Abstract
The nucleus in eukaryotic cells is the site for genomic functions such as RNA transcription, DNA replication, and DNA repair/recombination. However, the nucleus is subjected to various mechanical forces associated with diverse cellular activities, including contraction, migration, and adhesion. Although it has long been assumed that the lamina structure, underlying filamentous mesh-work of the nuclear envelope, plays an important role in resisting mechanical forces, the involvement of compact chromatin in mechanical resistance has also recently been suggested. However, it is still unclear how chromatin functions to cope with the stresses. To address this issue, we studied the mechanical responses of human cell nuclei by combining a force measurement microscopy setup with controlled biochemical manipulation of chromatin. We found that nuclei with condensed chromatin possess significant elastic rigidity, whereas the nuclei with a decondensed chromatin are considerably soft. Further analyses revealed that the linker DNA and nucleosome-nucleosome interactions via histone tails in the chromatin act together to generate a spring-like restoring force that resists nuclear deformation. The elastic restoring force is likely to be generated by condensed chromatin domains, consisting of interdigitated or “melted” 10-nm nucleosome fibers. Together with other recent studies, it is suggested that chromatin functions not only as a “memory device” to store, replicate, and express the genetic information for various cellular functions but also as a “nuclear spring” to resist and respond to mechanical forces.
Keywords: nuclear stiffness, cohesin, Mg2+, lamin, chromosome
Significance.
The nucleus in eukaryotic cells is the site for genomic functions such as RNA transcription, DNA replication, and DNA repair/recombination. However, the nucleus is continually subjected to various mechanical forces. How can the nucleus cope with such forces? Recent studies including ours have suggested that chromatin functions not only as a “memory device” to store, replicate, and express the genetic information for various cellular functions but also as a “nuclear spring” to resist and respond to mechanical forces.
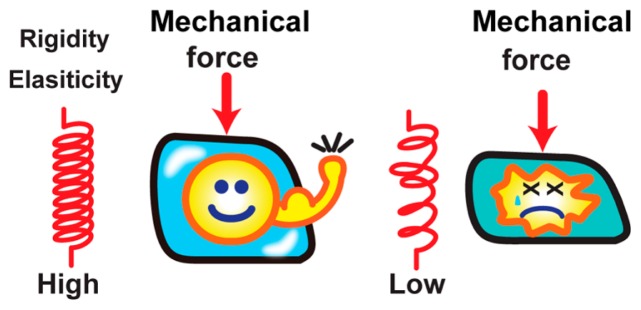
Our human body is composed of ~40 trillion cells [1]. Each cell contains ~2 m of genomic DNA in a small cell nucleus of an approximately 10 μm diameter (a volume of only ~100 fL–1 pL). The cell nucleus is the site of genomic functions such as RNA transcription, DNA replication, and DNA repair/recombination in eukaryotes [2]. While maintaining its structural and functional stability, the nucleus is subjected to various mechanical forces (Fig. 1) [3–6]. The stress is especially prominent in skeletal and cardiac muscle cells, whose nuclei are exposed to contractile forces generated by cells [5], and also in migrating cells, which penetrate to distant tissues by squeezing their bodies through small spaces [7]. Failures in resisting such mechanical stresses result in substantial damage to genomic DNA, which subsequently perturbs its function and has been linked to tumorigenesis and cell death (Fig. 1) [8–10]. The nucleus thus requires a mechanism(s) to resist and respond to these mechanical forces.
Figure 1.
Resisting mechanical stress. The cell is continually subjected to various mechanical forces (left). Failures in resisting such mechanical stress can cause cell abnormality such as cell death (right). The nucleus requires mechanism(s) to resist and respond to the mechanical force. The illustrations were reproduced from [67] with modifications.
In the stress resistance of nuclei, it has long been thought that the filamentous meshwork structure of the nuclear envelope, including lamin A/C, lamin B, and other inner nuclear membrane proteins, play an important role [11,12]. Indeed, their mutations often lead to loss of nuclear integrity and cause a variety of human genetic disorders: the so-called “laminopathies” such as Emery-Dreifuss muscular dystrophy and Hutchinson-Gilford progeria syndrome.
