Abstract
Purpose
The primary aim of this study was to determine the feasibility and effectiveness of an 8-week tongue-strengthening intervention protocol for seniors with mild to moderately severe cognitive impairment in the long-term care setting. Outcome measures of interest included tongue strength, mealtime duration, and food intake.
Method
In this pre–post group study of treatment outcomes, data were collected from 7 adults (aged 84–99 years). Participants were observed across a series of mealtimes to determine mealtime duration and intake before and after 16 treatment sessions. During therapy, participants performed isometric strength exercises and tongue pressure accuracy tasks using the Iowa Oral Performance Instrument (model number 2.1, IOPI Medical). Differences in tongue strength as a function of treatment were explored between the first 3 and final 3 sessions using univariate repeated-measures analysis of variance. Single-subject methods were used to explore baseline and posttreatment data for measures of mealtime function.
Results
Anterior and posterior tongue strength increased significantly with therapy. There were no changes in mealtime function.
Conclusions
This study shows proof of concept that some older adults with cognitive impairment are able to participate in a tongue-strengthening intervention and achieve improvements in tongue strength. Failure to find evidence of associated changes of mealtime function suggests that mealtime measures may not be directly sensitive to changes in tongue strength.
Advancing age is associated with major changes in body composition, including sarcopenia in the limb musculature, which is an age-related loss in skeletal muscle (Cohn et al., 1980). The consequences of sarcopenia are decreased strength and aerobic capacity, leading to reduced functional capacity (Bassey, Morgan, Dallosso, & Ebrahim, 1989). Physical exercise and resistance training of the limb muscles has been shown to yield large increases in strength in older adults, minimizing or reversing physical frailty among very old individuals living in long-term care (LTC; Evans, 1997). Age-related diminishment in strength, mobility, and endurance is also evident in the tongue (Nicosia et al., 2000; Robbins, Levine, Wood, Roecker, & Luschei, 1995) and could plausibly lead to both speech and swallowing deficits. In terms of speech, reduced tongue strength is seen in some forms of dysarthria (Dworkin & Aronson, 1986). In terms of swallowing, research has shown that objective measures of reduced tongue pressure are associated both with oral phase swallowing impairments, such as difficulties with bolus manipulation, mastication, and clearance (Clark, Henson, Barber, Stierwalt, & Sherrill, 2003), and with aspiration (entry of foreign material into the airway during swallowing; Butler et al., 2011).
According to the World Health Organization (2016), there were 47.5 million people living with dementia worldwide in 2016, and there are 7.7 million new cases diagnosed every year. Many of these individuals end up in LTC; recent research has suggested that approximately 65% of LTC residents have a confirmed diagnosis of dementia (H. K. Keller et al., 2017). Dysphagia (swallowing impairment) is a known comorbidity in those with dementia (Easterling & Robbins, 2008), and when present, dysphagia predisposes individuals with dementia to dehydration, malnutrition, weight loss, and aspiration pneumonia (Hudson, Daubert, & Mills, 2000; Mendez, Friedman, & Castell, 1991; Mion, McDowell, & Heaney, 1994). Given these numbers and the growing number of elderly people, it is critical that we find viable solutions to swallowing disorders for those residing in LTC.
In this article, we describe the results of a proof-of-principle study exploring the feasibility and benefits of a tongue pressure training intervention to address tongue weakness among seniors residing in LTC. Given that research has suggested that there is insufficient evidence to support the use of oral motor exercises, such as tongue strength training, to improve speech (McCauley, Strand, Lof, Schooling, & Frymark, 2009), the current study focused on outcome measures associated with swallowing function, specifically measures of mealtime duration and nutritional intake.
Tongue pressure training involves the repeated generation of lingual-palatal pressure by squeezing a small air-filled bulb. This treatment approach is based on principles of exercise-dependent neuroplasticity (Kleim & Jones, 2008), specifically the “use it and improve it” principle and concepts from sports medicine regarding repetition, exercise load, and intensity (Burkhead, Sapienza, & Rosenbek, 2007). Studies have shown that tongue pressure training is an effective intervention for reduced tongue strength in healthy individuals (Robbins et al., 2007), those with dysphagia after stroke (Steele et al., 2016) and acquired brain injury (Steele et al., 2013). However, tongue pressure training has not been explored in the LTC population or in individuals in the early stages of dementia. A previous pilot study in LTC residents without a confirmed diagnosis of dysphagia found that signs of dysphagia on a swallow screening test (Shephard, 2007) were more common in individuals with reduced tongue strength and that there were significant associations between reduced tongue strength, longer meal durations, and reduced food consumption (Namasivayam, Steele, & Keller, 2016). On the basis of these findings, it can be hypothesized that interventions that are effective at improving tongue strength may improve swallowing function, promote better mealtime function, and improve nutritional intake in LTC.
In this study, the primary aim was to determine the feasibility and effectiveness of an 8-week tongue-strengthening intervention protocol for seniors with mild to moderately severe cognitive impairment in the LTC setting. We hypothesized that adults enrolled in the study would demonstrate significant improvements in tongue strength as a result of treatment. In line with the typical course of research development, as cited by the American Speech-Language-Hearing Association's National Center for Evidence-Based Practice in Communication Disorders (Wheeler-Hegland et al., 2009), we decided to determine the impact of tongue strength training intervention in individuals with seemingly normal swallow function as a first step, before considering a study in individuals with dysphagia. A second objective was to determine whether measures of mealtime function, such as mealtime duration or food and drink intake, would change in association with improvements in tongue strength in elderly LTC residents. Demonstration of such proof-of-principle relationships was considered an important precursor to the use and selection of mealtime parameters as outcome measures for future studies.
Method
This study received human subjects approval from the local institutional research ethics board.
Participants
A sample of eight residents (two men, six women; mean age of 91 years, range 84–99 years) was enrolled in the study at an LTC facility in Toronto, Canada. All residents in the home received at least 2 hr per day of nursing care due to dependence in activities of daily living (e.g., bathing, toileting, etc.), and this differentiated them from residents of retirement homes who generally do not require any nursing care. Potential participants were identified and initially approached by care staff based on the following inclusion criteria: (a) over the age of 65 years; (b) able to follow simple, three-step directions due to the complexity of the intervention; (c) able to commit to 8 weeks of an intervention, scheduled twice per week; and (d) not known to exhibit signs of swallow difficulties. The assumption of no swallowing impairment was subjective; swallowing function was not formally assessed. Residents were excluded if they (a) were medically unstable (i.e., acute care hospitalization within the month prior to enrollment), (b) were on a short-term admission (e.g., respite care), (c) required tube feeding, (d) were receiving palliative care, and/or (e) had advanced directives that excluded them from research. The eight residents who were enrolled in the study came from two very similar units in the LTC facility, which housed residents of similar cognitive status and had the same dining room setup and menus. Residents were recruited from two units due to difficulties in finding substitute decision makers to sign consent forms from a single unit. There was no reason to believe that residents from either unit would differ significantly; participants simply resided on two different floors within the LTC home.
