Abstract
Purpose
Some boys with autism spectrum disorder (ASD) and boys with fragile X syndrome and a codiagnosis of ASD (FXS+ASD) have impairments in expressive grammatical abilities. The current study compared grammatical performance in these 2 groups of school-age boys.
Method
Thirty-seven boys similar on mean length of utterance participated in the current study (FXS: n = 19, ASD: n = 18). Participants completed an ASD assessment, nonverbal IQ testing, and conversation language samples. Convergent validity of a sentence imitation task with a norm-referenced assessment of grammar was examined in addition to divergent validity of the measures with nonverbal IQ and vocabulary comprehension and production.
Results
The boys with ASD outperformed the boys with FXS+ASD on the norm-referenced assessment of “be,” and effect sizes indicate that the boys with ASD had better performance on past tense probes on the sentence imitation task and “do” on the norm-referenced assessment. The two measures of grammar had good convergent validity except for copula and auxiliary “be” and “do.” Grammatical performance was not correlated with nonverbal IQ, and trends indicate a relationship between vocabulary and grammar.
Conclusions
Despite being similar on mean length of utterance, there were group differences on grammatical performance. The sentence imitation task had good convergent validity with a norm-referenced assessment of grammar for the third-person singular and past tense probes and therefore could be an inexpensive and valid tool to use clinically for these populations. Future research should continue to refine this task, particularly for the probes with high rates of unscorable responses (i.e., “be” and “do”).
The use of spoken language is a fundamental aspect of human communication, and impairments at any level can result in lifelong struggles. In neurodevelopmental disorders, language is not only impaired but can also distinguish the phenotypes of different disorders (Finestack, Sterling, & Abbeduto, 2013; Haebig, Sterling, & Hoover, 2016). Comparative studies, including disorders of known and unknown etiology with similar behavioral phenotypes, are important both for clinical purposes as well as from a biological perspective. Children with autism spectrum disorder (ASD) present with varying language abilities, ranging from significant language impairments to age-appropriate language abilities outside of pragmatics (see Boucher, 2012, for a review). There are children with ASD who have impairments in the rate and acquisition of language, including grammar. Given the heterogeneity in ASD, some research has focused on subgroups within ASD, focusing on children with and without language impairments beyond pragmatics, to understand the full spectrum of abilities (Kjelgaard & Tager-Flusberg, 2001; Roberts, Rice, & Tager-Flusberg, 2004; Tager-Flusberg & Joseph, 2003). Fragile X syndrome (FXS), an inherited genetic disorder, is an excellent comparison group for children with ASD given the overlap in the behavioral phenotype, including impairments in grammar (Estigarribia, Roberts, Sideris, & Price, 2011; Finestack et al., 2013; Martin, Losh, Estigarribia, Sideris, & Roberts, 2013; Sterling, Rice, & Warren, 2012). The current study included boys with ASD and boys with FXS+ASD to determine if boys with FXS+ASD have a unique grammatical profile compared to idiopathic ASD.
Impairments in the grammatical system not only are indicative of language disorders but also have a foundational role in academic skills, including reading and writing. Tense marking is one aspect of grammar that has been extensively studied in other child language disorders. It is a property of grammar involving tense and agreement markers on verbs, including past tense, auxiliary and copula “be,” auxiliary “do,” and third-person singular –s, as in “she walks home.” Although studies have evaluated tense marking in FXS compared to idiopathic ASD, they have relied on measures derived from language samples instead of norm-referenced assessments and experimental tasks. This is problematic, given the variability in performance on assessment measures noted in both FXS and ASD in multiple domains of the language system (Finestack et al., 2013; Kover, Davidson, Sindberg, & Ellis Weismer, 2014). There is a major gap in the literature surrounding the question of the impact of ASD on FXS with respect to grammar; thus, the potential impact an ASD codiagnosis has on this aspect of language is unclear. Comparisons between neurodevelopmental disorders with similar language profiles are essential for understanding grammatical impairments in idiopathic ASD and FXS+ASD. The current study compared performance on two different assessments of tense marking in boys with ASD compared to boys with FXS+ASD.
FXS is a single-gene disorder caused by a mutation on the FMR1 gene on the long arm of the X chromosome (Verkerk et al., 1991). The mutations on the FMR1 gene disrupt the production of a protein that is critical for normal brain development and functioning, resulting in pervasive developmental delays for male individuals, including intellectual disability, language delays, hyperactivity, social anxiety, and, in a significant number of individuals, a codiagnosis of ASD (Bailey et al., 1998; Budimirovic & Kaufmann, 2011; Demark, Feldman, & Holden, 2003; Harris et al., 2008; Sterling et al., 2012; Warren, Brady, Sterling, Fleming, & Marquis, 2010). Given the X-linked nature of FXS, the syndrome impacts male and female individuals differently. In order to control for these gender differences, we focused solely on male individuals.
There is a significant overlap in the behavioral phenotype of FXS and idiopathic ASD, and many boys with FXS meet diagnostic criteria for ASD. However, there is a debate in the literature as to the nature of this codiagnosis. Some argue that individuals with FXS who meet criteria for ASD differ in important and critical ways, and thus, the underlying neurobiology and subsequent impairments in behavior result from separate underlying processes (Abbeduto, McDuffie, & Thurman, 2014). Others argue that although FXS and ASD are separate disorders, when co-occurring they have an additive and unique impact on development in FXS (Bailey, Hatton, Skinner, & Mesibov, 2001; Hernandez et al., 2009). Despite this debate as to the true nature of ASD in FXS, it is well documented that a significant number of boys with FXS meet the criteria for a codiagnosis of ASD on gold standard diagnostic measures, including the Autism Diagnostic Observation Schedule–Second Edition (ADOS-2; Lord et al., 2012) and the Autism Diagnostic Interview–Revised (Rutter, Le Couteur, & Lord, 2003). The rates range from 27% to 81%, depending on the ages of the children and methods used (Clifford et al., 2007; Garcia-Nonell et al., 2008; Hall, Lightbody, Hirt, Rezvani, & Reiss, 2010; Harris et al., 2008; Klusek, Martin, & Losh, 2014). These high rates of ASD within FXS have raised questions about the overlap between the disorders, including investigations of similarities/differences in language abilities. These comparisons provide a wealth of information in terms of patterns of language impairment that are specific to one disorder or represent areas of overlap between disorders.
Grammar in FXS
There is a growing body of literature on grammar in FXS. Several studies have found that grammatical abilities in general are below mental age expectations in boys with FXS (Estigarribia et al., 2011; Finestack & Abbeduto, 2010; Roberts et al., 2007). These studies primarily utilized analysis procedures derived from language samples in place of experimental tasks and/or standardized language assessments. These studies were critically important for providing an in-depth understanding of spontaneous language abilities but were limited given the lack of specific prompts focused on grammatical targets. A handful of studies including standardized assessments have reported similar findings. Sterling et al. (2012) investigated tense marking, specifically third-person singular and past tense marking in school-age boys with FXS using the Test of Early Grammatical Impairment (TEGI; Rice & Wexler, 2001), a norm-referenced assessment designed to evaluate tense marking in young children. The boys with FXS performed below expectations based on receptive vocabulary ability on third-person singular and past tense probes. Mean length of utterance (MLU), a measure of grammatical complexity, was significantly correlated with percent correct on third-person singular and irregular past tense responses. Nonverbal IQ was not significantly related to tense marking. The study was limited to third-person singular and past tense markers, only used the TEGI, and did not provide a careful characterization of ASD symptoms, leaving open the question of the impact of an ASD codiagnosis on tense marking.