Chromatin, which consists of nucleosomes with long genomic DNA wrapped around core histones [13] and other associated proteins, has recently been suggested to contribute to the mechanical properties of the nucleus as a non-genetic function [14]. Unlike conventional textbook models, recent studies have suggested that, within the nucleus, the nucleosome fibers are irregularly folded into higher-order structures [15–24] such as interdigitated fibers, which resemble a “polymer melt” (i.e. liquid-like state of entangled polymers) [25], and which undergo compacted domain/fiber-like organization [26–31]. However, it has been unclear how such an irregularly-folded chromatin supports the nuclear structure and resists mechanical stress.
Several biophysical measurements have shown that nuclei with condensed chromatin possess substantial mechanical resistance [32–37]. However, the direct consequence of chromatin compaction on nuclear rigidity is unknown, because there is no effective method to quantitatively analyze chromatin’s mechanical contribution, together with its changing compaction levels. To address this issue, we developed an assay that combines a force measurement microscopy setup [38, 39] with controlled biochemical manipulation of chromatin [40] to study the mechanical responses of human cell nuclei. In this review article, we summarize our recent findings and discuss the mechanisms regulating nuclear mechanics, along with related studies recently published.
Measurement of nuclear rigidity
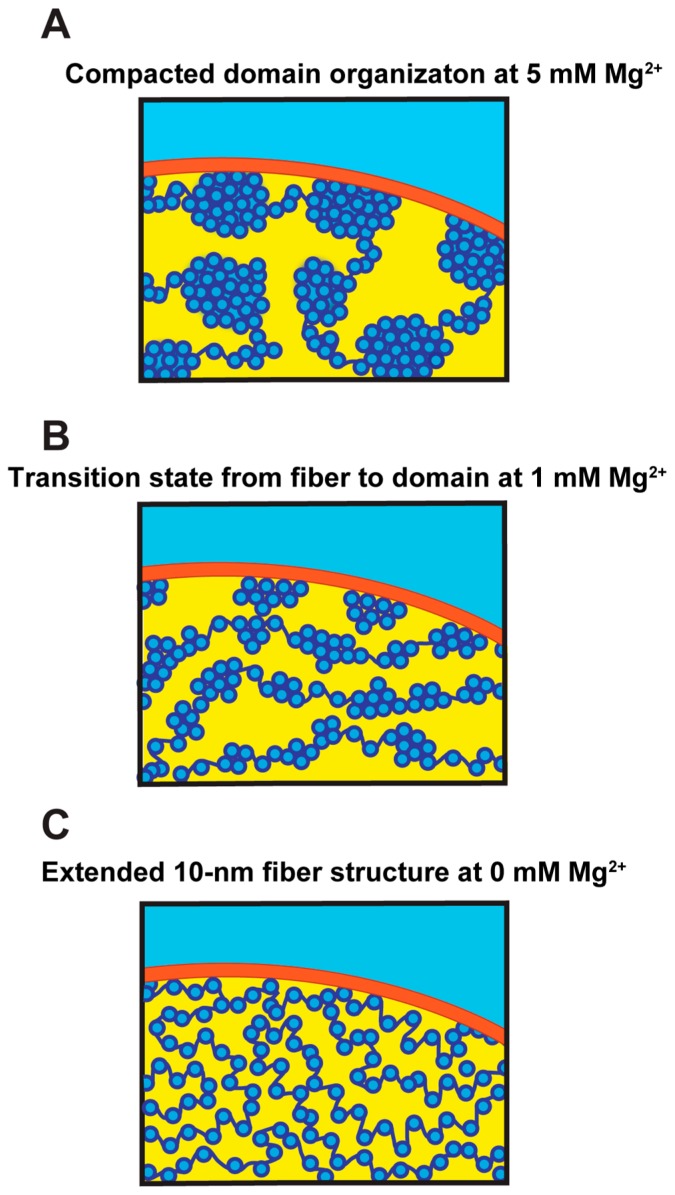
The nuclei that we examined were isolated from HeLa cells [41]. Chromatin has a net negative charge and is, by itself, stretched like “beads on a string” by electrostatic repulsion, and Mg2+decrease the charge and repulsion. The compaction states of chromatin in the nuclei can be controlled by altering the Mg2+ concentration in the environment [42,43]. For example, at 5 mM Mg2+, chromatin becomes condensed and forms chromatin domains, whereas it decondenses in the absence of Mg2+ (Fig. 2).
Figure 2.