Once a potential participant was identified, an intake session was scheduled, during which maximum isometric tongue pressures (MIPs) were screened using the Iowa Oral Performance Instrument (IOPI; model number 2.1, IOPI Medical; www.iopimedical.com) to confirm the presence of tongue weakness. To be included in the study, residents were required to demonstrate an MIP of less than 40 kPa on at least three out of 10 baseline measures, with half of these collected at the anterior and half at the posterior palate. The cutoff of 40 kPa was chosen based on the lower normative tongue strength boundary reported by Fei and colleagues (2013) for healthy adults over the age of 60 years. While measuring baseline values, the ability to follow three-step directions was also confirmed. All residents were asked to open their mouth (Step 1), keep the IOPI bulb on their tongue (Step 2), and squeeze the bulb against the roof of their mouths (Step 3). Ability to follow these directions was used as a proxy indicator of sufficient cognition and ability to participate in the experimental treatment. Once eligibility was confirmed, written consent to participate was obtained from the participant or their substitute decision maker. Age, gender, score on the Cognitive Performance Scale (CPS), and major medical diagnoses were collected from the health records of each participant posttreatment. The CPS combines information on memory impairment, level of consciousness, and executive function and has been shown to be highly correlated with the Mini-Mental State Examination (Hartmaier et al., 1995). Scores range from 0 (intact) to 6 (very severe impairment; Morris et al., 1994).
Measures of Tongue Strength
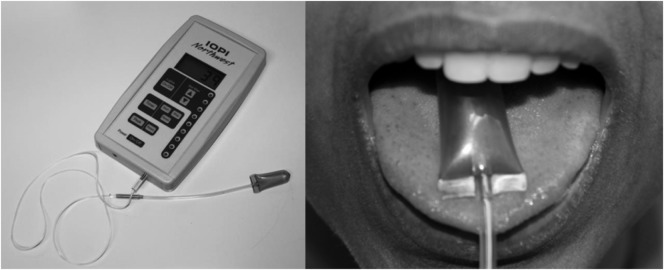
The IOPI was used both for tongue pressure measurement and for monitoring tongue pressure values during treatment sessions. The IOPI is a handheld manometry device, with a teaspoon-sized, air-filled bulb, which is placed on the upper surface of the tongue (Robbins et al., 2005; see Figure 1). Anterior placement means that the flat front end of the bulb is positioned just behind the teeth. Posterior placement involves aligning the flat front end of the bulb with the anterior edge of the first molar tooth (Gingrich, Stierwalt, Hageman, & LaPointe, 2012). When compressed between the tongue and palate, the amount of displaced air is registered in kilopascals on the device monitor. Participants were given visual biofeedback regarding their MIPs throughout their baseline intake measurement to encourage them to try to generate the strongest pressures possible.
Figure 1.
The Iowa Oral Performance Instrument (IOPI), a hand-held device that measures tongue pressure, is depicted on the left, and the anterior placement of an IOPI tongue pressure bulb is depicted on the right.
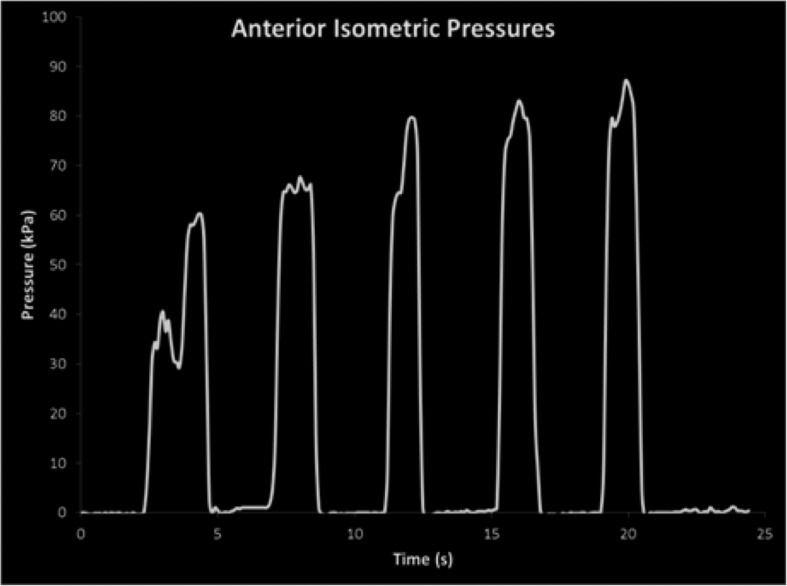
In order to provide biofeedback, the pressure signal was exported from the data out port on the IOPI device and displayed as a waveform on a computer screen (see Figure 2). Positive reinforcement was also directly provided by the clinician in a manner that was interpretable to each participant by telling them the pressure value registered on the IOPI device. The effectiveness of feedback was not monitored, but it was generally well received and unique to each participant.
Figure 2.
Waveform data and biofeedback from tongue-strengthening protocol provided to participants.
Measures of Mealtime Function
Measurements of mealtime function were collected through repeated meal observations. A single set of mealtime observations involved two research assistants (RAs) observing residents at two regularly scheduled meals (one lunch and one dinner) in the facility dining room. Both meal observations were scheduled in the same week, and the RAs were blinded to any information regarding the resident's tongue strength. RAs entered the dining area before the scheduled start of the meal and remained there until after the residents had left after completing their meal. They sat in a corner away from the residents so as not to distract them but positioned themselves in order to be able to clearly see each resident they were observing. There was no interaction between the RAs and the residents. The two RAs present for each observation were allocated two residents to focus on but were privy to the happenings around the dining room, including the other residents being observed. Following each observation, the RAs convened to discuss any issues that arose and capture any qualitative comments that might have affected scoring.
Mealtime duration was recorded as the interval of time when the resident was at their table with food and/or fluid in front of them for consumption. The end of meal was captured as the time when the resident left the dining area and did not return or when no food/fluid remained in front of the resident (i.e., the resident ate everything on their meal tray). If the resident left the room and did not return, the time at which the resident got up from the table was considered the end of the meal. If the resident left the dining room briefly but then returned to their table to continue eating or if the resident stopped eating for a brief period of time, this time was subtracted from mealtime duration in order to accurately capture the time spent eating.
The amount of food eaten was determined by weighing each resident's food tray with a kitchen scale prior to the resident beginning their meal and after the resident had finished their meal. Reference weights for all service ware were collected at the beginning of the study so that the true weight of consumed food and liquid could be determined.