Haebig et al. (2016) extended this work to include copula and auxiliary “be,” auxiliary forms of “do,” third-person singular, and past tense markers. They compared the performance of school-age boys with FXS to children with specific language impairment (SLI) and language-matched children with typical development. SLI provided an interesting comparison group, given that deficits in tense marking are a hallmark clinical feature in SLI, and similar deficits are well documented in ASD and children with Down syndrome (DS). The boys with FXS were significantly better on third-person singular, past tense, “be,” and “do” compared to the children with SLI. They performed similarly to their MLU-matched peers with typical development indicating performance in line with MLU expectations, which was a different profile compared to the children with SLI. This finding was at odds with previous studies that found a mismatch between grammatical abilities on norm-referenced tasks compared to MLU expectations (Finestack et al., 2013). Extending these comparisons to other neurodevelopmental disorders with similar MLUs such as idiopathic ASD is an important next step to identify areas of overlap or differences in the language phenotype and understand the relationship between MLU and tense marking.
Grammar in ASD
Historically, grammar has been relatively understudied in ASD, in part because early reports found no difference in skills compared to age, IQ, and language-matched comparison groups (for a review, see Boucher, 2012). The heterogeneity of language skills has since been well documented, and current research has focused on identifying subgroups of children with different levels of impairment. Eigsti and colleagues examined grammar measured during a free-play session in 5-year-old children with ASD, matched on developmental age to children with developmental delay and children with typical development (Eigsti, Bennetto, & Dadlani, 2007). The samples were transcribed, and they scored the Index of Productive Syntax (IPSyn; Scarborough, 1990), a measure of grammatical development derived from language samples. They found that the children with ASD had significantly lower MLUs compared to the children with developmental delay and typical development and lower IPSyn total scores, as well as the question/negations and noun phrases subscales. The authors also found that, although the children with ASD had significant deficits in grammatical skills, they demonstrated a relative strength in their lexical skills as indexed by number of word types and diversity of lexical items compared to their peers. Although their analyses were restricted to information derived from language samples, it provided solid evidence for grammatical impairments in young children with ASD.
In terms of more specific grammatical abilities, Roberts et al. (2004) examined two tense markers (i.e., third-person singular and past tense) in school-age children with ASD. They found that children with ASD who had a language impairment made significantly fewer correct responses on both tense markers compared to children with ASD and normal language or borderline impairment. Nonverbal IQ scores were not correlated with third-person singular responses. Nonverbal IQ was correlated with past tense scores but only accounted for 13% of the variance. The authors found significant variability in the sample, with some children with ASD and low IQs who had higher rates of correct responses and some children with higher IQs and low rates of correct scores. This study demonstrated the range of impairments in children with ASD and the complexity of accounting for these differences. Language comprehension was a more powerful predictor compared to nonverbal IQ, yet there was still variability within participants. Roberts et al. (2004) examined two aspects of tense marking in one context, leaving open the question of other aspects of tense marking and contextual differences.
Assessment Method and Context
The issue of assessment method and context is particularly compelling in disorders with multiple comorbidities, including FXS and ASD. Boys with FXS not only struggle with language impairments but also have intellectual disability, social anxiety, and attention-deficit/hyperactivity disorder, all of which could have a significant impact on assessment performance (Bailey et al., 2001; Kover, Pierpont, Kim, Brown, & Abbeduto, 2013; Skinner et al., 2005). Studies have found differences in language production in FXS and ASD in tense marking, and therefore it is imperative that studies use a variety of assessment contexts to explore the impact of contextual effects. However, many studies have relied on a single measure of grammar and therefore might not be providing a true picture of the strengths and weaknesses within these two disorders.
Finestack et al. (2013) examined performance on expressive grammar on a standardized test compared to a conversation language sample in children with FXS, DS, and typical development matched on mental age. They found that the participants with DS had a flat profile of impairment, in that they were below mental age expectations on both MLU and a standardized assessment. However, the participants with FXS were at mental age expectations on the standardized assessment, but below age expectations on MLU. The individuals with DS in the study appeared to be less sensitive to task differences in performance compared to FXS. That study did not include individuals with FXS+ASD, and therefore, it is unknown if these findings can be generalized or if they are specific to FXS only.
Research on children with idiopathic ASD has provided a similar picture. The context from which the language sample is drawn can result in significant differences in the type and complexity of language elicited. Losh and Capps (2003) compared the language elicited from a personal narrative compared to storybook narrative retells in 8- to 14-year-old children with ASD compared to children with typical development. The children with ASD had less complex syntax in retelling a personal narrative compared to peers. They also used a more restricted range of complex syntax during the personal narratives, but there were no group differences in syntax complexity during the storybook narrative retell. The personal narratives were conversational in nature and thus point to an important contextual difference for children with ASD. Kover et al. (2014) continued this work on contextual differences within conversational language samples, comparing language produced from the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999; Lord et al., 2012) to both parent–child and examiner–child language samples in preschool children with ASD. They found that the children had the highest MLUs in the examiner–child language sample compared to parent–child play and the ADOS. Children produced fewer requests, comments, and turn-taking in the ADOS compared to both examiner–child and parent–child samples. This is important, given that many studies in FXS have relied on the ADOS as the sole language-sampling context. Although the ADOS provides important information about the child's ASD status and language use in this specific context, the language produced during the test might not generalize to performance on standardized tests and conversation language samples. Conversation language samples are used broadly both in clinical work and in research, and therefore it is imperative to evaluate grammar produced in a conversation language sample.
Sentence imitation tasks are an assessment method used to elicit specific grammatical structures. Sentence imitation tasks are used in the child language disorders literature, given their ease in administration and effectiveness in identifying problematic structures (Abel, Rice, & Bontempo, 2015; Hoover, Storkel, & Rice, 2012). As compared to language sample analysis, sentence imitation tasks afford more efficiency in eliciting multiple instances of targeted grammatical morphemes. One study found that performance on a sentence imitation task was a better psycholinguistic marker for children with SLI than a past tense and third-person singular cloze procedure task (Conti-Ramsden, Botting, & Faragher, 2001). There are no studies to our knowledge that have used sentence imitation in FXS+ASD or examined the convergent validity of sentence imitation with a norm-referenced assessment of grammar in FXS or ASD.
Children with ASD demonstrate atypical patterns of imitation skills, raising the question of the utility of sentence imitation tasks in this group. One study compared different types of verbal working memory tasks in ASD and found that children with ASD had poorer performance on a sentence imitation task compared to nonword repetition and digit span tasks. These results indicate that imitation tasks, including language, are more difficult for children with ASD (Gabig, 2008). Heimann and colleagues asked children with ASD to complete an elicited and deferred imitation task and measured spontaneous imitation (Heimann, Nordqvist, Strid, Connant Almrot, & Tjus, 2016). The children with ASD had a significant weakness in elicited imitation compared to peers with DS and peers with typical development. The authors suggested that some children with ASD have a significant weakness in imitation, even when given explicit instructions. These studies included younger children with ASD and did not include children with co-occurring FXS.
Current Study
The purpose of the current study was to examine tense marking in boys with ASD and boys with FXS+ASD. We extended previous work to include additional tense markers (e.g., “be” and “do”) as well as different methods of assessment. The secondary purpose of this study was to evaluate the convergent validity of a newly developed sentence imitation task with a well-established norm-referenced assessment of tense marking. We examined the discriminant validity of measures of tense marking with nonverbal IQ, chronological age, and receptive and expressive vocabulary. Given that vocabulary is often correlated with measures of grammar, we felt that it was important to examine the extent of overlap between vocabulary and tense marking.
The specific research questions were as follows:
Do children with ASD have a similar profile of tense marking compared to children with FXS+ASD with similar language abilities?
How effective is an experimental sentence imitation task? Does it have good convergent validity with a well-established measure (i.e., the TEGI)?