Chromatin organization in the cell nucleus. Schematics showing possible folding of the nucleosome fibers in the nucleus at different Mg2+ concentrations. Dark blue, DNA; light blue, core histones; red, nuclear envelope. (A) At 5 mM Mg2+, the nucleosome fibers are irregularly folded and assemble into compacted domains. (B) At 1 mM Mg2+, the nucleosome is in a transition state. (C) At 0 mM Mg2+, the nucleosome fibers exhibit an elongated beads-on-a-string structure. The illustrations were reproduced from [41] with a minor modification.
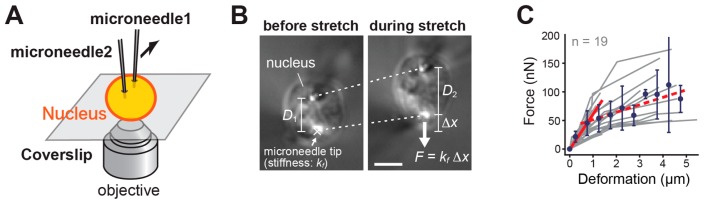
For measuring their micromechanics, single nuclei were captured using a pair of thin glass microneedles (diameter: ~1 μm). They were then stretched by moving one micro-needle away from the other (Fig. 3A) [41]. This induced an extensional deformation (dotted lines, Fig. 3B) and the development of a resisting force in the nuclei. The force (F) could be estimated according to the Hooke’s law, based on the bend of the flexible microneedle tip (Δx, Fig. 3B) and its pre-calibrated stiffness (kf) [41]. Nuclear rigidity was examined based on the developed force and resultant deformation, under various biochemical conditions of chromatin (Fig. 2).
Figure 3.
Quantifying nuclear mechanical response. (A) Microneedle-based setup used for examining the mechanical responses of nuclei. (B) Bright field images of a nucleus isolated from HeLa cells and deformed by using a pair of microneedles. The upper microneedle was moved upward to stretch the nucleus, resulting in an application of stretching force (F) whose magnitude could be estimated based on the bent of the force-calibrated microneedle tip (Δx; stiffness; kf) from its equilibrium (marked as ×). Dotted lines show changes in positions of the microneedle tips, which were used to estimate the extent of nuclear deformation (D2 – D1). Scale bar, 5 μm. (C) Force-deformation plots obtained at Mg2+ levels of 5 mM (n = 19). Data from individual nuclei (gray lines) were pooled for 0.5 μm bins and averaged (bars represent SD). The slope is 63.1 (red solid line; R2 > 0.97). The red broken line shows the region of predominant viscous deformation (slope: 14.5). The observed two-phase response might be related to those observed by Stephan et al. [54]. The illustration and data were reproduced from [41] with modifications.
We found that the nuclear rigidity depended on Mg2+ concentration in the buffer to which the nuclei were exposed. At 5 mM Mg2+, when chromatin is assembled into highly compacted domains (Fig. 2A), the nuclei showed an elastic deformation in the submicron range (~50 nN/μm), and restored their original shape as soon as the applied force was removed (solid line, Fig. 3C). On the other hand, the nuclei became more softened as a larger force was applied (~100 nN) ( dotted line, Fig. 3C) [41]. Typical force magnitudes that cells generate and are subjected to are on the order of nN. Therefore, nuclei with condensed chromatin possess considerable elastic rigidity and can maintain their shape against cellular-scale forces.
At 1 mM Mg2+, chromatin is slightly decondensed and forms an intermediate structure between the chromatin fibers and domains (Fig. 2B). The rigidity of nuclei, measured at the elastic small deformation range, was >3-fold smaller at 1 mM Mg2+ than at 5 mM Mg2+ (~15 nN/μm) [41]. When exposed to 1 mM EDTA (i.e., at ~0 mM Mg2+), the nuclei were swollen (>200%) with highly extended nucleosome fibers, which repulsed to each other (Fig. 2C). The rigidity of nuclei with the decondensed chromatin was further reduced to ~5 nN/μm, which was ~10-fold lower than the value at 5 mM Mg2+ [41]. Notably, the Mg2+-dependent change in nuclear rigidity was reversible, as nuclei, which were once swollen in 1 mM EDTA and then exposed to 5 mM Mg2+ buffer, regained their initial rigidity [41].
