ABSTRACT
Background
Fecal microbiota transplantation (FMT) is a highly effective therapy for recurrent Clostridium difficile infection (R-CDI). Despite its excellent efficacy, it is still not a routine procedure in most European centers. FMT has not been widely used in Spain to date. We describe our experience with FMT, including a novel approach based on oral fecal capsules.
Methods
We analyzed a prospectively recorded case series of patients with R-CDI treated with FMT at a single center (June 2014-July 2017). Primary outcome was defined as resolution of CDI without recurrence in a two-month period. FMT was administered via colonoscopy, nasojejunal tube, or oral capsules. All stool donors were rigorously screened.
Results
FMT was performed in 13 patients with R-CDI. Median age was 75.0 years and 76.9% were females. Six FMT were performed via nasojejunal tube, 5 via oral capsules, and 2 by colonoscopy. There were no procedure-related adverse events, except for bacteremia in one patient. During follow-up, R- CDI was observed in one patient at one month after FMT. The primary resolution rate was 83.3% and the overall resolution rate was 91.7%. FMT by capsules achieved a 100% resolution rate, colonoscopy 100%, and nasojejunal tube 80.0%.
Conclusions
In our cohort, FMT proved to be safe and effective, even in high risk patients. Oral administration in capsules also proved to be safe, well-tolerated, and highly effective for R-CDI. In our experience, the FMT capsule formulation seems feasible in the routine of a hospital. This administration method will allow FMT to be more widely used.
Keywords: C, difficile, fecal microbiota transplantation, bacteriotherapy, capsule, gut microbiota
RESUMEN
Introducción
El trasplante de microbiota fecal (TMF) es una terapia altamente efectiva para la infección recurrente por Clostridium difficile (R-ICD). A pesar de su excelente eficacia, todavía no es un procedimiento de rutina en la mayoría de los centros europeos. El TMF no ha sido ampliamente utilizado en España hasta la fecha. Describimos nuestra experiencia con TMF, incluida una aproximación novedosa basada en cápsulas orales fecales.
Métodos
Analizamos un registro prospectivo de casos de pacientes con R-ICD tratados con TMF en un solo centro (junio de 2014 a julio de 2017). La resolución primaria se definió como la resolución de la ICD sin recurrencia en un período de dos meses. TMF se administró mediante colonoscopia, sonda nasoyeyunal o cápsulas orales. Todos los donantes de heces fueron cribados rigurosamente.
Resultados
Se realizó TMF en 13 pacientes con R-CDI. La mediana de edad fue de 75,0 años y el 76,9% fueron mujeres. Se realizaron seis TMF mediante sonda nasoyeyunal, 5 mediante cápsulas orales y 2 mediante colonoscopia. No hubo eventos adversos relacionados con el procedimiento, a excepción de una bacteriemia en un paciente. Durante el seguimiento, se observó una R-ICD en un paciente al mes después del TMF. La tasa de resolución primaria fue del 83,3% y la tasa de resolución general fue del 91,7%. El TMF realizado mediante cápsulas alcanzó una tasa de resolución del 100%, por colonoscopia un 100% y por sonda nasoyeyunal un 80,0%.
Conclusiones
En nuestra cohorte, el TMF demostró ser seguro y efectivo, incluso en pacientes de alto riesgo. La administración oral en cápsulas también demostró ser segura, bien tolerada y altamente efectiva para la R-ICD. En nuestra experiencia, la formulación del TMF en cápsula parece factible en la rutina de un hospital. Este método de administración permitirá que el TMF sea más ampliamente utilizado.
Palabras clave: C, difficile, trasplante de microbiota fecal, bacterioterapia, cápsulas, microbiota intestinal
INTRODUCTION
Clostridium difficile infection (CDI) is the leading cause of hospital-acquired diarrhea in developed countries [1-4]. Metronidazole and vancomycin have been the primary treatment options in the management of CDI for the last few decades. Even though other antibiotics and monoclonal antibodies were recently approved for treatment of CDI [5, 6], the need for novel strategies is evident owing to therapeutic failure, vancomycin-resistant enterococci, high percentages of recurrences, and high costs in the case of newer drugs [5, 7].
