Abstract
Purpose
The goal was to evaluate the potential effects of increasing hearing loss and advancing age on spectral envelope perception.
Method
Spectral modulation detection was measured as a function of spectral modulation frequency from 0.5 to 8.0 cycles/octave. The spectral modulation task involved discrimination of a noise carrier (3 octaves wide from 400 to 3200 Hz) with a flat spectral envelope from a noise having a sinusoidal spectral envelope across a logarithmic audio frequency scale. Spectral modulation transfer functions (SMTFs; modulation threshold vs. modulation frequency) were computed and compared 4 listener groups: young normal hearing, older normal hearing, older with mild hearing loss, and older with moderate hearing loss. Estimates of the internal spectral contrast were obtained by computing excitation patterns.
Results
SMTFs for young listeners with normal hearing were bandpass with a minimum modulation detection threshold at 2 cycles/octave, and older listeners with normal hearing were remarkably similar to those of the young listeners. SMTFs for older listeners with mild and moderate hearing loss had a low-pass rather than a bandpass shape. Excitation patterns revealed that limited spectral resolution dictated modulation detection thresholds at high but not low spectral modulation frequencies. Even when factoring out (presumed) differences in frequency resolution among groups, the spectral envelope perception was worse for the group with moderate hearing loss than the other 3 groups.
Conclusions
The spectral envelope perception as measured by spectral modulation detection thresholds is compromised by hearing loss at higher spectral modulation frequencies, consistent with predictions of reduced spectral resolution known to accompany sensorineural hearing loss. Spectral envelope perception is not negatively impacted by advancing age at any spectral modulation frequency between 0.5 and 8.0 cycles/octave.
It is well known that coincident with the aging process is a degradation in auditory function at multiple levels within the auditory pathway. Physiological changes at the periphery often include a reduction in auditory nerve activity (Schmiedt, Mills, & Boettcher, 1996) and metabolic changes at the level of stria vascularis (Pauler, Schuknecht, & White, 1988; Schulte & Schmiedt, 1992), both of which degrade audibility and spectral tuning (for a review, see Chisolm, Willott, & Lister, 2003). Further, these deficits propagate to the central auditory system via degraded input and in the form of peripherally induced central changes that are comorbid with age-related changes in the central auditory system (Ouda, Profant, & Syka, 2015). Typical presbycusis is associated with reduced audibility (Allen & Eddins, 2010), poorer binaural and spatial hearing abilities (Eddins & Hall, 2010), and declines in temporal processing (Ozmeral, Eddins, Frisina, & Eddins, 2016). In addition, those suffering from age-related hearing loss (ARHL) often experience a difficulty in understanding speech in quiet or in the presence of competing background sounds (Humes & Dubno, 2010).
Reduced spectral resolution is one hallmark of sensorineural hearing loss (SNHL) and is thought to reflect a loss of peripheral tuning to audio frequency (Evans, 1975; Evans & Harrison, 1976; Glasberg & Moore, 1986; Henry, Turner, & Behrens, 2005). Spectral resolution is most often measured in terms of the masking effect that a sound at one audio frequency has on a simultaneous sound at another audio frequency. One consequence of reduced spectral resolution is a reduction in the sharpness of spectral peaks and, often, a decrease in the level variation between closely spaced spectral peaks and valleys. This can have a negative impact on the encoding and perception of spectrally complex signals, such as speech. Potential changes in the internal representation of the stimulus spectrum include (a) reduced spectral contrast (Leek, Dorman, & Summerfield, 1987), (b) lateral (upward and downward) spread of excitation and masking (Martin & Pickett, 1971), and (c) altered lateral suppression and/or adaptation of lateral suppression (Leshowitz & Lindstrom, 1977; Wightman, McGee, & Kramer, 1977). These changes have the greatest impact on speech perception in competing backgrounds (Baer & Moore, 1994; Nejime & Moore, 1997). With respect to typical aging, spectral resolution may be worse for elderly individuals than younger individuals (Glasberg, Moore, Patterson, & Nimmo-Smith, 1984; Peters & Moore, 1992); however, Lutman, Gatehouse, and Worthington (1991) reported only a very slight age-related decrease in spectral resolution when subjects were matched for hearing loss, and others have not found age to impact spectral resolution at all (Hopkins & Moore, 2011; Peters & Moore, 1992). From this, one may conclude that changes in spectral resolution in older individuals are more closely related to changes in hearing sensitivity than changes in age.
Related to but, in some cases, quite separate from spectral resolution is spectral envelope perception or the ability to compare and encode intensity variations across a range of audio frequencies. Whereas spectral resolution emphasizes the degree to which proximal spectral features are resolved, spectral envelope perception can be thought of as a more global concept that encompasses spectral resolution for proximal and distal spectral features. Spectral envelope perception has been measured using several psychoacoustic tasks, but most common have been spectral profile analysis (e.g., Green & Nguyen, 1988) and spectral modulation detection (in cycles/octave; Eddins & Bero, 2007). Similar to spectral resolution, spectral envelope perception also is worse for listeners with SNHL than listeners with normal hearing (NH; Lentz, 2006; Lentz & Leek, 2002, 2003; Summers & Leek, 1994). Such data indicate that spectral resolution declines markedly with hearing loss and not with aging alone, but the question remains as to whether or not spectral envelope perception deteriorates with age in a manner that is largely independent of SNHL.
