Abstract
Background:
Fibromyalgia is a chronic musculoskeletal pain condition that is often associated with sleep disturbances and fatigue. The pathophysiology of fibromyalgia is not understood, but indirect evidence suggests a central dysfunction of the nociceptive modulating system. The aim of this study was to evaluate whether quantitative sensory testing detects a change in pain thresholds in fibromyalgia patient receiving pregabalin treatment.
Methods:
A total of 25 patients were recruited for the study and received routine pregabalin, but only 14 patients completed the treatment. Assessment of pressure pain thresholds and changes in conditioned pain modulation using ischaemic pain as a conditioning stimulus were measured at baseline and every 4 weeks for 12 weeks. Fibromyalgia impact questionnaire, PainDETECT and SF-12 were also completed.
Results:
Patients with fibromyalgia demonstrated a less-efficient conditioned pain modulation at baseline. An efficient conditioned pain modulation was observed at 1 month and this was maintained until the final visit. Pressure pain thresholds (PPTs) showed a significant improvement from baseline. Patients also reported a similar magnitude of improvements in PainDETECT, fibromyalgia impact questionnaire (FIQ) and its impact on daily life and change in outcome for SF-12.
Conclusion:
This pilot study reports an increase in PPTs and improved conditioned pain modulation response after commencing pregabalin, which was maintained at 12 weeks, and this was supported by positive pain scores. Pregabalin is a licenced treatment for fibromyalgia in Europe, and its response to central sensitisation, particularly ‘dynamic responses’, has not been reported. We conclude that pregabalin has the potential to reduce peripheral and central sensitisation in patients with fibromyalgia, as measured using quantitative sensory testing.
Keywords: Fibromyalgia, conditioned pain modulation, quantitative sensory testing
Introduction
Fibromyalgia (FM) syndrome is a chronic musculoskeletal pain condition that is linked with sleep disturbances and fatigue. The syndrome is defined by the presence of mechanical hyperalgesia felt in deep tissues, and this has been suggested to be associated to central sensitisation, which may result from both peripheral and central mechanisms. Several studies have demonstrated a reduced or inefficient conditioned pain modulation (CPM) in FM,1–3 and this was also confirmed by de Souza4 suggesting that those FM patients with depression have a less-efficient CPM than those without depression.
FM is common, occurring in 2–5% of the population, more often in women than men and usually with profound impact on activities of daily living and productivity.5–8 Diagnosis is generally made according to the 2016 fibromyalgia diagnostic criteria from the American College of Rheumatology (ACR).9,10 However, the multi-dimensional nature of FM makes diagnosing this condition long and complicated, as other possible diagnoses must first be eliminated both medical and psychiatric before a positive diagnosis can be given.
It is increasingly recognised that medicines typically provide a modest response in half or fewer of FM patients treated, which is true in acute pain, neuropathic pain, migraine and osteoarthritis, and the ability to evaluate the effectiveness of these treatments remains poor. Uni-dimensional outcome measure, such as visual analogue scale (VAS), provides a crude integrated measure of a total pain experience but does not identify specific pain mechanisms or differential response of an individual mechanism to a particular treatment. Previous studies using duloxetine in patients with painful diabetic neuropathy recorded that those with malfunctioning pain modulation – less-efficient CPM – seem to benefit from drugs which augment descending inhibitory pain control than those with an efficient CPM.11
CPM refers to the observation that the activity of multi-receptive neurons within the spinal cord can be strongly modulated by an intense pain stimulus outside their peripheral receptive field. The response involves one of the main supraspinal pain inhibitory pathways in the central nervous system; this is known to be impaired in FM neuropathic pain. Diffuse noxious inhibitory control (DNIC) represents a neurophysiologically well-established model of endogenous pain modulation in both animal laboratories as well as in humans.12,13 The term ‘conditioned pain modulation’ for human application of the paradigm of DNIC was applied for human research, and this can be measured clinically by providing a distracting but significant second stimulus, which in practice may involve the sufficient inflation of a blood pressure cuff or immersion in ice water (cold pressor test) or hot water on a different body part than the one on which the pain perception testing is being performed. In a physiologically normal state, the induction of a second stimulus would increase pressure pain threshold (PPT) scores. In chronic pain conditions such as FM, the PPT scores would decrease after the second stimulus was applied, reflecting the lack of a DNIC response.14
Quantitative sensory testing (QST) is a powerful tool for explaining pain mechanisms in a variety of clinical and research conditions such as diabetic neuropathy, spinal cord injury, osteoarthritis15 and FM. This method allows us to measure thresholds for mechanical detection, vibration detection and cool and hot pain sensations and may enable us to evaluate treatments and measure severity of FM as a marker for disease progression. Efficacy outcomes in clinical trials of FM typically employ standardised questionnaire measures, but tests such as QST are yet to be utilised in measuring efficacy of treatments. Pregabalin is a Food and Drug Administration (FDA)-approved drug treatment for FM acting on central and peripheral neuropathic pain. The goals of this study were to assess how pregabalin affects sensory processing in patients with FM and examine whether QST alters over time with the treatment of pregabalin.