Fecal microbiota transplantation (FMT) has proven to be a safe and effective treatment for refractory or relapsing CDI, although its use has been limited by practical barriers such as donor selection, product preparation, storage, delivery, and patient reluctance [8-10].
The methods of administration used, mainly colonoscopic delivery and nasoduodenal infusion are inconvenient both for patients and for healthcare facilities.This problem could be solved using a stable encapsulated preparation of standardized fecal microbiota that can be easily administered in daily clinical practice. In this sense, several groups have reported cases treated with encapsulated preparations that have proven as efficacious as other routes [11, 12].
Despite the enormous potential of FMT, its use in clinical practice continues to be very restricted in many European countries. FMT has not been widely used in Spain to date, with few published series [13]. Here, we describe our experience with FMT for treatment of recurrent CDI, including the novel approach based on orally administered fecal capsules.
MATERIAL AND METHODS
Setting, design, and study population. Our institution is a large teaching hospital with 1,550 beds. The clinical microbiology laboratory receives samples from patients hospitalized at our center and from all the outpatient institutions in our catchment area. Systematic testing for toxigenic C. difficile was performed on all diarrheic stool samples from patients aged >2 years.
Definitions. A CDI episode was defined as the presence of a positive result for toxigenic C. difficile testing and the presence of diarrhea (≥3 unformed stools in 24 hours) or colonoscopic findings demonstrating pseudomembranous colitis.
Severity of CDI was defined according to the guidelines of the Society of Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) [7].
An episode was considered a recurrence (R-CDI) if after recovery from a previous episode (at least 3 days without diarrhea and clinical improvement), symptoms returned and a stool sample separated from the former by between 15 and 60 days proved to be positive for toxigenic C. difficile. Episodes occurring more than 60 days after the previous one were not considered recurrences but new episodes.
Death was considered CDI-related when it was not attributable to other unrelated causes and occurred within 10 days of the CDI diagnosis and/or was due to well-known complications of CDI.
Primary resolution was defined as resolution of diarrhea without recurrence of CDI within 2 months after FMT; overall resolution was defined as resolution of diarrhea without recurrence of CDI within 2 months after a further FMT.
FMT procedure
Donor
A questionnaire based on the Food and Drug Administration (FDA) donor history questionnaire for blood and blood products was completed. Additional questionnaires were completed on gastrointestinal comorbidities (inflammatory bowel disease, irritable bowel syndrome, constipation or chronic diarrhea, history of major gastrointestinal surgery, gastrointestinal cancer, or polyposis) and factors that affect or may affect the composition of the intestinal microbiota (metabolic syndromes, systemic autoimmune diseases, atopic diseases [asthma, eczema, eosinophilic alterations of the digestive tract], and chronic pain syndromes). Donors were also asked about the recent intake of potent allergens when the donation was for a known recipient.
If donors passed these questionnaires, they were screened for human immunodeficiency virus (HIV), hepatitis A, human T-lymphotropic virus (HTLV) I/II, hepatitis B, hepatitis C, and syphilis in serum. Donor feces was screened for ova and parasites, Giardia antigen, C. difficile, Listeria, Vibrio, Campylobacter, Yersinia, Salmonella, Shigella, E. coli O157 H7 , Helicobacter pylori antigen , Rotavirus, Norovirus, Adenovirus, vancomycin-resistant Enterococcus, methicillin-resistant S. aureus, and extended-spectrum beta-lactamase–producing and carbapenem-resistant gram-negative microorganisms. Donors were reassessed for signs and symptoms of infectious diseases in the lapse between screening results and stool donation.
