Abstract
Purpose
Dysarthria is a consequence of multiple sclerosis (MS) that can co-occur with cognitive impairment. Clinical management thus requires understanding the separate and combined effects of dysarthria and cognitive impairment on functional communication in MS. This study compared perceptual measures of intelligibility and speech severity that capture functional communication deficits for 4 operationally defined groups with MS. The relationship between communication participation and perceptual measures was also examined.
Method
Forty-eight adults with MS and 12 healthy controls participated. Cognitive testing and dysarthria diagnosis determined group assignment: (a) MS with cognitive impairment (MSCI), (b) MS with a diagnosis of dysarthria and no cognitive impairment (MSDYS), (c) MS with dysarthria and cognitive impairment (MSDYS + CI), and (d) MS without dysarthria or cognitive impairment (MS). Sentence Intelligibility Test scores, scaled speech severity obtained from the “Grandfather Passage,” and Communication Participation Item Bank (CPIB) scores were analyzed.
Results
Sentence Intelligibility Test scores approached 100% for all groups. Speech severity was greater for the MSDYS + CI and MSDYS groups versus controls. CPIB scores were greatest for the MSDYS + CI group and were not significantly correlated with either perceptual measure.
Conclusions
The CPIB and speech severity were sensitive to aspects of communication problems for some groups with MS not reflected in a measure of sentence intelligibility. Findings suggest the importance of employing a variety of measures to capture functional communication problems experienced by persons with MS.
Multiple sclerosis (MS) is an acquired, complex progressive disease of the central nervous system. Common symptoms include sensorimotor changes impacting bulbar and spinal function (e.g., speech and walking) as well as problems with cognition, coordination, vision, fatigue, depression, and pain. Although typically mild, communication problems are common in MS (Yorkston & Baylor, 2012). However, even a mild communication problem can have severe negative consequences for leisure activities, social relationships, and employment (Walshe, 2011). Variables with the potential to impact an individual's ability to gain and maintain employment are of particular concern in MS because the disease affects people during a time of major career development (Benedict et al., 2005; Rao, Leo, Bernardin, & Unverzagt, 1991).
Communication problems associated with MS are more diverse than first suggested by Charcot (Yorkston et al., 2003). Dysarthria has long been linked to MS, but there is now a growing appreciation for the contribution of cognitive limitations to problems with communication, particularly in recent studies investigating the impact of communication difficulties on participation in everyday life situations (Yorkston & Baylor, 2012; Yorkston, Baylor, & Amtmann, 2014). Prevalence estimates for dysarthria range from approximately 25% to 50% (Darley, Brown, & Goldstein, 1972; Hartelius, Runmarker, & Andersen, 2000; Hartelius & Svensson, 1994; Yorkston et al., 2003). Classic studies suggest a mixed spastic–ataxic dysarthria with prominent deviant perceptual characteristics of impaired loudness control, imprecise articulation, and vocal harshness (Darley et al., 1972). Upwards of 70% of individuals with MS experience problems in the cognitive domains of memory, attention, speed of information processing, or executive function that can contribute to high-level, nonaphasic language deficits such as difficulties with word finding and verbal fluency that are undetectable using standard language tests (e.g., Laakso, Brunnegard, Hartelius, & Ahlsen, 2000; Murdoch & Lethlean, 2000a, 2000b; Rao et al., 1991). Dysarthria may also frequently co-occur with cognitive impairment (Yorkston et al., 2003). Clinical management of communication problems in MS thus requires understanding the separate and combined effects of dysarthria and cognitive impairment on functional communication. As discussed in the following section, however, relevant studies employing psychometrically sound measures sensitive to cognitive impairment in MS are lacking.
Studies investigating motor speech problems in MS rarely include a rigorous measure of cognitive function. When dysarthria studies of MS have assessed cognition, screening tools or dementia instruments not intended for MS have typically been employed (e.g., Mackenzie & Green, 2009; Tjaden & Wilding, 2011; Wallace & Holmes, 1993). These types of assessments may lack sensitivity to subtle cognitive deficits in MS (Rao, 1995). For this reason, tests comprising the Minimal Assessment of Cognitive Function in MS (MACFIMS; Benedict et al., 2002) are considered to be ideally suited to assess cognitive function in MS (Strober et al., 2009). A recent study by Feenaughty, Tjaden, Benedict, and Weinstock-Guttman (2013) is one of the few MS speech studies in which tests from the MACFIMS, rather than a screening test, were used to assess cognitive function. Feenaughty et al. (2013) investigated differences in speech and articulatory rates and pause characteristics in reading aloud and narratives for 20 individuals with MS and 10 healthy talkers. Speakers with MS comprised high- and low-performance groups on the basis of neuropsychological tests of executive function and processing efficiency selected from the MACFIMS test battery. Pauses in spontaneous speech were the primary factor distinguishing speakers with relatively better cognition from those with relatively poorer cognition in MS. It was concluded that cognitive abilities, particularly information-processing efficiency, may be related to global timing characteristics during connected speech for individuals with MS. In addition, it was suggested that assumptions concerning the cognitive demands of reading aloud compared with spontaneous speech may need to be reconsidered for individuals with impaired cognition. However, it is not well understood how impaired cognition alone or combined with dysarthria affects speech behavior during oral reading.
Within the World Health Organization's (2009) International Classification of Function, Disability, and Health (ICF) framework, levels of assessment and intervention are categorized on the basis of impairment of body functions and structures, activity limitations, and participation restrictions in life events. As discussed in the following paragraphs, intelligibility, perceived speech severity, and communication participation were of interest in this study because these constructs reflect functional communication spanning the activity and participation domains of the ICF and also because these constructs are frequently the focus of clinical management of communication problems in MS and published tools facilitate their ease of use and implementation by clinicians and researchers (see Tjaden, Sussman, & Wilding, 2014; Yorkston & Baylor, 2012; Yorkston et al., 2014).
Intelligibility, as well as related perceptual constructs such as speech severity or naturalness, captures communication limitations in the activity domain of the ICF. Thus, intelligibility measures the functional impact of dysarthria on communication and overall dysarthria severity (Duffy, 2013; Weismer, 2008). The Sentence Intelligibility Test (SIT; Yorkston, Beukelman, & Tice, 1996) is one of the most widely used published tests for assessing intelligibility in dysarthria (Duffy, 2013). Intelligibility may be unaffected for individuals with MS with mild dysarthria, and as previously noted, communication problems in MS are typically mild (Yorkston & Baylor, 2012). An intelligibility measure such as the SIT therefore may fail to fully capture a mild speech disorder owing to ceiling effects (Yorkston & Beukelman, 1981). Visual analog scaling of speech severity or related perceptual constructs such as articulatory precision, however, are sensitive to mild dysarthria in MS and thus can supplement information regarding functional communication provided by the gold standard perceptual measure of intelligibility (Fletcher & McAuliffe, 2017; Sussman & Tjaden, 2012; Tjaden et al., 2014).
Whether cognitive limitations in MS bear on listeners' perceptions of functional communication, as indexed by speech severity or the closely related construct of intelligibility, is not well understood. Studies reporting a correlation between intelligibility and scores on cognitive instruments in MS do not shed light on the issue because participants in these studies had both motor speech (i.e., dysarthria) and cognitive impairment (Mackenzie & Green, 2009; Wallace & Holmes, 1993). More recently, Rodgers, Tjaden, Feenaughty, Weinstock-Guttman, and Benedict (2012) reported SIT and cognitive test scores from the MACFIMS for 25 healthy control speakers and 50 individuals with MS. Cognitive test results indicated significantly better performance for controls with medium to large effect sizes depending on the particular test, but SIT scores did not differ for the two groups. An independent clinical diagnosis of dysarthria was not obtained for participants with MS. Thus, it is unclear whether the equivalent intelligibility for the control group and the group with MS reflects a ceiling effect of mild dysarthria or the absence of dysarthria. Atypical speech behaviors such as longer, more frequent silent pauses that may indicate cognitive-based difficulties with word finding in MS have been linked to perceptions of reduced speaker competence (MacGregor, Corley, & Donaldson, 2010) and ability to perform in the workplace (LaRocca, Kalb, & Gregg, 1995; Rao et al., 1991; Yorkston, Klasner, & Swanson, 2001). Thus, it might be speculated that cognitive impairment alone or in combination with mild dysarthria in MS may be reflected in perceptual judgments of overall speech severity, even when intelligibility is at ceiling.