Methods
Study design
This was a single-centre, prospective, open-label pilot study carried out at the Pain and Anaesthesia Research Centre at St Bartholomew’s Hospital, Barts Health NHS Trust, London, United Kingdom. The study was approved by the local ethics committee (13/LO/0052) and was conducted in accordance with the Declaration of Helsinki with written informed consent obtained from the participants. There was no external funding or conflicts of interest within the team.
Participants
A total of 25 patients were recruited for the study and diagnosis was made by the pain consultant or rheumatologist. Inclusion criteria included age ≥18, diagnosis of FM as defined by the recently revised criteria of the ACR9,10 and patients with pain score of more than 4 on VAS. Patients had not taken pregabalin or participated in cognitive behavourial therapy/pain rehabilitation or psychological support prior to starting the study.
Baseline QST measurements and questionnaires were measured at baseline and repeated every 4 weeks up to 12 weeks of pregabalin treatment (Figure 1).
Figure 1.
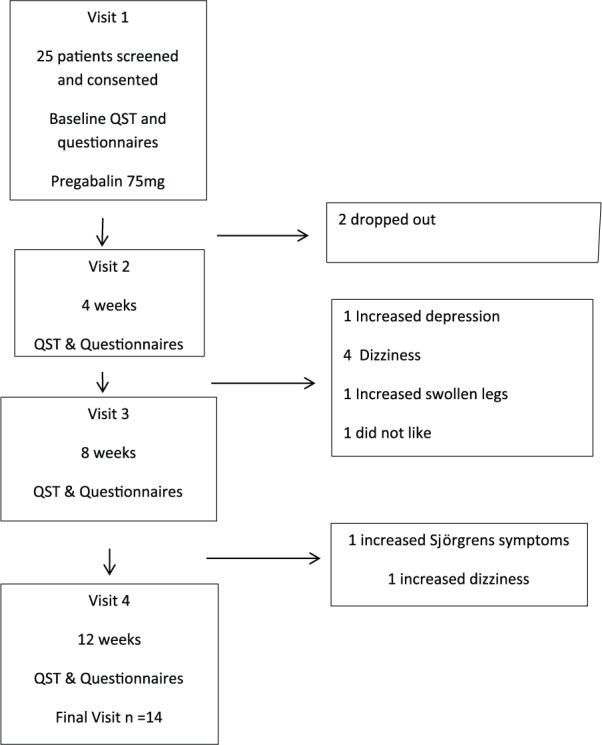
Flow diagram showing the study scheme.
All QST measurements were taken by a single, trained operator to ensure consistency and reproducibility of the results (T.W.). All visits took place in clinic at Barts NHS Trust.
Study procedures
QST: static measures
The test measured sensitivity of peripheral pain pathways to increasing mechanical pressure. A hand-held pressure algometer (Algometer type II, Somedic Production AB, Sweden, diameter contact tip 10 mm; cover 2-mm thick rubber; standardised and constant speed of pressure increase of 0.3 kg/s) was used to measure PPTs at a standardised point – middle part of the right quadriceps femoris muscle (midway between the groin and the apex of patella). This was then marked and recorded. A standardised speed of pressure increase of 0.3 kg/s was kept constant during pressure application to the point when perception changed from pressure to pain (PPT), and the patient then pressed a button to stop the procedure. A mean of the three measures were recorded.
QST: dynamic measures
CPM using the ischaemic arm technique: ischaemic compression of the left arm was used as the conditioning stimulus for evoking CPM (14 patients). The pressure cuff was inflated above systolic pressure (200 mm Hg) for 10 minutes, or until a visual analogue scale (VAS) rating of 6/10 cm was achieved, (where 0 cm represented ‘no pain’ and 10 cm represented ‘maximal pain’). Repeat PPTs were measured on the standardised point (above) while the cuff was in situ. The arm cuff was released once PPT and cuff pain assessments were completed (maximum of 10 minutes).
