Abstract
B-cell lymphomas represent a diverse group of neoplasms classified primarily by histopatholgy and are often challenging to accurately diagnose. Despite having been recognized less than 20 years ago, microRNAs (miRNAs) have emerged as one of the most promising class of cancer molecular biomarkers and are particularly attractive as they can be readily detected in formalin-fixed paraffin-embedded biopsy material and biological fluids such as blood. Many of the identified B-cell lymphoma miRNA biomarkers also play crucial regulatory roles in normal B-cell development. Below we consider the identity, function, and biomarker potential of miRNAs in B-cell lymphoma and most importantly the barriers that remain to be overcome if they are really to become part of routine clinical practice.
Keywords: microRNA, B-cell lymphoma, non-Hodgkin lymphoma, Hodgkin lymphoma, biomarker, liquid biopsies
Introduction
The first discovery of what we now know as microRNAs (miRNAs) came in 1993 from the laboratories of Victor Ambros in Dartmouth College and Gary Ruvkun in Harvard. They simultaneously published a description of lin-4, a previously identified locus in Caenorhabditis elegans involved in developmental timing, that appeared to have a direct function without encoding for a protein.1,2 Things went quiet for the next 7 years, until the Ruvkun lab identified, let-7a, a second sequence from C elegans, with similar properties to lin-4.3 Unlike lin-4, however, the sequence of let-7 was found to be highly conserved in eukaryotic genomes and it was realized that many similar sequences were present in the genomes of higher species. The first use of the term miRNA was made in 2001 by Lee and Ambros in a publication where they identified a further 15 C elegans miRNAs.4 Since that time, there have been more than 25 000 miRNAs identified in over 200 different species (http://www.mirbase.org), including more than 2500 human miRNAs.5,6
MicroRNAs are short non-coding (nc)RNAs of 18 to 24 nucleotides in length that bind to regions of complementarity generally located in the 3ʹ-UTR (untranslated region) of target genes. They primarily act as inhibitor molecules causing post-transcriptional inhibition or degradation, although in some instances, they may also act as gene activators.7 It is estimated that two-thirds of human genes are directly regulated by miRNAs,8 and as a consequence, miRNAs are involved in most, if not all, cellular processes under physiological conditions. Moreover, dysfunctional expression of miRNAs appears to be a hallmark of all cancer types,9,10 including B-cell lymphomas that are the focus of this review.
Lymphoma is a cancer of the lymphatic system arising from B cells or T cells that represents the fifth most common cancer type worldwide, affecting more than a million people. Lymphomas are a heterogeneous group of cancers that vary in presentation, prognosis, and pathogenesis. In the latest version of World Health Organization (WHO) classification, there were more than 100 different lymphoma types listed, most of which were B-cell lymphomas, but which can have very different clinical characteristics and treatment regimens.11 As a consequence, correct classification of a given lymphoma is often challenging, and therefore there is a clear clinical need for better biomarkers for these diseases. MicroRNAs are particularly attractive candidates as biomarkers, as their expression can classify different tumours according to their diagnosis, subtype, and stage more accurately than messenger RNA expression profiles.12 Moreover, due to their intrinsic stability, they can be reliably detected in routinely prepared formalin-fixed paraffin-embedded (FFPE) tissue. This stability also means they are readily detected in biological fluids such as blood, which has led to a great deal of interest in the use of miRNAs as biomarkers in liquid biopsies discussed below.
MiRNAs as lymphoma liquid biopsy biomarkers
Currently, the gold standard of B-cell lymphoma diagnosis depends on the histopathologic examination of surgically excised biopsy material. This procedure, however, is expensive, invasive, uncomfortable, and can be risky for patients. Therefore, there has been a great interest in the development of non-invasive cancer biomarkers, also known as liquid biopsies. MicroRNAs hold a great promise in this area, as not only can they be extracted from frozen and paraffin-embedded tissue but also from many different body fluids including blood,13,14 urine,15 saliva,16,17 sputum,18,19 amniotic fluid, and even from tears.20
Most of the attention has been focused circulating miRNAs in blood, either in whole plasma or within circulating extracellular vesicles such as exosomes.21,22 The first report of miRNAs in the blood of B-cell lymphomas, or indeed any cancer, came in 2007.23 We found that levels of miR-21, miR-155, and miR-210 in the serum samples of patients with diffuse large B-cell lymphoma (DLBCL) compared with healthy controls were higher suggesting their usefulness as biomarkers.24 Since this time, there have been many follow-up studies in blood of patients with lymphoma as described below and in Table 1.
Table 1.
List of major miRNAs identified as biomarkers in B-cell malignancies.
| Lymphoma | Biomarker | miRNA | Sample | References |
|---|---|---|---|---|
| HL | Diagnostic | miR-155 | Cell lines | van den berg et al25 and Metzler et al26 |
| 23-miRNA signature | Cell lines | Gibcus et al27 | ||
| 25-miRNA signature | Tissue | Navarro et al28 | ||
| 134- and 100-miRNA signature | Cell lines and tissue | Sanchez-Espiridion et al29 | ||
| miR-9-2 (methylation) | Tissue | Ben Dhiab et al30 | ||
| Prognostic | miR-135a | Tissue and cell lines | Navarro et al31 | |
| miR-21, miR-30e/d, and miR-92b | Tissue | Sanchez-Espiridion et al29 | ||
| miR-124a (methylation) | Tissue | Ben Dhiab et al32 | ||
| CLL | Diagnostic | miR-15a/16 cluster | PBMCs and cell lines | Calin et al33 |
| miR-7, miR-182, and miR-320c/d | PBMCs and cell lines | Blume et al34 | ||
| miR-29 | PBMCs and cell lines | Pekarsky et al35 | ||
| miR-151 | Serum (EV) | Caivano et al36 | ||
| miR-34a, miR-31, miR-155, miR-150, miR-15a, miR-29a | Serum | Filip et al37 | ||
| miR-192 | PBMCs | Fathullahzadeh et al38 | ||
| Prognostic | miR-181b | PBMCs | Visone et al39 | |
| miR-21 | PBMCs | Rossi et al40 | ||
| miR-155 | PBMCs | Cui et al41 | ||