What is the discriminant validity of the sentence imitation task and TEGI with nonverbal IQ, receptive and expressive vocabulary, and chronological age?
We predicted that both groups would demonstrate deficits in tense marking, given the previous literature. We hypothesized that the sentence imitation task would have good convergent validity with the TEGI. We predicted that the probe scores would be significantly correlated with receptive and expressive vocabulary, but not with nonverbal IQ, indicating good discriminant validity with general cognitive skills but overlap with vocabulary.
Method
Participants
Thirty-seven children participated in the current study (FXS: n = 19, ASD: n = 18). FXS was confirmed via previous molecular genetic testing, and boys with idiopathic ASD had previous genetic testing to rule out FXS. The boys with idiopathic ASD had a community diagnosis of ASD, and this was confirmed during the current study using the same methods applied to identify ASD in the group with FXS.
To be included in the current study, children had to be monoglingual and standard American English speakers and pass the phonological probe in the TEGI (Rice & Wexler, 2001). The probe assesses word final /s z t d/, all necessary for grammatical tense markers. Fifteen of the boys with ASD were White, two reported more than one race (African American and White), and one did not report ethnicity. Two boys with ASD did not report ethnicity; the other 16 were non-Hispanic. Sixteen boys with FXS were White, one was African American, one was American-Indian and White, and one reported other. Two of the boys with FXS were Hispanic and 17 were non-Hispanic.
Group Comparisons
Participants were drawn from a larger study on language in boys with FXS and idiopathic ASD (Haebig & Sterling, 2017; Haebig et al., 2016). For the present analysis, groups were similar on MLU, t(35) = 1.12, p = .272, d = .36, variance ratio = .58, following the guidelines outlined in Kover and Atwood (2013). The range of MLU for the boys with FXS+ASD was between 1.92 and 6.42 (mean = 3.78), and the range for the boys with ASD was 1.81–6.62 (mean = 4.30); thus, the ranges were also similar. MLU-based comparisons are common in the SLI literature and neurodevelopmental disorders (Haebig et al., 2016; Hoover et al., 2012; Leonard & McGregor, 1992; Rice, Buhr, & Nemeth, 1990; Rice, Redmond, & Hoffman, 2006). By using MLU as the comparison variable, findings from this study could be benchmarked within the broader literature on specific aspects of tense marking that have been examined in other language disorders. Although MLU is most important for less complex grammar (MLU < 4.0), the boys in this study had average MLUs around 4.0. By including groups that are similar on MLU, this allowed for an examination of patterns of grammatical development while controlling for important confounds such as utterance length. The groups were also similar on ADOS severity scores, t(35) = .34, p = .733, d = .14, variance ratio = .55.
Procedure
Participants participated in a 1-day session at the Waisman Center. Parents provided written informed consent, and children provided assent. Study procedures were approved by the institutional review board. Participants were given breaks as needed. Children completed language assessments, a nonverbal IQ test, and an ASD diagnostic battery (see Table 1).
Table 1.
Participant characteristics.
| Participant variable | ASD |
FXS |
t (df) | p | d | Variance ratio | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Chronological age | 13.40 | 2.00 | 12.12 | 2.17 | 1.93 (35) | .061 | 0.61 | 1.17 |
| MLU a | 4.30 | 1.60 | 3.78 | 1.22 | 1.12 (35) | .272 | 0.37 | 0.58 |
| ASD severity score b | 7.22 | 2.24 | 7.00 | 1.67 | 0.34 (35) | .733 | 0.11 | 0.55 |
| Nonverbal IQ c | 71.22 | 19.88 | 48.89 | 8.09 | 4.43 (22) | .000 | 1.47 | 0.17 |
| PPVT-4 standard score | 74.39 | 16.70 | 63.68 | 12.57 | 2.21 (35) | .034 | 0.72 | 0.57 |
| EVT-2 standard score | 78.89 | 17.90 | 65.74 | 10.35 | 2.76 (35) | .009 | 0.90 | 0.33 |
Note. ASD = autism spectrum disorder; FXS = fragile X syndrome; MLU = mean length of utterance; PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; EVT-2 = Expressive Vocabulary Test–Second Edition.
Morpheme level MLU from conversation language samples.
Autism Diagnostic Observation Schedule (Lord et al., 1999, 2012).
Nonverbal IQ from brief IQ score from the Leiter International Performance Scale–Revised (Roid & Miller, 1997).
Assessments
Nonverbal IQ
Participants completed the Leiter International Performance Scale–Revised (Roid & , 1997) to measure nonverbal cognitive abilities. Four subtests comprise the brief IQ standard score: Figure Ground, Form Completion, Repeated Patterns, and Sequential Order. Standard scores were calculated based on a mean of 100 and a standard deviation of 15.
Vocabulary
Vocabulary comprehension was assessed using the Peabody Picture Vocabulary Test–Fourth Edition (PPVT-4; Dunn & Dunn, 2007). Children pointed to a visual representation of a word spoken by an examiner. Vocabulary production was assessed using the Expressive Vocabulary Test–Second Edition (EVT-2; Williams, 2007). Children were shown a picture and asked to label it or produce a synonym for it. The PPVT-4 and EVT-2 are norm-referenced tests that yield standard scores based on a mean of 100 and a standard deviation of 15.
ASD Assessment
ASD symptoms were measured using the ADOS and the ADOS-2 (Lord et al., 1999, 2012). The ADOS is a semistructured assessment that evaluates the core impairments associated with ASD. An examiner who was research-reliable or training to be research-reliable (with a research-reliable examiner present for live scoring and coding) administered the appropriate ADOS module according to the child's language level. Nine children with FXS were given Module 2, and nine children with FXS were given Module 3. Five children with ASD were given Module 2, 12 children were given Module 3, and one participant was given Module 1. Four research reliable ADOS examiners participated in the scoring and/or coding, all of whom had prior clinical experience with children with neurodevelopmental disorders. All boys with FXS exceeded cutoff scores for autism or ASD on the ADOS. The ADOS has been used as the sole method of classifying ASD in FXS in several published studies (e.g., Dissanayake, Bui, Bulhak-Paterson, Huggins, & Loesch, 2009; Loesch et al., 2007; Martin et al., 2013; Price et al., 2008). The ADOS was used to measure ASD severity in the boys with idiopathic ASD. We used the autism severity scoring algorithm as a measure of calibrated severity scores, which facilitates ASD symptom level comparisons across the different modules of the ADOS (Gotham, Pickles, & Lord, 2009).
Conversation Language Sample
Each child participated in a 10-min conversation language sample. The examiner followed a semistructured list of conversation topics designed for school-age children and adolescents (Berry-Kravis et al., 2013) using standard language elicitation techniques. These included open-ended questions, comments, and minimizing the use of yes/no questions. Parents provided several favorite topics prior to the visit, and the conversation sample began and ended with these topics. The samples were transcribed and coded using the procedures outlined in Systematic Analysis of Language Transcripts (Miller, Andriacchi, & Nockerts, 2011). Trained undergraduate and graduate students completed the primary and reliability coding. Utterances were segmented by communication units or c-units, and only complete and intelligible utterances were used to calculate morpheme level MLU. A second, independent transcriber completed a random sample of files for reliability. We completed line-by-line reliability checks on 30% of the sample (27.8% of transcripts for children with ASD and 31.5% of transcripts for children with FXS). Utterance segmentation agreement was 85%, intelligibility was 95%, bound morphemes was 85%, and the number of words was 86%.