Questionnaires
A sensory symptom profile of the patient was elicited at baseline and 12 weeks visit using the individual questions from the PainDETECT. A total of seven different sensory symptom profiles were evaluated including burning, tingling, touching, electric shock, heat or cold, numbness and pressure pain sensation and were graded between 0 and 5 (0: no sensation, 1–2: mild sensation, 3: moderate sensation and 4–5: severe sensation). Brief Pain Inventory, FM impact questionnaire and 12 Item short form health survey were also assessed at the same time.
Routine treatment of pregabalin 75 mg twice daily was prescribed for the duration of 12 weeks.
Statistical analysis
All data were analysed using the STATA 14.2 software. Mean and medians (standard error), 95% confidence interval were calculated where appropriate. Normality and symmetry of the data was checked by visual inspection of the histogram and box plot, and a paired t-test was used
Results
Baseline characteristics of patients
The baseline characteristics of all 25 patients are summarised in Table 1. FM patients reported a mean numerical rating score of 8.25 ± 0.33 at baseline. 16% (4 patients) of patients had an efficient CPM as compared to 84% (21 patients) who demonstrated an inefficient CPM at baseline. PPT was found to be low at 193 ± 22.61 KPa. Other characteristics are outlined in Table 1.
Table 1.
Baseline characteristics of all the patients included in the study n = 25.
| Baseline characteristics (n = 25) | |
|---|---|
| Age (mean ± SD) | 46.7 ± 10.5 years |
| Female:male | 24:1 |
| NRS (mean ± SE) (CI; median) | 8.25 ± 0.33 (7.58–8.92); 8 |
| PPT (KPa) (mean ± SE) (CI; median) | 193 ± 22.61 (146.95–240.28); 156.33 |
| CPM (KPa) (mean ± SE) (CI; median) | 19.02 ± 8.5 (1.46–36.57); 25 |
| CPM (normal:abnormal), n (%) | 4 (16):21 (84) |
| Burning (mean ± SE) (CI; median) | 3.4 ± 0.34 (2.73–4.15); 4 |
| Tingling (mean ± SE) (CI; median) | 3.6 ± 0.28 (3.01–4.18); 4 |
| Touching (mean ± SE) (CI; median) | 2.96 ± 0.34 (2.25–3.67); 4 |
| Electric shock (mean ± SE) (CI; median) | 4.04 ± 0.32 (3.38–4.70); 5 |
| Heat or cold (mean ± SE) (CI; median) | 3.52 ± 0.29 (2.91–4.13); 4 |
| Numbness (mean ± SE) (CI; median) | 3.84 ± 0.27 (3.27–4.40); 4 |
| Pressure pain (mean ± SE) (CI; median) | 3.67 ± 0.25 (3.16–4.18); 4 |
PPT: pressure pain threshold; CPM: conditioned pain modulation; SE: standard error; CI: confidence interval; SD: standard deviation; NRS, numerical rating score.
Of the 25 patients, 11 patients dropped out of the study (Figure 1). The characteristics of the patients who dropped out of the study and that of the patients who completed the study at 3 months are summarised in Table 2.
Table 2.
Characteristics of patients who dropped out of the study and the patients who completed the study at 3 months.
| Baseline characteristics | Drop outs | Patients who continued 3 months study | p value, (t-test) |
|---|---|---|---|
| Female:male | 11 | 13:1 | |
| NRS (mean ± SE; CI) | 7.6 (0.54; 6.37–8.83) | 8.7 (0.37; 7.92–9.51) | 0.31 |
| PPT (KPa) (mean ± SE; CI) | 233 (40.46; 142.82–323.16) | 162.67 (22.99; 113–212.33) | 0.11 |
| CPM (KPa) (mean ± SE; CI) | 10.6 (16.2; –25.5 to 46.73); | 25.62 (8.5; 7.2–43.96) | 0.51 |
| CPM (normal:abnormal), n (%) | 3 (27):8 (73) | 1 (7):13 (93) | |
| Burning (mean ± SE; CI) | 3.18 (0.57; 1.91–4.45) | 3.64 (0.43; 2.7–4.6) | 0.55 |
| Tingling (mean ± SE; CI) | 3.55 (0.49; 2.45–4.64) | 3.64 (0.34; 2.9–4.4) | 0.89 |
| Touching (mean ± SE; CI) | 3 (0.57; 1.73–4.24) | 2.92 (0.44; 1.98–3.87) | 0.69 |
| Electric shock (mean ± SE; CI) | 4.18 (0.44; 3.19–5.15) | 3.93 (0.46; 2.93–4.93) | 0.76 |
| Heat or cold (mean ± SE; CI) | 3.5 (0.41; 2.6–4.5) | 3.5 (0.44; 2.5–4.5) | 0.92 |
| Numbness (mean ± SE; CI) | 3.6 (0.43; 2.7–4.6) | 4 (0.36; 3.22–4.78) | 0.37 |
| Pressure pain (mean ± SE; CI) | 3.7 (0.33; 2.9–4.6) | 3.64 (0.36; 2.87–4.12) | 0.98 |
PPT: pressure pain threshold; CPM: conditioned pain modulation; SE: standard error; CI: confidence interval; NRS, numerical rating score.