| miR-708 | PBMCs and cell lines | Baer et al42 | ||
| miR-150 | Cell lines and serum | Stamatopoulos et al43 | ||
| miR-150 and miR-155 | Blood cells | Georgiadis et al44 | ||
| miR-17~92 cluster | PBMCs | Bomben et al45 | ||
| 13-miRNA signature | PBMCs and cell lines | Calin et al46 | ||
| Predictive | miR-181b | PBMCs | Rossi et al40 | |
| miR-155 | PBMCs | Ferrajoli et al47 | ||
| miR-21*, miR-148a, and miR-222 | PBMCs and cell lines | Ferracin et al48 | ||
| DLBCL | Diagnostic | miR-21, miR-155, and miR-210 | Serum | Lawrie et al24 |
| 12-miRNA signature | Tissue | Roehle et al49 | ||
| 15-miRNA signature | Tissue | Lawrie et al50 | ||
| 12-miRNA signature | Tissue | Caramuta et al51 | ||
| miR-155, miR-221, miR-222, miR-21, miR-363, miR-518a, miR-181a, miR-590, miR-421, and miR-324 | Cell lines | Lawrie et al52 | ||
| miR-155 and miR-146a | Tissue | Zhong et al53 | ||
| 27-miRNA signature | Tissue and cell lines | Iqbal et al54 | ||
| miR-124, miR-532, miR-122, miR-128, miR-141, miR-145, miR-197, miR-345, miR-424, and miR-425 | Plasma and exosomes | Khare et al55 | ||
| miR-34a, miR-323b, and miR-431 | Serum | Meng et al56 | ||
| Prognostic | miR-21 | Serum | Lawrie et al24 | |
| miR-155 and miR-146a | Tissue | Zhong et al53 | ||
| miR-22 | Serum | Marchesi et al57 | ||
| miR-155 | Tissue and cell lines | Iqbal et al54 | ||
| miR-20a and miR-30d | Tissue | Pillar et al58 | ||
| miR-155 | Tissue and cell lines | Zhang et al59 | ||
| miR-17~92 cluster | Tissue and cell lines | Tagawa et al60 | ||
| miR-34a | Tissue | He et al61 | ||
| miR-27b | Tissue | Jia et al62 | ||
| miR-21 | Cell lines | Gu et al63 | ||
| miR-21 | Tissue | Lawrie et al24 and Zheng et al64 | ||
| Predictive | miR-27a, miR-142, miR-199b, miR-222, miR-302, miR-330, miR-425, and miR-519 | Tissue | Lawrie et al50 | |
| miR-155 and miR-146a | Tissue | Zhong et al53 | ||
| miR-21 | Cell lines | Gu et al63 and Bai et al65 | ||
| miR-224, miR-455, miR-1236, miR-33a, and miR-520d | Serum | Song et al66 | ||
| miR-125b and miR-130a | Tissue and blood | Yuan et al67 | ||
| miR-199a and miR-497 | Tissue and cell lines | Troppan et al68 | ||
| miR-370, miR-381, and miR-409 | Tissue and cell lines | Leivonen et al69 | ||
| FL | Diagnostic | miR-9 and miR-155 | Tissue | Roehle et al49 |
| miR-217, miR-221, miR-222, miR-223, let-7i, and let-7b | Tissue | Lawrie et al50 | ||
| miR-31 and miR-17 | Tissue | Thompson et al70 | ||
| 17-miRNA signature | Tissue | Leich et al71 | ||
| 44-miRNA signature | Tissue | Wang et al72 | ||
| miR-494 | Tissue | Arribas et al73 | ||
| 66-miRNA signature | Bone marrow smears | Takei et al74 | ||
| Predictive | 23-miRNA signature | Tissue | Wang et al72 | |
| BL | Diagnostic | miR-23a, miR-26a, miR-29b, miR-30d, miR-146a, miR-146b, miR-155, and miR-221 | Tissue | Lenze et al75 |
| miR-34b | Cell lines and tissue | Leucci et al76 | ||
| 22-miRNA signature | Tissue | Hezaveh et al77 | ||
| miR-155, miR-21, and miR-26a | Needle aspirates | Zajdel et al78 | ||
| miR-29 family | Cell lines and tissue | Robaina et al79 and De Falco et al80 | ||
| miR-513a | Tissue | De Falco et al80 | ||
| miR-628 | Tissue | De Falco et al80 | ||
| miR-9* | Tissue | Onnis et al81 | ||
| 39-miRNA signature | Tissue | Robertus et al82 | ||
| 19-miRNA signature | Tissue | Di Lisio et al83 | ||
| 49-miRNA signature | Tissue | Oduor et al84 | ||
| miR-181b | Cell lines and tissue | Li et al85 | ||
| MCL | Diagnostic | miR-15/16 and miR-17~92 | Cell lines | Chen et al86 and Deshpande et al87 |
| 95-miRNA signature | Tissue | Iqbal et al88 | ||
| Prognostic | miR-15b | Tissue | Arakawa et al89 | |
| miR-129, miR-135, miR-146a, miR-424, miR-450, and miR-222 | Tissue | Iqbal et al88 | ||
| miR-17, miR-18a, miR-19b, and miR-92a (miR-17~92 cluster) | Tissue | Roisman et al90 | ||
| miR-29 | Cell lines and tissue | Zhao et al91 | ||
| miR-20b | Cell lines and tissue | Di Lisio et al92 | ||
| miR-18b | Cell lines and tissue | Husby et al93 | ||
| miR-223 | PBMCs and cell lines | Zhou et al94 | ||
| SMZL | Diagnostic | miR-29a, miR-29b-1, miR-96, miR-129, miR-182, miR-183, miR-335, and miR-593 | Tissue | Watkins et al95 |
| miR-127, miR-139, miR-335, miR-411, miR-451, and miR-486 | Tissue | Bouteloup et al96 | ||
| MALT | Diagnostic | 27-miRNA signature | Tissue | Thorns et al97 |
| miR-142, miR-155, and miR-203 | Tissue | Fernandez et al98 | ||
| Prognostic | miR-142 and miR-155 | Tissue | Liu et al99 |
Abbreviations: BL, Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; miRNA, microRNA; MALT, mucosa-associated lymphoid tissue; MCL, mantle cell lymphoma; PBMCs, peripheral blood mononuclear cells; SMZL, splenic marginal zone lymphoma.
*the minor strand of the mature form of the miRNA
Aberrant Expression of miRNAs in B-cell Lymphoma
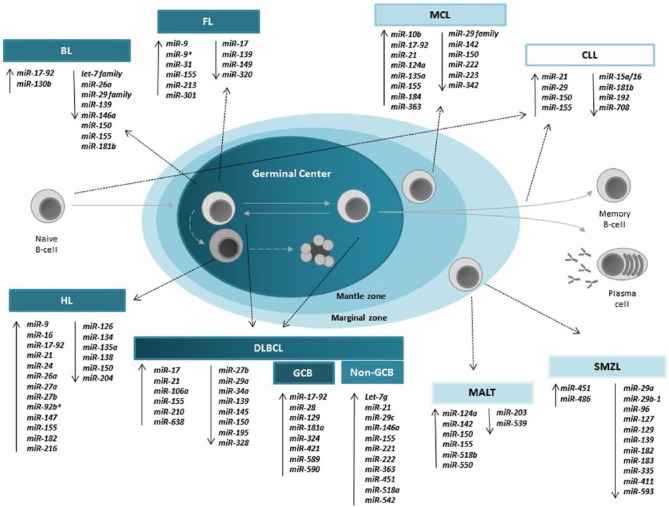
Many of the miRNAs that have been identified as lymphoma biomarkers (Figure 1 and Table 1) also play key roles in normal B-cell lymphopoiesis. Frequently, these aberrantly expressed biomarker miRNAs also appear to be key drivers of lymphomagenesis.100,101 For example, miR-155 controls germinal centre (GC) development by controlling immunoglobulin production, after activation of the B-cell receptor (BCR), and is a requirement for high-affinity antibody formation.102,103 However, when overexpressed in a transgenic mouse model, the mice developed a high-grade lymphoma similar to DLBCL.104 In a similar manner, the miR-17~92 controls pro–B-cell to pre–B-cell development via targeting of the proapoptotic protein BIM,105 but when overexpressed in a murine MYC model, increased the aggressiveness of B-cell lymphomas.106,107 MiR-21 that targets tumour suppressor molecules including PTEN and PDCD4,108,109 when overexpressed in mice resulted in formation of B-cell lymphomas.110 MiR-34a controls the transition of pro- to pre-B cell in haematopoietic stem cells via FOXP1 and SIRT1 targeting,111,112 and overexpression of this miRNA in mice abrogated lymphoma formation in a xenotransplant model.
Figure 1.
Schematic diagram of the major B-cell lymphoma miRNA biomarkers that have been identified and their relationship to B-cell development. BL indicates Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; miRNA, microRNA; MALT, mucosa-associated lymphoid tissue; MCL, mantle cell lymphoma; PBMCs, peripheral blood mononuclear cells; SMZL, splenic marginal zone lymphoma.
