Abstract
Background:
Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACE-I/ARB) improve outcomes in patients with heart failure and reduced left-ventricular (LV) systolic function. However, these medications can cause a rise in serum creatinine and their benefits in patients with HF accompanied by kidney disease are less certain.
Objective:
To characterize associations between estimated glomerular filtration rate (eGFR), patterns of ACE-Is and ARBs use, and 1-year survival following hospitalization for heart failure (HF).
Design:
We formed a retrospective cohort study of patients admitted with HF and followed HF medication prescriptions using the pharmaceutical information network, stratified by discharge eGFR.
Setting:
Cardiology services in 3 centers in Southern Alberta, Canada.
Patients:
The study cohort included patients admitted to hospital with a clinical diagnosis of HF.
Measurements:
eGFR was determined from inpatient laboratory data prior to discharge. Outpatient prescription data prior to and following the index hospitalization was obtained using the Pharmaceutical Information Network of Alberta and survival was determined from provincial vital statistics.
Methods:
Characteristics of the HF cohort were obtained from the Admissions Module of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) database. Multivariable Cox proportional hazards models were used to evaluate the association between time-varying ACE-I/ARB use, and mortality, and to test whether eGFR modified this association.
Results:
Totally, 1404 patients were included. Within the first 3 months following discharge, ACE-I/ARBs were used in 71%, 67%, 62%, and 52% for those with eGFR > 90, 45-89, 30-44, and < 30 mL/min/1.73 m2, respectively, with differences in use persisting after 1 year of follow-up. Patients with eGFR < 45 mL/min/1.73 m2 had significantly lower rates of ACE-I/ARB use following hospitalization. In adjusted models, ACE-I/ARB use following discharge was associated with 25% lower risk of mortality (Hazard Ratio [HR]: 0.75, 95% confidence interval [CI]: 0.61-0.92; P < 0.01), without evidence that this association differed by eGFR (P = 0.75).
Limitations:
LV function measurements were not available for the cohort. Due to the observation design of the study, treatment-selection bias may be present.
Conclusion:
Patients with HF and reduced eGFR at time of hospital discharge were less likely to receive ACE-I/ARB despite these medications being associated with lower mortality independent of eGFR. These findings demonstrate the need for further research on strategies for safe use of ACE-I and ARB in patients with HF and kidney disease.
Keywords: heart failure, kidney disease, ACE inhibitors, angiotensin receptor blockers
Abrégé
Contexte:
Les inhibiteurs de l’enzyme de conversion de l’angiotensine (IECA) et les antagonistes des récepteurs de l’angiotensine (ARA) améliorent les résultats des patients atteints d’insuffisance cardiaque (IC) et d’une fonction systolique réduite du ventricule gauche. Ces médicaments peuvent cependant provoquer une hausse de la créatinine sérique et leurs bienfaits pour les patients atteints d’IC et de néphropathie sont plus incertains.
Objectif:
L’étude visait à caractériser l’association entre le débit de filtration glomérulaire estimé (DFGe), les schémas d’utilisation des IECA/ARA, et la survie sur un an à la suite d’une hospitalisation pour IC.
Conception de l’étude:
Nous avons procédé à une étude de cohorte rétrospective à partir des données du réseau d’information pharmaceutique. La cohorte était constituée de patients admis pour IC et ayant suivi un traitement pour cette affection. La cohorte a été stratifiée sur la base du DFGe des patients à leur sortie de l’hôpital.
Cadre:
Le département de cardiologie de trois centres hospitaliers du sud de l’Alberta (Canada).
Sujets:
La cohorte était constituée de patients admis à la suite d’un diagnostic d’IC.
Mesures:
Le DFGe a été déterminé en consultant les résultats de laboratoire des patients hospitalisés avant leur départ. L’information sur les prescriptions avant et après l’hospitalisation a été obtenue grâce au réseau d’information pharmaceutique de l’Alberta, et le taux de survie a été déterminé à l’aide des statistiques de vie de la province.
Méthodologie:
Les caractéristiques des patients ont été obtenues grâce au module d’admission de la base de données APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease). Des modèles multivariés des risques proportionnels de Cox ont été employés pour évaluer l’association entre l’utilisation variable des IECA/ARA dans le temps et le taux de mortalité; de même que pour vérifier si le DFGe avait une incidence sur cette association.
Résultats:
Au total, 1404 patients ont été inclus à l’étude. Dans les trois mois suivant l’hospitalisation, les taux de prescriptions des IECA/ARA variaient entre les différentes strates de DFGe de la cohorte et s’établissaient à 71% (DFGe > 90 mL/min/1.73 m2), 67% (DFGe entre 45 et 89 mL/min/1.73 m2), 62% (DFGe entre 30 et 44 mL/min/1.73 m2), et 52% (DFGe < 30 mL/min/1.73 m2); et ces différences ont persisté après un an de suivi. Les patients dont le DFGe était inférieur à 45 mL/min/1.73 m2 présentaient des taux d’utilisation des IECA/ARA significativement inférieurs après leur séjour à l’hôpital. Dans les modèles ajustés, l’utilisation des IECA/ARA à la sortie de l’hôpital a été associée à un risque inférieur de 25% de la mortalité (RR: 0.75; IC 95%: 0.61-0.92; P < .01), sans preuve que cette association diffère selon le DFGe (P = .75).
