Abstract
Purpose:
Although stereotactic body radiation therapy is one of the standard treatments for stage I nonsmall cell lung cancer, in the case of central tumors it carries the risk of severe adverse events for serial organs. Accelerated hypofractionated radiotherapy is considered a reasonable alternative to treat central tumors. We have been treating central tumors with accelerated hypofractionated radiotherapy using a 75 Gy/25 fr/5 weeks regimen, and we compared the results with those of stereotactic body radiation therapy using 48 Gy/4 fr/1 week.
Methods:
Patients with central tumors and/or unfit for 1-hour fixation were candidates for accelerated hypofractionated radiotherapy. Based on the proximity to the biologically effective dose at 10 Gy, above accelerated hypofractionated radiotherapy regimen was adopted.
Results:
From October 2003 to December 2010, 159 patients, who received either accelerated hypofractionated radiotherapy (103 cases) or stereotactic body radiation therapy (56 cases), were included in the analysis. In the accelerated hypofractionated radiotherapy group, 40 (39%) cases were central tumors, whereas all cases were peripheral tumors in the stereotactic body radiation therapy group. Overall 5-year local control and survival rates were 81.9% (95% confidence interval 73.6%-90.1%) and 46.5% (95% confidence interval 36.7%-56.2%), respectively for the accelerated hypofractionated radiotherapy group, and 75.4% (95% confidence interval 63.0%-87.8%) and 44.6% (95% confidence interval 31.6%-57.7%), respectively for the stereotactic body radiation therapy group (n.s.). Among central tumors, ultracentral tumors (21 cases) and the remaining central tumors (19 cases) were similar in both local control and survival. On multivariate analysis, hazard ratios for accelerated hypofractionated radiotherapy versus stereotactic body radiation therapy were <1 for both local control and survival. Pulmonary toxicity was similar in both groups. No serial organ toxicity was observed for central tumors.
Conclusions:
Accelerated hypofractionated radiotherapy with a 75 Gy/25 fr/5 weeks regimen is promising in that it can obtain similar local control and survival results to stereotactic body radiation therapy, and it can control both central and peripheral tumors without any serial organ toxicities. Based on these results, prospective multicenter trials are worth conducting, especially for ultracentral tumors.
Keywords: Stage I nonsmall cell lung cancer, accelerated hypofractionated radiotherapy, SBRT, central tumor, ultracentral tumor
Introduction
Stereotactic body radiation therapy (SBRT) has become one of the standard treatments for early stage nonsmall cell lung cancer (NSCLC) because of its high local control rate and low complication rate.1–7 National Comprehensive Cancer Network Guidelines8 recommend SBRT, not only for inoperable patients, but also for high-risk patients or those who refuse surgery.
One critical issue exists, however, in that high dose-per-fraction SBRT (anything over 12 Gy) is hazardous for centrally located tumors, because of the toxicity for serial organs, such as the bronchus, major arteries, esophagus and so on.9 For such cases, efforts have been made to decrease the fractional dose from 20 Gy to 7.5 to 10 Gy,10–12 which are still called SBRT and whose biologically effective dose at 10 Gy (BED10) s still maintain 100 Gy, or to decrease the fractional dose to 2.5 to 4 Gy,13–16 using an increased number of these lower-dose fractions (accelerated hypofractionated radiotherapy [AHRT]).
In our institution, with the aim of not decreasing the local control effect, we set the total dose of AHRT at 75 Gy in 25 fractions (BED10: 97.5 Gy) in order to match the BED10 of SBRT of 48 Gy in 4 fractions (105.6 Gy), the schedule generally adopted in our country at that time. This approach greatly reduces the BED of late responding tissues (BED3) from 240 to 150 Gy, and in doing so, almost clears the dose constraint for serial organs, such as the bronchial trees and great vessels. In this article, the results of AHRT and SBRT for stage I NSCLC are compared, and the associated prognostic factors are analyzed.
Methods
Patients
After ethics committee approval (Approval number 1151) was obtained, all patients with a diagnosis of stage IA or IB, treated between October 2003 and December 2010 with curative intent at our hospital, were identified. Patients who received AHRT of 75 Gy/25 fr/5 weeks (103 cases) or SBRT (mainly 48 Gy/4 fr/1 week; 84 cases) were included.
All patients were inoperable, high-risk operable (with the referring thoracic surgeons or pulmonologists preferring radiation therapy to surgery), or had refused surgical intervention (operable). All but 1 case had a histological diagnosis of NSCLC, and all underwent physical examinations and computed tomography (CT), brain magnetic resonance imaging, and positron emission tomography-CT scans. Exclusion criteria included recurrent lung cancers and metastatic lung cancers. Exclusion criteria for SBRT were central tumors, which did not satisfy the dose constraints for mediastinal organs, or cases unfit for enduring body-frame fixation. These cases were treated by AHRT.