Although the effects of advancing age on spectral envelope perception are unknown, the effects of age on temporal envelope perception are well established (Humes et al., 2012). The adoption of a systems analysis approach involving measurement of modulation detection thresholds over a wide range of modulation frequencies has been widely successful in the study of auditory temporal envelope processing (Bacon & Viemeister, 1985; Eddins, 1993; Viemeister, 1979). Analogous methods can be used to study auditory spectral envelope perception. Spectral modulation detection thresholds may be measured as a function of spectral modulation frequency, resulting in a spectral modulation transfer function (SMTF). Such an approach has been used successfully to explore spectral shape performance in a number of previous reports (Bernstein & Green, 1988; Chi, Gao, Guyton, Ru, & Shamma, 1999; Summers & Leek, 1994; Supin, Popov, Milekhina, & Tarakanov, 1999). Eddins and Bero (2007) have shown in listeners with NH that the SMTF is bandpass in shape and is largely independent of carrier bandwidth and carrier frequency region. They interpreted the bandpass shape of the SMTF as a combination of limited spectral resolution, resulting in the greatest reduction in effective modulation depth at higher modulation frequencies, and limited across-channel spectral shape perception, resulting in progressively poorer modulation detection at lower modulation frequencies as the successive modulation peaks and valleys are spaced farther apart.
Listeners with SNHL may have elevated SMTFs, reflecting a reduction in the internal or effective spectral modulation depth as a result of reduced spectral resolution (Summers & Leek, 1994). Data from that study are limited to spectral modulation frequencies of 1 cycle/octave and above but clearly show the effect of SNHL on these moderate to high spectral modulation frequencies. Spectral modulation detection by listeners with cochlear implants is even more severely limited by their poor spectral resolution. In that population, spectral modulation detection thresholds are strongly correlated with speech perception, including vowel and consonant identification (Saoji, Litvak, Spahr, & Eddins, 2009). The effect of limited spectral resolution associated with SNHL should have the greatest negative effect on the detection of higher frequency spectral modulation because the modulation peaks and valleys are spaced more closely and limited spectral resolution will markedly reduce the effective modulation depth. As spectral modulation frequency is decreased, the reduced spectral resolution associated with SNHL should have a monotonically diminishing negative effect on the effective modulation depth because the spacing of adjacent peaks and valleys in the modulation become spaced farther and farther apart. Spectral envelope perception at lower spectral modulation frequencies is the primary basis for spectral feature extraction important for vowel identification and discrimination (e.g., Vanveen & Houtgast, 1985) and sound localization in the vertical plane (e.g., Qian & Eddins, 2008). The degree to which SNHL will have a significant impact on spectral modulation detection at these low modulation frequencies is not known at this time.
Although it is well established that spectral envelope perception is generally worse for listeners with hearing impairment (HI) than for listeners with NH (Lentz & Leek, 2002, 2003; Shrivastav, Humes, & Kewley-Port, 2006; Summers & Leek, 1994), comparisons have been restricted to younger subjects with NH and older subjects with HI, confounding age and peripheral hearing loss (e.g., Shrivastav et al., 2006). In this study, we seek to establish the effects of age on spectral envelope perception by systematically teasing out the age and hearing loss parameters using the spectral modulation detection method. The detection of sinusoidal spectral modulation superimposed on a noise carrier relies on several important auditory perceptual processes, including intensity coding, spectral resolution, and across-frequency comparisons of intensity. The principal aim of this study is to determine how spectral envelope perception varies with age and ARHL. Because the evidence associating advancing age with reduced spectral resolution is weak at best and there is no a priori reason to suspect that advancing age negatively affects simple acoustic pattern perception, the hypotheses to be evaluated here are that (a) aging alone will have no impact on the shape or sensitivity of the SMTF, (b) SNHL should lead to little or no impact on spectral modulation detection thresholds at low modulation frequencies, and (c) SNHL should have progressively increasing effect on spectral modulation detection at higher spectral modulation thresholds. A corollary to these hypotheses is that, with increasing degree of SNHL and progressively reduced spectral resolution, the bandpass SMTF should have a progressively steeper high-frequency slope as the degree of SNHL increases, while age should not impact the shape or sensitivity of the SMTF.
Method
Subjects
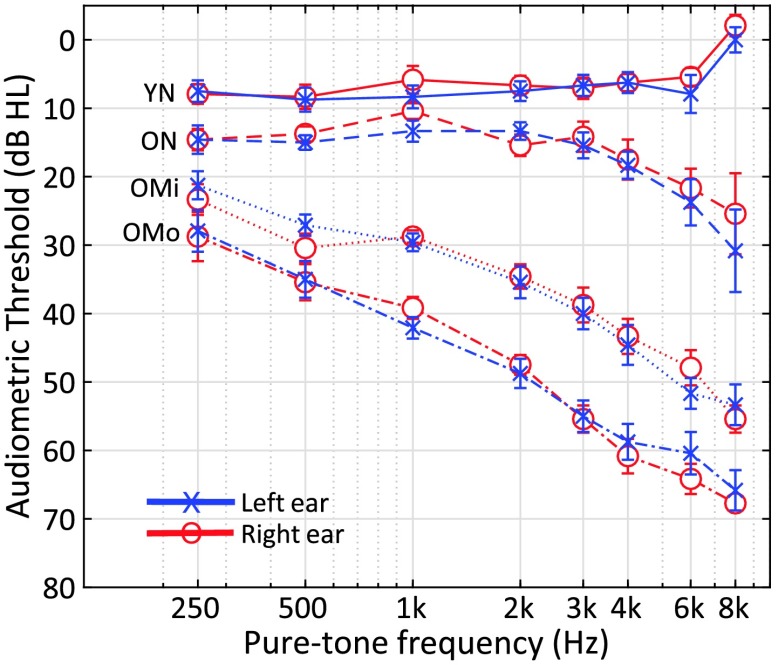
A total of 48 subjects consented to volunteer for the study following the procedures approved by university institutional review board and were selected on the basis of age, sex, and hearing status. Subjects were paid for their participation. Hearing loss, when present, was categorized as sensorineural based on air and bone conduction thresholds and screening tympanometry (Y, 226 Hz). Subjects with a history of head injury, seizures, stroke, Ménière's disease, vertigo, mastoiditis, or ear surgery were excluded from participation. The 48 subjects were separated into four groups of 12 as follows: Young listeners with NH were between 19 and 33 years of age and had pure-tone thresholds less than or equal to 25 dB HL (i.e., decibels hearing level) between 250 and 8000 Hz. Older listeners with NH were between 61 and 71 years of age and had pure-tone thresholds less than or equal to 25 dB HL from 250 to 8000 Hz. Older listeners with mild hearing loss (OMi) were between 63 and 82 years of age, had pure-tone thresholds at 2000 Hz between 30 and 40 dB HL, and a gently sloping audiogram between 250 and 8000 Hz. Older listeners with moderate (OMo) hearing loss were between 65 and 92 years old, had pure-tone thresholds at 2000 Hz between 45 and 55 dB HL, and a moderately sloping audiogram between 250 and 800 Hz. Figure 1 shows the mean audiometric profile by group, and means and standard deviations are summarized in Table 1.