Limites:
Les mesures de la fonction ventriculaire gauche n’étaient pas disponibles pour la cohorte. De plus, en raison de sa nature observationnelle, l’étude pourrait comporter des biais relatifs au choix du traitement.
Conclusion:
Les patients atteints d’IC et dont le DFGe était faible au moment du congé étaient moins susceptibles de se voir prescrire des IECA/ARA, bien que ces médicaments soient associés à de plus faibles taux de mortalité indépendamment de la valeur du DFGe. Ces résultats démontrent la nécessité de poursuivre la recherche de stratégies permettant une utilisation sûre des IECA/ARA chez les patients atteints de néphropathie et d’insuffisance cardiaque.
What was known before
Patients with heart failure often also have kidney disease. Many large trials of pharmacotherapies for heart failure, including those for ACE-I and ARB, did not include patients with significant kidney dysfunction and so use of these medications in this population has remained controversial.
What this adds
In this observational study, the use of ACE-I or ARB was significantly lower in patients with reduced kidney function after a recent hospitalization for heart failure. However, ACE-I or ARB use was associated with a 25% lower adjusted relative risk of 1-year mortality, and this association was consistently observed across all levels of kidney function.
Introduction
Heart failure (HF) is one of the most common cardiovascular syndromes, with a prevalence of approximately 2% in North American adults older than 45 years of age, and a lifetime risk of over 20%.1 HF is characterized by periodic exacerbations with nearly 1 million hospitalizations for HF in the United States each year.1 HF is associated with significant mortality, with survival estimates of 50% and 10% at 5 and 10 years, respectively. The use of evidence-based pharmacotherapy is crucial to improve the outcomes and costs of caring for HF.2 There is strong evidence that Angiotensin converting enzyme inhibitors (ACE-Is)3,4 or angiotensin receptor blockers (ARBs)5 improve survival in patients with HF with reduced left-ventricular ejection fraction (LVEF).
Reduced kidney function is prevalent in over half of patients with HF and is an independent risk factor for hospitalization, and mortality.6,7 However, the optimal management of patients with coexisting kidney disease is controversial because most trials of ACE-I and ARBs excluded patients with moderate to severely reduced kidney function. Furthermore, worsening renal function accompanying HF exacerbations may lead physicians to avoid these medications. There is little information on contemporary patterns of ACE-I/ARB use and outcomes in patients with HF according to kidney function.2
We examined pharmacy-prescribing data to characterize patterns of ACE-I/ARB use according to kidney function in a cohort of patients recently hospitalized for HF. We also examined the relationship between ACE-I/ARB use and survival following a HF hospitalization and explored whether this association differed by the level of discharge kidney function.
Methods
Study Population
We formed our study cohort from the Cardiac Admissions Module of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) database. The Admissions Module of APPROACH prospectively collects demographics, clinical data, comorbidities, treatment information including admission and discharge medications, and vital statistics from patients hospitalized under the care of cardiology services in 3 centers in Southern Alberta, Canada.8 Our cohort included adults admitted between January 1, 2008, and September 31, 2012, with a clinical diagnosis of HF at time of discharge identified by the attending physician. To be included in the cohort, patients required a serum creatinine before or during their admission. Patients who died during their index hospitalization or were discharged to an extended care facility were excluded.
Measurements of Kidney Function
We obtained serum creatinine measurements from the Alberta Health Services provincial laboratory data repository. The last creatinine prior to discharge was used to estimate Glomerular Filtration Rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).9 Patients were stratified based on Kidney Disease Improving Global outcomes (KDIGO) eGFR categories for kidney disease; eGFR ⩾ 90, 60-89, 45-59, 30-44, and < 30 mL/min/1.73 m2.
Measurement of Medication Prescriptions
We obtained outpatient prescription drug data prior to and following the index hospitalization using the Pharmaceutical Information Network (PIN) for the province of Alberta. Alberta’s community pharmacies are mandated to contribute their drug dispensing data to the PIN, and approximately 96% of the drug dispensations from community pharmacies are available in this system. Medications used during hospital admission were obtained from the APPROACH Admissions Module. We categorized individual drug records into classes: ACE-I/ARBs, beta-blockers, loop-diuretics, and mineralocorticoid receptor antagonists (MRA). We characterized medication use within fixed time periods relative to the index HF hospitalization, including within the year prior to admission, during hospitalization, 1 month, 3 months, and 1-year post discharge based on one or more records of dispensing within these time periods.