Central tumors were defined as those whose clinical target volume (CTV) was located within 2 cm from the bronchial tree and/or within 2 cm from mediastinal structures. Ultracentral tumors were defined as those whose CTV faced the lobar bronchus, esophagus, or major vessels and/or whose planning target volume (PTV) was overlapping the trachea or main bronchus.
Dose constraints of both treatment techniques are listed in Table 1. Especially in AHRT, the dose constraints were made by converting BED 3s from available data at that time, such as the JCOG0403 study, and they were restricted well below those of SBRT.
Table 1.
Dose Constraints.
| PRV (Planning Organ at Risk Volume) | Volume | Dose Constraints | Avoidance Endpoint | |
|---|---|---|---|---|
| AHRT | SBRT | |||
| Spinal cord | Max | 45 Gy/25 fr | 25 Gy/4 fr | Myelitis |
| Esophagus | <5 mL | 55 Gy/25 fr | 30 Gy/4 fr | Stenosis/fistula |
| Heart/ pericardium | <10 mL | 60 Gy/25 fr | 32 Gy/4 fr | Pericarditis |
| Great vessels (aorta) | <10 mL | 70 Gy/25 fr | 35 Gy/4 fr | Aneurysm |
| Great vessels (pulmonary artery) | <1 mL | 75 Gy/25 fr | 40 Gy/4 fr | Aneurysm |
| <10 mL | 70 Gy/25 fr | 35 Gy/4 fr | ||
| Great vessels (superior vena cava, pulmonary vein) | <10 mL | 70 Gy/25 fr | 35 Gy/4 fr | Stenosis/fistula |
| Trachea/bronchus | <1 mL | 75 Gy/25 fr | 40 Gy/4 fr | Stenosis/fistula |
| <10 mL | 70 Gy/25 fr | 35 Gy/4 fr | ||
| Lung (bil. lung - PTV) | <100 mL | 60 Gy/25 fr | 40 Gy/4 fr | Pneumonitis |
Treatment
All patients underwent CT-based planning. Stereotactic body radiation therapy patients were immobilized using Elekta’s Body Frame, while the immobilization device was not used for AHRT cases. Internal target volume was set using a long-time CT scan to cover all tumor points that moved as the result of respiration plus additional margins of up to 5 mm. Furthermore, an additional margin of 5 mm was set to account for patient setup (Setup margin). A leaf margin of around 5 mm was then added.
At the beginning of this study, the Clarkson Method, with the prescription point being the isocenter for both techniques, was adopted. Since the beginning of 2007, the Superposition Method was adopted for calculation, with the prescription point being the isocenter. From the beginning of 2009, we began to prescribe in the periphery of the tumor for SBRT cases, whereas for AHRT cases we did not change the prescription point. In all cases, heterogeneity correction was performed.
The energy of the photon beam was 6 MV. For SBRT, 8 ports were used and for AHRT 10 ports were used. Elective nodal irradiation was omitted in both techniques. In regard to treatment verification, orthogonal X-ray films were taken at every treatment for SBRT and once every 1 to 2 weeks for AHRT. Repositioning was performed if the positioning error reached 5 mm.
Follow-Up
Patients were assessed by interview, physical examination, blood tests, and chest CT scans at every follow-up visit. Typically, patients were evaluated for response by CT scan 1 month following the completion of treatment. Two months later, the next CT was evaluated. Subsequently, patients underwent CT examination every 3 months, up until 3 years, and every 4 to 6 months thereafter. Positron emission tomography-CT was performed when recurrence was suspected. At each follow-up visit, patients were screened for acute and late toxicities and graded according to the NCI Common Terminology Criteria for Adverse Effects, version 4.0.
Local failure was defined as progression of disease within the known and treated lung parenchymal lesions on CT imaging of the thorax. Confirmation by PET scanning was usually performed and biopsies were conducted in selected cases. Regional failure was defined as failure within the regional lymph node stations. Distant failure was defined as failure beyond the locoregional areas.
Statistics
Continuous variables were compared across groups using Student t tests, while frequencies were assessed using either the χ2 test or Fisher exact test. The overall survival and local control rates were estimated using the Kaplan-Meier method. The log-rank test and generalized Wilcoxon’s test were was used to test significance. The Cox proportional hazards model was used for multivariate analyses.