Consistent with this mechanical reversibility, SDS-PAGE analysis revealed no obvious differences in the abundance of major nuclear components, including core and linker histones, over the tested perturbation conditions [41]. A western blot analysis also confirmed that putative interphase DNA-crosslinking proteins cohesin, [44,45], CTCF [46], condensin II [47], and inner nuclear membrane protein lamin A/C [11,12] remained intact [41]. Together, we concluded that the mechanical rigidity of nuclei altered upon Mg2+-dependent chromatin compaction.
Nucleosome-nucleosome interactions via histone tails and linker DNA in chromatin regulate nuclear rigidity
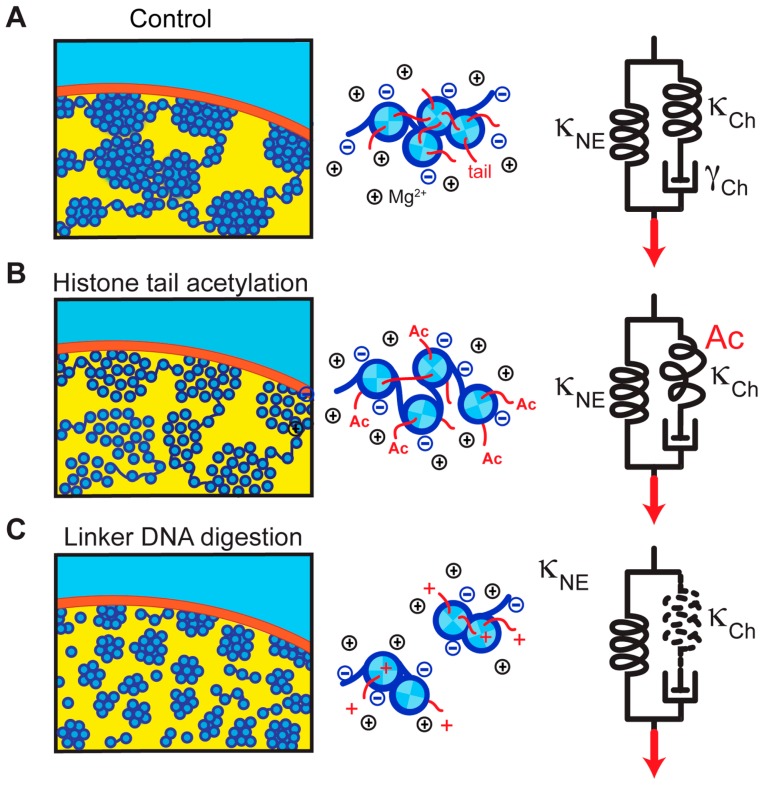
Previous studies reported that treatment of cells with the histone deacetylase inhibitor, trichostatin A (TSA) [48], resulted in decondensed chromatin, most likely because the nucleosome-nucleosome interactions, which are mediated by binding of the histone H4 and H3 tails to the neighboring nucleosomes, were weakened (Fig. 4A and B) [20,31,49,50]. To examine its effect on nuclear mechanics, histone-acetylated nuclei were prepared by treating cells with TSA followed by the same isolation protocol used as mentioned above. We found that histone-acetylated nuclei at 5 mM Mg2+ were significantly softer (~25 nN/μm) than untreated control nuclei [41]. Therefore, the nuclear rigidity depended on nucleosome-nucleosome interactions mediated by histone tails (Fig. 4A and B) [50–52].
Figure 4.
Model for the nuclear mechanical response. (Left panels) Possible chromatin folding architecture within a nucleus. DNA (dark blue) is wrapped around core histones (light blue), forming compacted domains at high Mg2+ levels (A). Histone tail acetylation weakens the nucleosome-nucleosome interaction and induces chromatin decompaction (B). Linker DNA digestion leads to disassembly of chromatin domains (C). (Middle panels) Molecular views of the nucleosomes. Nucleosomes are packed at high Mg2+ levels and provide spring-like elasticity (A). Histone tail acetylation (Ac) weakens the internucleosomal interaction, resulting in a smaller restoring force (B). Linker DNA digestion disrupts the connection between nucleosomes, and no restoring force is generated (C). (Right panels) Model of nuclear mechanics, which is composed of two parallel units: an elastic spring attributable to the nuclear envelope (κNE), and a series connection of a spring and a viscous dashpot attributable to chromatin (κCh and γCh, respectively) (A). Histone tail acetylation lowers the stiffness of κCh (B). Linker DNA digestion leads to its complete loss (C). Neither of these perturbations alters κNE, which can be linked to lamin-based structures underlying the nuclear envelope [11,12,53,54]. The illustrations were reproduced from [41] with modifications.