Statistical Analyses
Continuous variables were summarized as median and interquartile range. Binary variables were summarized as absolute number and proportion. We compared participant characteristics based on discharge eGFR using analysis of variance (ANOVA) for continuous variables and a chi-squared test for categorical variables. We also used chi-squared tests to examine differences in the proportion of patients using ACE-I/ARB or other cardiovascular medications according to eGFR category within each of the specified time periods.
Patients were followed from their date of hospital discharge until the study end date (April 30, 2014) or death. We examined the associations between ACE-I/ARB use and mortality after hospital discharge using Cox-proportional hazards models, with adjustment for age, sex, eGFR, BMI, smoking status and comorbidities including diabetes mellitus, hypertension, hyperlipidemia, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, cerebrovascular disease, liver disease, gastrointestinal disease, malignancy, psychiatric disorder, alcohol abuse, beta-blocker, loop-diuretic, and spironolactone use. These models incorporated ACE-I/ARB exposure as a time-varying exposure, in which time at risk prior to the first identified ACE/ARB prescription was attributed to untreated status and time following ACE-I/ARB prescription was attributed to treated status. We tested interactions between ACE-I/ARB use and eGFR category and used the model including the interaction terms to obtain stratum specific estimates by eGFR category. The proportional hazards assumption was tested using Schoenfeld residuals and was satisfied for all models. We performed all analyses using SAS (version 9.3; SAS Institute Inc., Cary, NC). The Conjoint Health Ethics Research Board at the University of Calgary approved this study.
Results
Cohort Formation and Patient Population
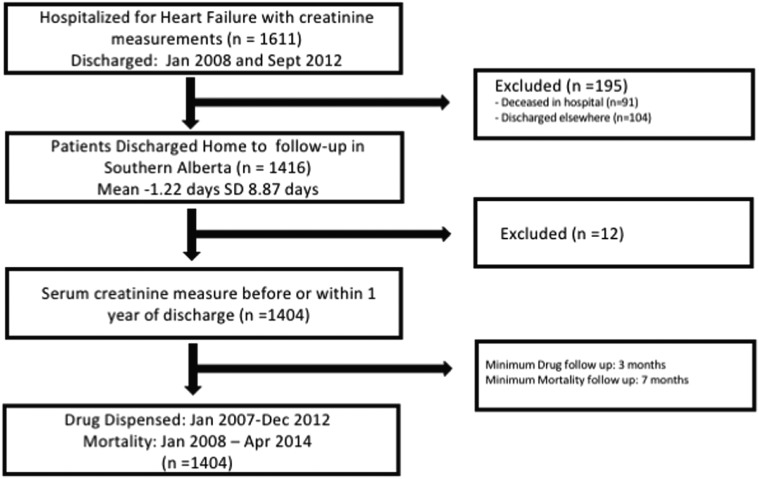
We identified 1611 hospitalized patients from the APPROACH database with a discharge diagnosis of HF and a serum creatinine measurement within the cohort entry period. After excluding 91 patients who died during their index hospitalization and 104 patients who were discharged to an extended care facility, there were 1404 patients remaining in the final cohort (Figure 1).
Figure 1.
Formation of the Southern Alberta cohort hospitalized with heart failure.
The median (interquartile range) duration between discharge date and the day of the last serum creatinine measurement used to define discharge eGFR was 0 (2.0) days. Table 1 shows the baseline characteristics of the cohort by discharge eGFR. Patients were predominately male in all eGFR categories, with male prevalence ranging from 57% to 68%. Cardiac risk factors (including older age, hypertension, diabetes and dyslipidemia) as well as established vascular disease (cerebrovascular disease and prior myocardial infarction) were more prevalent with lower eGFR.
Table 1.
Baseline Characteristics of the Southern Alberta Cohort Hospitalized With Heart Failure, According to Kidney Function at Time of Discharge.