Results
Patient Characteristics
From October 2003 to December 2010, 187 patients with stage I NSCLC were treated with either SBRT (84 cases) or AHRT (103 cases). However, among the SBRT cases, 28 were excluded from the analysis because of the dose difference due to the difference in calculation method. Thus, 56 cases were analyzed in the SBRT group. Table 2 shows the patients’ characteristics. As stated previously, patients with central tumors were treated with AHRT, so there was a significant difference between the 2 techniques in regard to the tumor site. In addition, since T2 cases had been treated with a 48 Gy/4 fr regimen by SBRT and local control was insufficient, a greater number of T2 cases tended to be treated with AHRT (P = .086). Consequently, tumor sizes were bigger in the AHRT group. Since squamous cell carcinoma arose more often in the periphery, these cancers were treated more often with SBRT. Fragile cases (high-risk operable and inoperable cases) accounted for 79.6% and 85.7% of cases in the AHRT group and SBRT group, respectively. The median follow-up period of the surviving patients was 7.8 years in the AHRT group and 10.6 years in the SBRT group.
Table 2.
Patient Characteristics.
| AHRT | SBRT | P Value | |
|---|---|---|---|
| No. of cases | 103 | 56 | |
| Age (range, median) | 53-93 median 78 | 49-91 median 79 | .367 |
| Gender (m / f) | 74/29 | 39/17 | .855 |
| PS (0/1/2/3) | (0/94/9/0) | (3/46/7/0) | .035 |
| Side (right/left) | 57/46 | 36/20 | .314 |
| Lobe (upper/middle/lower) | 71/4/28 | 32/2/22 | .312 |
| Site (peripheral/ central) | 60/43 | 56/0 | <.01 |
| Size in mm (median/mean/ | 30/29.9 ± 7.7 | 25/25.7 ± 7.9 | .0013 |
| range) | 12 ∼ 50 | 14 ∼ 44 | |
| T-factor T1/T2 | 61/42 | 41/15 | .086 |
| Histology adenocarcinoma | 67 | 34 | |
| Squamous cell carcinoma | 22 | 18 | |
| LCNEC | 0 | 1 | |
| NSCLC NOS | 15 | 2 | |
| Unprovena | 0 | 1 | .024 |
| Operability | |||
| Operable | 21 | 8 | |
| High risk operable | 30 | 27 | |
| Inoperable | 52 | 21 | .059 |
| Risk factor | |||
| Age | 18 | 20 | |
| Pulmonary | 61 | 31 | |
| Cardiac | 9 | 8 | |
| CNS | 9 | 4 | |
| Renal | 2 | 0 | |
| Hepatic | 1 | 1 | |
| AAA | 0 | 2 | |
| Refusal | 18 | 11 | .223 |
Abbreviations: AAA, abdominal aortic aneurysm; AHRT, accelerated hypofractionated radiotherapy; LCNEC, large cell neuroendocrine cancer; NOS, not otherwise specified; NSCLC, nonsmall cell lung cancer; PS, performance status.
aUnproven: One case in the SBRT group was omitted biopsy owing to severe emphysema. But the patient’s tumor revealed SUV-max of 8.0 in the PET-CT and thus was included in the group.
Among AHRT cases, 40 had central tumors. Central tumors were divided into central tumors and ultracentral tumors. There were 19 central tumors and 21 ultracentral tumors.
Local Control
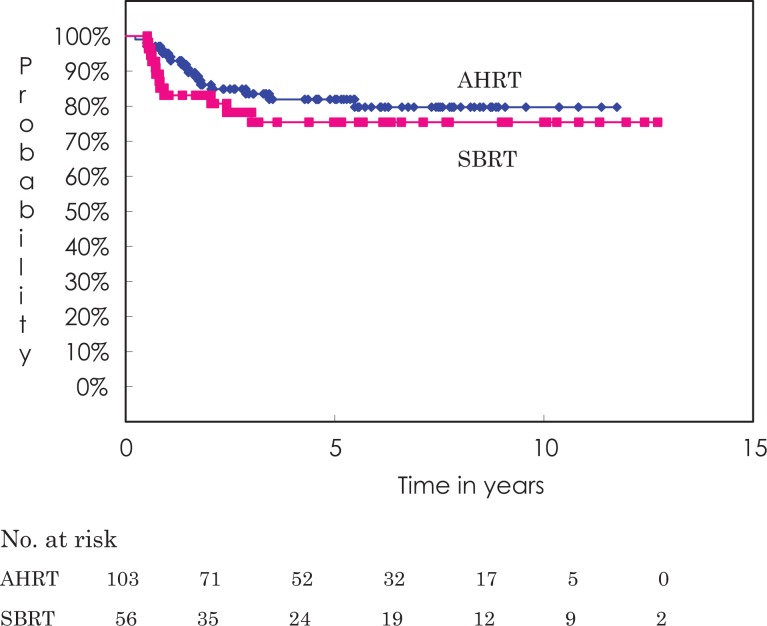
Figure 1 shows the local control curves for both techniques. Overall local control rates at 3 and 5 years were 83.5% (95% confidence interval [CI] 75.8%-91.2%) and 81.9% (95% CI 73.8%-90.1%), respectively, for the AHRT group and 78.2% (95% CI 66.6%-89.8%) and 75.4% (95% CI 63.0%-87.8%), respectively, for the SBRT group (P = .533). Table 3 shows the local control rates for both patient groups by prognostic categories. In both groups, adenocarcinomas obtained better local control than squamous cell carcinomas. Interestingly, there were no differences between T1 and T2 tumors.