In addition to the miRNAs mentioned above, miR-181 has long been recognized as a key regulator of GC B-cell differentiation,113,114 along with miR-150 that inhibits MYB downregulation.115 The GC B cells are characterized by expression of markers BCL6, CD10, HGAL, and LMO2, as well as the absence of activated B-cell markers such as IRF4, PRDM1/BLIMP1, and XBP1. These transcription factors are also regulated at the level of miRNAs. For example, BCL6 is regulated by miR-30 family, miR-9 and let-7a,116 whereas miR-155 regulates expression of HGAL and CD10 protein expression,117,118 and miR-223 regulates expression of LMO2.119 In contrast, miR-125b and miR-155 regulate expression of the activated B-cell markers, IRF4 and PRDM1.116,120
The cause of aberrant miRNA expression in lymphoma (and other cancers) can result from many genomic events, such as chromosomal aberrations, epigenetic modifications, mutations in the sequence of miRNAs or their promoter regions, or factors that regulate synthesis or function of miRNAs (for further details see the work by Croce121). Below, we discuss the aberrantly expressed miRNAs in different B-cell lymphoproliferative diseases that could facilitate the diagnosis, prognosis, and prediction of treatment response.
Chronic lymphocytic leukaemia
Chronic lymphocytic leukaemia (CLL) is the most common haematologic malignancy worldwide122 and was the first haematologic malignancy, or indeed any cancer to be associated with aberrant miRNA expression when in 2002, George Calin and colleagues reported that the frequently (55%) deleted locus, 13q14, encodes for the miR-15a/16-1 cluster, and that these miRNAs were downregulated in most of the patients with 13q(del) CLL.33 These miRNAs act as tumour suppressors in CLL through targeting of the anti-apoptotic BCL2 protein123 and the tumour suppressor TP53.124 In contrast, miR-7-5p, miR-182-5p, and miR-320c/d are regulated by p53 in CLL.34 Epigenetic silencing of the miR-15a/16-1 cluster is observed in 30% to 35% of patients with CLL, a feature mediated through HDAC1-3 overexpression,125 suggesting that these patients might benefit from HDAC-inhibitor–based therapies. However, murine models of the 13q14 deletion suggest that other factors also contribute to the aggressiveness of the disease.126 Furthermore, the closely related miR-15b/16-2 cluster also appears to modulate genes involved in proliferation and anti-apoptotic pathways.127
Similar to miR-15a/16-1, miR-181b is also typically downregulated in CLL, and low expression of this miRNA has been related to poor prognostic outcome.39 Consistent with this phenotype, levels of miR-181b correlate with treatment-free survival in CLL.40
In contrast, miR-155 is overexpressed in CLL but was found to be lower in patients who responded to therapy compared with refractory patients,47 suggesting its usefulness as a predictive biomarker for CLL. MiR-29 is also overexpressed in both indolent and aggressive CLL, when compared with normal counterpart, but its expression was found to be lower in aggressive CLL.35 When miR-29 was overexpressed in murine B cells, the animals developed an indolent-type form of CLL.128
MicroRNA expression profiling has been used to distinguish between aggressive and indolent CLLs, with high levels of miR-21 and miR-155 being associated with a higher mortality rate.40,41 In contrast, upregulation of miR-708 has been associated with a favourable prognostic outcome for patients with CLL that was shown to be linked to a reduction in the nuclear factor κB signalling pathway.42 The proliferation status of a subset of peripheral blood cells–unmutated patients with CLL was linked with miR-22 overexpression via inhibition of PTEN and PI3K/AKT activation.129
Recently, it has been described that low levels of miR-150 in tumour cells or alternatively high levels of this miRNA in (circulating) serum are related to poor prognosis in CLL.43 In another study, levels of both miR-150 and miR-155 in the blood were associated with the prognostic outcome of CLL.44 Moreover, high levels of miR-155 in extracellular vesicles derived from the serum samples of patients with CLL were found compared with healthy controls.36 Filip et al37 found that the serum of patients with CLL had higher levels of miR-34a, miR-31, miR-155, miR-150, miR-15a, and miR-29a than controls. Another study showed that levels of miR-192 in peripheral blood mononuclear cells (PBMCs) are downregulated in patients with CLL compared with controls, suggesting that this miRNA could be a diagnostic biomarker for early stage of CLL.38 In CLL, proliferation centres, considered to drive the disease and play a role in progression of disease, had high levels of miR-155 and miR-92 and low levels of miR-150.130
Hodgkin lymphoma
Hodgkin lymphoma (HL), first described in 1832 by Thomas Hodgkin,131 is one of the most frequent lymphomas, accounting for 1% of total cancers worldwide. The defining characteristic of HL is that neoplastic cells typically account for less than 1% of the tumour mass.132 Tumour cells in classical HL (cHL), known as Hodgkin and Reed-Sternberg (HRS) cells, lack functional BCR expression or typical B-cell markers and instead express CD15 and CD30 cell surface markers.133,134 Anke van den Berg’s lab was the first to identify miRNAs in HL, when they observed in 2003 that the non-coding BIC locus, subsequently found to encode for miR-155, was overexpressed in HL cell lines.25,26 Since this time, miR-155 has been shown to target several genes in HL cells including DET1 and NIAM, among others.135
Apart from this miRNA, several others have been implicated in HL including miR-135a which was the first miRNA to be associated with survival in HL.31 The patients with HL with low levels of miR-135a had shorter disease-free survival than those with high levels of this miRNA. JAK2 is directly targeted by miR-135a, and the overexpression of this miRNA increases apoptotic levels and decreases cell growth via Bcl-xL inhibition.31 In addition, let-7 and miR-9 inhibition has been shown to block plasma cell differentiation, by decreasing levels of PRDM1/BLIMP1, as well as targeting Dicer and HuR.136 In a complementary study, inhibition of miR-9 was observed to hamper cytokine production and consequent inflammatory cell attraction in HL cell lines.137
A 25-miRNA signature that could differentiate between cHL and reactive lymph nodes was identified by Navarro et al28 using chromogenic in situ hybridization. Gibcus et al27 compared the expression of miRNAs between different HL cell lines and other B-cell lymphoma cell lines and described a 23-miRNA signature for HL, which included the overexpression of miR-17~92 cluster, miR-16, miR-21, miR-24, and miR-155 along with the downregulation of miR-150. Using microarrays, another group identified 134 differentially expressed miRNAs in HL cell lines and an overlapping signature of 100 miRNAs differentially expressed in tumour samples.29 Moreover, they observed that the levels of miR-21, miR-30e, miR-30d, and miR-92b could differentiate patients with HL according to prognostic risk groups. Epigenetic modifications of miRNA sequences have also been associated with HL including hypermethylation of miR-124a which was associated with more aggressive HL,32 and miR-9-2 methylation which is a common feature of this disease.30 Navarro et al138 recently observed that miR-34a and miR-203 are frequently methylated in HL cells. It has been recently found that the alteration of miRNAs related to the regulation of antioxidant enzymes is associated with an aggressive outcome of the disease.139 In plasma, the levels of miR-494, miR-1973, and miR-21 were higher in patients with HL than controls,21 and in another study, levels of miR-24, miR-127, miR-21, miR-155, and let-7a were higher in purified plasma exosomes from patients with HL than disease controls.22
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma is the most common B-cell lymphoma in Western countries, accounting for around 20% to 30% of cases.11 Thanks to the routine implementation of R-CHOP therapy, the survival of patients with DLBCL has been greatly improved; however, a third of patients still relapse or have a refractory disease.140 Diffuse large B-cell lymphoma is a heterogeneous disease both at the clinical and molecular level, with the existence of at least 2 different molecular subtypes: GC B-cell like (GC-DLBCL) and activated B-cell like (ABC-DLBCL).141 These subtypes are also distinguishable at the miRNA profile level with ABC-type lymphoma being associated with high expression of miR-21, miR-146a, miR-155, miR-221, and miR-363, and GCB-type DLBCL with high expression of miR-421 and the miR-17~92 cluster.49-53,142 It has been described that miRNAs can predict differences between DLBCL and follicular lymphoma (FL)49,50 or DLBCL and Burkitt lymphoma (BL).54,75 Central nervous system (CNS) relapse is a complication of DLBCL that occurs in approximately 5% of patients, associated with low survival, miR-20a and miR-30d are correlated with CNS relapse in patients with DLBCL and therefore could be used for patient stratification.58