TEGI
The TEGI is a norm-referenced measure used to identify and assess grammatical deficits in young children (Rice & Wexler, 2001). It has been used in research with children with specific language impairment as well as children with FXS (Bishop, Adams, & Norbury, 2006; Haebig et al., 2016; Redmond, Thompson, & Goldstein, 2011; Sterling et al., 2012). We administered all probes from the TEGI: phonological probe screener, third-person singular, past tense, and be/do probes. The probes elicited responses in an obligatory context for the target grammatical form. For both the third-person singular and past tense probes, children were shown a picture and then asked to generate a sentence using the target structure (e.g., picture of a dentist; target answer: A dentist cleans your teeth). The past tense probe included both regular and irregular forms of verbs. The be/do probe involved a puppet and some manipulatives. The child is prompted to ask the puppet questions about the main characters of the story (e.g., the examiner says: “I wonder if the robots are thirsty. Ask the puppet if the robots are thirsty.” Target response: Are the robots thirsty?; Rice & Wexler, 2001). 1 Responses were scored online, and then the examiner verified scores from the audio recording. A second, independent coder listened to all recordings and noted any possible errors. Consensus coding was used for any disagreements. Reliability was 98.11% for third-person singular, 97.97% for past tense, and 94.43% for “be/do.”
Scoring was based on the TEGI manual, and scores are presented as percentages. Scores are based on responses to scorable items in obligatory context and not necessarily all the items on the probe. For example, the third-person singular probe has 10 items, and only verbs that carry an overt grammatical tense marker are included in the score (e.g., correct: A ballerina dances; incorrect: A pilot fly a plane). Children might provide a modal such as “can,” which does not carry an overt tense marker. This is considered an unscorable response. If the child had seven responses including verbs that carry overt tense markers, then the percentage would be calculated based on those seven responses. If the child produced five of the seven responses correctly and two incorrectly, this would yield a score of 5/7, which is 71.4% correct. Unscorable responses were given a separate code and analyzed separately.
Sentence Imitation Task
Participants listened to prerecorded instructions and sentences containing targeted tense markers: third-person singular –s, regular and irregular past tense verbs, and “be” and “do.” The task was developed for this project based on a task used for children with SLI (Hoover et al., 2012). The previous task only included third-person singular verbs. We expanded the task including past tense “be” and “do.” Participants were first presented with instructions and two practice items. Sentences were four to six words in length and recorded by a female native speaker of standard American English. There were 30 sentences: eight sentences for third-person singular –s, 10 sentences for past tense (five for regular past tense and five for irregular past tense), eight sentences for “be,” and four sentences for “do.” The original focus of the project was on third-person singular and past tense –ed. Sentences for “be” and “do” were added for preliminary data. Given concerns about testing length and participant fatigue and the desire for the task to be clinically relevant, we opted to keep this task as short as possible. Responses were scored online and verified via audio recordings. Coding followed the same procedures as the TEGI, including percent correct out of the total number of correct and incorrect responses. Reliability was completed by a second, independent coder and was 99.01%. Incorrect responses were coded for items omitting the obligatory tense marker. Unscorable responses were given a separate code and analyzed separately. Examples of unscorable responses included dropping the subject (e.g., target: The teacher reads a story, participant response: reads a story) or providing a yes/no answer to the target (e.g., target: Are the children playing, participant response: yes).
Data Analysis
The first research question focused on the pattern of tense markers in FXS and idiopathic ASD across different measurement contexts. We completed a multivariate analysis of variance (MANOVA) with dependent variables from the sentence imitation task and the TEGI probe scores (third-person singular, regular and irregular past tense, “be” and “do” scores), with group as the fixed factor. We then completed a series of t tests as planned follow-ups to determine the significant differences between the groups. Given the number of comparisons, we used a Holm–Bonferroni correction (Gaetano, 2013). We included effect size estimates along with the t tests and interpreted the values as .20 = small, .50 = medium, and .80 = large (Cohen, 1988). Given the large difference in nonverbal IQ between the two groups, we considered using nonverbal IQ as a covariate. In following best-practice guidelines, we first ran correlations to examine the relationship between nonverbal IQ and the dependent variables (Storkel, Maekawa, & Hoover, 2010; Tabachnick & Fidell, 2001). We completed correlations in two steps, first separating the groups by diagnosis and then collapsing the groups to be sensitive to power issues. Nonverbal IQ was not correlated with any of the dependent variables when the groups were separated and only minimally correlated with irregular past tense when the groups were combined. There are methodological and theoretical concerns with using nonverbal IQ as a covariate in neurodevelopmental research as outlined in Dennis et al. (2009). The concern is that adjusting for nonverbal IQ in clinical populations where intellectual disability is a key part of the disorder results in findings that are difficult to interpret, given that intellectual disability is inseparable from the disorder. Given the lack of a relationship with the dependent variables and the concerns noted in the literature, we elected not to control for nonverbal IQ (Dennis et al., 2009; Kover & Atwood, 2013; Miller & Chapman, 2001; Tupper & Rosenblood, 1984).
The second research question examined convergent validity of the sentence imitation task with the TEGI based on the multitrait–multimethod approach (Campbell & Fiske, 1959). We were interested in the effectiveness of the task, and scores were analyzed based on correct, incorrect, and unscorable responses to verify that the percent correct scores represented the majority of item responses and not only a few responses with the majority unscorable. We also examined the discriminant validity with the sentence imitation and TEGI probe scores with nonverbal IQ, receptive and expressive vocabulary, and chronological age. Given the larger number of correlations, we used a Bonferroni correction and reported all p values.
Results
Research Question 1: Between-Groups Comparison of Tense Marking
A MANOVA was used to compare performance across all probes from the sentence imitation and TEGI for boys with ASD and boys with FXS+ASD. Prior to completing the MANOVA, we completed a preliminary screening of the data and found that the “be” and “do” probes on the sentence imitation task had ceiling effects and therefore were excluded for the MANOVA. There was a significant effect of group on the language measures, F(9,18) = 3.43, p < .05, Wilk's Λ = .40, partial η2 = .60. This finding was followed up using independent planned t tests to compare performance across the different measures for each language variable. The results are summarized in Table 2. After applying a Holm–Bonferroni correction, the boys were significantly different on the “be” probe of the TEGI, with the boys with ASD outperforming the boys with FXS. There were large effect sizes for regular past tense and irregular past tense on the sentence imitation task and for the “do” probe on the TEGI. The boys with ASD had higher scores on these probes compared to the boys with FXS. There was a medium effect size for regular past tense on the TEGI, and again the boys with ASD had higher scores.
Table 2.
Group differences on sentence imitation task (SIT) and Test of Early Grammatical Impairment (TEGI) probes.
| TEGI and SIT variable | ASD |
FXS |
t (df) | p b | p′ b | d | ||
|---|---|---|---|---|---|---|---|---|
| Mean a | SD | Mean a | SD | |||||
| 3S SIT | 98.61 | 4.04 | 90.56 | 23.12 | 1.49 (19) | .152 | .760 | 0.49 |
| 3S TEGI | 94.76 | 12.62 | 86.36 | 23.51 | 1.34 (35) | .188 | .760 | 0.45 |
| Regular past tense SIT | 62.87 | 37.82 | 28.13 | 38.16 | 2.66 (32) | .012 | .096 | 0.91 |
| Regular past tense TEGI | 78.65 | 24.32 | 59.05 | 32.91 | 1.97 (33) | .057 | .342 | 0.68 |
| Irregular past tense SIT | 97.78 | 6.47 | 84.71 | 17.60 | 2.88 (20) | .009 | .081 | 0.99 |
| Irregular past tense TEGI | 60.54 | 36.99 | 45.12 | 25.92 | 1.43 (28) | .163 | .760 | 0.48 |
| BE SIT | 100.00 | 0.00 | 99.07 | 3.92 | 1.00 (17) | .331 | .760 | 0.34 |
| BE TEGI | 99.12 | 2.54 | 79.31 | 25.73 | 3.34 (18) | .004 | .040 | 1.08 |
| DO SIT | 98.61 | 5.89 | 100.00 | 0.00 | 0.57 (22) | .575 | .760 | 0.33 |
| DO TEGI | 79.02 | 36.06 | 43.78 | 35.38 | 2.68 (28) | .012 | .096 | 0.99 |
Note. ASD = autism spectrum disorder; FXS = fragile X syndrome; 3S = third-person singular; BE = auxiliary “be” verbs; DO = auxiliary “do” verbs.