| Baseline characteristics | eGFR ⩾ 90 (N = 141) | 60 < eGFR < 90 (N = 468) | 45 < eGFR < 60 (N = 329) | 30 < eGFR < 45 (N = 254) | eGFR < 30 (N = 212) | P value | Overall (N = 1404) |
|---|---|---|---|---|---|---|---|
| Sex (N(%), male) | 94 (66.67) | 319 (68.16) | 205 (62.31) | 146 (57.48) | 123 (58.02) | .019 | 887 (63.18) |
| Median age (IQR) | 58.53 (50.34, 64.46) | 68.34 (59.89, 77.85) | 75.15 (67.39, 82.01) | 79.57 (72.14, 84.07) | 76 .91 (67.88, 82.81) | <.0001 | 72.86 (62.56, 80.72) |
| eGFR (median, IQR) | 97.97 (93.67, 105.04) | 72.64 (65.98, 81.53) | 52.53 (49.22, 55.92) | 38.40 (34.59, 41.67) | 21.96 (15.72, 26.63) | <.0001 | 55.35 (39.26, 74.21) |
| Diabetes (N(%) | 44 (31.21) | 131 (27.99) | 118 (35.87) | 115 (45.28) | 124 (58.49) | <.0001 | 532 (37.89) |
| Hypertension | 68 (48.23) | 280 (59.83) | 233 (70.82) | 176 (69.29) | 181 (85.38) | <.0001 | 938 (66.81) |
| Death during follow-up | 34 (24.11) | 140 (29.91) | 140 (42.55) | 146 (57.48) | 132 (62.26) | <.0001 | 592 (42.17) |
| Blood pressure (mean, SD) (mmHG) | 1.63 (1.49, 1.80) | 1.63 (1.48, 1.85) | 1.75 (1.57, 2.00) | 1.78 (1.61, 2.05) | 1.83 (1.56, 2.11) | 1.71 (1.51, 1.96) | |
| Heart rate (mean, SD) (beats/min) | 96 (76, 113) | 91 (74, 110) | 84 (69, 100) | 79 (68, 96) | 77 (65.0) | <.0001 | 89.27 (25.93) |
| Hyperlipidemia | 79 (56.03) | 256 (54.70) | 185 (56.23) | 151 (59.45) | 149 (70.28) | .003 | 820 (58.40) |
| BMI (Mean, SD) | 28.96 (24.30, 35.99) | 28.40 (24.90, 32.98) | 28.41 (25.39, 33.91) | 27.41 (23.50, 31.65) | 27.68 (24.49, 32.46) | .11 | 30.05 (13.90) |
| Liver disease | 5 (3.55) | 7 (1.50) | 9 (2.74) | 1 (0.39) | 7 (3.30) | .09 | 29 (2.07) |
| GI disease | 4 (2.84) | 38 (8.12) | 34 (10.33) | 36 (14.17) | 34 (16.04) | .0002 | 146 (10.40) |
| Smoking | <.001 | ||||||
| Unknown | 25 (17.73) | 116 (24.79) | 99 (30.09) | 58 (22.83) | 42 (19.81) | 340 (24.22) | |
| Never | 36 (25.53) | 93 (19.87) | 73 (22.19) | 79 (31.10) | 67 (31.60) | 348 (24.79) | |
| Current | 42 (29.79) | 91 (19.44) | 35 (10.64) | 24 (9.45) | 23 (10.85) | 215 (15.31) | |
| Former | 38 (26.95) | 168 (35.90) | 122 (37.08) | 93 (36.61) | 80 (37.74) | 501 (35.68) | |
| Prior Infarction | 28 (10.04) | 111 (23.72) | 91 (27.66) | 104 (40.94) | 88 (41.51) | <.0001 | 422 (30.06) |
| Prior PCI | 20 (14.18) | 73 (15.60) | 58 (17.63) | 72 (28.35) | 65 (30.66) | <.0001 | 288 (20.51) |
| Prior CABG | 12 (8.51) | 68 (14.53) | 64 (19.45) | 58 (22/83) | 50 (22.58) | .0003 | 252 (17.95) |
| Peripheral Vascular | 2 (1.42) | 23 (4.91) | 25 (7.60) | 16 (6.30) | 23 (10.85) | .004 | 89 (6.34) |
| Cerebrovascular | 4 (2.84) | 43 (9.19) | 33 (10.03) | 32 (12.60) | 32 (15.09) | .003 | 144 (10.26) |
| Malignancy | 9 (6.38) | 23 (4.91) | 16 (4.86) | 23 (9.06) | 13 (6.13) | .019 | 84 (5.98) |
| Psychiatric history | 8 (5.67) | 12 (2.56) | 12 (3.65) | 9 (3.54) | 10 (4.72) | .417 | 51 (3.63) |
| History of alcoholism | 17 (12.06) | 36 (7.69) | 12 (3.65) | 3 (1.18) | 11 (5.19) | <.0001 | 79 (5.63 |
Note. eGFR = estimated glomerular filtration rate; BMI = body mass index; IQR = interquartile range; CABG = Coronary artery bypass grafting; GI = gastrointestinal; PCI = Percutaneous Coronary Intervention.