The participation domain of the ICF refers to a person's involvement in life situations. Communication participation is defined as taking part in situations where knowledge, information, ideas, or feelings are exchanged (Eadie et al., 2006). The recently developed Communication Participation Item Bank (CPIB; Baylor et al., 2013; Eadie et al., 2006) captures self-reported restrictions in the participation domain of the ICF. Yorkston et al. (2014) investigated symptoms associated with MS that may predict restrictions in communication, as measured using the CPIB. Over 200 individuals with MS reporting problems with communication completed the CPIB, provided demographic information, and self-reported symptom-related variables such as speech severity and cognitive–communication skills. Greater restrictions in communication participation were associated with self-report of more cognitive problems, more severe speech symptoms, reduced mobility or activity, and higher levels of education. As noted by the authors, studies including measures to quantify speech severity and cognitive function are needed to advance understanding of how these variables may relate or contribute to communication participation. Indeed, studies of Parkinson's disease and traumatic brain injury employing perceptual measures to quantify speech intelligibility suggest a complex relationship between the activity and participation domains of the ICF, such that even individuals with mildly reduced speech intelligibility may experience severe restrictions in communication participation (Donovan, Kendall, Young, & Rosenbek, 2007; Dykstra, Hakel, & Adams, 2007; McAuliffe, Carpenter, & Moran, 2010).
Effective clinical management of communication problems in MS requires understanding the separate and combined effects of dysarthria and cognitive impairment on functional communication, as indicated by measures corresponding to the activity and participation domains of the ICF. As indicated in the previous review, progress has been made but methodological limitations in prior research suggest that additional studies are warranted. Thus, the purpose of this study was to compare measures of speech intelligibility and perceived speech severity (Sussman & Tjaden, 2012) as well as a patient-reported measure of communication participation for four operationally defined groups of speakers with MS: (a) MS with cognitive impairment (MSCI), (b) MS with clinically diagnosed dysarthria and intact cognition (MSDYS), (c) MS with comorbid dysarthria and cognitive impairment (MSDYS + CI), and (d) MS without dysarthria or cognitive impairment (MS). Relationships among measures also were of interest. Healthy controls (CON) were included for comparison. The following research questions were addressed:
Do perceptual measures of intelligibility and speech severity differ among groups?
Does communication participation differ among groups with MS?
What is the relationship between perceptual measures and communication participation for groups with MS?
Method
Participants
A new cohort of speakers with MS who did not participate in our prior investigations (Feenaughty et al., 2013; Rodgers et al., 2012) was recruited for study using flyers distributed at Buffalo General Hospital, MS support groups, and advertisements in an MS newsletter distributed in western New York. Healthy speakers were recruited using flyers posted at the University at Buffalo. All speakers signed an informed consent form approved by the Social and Behavioral Sciences Institutional Review Board at the University at Buffalo.
Sixty speakers were studied including 48 community-dwelling individuals reporting a neurological diagnosis of MS and 12 sex-matched healthy controls. Speakers with MS ranged in age from 26 to 67 years (M = 52 years, SD = 10 years). Healthy talkers ranged in age from 40 to 60 years (M = 52 years, SD = 6 years). All participants were native speakers of Standard American English; reported normal or corrected-to-normal vision, no hearing problems or use of a hearing aid, and no history of or current substance abuse; and passed a pure-tone hearing screening at 40 dB for octave frequencies ranging from 500 to 4000 Hz, in at least one ear (American National Standards Institute, 2004). Speakers with MS also reported no other major neurological disorder or history of neuropsychiatric disease, no use of corticosteroids for the relapse of MS within eight weeks of the experiment, and no recent medication changes for the treatment of MS or symptoms related to MS within 12 weeks of testing. Forty speakers with MS reported a relapsing-remitting disease course, whereas six speakers with MS reported a secondary progressive disease course. The two remaining speakers with MS reported a progressive course of MS, one reported a primary progressive course, but the final speaker with MS did not report whether the course was secondary or primary progressive. Participants were taking a variety of medications for the treatment of MS and for associated symptoms. No one was receiving speech therapy at the time of this study. Neuropsychological tests and dysarthria measures used to assign each participant with MS to four operationally defined groups are described in the following sections.
Neuropsychological Testing and Scoring Procedures to Determine Speaker Groups
Four psychometrically sound neuropsychological tests from the MACFIMS (Benedict et al., 2002) were used to assess cognitive function and subsequently assign participants with MS to groups with and without cognitive impairment. The 3-second version of the Paced Auditory Serial Addition Test (Gronwall, 1977; Rao et al., 1991) and the oral version of the Symbol Digit Modalities Test (Rao et al., 1991; Smith, 1982) measured cognitive processing speed and working memory. Scores from the 3-second version of the Paced Auditory Serial Addition Test and the Symbol Digit Modalities Test were subsequently averaged to provide a composite measure of information-processing speed and efficiency (see Parmenter, Testa, Schretlen, Weinstock-Guttman, & Benedict, 2010). The California Verbal Learning Test–Second Edition (Delis, Kramer, Kaplan, & Ober, 2000) measured auditory–verbal episodic memory and learning. The Delis–Kaplan Executive Function System Test (Delis, Kaplan, & Kramer, 2001) measured executive function. The total number of words recalled after a long delay (~25 min) from the California Verbal Learning Test–Second Edition and the total number of correct sorts from the Delis–Kaplan Executive Function System Test were variables of interest.
Total raw scores from each cognitive test were normalized for a speaker's age, years of education, and gender using a regression-based procedure yielding z scores to limit demographic biases (Parmenter et al., 2010; see also review in Amato et al., 2013). Score normalization has been shown to be valid for use with outcome measures obtained from the MACFIMS (Parmenter et al., 2010). Subsequent to normalization, a universally accepted cutoff score (i.e., ≤ −1.50) in the MS literature was used to determine mutually exclusive speaker groups (Benedict et al., 2006). Table 1 summarizes characteristics of the operationally defined speaker groups. As indicated in Table 1, only participants with MS with a z score of ≤ −1.50 in at least one cognitive domain on neuropsychological tests comprised the MSCI group, as a z score ≤ −1.50 is consistent with a clinical diagnosis of at least a mild cognitive impairment (Benedict et al., 2006). Participants with clinically diagnosed dysarthria, as determined by three speech-language pathologists (SLPs), comprised the MSDYS group. Finally, participants with a z score of ≤ −1.50 on neuropsychological tests and a clinical diagnosis of dysarthria comprised the MSDYS + CI group. Table 1 also summarizes years of education and additional measures used to further characterize the speaker groups.
Table 1.
Means and standard deviations are reported for characteristics of the operationally defined speaker groups.
| Group | Cognitive function (z score) | Age (years) |
Years post diagnosis |
Years of education |
Depression (BDI-FS) |
Fatigue (FSS) |
Disease severity (EDSS) |
|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD); median | ||
| MSDYS + CI | ≤ −1.50 | 52 (9) | 19 (13) | 16 (2) | 4 (5) | 4 (1) | 4 (1); 4.5 a |
| MSDYS | > −1.50 | 56 (10) | 19 (14) | 14 (2) | 2 (2) | 4 (1) | 5 (1); 6.0 |
| MSCI | ≤ −1.50 | 49 (9) | 11 (11) | 19 (3) | 4 (1) | 3 (4) | 4 (1); 4.0 a |
| MS | > −1.50 | 55 (6) | 15 (6) | 14 (3) | 4 (1) | 1 (1) | 4 (1); 3.5 |
| CON | > −1.50 | 52 (6) | — | 16 (2) | 1 (1) | 2 (1) | — |
Note. Median values are also reported for disease severity. A normalized z score ≤ −1.50 indicated a cognitive deficit in a given cognitive domain. A z score > −1.50 indicated cognition within normal limits. EDSS indicates the mean disease severity of patient and neurologist or trained assistant who administered EDSS for speakers identified at Buffalo General Hospital. BDI-FS = Beck Depression Inventory–Fast Screen; FSS = Fatigue Severity Scale; EDSS = Expanded Disability Status Scale; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy talkers.
An EDSS score was not available for 1 speaker in the MSCI group and 1 speaker in the MSDYS + CI group. Thus, values are based on a total of 11 speakers for these groups.
Speech Tasks and Procedures for Clinical Dysarthria Diagnosis to Determine Speaker Groups
Each speaker was audio-recorded producing a variety of speech tasks that were used to determine speaker groups and are explained in more detail below. Speakers were randomly assigned to one of three task orders to elicit speech samples. Within each task order, speech tasks were randomized. Audio-recorded data files were assigned a numeric code to blind group membership. Speech samples were audio-recorded in a sound-treated room using a Countryman E610P5L2 ear-mounted microphone placed 6 cm from the center of the speaker's upper lip. The acoustic signal was preamplified and digitized at a sampling rate of 22.05 kHz (Boersma & Weenink, 2012). For a given speaker, audio-recorded speech samples and administration of neuropsychological tests were obtained on the same day.
