee C, Sun H, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–51. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viedt C, Dechend R, Fei J, et al. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol. 2002;13:1534–47. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- 12.Hosaka K, Rojas K, Fazal HZ, et al. The monocyte chemotactic protein-1 (MCP-1)-interleukin-6 (IL-6)-osteopontin (OPN) pathway of intra-aneurysmal tissue healing. Stroke. 2017;48:1052–60. doi: 10.1161/STROKEAHA.116.015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–93. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126:2151–66. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Mordechai T, Holbova R, Landa-Rouben N, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Li J, Peng X, et al. Geraniin inhibits LPS-induced THP-1 macrophages switching to M1 phenotype via SOCS1/NF-κB pathway. Inflammation. 2016;39:1421–33. doi: 10.1007/s10753-016-0374-7. [DOI] [PubMed] [Google Scholar]
- 17.Geifman N, Rubin E. The mouse age phenome knowledgebase and disease-specific inter-species age mapping. PLoS ONE. 2013;8:e81114. doi: 10.1371/journal.pone.0081114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adibi A, Eesa M, Wong JH, et al. Combined endovascular coiling and intra-aneurysmal allogeneic mesenchymal stromal cell therapy for intracranial aneurysms in a rabbit model: a proof-of-concept study. J Neurointerv Surg. doi: 10.1136/neurintsurg-2016-012520. Published Online First: 7 Jul 2016. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Yang L, Chen Y, et al. Controlled release of osteopontin and interleukin-10 from modified endovascular coil promote cerebral aneurysm healing. J Neurol Sci. 2016;360:13–17. doi: 10.1016/j.jns.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Gao Y, Sun X, et al. Acceleration of aneurysm healing by P (DLLA-co-TMC)-coated coils enabling the controlled release of vascular endothelial growth factor. Biomed Mater. 2014;9:045004. doi: 10.1088/1748-6041/9/4/045004. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Lu Z, Chen C, et al. Mesenchymal stem cells and endothelial progenitor cells accelerate intra-aneurysmal tissue organization after treatment with SDF-1α-coated coils. Neurol Res. 2016;38:333–41. doi: 10.1080/01616412.2016.1164433. [DOI] [PubMed] [Google Scholar]
- 22.Sano H, Toda M, Sugihara T, et al. Coils coated with the cyclic peptide SEK-1005 accelerate intra-aneurysmal organization. Neurosurgery. 2010;67:984–91. doi: 10.1227/NEU.0b013e3181eb95da. discussion 92. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Kataoka H, Ishibashi R, et al. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40:942–51. doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- 24.Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–8. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinjikji W, Ding YH, Kallmes DF, et al. From bench to bedside: utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J Neurointerv Surg. 2016;8:521–5. doi: 10.1136/neurintsurg-2015-011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moftakhar R, Xu F, Aagaard-Kienitz B, et al. Preliminary in vivo evaluation of a novel intrasaccular cerebral aneurysm occlusion device. J Neurointerv Surg. 2015;7:584–90. doi: 10.1136/neurintsurg-2014-011179. [DOI] [PubMed] [Google Scholar]
- 27.Fahed R, Gentric JC, Salazkin I, et al. Flow diversion of bifurcation aneurysms is more effective when the jailed branch is occluded: an experimental study in a novel canine model. J Neurointerv Surg. 2017;9:311–15. doi: 10.1136/neurintsurg-2015-012240. [DOI] [PubMed] [Google Scholar]
- 28.Aquarius R, Smits D, Gounis MJ, et al. Flow diverter implantation in a rat model of sidewall aneurysm: a feasibility study. J Neurointerv Surg. 2017;2017 doi: 10.1136/neurintsurg-2016-012878. Published Online First: 8 Feb 2017. [DOI] [PubMed] [Google Scholar]
- 29.Greim-Kuczewski K, Berenstein A, Kis S, et al. Surgical technique for venous patch aneurysms with no neck in a rabbit model. J Neurointerv Surg. 2017;2017 doi: 10.1136/neurintsurg-2016-012955. Published Online First: 8 Feb 2017. [DOI] [PubMed] [Google Scholar]
- 30.Boileau X, Zeng H, Fahed R, et al. Bipolar radiofrequency ablation of aneurysm remnants after coil embolization can improve endovascular treatment of experimental bifurcation aneurysms. J Neurosurg. 2016:1–8. doi: 10.3171/2016.3.JNS152871. [DOI] [PubMed] [Google Scholar]