Mean based on percent correct of scorable responses (i.e., response provided in an obligatory context and contains a verb that overtly marks finiteness).
p refers to the uncorrected p values. p′ refers to p values after applying a Holm–Bonferroni correction (Gaetano, 2013).
Research Question 2: Sentence Imitation Task Compared to TEGI
We examined the data in the sentence imitation task and TEGI in two ways. First, we completed correlations between the probes of the TEGI and sentence imitation task with all participants to examine convergent validity and discriminant validity between the groups. Given our sample size, we collapsed the groups. The correlation matrix is presented in Table 3. We used a Bonferroni correction, and therefore, the adjusted p value was set at p < .0001.
Table 3.
Convergent validity between sentence imitation task (SIT) and Test of Early Grammatical Impairment (TEGI).
| SIT and TEGI variable | SIT 3S | SIT RPT | SIT IPT | SIT BE | SIT DO | TEGI 3S | TEGI RPT | TEGI IPT | TEGI BE | TEGI DO |
|---|---|---|---|---|---|---|---|---|---|---|
| SIT 3S | 1 | |||||||||
| SIT RPT | .266 | 1 | ||||||||
| SIT IPT | .138 | .388* | 1 | |||||||
| SIT BE | .476** | 0 | .297 | 1 | ||||||
| SIT DO | −.063 | .098 | −.079 | 0 | 1 | |||||
| TEGI 3S | .757*** | .279 | .403* | −.102 | −.083 | 1 | ||||
| TEGI RPT | .445* | .596*** | .413* | −.009 | .109 | .670*** | 1 | |||
| TEGI IPT | .438* | .468* | .636*** | .260 | −.045 | .683** | .732** | 1 | ||
| TEGI BE | .755** | .415* | .218 | −.100 | −.110 | .729** | .739** | .526** | 1 | |
| TEGI DO | .095 | .480* | .347 + | .043 | −.170 | .501* | .708** | .592** | .599** | 1 |
Note. 3S = third-person singular; RPT = regular past tense; IPT = irregular past tense; BE = auxiliary and copula “be” verbs; DO = auxiliary “do” verbs.
p < .050.
p < .010.
p <.001.
p < .060.
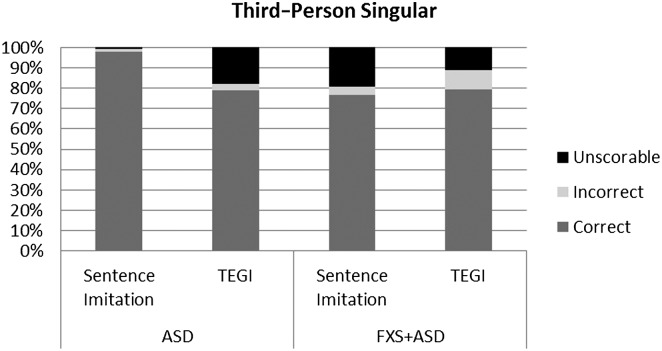
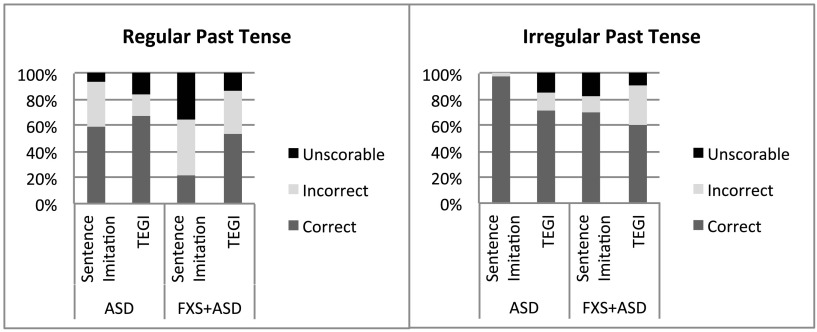
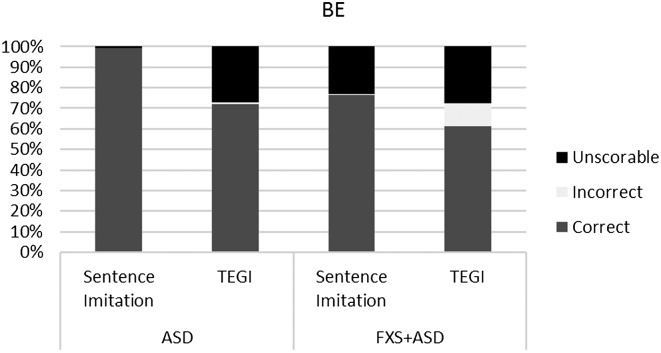
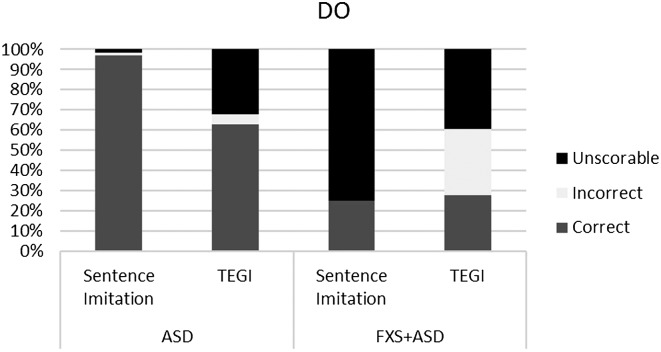
In line with other studies, we analyzed participant responses and recalculated percentages based on the response type (Haebig et al., 2016; Roberts et al., 2004; Sterling et al., 2012). This level of analysis allows us to examine the robustness of each probe score. The goal was merely to compare the type and amount of responses in order to verify that the probe scores were valid and not based on only one or two items with a majority of unscorable responses. Figures 1 –4 show the breakdown of unscorable, correct, and incorrect responses for the individual probe scores.
Figure 1.
Comparison of correct, incorrect, and unscorable responses for third-person singular between the Test of Early Grammatical Impairment (TEGI) and the sentence imitation task for boys with autism spectrum disorder (ASD) and boys with fragile X syndrome and ASD (FXS+ASD).
Figure 2.
Comparison of correct, incorrect, and unscorable responses for regular (displayed on left) and irregular past tense (displayed on right) verbs between the Test of Early Grammatical Impairment (TEGI) and the sentence imitation task for boys with autism spectrum disorder (ASD) and boys with fragile X syndrome and ASD (FXS+ASD).
Figure 3.
Comparison of correct, incorrect, and unscorable responses for copula and auxiliary “be” verbs (BE) between the Test of Early Grammatical Impairment (TEGI) and the sentence imitation task for boys with autism spectrum disorder (ASD) and boys with fragile X syndrome and ASD (FXS+ASD).
Figure 4.
Comparison of correct, incorrect, and unscorable responses for “do” verbs (DO) between the Test of Early Grammatical Impairment (TEGI) and the sentence imitation task for boys with autism spectrum disorder (ASD) and boys with fragile X syndrome and ASD (FXS+ASD).