Stool processing for FMT
To be accepted for processing, stools had to be properly collected from suitable donors (preferably within <6 hours after evacuation, always within 24 hours of evacuation), not contaminated (with water, urine, or blood), and complete (acceptable minimum, 50 grams)
A fecal suspension was generated in saline without preservatives, using a commercial blender during the study period (2014-August 2016); a Stomacher 4000 was used to homogenize samples from September 2016 onward. The fecal suspension was filtered, centrifuged, and resuspended in concentrated form in sterile saline with 12.5% glycerol as cryoprotectant for frozen forms.
For the capsule formulation, the fecal suspension was further centrifuged to increase the concentration. The bacterial pellet was then resuspended with saline and 12.5% glycerol. The resulting suspension was double-encapsulated in hypromellose capsules (sizes 0/00, Capsugel, Cambridge, MA, USA) and stored at –80°C.
Recipient
Antibiotic treatment was discontinued at least 48 hours before the FMT procedure. On the day before the procedure, all patients received a light dinner and those receiving FMT via nasojejunal also received omeprazole 40 mg. After a 12-hour fast, the donor’s intestine was prepared with Bohm evacuating solution (4L Macrogol 4000). On the morning of the procedure in the case of nasojejunal delivery, an x-ray was performed to verify that the probe was correctly inserted and a further 40 mg of omeprazole was administered. A routine blood analysis was also performed on all cases the morning before the procedure.
After the procedure, a 4-6 hour fast was recommended. From 2017, blood cultures and blood analysis were performed systematically on the first and second day after the FMT procedure. Follow-up has been continuous since then.
Clinical data. The demographic data collected included age, sex, and ethnicity. Clinical data regarding the underlying condition were recorded using the McCabe and Jackson score for prognosis of underlying diseases; comorbidity was graded according to the Charlson index [14, 15]. As a referral center, some of the patients included in this case series received medical care and treatment at other medical facilities prior to receiving FMT at our institution. We made every effort to obtain the medical records and previous treatment courses.
The clinical data recorded for the CDI episodes were severity of the CDI episode, and antibiotic treatment for the CDI episode. Outcomes such as treatment failure, recurrence, mortality, CDI-related mortality, FMT, and FMT procedure–related mortality were also recorded.
Microbiome analysis. Selected samples from donors and recipients underwent microbiome analysis. Total DNA was extracted from fecal samples using the QIAamp DNA Stool mini kit (QIAGEN; Hilden, Germany) according to the manufacturer’s protocol and including a homogenization step. The sample was homogenized in FastPrep-24 (MPBio, California, USA) with Lysing Matrix E tubes (MPBio, California, USA) twice at 6.5 m/s for 45 seconds. The hypervariable V4 region of the 16s rRNA gene was amplified by PCR with 515-806 primers tailed with sequences to incorporate Illumina flow cell adapters and indexing barcodes.
Primer dimers and low-molecular-weight products were removed using Agencourt Ampure Beads (Beckman Coulter, California, USA), and samples were quantified and quality checked for amplicon size using the Agilent TapeStation (Agilent; California, USA). Amplicons were equimolar pooled and sequenced (2 × 250) on a Miseq platform (Illumina; California, USA) according to standard protocols. Mothur’s bioinformatic pipeline was followed for data analysis.
Data analysis. Data were analyzed using PASW Statistics for Windows, Version 18.0 (SPSS Inc, Illinois, USA). Qualitative variables appear with their frequency distribution. Quantitative variables are expressed as the median and interquartile range (IQR). Groups were compared using the Fisher exact test for categorical variables and the Mann-Whitney or t test for continuous variables. A p value <0.05 was considered significant.
Ethical Issues. This study was approved by the Ethics Committee of Hospital General Universitario Gregorio Marañón (number MICRO.HGUGM.2016-029). Informed consent for FMT was obtained from all patients as well as from donors. Since FMT policy and legislation differ from country to country, we consulted the Spanish Agency of Medicines and Medical Devices (AEMPS), which is the national authority for regulating pharmaceutical products. The AEMPS noted that FMT does not fall under the category of pharmaceutical products. The Spanish National Transplant Organization (ONT) was also consulted, and did not consider the procedure a tissue or organ transplant.