As noted above, overexpression of miR-155 in mice is enough to cause development of a high-grade lymphoma, similar to DLBCL.143 Indeed, when the same authors used an inducible expression system, removal of the miR-155 stimulus was sufficient to allow complete recovery of affected mice.104 MiR-155 has also been linked with metastasis and prognosis in patients with DLBCL.59 Apart from miR-155 overexpression, low expression of both miR-34a and miR-27b expression has also been linked with a worse prognostic outcome for patients with DLBCL.61,62 In addition, low levels of miR-21 have been linked with shorter relapse-free survival in both tumour tissue50 and in serum from patients.24,66 As a consequence, levels of this miRNA have been proposed to act as an independent prognostic factor in DLBCL.64 It has been suggested that miR-21 may contribute to increase viability and reduce apoptotic levels of tumour cells through targeting BCL2 and PTEN.144,145 Furthermore, miR-21 inhibition leads to an increase in the sensitivity of DLBCL cell lines to CHOP treatment and reduces tumour cell proliferation and invasion.63,65
Several studies have looked at the association between miRNA expression and prognostic outcome in R-CHOP-treated patients with DLBCL. Our study found that levels of miR-27a, miR-142, miR-199b, miR-222, miR-302, miR-330, miR-425, and miR-519 were linked with overall survival.50 More recently, miR-125b and miR-130a were associated with resistance to R-CHOP in DLBCL,67 and high expression of miR-155 has also been linked to treatment failure.54 In vitro, overexpression of miR-199a and miR-497 resulted in increased sensitivity to rituximab, vincristine, and doxorubicin, drugs present in R-CHOP regimen.68 Overexpression of miR-370-3p, miR-381-3p, and miR-409-3p also increased sensitivity to rituximab and doxorubicin.69
Outside of the tumour itself, we observed that levels of miR-21, miR-155, and miR-210 in the serum samples of patients with DLBCL were differentially expressed when compared with serum samples from healthy controls.24 Subsequent studies using plasma also observed increased levels of miR-124 and miR-532-5p along with decreased levels of miR-122, miR-128, miR-141, miR-145, miR-197, miR-345, miR-424, and miR-425.55 Fang et al146 found that miR-15a, miR-16, miR-29c, and miR-155 were upregulated and miR-34a was downregulated in the serum samples of patients with DLBCL, and more recently Yuan et al67 found a good correlation between circulating levels of 8 miRNAs and their matched FFPE samples. High expression of serum miR-22 was associated with poor prognostic outcome.57 Recently, next-generation sequencing (NGS) technology was used to identify 51 miRNAs that were differentially expressed in the serum samples of patients with DLBCL compared with control serum samples.56 Three of these were validated by quantitative reverse transcription-polymerase chain reaction in a validation cohort. MiR-34a-5p was upregulated, whereas miR-323-3p and miR-431-5p were downregulated.
Follicular lymphoma
Follicular lymphoma is the most common indolent B-cell lymphoma worldwide, and despite being essentially incurable, it has a median overall survival of ~20 years. However, nearly a third of patients with FL will suffer histologic transformation into a high-grade lymphoma often termed transformed FL (tFL), that is morphologically indistinguishable from DLBCL, with a much worse prognosis than the antecedent FL.147,148 We identified a signature of 6 miRNAs (miR-223, miR-217, miR-222, miR-221, and let-7i and let-7b) that could distinguish between de novo DLBCL and tFL.50 Subsequently, miR-31 and miR-17-5p have also been identified as being differentially expressed between FL and tFL.70
The t(14;18) translocation resulting in the constitutive expression of the anti-apoptotic BCL2 protein is the genetic hallmark of more than 90% of FL cases.149 Using microarrays, a signature of 17 miRNAs was identified when comparing t(14; 18)-positive and t(14; 18)-negative FL cases. Downregulation of miR-16, miR-26a, miR-101, miR-29c, and miR-138 was associated with changes in the expression of target genes related to cell cycle control, apoptosis, and B-cell differentiation.71 It has been demonstrated that miRNA expression differs between pathogenic and non-neoplastic tissue, such as miR-9 and miR-155.49 Another study found a subset of 44 miRNAs which discriminates between FL and follicular hyperplasia, and the same study also described a 23-miRNA signature that was associated with an improved response to chemotherapy.72 Moreover, miR-494 was found overexpressed in FL compared with a potentially confounding diagnosis of nodal marginal zone lymphoma.73
Finally, one study analysed bone marrow smears from patients with FL and showed that 39 miRNA were decreased and 27 miRNA were increased significantly; among these, miR-451 showed the greatest decrease and miR-338-5p the greatest increase in patients with FL.74
Burkitt lymphoma
Burkitt lymphoma most commonly affects children and adolescents and is a highly aggressive lymphoma with a very poor prognosis that often involves extra-nodal sites. Burkitt lymphoma is characterized by overexpression of the MYC oncogene and is associated with the t(8:14) translocation in most of the cases (>90%).11 However, there are few cases that lack the t(8:14) translocation but have MYC overexpressed.76 The authors suggest that miR-34b could be responsible for MYC overexpression in these cases.76 In further studies, additional miRNAs have been identified as being differentially expressed between t(8:14)-positive and t(8:14)-negative cases by downregulation of miR-29 family members,79,80 miR-981 and miR-34b,76 and upregulation of miR-513a-5p and miR-628-3p.77,80 Furthermore, levels of MYC-regulated miRNAs, such as the let-7 family, miR-155, miR-146a, miR-29, and the miR-17~92 cluster, can distinguish BL from other B-cell lymphoma types.75,81-83,150 Recently, NGS was used to identify 49 differentially expressed miRNAs between BL cases and normal GC B cells, many of which can target MYC.84 Furthermore, miR-181b was found downregulated in BL cases, and the authors propose that it may function as a tumour suppressor.85 In an earlier study, significantly lower expression of miR-155, miR-21, and miR-26a was observed between classical BL and cases with intermediate features between BL and DLBCL (DLBCL/BL).78
Most of the endemic BL cases (>90%) are associated with Epstein-Barr virus (EBV) infection11,151 that has been shown to regulate several miRNAs, including miR-21, miR-146a, miR-155, miR-10a, and miR-127 in BL cases.152–155 In addition, EBV itself encodes for miRNAs that can interfere and compete with endogenous expression of miRNAs.156,157 In paediatric BL levels of cplasma, miR-21 and miR-23a were associated with both diagnosis and prognosis.158
Mantle cell lymphoma
Mantle cell lymphoma (MCL) accounts for 5% to 10% of non-Hodgkin lymphomas159 and has the worst prognosis of any B-cell lymphoma.160,161 Nearly all MCL (>90%) cases contain the t(11:14) translocation leading to overexpression of cyclin D1 (CCND1).162,163 It has been demonstrated that miR-15/16 and miR-17~92 are involved in CCND1 deregulation.86,87 The former miRNA (miR-15b) additionally involved in the transformation of classical to aggressive MCL.89 A miRNA signature of 95 miRNAs was identified that could differentiate between differing clinical subtypes of MCL.88,90 Low miR-29 together with high miR-20b and miR-18b levels; high expression of miR-129, miR-135, miR-146a, miR-424, and miR-450; and low expression of miR-222 or low miR-223 levels have been associated with poor prognosis in MCL.88,91–94
Other B-cell lymphomas
Splenic marginal zone lymphoma (SMZL) is a rare indolent B-cell lymphoproliferative disorder characterized by the 7q32 deletion. It has been demonstrated that this chromosomal aberration triggers the downregulation of 8 miRNAs (miR-29a, miR-29b1, miR-96, miR-129, miR-182, miR-183, miR-335, and miR-593) in SMZL cases.95 MiR-127, miR-139, miR-335, and miR-411 were also found downregulated in SMZL cases, whereas miR-451 and miR-486 were upregulated.96
Mucosa-associated lymphoid tissue (MALT) lymphoma is a multifocal disease that involves the MALT, frequently of the stomach, and is frequently associated with chronic inflammation as a result of Helicobacter pylori infection.11 On one hand, a signature of 27 miRNAs has been identified that can distinguish between gastritis and MALT lymphoma cases.97,98 On the other hand, miR-142 and miR-155 were found overexpressed in MALT lymphoma lesions compared with surrounding non-tumour mucosae. The expression levels of miR-142-5p and miR-155 were significantly increased in MALT lymphomas resistant to H pylori eradication than in cases showing complete remission after H pylori eradication. The expression levels of miR-142-5p and miR-155 were also associated with the clinical courses of gastric MALT lymphoma cases.99
Discussion and Future Directions
Despite the rapid growth of literature proposing miRNAs as B-cell lymphoma biomarkers, we are still far from the clinical implementation. Most of the miRNA biomarker studies to date are single centre with a retrospective design, with not enough power in most cases (Table 1). As a consequence, many reports are non-overlapping or even contradictory. These differences are probably due to variation in the handling of the material and the technical methodology used in each study.