Figure 1.
Local control curve by treatment method. The 5-year local control rate is 81.9% for the AHRT group and 75.4% for the SBRT group (not significantly different). AHRT indicates accelerated hypofractionated radiotherapy; SBRT, stereotactic body radiation therapy.
Table 3.
Local Control Rates by Category.
| AHRT | SBRT | |||||
|---|---|---|---|---|---|---|
| Category | 5yLCR (%) | 95%Cl (%) | P Value | 5yLCR (%) | 95%Cl (%) | P Value |
| Overall | 81.9 | 73.8-90.1 | .533 | 75.4 | 63.0-87.8 | |
| Central | 80.4 | 67.0-93.8 | .685 | |||
| Peripheral | 83.1 | 72.9-93.2 | 75.4 | 63.0-87.8 | ||
| Male | 76.6 | 65.5-87.7 | .167 | 68.4 | 51.4-85.4 | .181 |
| Female | 92.9 | 83.3-100 | 88.2 | 72.9-100.0 | ||
| T1 | 80.7 | 69.7-91.6 | .952 | 77.5 | 63.8-91.5 | .459 |
| T2 | 83.7 | 71.5-95.8 | 69.3 | 43.8-94.9 | ||
| Inoperable | 86.8 | 76.8-96.9 | .131 | 66.7 | 44.3-89.1 | .525 |
| HR operable | 68.7 | 50.5-86.9 | 81.5 | 64.3-98.7 | ||
| Operable | 89.2 | 75.0-100.0 | 75.0 | 45.0-100.0 | ||
| Upper lobe | 83.9 | 77.6-93.2 | .191 | 78.8 | 63.4-94.2 | .312 |
| Lower lobe | 77.3 | 61.3-93.4 | 70.2 | 50.0-90.4 | ||
| Rt side | 83.2 | 72.3-94.0 | .534 | 72.3 | 55.2-89.5 | .861 |
| Lt side | 80.4 | 68.0-92.7 | 78.3 | 59.4-97.2 | ||
| PS0-1 | 85.0 | 77.1-93.0 | .002 | 74.2 | 60.7-87.7 | .730 |
| PS2-3 | 45.7 | 8.7-82.8 | 85.7 | 59.8-100.0 | ||
| Age ≤ 79 | 80.4 | 70.3-90.5 | .365 | 82.3 | 68.1-98.5 | .288 |
| Age ≥ 80 | 85.5 | 72.2-98.8 | 71.9 | 52.4-91.4 | ||
| Adenoca | 87.4 | 78.5-96.2 | .066 | 86.9 | 74.9-99.0 | .017 |
| SQCCa | 75.6 | 54.3-90.3 | 51.2 | 26.3-76.1 | ||
| Others | 66.0 | 41.7-90.3 | 100.0 | 100.0-100.0 | ||
Abbreviations: AHRT, accelerated hypofractionated radiotherapy; Adenoca, adenocarcinoma; HR, high risk; Lt, left side; LCR, local control rate; SQCCa, squamous cell carcinoma; Rt, right side; SBRT, stereotactic body radiation therapy.
For central tumors, local control rates at 3 and 5 years were 84.2% (95% CI 72.5%-96.0%) and 80.4% (95% CI 67.0%-93.8%), respectively. Three and 5-year local control rates were 95.0% (95% CI 85.4%-100%) and 81.5% (95% CI 62.2%-100%), respectively for ultracentral tumors and 95.0% (95% CI 85.4%-100%) and 73.3% (95% CI 50.3%-96.3%), respectively for the remaining central tumors (P = .414).