Three certified SLPs made a clinical diagnosis of dysarthria on the basis of a consensus, auditory perceptual approach, as in previously published studies (e.g., Keintz, Bunton, & Hoit, 2007; Tjaden & Wilding, 2004). These perceptual judgments of dysarthria were based on clinical audio recordings of a variety of speech tasks including vowel prolongation, diadochokinesis, and sentences from the SIT (Yorkston et al., 1996) as well as the “Grandfather Passage” and a short interval of spontaneous speech. As described in the Experimental Perceptual Measures and Procedures section, SIT scores and the “Grandfather Passage” were two of the speech tasks examined in this study. Speech samples were presented in a quiet room. Before listening to the stimuli, the speaker's age, gender, years of education, neurological diagnosis, and disease course were provided to the SLPs. Descriptive statistics reported in Table 1 also reflect self-reported clinical measures of depression and fatigue including the Beck Depression Inventory–Fast Screen (Beck, Steer, & Brown, 2000) and the Fatigue Severity Scale (Krupp, LaRocca, Muir-Nash, & Steinberg, 1989). The patient-administered version of the Expanded Disability Status Scale (Kurtzke, 1983) provided a measure of overall disease severity. Appendix A reports the deviant perceptual speech and voice characteristics to further describe speakers in the MSDYS and MSDYS + CI groups.
Evaluation of Speaker Group Characteristics
Each speaker group contained eight women and four men as MS affects at least twice as many women than men. Speakers comprising the MSDYS group ranged in age from 35 to 67 years (M = 56 years, SD = 10 years); the MSDYS + CI group, from 37 to 66 years (M = 52 years, SD = 9 years); the MSCI group, from 26 to 54 years (M = 43 years, SD = 9 years); and the MS group, from 44 to 63 years (M = 55 years, SD = 6 years; see Table 1). Analysis of variance (ANOVA) indicated group differences in age, F(4, 55) = 4.743, p < .01, and education, F(4, 55) = 3.707, p < .05, but not years postdiagnosis, F(3, 44) = 1.309, p > .05. Post hoc tests confirmed that the MSCI group was significantly younger than the MSDYS (p = .009) and MS (p = .002) groups. The MSCI group also had significantly more years of education compared with the MSDYS (p = .018) and MS (p = .014) groups, but not the MSDYS + CI and CON groups.
Table 2 (upper panel) reports mean z scores and standard deviations for neuropsychological testing by group and cognitive domain. ANOVA results for neuropsychological testing and significant post hoc comparisons among speaker groups are also summarized in Table 2 (lower panel). ANOVA confirmed that the MSCI and MSDYS + CI groups had similar levels of impairment in all cognitive domains tested, as expected. However, the MSDYS and MSDYS + CI groups did not differ significantly on executive function, despite the fact that speakers who comprised the MSDYS group had executive function test scores within normal limits. Finally, there were no significant differences in fatigue, depression, or disease severity among the groups with MS (see Table 1). Thus, on average, all speakers with MS in the current study had varying levels of disability in at least a single neurological domain and reported minimal symptoms of depression and moderate levels of fatigue as indexed by the Expanded Disability Status Scale, the Beck Depression Inventory–Fast Screen, and the Fatigue Severity Scale, respectively.
Table 2.
Means and standard deviations are reported by speaker group and neuropsychological domain as well as summarized analysis of variance results and the direction of the effect of significant post hoc tests comparing neuropsychological measures among speaker groups.
| Group | IP efficiency (PASAT-3 and SDMT) |
Verbal memory and learning (CVLT-II) |
Executive function (DKEFS) |
|
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| MSDYS + CI | −1.96 (−0.76) | −0.96 (0.85) | −1.18 (1.03) | |
| MSDYS | −0.17 (0.47) | 0.76 (1.12) | 0.01 (0.71) | |
| MSCI | −1.16 (1.05) | −0.94 (1.55) | −1.44 (1.39) | |
| MS | 0.27 (0.58) | 0.90 (0.67) | 0.61 (1.07) | |
| CON |
0.27 (0.80) |
0.67 (0.82) |
−0.08 (0.80) |
|
|
Neuropsychological measures |
Source |
ANOVA |
Post hoc comparisons (significant differences) |
|
| Variable |
F |
p |
Comparison |
|
| IP efficiency | Group | 19.859 | .001* | MSCI < MSDYS |
| MSCI < MS | ||||
| MSCI < CON | ||||
| MSDYS + CI < MSDYS | ||||
| MSDYS + CI < MS | ||||
| MSDYS + CI < CON | ||||
| Verbal memory and learning | Group | 9.812 | .001* | MSCI < MSDYS |
| MSCI < MS | ||||
| MSCI < CON | ||||
| MSDYS + CI < MSDYS | ||||
| MSDYS + CI < MS | ||||
| MSDYS + CI < CON | ||||
| Executive function | Group | 8.442 | .001* | MSCI < MSDYS |
| MSCI < MS | ||||
| MSCI < CON | ||||
| MSDYS + CI < MS | ||||
| MSDYS + CI < CON | ||||
Note. IP = information processing; PASAT-3 = 3-second version of the Paced Auditory Serial Addition Test; SDMT = Symbol Digit Modalities Test; CVLT-II = California Verbal Learning Test–Second Edition; DKEFS = Delis–Kaplan Executive Function System Sorting Test; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy controls; ANCOVA = analysis of covariance.
p < .05.
Experimental Perceptual Measures and Procedures
The SIT (Yorkston et al., 1996) quantified sentence intelligibility. With the exception of three speakers for whom a fourth SLP judged intelligibility, the same certified SLPs who diagnosed dysarthria and judged dysarthria severity (see Appendix A) also judged sentence intelligibility, as in previous studies (e.g., Feenaughty et al., 2013; Sussman & Tjaden, 2012). Six hundred sixty sentences (60 speakers × 11 sentences = 660 total sentences) were orthographically transcribed following standard SIT procedures over multiple sessions lasting about 1 hr in duration. Percent correct scores reflecting the combined average of the three SLPs for each speaker were used in the statistical analyses.
The stimuli of interest to obtain judgments of speech severity were audio recordings of participants' reading aloud the “Grandfather Passage” (Duffy, 2013). The validity of similar reading tasks has been demonstrated when evaluating speech behaviors in relation to cognitive abilities (e.g., De Looze et al., 2017; Feenaughty et al., 2013; Rodgers et al., 2012; Yunusova et al., 2016). the “Grandfather Passage” was also selected to elicit longer connected speech samples for greater face validity, to impose similar linguistic demands to compare across speaker groups, and because it is frequently used for research purposes and during clinical evaluations of connected speech function (Duffy, 2013). Reading aloud was also preferred because it may be more challenging than previously assumed for individuals with impaired cognition (Feenaughty et al., 2013; Rodgers et al., 2012). Before audio-recording the stimuli, the investigator read the passage aloud while a speaker followed along in silence to familiarize the speaker with the content of the passage. Then, speakers were instructed to read aloud the passage from a printed script and to keep reading if they missed or skipped a word. The average length of time that it took for all speakers in the current study to read aloud the “Grandfather Passage” was 43 s.
Listeners, Listening Task, and Procedure
Ten listeners were recruited to perform the perceptual judgments of speech severity for the reading task for all groups, except the MSDYS + CI group. All listeners were female and between the ages of 19 and 32 years (M = 23 years, SD = 3 years). Because speech severity ratings for the MSDYS + CI group were obtained at a later time, 10 new listeners were recruited, as in similar studies (e.g., Lam & Tjaden, 2013; Sussman & Tjaden, 2012). Listeners who judged the MSDYS + CI group included two men and eight women ranging in age from 20 to 33 years (M = 23 years, SD = 4 years). All 20 listeners spoke Standard American English; achieved at least a high school diploma or equivalent; reported normal speech-language function, adequate visual acuity for reading, and minimal familiarity with speech-language disorders resulting from neurologic diseases (e.g., stroke, MS); and passed a hearing screening administered bilaterally at 20 dB HL for octave frequencies ranging from 250 to 8000 Hz (American National Standards Institute, 2004).
Each listener was randomly assigned to one of three randomly generated stimuli orders. Although judgments of speech severity were obtained for a variety of speech tasks, listener judgments of speech severity for the reading passage were of interest. Within each stimuli order, the speech tasks were blocked by speaker and counterbalanced to minimize order effects. Listeners were seated in front of a computer screen in a sound-treated room, and stimuli were presented over headphones at a comfortable listening level (M = 7 dB SPL, SD = 2 dB SPL). GoldWave (Craig, 2013) was used to make gain adjustments to equate perceptual stimuli for average root-mean-square voltage across speakers and tasks (Lam & Tjaden, 2013). This procedure minimized possible variation in sound pressure level among speakers during audio recordings to eliminate the influence of audibility on listener judgments (Kim & Kuo, 2012). Root-mean-square values were inspected to confirm whether gain manipulations of speech samples that contained longer pauses did not increase sound pressure level in an excessive manner.