The choice of the starting material (whole blood, PBMCs, serum, plasma, fresh of FFPE biopsy material) is of vital importance for the experimental design as it will generate different expression profiles.164-166 Sample collection and handling procedures are also crucial, and in the case of liquid biopsies, they should be optimized to reduce the time between phlebotomy and processing and to avoid excessive haemolysis which could lead major differences in the levels of miRNAs.167–169
It should also be taken into account that differences in the miRNA purification procedure are a source of variability.170 In addition, miRNA detection technique (qRT-PCR, microarrays, or NGS), along with the lack of a standard approach to normalization or a suitable endogenous reference gene for miRNA studies, can influence results significantly.13,24,171–175 It is therefore necessary to establish a standardized approach to miRNA biomarker studies alongside a systematic and comprehensive comparison of these confounding factors to ensure that the potential of these molecules is effectively realized in the clinic and live up to the hyperbole.
Acknowledgments
We apologize to the authors of the many studies who were not included in this review due to space limitations.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This work is supported by grants from the Ikerbasque Foundation for Science, Ministerio de Economía y Competitividad of Spanish central government and FEDER funds (PI12/00663, PIE13/00048, DTS14/00109, PI15/00275), Departamento Desarrollo Económico y Competitividad y Departamento de Sanidad of Basque government, Asociación Española Contra el Cancer (AECC), and the Diputación Foral de Gipuzkoa (DFG).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CS and EA contributed equally to this work.
ORCID iD: Esther Arnaiz  https://orcid.org/0000-0001-7838-4575
https://orcid.org/0000-0001-7838-4575
References
- 1. Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. [DOI] [PubMed] [Google Scholar]
- 2. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [DOI] [PubMed] [Google Scholar]
- 3. Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. [DOI] [PubMed] [Google Scholar]
- 4. Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. [DOI] [PubMed] [Google Scholar]
- 5. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 8. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vannini I, Fanini F, Fabbri M. Emerging roles of microRNAs in cancer. Curr Opin Genet Dev. 2018;48:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez-Mercado M, Manterola L, Larrea E, et al. The circulating transcriptome as a source of non-invasive cancer biomarkers: concepts and controversies of non-coding and coding RNA in body fluids. J Cell Molec Med. 2015;19:2307–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. [DOI] [PubMed] [Google Scholar]
- 13. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. [DOI] [PubMed] [Google Scholar]
- 15. Hanke M, Hoefig K, Merz H, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28:655–661. [DOI] [PubMed] [Google Scholar]
- 16. Park NJ, Zhou H, Elashoff D, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michael A, Bajracharya SD, Yuen PS, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie Y, Todd NW, Liu Z, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu L, Todd NW, Xing L, et al. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones K, Nourse JP, Keane C, Bhatnagar A, Gandhi MK. Plasma microRNA are disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2014;20:253–264. [DOI] [PubMed] [Google Scholar]
- 22. van Eijndhoven MA, Zijlstra JM, Groenewegen NJ, et al. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI Insight. 2016;1:e89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawrie CH. MicroRNA expression in lymphoma. Exp Opin Biol Ther. 2007;7:1363–1374. [DOI] [PubMed] [Google Scholar]
- 24. Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. [DOI] [PubMed] [Google Scholar]
- 25. van den Berg A, Kroesen BJ, Kooistra K, et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. [DOI] [PubMed] [Google Scholar]
- 26. Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer. 2004;39:167–169. [DOI] [PubMed] [Google Scholar]
- 27. Gibcus JH, Tan LP, Harms G, et al. Hodgkin lymphoma cell lines are characterized by a specific miRNA expression profile. Neoplasia. 2009;11:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navarro A, Gaya A, Martinez A, et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008;111:2825–2832. [DOI] [PubMed] [Google Scholar]
- 29. Sanchez-Espiridion B, Martin-Moreno AM, Montalban C, et al. MicroRNA signatures and treatment response in patients with advanced classical Hodgkin lymphoma. Br J Haematol. 2013;162:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben Dhiab M, Ziadi S, Louhichi T, Ben Gacem R, Ksiaa F, Trimeche M. Investigation of miR9-1, miR9-2 and miR9-3 methylation in Hodgkin lymphoma. Pathobiology. 2015;82:195–202. [DOI] [PubMed] [Google Scholar]
- 31. Navarro A, Diaz T, Martinez A, et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood. 2009;114:2945–2951. [DOI] [PubMed] [Google Scholar]
- 32. Ben Dhiab M, Ziadi S, Ksiaa F, et al. Methylation of miR124a-1, miR124a-2, and miR124a-3 in Hodgkin lymphoma. Tumour Biol. 2015;36:1963–1971. [DOI] [PubMed] [Google Scholar]
- 33. Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Nat Acad Sci U S A. 2002;99:15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blume CJ, Hotz-Wagenblatt A, Hullein J, et al. p53-dependent non-coding RNA networks in chronic lymphocytic leukemia. Leukemia. 2015;29:2015–2023. [DOI] [PubMed] [Google Scholar]
- 35. Pekarsky Y, Santanam U, Cimmino A, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590-11593. [DOI] [PubMed] [Google Scholar]
- 36. Caivano A, La Rocca F, Simeon V, et al. MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies – a short report. Cell Oncol. 2017;40:97–103. [DOI] [PubMed] [Google Scholar]
- 37. Filip AA, Grenda A, Popek S, et al. Expression of circulating miRNAs associated with lymphocyte differentiation and activation in CLL-another piece in the puzzle. Ann Hematol. 2017;96:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fathullahzadeh S, Mirzaei H, Honardoost MA, Sahebkar A, Salehi M. Circulating microRNA-192 as a diagnostic biomarker in human chronic lymphocytic leukemia. Cancer Gene Ther. 2016;23:327–332. [DOI] [PubMed] [Google Scholar]
- 39. Visone R, Veronese A, Balatti V, Croce CM. MiR-181b: new perspective to evaluate disease progression in chronic lymphocytic leukemia. Oncotarget. 2012;3:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi S, Shimizu M, Barbarotto E, et al. microRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood. 2010;116:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui B, Chen L, Zhang S, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baer C, Oakes CC, Ruppert AS, et al. Epigenetic silencing of miR-708 enhances NF-κB signaling in chronic lymphocytic leukemia. Int J Cancer. 2015;137:1352–1361. [DOI] [PubMed] [Google Scholar]
- 43. Stamatopoulos B, Van Damme M, Crompot E, et al. Opposite prognostic significance of cellular and serum circulating microRNA-150 in patients with chronic lymphocytic leukemia. Molec Med. 2015;21:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Georgiadis P, Liampa I, Hebels DG, et al. Evolving DNA methylation and gene expression markers of B-cell chronic lymphocytic leukemia are present in pre-diagnostic blood samples more than 10 years prior to diagnosis. BMC Genomics. 2017;18:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bomben R, Gobessi S, Dal Bo M, et al. The miR-17 approximately 92 family regulates the response to Toll-like receptor 9 triggering of CLL cells with unmutated IGHV genes. Leukemia. 2012;26:1584–1593. [DOI] [PubMed] [Google Scholar]