Survival
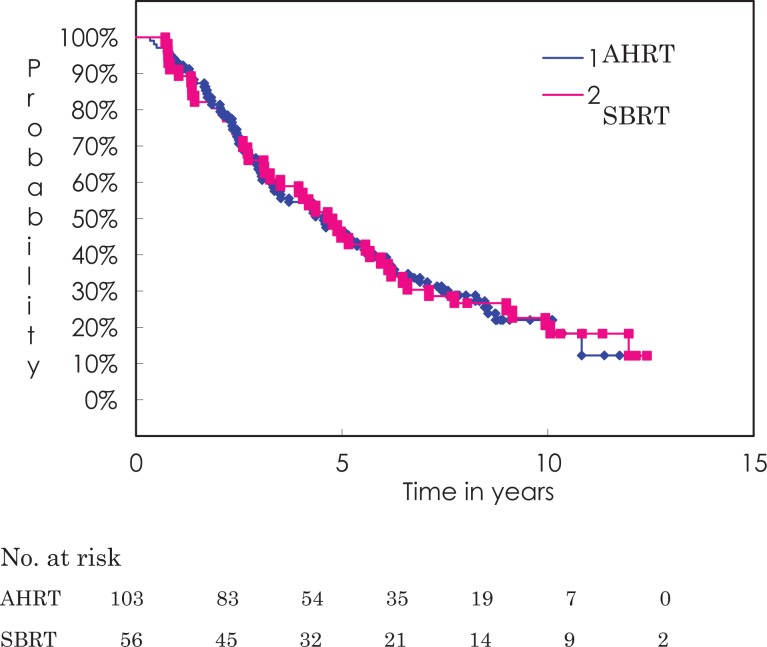
Figure 2 shows the overall survival curves for both treatment techniques. Overall survival rates at 3 and 5 years were 63.6% (95% CI 54.3%-73.0%) and 46.5% (95% CI 36.7%-56.2%) for the AHRT group and 66.1% (95% CI 53.7%-78.5%) and 44.6% (95% CI 31.6%-57.7%) for the SBRT group (P = .908). Table 4 shows the overall survival rates for both treatment groups by prognostic categories. In both groups, women had better overall survival than men. In the AHRT group, operable cases fared marginally better than inoperable cases or high-risk operable cases (P = .112), whereas in the SBRT group, there was no significant difference. In the AHRT group, favorable performance status (PS) cases fared better than unfavorable PS cases (P = .003), whereas in the SBRT group, there was no significant difference.
Figure 2.
Overall survival curve by treatment method. The 5-year overall survival rate and median survival time are 46.5% and 54.7 months, respectively, for the AHRT group and 44.6% and 57.3 months, respectively, for the SBRT group (not significantly different). AHRT indicates accelerated hypofractionated radiotherapy; SBRT, stereotactic body radiation therapy.
Table 4.
Overall Survival Rates by Category.
| AHRT | SBRT | |||||||
|---|---|---|---|---|---|---|---|---|
| Category | 5ySR (%) | 95%Cl (%) | MST months | P Value | 5ySR (%) | 95%Cl (%) | MST months | P Value |
| Overall | 46.5 | 36.7-56.2 | 54.7 | .777 | 44.6 | 31.6-57.7 | 57.3 | |
| Central | 46.7 | 31.0-62.3 | 51.6 | .336 | ||||
| Peripheral | 46.3 | 33.8-58.1 | 54.7 | 44.6 | 31.6-57.7 | 57.3 | ||
| Male | 37.3 | 26.1-48.6 | 42.2 | .001 | 35.9 | 20.8-51.0 | 42.0 | .078 |
| Female | 69.0 | 52.1-85.8 | 82.7 | 64.7 | 42.0-87.4 | 74.3 | ||
| T1 | 48.4 | 35.7-61.0 | 55.4 | .467 | 48.8 | 33.5-64.1 | 59.7 | .288 |
| T2 | 43.6 | 28.3-58.9 | 52.3 | 33.3 | 9.5-57.2 | 47.4 | ||
| Inoperable | 40.5 | 26.8-56.2 | 51.3 | .112 | 38.1 | 17.3-58.9 | 48.6 | .466 |
| HR operable | 38.7 | 21.0-56.4 | 36.5 | 44.4 | 25.7-63.2 | 57.3 | ||
| Operable | 71.4 | 52.1-90.8 | 74.4 | 62.5 | 29.0-96.0 | 109.7 | ||
| Upper lobe | 47.9 | 36.4-59.5 | 55.4 | .761 | 47.1 | 30.3-63.9 | 59.7 | .176 |
| Lower lobe | 42.9 | 24.5-61.2 | 51.3 | 40.9 | 20.4-61.5 | 52.3 | ||
| Rt side | 47.4 | 34.4-60.3 | 52.3 | .962 | 38.9 | 23.0-54.8 | 48.6 | .192 |
| Lt side | 45.0 | 30.1-59.9 | 54.7 | 55.0 | 33.2-76.8 | 68.1 | ||
| PS0-1 | 49.9 | 39.7-60.2 | 55.4 | .003 | 44.9 | 30.6-66.0 | 59.7 | .337 |
| PS2-3 | 11.1 | 0.0-31.6 | 29.9 | 42.9 | 6.2-79.5 | 50.3 | ||
| Age ≤ 79 | 44.5 | 32.7-56.3 | 51.4 | .891 | 48.4 | 36.7-63.3 | 62.3 | .337 |
| Age ≥ 80 | 50.5 | 33.2-67.8 | 60.6 | 40.0 | 20.8-59.2 | 48.6 | ||
| Adenoca | 51.0 | 38.9-63.1 | 60.6 | .255 | 50.0 | 33.2-66.8 | 61.9 | .429 |
| SQCCa | 32.0 | 11.5-52.5 | 32.9 | 33.3 | 11.6-55.1 | 57.3 | ||
| Others | 46.7 | 21.4-71.9 | 40.0 | 50.0 | 1.0-99.0 | 74.3 | ||
Abbreviations: AHRT, accelerated hypofractionated radiotherapy; Adenoca, adenocarcinoma; HR, high risk; LCR, local control rate; Lt, leftside; Rt, right isde; SQCCa, squamous cell carcinoma; SBRT, stereotactic body radiation therapy
For central tumors, overall survival rates at 3 and 5 years were 67.4% (95% CI 53.3%-81.4%) and 48.1% (95% CI 33.0%-63.2%), respectively. Three and 5-year overall survival rates were 61.9% (95% CI 41.1%-82.7%) and 42.9% (95% CI 21.7%-64.0%), respectively for ultracentral tumors and 73.7% (95% CI 53.9%-93.5%) and 51.0% (95% CI 28.0%-74.0%), respectively for the remaining central tumors (P = .456).