Figure 1.
Average audiometric pure-tone air conduction thresholds (left ear, test ear) for the four subject groups: young normal hearing (YN), older normal hearing (ON), older with mild hearing loss (OMi), and older with moderate hearing loss (OMo) in audiogram format.
Table 1.
Audiometric profile (age and pure-tone average for frequencies 0.5–2 kHz [PTA2k] in decibels hearing level) of the four test groups, including young normal hearing (YN), older normal hearing (ON), older with mild hearing loss (OMi), and older with moderate hearing loss (OMo).
| Group | Age (years) | PTA2k (dB HL) |
|---|---|---|
| YN (n = 12) | M = 22.9, SD = 4.4 | M = 7.6, SD = 4.8 |
| ON (n = 12) | M = 66.6, SD = 3.1 | M = 13.5, SD = 2.9 |
| OMi (n = 12) | M = 70.7, SD = 6.3 | M = 31.0, SD = 3.3 |
| OMo (n = 12) | M = 80.3, SD = 7.8 | M = 41.3, SD = 4.9 |
Stimuli
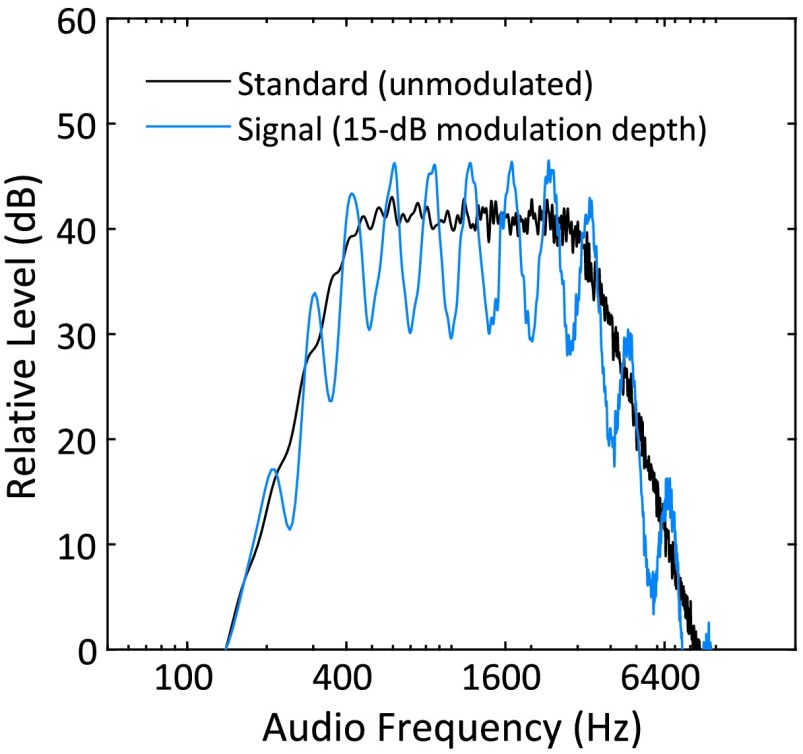
The stimuli used here represent a subset of those reported by Eddins and Bero (2007). Band-limited Gaussian noise carriers with a flat spectrum were filtered with a Butterworth filter with nominal cutoff frequencies of 400 to 3200 Hz and a slope of −24 dB/octave outside that passband (standard). In the signal interval, spectral modulation was introduced by imposing a sinusoidal spectral envelope across a logarithmic (log2 or octave scale) audio frequency axis, grossly reflecting the tonotopic nature of the auditory system. Spectral modulation frequency was measured in units of cycles per octave such that successive peaks and valleys were separated by multiples or fractions of an octave. Importantly, the starting phase of the sinusoidal spectral envelope was random (0 to 2π radians) from stimulus to stimulus. As a result, peaks and valleys were randomly located along the audio frequency (cochlear place) axis. This procedure minimized the utility cues associated with overall pitch, local intensity, and local (peripheral) adaptation during the detection task (analogous to random level variation, e.g., Green, 1993). The modulation depth was computed as the decibel difference between spectral peaks and valleys. Examples of an unmodulated standard (black curve) and modulated signal stimulus (15-dB depth; blue curve) are shown in Figure 2. Stimulus durations were 400 ms, including a 10-ms, cosine-squared, rise–fall window.
Figure 2.
Spectrum level (decibel relative) by frequency for test stimulus with 15-dB spectral modulation depth (blue) and the smooth standard superimposed (black).