Patterns of ACE-I/ARB Use According to Kidney Function
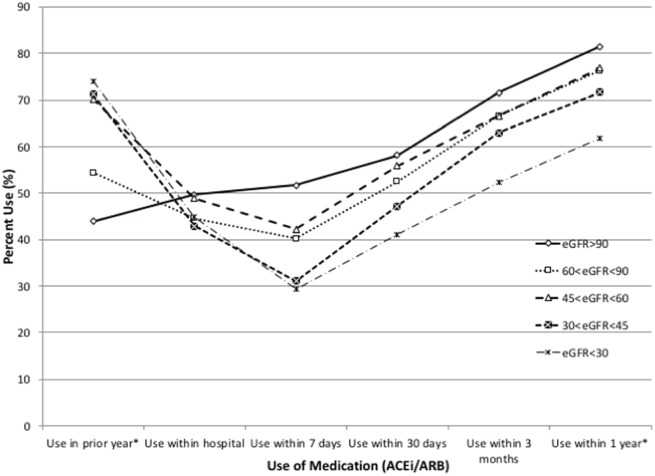
Patients in all eGFR categories showed increasing ACE-I/ARB use over time following hospital discharge; however, the proportion of patients using an ACE-I/ARB by significantly differed by eGFR at 30 days (P = .002), 3 months (P < .001), and 1 year (P < .001), and compared to those with eGFR > 90 mL/min/1.73 m2, was lower for those with eGFR 30-44 and < 30 mL/min/1.73 m2 (Figure 2). The proportion receiving an ACE-I/ARB over 1 year after hospital discharge exceeded 70% and met or exceeded the proportion of use before the index hospitalization for each of the groups with eGFR > 30ml/min/1.73 m2, while for those with eGFR < 30 mL/min/1.73 m2, the proportion using an ACE-I/ARB declined significantly from 74.0% in the year before admission, to 61.8% at 1 year after discharge (P < .0001).
Figure 2.
Percentage of patients in the Southern Alberta cohort hospitalized with heart failure receiving an ACE-I/ARB at varying times before and after hospital admission, according to discharge eGFR.
Note. ACE-I = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blockers; eGFR = estimated glomerular filtration rate.
Comparisons With Other Cardiovascular Medication Use
Contrary to the pattern seen with ACE-I/ARB, beta-blocker use following hospital discharge did not significantly differ by eGFR level at 30 days, 3 months, or 1 year following hospital discharge. However, loop-diuretic use significantly differed at 3 months (P = .009) and 1 year (P = .002), and was higher among patients with eGFR 30-44 and < 30 than those with eGFR > 90 mL/min/1.73 m2 (Supplementary Figures 1-3). Similar to the pattern seen with ACE-I/ARB, use of MRA significantly differed at 30 days (P < .0001), 3 months (P = .002), and 1 year (P < .0001), and was lowest among patients with eGFR <30 mL/min/1.73 m2(Supplementary Figure 2).
Relationship between ACE-I/ARB use and mortality
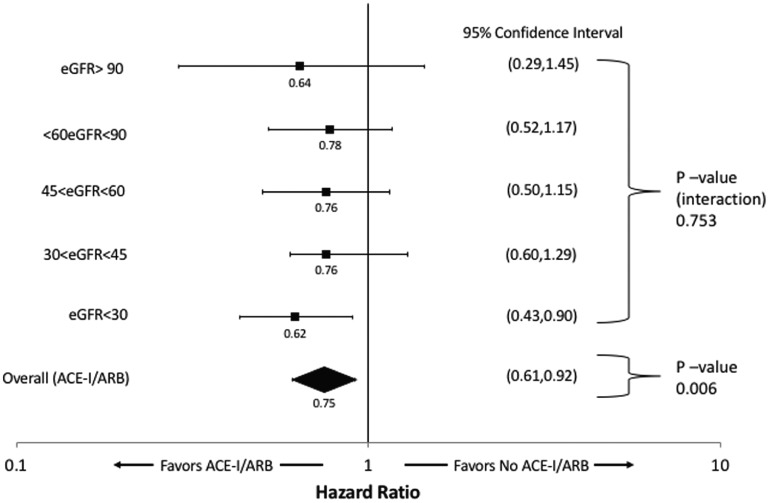
Over a median follow-up of 2.2 years, 465 (33.1%) patients in the cohort died. In unadjusted Cox-proportional hazards models accounting for time-varying medication exposure, lower levels of discharge eGFR were associated with incrementally higher mortality (Table 2), while ACE-I/ARB use was associated with a 27% lower risk of death (Hazard Ratio [HR] of 0.73, 95% Confidence Interval [CI]: 0.60-0.88, P = .009). ACE-I/ARB use remained associated with a 25% lower risk of death in the multivariable Cox proportional hazards model accounting for time-varying medication exposure (adjusted HR 0.75, 95% CI: 0.61,-0.91, P = .006). There was no significant difference in the association between ACE-I/ARB use and mortality at different levels of discharge eGFR (p-interaction 0.753). The overall and eGFR stratum specific estimates are shown in Figure 3.
Table 2.