RESULTS
During the study period, the microbiology laboratory identified 1,447 patients with a CDI episode, which was recurrent in 236 (16.3%). Further recurrences were detected, as follows: 65 patients had 2 R-CDI episodes, 13 had 3 R-CDI episodes, 4 had 4 R-CDI episodes, 2 had 5 R-CDI episodes, and 1 had 6 R-CDI episodes. FMT for patients with R-CDI was performed in 13 cases: 9 from our center and 4 who were referred to us from other centers. Our FMT program was initially designed for patients with 3 or more R-CDI episodes until March of 2017, when we decided to include patients with 2 R-CDI episodes.
The demographic and clinical characteristics of the CDI patients who underwent FMT are shown in table 1. Most patients were female (76.9%), and the median age was 75.0 years. The most frequent underlying disease was malignancy (53.8%), followed by endocrine disease (38.5%). The median Charlson score was 3.0 (IQR 1.0-5.5).
Table 1.
Demographic and clinical characteristics of patients with Clostridium difficile infection treated with fecal microbiota transfer
| Patient number | Age | Sex | Referred patient | Underlying conditions categories | McCabe Jackson | Charlson Comorbidity Index | Number of R-CDI episodes |
|---|---|---|---|---|---|---|---|
| 1 | 84 | F | NO | Malignancy, cardiovascular, liver | 2 | 6 | 7 |
| 2 | 82 | M | NO | Malignancy, cardiovascular, respiratory, gastrointestinal, hematologic, rheumatologic, nephro-urologic | 3 | 7 | 3 |
| 3 | 93 | F | NO | Cardiovascular, endocrine, nephro-urologic | 3 | 2 | 4 |
| 4 | 58 | F | YES | Cardiovascular, gastrointestinal, liver | 3 | 2 | 3 |
| 5 | 75 | M | NO | Malignancy, nephro-urologic | 3 | 1 | 4 |
| 6 | 24 | M | NO | Gastrointestinal | 3 | 1 | 3 |
| 7 | 60 | F | NO | Malignancy, liver | 2 | 5 | 2 |
| 8 | 72 | F | NO | Malignancy, liver, endocrine | 2 | 5 | 4 |
| 9 | 70 | F | YES | Solid organ transplant, endocrine | 3 | 3 | 2 |
| 10 | 62 | F | YES | Malignancy | 3 | 6 | 3 |
| 11 | 89 | F | NO | Endocrine, metabolic | 3 | 1 | 2 |
| 12 | 90 | F | NO | Malignancy, respiratory, endocrine, nephro-urologic | 3 | 4 | 3 |
| 13 | 93 | F | YES | Rheumatologic | 3 | 0 | 2 |
R-CDI. Recurrent Clostridium difficile infection; F. female; M. male.
The median number of CDI previous recurrences was 3.0 (IQR 2.0-4.0). In patients that were not transferred from other centers, most of the initial CDI episodes were considered mild to moderate (66.7%), 22.2% were considered severe, and 11.1% severe complicated. Three cases were due to C. difficile ribotype 027. We found no significant differences with respect to primary resolution rates (p=0.464) or overall resolution rates (p=1.000) between episodes caused by C. difficile ribotype 027 and non-027 ribotypes.
As for previous treatments for CDI, the initial episodes were treated with metronidazole (53.8%), 2 were treated with a combination of metronidazole and vancomycin, 3 were treated with vancomycin taper, and 1 with vancomycin alone. The most frequent treatments for R-CDI were vancomycin taper and fidaxomicin. Antibiotic treatments for R-CDI episodes are summarized in table 2.
Table 2.