Listeners judged speech severity using a computerized visual analog scale (VAS) following procedures described in Sussman and Tjaden (2012). Printed instructions were read aloud, and the printed script was provided to each listener to reference during the experiment (for script, see Appendix B). Perceptual judgments were made without knowledge of a speaker's identity and group membership. Listeners were instructed to scale their overall impression of speech naturalness and prosody for each speech sample by focusing on voice quality, resonance, articulatory precision, and speech rhythm and timing characteristics (for recent studies using similar paradigms and naive listeners, see Anand & Stepp, 2015; Stipancic, Tjaden, & Wilding, 2016; Sussman & Tjaden, 2012). Listeners were also instructed to pay attention to within- and between-speaker differences but to ignore intelligibility. Speech severity was judged for each speech sample on a continuous 150-mm vertical scale displayed on a computer. End points were labeled no impairment at the bottom and severe impairment at the top. Using a custom computer program, the position of the indicator was translated to scores between 0 and 1. An average estimate of speech severity for each speaker and speech task was obtained for use in the statistical analysis. Before beginning the experiment, listeners were presented with three practice trials in the presence of the investigator to become familiar with the task and to ensure that they understood how to use the VAS. Practice trials were composed of speech samples produced by speakers with MS and control speakers who were not included in this study.
Listener Reliability
Each listener judged 20% of the stimuli twice to confirm intrajudge reliability. All listeners achieved reliability of r = .70 or greater as indexed by Pearson correlation coefficient (Tjaden et al., 2014). Interjudge reliability was evaluated using the intraclass correlation coefficient (Neel, 2009). The average measure intraclass correlation coefficient was .90 (SD = .08, p < .001) for the group of 10 listeners who judged speech severity for the MSCI, MSDYS, MS, and CON groups and .96 (SD = .06, p < .001) for the MSDYS + CI group, who were judged by the second group of 10 listeners. Listener intrajudge and interjudge reliability meets or exceeds reliability in similar studies (Sussman & Tjaden, 2012; Tjaden et al., 2014).
CPIB
The CPIB (Baylor et al., 2013) was used to describe communication satisfaction from the speaker's perspective. Items comprising the CPIB ask about how the patient's condition interferes with a variety of everyday speaking situations (e.g., Does your condition interfere with talking to people you know?). The survey yields total summary scores ranging from 0 to 30. Summary scores were subsequently converted to standard T scores following the CPIB short-form procedures. T scores range from 24.20 to 71.00. A T score value of 71.00 is indicative of no participation restrictions, whereas a score of 24.20 reflects significant participation limitations.
Statistical Analyses
Standard, descriptive, and parametric statistics were performed for each dependent variable using SPSS Version 21. A linear mixed-model analysis of covariance (ANCOVA) using age as a covariate was used to test group differences. Bonferroni-adjusted p values were used to determine statistical significance of post hoc tests. Nonsignificant group differences were examined using linear contrast trend analysis. Relationships between communication participation and perceptual measures were examined using correlation analysis between and within operationally defined groups with MS. Finally, analyses (ANCOVA, correlation) were also conducted to investigate measures of speaking rate to determine whether differences in speech and articulation rates distinguished speaker groups with and without dysarthria.
Results
Perceptual Measures
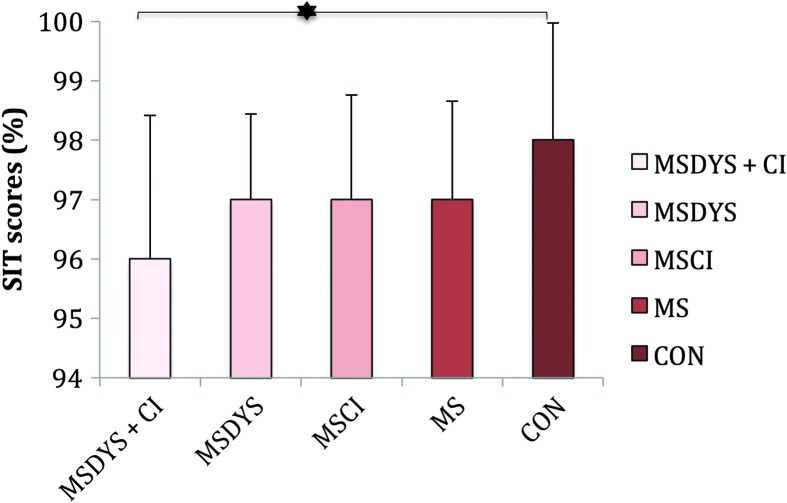
Table 3 summarizes the group results. Figure 1 indicates group means and standard deviations for SIT scores in percent. Figure 1 indicates significant differences among groups, F(4, 55) = 2.732, p = .03, ηp 2 = .16. Bonferroni post hoc tests indicated significantly poorer intelligibility for the MSDYS + CI group compared with the CON group (p = .03). This finding was not considered to be clinically meaningful, as all groups demonstrated 96% or greater average sentence intelligibility (Yorkston & Beukelman, 1981). There were no significant differences between the groups with MS.
Table 3.
Summary of group results.
| Group | SIT scores (%) | Speech severity (VAS) | Communication participation (CPIB T score; logit score) |
|---|---|---|---|
| MSDYS + CI | 97 | 0.49 | 50.07; 0.005 |
| MSDYS | 96 | 0.58 | 56.45; 0.643 |
| MSCI | 97 | 0.36 | 57.98; 0.798 |
| MS | 97 | 0.35 | 60.16; 1.016 |
| CON | 98 | 0.23 | — |
Note. Means are reported for SIT scores, speech severity, and the 10-item CPIB short form. VAS speech severity scores range from 0 to 1. Higher scaled scores indicate greater severity. Ten-item CPIB T scores (standard scores) range from 24.20 to 71.00, and logit scores range from −2.58 to 2.10. Higher T scores and logit scores are better. SIT = Sentence Intelligibility Test; VAS = visual analog scale; CPIB = Communication Participation Item Bank; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy controls.
Figure 1.
SIT scores in percent are reported for the MSDYS + CI, MSDYS, MSCI, MS, and CON groups. The error bars indicate standard deviations. The asterisk indicates a significant post hoc comparison at the p < .05 level. SIT = Sentence Intelligibility Test; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy controls.
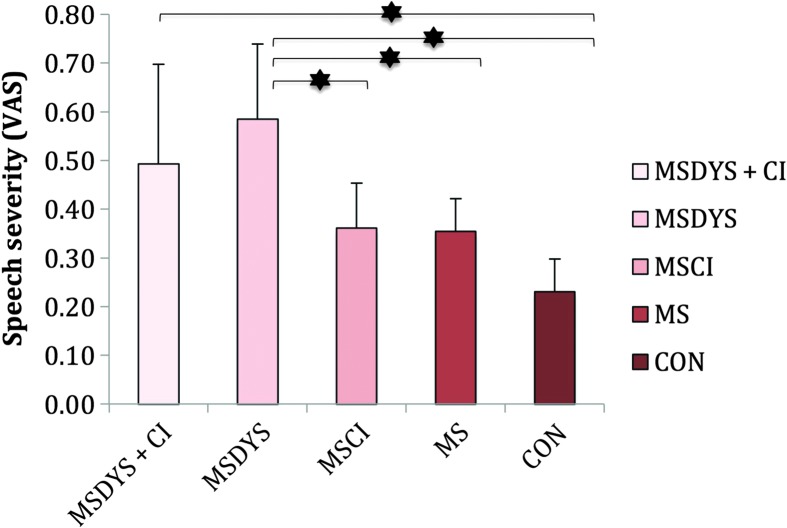
Mean judgments of speech severity and standard deviations are reported in Figure 2. As indicated in Figure 2, significant differences were demonstrated among speaker groups, F(4, 55) = 12.358, p < .001, ηp 2 = .47. Post hoc comparisons indicated that the MSDYS + CI group was significantly more severe compared with the CON group (p < .001). The MSDYS group was significantly more severe compared with all the other groups—MS, p = .001; MSCI, p = .014; and CON, p < .001—except the MSDYS + CI group (p = .88). Group differences were considered to be clinically meaningful, as the average difference in magnitude for the MSDYS and MSDYS + CI groups relative to controls was 0.26 and 0.35 scale points, respectively.
Figure 2.
Judgments of speech severity are reported for the MSDYS + CI, MSDYS, MSCI, MS, and CON groups. The error bars indicate standard deviations. The asterisks indicate a significant post hoc comparison at the p < .05 level. Higher scores indicate greater severity. VAS = visual analog scale; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy controls.
Communication Participation Measure
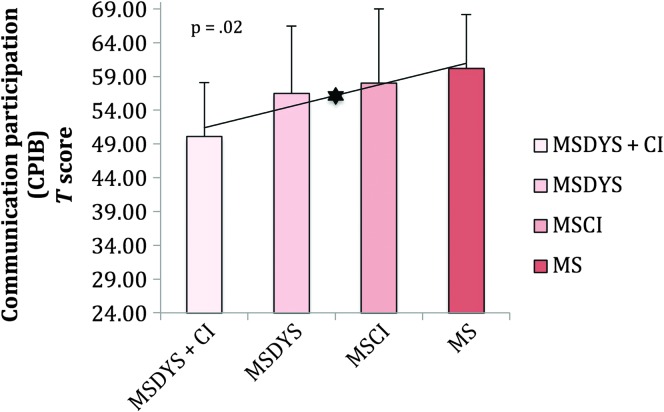
Figure 3 reports means and standard deviations for the CPIB T scores. Figure 3 indicates no significant group differences (p = .08). The MSDYS + CI group reported the greatest restrictions in communication participation compared with all other groups. A significant linear trend across groups with MS (p = .02) emerged when speaker groups were ordered to examine the influence of comorbid cognitive limitations and dysarthria beyond a clinical diagnosis of dysarthria on communication participation. The MSCI group was included in the figure for completeness.