Associations between level of discharge eGFR and mortality for the Southern Alberta cohort hospitalized with heart failure.
| Variable | Adjusted hazard ratio (95% CI) | P value | Unadjusted hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| GFR⩾90 | 1.30 (0.88-1.91) | .201 | 0.83 (0.57-1.21) | .327 |
| 60<eGFR<90 | Ref | — | — | — |
| 45<eGFR<60 | 1.21 (0.95-1.53) | .123 | 1.46 (1.15-1.21) | .002 |
| 30<eGFR<45 | 1.49 (1.16-1.91) | .002 | 2.29 (1.81-2.89) | <.001 |
| eGFR<30 | 2.01 (1.54-2.61) | <.0001 | 2.69 (2.11-3.42) | <.001 |
Note. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate.
Figure 3.
Adjusted hazard ratios with 95% confidence intervals for the association between ACE-I/ARB use and all-cause mortality in Southern Alberta cohort hospitalized with heart failure, stratified by discharge eGFR, and for the overall cohort.
Note. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate.
Discussion
In this cohort study, we found that patients with HF and all levels of kidney function had an increase in use of ACE-I/ARB in the year after hospital discharge, with the proportion exceeding 70% at 1 year. However, we also observed that reduced kidney function was associated with significantly lower use of ACE-I and ARB, which persisted over that time frame. This pattern significantly differed from that of beta-blocker use. Furthermore, we observed a 25% lower adjusted relative risk of 1-year mortality associated with ACE-I or ARB use among patients with HF, with no difference in the association at different levels of discharge eGFR.
Our finding that ACE-I/ARB use is associated with improved survival in HF is similar to findings from other observational studies and clinical trials. The landmark studies of Left Ventricular Dysfunction10 (SOLVD) and the Survival And Ventricular Enlargement11 (SAVE) demonstrated 4.5% and 14% absolute reductions in all-cause death with ACE-I/ARB use in systolic HF. Subsequent subgroup analysis of the SOLVD trial found that enalapril remained associated with reduced all-cause mortality among patients with moderate and severe renal dysfunction without adverse kidney effects, and no statistical interaction between eGFR level and enalapril treatment was found in the trial.12 Similarly in the CHARM-Alternative trial, ARB treatment lead to a 23% relative risk reduction for cardiovascular death or hospital admission for HF in patients with systolic dysfunction intolerant of ACE-I.13
Our findings are similar to the results of other cohort studies showing improved outcomes with the use of ACE-I/ARB in patients with HF and moderate to severe CKD.14-18 A retrospective analysis of the Minnesota Heart Survey reported lower mortality for patients treated with ACE-I or ARBs across all stages of CKD, excluding patients receiving dialysis.19 Moreover a large retrospective cohort study evaluating elderly patients with LVEF <40% found that prescription of ACE-I upon hospital discharge was associated with the largest reduction in mortality among patients with the most severe renal dysfunction (creatinine > 265 μmol/L).14 Similar findings have also been observed in the subspecialty HF care setting in Canada, including an Alberta heart function clinic.20 Most recently, data from the Swedish Heart Failure Registry also reported increased mortality in patients with lower eGFR, but lower relative risks of death in those who received target doses of guideline-directed HF medical therapy including ACE-I, ARB, beta-blockers (BB), and mineralocorticoids receptor antagonists (MRA).21-23
Patients with kidney disease have been frequently excluded from large multicenter cardiovascular therapeutics trials, with one review noting their exclusion from 56% of major clinical trials. Moreover, only 5% of original trial publications reported the proportion of patients with CKD and 10% reported baseline renal function, further raising uncertainty about the generalizability of findings to patients with CKD.24 Our findings add to the current literature, by quantifying the current disparity in use of ACE-I/ARB in HF patients with reduced kidney function and by demonstrating similar associations with improved survival in these patients.