Patients’ antibiotic treatments for recurrent Clostridium difficile infection episodes previous to fecal microbiota transfer
| Patient number | 1st R-CDI treatment | 2nd R-CDI treatment | 3rd R-CDI treatment | 4th R-CDI treatment | 5th R-CDI treatment | 6th R-CDI treatment | 7th R-CDI treatment |
|---|---|---|---|---|---|---|---|
| 1 | Vancomycin | Vancomycin taper | Vancomycin taper | Fidaxomicin | Vancomycin taper | Metronidazole | Vancomycin taper |
| 2 | Vancomycin taper | Fidaxomicin | Vancomycin | ||||
| 3 | Vancomycin taper | Vancomycin taper | Fidaxomicin | Vancomycin | |||
| 4 | Metronidazole | Vancomycin | Fidaxomicin, vancomycin taper | ||||
| 5 | Vancomycin, vancomycin taper | Combined metronidazole and Vancomycin, Vancomycin taper | Fidaxomicin | Fidaxomicin, Tapered vancomycin | |||
| 6 | Vancomycin taper | Metronidazole, vancomycin taper | Fidaxomicin | ||||
| 7 | Fidaxomicin | Vancomycin taper | |||||
| 8 | Vancomycin taper | Fidaxomicin | Vancomycin taper | Vancomycin taper | |||
| 9 | Vancomycin taper | Fidaxomicin | |||||
| 10 | Vancomycin | Fidaxomicin | Metronidazole | ||||
| 11 | Fidaxomicin | Vancomycin taper | |||||
| 12 | Vancomycin | Fidaxomicin | Vancomycin taper | ||||
| 13 | Fidaxomicin | Vancomycin taper |
R-CDI. Recurrent Clostridium difficile infection. Metronidazole: Metronidazole 500mg/8 hours 10 days; Vancomycin: Vancomycin 125mg/6 hours 10days; Vancomycin taper: Vancomycin in tapered regimen for a minimum of 6-8 weeks; Fidaxomicin: Fidaxomicin 200mg/12hours 10 days
Donors were family members in 30.8% of cases and unrelated healthy donors from our stool bank in 69.2%. Two procedures (15.4%) were performed with fresh donations, and 84.6% were performed from frozen preparations. Data regarding the FMT specifications are shown in table 3. Six FMT were performed via nasojejunal tube, 5 were performed via oral capsules, and 2 were performed via colonoscopy.
Table 3.
Fecal microbiota procedure and patients’ outcome
| Patient number | Number of FMT | Administration | Fresh or frozen | Quantity | FMT- related complications | Primary outcome resolution | Secondary outcome (overall resolution) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | Nasojejunal | Fresh | 200ml | NA* | NA* | NA* |
| 2 | 1 | Nasojejunal | Fresh | 200ml | NO | YES | YES |
| 3 | 1 | Nasojejunal | Frozen | 200ml | NO | YES | YES |
| 4 | 1 | Nasojejunal | Frozen | 120ml | NO | YES | YES |
| 5 | 1 | Nasojejunal | Frozen | - | NO | YES | YES |
| 6 | 1 | Nasojejunal | Frozen | - | NO | NO | NO** |
| 7 | 1 | Colonoscopy | Frozen | 300ml | YES (E. coli bacteremia) | YES | YES |
| 8 | 1 | Colonoscopy | Frozen | 200ml | NO | YES | YES |
| 9 | 1 | Oral capsules | Frozen | 15 capsules/day; 2 days | NO | YES | YES |
| 10 | 1 | Oral capsules | Frozen | 15 capsules/day; 2 days | NO | YES | YES |
| 11 | 1 | Oral capsules | Frozen | 15 capsules/day; 2 days | NO | YES | YES |
| 12 | 2 | Oral capsules | Frozen | 15 capsules/day; 2 days | NO | NO | YES |
| 13 | 1 | Oral capsules | Frozen | 15 capsules/day; 2 days | NO | YES | YES |
NA*: Not applicable, the patient died of causes not related to FMT within a week of FMT, and outcome could not be assessed. NO**: The patient did not receive any additional transplants.