Study Design
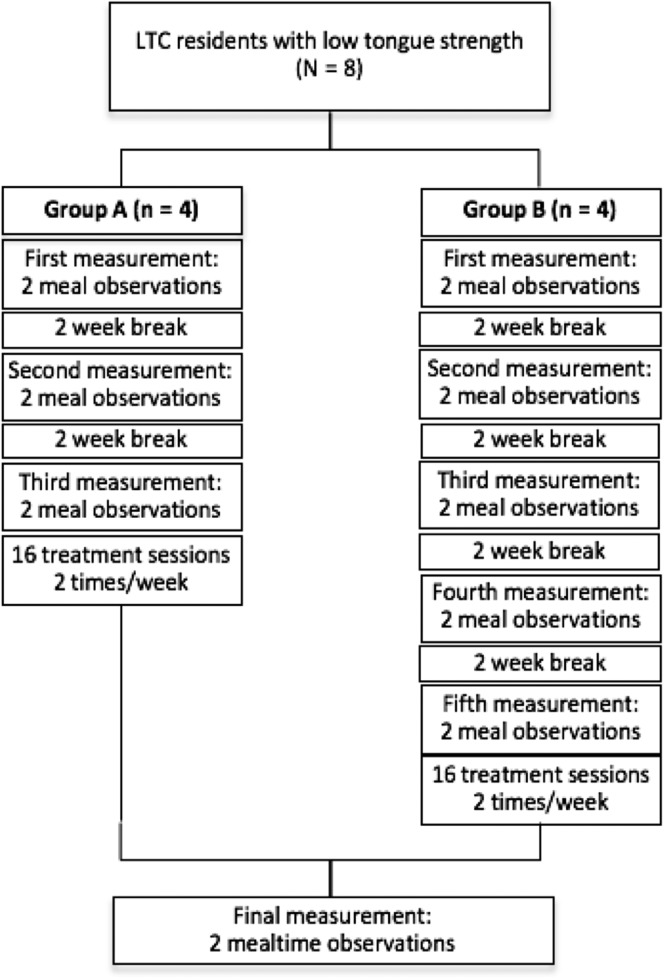
Because of the various day programs being held in the home, the intervention could only be conducted within a 3-hr window in the morning 2 days per week, which only guaranteed that four residents could complete the intervention on a given day. For ease of scheduling for both the clinician and the LTC residents, the sample was divided into two groups based on their unit of origin: one unit was randomly selected to be an early treatment group (Group A), whereas the other was assigned to be the later group (Group B). In addition to measuring changes in tongue strength across the 8-week intervention, a multiple-baseline design was used to monitor any associated changes in mealtime function. As shown in Figure 3, three sets of meal observations (a total of six meals) were completed over a 6-week baseline phase for participants in Group A, with each set of observations scheduled approximately 2 weeks apart. For participants in Group B, these same meal observations were completed, but the baseline phase continued for a further 4 weeks, yielding a total of five sets of baseline mealtime observations (10 meals). The continuation of the meal observations ensured that the participants in the delayed treatment group remained stable until beginning treatment. For both groups, the intervention phase comprised 16 treatment sessions, scheduled twice per week over 8 weeks. Group A reached the end of intervention 4 weeks prior to Group B. A final set of two mealtime observations was completed for each group in the week following the end of treatment.
Figure 3.
Study flow from the point of enrollment.
Tongue Strength Training Intervention
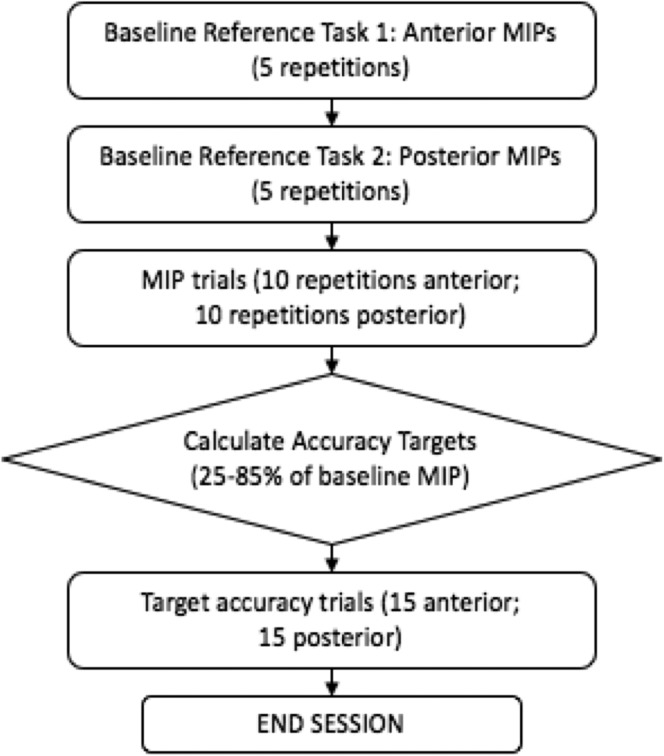
Individual tongue-strengthening intervention sessions (up to 40 min in length) were conducted by two licensed speech-language pathologists, who were blinded to the mealtime observation data. All participants received the same tongue pressure strength and accuracy training (TPSAT) program. This protocol was chosen based on evidence from two recent studies by Steele et al. (2013, 2016), who found the protocol to be effective for improving tongue strength in adults with dysphagia following acquired brain injury or stroke. As shown in Figure 4, the TPSAT protocol is divided evenly between tasks focusing on the anterior tongue and the posterior tongue. Exercise for both parts of the tongue is considered important due to the different functions played by these regions of the tongue in swallowing. Specifically, the anterior tongue is used for formation, placement, and manipulation of the bolus in the oral cavity during swallowing, whereas the posterior tongue is responsible for containment of the bolus in the oral cavity and propulsion into the pharynx (Hiiemae & Palmer, 1999). In the TPSAT protocol, half of the exercises in each bulb position comprised isometric pressure tasks, for which the participant was instructed to press the bulb to the roof of their mouth as hard as possible. The remaining exercises consisted of accuracy tasks, where the participant was instructed to try to generate precise pressures in either the anterior or posterior bulb location. The target amplitudes for the accuracy task were randomly selected by a computer program, falling between 25% and 85% of the participant's maximum pressure range, measured during the first five isometric strength exercises in the session for each bulb location. Visual biofeedback was provided for each pressure trial for each participant in the same fashion as the feedback given during the baseline measurements, as explained previously. The clinician also provided verbal feedback by providing encouraging comments, such as “Well done! Your pressure was X, and close to the target” or “That was a nice try. Your pressure was X which is about half of what we are hoping for. See if you can get closer on the next try.” There was no home practice component outside of the face-to-face therapy sessions with the speech-language pathologist.
Figure 4.
Flowchart of a tongue pressure strength and accuracy training session.