There were no significant differences in the clinical characteristics between those who dropped out and those who completed the 12-week treatment of pregabalin.
PPTs significantly improved from baseline 162.6 ± 22.9 to Visit 1 (4 weeks treatment) 206 ± 39.2 (p < 0.02), and this further increased from baseline to Visit 3 (12 weeks) 227.6 ± 35.33 (p < 0.006). Significant improvements were found at all time-points in CPM efficiency from baseline (Table 3), and this was supported by significant improvement in NRS from baseline to 12 weeks of treatment. Further significant improvements were found in most sensory profiles except pressure and electric shock parameters.
Table 3.
The effect of pregabalin on the various psychosensory profiles of patients who completed the study.
| Characteristics of patients who continued 3 months study, n = 14 | Baseline | V1 (4 weeks) | V2 (8 weeks) | V3 (12 weeks) |
|---|---|---|---|---|
| PPT (KPa) (mean ± SE) (median) | 162.6 (22.99) 140.83 | 206, 154.04; (p = 0.02) | 199.46 (28.82), 209.25; (p = 0.07) | 227.58 (35.33), 184; (p = 0.006) |
| CPM (KPa) (mean ± SE) (median) | 25.62 (8.5); 26.3 | −82.13 (19.6), –71.5; (p = 0.0015) | −62.34 (15.32), –56.66; (p = 0.0088) | −101.81 (20.77), –103.59; (p = 0.0023) |
| NRS (mean ± SE) (median) | 8.7 (0.37); 9 | 6 (0.61), 7; (p = 0.0019) | ||
| Burning (mean ± SE) (median) | 3.64 (0.43); 4 | 1.79 (0.35), 2; (p = 0.0088) | ||
| Tingling (mean ± SE) (median) | 3.64 (0.34); 4 | 1.86 (0.29), 2; (p = 0.0047) | ||
| Touching (mean ± SE) (median) | 2.92 (0.44); 3 | 1.36 (0.34), 1; (p = 0.01) | ||
| Electric shock (mean ± SE) (median) | 3.93 (0.46); 4.5 | 2.46 (0.31), 3; (p = 0.07) | ||
| Heat or cold (mean ± SE) (median) | 3.5 (0.44); 3.5 | 1.71 (0.44), 1.5; (p = 0.004) | ||
| Numbness (mean ± SE) (median) | 4 (0.36); 4 | 2 (0.41), 2; (p = 0.002) | ||
| Pressure pain (mean ± SE) (median) | 3.64 (0.36); 4 | 2.64 (0.46), 3; (p = 0.27) | ||
| FIQ baseline - 12 weeks | 60.1 ± 11.9 | 39.4 ± 18.4; p = 0.006 |
PPT: pressure pain threshold; CPM: conditioned pain modulation; SE: standard error; FIQ: fibromyalgia impact questionnaire; NRS: numerical rating score.
PPT, CPM, NRS and other psychosensory profile means all significantly improved (t-test).
Discussion
FM is challenging to treat and has significant impact on patients and their health-related quality of life. Pregabalin is a licenced treatment for FM in Europe and an FDA-approved treatment option. The central sensitisation response particularly ‘dynamic responses’ to pregabalin has not been reported before. This is the first study demonstrating improvement in peripheral and central sensitisation as measured by QST in patients with FM following treatment with pregabalin.