We also identified a role of linker DNA in regulating nuclear rigidity. This was achieved by treating isolated nuclei with a restriction endonuclease HaeIII to cut the nuclear DNA into small fragments, followed by exposure to a stretching force (Fig. 4A and C). These nuclei appeared to have >3-fold softer rigidity than untreated control samples (~17 nN/μm; versus >55 nN/μm at 5 mM Mg2+) [41].
Notably, the rigidity of nuclei with biochemically perturbed chromatin, such as those treated with histone deacetylase inhibitor or with HaeIII, was not decreased further when the Mg2+ concentration was reduced (~15–20 nN/μm at 1 mM Mg2+) [41]. We suggest that this residual “baseline” rigidity is derived from lamin-based structures underlying the nuclear envelope [11,12,53,54]. As these biochemical perturbations of chromatin showed little effect on nuclear composition, the nucleosome-nucleosome interactions via histone tails play a major role in regulating the nuclear mechanical response (Fig. 4).
The mechanical properties of the isolated nuclei appeared to have the conserved characteristics of the nuclei in living cells. This was verified by inserting very thin microneedle tips (<1 μm diameter) into the living cell’s interior and capturing the single nucleus in situ, without perturbing the overall physiology of the cell. The measured rigidity of the nuclei was approximately linear over a range of deformations (several μm range) with a stiffness value of ~25 nN/μm [41]. Furthermore, treatment of cells with TSA resulted in a marked decrease of the nuclear rigidity (~10 nN/μm), as observed in vitro [41]. Notably, the rigidities of the cell cortex and cytoplasm were relatively small (~5 nN/μm), as measured by inserting the probe tips into the cell’s interior but outside the nucleus [41].
These findings led us to propose a simple model for the mechanical response of the nucleus to applied force (right panel, Fig. 4). In this model, the mechanical resistance of the nucleus is governed by two linear mechanical springs that are arranged in parallel [41]; one is attributable to chromatin (κCh) and the other to nuclear envelope structures such as lamins (κNE) [11,12,53,54]. The spring, κCh, engages in a series connection to a viscous dashpot element (γch) whose contribution becomes significant when chromatin fibers are compacted and subjected to an extremely large distortion (right panel, Fig. 4).
Recently, Stephens et al. also addressed a similar issue by applying a micro-manipulation technique to deform isolated nuclei [54]. They found two distinct mechanical regimes; an initial “short-extension” response was followed by a “long-extension” response with higher stiffness to force [54]. The first response was due to chromatin, shown by experiments involving digestion of DNA. The second response was due to lamins (lamin A/C and lamin B1), which was shown by their knockdown experiments and by using nuclei with naturally low levels of lamins. They showed that chromatin governs elastic deformations of the nucleus under small extensions (<3 μm), while lamins govern the elastic deformation under larger extensions [54]. The authors also provided a computational model consisting of a polymeric shell (lamins) and crosslinked polymer interior (chromatin) to explain the observed mechanical responses [54]. Overall, their results are consistent with ours, except for the following two points: in their model, the two elastic springs are nonlinear and each acted at different deformation magnitude, and the values of spring stiffness are >10-fold smaller than ours [41]. Possible explanations for these differences might involve the buffer composition such as the concentration of divalent cations, as well as the micromanipulation systems used.
Notably, more recent studies showed that an abnormal appearance of nuclear deformations called “nuclear blebs” were often associated with changes in chromatin stiffness [55]; also see [36]. Hence, chromatin tethering to the nuclear lamina/envelope might also be an important factor in maintaining mechanical stability of the nucleus [56]; see also [36,37].
Chromatin functions as a spring
Our analysis revealed that the linker DNA and nucleosome-nucleosome interactions via histone tails act together to generate a restoring force that resists nuclear deformation (Fig. 4) [41]. The elastic restoring force of this nuclear spring is likely to be generated by compact chromatin domains [26–31], consisting of interdigitated or the “melted” 10 nm nucleosome fibers [25], which provide the biological relevance of irregular folding found in chromatin in various cells. Furthermore, considering that condensed chromatin is more resistant to radiation damage/chemical attack than its extended form [40], the condensed chromatin domains might have an evolutionary advantage in maintaining genomic DNA integrity as a nongenetic function, which has not been well-studied or well-appreciated.