Analysis
Tongue Strength Measures
To determine whether tongue strength improvements were seen in this study, we compared MIP values for the first three and final three treatment sessions. The MIP data from all participants across these six sessions were initially pooled in order to identify any extreme outliers (i.e., values exceeding the third quartile by a full interquartile range (Bryman & Cramer, 1996; Gingrich, Stierwalt, Hageman, & LaPointe, 2012) and remove these from the data. For the anterior MIPs, outliers were considered to be any values over 64 kPa, and outliers for the posterior MIPs were considered to be any values over 61 kPa. A total of six data points were removed from the anterior MIP data, and none were removed from the posterior MIP data. These outliers were placed by missing values.
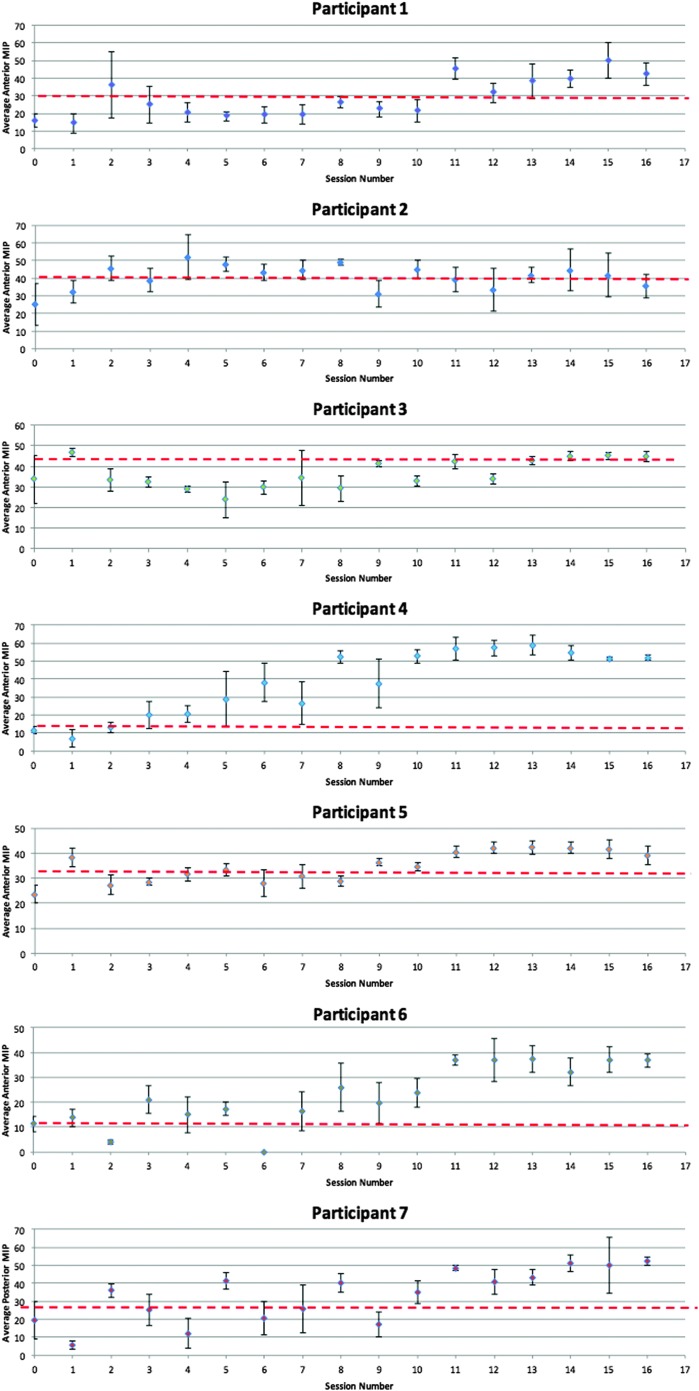
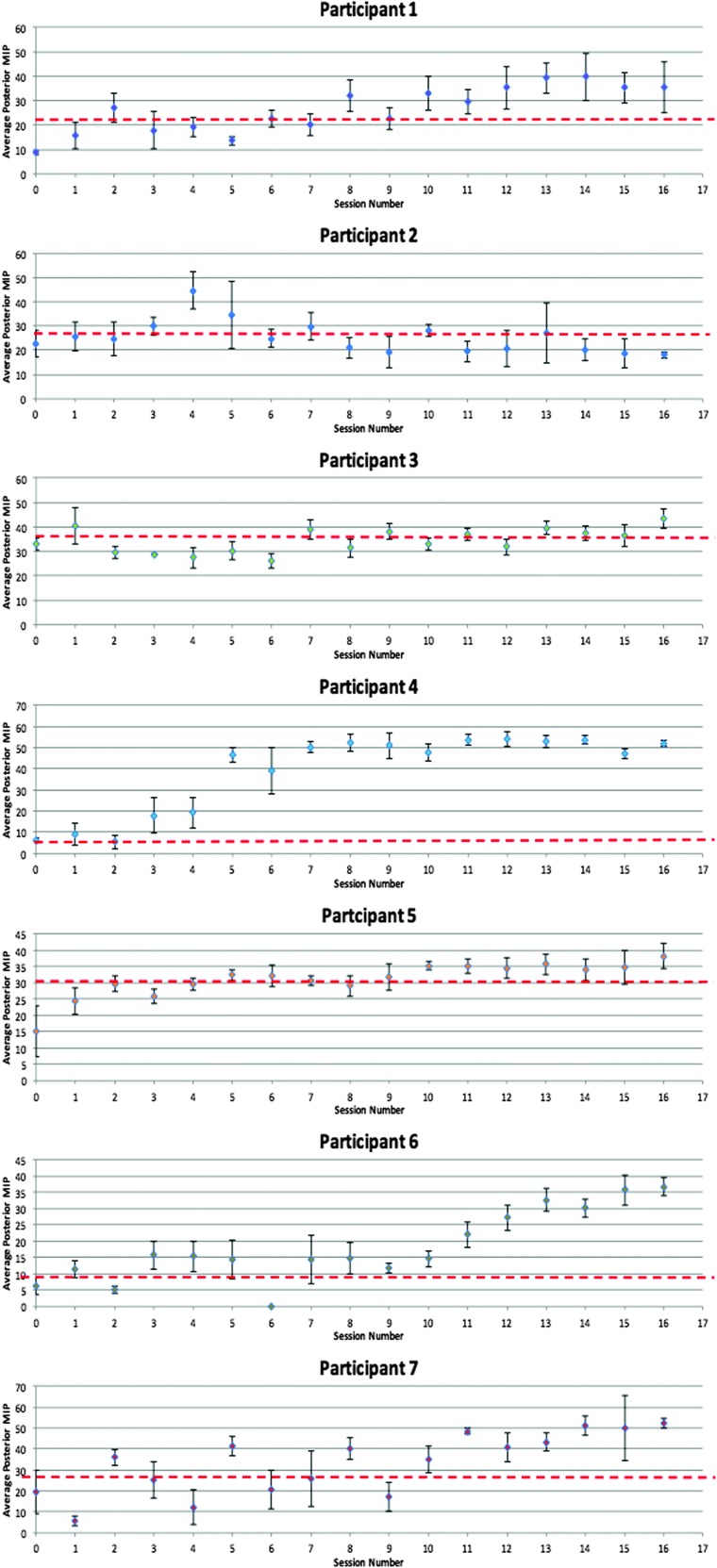
Within-participant changes in tongue strength were monitored across the entire course of treatment using a control chart method, as illustrated in Figures 5 and 6. For each treatment session, mean values for either the anterior or posterior strength tasks were plotted, with standard deviations represented by error bars. The first three data points (intake and first two treatment sessions) constituted the baseline reference range to which subsequent data were compared. An a priori threshold for identifying improvements in tongue strength was established, based on a medium effect size calculation (Cohen's d = 0.5). This is represented on the control charts as a broken red line. Cohen's d can be interpreted as showing a small effect size for values of < 0.5, medium effect size for values of 0.5–0.8, and large effect size for values of > 0.8 (Dunlap, Cortina, Vaslow, & Burke, 1996; Joe & Heather, 2003). In order to conclude that an improvement in MIP values had occurred, we required evidence of three or more consecutive data points falling above the medium effect size threshold and the subsequent points remaining there.