The driver for disparity in use of ACE-I/ARB that we observed in patients with HF and kidney disease is uncertain. The difference may be attributable to clinicians’ concerns regarding the applicability of current evidence to the CKD and HF population since previous large trials excluded patients with severe renal dysfunction and existing recommendations have been based on secondary analyses of these trials.20 Furthermore, ACE-I/ARB avoidance may result from apparent safety concerns such as hyperkalemia, hypotension, or a rise in serum creatinine, which frequently accompany decompensated HF.25,26 However, small rises in serum creatinine in response to ACE-I/ARB have been associated with long-term preservation of kidney function in CKD, and a lack of prognostic significance in HF as they may occur due to functional effects on eGFR without evidence of structural kidney injury.27-29 Hyperkalemia is a justifiable safety concern with ACE-I/ARB use, as ACE-I/ARB are the most common medication associated with hyperkalemia, leading to discontinuation or dose reduction in 29% of patients.30 However, elevations of serum potassium were usually modest (<1 mEq/L) with life threatening hyperkalemia occurring rarely. Higher baseline serum potassium, renal dysfunction, and use of multiple RAAS antagonists have been associated with a higher risk of hyperkalemia, suggesting that strategies to predict the risks of clinically significant hyperkalemia are possible.20,31 Furthermore, randomized controlled trials of ACE-I/ARB in patients with early stage and advanced proteinuric CKD have demonstrated that these agents can be used effectively and safety in most patients across the stages of CKD.32,33
Strengths of our study include the detailed characterization of a large cohort of HF patients combined with prospectively ascertained clinical, laboratory, and medication information. Additionally, we captured dispensed prescriptions as opposed to medications reported by patients or on discharge summaries which may not reflect community prescriptions. However, there are several important limitations to our study. First, due to its observational design, our study is susceptive to treatment-selection bias, whereby patients at highest risk for poor outcomes may not receive ACE-I/ARB. However, the relationship between treatment and outcomes that we observed is in keeping with benefits reported from randomized trials. Second, we characterized patients based on discharge eGFR, which did not allow us to identify contributions due to chronic kidney disease versus acute kidney injury. However, fluctuations in kidney function are common in the setting of HF, and use of the most recent measure of kidney function is relevant to clinical decision making about outpatient medication prescribing upon hospital discharge. Third, we did not have reliable data on LV function and this was excluded from our analysis. Inclusion in our cohort did not depend of LVEF measurement, and therefore our study includes patients with Heart Failure with Reduced Ejection Fraction (HFrEF) and Heart Failure with preserved Ejection Fraction (HFpEF). Although there is stronger evidence for the use of ACE-I and ARB in HFrEF than HFpEF, benefits of ACE-I/ARB therapy in HFpEF include lower risk of hospitalization observed in the Candesartan in Heart Failure Reduction in Mortality CHARM-Preserved trial34 and the Perindopril in Elderly People with Chronic Heart Failure Study.35 Fourth, we did not have information surrounding the cause of HF exacerbation.
Importantly, we lacked information on adverse medication safety events such as hyperkalemia, acute kidney injury, and hypotension, which may have contributed to lower use of ACE-I/ARB in participants with reduced kidney function. Observational studies have demonstrated an increasing incidence of hyperkalemia with ACE-I/ARB in CKD; however, there has been wide variation in estimates of frequency, with incidence as low as 2.8% in some studies36 and as high as 51% in other studies of patients with stage G5 CKD.37 In the largest recent observational study the risk of hyperkalemia (serum potassium ⩾ 5.5mM) was 7.7% in patients with CKD treated with an ACE-I or ARB.38 Recommendations in guidelines for HF highlight that hyperkalemia may prompt a switch from ACE-I/ARB therapy to other vasodilators when persistent hyperkalemia despite dietary intervention, dosage reduction, and removal of other agents known to increase serum potassium.39 Worsening kidney function may also prompt a switch from ACE-I/ARB therapy to other agents when reduced kidney function persists despite modification of dose, rechallenge, and removal of other potential nephrotoxic agents, or when there is a concern it may precipitate need for renal replacement therapy. These issues likely contribute to the lower us of ACE-I and ARB in patients with moderate to severely reduced kidney function in our study.
In conclusion, we identified lower use ACE-I and ARB after hospitalization with HF in patients with moderate to severely reduced kidney function. Nonetheless, we observed lower mortality among patients who received ACE-I and ARBs with no evidence of a significant modification of this associations across different eGFR strata. Further research is needed to develop and test strategies to support the safe use of ACE-I and ARB in patients with HF and kidney disease.
Clinical Perspectives
Patients with HF often develop coexisting kidney disease. Many large trials of pharmacotherapies for HF, including those for ACE-I and ARB, did not include patients with significant renal dysfunction and so use of these medications in this population has remained controversial. In this observational study, the use of ACE-I or ARB was significantly lower in patients with reduced kidney function after a recent hospitalization for HF. However, ACE-I or ARB use was associated with a 25% lower adjusted relative risk of 1-year mortality, and this association was consistently observed across all levels of kidney function.
Translational Outlook
Current prescribing patterns and outcomes associated with ACE-I/ARB use point to the need for further research on strategies for safe use of ACE-I and ARB in patients with HF and advanced coexisting kidney disease.
Supplemental Material
Supplemental material, Supplementary_Figure_1,2_and_3_(1) for Kidney Function, ACE-Inhibitor/Angiotensin Receptor Blocker Use, and Survival Following Hospitalization for Heart Failure: A Cohort Study by Michael H. Chiu, Robert J. H. Miller, Rebecca Barry, Bing Li, Bryan J. Har, Stephen B. Wilton, Merril Knudtson, Jonathan G. Howlett and Matthew T. James in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study is based, in part, on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor Alberta Health or Alberta Health Services express any opinion in relation to this study.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was obtained from the Conjoint Health Research Ethics Board of the University of Calgary, who granted waiver of patient consent for the study.
Consent for Publication: All authors reviewed the final manuscript and provided consent for publication.