- 46. Calin GA, Ferracin M, Cimmino A, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. [DOI] [PubMed] [Google Scholar]
- 47. Ferrajoli A, Shanafelt TD, Ivan C, et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood. 2013;122:1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferracin M, Zagatti B, Rizzotto L, et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol Cancer. 2010;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roehle A, Hoefig KP, Repsilber D, et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol. 2008;142:732–744. [DOI] [PubMed] [Google Scholar]
- 50. Lawrie CH, Chi J, Taylor S, et al. Expression of microRNAs in diffuse large B cell lymphoma is associated with immunophenotype, survival and transformation from follicular lymphoma. J Cell Molec Med. 2009;13:1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caramuta S, Lee L, Ozata DM, et al. Role of microRNAs and microRNA machinery in the pathogenesis of diffuse large B-cell lymphoma. Blood Cancer J. 2013;3:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lawrie CH, Saunders NJ, Soneji S, et al. MicroRNA expression in lymphocyte development and malignancy. Leukemia. 2008;22:1440–1446. [DOI] [PubMed] [Google Scholar]
- 53. Zhong H, Xu L, Zhong JH, et al. Clinical and prognostic significance of miR-155 and miR-146a expression levels in formalin-fixed/paraffin-embedded tissue of patients with diffuse large B-cell lymphoma. Exp Ther Med. 2012;3:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iqbal J, Shen Y, Huang X, et al. Global microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphoma. Blood. 2015;125:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khare D, Goldschmidt N, Bardugo A, Gur-Wahnon D, Ben-Dov IZ, Avni B. Plasma microRNA profiling: exploring better biomarkers for lymphoma surveillance. PLoS ONE. 2017;12:e0187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meng Y, Quan L, Liu A. Identification of key microRNAs associated with diffuse large B-cell lymphoma by analyzing serum microRNA expressions. Gene. 2018;642:205–211. [DOI] [PubMed] [Google Scholar]
- 57. Marchesi F, Regazzo G, Palombi F, et al. Serum miR-22 as potential non-invasive predictor of poor clinical outcome in newly diagnosed, uniformly treated patients with diffuse large B-cell lymphoma: an explorative pilot study. J Exp Clin Cancer Res. 2018;37:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pillar N, Bairey O, Goldschmidt N, et al. MicroRNAs as predictors for CNS relapse of systemic diffuse large B-cell lymphoma. Oncotarget. 2017;8:86020–86030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang J, Wei B, Hu H, et al. Preliminary study on decreasing the expression of FOXP3 with miR-155 to inhibit diffuse large B-cell lymphoma. Oncol Lett. 2017;14:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tagawa H, Karube K, Tsuzuki S, Ohshima K, Seto M. Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development. Cancer Sci. 2007;98:1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. He M, Gao L, Zhang S, et al. Prognostic significance of miR-34a and its target proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and DLBCL. Gastric Cancer. 2014;17:431–441. [DOI] [PubMed] [Google Scholar]
- 62. Jia YJ, Liu ZB, Wang WG, et al. HDAC6 regulates microRNA-27b that suppresses proliferation, promotes apoptosis and target MET in diffuse large B-cell lymphoma. Leukemia. 2017;32:703–711. [DOI] [PubMed] [Google Scholar]
- 63. Gu L, Song G, Chen L, et al. Inhibition of miR-21 induces biological and behavioral alterations in diffuse large B-cell lymphoma. Acta Haematol. 2013;130:87–94. [DOI] [PubMed] [Google Scholar]
- 64. Zheng Z, Li X, Zhu Y, Gu W, Xie X, Jiang J. Prognostic significance of miRNA in patients with diffuse large B-cell lymphoma: a meta-analysis. Cell Physiol Biochem. 2016;39:1891–1904. [DOI] [PubMed] [Google Scholar]
- 65. Bai H, Wei J, Deng C, Yang X, Wang C, Xu R. MicroRNA-21 regulates the sensitivity of diffuse large B-cell lymphoma cells to the CHOP chemotherapy regimen. Int J Hematol. 2013;97:223–231. [DOI] [PubMed] [Google Scholar]
- 66. Song G, Gu L, Li J, et al. Serum microRNA expression profiling predict response to R-CHOP treatment in diffuse large B cell lymphoma patients. Ann Hematol. 2014;93:1735–1743. [DOI] [PubMed] [Google Scholar]
- 67. Yuan WX, Gui YX, Na WN, Chao J, Yang X. Circulating microRNA-125b and microRNA-130a expression profiles predict chemoresistance to R-CHOP in diffuse large B-cell lymphoma patients. Oncol Lett. 2016;11:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Troppan K, Wenzl K, Pichler M, et al. miR-199a and miR-497 are associated with better overall survival due to increased chemosensitivity in diffuse large B-cell lymphoma patients. Int J Molec Sci. 2015;16:18077–18095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leivonen SK, Icay K, Jantti K, et al. MicroRNAs regulate key cell survival pathways and mediate chemosensitivity during progression of diffuse large B-cell lymphoma. Blood Cancer J. 2017;7:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thompson MA, Edmonds MD, Liang S, et al. miR-31 and miR-17-5p levels change during transformation of follicular lymphoma. Human Pathol. 2016;50:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leich E, Zamo A, Horn H, et al. MicroRNA profiles of t(14;18)-negative follicular lymphoma support a late germinal center B-cell phenotype. Blood. 2011;118:5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang W, Corrigan-Cummins M, Hudson J, et al. MicroRNA profiling of follicular lymphoma identifies microRNAs related to cell proliferation and tumor response. Haematologica. 2012;97:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arribas AJ, Campos-Martin Y, Gomez-Abad C, et al. Nodal marginal zone lymphoma: gene expression and miRNA profiling identify diagnostic markers and potential therapeutic targets. Blood. 2012;119:e9–e21. [DOI] [PubMed] [Google Scholar]
- 74. Takei Y, Ohnishi N, Kisaka M, Mihara K. Determination of abnormally expressed microRNAs in bone marrow smears from patients with follicular lymphomas. Springerplus. 2014;3:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leucci E, Cocco M, Onnis A, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216:440–450. [DOI] [PubMed] [Google Scholar]
- 77. Hezaveh K, Kloetgen A, Bernhart SH, et al. Alterations of microRNA and microRNA-regulated messenger RNA expression in germinal center B-cell lymphomas determined by integrative sequencing analysis. Haematologica. 2016;101:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zajdel M, Rymkiewicz G, Chechlinska M, et al. miR expression in MYC-negative DLBCL/BL with partial trisomy 11 is similar to classical Burkitt lymphoma and different from diffuse large B-cell lymphoma. Tumour Biol. 2015;36:5377–5388. [DOI] [PubMed] [Google Scholar]
- 79. Robaina MC, Mazzoccoli L, Arruda VO, et al. Deregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp Molec Pathol. 2015;98:200–207. [DOI] [PubMed] [Google Scholar]
- 80. De Falco G, Ambrosio MR, Fuligni F, et al. Burkitt lymphoma beyond MYC translocation: N-MYC and DNA methyltransferases dysregulation. BMC Cancer. 2015;15:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Onnis A, De Falco G, Antonicelli G, et al. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PLoS ONE. 2010;5:e12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Robertus JL, Kluiver J, Weggemans C, et al. MiRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphoma. Br J Haematol. 2010;149:896–899. [DOI] [PubMed] [Google Scholar]