Irregular folding of chromatin [17,21,23,57–60] can also contribute to mitotic chromosomal integrity. Indeed, isolated mitotic chromosomes behave like interphase chromatin in the presence and absence of salt. Without Mg2+, the chromosomes are highly swollen and the chromosomal fibers are stretched into 10 nm-like fibers, whereas in the presence of Mg2+, the chromosomes are highly condensed [57,61–63]. Consistent with findings of interphase nuclei described in this paper, the mechanical rigidity of isolated mitotic chromosomes is highly sensitive to nuclease treatment [64] and salt concentrations [65]. Notably, a recent study reported that levels of free Mg2+ increased during mitosis [43]. This is especially advantageous for chromosome segregation and transmission processes in mitosis, during which chromosomes are subjected to significant pulling and shearing stresses on the order of nN [43,66].
Conclusions
We highlighted several recent studies indicating that highly condensed chromatin provides mechanical elasticity to the nucleus. Chromatin functions as not only a “memory device” to store, replicate, and express the genetic information for various cellular functions, but also as a “nuclear spring” to resist and respond to mechanical force.
Acknowledgements
We thank the Maeshima and Shimamoto laboratory members for helpful discussions and support. This work was supported by JSPS grant (16H04746), a Japan Science and Technology Agency CREST grant (JPMJCR15G2), and Takeda Science Foundation to K. M, and AMED-PRIME, JSPS grant (15K14515), JST Disseminate Tenure-Track System, and The Takeda Science Foundation to Y. S.
Footnotes
Conflicts of Interest
All the authors declare that they have no conflicts of interest.
Author Contribution
K. M, S. T., and Y. S. wrote the manuscript.
References
- 1.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463–471. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell, Fifth Edition. Garland Science; USA: 2007. [Google Scholar]
- 3.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 4.Hampoelz B, Lecuit T. Nuclear mechanics in differentiation and development. Curr Opin Cell Biol. 2011;23:668–675. doi: 10.1016/j.ceb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Davidson PM, Lammerding J. Broken nuclei--lamins, nuclear mechanics, and disease. Trends Cell Biol. 2014;24:247–256. doi: 10.1016/j.tcb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwerger M, Ho CY, Lammerding J. Nuclear mechanics in disease. Annu Rev Biomed Eng. 2011;13:397–428. doi: 10.1146/annurev-bioeng-071910-124736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–1121. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 10.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, et al. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012;28:464–471. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenbaum Y, Medalia O. Lamins: the structure and protein complexes. Curr Opin Cell Biol. 2015;32:7–12. doi: 10.1016/j.ceb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 14.Bustin M, Misteli T. Nongenetic functions of the genome. Science. 2016;352:aad6933. doi: 10.1126/science.aad6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joti Y, Hikima T, Nishino Y, Kamada F, Hihara S, Takata H, et al. Chromosomes without a 30-nm chromatin fiber. Nucleus. 2012;3:404–410. doi: 10.4161/nucl.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fussner E, Strauss M, Djuric U, Li R, Ahmed K, Hart M, et al. Open and closed domains in the mouse genome are configured as 10-nm chromatin fibres. EMBO Rep. 2012;13:992–996. doi: 10.1038/embor.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan L, Ladinsky MS, Jensen GJ. Chromatin in a marine picoeukaryote is a disordered assemblage of nucleosomes. Chromosoma. 2013;122:377–386. doi: 10.1007/s00412-013-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanborn AL, Rao SS, Huang SC, Durand NC, Huntley MH, Jewett AI, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci USA. 2015;112:E6456–6465. doi: 10.1073/pnas.1518552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh TH, Weiner A, Lajoie B, Dekker J, Friedman N, Rando OJ. Mapping Nucleosome Resolution Chromosome Folding in Yeast by Micro-C. Cell. 2015;162:108–119. doi: 10.1016/j.cell.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricci MA, Manzo C, Garcia-Parajo MF, Lakadamyali M, Cosma MP. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell. 2015;160:1145–1158. doi: 10.1016/j.cell.