Figure 3.
Communication Participation Item Bank (CPIB) T scores are reported for the MSDYS + CI, MSDYS, MSCI, and MS groups. The error bars indicate standard deviations. The asterisk indicates a significant linear trend at the p < .05 level. Lower scores indicate greater communication participation restrictions. MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment.
Relationships Between Perceptual Measures and Communication Participation for Groups With MS
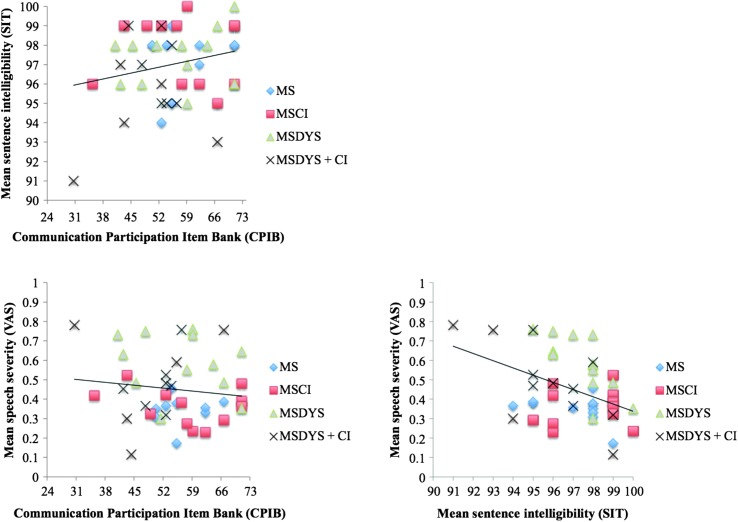
As indicated in the lower right panel of Figure 4, speech severity and mean SIT scores were significantly correlated when data from all speaker groups with MS were combined (Pearson r = −.436, p < .01). In contrast, speech severity and mean SIT scores (see Figure 4, lower and upper left panels) were not significantly correlated with CPIB T scores (Pearson r = −.134, p > .05 and Pearson r = .230, p > .05, respectively). When correlations were conducted within each speaker group, speech severity and mean SIT scores were not significantly correlated with CPIB T scores (p > .05), as indicated in Table 4.
Figure 4.
Scatter plots relating Communication Participation Item Bank (CPIB) T scores and mean sentence intelligibility and speech severity are reported for speaker groups with MS. The relationship between mean sentence intelligibility test scores and speech severity is also reported. Linear regression functions have been fit to the data collapsed across all groups with MS. Each symbol corresponds to an individual speaker with MS. SIT = Sentence Intelligibility Test; VAS = visual analog scale; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment.
Table 4.
Pearson correlation coefficients are reported between Communication Participation Item Bank (CPIB) T scores and the perceptual measures (Sentence Intelligibility Test [SIT] scores and scaled speech severity) within each operationally defined speaker group with MS.
| SIT scores (%) |
Speech severity (VAS) |
|
|---|---|---|
| Pearson r | Pearson r | |
| CPIB (T score) | ||
| MSDYS + CI | .13 | .25 |
| MSDYS | .26 | −.25 |
| MSCI | −.07 | −.26 |
| MS | .19 | .25 |
Note. Speech severity and SIT scores were not significantly correlated with CPIB T scores at the alpha level of p < .05. VAS = visual analog scale; MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment.
Analysis of Speaking Rate Measures and Sentence Intelligibility Scores
Because SIT scores did not differentiate groups with and without dysarthria, analyses were conducted to investigate whether differences in speaking rates obtained from a reading passage, rather than the SIT, distinguished groups with and without dysarthria. SIT scores and speech rates were not significantly correlated for the MSDYS (Pearson r = .31, p > .05) or MSDYS + CI (Pearson r = .19, p > .05) group. ANCOVA further indicated significant group differences in speech rate, F(5, 54) = 9.66, p < .001, ηp 2 = .47. Post hoc contrasts indicated that the MSDYS + CI group had significantly slower speech rates compared with all the other groups (MS, p < .001; MSCI, p < .001; MSDYS, p < .05; and CON, p < .001). With the exception of the MSDYS + CI–MSDYS contrast (p = .16), the pattern of results was similar for articulation rate. Appendix C reports a summary of means and standard deviations for speech and articulation rates.
Discussion
The purpose of this study was to determine if perceptual measures of intelligibility and speech severity, as well as a measure of communication participation, differed for four speaker groups with MS, as captured by SIT scores and scaled speech severity and the CPIB, respectively. Results indicated that scaled speech severity, but not SIT scores, differentiated some speaker groups with MS. In addition, results suggested that speakers with comorbid clinical diagnoses of dysarthria and cognitive limitations were likely to experience greater restrictions in communication participation than individuals with MS who experienced a deficit in a single modality (i.e., dysarthria or impaired cognition). Finally, communication participation was not significantly correlated with SIT scores and the scaled speech severity measure that are thought to reflect the activity level of the ICF. These findings are considered in more detail below for each research question.
Research Question 1: Do Perceptual Measures of Intelligibility and Speech Severity Differ Among Groups?
SIT scores did not differentiate speaker groups with MS in a meaningful way (see Figure 1). In other words, mean intelligibility scores were concentrated at the upper limit of the intelligibility continuum, despite the fact that speakers who comprised the MSDYS and MSDYS + CI groups had clinical diagnoses of dysarthria as judged by three certified SLPs (see Table 3). Furthermore, SIT scores were not affected by cognitive limitations (MSCI group) or by the combination of cognitive deficits and mild dysarthria (MSDYS + CI group), although the statistical analysis indicated a significant difference between the MSDYS + CI and CON groups for whom SIT scores approached 100%.
Speakers with mild dysarthria are difficult to distinguish from healthy talkers on the basis of percent correct scores obtained from the SIT. When SIT scores are supplemented by measures of speaking rate, speakers with mild dysarthria may be differentiated from controls (Yorkston & Beukelman, 1981; Yorkston, Beukelman, Strand, & Hakel, 2010). Because SIT scores did not differentiate groups with and without dysarthria, analyses were conducted to consider whether differences in speech rate (syllables per second) distinguished speaker groups. Although statistical analyses indicated significant group differences in speech and articulation rates, neither speech rate nor articulation rate clearly distinguished speaker groups with and without mild dysarthria.
Although measures of intelligibility have a long history of differentiating dysarthria severity levels (mild, moderate, and severe), ceiling effects may have decreased the SIT's ability to capture the level of functional communication for individuals with MS in this study. The fact that cognitive limitations alone or in combination with mild dysarthria were not well captured by sentence intelligibility scores also suggests that auditory perceptual measures such as the SIT are not sensitive to mild cognitive impairment in MS, when the number of words transcribed correctly is used to derive percent correct scores. Thus, when speakers are highly intelligible, supplementary perceptual measures may be necessary to detect the presence of functional communication impairment as an effect of mild dysarthria (e.g., Fletcher & McAuliffe, 2017; Sussman & Tjaden, 2012).
In contrast to the finding that SIT scores did not differ among speaker groups with MS, significant group differences were indicated for speech severity (see Figure 2), particularly for the MSDYS group versus the MSCI, MS, and CON groups. The finding that the MSDYS and MSDYS + CI groups, with relatively preserved intelligibility, were perceived to be more severe than the healthy talkers with similar levels of intelligibility suggests that the perceptual construct of speech severity was sensitive to functional communication limitations of speakers even with mild dysarthria. Because speakers who comprised the MSDYS + CI group also had cognitive limitations, it may be speculated that limited memory, processing speed, executive function skills, or any combination of these problems may have partially contributed to speech severity judgments. However, it is more plausible that the deviant speech characteristics associated with mild dysarthria accounted for this later finding (see Table 3), despite the modest trend for the MSCI and MS groups to be more severe than controls.
The perceptual results may have been influenced by methodological factors. Consistent with Sussman and Tjaden (2012), speech severity judgments were generated using a reading passage and were judged by naive listeners, whereas sentences transcribed by certified SLPs were used to generate SIT scores. In this manner, impressions of speech severity and intelligibility were obtained from listeners who not only differed in experience level but also judged different speech stimuli. Scaled estimates of speech severity for the “Grandfather Passage” obtained from expert listeners (SLPs) have been shown to be more severe compared with those obtained from naive listeners (Sussman & Tjaden, 2012). Nonetheless, significant group differences between the MSDYS + CI and CON groups may suggest that the construct of speech severity may be better suited to gauge functional communication impairment when mild comorbid limitations are anticipated in MS.
Research Question 2: Does Communication Participation Differ Among Groups With MS?