Availability of Data and Materials: Data is not available for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: APPROACH was initially funded with a grant from the W. Garfield Weston Foundation and has also received contributions from Alberta Health and Wellness and the following industry sponsors: Merck Frosst Canada Inc., Eli Lily Canada Inc., Roche Canada, Bristol-Myers Squibb, and Philips Medical Systems Canada. The ongoing operation of the APPROACH project has been made possible by support from Alberta Health Services (Calgary Zone, Edmonton Zone), the Libin Cardiovascular Institute of Alberta and the Mazankowski Alberta Heart Institute.
Supplemental Material: The supplemental material for this article is available online.
References
- 1. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel UD, Hernandez AF, Liang L, et al. Quality of care and outcomes among patients with heart failure and chronic kidney disease: a Get With the Guidelines—Heart Failure Program study. Am Heart J. 2008;156:674-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). the CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429-1435. [DOI] [PubMed] [Google Scholar]
- 4. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302. [DOI] [PubMed] [Google Scholar]
- 5. Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675. [DOI] [PubMed] [Google Scholar]
- 6. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592-2600. [DOI] [PubMed] [Google Scholar]
- 7. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422-430. [DOI] [PubMed] [Google Scholar]
- 8. Ghali W, Knudtson M. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol. 2000;16:1225-1230. [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302. [DOI] [PubMed] [Google Scholar]
- 11. Tokmakova MP, Skali H, Kenchaiah S, et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) Study. Circulation. 2004;110:3667-3673. [DOI] [PubMed] [Google Scholar]
- 12. Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, Sarnak MJ, Levin A. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244-253. [DOI] [PubMed] [Google Scholar]
- 13. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. The Lancet. 2003;362:772-776. [DOI] [PubMed] [Google Scholar]
- 14. Frances CD, Noguchi H, Massie BM, Browner WS, McClellan M. Are we inhibited? renal insufficiency should not preclude the use of ACE inhibitors for patients with myocardial infarction and depressed left ventricular function. Arch Intern Med. 2000;160:2645-2650. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002;50:1659-1666. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed A, Love TE, Sui X, Rich MW. Effects of angiotensin-converting enzyme inhibitors in systolic heart failure patients with chronic kidney disease: a propensity score analysis. J Card Fail. 2006;12:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629-636. [DOI] [PubMed] [Google Scholar]
- 18. Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587-1592. [DOI] [PubMed] [Google Scholar]
- 19. Berger AK, Duval S, Manske C, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. American Heart Journal. 2007;153:1064-1073. [DOI] [PubMed] [Google Scholar]
- 20. McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004-1009. [DOI] [PubMed] [Google Scholar]
- 21. Ouwerkerk W, Voors AA, Anker SD, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38:1883-1890. [DOI] [PubMed] [Google Scholar]
- 22. Löfman I, Szummer K, Hagerman I, Dahlström U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3:e000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail. 2017;19:1606-1614. [DOI] [PubMed] [Google Scholar]
- 24. Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377-1384. [DOI] [PubMed] [Google Scholar]
- 25. Bart BA, Gattis WA, Diem SJ, O’Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79:1118-1120. [DOI] [PubMed] [Google Scholar]
- 26. Damman K, Valente MA, Voors AA, O’Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455-469. [DOI] [PubMed] [Google Scholar]
- 27. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693. [DOI] [PubMed] [Google Scholar]
- 28. Acquarone N, Castello C, Antonucci G, Lione S, Bellotti P. Pharmacologic therapy in patients with chronic heart failure and chronic kidney disease: a complex issue. J Cardiovasc Med. 2009;10:13-21. [DOI] [PubMed] [Google Scholar]
- 29. Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang AR, Sang Y, Leddy J, et al. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2016;67:1181-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desai A. Hyperkalemia associated with inhibitors of the renin-angiotensin-aldosterone system. Circulation. 2008;118:1609-1611. [DOI] [PubMed] [Google Scholar]
- 32. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131-140. [DOI] [PubMed] [Google Scholar]
- 33. Sharma P, Blackburn RC, Parke CL, McCullough K, Marks A, Black C. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database Syst Rev. 2011:CD007751. [DOI] [PubMed] [Google Scholar]
- 34. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781. [DOI] [PubMed] [Google Scholar]
- 35. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345. [DOI] [PubMed] [Google Scholar]
- 36. Johnson ES, Weinstein JR, Thorp ML, et al. Predicting the risk of hyperkalemia in patients with chronic kidney disease starting lisinopril. Pharmacoepidemiol Drug Saf. 2010;19:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Einhorn L, Zhan M, Hsu V. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342-1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_1,2_and_3_(1) for Kidney Function, ACE-Inhibitor/Angiotensin Receptor Blocker Use, and Survival Following Hospitalization for Heart Failure: A Cohort Study by Michael H. Chiu, Robert J. H. Miller, Rebecca Barry, Bing Li, Bryan J. Har, Stephen B. Wilton, Merril Knudtson, Jonathan G. Howlett and Matthew T. James in Canadian Journal of Kidney Health and Disease