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Lim HH, Shi J, Tamura S, Maeshima K, Surana U, et al. Budding yeast chromatin is dispersed in a crowded nucleoplasm in vivo. Mol Biol Cell. 2016;27:3357–3368. doi: 10.1091/mbc.E16-07-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeshima K, Ide S, Hibino K, Sasai M. Liquid-like behavior of chromatin. Curr Opin Genet Dev. 2016;37:36–45. doi: 10.1016/j.gde.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, O’Shea CC. ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science. 2017;357:eaag0025. doi: 10.1126/science.aag0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risca VI, Denny SK, Straight AF, Greenleaf WJ. Variable chromatin structure revealed by in situ spatially correlated DNA cleavage mapping. Nature. 2017;541:237–241. doi: 10.1038/nature20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen JC, Connolly M, McDonald CJ, Pan A, Pryamkova A, Ray K, et al. The 10-nm chromatin fiber and its relationship to interphase chromosome organization. Biochem Soc Trans. 2018;46:67–76. doi: 10.1042/BST20170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Kireev I, Plutz M, Ashourian N, Belmont AS. Large-scale chromatin structure of inducible genes: transcription on a condensed, linear template. J Cell Biol. 2009;185:87–100. doi: 10.1083/jcb.200809196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker J, Heard E. Structural and functional diversity of Topologically Associating Domains. FEBS Lett. 2015;589:2877–2884. doi: 10.1016/j.febslet.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markaki Y, Gunkel M, Schermelleh L, Beichmanis S, Neumann J, Heidemann M, et al. Functional nuclear organization of transcription and DNA replication: a topographical marriage between chromatin domains and the interchromatin compartment. Cold Spring Harb Symp Quant Biol. 2010;75:475–492. doi: 10.1101/sqb.2010.75.042. [DOI] [PubMed] [Google Scholar]
- 29.Smallwood A, Ren B. Genome organization and long-range regulation of gene expression by enhancers. Curr Opin Cell Biol. 2013;25:387–394. doi: 10.1016/j.ceb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozaki T, Imai R, Tanbo M, Nagashima R, Tamura S, Tani T, et al. Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging. Mol Cell. 2017;67:282–293.e7. doi: 10.1016/j.molcel.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J. 2005;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause M, Te Riet J, Wolf K. Probing the compressibility of tumor cell nuclei by combined atomic force-confocal microscopy. Phys Biol. 2013;10:065002. doi: 10.1088/1478-3975/10/6/065002. [DOI] [PubMed] [Google Scholar]
- 35.Mazumder A, Roopa T, Basu A, Mahadevan L, Shivashankar GV. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys J. 2008;95:3028–3035. doi: 10.1529/biophysj.108.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furusawa T, Rochman M, Taher L, Dimitriadis EK, Nagashima K, Anderson S, et al. Chromatin decompaction by the nucleosomal binding protein HMGN5 impairs nuclear sturdiness. Nat Commun. 2015;6:6138. doi: 10.1038/ncomms7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiner SM, Koo PK, Zhao Y, Mochrie SG, King MC. The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat Commun. 2015;6:7159. doi: 10.1038/ncomms8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimamoto Y, Maeda YT, Ishiwata S, Libchaber AJ, Kapoor TM. Insights into the micromechanical properties of the metaphase spindle. Cell. 2011;145:1062–1074. doi: 10.1016/j.cell.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimamoto Y, Kapoor TM. Microneedle-based analysis of the micromechanics of the metaphase spindle assembled in Xenopus laevis egg extracts. Nat Protoc. 2012;7:959–969. doi: 10.1038/nprot.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takata H, Hanafusa T, Mori T, Shimura M, Iida Y, Ishikawa K, et al. Chromatin compaction protects genomic DNA from radiation damage. PLoS ONE. 2013;8:e75622. doi: 10.1371/journal.pone.0075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimamoto Y, Tamura S, Masumoto H, Maeshima K. Nucleosome-nucleosome interactions via histone tails and linker DNA regulate nuclear rigidity. Mol Biol Cell. 2017;28:1580–1589. doi: 10.1091/mbc.E16-11-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeshima K, Rogge R, Tamura S, Joti Y, Hikima T, Szerlong H, et al. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. EMBO J. 2016;35:1115–1132. doi: 10.15252/embj.201592660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeshima K, Matsuda T, Shindo Y, Imamura H, Tamura S, Imai R, et al. A Transient Rise in Free Mg2+ Ions Released from ATP-Mg Hydrolysis Contributes to Mitotic Chromosome Condensation. Curr Biol. 2018;28:444–451.e6. doi: 10.1016/j.cub.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 45.Uhlmann F. SMC complexes: from DNA to chromosomes. Nat Rev Mol Cell Biol. 2016;17:399–412. doi: 10.1038/nrm.2016.30. [DOI] [PubMed] [Google Scholar]