At the time of writing, the chart analysis revealed the median follow-up time to be 6 months (IQR 6-17 months). One of the patients died of causes not related to FMT within a week of treatment and was therefore not included in the analysis of FMT outcome since it was not possible to assess the outcome. The primary resolution rate was 83.3% (10/12). The 2 patients who were considered to have R-CDI or in whom R-CDI could not be ruled out developed diarrhea within 2 months after FMT. One had a toxigenic C. difficile–positive stool sample in the context of an underlying inflammatory bowel disease and was treated with vancomycin taper. In the other patient, vancomycin was initiated prior to obtaining a fecal sample for testing, an additional FMT was performed and no further recurrences were observed, yielding an overall resolution rate of 91.7%. FMT-related complications occurred in only 1 patient. In this patient, there were no complications in the following 48 hours after the fecal transplant. However, at 72 hours post-transplant, the patient presented with generalized abdominal pain and subsequently a febrile peak of 38ºC, blood cultures were extracted, which were positive for E. coli. The patient was successfully treated and was discharged on the day +10 post-transplant.
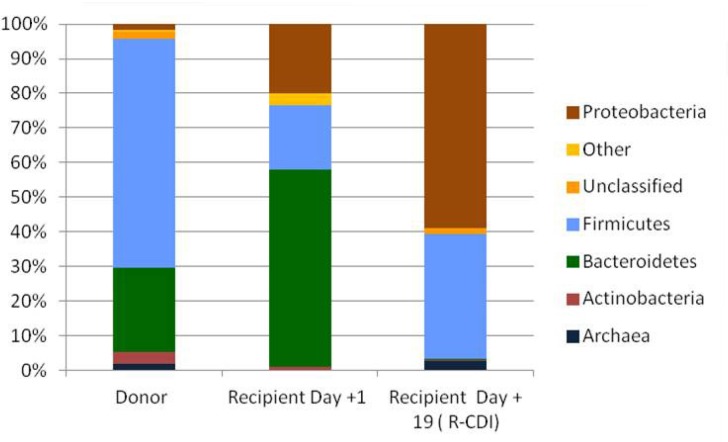
Figure 2 shows the results of the microbiome analysis that was performed in the donor stool and in the recipient’s stool (patient #12) soon after FMT (day +1), as well as in a stool sample after the patient developed diarrhea (day +19). As can be seen, patient #12 did not seem to have engrafted the donation, and the microbiota pattern was altered within the episode of R-CDI diarrhea, with an increase in the amount of Proteobacteria and a decrease in Bacteroidetes.
Figure 1.
Oral capsules for fecal transplant
Figure 2.
Microbiome analysis for patient #12 and its donor
DISCUSSION
We report our experience and results for 13 FMT-treated patients with significant comorbidities, 5 of whom were treated with a capsule formulation. Of the 13 patients with R-CDI, FMT was successful in 12 (91.7%). These results are similar to those found in the literature, with most studies reporting overall cure rates above 90% [16].
Most of the cases in the present study were performed with frozen material. Selecting frozen FMT is now a reasonable option owing to its convenience and data showing that this approach is as effective as fresh FMT. A recent meta-analysis showed that frozen FMT was as effective as fresh FMT, both for primary resolution and overall effective rate (95%) [17].
The few studies that describe FMT in the form of capsules [11, 18, 19] report an overall resolution rates above 90%. In our series, we observed a primary resolution rate of 80% and an overall resolution rate of 100% with frozen capsules. A very recent study by Kao et al. compared capsules with colonoscopic delivery, although no significant differences were observed between the groups, with a 96.2% resolution rate in both the capsule group (51/53) and the colonoscopy group (50/52). The FMT capsule delivery group had a better rating in terms of the pleasantness of the FMT procedure compared to colonoscopic delivery [12].