Limitations exclusively related to dysarthria, cognitive performance, or a clinical diagnosis of MS did not strongly influence communication participation as indexed by the CPIB (see Figure 3). This finding may be explained by the overall mild dysarthria severity and cognitive limitations experienced by many of the speakers who comprised the MSDYS and MSCI groups. For the MSDYS and MSCI groups, as well as speakers in the MS group, the implication may also be that other disease-related symptoms such as fatigue did not restrict participation. However, the significant linear trend across groups with MS suggests that, as the level of impairment increased toward comorbidity, the level of communication participation restriction increased proportionally. Results suggest that speakers with more involved or complex impairment (i.e., with comorbid clinical diagnoses of dysarthria and cognitive limitations) are likely to experience greater restrictions in communication participation than individuals with MS who experience a deficit in a single modality (i.e., dysarthria or impaired cognition).
To better understand the nuances between CPIB scores for the groups with MS as indicated by the significant linear trend (see Table 3), a closer look at the distribution of CPIB scores for the 12 speakers within each group with MS was undertaken. Inspection of the data revealed that speakers who comprised the MS, MSDYS, and MSCI groups had a wide range of CPIB scores. In contrast, speakers comprising the MSDYS + CI group had CPIB scores that were compressed within the middle of the scale. According to the 10-item CPIB, theta or logit scores can range from −2.58 (very restricted participation) to 2.10 (very good participation; Baylor et al., 2013). In the current study, CPIB scores for the MSDYS + CI group ranged from −1.76 to 1.67, with most scores clustered in the middle of the scale suggesting moderate participation restrictions for 10 of 12 speakers within this group. Yorkston et al. (2014) reported CPIB scores from the 46-item CPIB that ranged from −1.82 to 2.61, for a subset of speakers with MS who felt that their speech sounded perceptually normal. The current findings support prior research suggesting restricted participation resulting from a variety of MS-related signs including mild dysarthria and cognitive limitations. Furthermore, the current study employing objective measures of cognitive function and dysarthria supports prior research that relied on patient self-report of problems with speech or cognition. Overall, the fact that speakers comprising the MSDYS + CI group reported the greatest restrictions in communication participation compared with all other groups advances our understanding of the separate and combined influences of dysarthria and cognitive limitations on participation.
Research Question 3: What Is the Relationship Between Perceptual Measures and the CPIB for Groups With MS?
The significant correlation between speech severity and SIT scores when data were pooled across all speakers with MS suggests that intelligibility indexed by the SIT and speech severity are not mutually exclusive perceptual constructs. That is, when SIT scores were better, speech severity judgments also were better (or less severe toward the zero end of the VAS). This finding is consistent with prior research suggesting that scaled estimates of speech severity were significantly correlated with sentence intelligibility scores for a different group of speakers with MS and speakers with Parkinson's disease (Sussman & Tjaden, 2012; Weismer, Jeng, Laures, Kent, & Kent, 2001). This finding also appears to support prior research suggesting that listeners may interpret the broad perceptual constructs of intelligibility and severity similarly, although methodological differences among studies exist, thus limiting direct comparisons and strong conclusions (e.g., Dagenais, Watts, Turnage, & Kennedy, 1999; Hustad, 2008; Sussman & Tjaden, 2012; Weismer et al., 2001).
Neither SIT scores nor speech severity judgments were significantly correlated with communication participation. These findings, as well as those from past studies, suggest a complex relationship between communication at the participation level of the ICF and the related perceptual constructs of intelligibility and speech severity that are thought to reflect the activity level of the ICF (Donovan et al., 2007; Dykstra et al., 2007; McAuliffe et al., 2010). Although SIT scores and scaled estimates of speech severity were significantly correlated, scaling speech severity of a longer connected speech sample may help to differentiate between separate and combined dysarthria and cognitive limitations that may restrict functional communication in MS.
To our knowledge, this is the first study to use quantitative rather than self-report measures of communication and neuropsychological function sensitive to even mild dysarthria and cognitive impairment in MS to examine the relationships between the perceptual measures of speech severity and SIT scores, and communication participation. Therefore, each perceptual measure and its relationship with communication participation were also examined separately for speakers within each group with MS. The strength and direction of the relationships varied depending on the measure and group (see Table 4). Nonetheless, results suggested that better speech intelligibility or severity was associated with less restricted communication participation. Notably, speakers comprising the MSDYS + CI group who were judged to have lower speech severity reported greater restrictions in communication participation, bearing in mind that only 12 speakers comprised the within-group correlations and correlations were not significant. This pattern could reflect the fact that CPIB questions ask about more ecological speaking situations than reading aloud. Future studies could investigate the relationship between restrictions in communication participation and judgments of speech severity for a more ecologically valid spontaneous speech task.
Caveats and Future Directions
Physiological variations, vision, and cognitive and psychosocial factors have been acknowledged to have an effect on speech performance including functional communication (Feenaughty et al., 2013; Lowit, Brendel, Dobinson, & Howell, 2006; Ramig, 1983; Yorkston et al., 2014). Although cognitive status alone did not play a large role in listener perceptions of sentence intelligibility and speech severity, future studies are needed to better understand the consequences of impaired cognition in MS and its impact on perceptual constructs such as intelligibility and speech severity. Specifically, future studies could investigate speakers who experience more severe cognitive impairment. Perceptual measures that gauge the quality and quantity of spoken language that may reflect high-level, nonaphasic communication problems combined with dysarthria are needed to determine the totality of functional limitations in MS. Future studies are also warranted to determine whether using similar speech tasks and listeners with similar levels of experience to obtain auditory perceptual impressions of sentence intelligibility and speech severity yields different results.
Conclusions
In clinical practice, recognition of comorbid conditions is important for effective and efficient treatment (Yorkston et al., 2007, 2003). Thus, it is critical to understand the total burden of combined and separate symptoms associated with MS contributing to reduce functional communication. Overall, results suggest that the degree of speech abnormality at the activity level may not share a one-to-one relationship with the degree to which persons are restricted in their ability to participate in social activities. Results also suggest that scaled estimates of speech severity were sensitive to aspects of speech impairment in MS that were not reflected in traditional measures of sentence intelligibility. The implication is that therapeutic interventions may be warranted for even persons with mild dysarthria with relatively preserved intelligibility who may be experiencing restricted communication participation. Given the significant linear trend results for communication participation, findings also suggest that further investigation of combined effects of mild dysarthria and cognitive limitations is needed to better understand how these more complex patterns of impairment may impact functional communication for persons with MS.
Acknowledgments
This research was funded by an ASHFoundation New Century Doctoral Scholarship (L. Feenaughty), the Mark Diamond Research Fund of the Graduate Student Association at the University at Buffalo (L. Feenaughty), and The State University of New York and National Institute on Deafness and Other Communication Disorders Grant R01DC004689 (K. Tjaden). Results were presented at the 2015 American Speech-Language-Hearing Association Annual Convention in Denver, Colorado.
Appendix A
Dysarthria Type, Severity, and Perceptual Speech Characteristics Are Reported for Participants Comprising the MSDYS and MSDYS + CI Groups
| Group | ID | Gender | Dysarthria type | Dysarthria severity | Deviant perceptual speech characteristics |
|---|---|---|---|---|---|
| MSDYS | 2 | F | Flaccid | Mild | Imprecise consonants, breathy |
| MSDYS | 5 | F | Spastic–ataxic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, breathy, strained–strangled, hypernasal, pitch breaks, audible inspiration, forced expiration |
| MSDYS | 6 | F | Spastic–ataxic | Mild | Irregular articulatory breakdown, imprecise consonants, harsh/strained, hypernasal, variable rate |
| MSDYS | 19 | F | Ataxic | Mild | Imprecise consonants, vowels distorted, low pitch, slow rate |
| MSDYS | 23 | F | Spastic–ataxic | Mild | Imprecise consonants, hypernasal, voice tremor, slow rate |
| MSDYS | 26 | F | Spastic | Moderate | Imprecise consonants, harsh/strained, low pitch |
| MSDYS | 55 | F | Ataxic | Mild | Imprecise consonants, harsh, slow rate |
| MSDYS | 56 | F | Spastic | Moderate | Strained–strangled, harsh, slow rate |
| MSDYS | 12 | M | Flaccid–spastic | Mild | Imprecise consonants, hypernasality, audible inspiration, nasal emission, variable rate |
| MSDYS | 17 | M | Spastic–ataxic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, harsh, hypernasal, monopitch, low pitch, slow rate |
| MSDYS | 33 | M | Ataxic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, phonemes prolonged, harsh |
| MSDYS | 48 | M | Spastic–ataxic | Mild | Imprecise consonants, irregular articulatory breakdown, strained–strangled, variable rate |
| MSDYS + CI | 4 | F | Spastic | Mild–moderate | Irregular articulatory breakdown, breathy, strained–strangled, voice tremor, pitch breaks, variable rate, short phrases |
| MSDYS + CI | 13 | F | Spastic–ataxic | Moderate | Irregular articulatory breakdown, imprecise consonants, vowels distorted, breathy, strained–strangled, intervals prolonged, slow rate |
| MSDYS + CI | 14 | F | Ataxic | Mild | Irregular articulatory breakdown, imprecise consonants |
| MSDYS + CI | 39 | F | Spastic–ataxic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, harsh, inappropriate silences, slow rate, short phrases |
| MSDYS + CI | 51 | F | Ataxic | Moderate | Imprecise consonants, insufficient loudness, slow rate |
| MSDYS + CI | 53 | F | Ataxic | Mild | Imprecise consonants, harsh, voice tremor, slow rate |
| MSDYS + CI | 57 | F | Spastic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, harsh, hypernasality, slow rate |
| MSDYS + CI | 62 | F | Ataxic | Mild–moderate | Irregular articulatory breakdown, imprecise consonants, harsh, slow rate, short phrases |
| MSDYS + CI | 15 | M | Spastic | Mild | Imprecise consonants, strained–strangled, pitch breaks, hyponasality, variable rate |
| MSDYS + CI | 29 | M | Spastic–ataxic | Mild | Irregular articulatory breakdown, imprecise consonants, strained–strangled, harsh |
| MSDYS + CI | 30 | M | Spastic–ataxic | Mild | Irregular articulatory breakdown, imprecise consonants, strained–strangled |
| MSDYS + CI | 49 | M | Spastic–ataxic | Mild | Imprecise consonants, monoloudness, insufficient loudness, slow rate |
Note. MSDYS = MS group with dysarthria and intact cognition; MSDYS + CI = MS group with dysarthria and cognitive impairment; F = female; M = male.