- 46.Ghirlando R, Felsenfeld G. CTCF: making the right connections. Genes Dev. 2016;30:881–891. doi: 10.1101/gad.277863.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirano T. Condensins: universal organizers of chromosomes with diverse functions. Genes Dev. 2012;26:1659–1678. doi: 10.1101/gad.194746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 49.Gorisch SM, Wachsmuth M, Toth KF, Lichter P, Rippe K. Histone acetylation increases chromatin accessibility. J Cell Sci. 2005;118:5825–5834. doi: 10.1242/jcs.02689. [DOI] [PubMed] [Google Scholar]
- 50.Kalashnikova AA, Porter-Goff ME, Muthurajan UM, Luger K, Hansen JC. The role of the nucleosome acidic patch in modulating higher order chromatin structure. J R Soc Interface. 2013;10 doi: 10.1098/rsif.2012.1022. 20121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Funke JJ, Ketterer P, Lieleg C, Schunter S, Korber P, Dietz H. Uncovering the forces between nucleosomes using DNA origami. Sci Adv. 2016;2:e1600974. doi: 10.1126/sciadv.1600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–237. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephens AD, Banigan EJ, Adam SA, Goldman RD, Marko JF. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol Biol Cell. 2017;28:1984–1996. doi: 10.1091/mbc.E16-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephens AD, Liu PZ, Banigan EJ, Almassalha LM, Backman V, Adam SA, et al. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol Biol Cell. 2018;29:220–233. doi: 10.1091/mbc.E17-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephens AD, Banigan EJ, Marko JF. Separate roles for chromatin and lamins in nuclear mechanics. Nucleus. 2018;9:119–124. doi: 10.1080/19491034.2017.1414118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eltsov M, Maclellan KM, Maeshima K, Frangakis AS, Dubochet J. Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc Natl Acad Sci USA. 2008;105:19732–19737. doi: 10.1073/pnas.0810057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDowall AW, Smith JM, Dubochet J. Cryo-electron microscopy of vitrified chromosomes in situ. EMBO J. 1986;5:1395–1402. doi: 10.1002/j.1460-2075.1986.tb04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishino Y, Eltsov M, Joti Y, Ito K, Takata H, Takahashi Y, et al. Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure. EMBO J. 2012;31:1644–1653. doi: 10.1038/emboj.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grigoryev SA, Bascom G, Buckwalter JM, Schubert MB, Woodcock CL, Schlick T. Hierarchical looping of zigzag nucleosome chains in metaphase chromosomes. Proc Natl Acad Sci USA. 2016;113:1238–1243. doi: 10.1073/pnas.1518280113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marsden MP, Laemmli UK. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979;17:849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- 62.Earnshaw WC, Laemmli UK. Architecture of metaphase chromosomes and chromosome scaffolds. J Cell Biol. 1983;96:84–93. doi: 10.1083/jcb.96.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell. 2003;5:323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 64.Poirier MG, Marko JF. Mitotic chromosomes are chromatin networks without a mechanically contiguous protein scaffold. Proc Natl Acad Sci USA. 2002;99:15393–15397. doi: 10.1073/pnas.232442599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poirier MG, Monhait T, Marko JF. Reversible hyper-condensation and decondensation of mitotic chromosomes studied using combined chemical-micromechanical techniques. J Cell Biochem. 2002;85:422–434. doi: 10.1002/jcb.10132. [DOI] [PubMed] [Google Scholar]
- 66.King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J Cell Sci. 2000;113:3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- 67.Shimamoto Y, Tamura S, Maeshima K. DNA Works as a Spring in the Cell! Seibutsu Butsuri. 2018;58:24–26. [Google Scholar]