Analysis of Prognostic Factors
Since there were large differences between the 2 treatment groups, multivariate analysis was performed. Table 5 shows the results of multivariate analysis for local control (on the left) and overall survival (on the right). In regard to local control, histology was the only significant prognostic factor (P = .009). Adenocarcinoma cases were better controlled than other histologies. There was no difference in local control between the treatment methods and the hazard ratio for AHRT was <1.
Table 5.
Results of Multivariate Analysis.
| Local Control | Overall Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | H.R. | 95% CI | H.R. | 95% CI | |||||
| Lower | Upper | P Value | Lower | Upper | P Value | ||||
| Site | Peripheral vs central | 1.1666 | 0.4174 | 3.2602 | .769 | 0.7251 | 0.4472 | 1.1758 | .192 |
| Technique | AHRT vs SBRT | 0.7665 | 0.3257 | 1.8039 | .543 | 0.9143 | 0.5845 | 1.4303 | .695 |
| Gender | Female vs male | 0.4296 | 0.1571 | 1.1748 | .100 | 0.4426 | 0.2809 | 0.6973 | <.001 |
| Age | (older) | 1.0120 | 0.9572 | 1.0701 | .874 | 1.0178 | 0.9919 | 1.0442 | .179 |
| PS | PS2-3 vs PS0-1 | 2.4070 | 0.8584 | 6.7492 | .095 | 1.5211 | 0.8213 | 2.8174 | .182 |
| Side | Left vs right | 1.0844 | 0.4869 | 2.4150 | .843 | 0.8597 | 0.5773 | 1.2803 | .457 |
| Lobe | Upper vs lower | 0.4907 | 0.2204 | 1.0922 | .081 | 0.8536 | 0.5631 | 1.2940 | .456 |
| Size | (larger) | 1.0156 | 0.9687 | 1.0648 | .521 | 1.0161 | 0.9926 | 1.0400 | .181 |
| Histology | Adenoca. vs others | 0.3580 | 0.1665 | 0.7697 | .009 | 0.8186 | 0.5630 | 1.1903 | .295 |
| Operability | Operable vs others | 0.7593 | 0.2487 | 2.3184 | .529 | 0.7051 | 0.4214 | 1.1797 | .183 |
Abbreviations: H.R., hazard ratio; 95% CI, 95% confidence interval.
In regard to overall survival, sex (P < .001) was the only significant prognostic factor. Female patients fared better than male patients. There was no significant difference in overall survival between the treatment methods, and the hazard ratio for AHRT was <1.
Toxicity
In regard to pulmonary toxicities, there were 5 Grade 3 pulmonary toxicities (5%) for AHRT cases and 1 Grade 3 pulmonary toxicity (2%) for SBRT cases (no significant difference). In the SBRT group, there was 1 Grade 4 case with stomach perforation due to her continuous use of nonsteroidal anti-inflammatory drugs, because the dose to the stomach was within the constraint of JCOG studies, which was salvaged by gastrectomy, and 1 Grade 3 case with cholecystitis, which required surgery. The cholecystitis was probably caused by the pressure of the Diaphragm Control attached to the Body Frame, because the patient’s gallbladder was completely out of the target volume. For central tumors, there were no major toxicities of serial organs.
Patterns of Failure and Causes of Death
Disease recurrence was observed in 47 (45.6%) cases and 28 (50.0%) cases in the AHRT and SBRT groups, respectively. In regard to the first site of failure, there were 14 and 12 local failures, 8 and 6 regional failures, 4 and 1 pleural failures, and 21 and 9 distant failures in the AHRT and SBRT groups, respectively. Regarding distant failure sites, the lung was the most frequent site (12 cases), followed by the liver (3), brain (3), adrenal glands (2), and bone (1) in the AHRT group. In the SBRT group, the lung was also the most frequent site (8 cases), followed by the brain (1). There was no difference in the pattern of failure between the 2 groups.