Stimulus generation, presentation, and response collection were executed using the SykofizX software suite (Tucker-Davis Technologies, TDT). Stimuli were routed to a TDT RP2 real-time processor, scaled via a programmable attenuator (TDT PA5) and output via headphone buffer (TDT HB6) to the left insert earphone (Etymotic, ER-2). Attenuator settings ensured that stimuli were presented at an overall level of 75 dB SPL as calibrated in an ear simulator (Bruel & Kjaer, model DB-100). The overall level of the modulated signal stimulus was equal to that of the standard.
Procedure
The modulation depth (peak to valley difference in decibel) was varied to determine the modulation detection threshold (minimum depth necessary to discriminate between the standard and signal stimuli). Modulation detection thresholds were acquired using a cued, two-interval, forced-choice procedure consisting of three listening intervals separated by 500 ms each. Test conditions included modulation frequencies of 0.5, 1, 2, 4, and 8 cycles/octave and were completed in a different random order for each subject. The threshold was estimated using an adaptive three-down, one-up adaptive tracking procedure that estimated a 79.4% threshold (Levitt, 1971). The initial modulation depth was 20 dB and was reduced in 5-dB steps for the first three reversals in the adaptive track, after which, the step size was reduced to 1 dB for the remainder of the track. Threshold for a track was taken as the average depth across the last even number of reversals excluding the first three reversals. Reported thresholds are based upon the average of three 75-trial runs. Testing was conducted in a double-walled, sound-attenuating chamber. All subjects had prior experience in at least one psychoacoustic task (temporal gap detection) during a previous intake session. Further, each subject completed three practice runs in the spectral modulation detection task for 1.5 cycles/octave, a spectral modulation frequency not included in this experimental design.
Results
Spectral Modulation Detection With Age and Hearing Loss
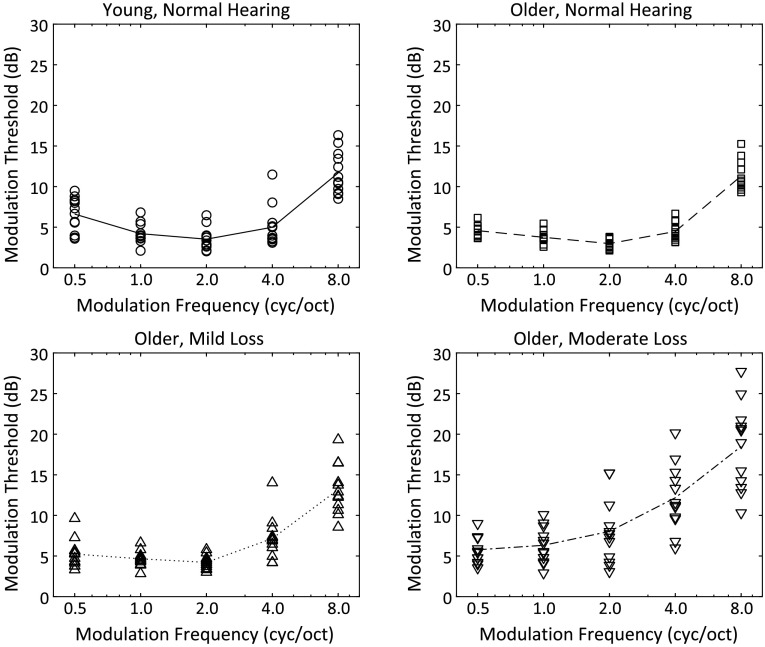
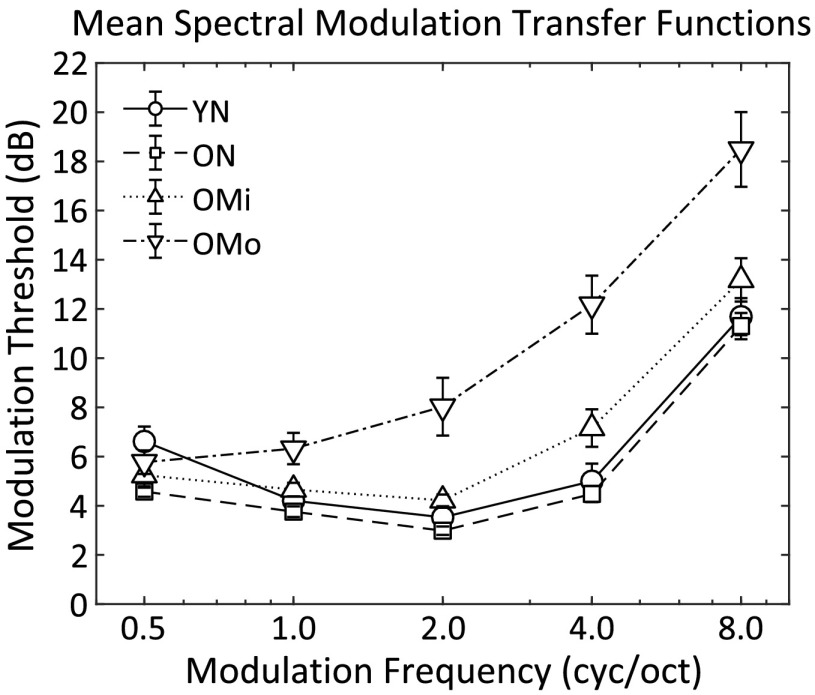
Spectral modulation detection thresholds are shown in Figure 3 (individual data) and Figure 4 (averaged by group) with thresholds (in decibels) on the ordinate and spectral modulation frequency (in cycles/octave) on the abscissa. The four threshold functions represent SMTFs for each of the four subject groups, indicated by the different symbols. The resulting SMTFs for the young normal hearing (YN), older normal hearing (ON), and OMi groups reflect a bandpass characteristic that is steeper on the high-frequency side of the function. SMTFs for the YN (circles) and ON (squares) groups essentially overlap. Each function shows a minimum threshold at 2 cyc/oct and a steeper high-frequency than low-frequency slope. The only modulation frequency at which threshold for the two groups tended to differ was at 0.5 cyc/oct, where the YN group had slightly higher modulation detection thresholds on average (6.6 dB) than the ON group (4.6 dB). Thresholds for the OMi group tended to be slightly higher than the YN or ON group at 2.0 cyc/oct and above. SMTFs for the OMi group show increased variability relative to the ON group, but otherwise, the pattern of thresholds as a function of modulation frequency was similar between the groups. Thresholds were clearly higher for the OMo group for modulation frequencies 1.0 cyc/oct and greater. Threshold variability was greatest among the subjects in this group, and the threshold functions tended to show more of a low-pass than a bandpass function. Furthermore, high-frequency thresholds were clearly worse for many of the OMo subjects than for subjects in the other three groups.