Research Question 3: Discriminant Validity of the Sentence Imitation Task and TEGI With Descriptive Variables
We completed correlations between the TEGI and sentence imitation probes and four descriptive variables: nonverbal IQ, receptive and expressive vocabulary, and chronological age. Results are reported in Table 4. Given our sample size, we collapsed the groups for this analysis. We again used a Bonferroni correction, and the adjusted p value was set at p < .0001.
Table 4.
Discriminant validity between sentence imitation task (SIT) and Test of Early Grammatical Impairment (TEGI) probe scores with descriptive variables.
| SIT and TEGI variable | Nonverbal IQ | PPVT-4 | EVT-2 | Chronological age |
|---|---|---|---|---|
| 3S SIT | .130 | .322 + | .134 | .290 |
| 3S TEGI | .110 | .332* | .224 | .337* |
| Regular past tense SIT | .264 | .091 | .202 | .272 |
| Regular past tense TEGI | .289 | .351* | .366* | .236 |
| Irregular past tense SIT | .416* | .250 | .297 | .117 |
| Irregular past tense TEGI | .267 | .379* | .432** | .370* |
| BE SIT | .165 | .115 | .114 | .034 |
| BE TEGI | .258 | .382* | .266 | .247 |
| DO SIT | −.076 | −.311 | −.129 | .030 |
| DO TEGI | .318 | .395* | .529** | .328 |
Note. PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; EVT-2 = Expressive Vocabulary Test–Second Edition; 3S = third-person singular; BE = auxiliary and copula “be” verbs; DO = auxiliary “do” verbs.
p < .050.
p < .010.
p < .060.
Discussion
Tense marking is an area of weakness for many children with language impairments. The amount of overlap between ASD and FXS+ASD in this aspect of language has not been studied to date, and therefore, this early-stage study provided interesting preliminary findings into patterns of similarities and differences between these two clinical groups. Given the small sample sizes, we included effect size estimates. The profile of strengths and weaknesses within the groups was similar, despite the boys with ASD outperforming the boys with FXS on the majority of the probes. Both groups of boys had excellent accuracy on the third-person singular probes, indicating an area of strength. Their scores on the “be” probe of the TEGI were quite high. However, both groups of boys struggled more with past tense verbs and “do.” It is important to note that the TEGI score for irregular past tense verbs marks overregularized verbs as incorrect (e.g., “She catched the ball” instead of “She caught the ball”). The TEGI includes a separate scoring that includes overregularizations as correct. We completed this scoring as well and found the boys with ASD were 81.79% correct (SD = 19.78) compared to original score of 60.54% and the boys with FXS+ASD were 61.27% correct (SD = 32.58), instead of 45.12%. Using these scores, the boys with ASD had higher scores than the boys with FXS+ASD, t(30) = 2.31, p = .028; d = .76; although with the adjusted p values, this was not significant.
Taken together, these results are striking given the developmental course of these grammatical morphemes in typical development. Based on Brown's stages of morphological development, children acquire irregular past tense verbs before third-person singular or auxiliary “be” (Brown, 1973). Regular past tense and third-person singular are both acquired in Stage IV, and auxiliary “be” and “do” are later acquired in Stage V. It seems that the boys in this study have a weakness with regular past tense verbs and “do” as measured by the TEGI, but a strength in third-person singular and copula and auxiliary “be.” The use of overregularizations for past tense verbs is a normal part of the development of these verbs; however, they are used relatively infrequently, and children quickly replace this form with the correct form (Marcus et al., 1992). As noted above, the boys in this study were using overregularizations with some frequency. It seems both groups of boys are in fact using this form, perhaps indicating a more immature understanding of the grammatical rule associated with irregular past tense verbs.
Although the two groups were similar on MLU and ASD severity, the boys with ASD outperformed the boys with FXS on the “be” probe of the TEGI, and effect sizes indicate that there were most likely differences in regular and irregular past tense on the sentence imitation task and the “do” probe of the TEGI and a medium effect size for the regular past tense probe of the TEGI. At first glance, it might seem that this was due to the significantly lower IQs in the group of boys with FXS+ASD. The discriminant validity data indicated that nonverbal IQ was only correlated with the irregular past tense probe on the sentence imitation task, providing evidence that the differences noted in these groups are not necessarily tightly linked with nonverbal IQ. Although the two groups of boys were quite different on nonverbal IQ, they were similar on MLU, indicating that grammatical skills as measured in this study via MLU, the TEGI, and the sentence imitation task are not explained by differences in nonverbal IQ.
In terms of the other discriminant validity data, after applying a Bonferroni correction, there were no significant correlations. However, the magnitude of the correlations and trend of the p values suggest that PPVT-4 and EVT-2 scores are related to the probes from the TEGI and sentence imitation task, which suggests an important link with vocabulary. In fact, the trends within the correlations indicate a possible relationship between PPVT-4 and all of the TEGI probes, but not the sentence imitation probes. Roberts et al. (2004) reported a significant correlation between vocabulary comprehension and tense markers in children with ASD. They did not find significant differences between their groups of children with low and high IQs, with the exception of irregular past tense scores. Future studies should continue to examine this relationship, because some children with ASD and FXS+ASD have an atypical profile of language, with vocabulary production exceeding comprehension (Haebig & Sterling, 2017). These studies provide evidence that children with ASD and FXS have grammatical skills that are related to vocabulary comprehension but not necessarily nonverbal IQ. We examined the convergent validity between the two assessments—a standardized measure of grammar and a sentence imitation task. The TEGI is a norm-referenced assessment that is sensitive and specific to the development of grammatical tense markers. Although sentence imitation tasks have been used in clinical populations, this was a first look at the use of this specific task in boys with FXS+ASD and idiopathic ASD. After applying a Bonferroni correction, we found significant correlations between the third-person singular, regular past tense, and irregular past tense probes from the sentence imitation task and the TEGI. The convergent validity for these two instruments was high, particularly for the third-person singular and irregular past tense probes, indicating the sentence imitation task is an appropriate measure for these targets. There were no significant correlations between the “be” probes or the “do” probes. The boys were near ceiling on the “be” and “do” probes for the sentence imitation task, although there were a high number of unscorable responses on the “do” probe in particular, thus influencing the results. The ceiling scores on the “be” probe suggest that the children in this study were too developmentally advanced for the items and that this probe perhaps would be more appropriate for younger children.
The two groups—idiopathic ASD and FXS+ASD—demonstrated a consistent profile across the two measures. The significant large correlations between the TEGI and sentence imitation task probes for the third-person singular and regular and irregular past tense probes indicate good convergent validity. When examining the types of responses in more depth, the participants for the most part were providing some sort of scorable response. This is important to determine, given that the TEGI scores reflect only the correct and incorrect items. Therefore, if there were a high number of unscorable items, the score might not be reflective of true ability. One notable exception was the high number of unscorable responses for the boys with FXS+ASD on the “do” probes in the sentence imitation and TEGI. The boys with ASD had more than 30% unscorable responses on the “do” probe of the TEGI, and the boys with FXS+ASD had even higher rates. Both the sentence imitation task and the TEGI required the participants to ask a question using the verb “do” (e.g., TEGI: Do the moon guys like apples?; sentence imitation task: Do the children like pizza?), whereas the third-person singular and past tense probes were in declarative format. The “be” probes had a mix of declarative and interrogative forms, perhaps partly explaining the higher rates of unscorable responses for the “be” probes. The ability to ask questions involves skills in pragmatic abilities, a core deficit in ASD and a major weakness in FXS. The literature on question asking in ASD consistently documents weaknesses in question asking in young children with ASD, as well as adolescents and adults (Koegel, Camarata, Valdez-Menchaca, & Koegel, 1998; Koegel, Koegel, Green-Hopkins, & Barnes, 2010). The difficulty in this grammatical form and high rate of unscorable responses could be a reflection of the poor pragmatic skills and overall difficulty with question asking in these clinical populations.