Regarding causes of death, 39 (38%) and 26 (46%) cases died of their treated lung cancer, 10 and 4 cases died of other cancers, 20 and 14 cases died of intercurrent diseases, and 7 and 2 cases died of unknown causes in the AHRT and SBRT groups, respectively (no significant differences).
Discussion
In our country, the most frequently used SBRT regimen at the beginning of this century, was 48 Gy/4 fr/1 wk.17,18 Due to the promising results of SBRT and hypofractionated radiotherapy reported by Onishi,19 we set the BED10 of hypofractionated radiotherapy at nearly 100 Gy, that is 75 Gy/25 fr/5 weeks, the BED10 of which is 97.5 Gy. Comparing this AHRT regimen with the SBRT regimen, overall survival rates and local control rates were quite similar, although the AHRT cohort had larger tumors and more T2 cases than the SBRT cohort. In addition, since central tumors are difficult to treat, treatment results are expected to be somewhat worse than those of peripheral tumors.
Multivariate analysis demonstrated that the hazard ratio of AHRT over SBRT was <1 for both local control and survival, meaning that AHRT neither increased the hazard of treatment failure nor worsened prognosis. In addition, central tumors were treated as well as peripheral tumors in terms of both local control and overall survival. This may indicate that BED10 was similar between AHRT and SBRT. Recently, we have adopted a higher dose regimen for SBRT, and therefore, we do not use AHRT for peripherally-located T2 tumors. Regarding adverse effects on serial organs, there were no severe toxicities in the 40 cases whose tumors were located in the central part of the lung during these long follow-up periods. Since dose constraints of the central serial organs for SBRT are much higher than for our AHRT regimen if they are converted to BED3, BED conversion may be a reasonable approach.
There have been several reports of the use of AHRT for the treatment of central tumors. Bogart et al reported the results of the CALGB39904 trial.13 They fixed the total radiotherapy dose at 70 Gy and reduced the number of fractions from 29 (BED10: 86.9 Gy) to 17 (BED10: 98.8 Gy) in 5 steps. Of the 39 patients they treated, 3 cases recurred locally, and the response rate was similar in every cohort. In addition, they stated that all patients tolerated the treatment well, and that overall median survival was 38.5 months. Yung et al also reported their experience with AHRT, in which they treated 60 patients with various fractionations.14 The majority (42/60) of the cases were treated with 60 Gy in 20 fractions. Their overall median survival time was 28 months, and the actuarial local recurrence rate was 20%. Lucas et al reported results of AHRT in comparison with SBRT for T1-2N0M0 NSCLC. They predominately used an AHRT schedule with 70.2 Gy in 26 fractions (BED10: 89.0 Gy) and an SBRT schedule with 54 Gy in 3 fractions (BED10: 151.2 Gy).15 They excluded T2 cases from SBRT and treated more central tumors with AHRT. Their results showed a 3-year local control rate of 87.7% for SBRT and 71.7% for AHRT, and a median survival time of 38.4 months for SBRT and 35 months for AHRT, without any severe adverse events. The authors concluded that, despite increased tumor sizes and central disease, AHRT had similar local control to SBRT. Judging from these reports and from our own experience, AHRT is no less capable of treating central tumors than SBRT. However the total dose of AHRT can be increased, the optimal regimen has not been established.
As the result of recent improvements in treatment planning and dose delivery, total doses used for SBRT have been increasing. Even for central tumors, lowering the fractional dose down to ∼7.5 Gy and increasing the total dose up to 60 Gy has been successful in avoiding adverse events of serial organs. Regarding SBRT for central tumors, there are already guidelines20 and review papers.21 Judging from the review papers, there was a paper reporting good results, such as Radiation Therapy Oncology Group (RTOG),22 but there are studies without many good results because of failing to deliver a sufficient dose to the target. By reducing the fractional dose, these studies might improve the treatment results. However, because of the development of SBRT, the role of AHRT might become less important over time. However, the adverse events affecting serial organs are so striking that safer treatment regimens should still be pursued. In this regard, our AHRT regimen can be used without significant concern. Furthermore, so-called ultracentral tumors, such as those attached to the main bronchus or the pulmonary arteries, carry higher risk with SBRT. For such tumors, researchers worldwide are searching for the ideal fractionation regimen.23 Our regimen might become one candidate because of the reasonably high local control rate and low toxicities.