Figure 5.
Anterior tongue maximum isometric pressures from baseline to the end of intervention for Participants 1–7. The broken red lines indicate the medium effect size threshold.
Figure 6.
Posterior tongue maximum isometric measures from baseline to the end of intervention for Participants 1–7. The broken red lines indicate the medium effect size threshold.
Groupwise investigations of differences in tongue strength were conducted in SPSS 24.0 using an alpha criterion of p < .05. Repeated-measures analyses of variance with a between-participants factor of group (A vs. B) and a within-participant factor of baseline observation number (n) were run for measures of tongue strength and all mealtime outcomes. Given the absence of group differences at baseline, differences in tongue strength as a function of treatment were explored for each tongue pressure parameter between the first three sessions and the final three sessions using univariate repeated-measures analysis of variance with a repeated factor of time point (mean baseline tongue strength vs. mean outcome tongue strength). Significant effects were further explored with post hoc analyses of effect size using Cohen's d.
Measures of Mealtime Function
Single-subject methods were used to explore baseline and posttreatment data for both measures of mealtime function. Means and standard deviations for mealtime durations and amount of food consumed (in grams and percentage of food served) were calculated from the data collected from the six or 10 baseline observations (depending on the group) and the two posttreatment observations. The amount of food and drink consumed at each mealtime observation was determined by subtracting the postmeal weight of the food tray from the corresponding premeal tray weight (i.e., premeal food tray − postmeal food tray = grams of food and drink consumed). The percentage of food and drink consumed was calculated by taking the premeal and postmeal weights of the food tray and subtracting from these the weights of all service ware in order to determine the true weight of the food and drink offered to and consumed by the resident. The weight of food and drink consumed was then divided by the weight of the food and drink offered and multiplied by 100 in order to yield the percentage intake at each meal (i.e., (grams of food and drink consumed/grams of food and drink offered) × 100 = percentage of food and drink consumed). Mealtime durations were calculated as total mealtime minus any prolonged periods of distractions.
Results
Complete data were available for only seven participants who completed all 16 treatment sessions and meal observations. The eighth participant became ill and was put on contact precautions after her first intervention session; she declined to resume the intervention once her health improved. Table 1 provides the demographic details for the seven participants who completed the study. They were enrolled an average of 2.9 years after admission into the LTC home. Three residents had a score of 1 on the CPS, indicating that their cognitive performance was borderline intact. Two residents had a CPS score of 3, indicating their cognition was moderately impaired, whereas the remaining two participants had a score of 4, indicating that their cognition was moderately to severely impaired. Six of the seven residents enrolled were drinking thin liquids at all meals, with one resident drinking honey-thick liquids. Five of the seven residents were eating all types of solid foods. Three residents were on minced solid diets.
Table 1.
Participant demographics.
| Participant number | Gender | Age | CPS score | Presence of dentures | Diet | Major medical diagnoses |
|---|---|---|---|---|---|---|
| 1 | F | 85 | 3 | N | Regular liquids, minced solids | Congestive heart failure, dementia, cardiovascular disease, hemiplegia left side |
| 2 | M | 95 | 4 | N | Regular liquids, minced solids | Aphasia, atherosclerotic heart disease, schizophrenia |
| 3 | F | 93 | 1 | N | Regular liquids and solids | Peripheral vascular disease, spastic hemiplegia |
| 4 | F | 91 | 3 | Y | Regular liquids and solids | Dementia, congestive heart failure |
| 5 | M | 86 | 4 | Y | Honey-thick liquids, minced solids | Parkinson's disease, aphasia, cardiovascular disease |
| 6 | F | 99 | 1 | N | Regular liquids and solids | Congestive heart failure |
| 7 | F | 84 | 1 | N | Regular liquids and solids | Dementia, COPD |
Note. CPS = Cognitive Performance Scale; F = female; M = male; N = no; Y = yes; COPD = chronic obstructive pulmonary disease.
There were no significant group differences at baseline for any of the parameters measured: anterior MIPs, F(1, 6) = 1.958, p = .221, posterior MIPs, F(1, 6) = 2.733, p = .159, mealtime duration, F(1, 6) = 1.61, p = .330, amount of food consumed in grams, F(1, 6) = 0.013, p = .91, and percentage of food intake, F(1, 6) = 1.812, p = .236. Moreover, there was good stability across repeated baseline meal observations, regardless of group, for all mealtime measures: mealtime duration, F(9, 31) = 0.857, p = .572; percentage of intake, F(9, 31) = 1.078, p = .406; amount consumed in grams, F(9, 31) = 1.064, p = .415. On the basis of these results, there was no reason to expect that the two participant groups would have different outcomes posttreatment.
Tongue Strength
At baseline, anterior MIPs for the entire sample had a mean value of 23 kPa (95% CI [15, 31 kPa]). Outcome anterior MIP measures (based on the last three intervention sessions) increased significantly to a mean value of 44 kPa (95% CI [40, 48 kPa]), F(1, 6) = 223.80, p < .001, Cohen's d = 2.31 (large). Similarly, posterior MIPs improved from a baseline mean value of 19 kPa (95% CI [12, 27 kPa]) to an outcome mean of 38 kPa (95% CI [30, 46 kPa]), F(1, 6) = 154.30, p < .001, Cohen's d = 1.81 (large). Post hoc analyses showed average gains of 21 kPa for anterior pressures (95% CI [11, 30 kPa]) and 19 kPa in the posterior position (95% CI [6, 31 kPa]). As can be seen in control charts of the single-subject data (see Figures 5 and 6), post hoc analyses also revealed that six of the seven residents who completed the tongue strength training intervention showed marked improvements in both anterior and posterior tongue strength. These six participants all achieved or surpassed the a priori definition of improvement, that is, displaying values that were stronger than those seen across the first three measurements by a value equal to or greater than a medium effect size threshold.