There are several limitations to the current study. Although the sample size is in line with published studies, our ability to analyze the data was limited. This early-stage, small-scale study served as the first step in evaluating aspects of tense marking in ASD and FXS+ASD. Our effect sizes indicate important differences between the two groups, but results must be replicated in larger studies. This study did not include a group of boys with FXS only or a group of boys matched on mental age without ASD or FXS. The inclusion of either group would allow the opportunity to dig deeper into the impact of ASD on FXS and the extent to which it uniquely impacts FXS versus what is observed in children with similar mental ages without an ASD diagnosis. The current study indicates important areas of overlap and differences, which should be tested in boys with FXS only and mental age matches. In addition, future studies should examine more complex grammatical abilities, such as through the use of the IPSyn, to allow for more in-depth comparisons in terms of spontaneous language (Scarborough, 1990). The current study represents only a snapshot in time, and future work should follow adolescents over time to understand how language continues to grow and how adults use grammar once they transition out of school services. This study excluded female individuals, and yet female individuals with ASD and FXS are understudied, particularly in terms of language skills. Future studies should evaluate language in female individuals.
There are important clinical implications from this study, in terms of the pattern of strengths and weakness in these grammatical tense markers. Although the sentence imitation task had high convergent validity and low rates of unscorable responses for the third-person singular and past tense probes, it had much higher rates of unscorable responses for the “be” and “do” probes and poor convergent validity for these two aspects of tense marking. It would not be an appropriate measure for “be” and “do” but could be used for third-person singular and past tense. Given its low cost in administration and scoring, it is an efficient measure to use these clinical populations. This is particularly important given the attention and hyperactivity difficulties commonly noted in children with FXS+ASD and idiopathic ASD. Nonverbal IQ indicated a trend toward significance with irregular past tense responses on the sentence imitation task, but no other probes. Clinicians should be aware that grammatical abilities are not necessarily tightly linked with intellectual abilities, but instead a unique deficit within the language phenotype of these clinical populations.
Acknowledgments
This research is supported in part by Grants R03 DC011616 (principal investigator: Sterling) and P30 HD03352 (principal investigator: Albee Messing), as well as start-up funds from the University of Wisconsin-Madison (awarded to Sterling). The author would like to thank the children and families who participated in this research. The author would also like to thank Leonard Abbeduto for his input on the design of the study; Susan Ellis Weismer and Jill Hoover for their thoughtful commentary on this article; Eileen Haebig for her key role in data collection, and transcription and coding of the data; and Susen Schroeder, Sara Kover, Courtney Venker, Rachel Brewer, Michelle Kletzien, Erin Schwartz, Courtney Ramczyk, Holly Erbstoesser, Laura Friedman, and Kirsten Larson for their assistance with, recruitment, transcription and data entry.
Funding Statement
This research is supported in part by Grants R03 DC011616 (principal investigator: Sterling) and P30 HD03352 (principal investigator: Albee Messing), as well as start-up funds from the University of Wisconsin-Madison (awarded to Sterling).
Footnote
TEGI test items reprinted with permission. Copyright © 2014 Mabel Rice & Kenneth Wexler.
References
- Abbeduto L., McDuffie A., & Thurman A. J. (2014). The fragile X syndrome-autism comorbidity: What do we really know? Frontiers in Genetics, 5, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel A. D., Rice M. L., & Bontempo D. E. (2015). Effects of verb familiarity on finiteness marking in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 58, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. B., Hatton D. D., Skinner M., & Mesibov G. (2001). Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders, 31, 165–174. [DOI] [PubMed] [Google Scholar]
- Bailey D. B., Mesibov G. B., Hatton D. D., Clark R. D., Roberts J. E., & Mayhew L. (1998). Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders, 28, 499–508. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E., Doll E., Sterling A., Kover S. T., Schroeder S. M., Mathur S., & Abbeduto L. (2013). Development of an expressive language sampling procedure in fragile X syndrome: A pilot study. Journal of Developmental and Behavioral Pediatrics, 34, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. V. M., Adams C. V., & Norbury C. F. (2006). Distinct genetic influences on grammar and phonological short-term memory deficits: Evidence from 6-year-old twins. Genes, Brain and Behavior, 5, 158–169. [DOI] [PubMed] [Google Scholar]
- Boucher J. (2012). Research review: Structural language in autistic spectrum disorder—characteristics and causes. The Journal of Child Psychology and Psychiatry, 53, 219–233. [DOI] [PubMed] [Google Scholar]
- Brown R. (1973). A first language: The early stages. Cambridge, MA: Harvard University Press. [Google Scholar]
- Budimirovic D. B., & Kaufmann W. E. (2011). What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience, 33, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., & Fiske D. W. (1959). Convergent and discriminant validation by the multitrait–multimethod matrix. Psychological Bulletin, 56, 81–105. [PubMed] [Google Scholar]
- Clifford S., Dissanayake C., Bui Q. M., Huggins R. M., Taylor A. K., & Loesch D. Z. (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders, 37, 738–747. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Conti-Ramsden G., Botting N., & Faragher B. (2001). Psycholinguistic markers for specific language impairment. The Journal of Child Psychology and Psychiatry, 42, 741–748. [DOI] [PubMed] [Google Scholar]
- Demark J. L., Feldman M. A., & Holden J. J. (2003). Behavioral relationship between autism and fragile x syndrome. American Journal of Mental Retardation, 108, 314–326. [DOI] [PubMed] [Google Scholar]
- Dennis M., Francis D. J., Cirino P. T., Russell S., Barnes M. A., & Fletcher J. M. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake C., Bui Q., Bulhak-Paterson D., Huggins R., & Loesch D. Z. (2009). Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50, 290–299. [DOI] [PubMed] [Google Scholar]
- Dunn L. M., & Dunn D. M. (2007). Peabody Picture Vocabulary Test–Fourth Edition. Bloomington, MN: NCS Pearson. [Google Scholar]
- Eigsti I. M., Bennetto L., & Dadlani M. B. (2007). Beyond pragmatics: Morphosyntactic development in autism. Journal of Autism and Developmental Disorders, 37, 1007–1023. [DOI] [PubMed] [Google Scholar]
- Estigarribia B., Roberts J. E., Sideris J., & Price J. R. (2011). Expressive morphosyntax in boys with fragile X syndrome with and without autism spectrum disorder. International Journal of Language & Communication Disorders/Royal College of Speech & Language Therapists, 46, 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack L. H., & Abbeduto L. (2010). Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. Journal of Speech, Language, and Hearing Research, 53, 1334–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack L. H., Sterling A. M., & Abbeduto L. (2013). Discriminating Down syndrome and fragile X syndrome based on language ability. Journal of Child Language, 40, 244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig C. S. (2008). Verbal working memory and story retelling in school-age children with autism. Language, Speech, and Hearing Services in Schools, 39, 498–512. [DOI] [PubMed] [Google Scholar]
- Gaetano J. (2013). Holm–Bonferroni sequential correction: An EXCEL calculator [Microsoft Excel Workbook].