- 83. Di Lisio L, Sanchez-Beato M, Gomez-Lopez G, et al. MicroRNA signatures in B-cell lymphomas. Blood Cancer J. 2012;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oduor CI, Kaymaz Y, Chelimo K, et al. Integrative microRNA and mRNA deep-sequencing expression profiling in endemic Burkitt lymphoma. BMC Cancer. 2017;17:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li JG, Ding Y, Huang YM, et al. FAMLF is a target of miR-181b in Burkitt lymphoma. Braz J Med Biol Res. 2017;50:e5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen RW, Bemis LT, Amato CM, et al. Truncation in CCND1 mRNA alters miR-16-1 regulation in mantle cell lymphoma. Blood. 2008;112:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Deshpande A, Pastore A, Deshpande AJ, et al. 3ʹUTR mediated regulation of the cyclin D1 proto-oncogene. Cell Cycle. 2009;8:3592–3600. [DOI] [PubMed] [Google Scholar]
- 88. Iqbal J, Shen Y, Liu Y, et al. Genome-wide miRNA profiling of mantle cell lymphoma reveals a distinct subgroup with poor prognosis. Blood. 2012;119:4939–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arakawa F, Kimura Y, Yoshida N, et al. Identification of miR-15b as a transformation-related factor in mantle cell lymphoma. Int J Oncol. 2016;48:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Roisman A, Huaman Garaicoa F, Metrebian F, et al. SOXC and miR17-92 gene expression profiling defines two subgroups with different clinical outcome in mantle cell lymphoma. Genes Chromosomes Cancer. 2016;55:531–540. [DOI] [PubMed] [Google Scholar]
- 91. Zhao JJ, Lin J, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Di Lisio L, Gomez-Lopez G, Sanchez-Beato M, et al. Mantle cell lymphoma: transcriptional regulation by microRNAs. Leukemia. 2010;24:1335–1342. [DOI] [PubMed] [Google Scholar]
- 93. Husby S, Ralfkiaer U, Garde C, et al. miR-18b overexpression identifies mantle cell lymphoma patients with poor outcome and improves the MIPI-B prognosticator. Blood. 2015;125:2669–2677. [DOI] [PubMed] [Google Scholar]
- 94. Zhou K, Feng X, Wang Y, et al. miR-223 is repressed and correlates with inferior clinical features in mantle cell lymphoma through targeting SOX11. Exp Hematol. 2018;58:27.e1–34.e1. [DOI] [PubMed] [Google Scholar]
- 95. Watkins AJ, Hamoudi RA, Zeng N, et al. An integrated genomic and expression analysis of 7q deletion in splenic marginal zone lymphoma. PLoS ONE. 2012;7:e44997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bouteloup M, Verney A, Rachinel N, et al. MicroRNA expression profile in splenic marginal zone lymphoma. Br J Haematol. 2012;156:279–281. [DOI] [PubMed] [Google Scholar]
- 97. Thorns C, Kuba J, Bernard V, et al. Deregulation of a distinct set of microRNAs is associated with transformation of gastritis into MALT lymphoma. Virchows Arch. 2012;460:371–377. [DOI] [PubMed] [Google Scholar]
- 98. Fernandez C, Bellosillo B, Ferraro M, et al. MicroRNAs 142-3p, miR-155 and miR-203 are deregulated in gastric MALT lymphomas compared to chronic gastritis. Cancer Genomics Proteomics. 2017;14:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu TY, Chen SU, Kuo SH, Cheng AL, Lin CW. E2A-positive gastric MALT lymphoma has weaker plasmacytoid infiltrates and stronger expression of the memory B-cell-associated miR-223: possible correlation with stage and treatment response. Modern Pathol. 2010;23:1507–1517. [DOI] [PubMed] [Google Scholar]
- 100. Johanson TM, Skinner JP, Kumar A, Zhan Y, Lew AM, Chong MM. The role of microRNAs in lymphopoiesis. Int J Hematol. 2014;100:246–253. [DOI] [PubMed] [Google Scholar]
- 101. Lawrie CH. MicroRNAs and lymphomagenesis: a functional review. Br J Haematol. 2013;160:571–581. [DOI] [PubMed] [Google Scholar]
- 102. Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vigorito E, Perks KL, Abreu-Goodger C, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Babar IA, Cheng CJ, Booth CJ, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Nat Acad Sci U S A. 2012;109:E1695–E1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Danielson LS, Reavie L, Coussens M, et al. Limited miR-17-92 overexpression drives hematologic malignancies. Leuk Res. 2015;39:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Craig VJ, Cogliatti SB, Imig J, et al. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood. 2011;117:6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yamanaka Y, Tagawa H, Takahashi N, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–3275. [DOI] [PubMed] [Google Scholar]
- 110. Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. [DOI] [PubMed] [Google Scholar]
- 111. Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712–715. [DOI] [PubMed] [Google Scholar]
- 112. Rao DS, O’Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity. 2010;33:48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. de Yebenes VG, Belver L, Pisano DG, et al. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med. 2008;205:2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Teng G, Hakimpour P, Landgraf P, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Nat Acad Sci U S A. 2007;104:7080–7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lin J, Lwin T, Zhao JJ, et al. Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin’s B-cell lymphomas. Leukemia. 2011;25:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dagan LN, Jiang X, Bhatt S, Cubedo E, Rajewsky K, Lossos IS. miR-155 regulates HGAL expression and increases lymphoma cell motility. Blood. 2012;119:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thompson RC, Herscovitch M, Zhao I, Ford TJ, Gilmore TD. NF-kappaB down-regulates expression of the B-lymphoma marker CD10 through a miR-155/PU.1 pathway. J Biol Chem. 2011;286:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Malumbres R, Sarosiek KA, Cubedo E, et al. Differentiation stage-specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood. 2009;113:3754–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gururajan M, Haga CL, Das S, et al. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int Immunol. 2010;22:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fabbri G, Dalla-Favera R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat Rev Cancer. 2016;16:145–162. [DOI] [PubMed] [Google Scholar]
- 123. Pekarsky Y, Balatti V, Croce CM. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ. 2018;25:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011;305:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sampath D, Liu C, Vasan K, et al. Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia. Blood. 2012;119:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lia M, Carette A, Tang H, et al. Functional dissection of the chromosome 13q14 tumor-suppressor locus using transgenic mouse lines. Blood. 2012;119:2981–2990. [DOI] [PubMed] [Google Scholar]
- 127. Lovat F, Fassan M, Gasparini P, et al. miR-15b/16-2 deletion promotes B-cell malignancies. Proc Nat Acad Sci U S A. 2015;112:11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Santanam U, Zanesi N, Efanov A, et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Nat Acad Sci U S A. 2010;107:12210–12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Palacios F, Prieto D, Abreu C, et al. Dissecting chronic lymphocytic leukemia microenvironment signals in patients with unmutated disease: microRNA-22 regulates phosphatase and tensin homolog/AKT/FOXO1 pathway in proliferative leukemic cells. Leuk Lymphoma. 2015;56:1560–1565. [DOI] [PubMed] [Google Scholar]