Mealtime Outcomes
Table 2 displays each participant's baseline and posttreatment data for mean mealtime duration, amount of food and drink consumed in grams, and food/drink consumed expressed as a percentage of the amount served (henceforth “percentage food and drink consumed”). The pretreatment group mean mealtime duration was 17.76 ± 8.52 min, and the posttreatment group mean mealtime duration was 18.75 ± 7.26 min. Four of the seven participants displayed shorter mealtime duration posttreatment; however, all improvements were small and fell within the group baseline standard deviation. Three participants had posttreatment mealtime durations that were shorter by less than 1 min compared to baseline (Participants 1, 3, and 6), and Participant 7's posttreatment mealtime duration was shorter by 2.36 min. Balancing these reductions in mealtime duration, the remaining three residents displayed slightly longer posttreatment mealtime duration, from about 1 min to almost 5 min, but these variations were also well within the standard deviation of the baseline measures.
Table 2.
Mean mealtime duration (MTD), amount of food and drink consumed, and percentage of food and drink intake.
| Participantnumber | MTD (min) |
Amount consumed (g) |
% of food intake |
|||
|---|---|---|---|---|---|---|
| Pre-tx |
Post-tx |
Pre-tx |
Post-tx |
Pre-tx |
Post-tx |
|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| 1 | 13 (4) | 12 (3) | 680 (50) | 406 (148) | 80 (12) | 61 (5) |
| 2 | 25 (7) | 27 (7) | 610 (107) | 250 (121) | 75 (16) | 40 (26) |
| 3 | 15 (6) | 15 (7) | 446 (79) | 579 (214) | 43 (12) | 65 (14) |
| 4 | 9 (3) | 10 (2) | 513 (89) | 560 (129) | 63 (23) | 73 (14) |
| 5 | 15 (3) | 19 (0) | 478 (74) | 403 (37) | 58 (10) | 60 (8) |
| 6 | 21 (7) | 21 (4) | 369 (79) | 364 (146) | 47 (8) | 43 (11) |
| 7 | 30 (11) | 27 (4) | 531 (128) | 396 (111) | 62 (12) | 55 (13) |
Note. Pre-tx = pre-treatment; post-tx = post-treatment.
Amount of food and drink consumed were 498.66 ± 124.41 g pretreatment and 422.34 ± 149.34 g posttreatment. The pretreatment group mean percentage food and drink consumed, based on the amount of food and drink provided, was 60.41% ± 15.06%, and posttreatment, the mean dropped slightly to 56.64% ± 14.57%. Inspection of the individual participant data showed increased posttreatment food intake compared to baseline for three residents. The greatest increase was seen in Participant 3, who consumed 133.43 g more food and drink posttreatment than at baseline (an average increase of 22% in intake from baseline). Five residents showed posttreatment decreases in amount of food consumed, with these differences ranging from 4.69 to 359.9 g (4%–35% decrease in food and drink intake from baseline). None of these numbers were significantly impacted by the inclusion of the resident drinking honey-thick liquids.
Discussion
This study provides a proof of principle that tongue pressure resistance training is effective for improving tongue strength among elderly residents living in LTC, many of whom present with cognitive impairment (as per their CPS scores). It is important to note that the residents in this study were enrolled on the basis of reduced tongue strength, and there was no formal assessment of swallowing function. This finding is similar to the results of previous studies in healthy seniors, stroke patients, and individuals with acquired brain injury (Robbins et al., 2005, 2007; Steele et al., 2013, 2016; Yeates, Molfenter, & Steele, 2008). Compared to these previous studies, a greater magnitude of increased tongue strength was observed in this study, regardless of bulb position. The average gain of 21 kPa anteriorly and 19 kPa posteriorly is similar to the average strength increase of 20 kPa reported by Steele and colleagues in a tongue pressure training study for patients after stroke (Steele et al., 2016) but slightly less than the gains reported by Robbins and colleagues for poststroke patients (Robbins et al., 2007). Not surprisingly, the mean baseline pressures of the residents in the current study were well below the reported norms for healthy, older adults, which are reported to range from 34 kPa (95% CI [29, 38 kPa]) in men and 28 kPa (95% CI [24, 32 kPa]) in women (Vanderwegen, Guns, Van Nuffelen, Elen, & De Bodt, 2013) to 55.5 kPa (95% CI [51, 60 kPa]) in a sample of both genders (Solomon, Robin, & Luschei, 2000), despite having similar methods. However, the significant increases in tongue strength seen over the course of this study increased the mean values for tongue strength posttreatment to measurements above the means and confidence intervals reported by Vanderwegen and colleagues for adults over the age of 80 (Vanderwegen et al., 2013). The extremely low initial tongue strength values seen in this sample may also suggest the presence of sarcopenia as hypothesized by Nicosia and colleagues (2000); if this is the case, the findings provide some support for the idea that tongue-strengthening intervention may reverse sarcopenia in the tongue musculature in a manner similar to strengthening interventions reported in the limb musculature (Evans, 1997).
The investigation of associated changes in mealtime outcomes was motivated by previous reports of correlations between low tongue strength and functional mealtime outcomes (Namasivayam et al., 2016). Despite large improvements in tongue strength, this study did not show convincing evidence of associated improvements in measures of mealtime function, such as mealtime duration and food and drink intake. These findings suggest that these functional mealtime measures may not be directly sensitive to changes in tongue strength. The small, heterogeneous sample and limited number of posttreatment meal observations in this study may have hindered our ability to detect changes in mealtime outcomes. A previous study performed in LTC that analyzed the effect of tongue strength on meal consumption suggested that mealtime durations over 62 min were associated with the presence of dysphagia risk based on a standard swallow screening procedure (Namasivayam et al., 2016). In the current study, all participants had baseline mealtime durations well under 62 min. This may have acted as a ceiling effect precluding the possibility of seeing change. Future studies should screen residents for the combination of low tongue strength, as well as reduced mealtime durations based on norms reported in the literature.
Changing only one factor (i.e., tongue strength) in a small number of individuals proved to be insufficient and likely underpowered to demonstrate an impact on measures of mealtime function and suggests that other factors are also likely to have been at play. Recent studies of nutritional intake in seniors residing in LTC suggests that a wide array of factors affect food intake, including distractibility, availability of eating assistance, depression, and lack of food choice (Namasivayam et al., 2016). Mealtime function may have also been affected by palatability of the food and general mood of the residents on any given day, which were factors not captured within the current study. All of these variables should be considered in future studies. It should also be noted that poor food intake in older adults is multifactorial and becomes a greater problem in the later stages of dementia (H. H. Keller, 2016). According to the residents' CPS scores, most of the residents were likely in the earlier stages of dementia. Cognitive status and communication status are other factors that should also be controlled in future studies.