- Garcia-Nonell C., Rigau Ratera E., Harris S. W., Hessl D., Ono M. Y., Tartaglia N., … Hagerman R. J. (2008). Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. American Journal of Medical Genetics, Part A, 146A, 1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K., Pickles A., & Lord C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haebig E., & Sterling A. (2017). Investigating the receptive-expressive vocabulary profile in children with idiopathic ASD and comorbid ASD and fragile X syndrome. Journal of Autism and Developmental Disorders, 47, 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haebig E., Sterling A. M., & Hoover J. R. (2016). Examining the language phenotype in children with typical development, specific language impairment, and fragile X syndrome. Journal of Speech, Language, and Hearing Research, 59, 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. S., Lightbody A. A., Hirt M., Rezvani A., & Reiss A. L. (2010). Autism in fragile X syndrome: A category mistake? Journal of the American Academy of Child & Adolescent Psychiatry, 49, 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. W., Hessl D., Goodlin-Jones B., Ferranti J., Bacalman S., Barbato I., … Hagerman R. J. (2008). Autism profiles of males with fragile X syndrome. American Journal of Mental Retardation, 113, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann M., Nordqvist E., Strid K., Connant Almrot J., & Tjus T. (2016). Children with autism respond differently to spontaneous, elicited and deferred imitation. Journal of Intellectual Disability Research, 60, 491–501. [DOI] [PubMed] [Google Scholar]
- Hernandez R. N., Feinberg R. L., Vaurio R., Passanante N. M., Thompson R. E., & Kaufmann W. E. (2009). Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Medical Genetics, Part A, 149, 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J. R., Storkel H. L., & Rice M. L. (2012). The interface between neighborhood density and optional infinitives: Normal development and specific language impairment. Journal of Child Language, 39, 835–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard M. M., & Tager-Flusberg H. (2001). An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes, 16, 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J., Martin G. E., & Losh M. (2014). Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. Journal of Intellectual Disability Research, 58, 940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel L. K., Camarata S., Valdez-Menchaca M., & Koegel R. L. (1998). Setting generalization of question-asking by children with autism. American Journal on Mental Retardation, 102, 346–357. [DOI] [PubMed] [Google Scholar]
- Koegel L. K., Koegel R. L., Green-Hopkins I., & Barnes C. C. (2010). Brief report: Question-asking and collateral language acquisition in children with autism. Journal of Autism and Developmental Disorders, 40, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover S., & Atwood A. (2013). Establishing equivalence: Methodological progress in group-matching design and analysis. American Journal on Intellectual and Developmental Disabilities, 118, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover S. T., Davidson M. M., Sindberg H. A., & Ellis Weismer S. (2014). Use of the ADOS for assessing spontaneous expressive language in young children with ASD: A comparison of sampling contexts. Journal of Speech, Language, and Hearing Research, 57, 2221–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover S. T., Pierpont E. I., Kim J.-S., Brown W. T., & Abbeduto L. (2013). A neurodevelopmental perspective on the acquisition of nonverbal cognitive skills in adolescents with fragile X syndrome. Developmental Neuropsychology, 38, 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L. B., & McGregor K. K. (1992). Grammatical morphology and speech perception in children with specific language impairment. Journal of Speech and Hearing Research, 35, 1076–1086. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Bui Q. M., Dissanayake C., Clifford S., Gould E., Bulhak-Paterson D., … Huggins R. M. (2007). Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience and Biobehavioral Reviews, 31, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P. C., & Risi S. (1999). Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., & Bishop S. (2012). Autism Diagnostic Observation Schedule–Second Edition (ADOS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Losh M., & Capps L. (2003). Narrative ability in high-functioning children with autism or Asperger's syndrome. Journal of Autism and Developmental Disorders, 33, 239–251. [DOI] [PubMed] [Google Scholar]
- Marcus G., Pinker S., Ullman M., Hollander M., Rosen T., & Xu Fei. (1992). Overregularization in language acquisition. Monographs for the Society of Research in Child Development, 57(4, Serial No. 228). [PubMed] [Google Scholar]
- Martin G. E., Losh M., Estigarribia B., Sideris J., & Roberts J. E. (2013). Longitudinal profiles of expressive vocabulary, syntax and pragmatic language in boys with fragile X syndrome or Down syndrome. International Journal of Language and Communication Disorders, 48, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., & Chapman J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Andriacchi K., & Nockerts A. (2011). Assessing language production using SALT software: A clinician's guide to language sample analysis. Madison, WI: SALT Software, LLC. [Google Scholar]
- Price J. R., Roberts J. E., Hennon E. A., Berni M. C., Anderson K. L., & Sideris J. (2008). Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research, 51, 3–15. [DOI] [PubMed] [Google Scholar]
- Redmond S. M., Thompson H. L., & Goldstein S. (2011). Psycholinguistic profiling differentiates specific language impairment from typical development and from attention-deficit/hyperactivity disorder. Journal of Speech, Language, and Hearing Research, 54, 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. L., Buhr J. C., & Nemeth M. (1990). Fast mapping word-learning abilities of language-delayed preschoolers. Journal of Speech and Hearing Disorders, 55, 33–42. [DOI] [PubMed] [Google Scholar]
- Rice M. L., Redmond S. M., & Hoffman L. (2006). Mean length of utterance in children with specific language impairment and in younger control children shows concurrent validity and stable and parallel growth trajectories. Journal of Speech, Language, and Hearing Research, 49, 793–809. [DOI] [PubMed] [Google Scholar]
- Rice M. L., & Wexler K. (1996). Toward tense as a clinical marker of specific language impairment in English-speaking children. Journal of Speech and Hearing Research, 39, 239–257. [DOI] [PubMed] [Google Scholar]
- Rice M. L., & Wexler K. (2001). Test of Early Grammatical Impairment. San Antonio, TX: The Psychological Corporation; Retrieved from https://cldp.ku.edu/rice-wexler-tegi [Google Scholar]
- Roberts J. A., Rice M. L., & Tager-Flusberg H. (2004). Tense marking in children with autism. Applied Psycholinguistics, 25, 429–448. [Google Scholar]
- Roberts J. E., Hennon E. A., Price J. R., Dear E., Anderson K. L., & Vandergrift N. A. (2007). Expressive language during conversational speech in boys with fragile X syndrome. American Journal on Mental Retardation, 112, 1–17. [DOI] [PubMed] [Google Scholar]
- Roid G., & Miller L. (1997). Leiter International Performance Scale–Revised. Wood Dale, IL: Stoelting. [Google Scholar]
- Rutter M., Le Couteur A., & Lord C. (2003). Autism Diagnostic Interview–Revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Scarborough H. S. (1990). Index of Productive Syntax. Applied Psycholinguistics, 11, 1–22. [Google Scholar]
- Skinner M., Hooper S., Hatton D. D., Roberts J. E., Mirrett P., Schaaf J., … Bailey D. B. (2005). Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics, 132A, 25–32. [DOI] [PubMed] [Google Scholar]
- Sterling A. M., Rice M. L., & Warren S. F. (2012). Finiteness marking in boys with fragile X syndrome. Journal of Speech, Language, and Hearing Research, 55, 1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkel H. L., Maekawa J., & Hoover J. R. (2010). Differentiating the effects of phonotactic probability and neighborhood density on vocabulary comprehension and production: A comparison of preschool children with and without phonological delays. Journal of Speech, Language, and Hearing Research, 53, 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B. G., & Fidell L. S. (2001). Using multivariate statistics (4th ed.). Boston, MA: Allyn & Bacon. [Google Scholar]
- Tager-Flusberg H., & Joseph R. M. (2003). Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London, 358, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper D. E., & Rosenblood L. K. (1984). Methodological considerations in the use of attribute variables in neuropsychological research. Journal of Clinical Neuropsychology, 6, 441–453. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J. M. H., Pieretti M., Sutcliffe J. S., Fu Y.-H., Kuhl D. P. A., Pizzuti A., … Warren S. T. (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell, 65, 905–914. [DOI] [PubMed] [Google Scholar]
- Warren S. F., Brady N. C., Sterling A., Fleming K., & Marquis J. (2010). Maternal responsivity predicts language development in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 115, 54–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. T. (2007). Expressive Vocabulary Test. Minneapolis, MN: Pearson Assessments. [Google Scholar]