Figure 3.
Individual spectral modulation transfer functions for the four listener groups. Line represents the mean of the 12 listeners in each group. cyc/oct = cycles/octave.
Figure 4.
Mean spectral modulation transfer functions for the four listener groups: young normal hearing (YN), older normal hearing (ON), older with mild hearing loss (OMi), and older with moderate hearing loss (OMo). Symbols represent the mean of 12 listeners in each group and error bars represent ±1 SEM. cyc/oct = cycles/octave.
To assess the potential relationships among modulation frequency and group, a two-factor repeated-measures analysis of variance was computed and revealed the main effects of modulation frequency, F(4, 176 = 216, p < .001, ηp 2 = .83, and listener group, F(3, 44 = 750, p < .001, ηp 2 = .52, and the interaction between the two, F(12, 176) = 8.42, p < .001, ηp 2 = .36. Post hoc group comparisons (with Bonferroni correction) showed significantly different SMTFs for the OMo group than the other three groups (p < .001 in all comparisons), especially at higher spectral modulation rates, which likely drove the significant interaction. Differences among the YN, ON, and OMi groups were not statistically significant.
In addition to the repeated-measures analysis of variance, a correlation analysis including all subjects was conducted to identify potential relationships among hearing threshold (pure-tone average between 250 and 2000 Hz in the stimulus ear [PTA2k]), age, and each of the modulation frequency thresholds (see Table 2). Although the older groups were designed to isolate the differences in the hearing threshold, it is clear from Tables 1 (right column) and 2 (Age-PTA2k intersection) that hearing threshold covaried with age, and both terms significantly correlated with observed thresholds in all conditions except the lowest modulation condition. Therefore, further regression analyses were conducted per spectral modulation condition, which served to model the observed thresholds based on both predictors: age and PTA2k (see Table 3). Results showed that, except for the 0.5-cyc/oct condition, between 28% and 51% of the variance could be explained by the respective regression model. Moreover, only the PTA2k term was found to have a significant effect on these models.
Table 2.
Correlation matrix with hearing threshold (pure-tone average for frequencies 0.5–2 kHz [PTA2k]), age, and spectral modulation thresholds at modulation frequencies (0.5, 1, 2, 4, and 8 cycles/octave [cyc/oct]).
| Measure | PTA2k | Age | 0.5 cyc/oct | 1 cyc/oct | 2 cyc/oct | 4 cyc/oct | 8 cyc/oct |
|---|---|---|---|---|---|---|---|
| PTA2k | 1.00 | .76*** | −.10 | .53*** | .65*** | .71*** | .62*** |
| Age | — | 1.00 | −.23 | .39** | .42** | .49*** | .46*** |
| 0.5 cyc/oct | — | 1.00 | .51*** | .16 | .06 | .32* | |
| 1 cyc/oct | — | 1.00 | .64*** | .58*** | .78*** | ||
| 2 cyc/oct | — | 1.00 | .88*** | .88*** | |||
| 4 cyc/oct | — | 1.00 | .81*** | ||||
| 8 cyc/oct | — | 1.00 |
Note. Asterisks indicate significant t tests for Pearson r:
p < .05.
p < .01.
p < .001.
Table 3.
Separate linear regression models per spectral modulation condition, with predictors: age and pure-tone average for frequencies 0.5–2 kHz (PTA2k), with statistical results (analysis of variance [ANOVA]).
| Condition (cycles/octave) | Intercept | Age | PTA2k | ANOVA |
|---|---|---|---|---|
| 0.5 (r2 = .067) | 6.75** | −0.280 | 0.170 | F(2, 45) = 1.6, p = .210 |
| 1 (r2 = .28) | 3.46** | −0.003 | 0.052** | F(2, 45) = 8.7, p = .001 |
| 2 (r2 = .43) | 2.50* | −0.022 | 0.129** | F(2, 45) = 17.2, p < .001 |
| 4 (r2 = .51) | 3.44* | −0.021 | 0.184** | F(2, 45) = 23.2, p < .001 |
| 8 (r2 = .39) | 9.55** | −0.003 | 0.158** | F(2, 45) = 14.2, p < .001 |
Note. Asterisk indicates significant t test for coefficient:
p < .01.
p < .001.