It is important to keep in mind that the residents in this proof-of-principle study were not enrolled on the basis of preexisting dysphagia or swallowing complaints. Residents were enrolled based on evidence of reduced tongue strength to see whether tongue strengthening was feasible in this population. The results confirm that tongue pressure training can be used to improve tongue strength in this population. It is possible that the intervention impacted swallowing function in ways that were not detected based on the measures selected for this study. Using videofluoroscopy, Steele et al. (2016) observed that tongue pressure resistance training was effective for reducing postswallow residue in the pharynx. Similarly, another study employing videofluoroscopy (Yeates et al., 2008) showed improvement in oral bolus control in the form of reduced spillage of the bolus into the pharynx prior to the initiation of the swallow. This sort of improvement has the potential to reduce the occurrence of aspiration and its sequelae based on the findings of a recent study performed by Rogus-Pulia and colleagues, who reported a 67% reduction in pneumonia diagnoses after older adults completed an 8-week tongue-strengthening intervention (Rogus-Pulia et al., 2016). Measurement of physiological swallowing outcomes was not explored in the current project but would be interesting to include in future studies of tongue-strength training among institutionalized seniors.
All of the mealtime factors explored in this study could also have been affected by the level of difficulty of the diet textures offered at meals. Diet texture may represent a scale of difficulty, but this has not yet been proven within the dysphagia literature. A study performed by Kays, Hind, Gangnon, and Robbins (2010) measured the tongue strength of older, healthy adults (aged 65–82 years) before and after consumption of a meal consisting of half a bagel with peanut butter, carrot sticks, and milk. The results showed that the activity of eating a meal can be tiring enough to cause reduced postmeal measures of tongue strength compared to premeal measures. In the current study, we were unable to control for the textures of foods served at meals. This is a factor that should be carefully monitored in future studies. Related considerations, which would be interesting to explore in future studies, include dental health and the presence of functional teeth or dentures, which are known to impact the time required for food intake (Krall, Hayes, & Garcia, 1998; Sheiham et al., 2001). Similarly, medications were not considered in this study but may affect both appetite and lethargy at meals, with consequences for measures of mealtime intake and duration.
In summary, there are several limitations to acknowledge regarding this study. The first is the very small, heterogeneous sample of LTC residents enrolled; results might differ with a larger sample size composed of more similar residents, and this should be considered in future studies. The residents in our study presented with a wide variety of diagnoses and also differed in terms of diet prescriptions, as seen in Table 1. Although the heterogeneity of the sample is likely to be representative of the LTC population, overall impairment due to each individual's combination of diagnoses may have affected results of both the tongue-strengthening therapy and mealtime function. It is possible that some residents performed better or worse as a consequence of their diagnoses. In hindsight, the single resident on thickened liquids should have been excluded from the study given that he was likely drinking honey-thick liquids due to swallowing difficulties. However, there was no upfront awareness of possible dysphagia in this participant or any other residents, as the clinician performing the intervention was blinded to the results of the mealtime observations. Moreover, modified liquid and/or food consistencies did not act as an exclusion criterion. The tongue pressure data and measures of mealtime function of the participant on thickened liquids were not significantly different from the other residents and did not skew the results of the current study.
The enrollment of residents based on low tongue strength alone is another limitation. The absence of a confirmed swallowing problem may have created a ceiling effect that limited the chances of seeing change in the dependent variables of mealtime function. Future studies should include a more detailed swallowing assessment to clearly delineate swallowing issues and changes in swallowing parameters as a treatment outcome and should be adequately powered to do so. The inclusion of a no-treatment control group would also be beneficial in future investigations to explore whether individuals who do not receive the intervention decline in their functional mealtime outcomes compared to residents who receive treatment. Another limitation of the current study was the limited number of posttreatment mealtime observations, which may not have been adequate to obtain a representative sample of each resident's mealtime performance compared to the longer pretreatment surveillance phase.
In conclusion, this proof-of-principle study has shown that tongue pressure resistance training can be used successfully in the LTC setting and that individuals with mild to moderately severe cognitive impairments are able to participate and achieve improvements in both anterior and posterior tongue strength. There was a clear difference between baseline and outcome tongue pressures for six out of seven LTC residents in this study, all of whom had some degree of dementia. However, despite these improvements in tongue pressures, we were unable to identify associated improvements in measures of mealtime function, such as mealtime duration and amount of food consumed. Future investigations of the effectiveness of tongue pressure resistance training should focus on a more homogeneous subset of the LTC population and include comprehensive swallowing assessment. A no-treatment control group would also be beneficial to confirm the degree to which change in measures of mealtime function can be expected as an outcome of tongue strength training.
Acknowledgments
We would like to acknowledge the support of our funding sources, including the Peterborough K. M. Hunter Charitable Foundation and the Annie Kirshenblatt Memorial Fund (funding awarded to Ashwini Namasivayam-MacDonald).
Funding Statement
We would like to acknowledge the support of our funding sources, including the Peterborough K. M. Hunter Charitable Foundation and the Annie Kirshenblatt Memorial Fund (funding awarded to Ashwini Namasivayam-MacDonald).