Management of these patients requires a combination of pharmacological and non-pharmacological modalities including exercise and cognitive behavioural therapy. Making a diagnosis of FM has a positive effect on its management, and patient education emphasising that the patient does not have a life-threatening or serious disease reduces anxiety. FM is a syndrome including many symptoms and comorbidities and therefore it is not surprising that no single pharmacological agent is capable of effectively addressing all of the potential symptoms of FM. Evidence also suggests that pharmacological treatment in FM generally shows only small to moderate effects. Non-pharmacological interventions report as having similar or smaller effects.16 In a randomised study using lidocaine and amitriptyline, no meaningful impact was found in FM patients.17 Other studies using lidocaine have found more positive results. In one study, daily intravenous lidocaine was used in patients for 6 days; however, this treatment plan would not be easy to use within clinical practice.18 Pregabalin has been approved by the US FDA for the management of FM. It shares with gabapentin a novel high-affinity drug-binding site, the calcium channel alpha2 – delta subunit.19 Pregabalin has been examined in several clinical trials and has been found to be effective and safe in treating FM20,21 and notably compared with placebo more often associated with clinical improvements in pain categories.22 The use of pregabalin on central sensitisation for pain control in chronic pancreatitis have demonstrated similar results with moderate inhibitory effects on central sensitisation, suggesting that QST may be of clinical use for monitoring pain treatments.23
Quantitative sensory testing is a measure of large and small afferent nerve fibre function, using psychophysical tests involving the skin, mucosa and muscle tissues. The tests used in QST can be broadly classified as either static or dynamic measurements. Static measurements depict a single point on the pain experience continuum, for example, the threshold determination of pressure when it becomes ‘painful’ to the participant (PPT response), or the threshold when a subject is able to detect temperature change and it becomes painful (heat and cold temperature threshold and painful response). The static measures have the advantage of using an easily defined endpoint, which is stable and reproducible in practice but represents only one part of the pain process.24 In contrast, dynamic QST measurements may be used to capture the endogenous pain modulatory process. These include tests of central integration such as temporal and spatial summation, or tests of descending control or CPM, such as the DNIC paradigm.25 Current evidence suggests that dynamic tests can be more suitable in predicting outcomes for pain interventions.26
In this study, we found that the patients at baseline were, except one patient, central sensitisation and after treatment with pregabalin this effect was reversed.
Findings from QST studies in patients with FM have demonstrated an increased pain response to painful stimuli5,27 and reduced tolerance in the tourniquet ischaemia test.28 This hyperalgesia is not restricted to the tender points but found widespread,29 proposing central sensitisation as a contributing pathophysiological factor.27
A limitation to this study was the high drop-out, with 25 consented and only 14 completing the 3-month treatment with pregabalin. Dizziness affected almost all of the 14 completed patients; however, most found that the benefit of reduced pain outweighed dizziness which over time or with a reduction in dose did subside. Five dropped out having felt that the dizziness had a disabling impact on their lives, one patient dropped out with unacceptable swelling of her legs and another withdrew after subjective reports of deterioration in her affective state. Other common side effects include weight gain and increased sleepiness. These however were well tolerated by most patients.
We speculate that the low retention rate was due to adverse events related to pregabalin, with dizziness being the most frequently reported; this drop-out rate is high compared to previously published studies.30 Future studies should draw on our experience and consider lower starting doses with dose titration to minimise adverse events, with closer monitoring from clinical staff during the initial treatment period.
This proof of principle study, using the application of QST, reports a gradual increase in PPTs and in the DNIC response with pregabalin, which was maintained at 12 weeks. This was also supported by positive pain scores. Large adequately powered studies comparing pregabalin with placebo or another active treatment for pain relief are required to investigate the role for routine dynamic QST assessments, to explore potential mechanisms through which pregabalin exerts its analgesic effect in FM patients. This is the first study demonstrating improvement in peripheral and central sensitisation as measured by QST in patients with FM following pregabalin treatment.
Acknowledgments
T.W. was responsible for conception, design, acquisition and analysis of data; drafting and revising of the article and approval of the article as written and responsibility for the content and completeness. The guarantor (T.W.) is the person willing to take full responsibility for the article, including for the accuracy and appropriateness of the reference list. This will often be the most senior member of the research group and is commonly also the author for correspondence.
Footnotes
Declaration of Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This study was approved by the NRES Committee London – City & East and Bristol Research Ethics Committee Centre (13/LO/0052).