Appendix B
Verbal and Printed Instructions for Listening Task Following Sussman and Tjaden (2012)
You will be hearing speech samples that, for the most part, are highly understandable. I want you to rate the OVERALL SEVERITY of the speech sample. Please pay attention to the following things when you listen to the speech samples including.
VOICE (quality – breathy, noisy, gurgly, pitch too high, pitch too low or OK);
RESONANCE (too nasal, not nasal in the right places, sounds like they have a cold or OK);
ARTICULATORY PRECISION (speech sounds are crisp or slurred or somewhere in between or OK); and
SPEECH RHYTHM (the timing of speech doesn't sound right or is OK).
In other words, pay attention to overall speech naturalness and prosody (melody and timing of speech).
DO pay attention to within speaker and between speaker differences.
DO NOT focus on the speaker's intelligibility or how understandable each sample is.
Scale your overall impression of the speech from NO IMPAIRMENT (at the bottom of the scale) to SEVERELY IMPAIRED (at the top).
Do you have any questions?
The experiment will start as soon as you click OK.
Appendix C
Means and Standard Deviations Are Reported for Speech and Articulation Rates for Each Operationally Defined Speaker Group
| Group | Speech rate (syllables/s) |
Articulation rate (syllables/s) |
|---|---|---|
| M (SD) | M (SD) | |
| MSDYS + CI | 2.94 (0.59) | 3.76 (0.58) |
| MSDYS | 3.49 (0.37) | 4.25 (0.45) |
| MSCI | 3.92 (0.29) | 4.79 (0.31) |
| MS | 3.95 (0.32) | 4.74 (0.34) |
| CON | 3.86 (0.33) | 4.63 (0.38) |
Note. MSDYS + CI = MS group with dysarthria and cognitive impairment; MSDYS = MS group with dysarthria and intact cognition; MSCI = MS group with cognitive impairment; MS = MS group without dysarthria or cognitive impairment; CON = healthy talkers.
Funding Statement
This research was funded by an ASHFoundation New Century Doctoral Scholarship (L. Feenaughty), the Mark Diamond Research Fund of the Graduate Student Association at the University at Buffalo (L. Feenaughty), and The State University of New York and National Institute on Deafness and Other Communication Disorders Grant R01DC004689 (K. Tjaden). Results were presented at the 2015 American Speech-Language-Hearing Association Annual Convention in Denver, Colorado.
References
- Amato M. P., Langdon D., Montalban X., Benedict R. H. B., DeLuca J., Krupp L. B., … Comi G. (2013). Treatment of cognitive impairment in multiple sclerosis: Position paper. Journal of Neurology, 260(6), 1452–1468. https://doi.org/10.1007/s00415-012-6678-0 [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. (2004). Specifications for audiometers (ANSI S3.6-2004). New York, NY: Author. [Google Scholar]
- Anand S., & Stepp C. E. (2015). Listener perception of monopitch, naturalness, and intelligibility for speakers with Parkinson's disease. Journal of Speech, Language, and Hearing Research, 58(4), 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor C., Yorkston K., Eadie T., Kim J., Chung H., & Amtmann D. (2013). The Communication Participation Item Bank (CPIB): Item bank calibration and development of a disorder-generic short form. Journal of Speech, Language, and Hearing Research, 56, 1190–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., & Brown G. K. (2000). Manual for the Beck Depression Inventory–Fast Screen for medical patients: Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benedict R. H., Bruce J. M., Dwyer M. G., Abdelrahman N., Hussein S., Weinstock-Guttman B., … Zivadinov R. (2006). Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Archives of Neurology, 63(9), 1301–1306. [DOI] [PubMed] [Google Scholar]
- Benedict R. H., Fischer J. S., Archibald C. J., Arnett P. A., Beatty W. W., Bobholz J., … Munschauer F. (2002). Minimal neuropsychological assessment of MS patients: A consensus approach. The Clinical Neuropsychologist, 16(3), 381–397. [DOI] [PubMed] [Google Scholar]
- Benedict R. H., Whlig E., Bakshi R., Fishman I., Munschauer F., Zivadinov R., & Weinstock-Gittman B. (2005). Predicting quality of life in multiple sclerosis: Accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. Journal of the Neurological Sciences, 231, 29–34. [DOI] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2012). Pratt: A system for doing phonetics by computer [computer program] (version 5.4.04). Retrieved from http://www.pratt.org/
- Craig C. (2013). GoldWave (Version 5.70) [sound editing program]. St. Johns, Canada: GoldWave Inc. [Google Scholar]
- Dagenais P., Watts C., Turnage L., & Kennedy S. (1999). Intelligibility and acceptability of moderately dysarthric speech by three types of listeners. Journal of Medical Speech-Language Pathology, 7, 91–96. [Google Scholar]
- Darley F. L., Brown J. R., & Goldstein N. P. (1972). Dysarthria in multiple sclerosis. Journal of Speech and Hearing Research, 15(2), 229–245. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., & Kramer J. H. (2001). Delis–Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., & Ober B. A. (2000). California Verbal Learning Test Manual, Adult Version (2nd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- De Looze C., Moreau N., Renié L., Kelly F., Ghio A., Rico A., … Petrone C. (2017). Effects of cognitive impairment on prosodic parameters of speech production planning in multiple sclerosis. Journal of Neuropsychology. Epub ahead of print. https://doi.org/10.1111/jnp.12127 [DOI] [PubMed] [Google Scholar]
- Donovan N. J., Kendall D. L., Young M. E., & Rosenbek J. C. (2007). The communicative effectiveness survey: Preliminary evidence of construct validity. American Journal of Speech-Language Pathology, 17(4), 335–347. [DOI] [PubMed] [Google Scholar]
- Duffy J. R. (2013). Motor speech disorders: Substrates, differential diagnosis, and management (3rd ed.). St. Louis, MO: Mosby Incorporated. [Google Scholar]
- Dykstra A. D., Hakel M. E., & Adams S. G. (2007). Application of the ICF in reduced speech intelligibility in dysarthria. Seminars in Speech and Language, 28(4), 301–311. [DOI] [PubMed] [Google Scholar]
- Eadie T. L., Yorkston K. M., Klasner E. R., Dudgeon B. J., Deitz J. C., Baylor C. R., … Amtmann D. (2006). Measuring communicative participation: A review of self-report instruments in speech-language pathology. American Journal of Speech-Language Pathology, 15(4), 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenaughty L., Tjaden K., Benedict R. H., & Weinstock-Guttman B. (2013). Speech and pause characteristics in multiple sclerosis: A preliminary study of speakers with high and low neuropsychological test performance. Clinical Linguistics & Phonetics, 27(2), 134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A., & McAuliffe M. (2017). Examining variation in treatment outcomes among speakers with dysarthria. Seminars in Speech and Language, 38(3), 191–199. [DOI] [PubMed] [Google Scholar]
- Gronwall D. M. A. (1977). Paced auditory serial addition task: A measure of recovery from concussion. Perceptual and Motor Skills, 44(2), 367–373. [DOI] [PubMed] [Google Scholar]
- Hartelius L., Runmarker B., & Andersen O. (2000). Prevalence and characteristics of dysarthria in multiple-sclerosis cohort: Relation to neurological data. Folia Phoniatrica et Logopaedica, 52, 160–177. [DOI] [PubMed] [Google Scholar]
- Hartelius L., & Svensson P. (1994). Speech and swallowing symptoms associated with Parkinson's disease and multiple sclerosis: A survey. Folia Phoniatrica et Logopaedica, 46, 9–17. [DOI] [PubMed] [Google Scholar]
- Hustad K. C. (2008). The relationship between listener comprehension and intelligibility scores for speakers with dysarthria. Journal of Speech, Language, and Hearing Research, 58, 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keintz C. K., Bunton K., & Hoit J. D. (2007). Influence of visual information on the intelligibility of dysarthric speech. American Journal of Speech-Language Pathology, 16, 222–234. [DOI] [PubMed] [Google Scholar]
- Kim Y., & Kuo C. (2012). Effect of level of presentation to listeners on scaled speech intelligibility of speakers with dysarthria. Folia Phoniatrica et Logopaedica, 64(1), 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L. B., LaRocca N. G., Muir-Nash J., & Steinberg A. D. (1989). The fatigues severity scale: Application to patients with multiple sclerosis and systematic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. (1983). Rating neurologic impairment in multiple sclerosis: An expanded disability scale (EDSS). Neurology, 33(11), 1444–1452. [DOI] [PubMed] [Google Scholar]