Few data have been reported on FMT in patients with significant comorbidities, as is the case with immunocompromised patients, and data on FMT performed on cirrhotic patients are even scarcer [20, 21]. Cirrhotic patients have been excluded from many studies owing to the suspected risk of infectious complications. Published data from case series to date suggest that FMT is safe and effective, even for immunocompromised patients [20, 22]. In our series, we included patients with significant underlying hematological diseases and cirrhotic patients. The outcome was generally favorable.
Although less is known about the application of FMT for the treatment of severe or complicated CDI, there is evidence supporting the use of FMT in these cases [23]. Lagier et al. [24] reported an outbreak of CDI due to ribotype 027 in Marseille that had been treated with early fecal transplantation. The authors observed that it was associated with a significantly reduced mortality rate [24]. In our series, we found no significant differences in outcome between episodes caused by ribotype 027 strains and ribotype non-027 strains with respect to the outcome of FMT, however we had a very limited number of cases due to ribotype 027.
Bacterial diversity among patients with recurrent CDI is noticeably lower than that of healthy subjects [25], with higher relative amounts of Proteobacteria and Firmicutes phyla, and lower relative abundance of Bacteroidetes [26]. Increased abundancy of Gammaproteobacteria has been associated with more recurrences [26-28]. We observed a similar situation in the patient who had R-CDI after 1 fecal transplant. The data from the microbiome analysis revealed that the recipient did not seem to have engrafted the donation and that the microbiota pattern was altered within the R-CDI diarrhea episode, with an increase in Proteobacteria to the detriment of Bacteroidetes.
Our study is limited by the fact that it is a single-center study with a limited number of patients. However, to the best of our knowledge, it is the first such report addressing the capsule FMT formulation in Spain.
In conclusion, in our cohort, FMT appeared to be a safe and effective treatment for R-CDI in patients with significant comorbidities. Delivery of FMT in capsules was as safe and effective as other FMT delivery methods and was well tolerated by patients. In our experience, it seems reasonable to include the FMT capsule formulation in the general hospital routine. Moreover, future directions for FMT should encompass the use of lyophilized FMT capsules that can be administered orally in an even more convenient therapeutic regimen.
ACKNOWLEDGMENTS
We thank Thomas O’Boyle for his help in the preparation of the manuscript, Raquel Navarro Rodríguez and Roberto Alonso for assisting in laboratory procedures, Viviana de Egea for clinical assistence, and Nuria Lozano for the bioinformatics analysis.
Some of the results of this study were previously presented in poster form at the “28th European Congress of Clinical Microbiology and Infectious Diseases” (21st-24th April, 2018; Madrid, Spain).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This study was financed by Fondo de Investigaciones Sanitarias (FIS), Research Project number PI13/00687 and PI16/00490, and by the European Regional Development Fund (FEDER) “A way of making Europe”.