Spectral Modulation Detection and Excitation Patterns
Simulations of spectral resolution often include computations of the internal excitation pattern (EP), which is based on estimates of auditory filter shape and gain in human subjects with and without hearing loss (Moore & Glasberg, 1987). Because hearing loss as measured by pure-tone threshold is often associated with a reduction in spectral resolution (Glasberg & Moore, 1986), variations in the spectral envelope of a stimulus should be smoothed to a greater extent in listeners with HI than in listeners with NH. To assess the degree to which the general shape of the SMTF and the specific differences in SMTF shape are limited by spectral resolution, EPs were computed with corrections for the earphone transfer function, the middle ear transfer function, and the specific hearing loss in each subject (Moore & Glasberg, 2004). This analysis is adopted and adapted from the earlier work of Summers and Leek (1994) and is based on the logic put forth in the introduction that limited spectral resolution alone should have progressively less and less impact on spectral modulation detection thresholds with decreasing spectral modulation frequency, leading to a low-pass SMTF. That shape was not observed in the threshold data, leading to the prediction that spectral resolution alone and, by extension, EPs should not be able to account for the bandpass shape of the observed SMTFs.
EPs were computed for an unmodulated standard and a sinusoidally modulated signal with a modulation depth corresponding to the threshold for each subject. Spectra were based on the average of 100 stimulus samples with a fixed modulation phase. A signal value representing the internal spectral modulation depth for each subject and modulation frequency was computed over the audio frequency range of 283 to 4525 Hz (.5 octave below and above the nominal passband of 400 to 3200 Hz). That single value was computed by first taking the difference between the EP for the signal and standard spectra for each listener and then taking the difference between the maximum and the minimum in that difference function. This value is referred to as the excitation pattern difference or EPD.
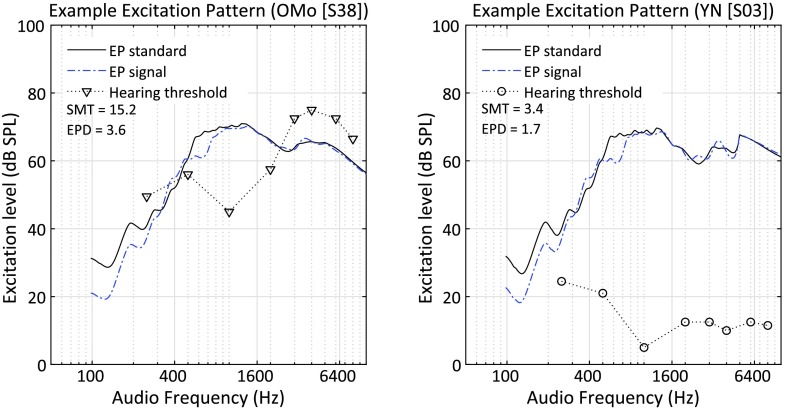
To illustrate the EP results, data for Subject S38 from the OMo group are shown in Figure 5, left panel, for a modulation frequency of 2 cyc/oct and a modulation depth corresponding to the threshold for that subject, or 15.2 dB. The resulting EPD was 3.6 dB. In comparison, the data for Subject S03 from the YN group is shown in the right panel for the same modulation frequency. The modulation depth corresponding to the threshold for S03 was 3.4 dB, and this value produced an EPD of 1.7 dB. If differences in the 2 cyc/oct thresholds for these two listeners (15.2 vs. 3.4 dB) could be accounted for by reduced spectral resolution alone and if the EP model accurately captures an individual's spectral resolution, then the EPDs should be the same for both listeners. Clearly, the EPDs differed by more than a factor of two. One interpretation is that thresholds were not strictly dependent on spectral resolution for this midfrequency condition.
Figure 5.
Excitation patterns (EP) for the unmodulated standard (solid black line) and the modulated signal (2 cycles/octave [cyc/oct]; dashed blue line) are shown for one subject, S38, from the older with moderate hearing loss (OMo) group, in the left panel and one subject, S03, from the young normal hearing (YN) group, in the right panel. Pure-tone hearing thresholds are indicated by asterisks according to the right vertical axis in each panel. SMT = spectral modulation threshold. EPD = excitation pattern difference.
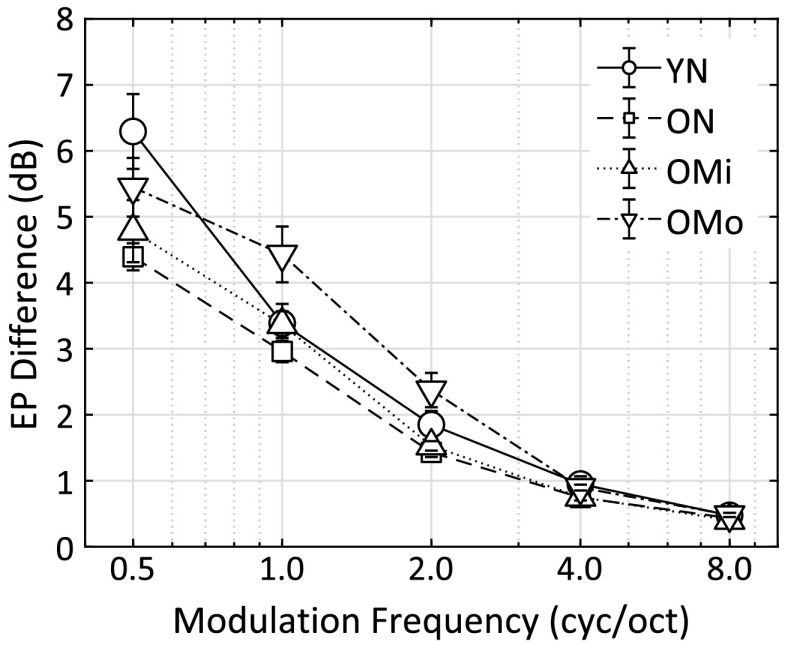
To better interpret the EPD values and to better capture the group differences, the same computations were completed for all subjects in all four subject groups and for all spectral modulation frequencies. The EPDs between the standard and signal interval (computed based on the subject- and condition-specific modulation detection threshold) are shown in Figure 6.