- Laakso K., Brunnegard K., Hartelius L., & Ahlsen E. (2000). Assessing high-level language in individuals with multiple sclerosis: A pilot study. Clinical Linguistics & Phonetics, 14(5), 329–349. [Google Scholar]
- Lam J., & Tjaden K. (2013). Intelligibility of clear speech: Effect of instruction. Journal of Speech, Language, and Hearing Research, 56(5), 1429–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca N. G., Kalb R. C., & Gregg K. (1995). A program to facilitate retention of employment among persons with multiple sclerosis. Work, 7(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Lowit A., Brendel B., Dobinson C., & Howell P. (2006). An investigation into the influences of age, pathology and cognition on speech production. Journal of Medical Speech-Language Pathology, 12, 253–262. [PMC free article] [PubMed] [Google Scholar]
- MacGregor L. J., Corley M., & Donaldson D. I. (2010). Listening to the sound of silence: Disfluent silent pauses in speech have consequences for listeners. Neuropsychologia, 48(14), 3982–3992. [DOI] [PubMed] [Google Scholar]
- Mackenzie C., & Green J. (2009). Cognitive–linguistic deficit and speech intelligibility in chronic progressive multiple sclerosis. International Journal of Language & Communication Disorders, 44, 401–420. [DOI] [PubMed] [Google Scholar]
- McAuliffe M. J., Carpenter S., & Moran C. (2010). Speech intelligibility and perceptions of communication effectiveness by speakers with dysarthria following traumatic brain injury and their communication partners. Brain Injury, 24(12), 1408–1415. [DOI] [PubMed] [Google Scholar]
- Murdoch B. E., & Lethlean J. B. (2000a). High-level language, naming and discourse abilities in multiple sclerosis. In Theodoros D. G. (Ed.), Speech and language disorders in multiple sclerosis (pp. 131–155). London, England: Whurr Publishers. [Google Scholar]
- Murdoch B. E., & Lethlean J. B. (2000b). Language disorders in multiple sclerosis. In Theodoros D. G. (Ed.), Speech and language disorders in multiples sclerosis (pp. 109–130). London, England: Whurr Publishers. [Google Scholar]
- Neel A. T. (2009). Effects of loud and amplified speech on sentence and word intelligibility in Parkinson disease. Journal of Speech, Language, and Hearing Research, 52(4), 1021–1033. [DOI] [PubMed] [Google Scholar]
- Parmenter B. A., Testa S. M., Schretlen D. J., Weinstock-Guttman B., & Benedict R. H. (2010). The utility of regression-based norms in interpreting the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). Journal of the International Neuropsychological Society, 16(1), 6–16. [DOI] [PubMed] [Google Scholar]
- Ramig L. A. O. (1983). Effects of physiological aging on speaking and reading rates. Journal of Communication Disorders, 16, 217–226. [DOI] [PubMed] [Google Scholar]
- Rao S. M. (1995). Neuropsychology of multiple sclerosis. Current Opinion in Neurology, 8, 216–220. [DOI] [PubMed] [Google Scholar]
- Rao S. M., Leo G. J., Bernardin L., & Unverzagt F. (1991). Cognitive dysfunction in multiple sclerosis: I. Frequency, patterns, and prediction. Neurology, 41(5), 685–691. [DOI] [PubMed] [Google Scholar]
- Rodgers J. D., Tjaden K., Feenaughty L., Weinstock-Guttman B., & Benedict R. H. (2012). Influence of cognitive function on speech and articulation rate in multiple sclerosis. Journal of the International Neuropsychological Society, 19(2), 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. (1982). Symbol Digit Modalities Test (SDMT) manual (revised). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Stipancic K. L., Tjaden K., & Wilding G. (2016). Comparison of intelligibility measures for adults with Parkinson's disease, adults with multiple sclerosis, and healthy controls. Journal of Speech, Language, and Hearing Research, 59(2), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober L., Englert J., Munschauer F., Weinstock-Guttman B., Rao S., & Benedict R. H. B. (2009). Sensitivity of conventional memory tests in multiple sclerosis: Comparing the Rao Brief Repeatable Neuropsychological Batter and the Minimal Assessment of Cognitive Function in MS. Multiple Sclerosis, 15, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Sussman J. E., & Tjaden K. (2012). Perceptual measures of speech from individuals with Parkinson's disease and multiple sclerosis: Intelligibility and beyond. Journal of Speech, Language, and Hearing Research, 55(4), 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., Sussman J. E., & Wilding G. E. (2014). Impact of clear, loud, and slow speech on scaled intelligibility and speech severity in Parkinson's disease and multiple sclerosis. Journal of Speech, Language, and Hearing Research, 57(3), 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., & Wilding G. E. (2004). Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research, 47(4), 766–783. [DOI] [PubMed] [Google Scholar]
- Tjaden K., & Wilding G. E. (2011). Effects of speaking task on intelligibility in Parkinson's disease. Clinical Linguistics & Phonetics, 25(2), 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G. L., & Holmes S. (1993). Cognitive–linguistic assessment of individuals with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 74, 637–643. [DOI] [PubMed] [Google Scholar]
- Walshe M. (2011). The psychological impact of acquired motor speech disorders. In Lowit A. & Kent R. D. (Eds.), Assessment of motor speech disorders. San Diego, CA: Plural. [Google Scholar]
- Weismer G. (2008). Speech intelligibility. In Ball M. J., Perkins M. R., Muller N., & Howard S. (Eds.), The handbook of clinical linguistics (pp. 568–582). Oxford, England: Blackwell. [Google Scholar]
- Weismer G., Jeng J., Laures J., Kent R., & Kent J. (2001). Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica, 53, 1–18. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2009). International Classification of Function, Disability and Health (ICF). Geneva, Switzerland: Author. [Google Scholar]
- Yorkston K. M., & Baylor C. (2012). Communication. In Finlayson M. (Ed.), Multiple sclerosis rehabilitation: From impairment to participation (pp. 277–288). Boca Raton, FL: CRC Press. [Google Scholar]
- Yorkston K. M., Baylor C., & Amtmann D. (2014). Communicative participation restrictions in multiple sclerosis: Associated variables and correlation with social functioning. Journal of Communication Disorders, 52, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K. M., Baylor C. R., Klasner E. R., Deitz J., Dudgeon B. J., Eadie T., … Amtmann D. (2007). Satisfaction with communicative participation as defined by adults with multiple sclerosis: A qualitative study. Journal of Communication Disorders, 40(6), 433–451. [DOI] [PubMed] [Google Scholar]
- Yorkston K. M., & Beukelman D. R. (1981). Communication efficiency of dysarthric speakers as measured by sentence intelligibility and speaking rate. Journal of Speech and Hearing Disorders, 46, 296–301. [DOI] [PubMed] [Google Scholar]
- Yorkston K. M., Beukelman D. R., Strand E. A., & Hakel M. (2010). Management of motor speech disorders in children and adults (3rd ed.). Austin, TX: Pro-Ed. [Google Scholar]
- Yorkston K., Beukelman D. R., & Tice R. (1996). Sentence intelligibility test. Lincoln, NE: Institute for Rehabilitation Science and Engineering at Madonna Rehabilitation Hospital. [Google Scholar]
- Yorkston K. M., Klasner E. R., Bowen J., Ehde D. M., Gibbons L. E., Johnson K., & Kraft G. (2003). Characteristics of multiple sclerosis as a function of the severity of speech disorders. Journal of Medical Speech-Language Pathology, 11(2), 73–84. [Google Scholar]
- Yorkston K. M., Klasner E. R., & Swanson K. M. (2001). Communication in context: A qualitative study of the experiences of individuals with multiple sclerosis. American Journal of Speech-Language Pathology, 10(2), 126–137. [Google Scholar]
- Yunusova Y., Graham N. L., Shellikeri S., Phuong K., Kulkarni M., Rochon E., … Green J. R. (2016). Profiling speech and pausing in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). PLoS One, 11(1), e0147573. [DOI] [PMC free article] [PubMed] [Google Scholar]