This study has some limitations. First, it was a single-institution study and retrospective in design. Therefore, selection bias cannot be avoided. However, multivariate analysis demonstrated that AHRT was not inferior to standard SBRT, and long-term follow-up analysis showed that AHRT is safe for serial organs located in the central part of the lung. Another limitation is that the SBRT regimen used in this study may be insufficient to control large tumors, although the 48 Gy/4 fr/1 week regimen is still used in our country. By way of comparison, the RTOG 0813 regimen used 50 Gy/5 fr, and the JROSG10-1 regimen used 60 Gy/8 fr for central tumors. Their BED10s (100 Gy and 105 Gy, respectively) are still lower than the BED10 for the 48 Gy/4 fr regimen. As such, we believe that the 48 Gy/4 fr regimen can be considered a standard approach at the present time.
To the best of our knowledge, this is the first report dealing with an AHRT regimen of 75 Gy/25 fr/5 weeks. This regimen is promising because it can provide similar local control and survival results to SBRT, and it can control central tumors, as well as peripheral tumors, without serial organ toxicities. Based on these results, a prospective multicenter trial of this regimen is worth conducting.
Acknowledgments
We thank Mr Satoshi Kitou and Mr Yujiro Nakajima for his devoted work on the preparation of the article.
Abbreviations
- AHRT
accelerated hypofractionated radiotherapy
- CTV
clinical target volume
- CT
computed tomography
- CI
confidence interval
- NSCLC
nonsmall cell lung cancer
- PS
performance status
- SBRT
stereotactic body radiation therapy
- SVC
superior vena cava.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by Grant in Aid from Japan Agency for Medical Research and Development. JP18ck0106303 and JP17ck0106302.
ORCID iD: Katsuyuki Karasawa, MD, PhD  http://orcid.org/0000-0003-3505-2655
http://orcid.org/0000-0003-3505-2655
Kei Ito, MD, PhD  http://orcid.org/0000-0001-5792-3795
http://orcid.org/0000-0001-5792-3795
References
- 1. Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 suppl 3):S94–S100. [DOI] [PubMed] [Google Scholar]
- 2. Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. [DOI] [PubMed] [Google Scholar]
- 3. Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011;81(5):1352–1358. [DOI] [PubMed] [Google Scholar]
- 4. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. J Am Med Assoc. 2010;303(11):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study, Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. [DOI] [PubMed] [Google Scholar]
- 6. Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68(1):72–77. [DOI] [PubMed] [Google Scholar]
- 7. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. NCCN guidelines for treatment of cancer by site 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.Accessed February 10, 2018
- 9. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 10. Radiation Therapy Oncology Group (rtog) Philadelphia, PA: RTOG; . Clinical trials > protocol table > study details > RTOG 0813 protocol information. Seamless phase I/II study of stereotactic lung radiotherapy (SBRT) for early stage, centrally located, non-small cell lung cancer (NSCLC) in medically inoperable patients. 2012; http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813. Accessed May 30, 2012.
- 11. Tekatli H, Senan S, Dahele M, et al. Stereotactic ablative radiotherapy (SABR) for central lung tumors: plan quality and long-term clinical outcomes. Radiother Oncol. 2015;117(1):64–70. [DOI] [PubMed] [Google Scholar]
- 12. Kimura T, Nagata Y, Harada H, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1). Int J Clin Oncol. 2017;22(5):849–856. [DOI] [PubMed] [Google Scholar]
- 13. Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yung T, Giuliani ME, Le LW, et al. Outcomes of accelerated hypofractionated radiotherapy in stage i non-small-cell lung cancer. Curr Oncol. 2012;19(4), e264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucas JT, Jr, Kuremsky JG, Soike M, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for stage 1 and node negative stage 2 non-small cell lung cancer (NSCLC). Lung Cancer. 2014;85(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soliman H, Cheung P, Yeung L, et al. Accelerated hypofractionated radiotherapy for early-stage non-small-cell lung cancer: long-term results. Int J Radiat Oncol Biol Phys. 2011;79(2):459–465. [DOI] [PubMed] [Google Scholar]
- 17. Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63(5):1427–1431. [DOI] [PubMed] [Google Scholar]
- 18. Onimaru R, Fujino M, Yamazaki K, et al. Steep dose–response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(2):374–381. [DOI] [PubMed] [Google Scholar]
- 19. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–1631. [DOI] [PubMed] [Google Scholar]
- 20. Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7(5):295–301. [DOI] [PubMed] [Google Scholar]
- 21. Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol. 2013;106(3):276–282. [DOI] [PubMed] [Google Scholar]
- 22. Bezjak A, Paulus R, Gasper LE, et al. Primary study endpoint analysis for NRG oncology/RTOG0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2016;94(1):5–6. [Google Scholar]
- 23. Baker S, Dahele M, Lagerwaard FJ, et al. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat Oncol. 2016;11(1):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]