References
- Bassey E. J., Morgan K., Dallosso H. M., & Ebrahim S. B. (1989). Flexibility of the shoulder joint measured as range of abduction in a large representative sample of men and women over 65 years of age. European Journal of Applied Physiology and Occupational Physiology, 58(4), 353–360. [DOI] [PubMed] [Google Scholar]
- Bryman A., & Cramer D. (1996). Quantitative data analysis with minitab: A guide to social scientists (pp. 288) London, England: Routledge. [Google Scholar]
- Burkhead L. M., Sapienza C. M., & Rosenbek J. C. (2007). Strength-training exercise in dysphagia rehabilitation: Principles, procedures, and directions for future research. Dysphagia, 22(3), 251–265. [DOI] [PubMed] [Google Scholar]
- Butler S. G., Stuart A., Leng X., Wilhelm E., Rees C., Williamson J., & Kritchevsky S. B. (2011). The relationship of aspiration status with tongue and handgrip strength in healthy older adults. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 66(4), 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. M., Henson P. A., Barber W. D., Stierwalt J. A. G., & Sherrill M. (2003). Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American Journal of Speech-Language Pathology, 12(1), 40–50. [DOI] [PubMed] [Google Scholar]
- Cohn S. H., Vartsky D., Yasumura S., Sawitsky A., Zanzi I., Vaswani A., & Ellis K. J. (1980). Compartmental body composition based on total-body nitrogen, potassium, and calcium. American Journal of Physiology-Endocrinology and Metabolism, 239(6), 524–530. [DOI] [PubMed] [Google Scholar]
- Dunlap W. P., Cortina J. M., Vaslow J. B., & Burke M. J. (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1(2), 170–177. [Google Scholar]
- Dworkin J. P., & Aronson A. E. (1986). Tongue strength and alternate motion rates in normal and dysarthric subjects. Journal of Communication Disorders, 19(2), 115–132. https://dx.doi.org/10.1016/0021-9924(86)90015-8 [DOI] [PubMed] [Google Scholar]
- Easterling C. S., & Robbins E. (2008). Dementia and dysphagia. Geriatric Nursing, 29(4), 275–285. [DOI] [PubMed] [Google Scholar]
- Evans W. (1997). Functional and metabolic consequences of sarcopenia. The Journal of Nutrition, 127(5 Suppl.), 998S–1003S. [DOI] [PubMed] [Google Scholar]
- Fei T., Polacco R. C., Hori S. E., Molfenter S. M., Peladeau-Pigeon M., Tsang C., & Steele C. M. (2013). Age-related differences in tongue-palate pressures for strength and swallowing tasks. Dysphagia, 28(4), 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich L. L., Stierwalt J. A. G., Hageman C. F., & LaPointe L. L. (2012). Lingual propulsive pressures across consistencies generated by the anteromedian and posteromedian tongue by healthy young adults. Journal of Speech, Language, and Hearing Research, 55(3), 960–972. [DOI] [PubMed] [Google Scholar]
- Hartmaier S. L., Sloane P. D., Guess H. A., Koch G. G., Mitchell C. M., & Phillips C. D. (1995). Validation of the Minimum Data Set Cognitive Performance Scale: Agreement with the Mini-Mental State Examination. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 50(2), M128–M133. [DOI] [PubMed] [Google Scholar]
- Hiiemae K. M., & Palmer J. B. (1999). Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia, 14(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Hudson H. M., Daubert C. R., & Mills R. H. (2000). The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia, 15(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Joe W. K., & Heather A. W. (2003). The incoporation of effect size in information technology, learning, and performance research. Information Technology, Learning, and Performance Journal, 21(1), 1–7. [Google Scholar]
- Kays S. A., Hind J. A., Gangnon R. E., & Robbins J. (2010). Effects of dining on tongue endurance and swallowing-related outcomes. Journal of Speech, Language, and Hearing Research, 53(4), 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. H. (2016). Improving food intake in persons living with dementia: Food intake and dementia. Annals of the New York Academy of Sciences, 1367(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Keller H. K., Carrier N., Slaughter S. E., Lengyel C., Steele C. M., Duizer L., … Villalon L. (2017). Prevalence and determinants of poor food intake of residents living in long term care. Journal of the American Medical Directors Association. Advance online publication. https://doi.org/10.1016/j.jamda.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Kleim J. A., & Jones T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225–S239. [DOI] [PubMed] [Google Scholar]
- Krall E., Hayes C., & Garcia R. (1998). How dentition status and masticatory function affect nutrient intake. The Journal of the American Dental Association, 129(9), 1261–1269. [DOI] [PubMed] [Google Scholar]
- McCauley R. J., Strand E., Lof G. L., Schooling T., & Frymark T. (2009). Evidence-based systematic review: Effects of nonspeech oral motor exercises on speech. American Journal of Speech-Language Pathology, 18(4), 343–360. [DOI] [PubMed] [Google Scholar]
- Mendez L., Friedman L. S., & Castell D. O. (1991). Swallowing disorders in the elderly. Clinics in Geriatric Medicine, 7(2), 215–230. [PubMed] [Google Scholar]
- Mion L. C., McDowell J. A., & Heaney L. K. (1994). Nutritional assessment of the elderly in the ambulatory care setting. Nurse Practitioner Forum, 5(1), 46–51. [PubMed] [Google Scholar]
- Morris J. N., Fries B. E., Mehr D. R., Hawes C., Phillips C., Mor V., & Lipsitz L. A. (1994). MDS Cognitive Performance Scale. Journal of Gerontology, 49(4), M174–M182. [DOI] [PubMed] [Google Scholar]
- Namasivayam A. M., Steele C. M., & Keller H. (2016). The effect of tongue strength on meal consumption in long term care. Clinical Nutrition (Edinburgh, Scotland), 35(5), 1078–1083. [DOI] [PubMed] [Google Scholar]
- Nicosia M. A., Hind J. A., Roecker E. B., Carnes M., Doyle J., Dengel G. A., & Robbins J. (2000). Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 55(11), M634–M640. [DOI] [PubMed] [Google Scholar]
- Robbins J., Gangnon R. E., Theis S. M., Kays S. A., Hewitt A. L., & Hind J. A. (2005). The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society, 53(9), 1483–1489. [DOI] [PubMed] [Google Scholar]
- Robbins J., Kays S. A., Gangnon R. E., Hind J. A., Hewitt A. L., Gentry L. R., & Taylor A. J. (2007). The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation, 88(2), 150–158. [DOI] [PubMed] [Google Scholar]
- Robbins J., Levine R., Wood J., Roecker E. B., & Luschei E. (1995). Age effects on lingual pressure generation as a risk factor for dysphagia. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 50(5), M257–M262. [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia N., Rusche N., Hind J. A., Zielinski J., Gangnon R., Safdar N., & Robbins J. (2016). Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. Journal of the American Geriatrics Society, 64(2), 417–424. [DOI] [PubMed] [Google Scholar]
- Sheiham A., Steele J. G., Marcenes W., Lowe C., Finch S., Bates C. J., … Walls A. W. (2001). The relationship among dental status, nutrient intake, and nutritional status in older people. Journal of Dental Research, 80(2), 408–413. [DOI] [PubMed] [Google Scholar]
- Shephard T. (2007). Dysphagia update: Evidence, tools, and practice. Paper presented at the International Stroke Conference, San Francisco, USA. [Google Scholar]
- Solomon N. P., Robin D. A., & Luschei E. S. (2000). Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech, Language, and Hearing Research, 43(1), 256–267. [DOI] [PubMed] [Google Scholar]
- Steele C. M., Bailey G. L., Polacco R. E. C., Hori S. F., Molfenter S. M., Oshalla M., & Yeates E. M. (2013). Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. International Journal of Speech-Language Pathology, 15(5), 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Bayley M. T., Peladeau-Pigeon M., Nagy A., Namasivayam A. M., Stokely S. L., & Wolkin T. (2016). A randomized trial comparing two tongue-pressure resistance training protocols for post-stroke dysphagia. Dysphagia, 31(3), 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwegen J., Guns C., Van Nuffelen G., Elen R., & De Bodt M. (2013). The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia, 28(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Wheeler-Hegland K., Ashford J., Frymark T., McCabe D., Mullen R., Musson N., … Schooling T. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part II—Impact of dysphagia treatment on normal swallow function. Journal of Rehabilitation Research and Development, 46(2), 185–194. [PubMed] [Google Scholar]
- World Health Organization. (2016, April). Dementia fact sheet. Retrieved from http://www.who.int/mediacentre/factsheets/fs362/en/ [Google Scholar]
- Yeates E. M., Molfenter S. M., & Steele C. M. (2008). Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: Three case reports. Clinical Interventions in Aging, 3(4), 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]