ORCID iD: Theresa Wodehouse  https://orcid.org/0000-0002-1686-1230
https://orcid.org/0000-0002-1686-1230
References
- 1. Staud R, Robinson ME, Vierck CJ, et al. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain 2003; 101(1–2): 167–174. [DOI] [PubMed] [Google Scholar]
- 2. Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003; 48(5): 1420–1429. [DOI] [PubMed] [Google Scholar]
- 3. Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005; 114(1–2): 295–302. [DOI] [PubMed] [Google Scholar]
- 4. de Souza JB, Potvin S, Goffaux P, et al. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain 2009; 25(2): 123–127. [DOI] [PubMed] [Google Scholar]
- 5. Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain 1994; 59: 45–53. [DOI] [PubMed] [Google Scholar]
- 6. Katz JD, Mamyrova G, Guzhva O, et al. Gender bias in diagnosing fibromyalgia. Gender Med 2010; 7(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 7. Yokota S, Kikuchi M, Miyamae T. Juvenile fibromyalgia: guidance for management. Pediatr Int 2013; 55(4): 403–409. [DOI] [PubMed] [Google Scholar]
- 8. Kashikar-Zuck S, Ting TV. Juvenile fibromyalgia: current status of research and future developments. Nat Rev Rheumatol 2014; 10(2): 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolfe F, Clauw DJ, Fittzcharles MA, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicentre criteria committee. Arthritis Rheum 1990; 33(20): 160–172. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010; 62(5): 600–610. [DOI] [PubMed] [Google Scholar]
- 11. Yarnitsky D, Granot M, Nahman-Averbuch H, et al. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012; 153(6): 1193–1198. [DOI] [PubMed] [Google Scholar]
- 12. Sprenger C, May A, Buchel C. Pain contra pain: the concept of DNIC. Schemerz 2010; 24(6): 569–574. [DOI] [PubMed] [Google Scholar]
- 13. Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010; 14(4): 339. [DOI] [PubMed] [Google Scholar]
- 14. Kosek E, Harrison P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 1997; 70: 41–51. [DOI] [PubMed] [Google Scholar]
- 15. Graven-Nielson T, Wodehouse T, Langford RM, et al. Normalization of widespread hyperesthesia and facilitated special summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012; 64(9): 2907–2916. [DOI] [PubMed] [Google Scholar]
- 16. Kim S, Slaven JE, Ang DC. Sustained benefits of exercise-based motivational interviewing, but only among nonusers of opioids in patients with fibromyalgia. J Rheumatol 2016; 44: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giraldes AL, Salomao R, Leal PC, et al. Effect of intravenous lidocaine combined with amitriptyline on pain intensity, clinical manifestations and the concentrations of IL-1, IL-6 and IL-8 in patients with fibromyalgia: a randomized double-blind study. Int J of Rheumatic Dis 2016; 19: 946–953. [DOI] [PubMed] [Google Scholar]
- 18. Raphael JH, Southall JL, Treharne GL, et al. Efficacy and adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome. BMC Musculoskeletal Dis 2002; 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin-calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain 2009; 142: 13–16. [DOI] [PubMed] [Google Scholar]
- 20. Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009; 144: 465–471. [DOI] [PubMed] [Google Scholar]
- 21. Straube S, Derry S, Moore RA, et al. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology 2010; 49: 706–715. [DOI] [PubMed] [Google Scholar]
- 22. Parsons B, Argoff CE, Clair A, et al. Improvement in pain severity category in clinical trials of pregabalin. J Pain Res 2016; 9: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouwense SA, Olesen SS, Drewes AM, et al. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS ONE 2012; 7(8): e42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hauser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin-meta-analysis of randomized controlled trials. Pain 2009; 145(1–2): 69–81. [DOI] [PubMed] [Google Scholar]
- 25. Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008; 136(1–2): 142–149. [DOI] [PubMed] [Google Scholar]
- 26. Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr Opin Anaes 2009; 22(3): 425–430. [DOI] [PubMed] [Google Scholar]
- 27. Kosek E, Ekholm J, Hansson P. Increased pressure pain sensibility in fibromyalgia patients is located deep to the skin but not restricted to muscle tissue. Pain 1995; 63: 335–339. [DOI] [PubMed] [Google Scholar]
- 28. Carli G, Suman AL, Biasi G, et al. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal. Pain 2002; 100: 259–269. [DOI] [PubMed] [Google Scholar]
- 29. Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitisation and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001; 91: 165–175. [DOI] [PubMed] [Google Scholar]
- 30. Derry S, Cording M, Wiffen PJ, et al. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev 2016; 9: CD011790. [DOI] [PMC free article] [PubMed] [Google Scholar]