Figure 6.
Average excitation pattern (EP) difference per spectral modulation frequency per group: young normal hearing (YN), older normal hearing (ON), older with mild hearing loss (OMi), and older with moderate hearing loss (OMo). cyc/oct = cycles/octave.
At 8 cyc/oct, despite wide variation in measured thresholds across groups (from 12 to 18 dB, i.e., right side of Figure 4), the EP differences from all subjects in all groups converge to a single value of about 0.4 dB (right side of Figure 6). Recall that the EP computations, based on Moore and Glasberg (2004), include a hearing loss parameter that adjusts the width of the underlying auditory filters based on threshold and an assumed distribution of outer hair cell loss proportional to hearing loss. The most parsimonious interpretation of this result is that the EP model fully captures the loss of spectral resolution at this modulation frequency, and when that is taken into account, the EPDs are nearly the same among subjects regardless of the degree of hearing loss or age. When intensity variations are proximal or dense along the audio frequency dimension, coding of these local intensity variations is limited by the frequency resolving power of the auditory system, effectively smoothing intensity variations between peaks and valleys of each modulation cycle. In the YN listener group, the upturn in the SMTF above about 4 cyc/oct should largely be due to this limitation. This result is consistent with the asymptotic EP differences shown by Summers and Leek (1994; their Figure 6) beyond about 6 cyc/oct (as shown in their Figure 6).
Discussion
If spectral resolutions were the sole determining factors for all spectral modulation detection thresholds between 0.5 and 8 cyc/oct, then the EP differences in Figure 6 computed from actual modulation detection thresholds should form a flat line fixed at about 0.4 dB across all modulation frequencies. Likewise, detection thresholds in Figures 3 and 4 would improve monotonically with decreasing modulation frequency, reflecting diminishing spectral smoothing as the spectral density decreases. However, it is clear that, as the modulation frequency decreases from right to left in Figure 6, the EP differences deviate progressively greater from a fixed internal spectral contrast, and the modulation detection thresholds in Figures 3 and 4 improve monotonically with decreasing modulation frequency from 8 to 2 cyc/oct (for the YN, ON, and OMi groups). Thus, as the peaks and valleys of the modulation function are more sparsely spaced, spectral resolution has progressively less impact on thresholds. With further decreases in modulation frequency (below 2 cyc/oct), thresholds do not improve monotonically or even plateau; they get worse. This is not in line with the notion that the SMTF reflects spectral resolution alone but is consistent with the explanation provided by Eddins and Bero (2007) that the auditory system has a limited ability to encode and compare intensity variations to form a coherent pattern as the spectral density (proximity of neighboring peaks in the modulated spectrum) decreases. The fact that spectral modulation detection thresholds do not depend strongly on the number of cycles (i.e., carrier bandwidth; Eddins & Bero, 2007) implies that the high-pass portion of the SMTF at low-modulation frequencies is not related to a multiple-looks phenomenon (cf. Summers & Leek, 1994; Viemeister & Wakefield, 1991) or an information-theoretic approach, which would assume better detection as the number of stimulated frequency channels increases. This combination of the effects of limited spectral resolution at higher modulation frequencies and limited ability to encode and compare intensity variations as those variations become more distal or sparse at lower modulation frequencies forms a duplex of overlapping limitations with the least impact around 2 cyc/oct in the YN, ON, and OMi groups. For the OMo group, the presumed impact of limited spectral resolution persisted to lower spectral modulation frequencies than for the YN, ON, and OMi groups. This result is consistent with previous studies that showed limited effects of HI when spectral features are sparsely spaced (Lentz & Leek, 2002, 2003) but clear effects of HI as the spectral density is increased (Lentz, 2006). These results provide strong support for the three hypotheses put forth in the introduction, namely, that SNHL has little impact on spectral modulation detection at lower modulation frequencies and a progressively increasing effect at higher spectral modulation frequencies, whereas aging alone has no discernable impact on the shape or sensitivity of the SMTF.
A clear relationship between spectral modulation detection and the perception of speech has been established (e.g., Liu & Eddins, 2008; Saoji et al., 2009; Vanveen & Houtgast, 1985). Although several studies have demonstrated relatively poorer performance on speech tasks with advancing age (for a review, see Humes et al., 2012), even in the absence of elevated pure-tone thresholds, the current data indicate that such poorer performance with age is not directly attributable to spectral modulation detection ability. From the perspective of basic auditory perception, poorer coding of temporal fine structure (e.g., Grose & Mamo, 2012) and temporal envelope (e.g., Ozmeral et al., 2016) cues are more likely the contributors to age-related declines in speech perception. Nevertheless, typical aging is associated with elevated pure-tone thresholds, and such changes in hearing sensitivity clearly can negatively impact spectral modulation detection ability as demonstrated by Summers and Leek (1994) and, again, in the current study. In this case, hearing loss likely contributes substantially to poorer coding of the spectral contrast, which is an important information-bearing feature of speech stimuli. Coding of similar cues is important for spatial processing of sounds distributed in the vertical plane (see Qian & Eddins, 2008).