REFERENCES
- 1.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012; 55 Suppl 2: S88-92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in europe: A systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012; 81: 1-14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Asensio A, Bouza E, Grau S, Rubio-Rodriguez D, Rubio-Terres C. [cost of clostridium difficile associated diarrhea in spain]. Rev Esp Salud Publica. 2013; 87: 25-33. doi: 10.4321/s1135-57272013000100004. [DOI] [PubMed] [Google Scholar]
- 4.Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M. Morbidity, mortality, and healthcare burden of nosocomial clostridium difficile-associated diarrhea in canadian hospitals. Infect Control Hosp Epidemiol. 2002; 23: 137-140. doi: 10.1086/502023. [DOI] [PubMed] [Google Scholar]
- 5.Debast SB, Bauer MP, Kuijper EJ. European society of clinical microbiology and infectious diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014; 20 Suppl 2: 1-26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017; 376: 305-317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of america (SHEA) and the infectious diseases society of america (IDSA). Infect Control Hosp Epidemiol. 2010; 31: 431-455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 8.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368: 407-415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 9.Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: A randomized, open-label, controlled pilot study. Clin Infect Dis. 2014; 58: 1515-1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moayyedi P, Yuan Y, Baharith H, Ford AC. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: A systematic review of randomised controlled trials. Med J Aust. 2017; 207: 166-172. [DOI] [PubMed] [Google Scholar]
- 11.Staley C, Hamilton MJ, Vaughn BP, Graiziger CT, Newman KM, Kabage AJ, et al. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017; 112: 940-947. doi: 10.1038/ajg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: A randomized clinical trial. JAMA. 2017; 318: 1985-1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Sanroman A, Rodriguez de Santiago E, Cobo Reinoso J, Del Campo Moreno R, Foruny Olcina JR, Garcia Fernandez S, et al. Results of the implementation of a multidisciplinary programme of faecal microbiota transplantation by colonoscopy for the treatment of recurrent Clostridium difficile infection. Gastroenterol Hepatol. 2017; 40: 605-614. doi: 10.1016/j.gastrohep.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40: 373-383. [DOI] [PubMed] [Google Scholar]
- 15.McCabe WR, Jackson G.G. Gram-negative bacteremia I. Etiology and ecology. Arch Intern Med. 1962; 110: 847–855. doi: 10.1001/archinte.1962.03620240029006. [DOI] [Google Scholar]
- 16.Chapman BC, Moore HB, Overbey DM, Morton AP, Harnke B, Gerich ME, et al. Fecal microbiota transplant in patients with Clostridium difficile infection: A systematic review. J Trauma Acute Care Surg. 2016; 81: 756-764. doi: 10.1097/ta.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 17.Tang G, Yin W, Liu W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory clostridium difficile infection: A meta-analysis? Diagn Microbiol Infect Dis. 2017; 88: 322-329. doi: 10.1016/j.diagmicrobio.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014; 312: 1772- 1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 19.Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, et al. Oral, frozen fecal microbiota transplant (fmt) capsules for recurrent Clostridium difficile infection. BMC Med. 2016; 14: 134. doi: 10.1186/s12916-016-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Bella S, Gouliouris T, Petrosillo N. Fecal microbiota transplantation (fmt) for Clostridium difficile infection: Focus on immunocompromised patients. J Infect Chemother. 2015; 21: 230-237. doi: 10.1016/j.jiac.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA et al. ,Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011; 9: 1044-1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman-Moraco RJ, Mehta AK, Lyon GM, Kraft CS. Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014; 14: 477- 480. doi: 10.1111/ajt.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beurden YH, Nieuwdorp M, van de Berg P, Mulder CJJ, Goorhuis A. Current challenges in the treatment of severe clostridium difficile infection: Early treatment potential of fecal microbiota transplantation. Therap Adv Gastroenterol. 2017; 10: 373-381. doi: 10.1177/1756283x17690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagier JC, Delord M, Million M, Parola P, Stein A, Brouqui P, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: A preliminary report. Eur J Clin Microbiol Infect Dis. 2015; 34: 1597-1601. doi: 10.1007/s10096-015-2394-x. [DOI] [PubMed] [Google Scholar]
- 25.Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013; 51: 2884-2892. doi: 10.1128/jcm.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert AM, Rogers MA, Ring C, Mogle J, Petrosino JP, Young VB, et al. Microbiome data distinguish patients with Clostridium difficile infection and non-c. Difficile-associated diarrhea from healthy controls. MBio. 2014; 5: e01021-01014. doi: 10.1128/mBio.01021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allegretti JR, Kearney S, Li N, Bogart E, Bullock K, Gerber GK, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther. 2016; 43: 1142-1153. doi: 10.1111/apt.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna S, Montassier E, Schmidt B, Patel R, Knights D, Pardi DS, et al. Gut microbiome predictors of treatment response and recurrence in primary clostridium difficile infection. Aliment Pharmacol Ther. 2016; 44: 715-727. doi: 10.1111/apt.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]