- 130. Szurian K, Csala I, Piurko V, Deak L, Matolcsy A, Reiniger L. Quantitative miR analysis in chronic lymphocytic leukaemia/small lymphocytic lymphoma – proliferation centres are characterized by high miR-92a and miR-155 and low miR-150 expression. Leukemia Res. 2017;58:39–42. [DOI] [PubMed] [Google Scholar]
- 131. Hodgkin T. On some morbid appearances of the absorbent glands and spleen. Med Chir Trans. 1832;17:68–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schmitz R, Stanelle J, Hansmann ML, Kuppers R. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol. 2009;4:151–174. [DOI] [PubMed] [Google Scholar]
- 133. Pileri SA, Ascani S, Leoncini L, et al. Hodgkin’s lymphoma: the pathologist’s viewpoint. J Clin Pathol. 2002;55:162–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. [DOI] [PubMed] [Google Scholar]
- 135. Slezak-Prochazka I, Kluiver J, de Jong D, et al. Inhibition of the miR-155 target NIAM phenocopies the growth promoting effect of miR-155 in B-cell lymphoma. Oncotarget. 2016;7:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nie K, Gomez M, Landgraf P, et al. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008;173:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Leucci E, Zriwil A, Gregersen LH, et al. Inhibition of miR-9 de-represses HuR and DICER1 and impairs Hodgkin lymphoma tumour outgrowth in vivo. Oncogene. 2012;31:5081–5089. [DOI] [PubMed] [Google Scholar]
- 138. Navarro A, Diaz T, Cordeiro A, et al. Epigenetic regulation of microRNA expression in Hodgkin lymphoma. Leuk Lymphoma. 2015;56:2683–2689. [DOI] [PubMed] [Google Scholar]
- 139. Karihtala P, Porvari K, Soini Y, Haapasaari KM. Redox regulating enzymes and connected microRNA regulators have prognostic value in classical Hodgkin lymphomas. Oxidative Med Cell Longevity. 2017;2017:2696071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Friedberg JW. New strategies in diffuse large B-cell lymphoma: translating findings from gene expression analyses into clinical practice. Clin Cancer Res. 2011;17:6112–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. [DOI] [PubMed] [Google Scholar]
- 142. Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Nat Acad Sci U S A. 2008;105:13520–13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Costinean S, Zanesi N, Pekarsky Y, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Nat Acad Sci U S A. 2006;103:7024–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Song J, Shao Q, Li C, et al. Effects of microRNA-21 on apoptosis by regulating the expression of PTEN in diffuse large B-cell lymphoma. Medicine. 2017;96:e7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu K, Du J, Ruan L. MicroRNA-21 regulates the viability and apoptosis of diffuse large B-cell lymphoma cells by upregulating B cell lymphoma-2. Exp Ther Med. 2017;14:4489–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Fang C, Zhu DX, Dong HJ, et al. Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann Hematol. 2012;91:553–559. [DOI] [PubMed] [Google Scholar]
- 147. Castellino A, Santambrogio E, Nicolosi M, Botto B, Boccomini C, Vitolo U. Follicular lymphoma: the management of elderly patient. Mediterr J Hematol Infect Dis. 2017;9:e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the BCL-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. [DOI] [PubMed] [Google Scholar]
- 150. Zhang J, Jima DD, Jacobs C, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113:4586–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nguyen L, Papenhausen P, Shao H. The role of c-MYC in B-cell lymphomas: diagnostic and molecular aspects. Genes. 2017;8:E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Godshalk SE, Bhaduri-McIntosh S, Slack FJ. Epstein-Barr virus-mediated dysregulation of human microRNA expression. Cell Cycle. 2008;7:3595–3600. [DOI] [PubMed] [Google Scholar]
- 153. Yin Q, McBride J, Fewell C, et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J Virol. 2008;82:5295–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Leucci E, Onnis A, Cocco M, et al. B-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expression. Int J Cancer. 2010;126:1316–1326. [DOI] [PubMed] [Google Scholar]
- 155. Oduor CI, Movassagh M, Kaymaz Y, et al. Human and Epstein-Barr virus miRNA profiling as predictive biomarkers for endemic Burkitt lymphoma. Front Microbiol. 2017;8:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. De Falco G, Antonicelli G, Onnis A, Lazzi S, Bellan C, Leoncini L. Role of EBV in microRNA dysregulation in Burkitt lymphoma. Semin Cancer Biol. 2009;19:401–406. [DOI] [PubMed] [Google Scholar]
- 157. Mundo L, Ambrosio MR, Picciolini M, et al. Unveiling another missing piece in EBV-driven lymphomagenesis: EBV-encoded microRNAs expression in EBER-negative Burkitt lymphoma cases. Front Microbiol. 2017;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li J, Zhai XW, Wang HS, Qian XW, Miao H, Zhu XH. Circulating microRNA-21, microRNA-23a, and microRNA-125b as biomarkers for diagnosis and prognosis of Burkitt lymphoma in children. Med Sci Monitor. 2016;22:4992–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Velders GA, Kluin-Nelemans JC, De Boer CJ, et al. Mantle-cell lymphoma: a population-based clinical study. J Clin Oncol. 1996;14:1269–1274. [DOI] [PubMed] [Google Scholar]
- 160. Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34:1256–1269. [DOI] [PubMed] [Google Scholar]
- 161. Inamdar AA, Goy A, Ayoub NM, et al. Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget. 2016;7:48692–48731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Brizova H, Kalinova M, Krskova L, Mrhalova M, Kodet R. Quantitative monitoring of cyclin D1 expression: a molecular marker for minimal residual disease monitoring and a predictor of the disease outcome in patients with mantle cell lymphoma. Int J Cancer. 2008;123:2865–2870. [DOI] [PubMed] [Google Scholar]
- 164. Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10:543–550. [DOI] [PubMed] [Google Scholar]
- 165. Gourzones C, Ferrand FR, Amiel C, et al. Consistent high concentration of the viral microRNA BART17 in plasma samples from nasopharyngeal carcinoma patients – evidence of non-exosomal transport. Virology J. 2013;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hu J, Wang Z, Tan CJ, et al. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation. 2013;95:991–999. [DOI] [PubMed] [Google Scholar]
- 167. McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57:833–840. [DOI] [PubMed] [Google Scholar]
- 168. Kirschner MB, Kao SC, Edelman JJ, et al. Haemolysis during sample preparation alters microRNA content of plasma. PLoS ONE. 2011;6:e24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pritchard CC, Kroh E, Wood B, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res. 2012;5:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kim YK, Yeo J, Kim B, Ha M, Kim VN. Short structured RNAs with low GC content are selectively lost during extraction from a small number of cells. Mol Cell. 2012;46:893–895. [DOI] [PubMed] [Google Scholar]
- 171. Bernardo BC, Charchar FJ, Lin RC, McMullen JR. A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ. 2012;21:131–142. [DOI] [PubMed] [Google Scholar]
- 172. McDermott AM, Kerin MJ, Miller N. Identification and validation of miRNAs as endogenous controls for RQ-PCR in blood specimens for breast cancer studies. PLoS ONE. 2013;8:e83718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE. 2009;4:e6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Friedman EB, Shang S, de Miera EV, et al. Serum microRNAs as biomarkers for recurrence in melanoma. J Transl Med. 2012;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Murata K, Furu M, Yoshitomi H, et al. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS ONE. 2013;8:e69118. [DOI] [PMC free article] [PubMed] [Google Scholar]