Conclusions
SMTFs estimated from spectral modulation detection thresholds represent a filter characteristic that is related to the internal auditory representation of the spectral contrast. Estimates of the internal spectral contrast in response to threshold-level modulation based on EP computations (as implemented by Moore & Glasberg, 2004) indicated that the differences among listener groups, particularly 4 cyc/oct and above, were due to changes in spectral resolution that typically accompany SNHL. Group and individual differences at lower spectral modulation frequencies are not explained by limited spectral resolution. Rather, the low-frequency side of the SMTF reflects a process governed by across-channel spectral envelope perception. Neither process appears to be influenced by advancing age, whereas modulation detection thresholds at the high-frequency side of the SMTF are negatively impacted by moderate SNHL. Intentional manipulation of lower spectral modulation frequencies can have a marked impact on the spectral envelope perception required for phoneme identification (Liu & Eddins, 2008) and sound localization in the vertical plane (Qian & Eddins, 2008), highlighting the relevance of the spectral modulation domain. While the current results indicate no age-related deficit in spectral shape perception, relatively long duration (400 ms) noise bursts were used here. Typical static spectral features in speech are much shorter than this, and it is possible that an age effect would occur for shorter stimuli or for temporally varying stimuli. Furthermore, pieces of evidence from both behavioral (Saoji & Eddins, 2007) and physiological studies (e.g., Kowalski, Depireux, & Shamma, 1996; Shamma, Fleshman, Wiser, & Versnel, 1993) indicate tuning to spectral modulation frequency, much like tuning to temporal modulation frequency. It may be that changes in tuning with age and/or hearing loss are more relevant to perceptual deficits than the broad modulation transfer function. In the context of ARHL, it is refreshing to note that the spectral envelope perception does not appear to be negatively impacted by age alone.
Acknowledgments
We gratefully acknowledge the support of the National Institute on Aging Grant P01 AG009524 Project 1, awarded to David A. Eddins, sub under Robert Frisina, and the National Institute on Deafness and Other Communication Disorders Grant R01 DC015051, awarded to Frederick Gallun, David A. Eddins, and Aaron Seitz, and helpful suggestions from Eric Hoover.
Funding Statement
We gratefully acknowledge the support of the National Institute on Aging Grant P01 AG009524 Project 1, awarded to David A. Eddins, sub under Robert Frisina, and the National Institute on Deafness and Other Communication Disorders Grant R01 DC015051, awarded to Frederick Gallun, David A. Eddins, and Aaron Seitz, and helpful suggestions from Eric Hoover.
References
- Allen P. D., & Eddins D. A. (2010). Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hearing Research, 264(1–2), 10–20. https://doi.org/10.1016/j.heares.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon S. P., & Viemeister N. F. (1985). Temporal modulation transfer functions in normal-hearing and hearing-impaired listeners. Audiology, 24(2), 117–134. [DOI] [PubMed] [Google Scholar]
- Baer T., & Moore B. C. (1994). Effects of spectral smearing on the intelligibility of sentences in the presence of interfering speech. The Journal of the Acoustical Society of America, 95(4), 2277–2280. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R., & Green D. M. (1988). Detection of changes in spectral shape—Uniform vs. non-uniform background spectra. Hearing Research, 34(2), 157–166. https://doi.org/10.1016/0378-5955(88)90103-7 [DOI] [PubMed] [Google Scholar]
- Chi T. S., Gao Y. J., Guyton M. C., Ru P. W., & Shamma S. (1999). Spectro-temporal modulation transfer functions and speech intelligibility. The Journal of the Acoustical Society of America, 106(5), 2719–2732. https://doi.org/10.1121/1.428100 [DOI] [PubMed] [Google Scholar]
- Chisolm T. H., Willott J. F., & Lister J. J. (2003). The aging auditory system: Anatomic and physiologic changes and implications for rehabilitation. International Journal of Audiology, 42(Suppl. 2), 2S3–2S10. [PubMed] [Google Scholar]
- Eddins D. A. (1993). Amplitude-modulation detection of narrow-band noise: Effects of absolute bandwidth and frequency region. The Journal of the Acoustical Society of America, 93(1), 470–479. https://doi.org/10.1121/1.405627 [Google Scholar]
- Eddins D. A., & Bero E. M. (2007). Spectral modulation detection as a function of modulation frequency, carrier bandwidth, and carrier frequency region. The Journal of the Acoustical Society of America, 121(1), 363–372. [DOI] [PubMed] [Google Scholar]
- Eddins D. A., & Hall J. W. (2010). Binaural processing and auditory asymmetries. Aging Auditory System, 34, 135–165. https://doi.org/10.1007/978-1-4419-0993-0_6 [Google Scholar]
- Evans E. F. (1975). The sharpening of cochlear frequency selectivity in the normal and abnormal cochlea. Audiology, 14(5–6), 419–442. [DOI] [PubMed] [Google Scholar]
- Evans E. F., & Harrison R. V. (1976). Proceedings: Correlation between cochlear outer hair cell damage and deterioration of cochlear nerve tuning properties in the guinea-pig. Journal of Physiology, 256(1), 43P–44P. [PubMed] [Google Scholar]
- Glasberg B. R., & Moore B. C. (1986). Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. The Journal of the Acoustical Society of America, 79(4), 1020–1033. [DOI] [PubMed] [Google Scholar]
- Glasberg B. R., Moore B. C., Patterson R. D., & Nimmo-Smith I. (1984). Dynamic range and asymmetry of the auditory filter. The Journal of the Acoustical Society of America, 76(2), 419–427. [DOI] [PubMed] [Google Scholar]
- Green D. M. (1993). Detecting changes in spectral shape of complex auditory signals: Profile analysis. The Journal of the Acoustical Society of America, 93(4), 2294 https://doi.org/10.1121/1.406493 [Google Scholar]
- Green D. M., & Nguyen Q. T. (1988). Profile analysis-detecting dynamic spectral changes. Hearing Research, 32(2–3), 147–163. [DOI] [PubMed] [Google Scholar]
- Grose J. H., & Mamo S. K. (2012). Frequency modulation detection as a measure of temporal processing: Age-related monaural and binaural effects. Hearing Research, 294(1–2), 49–54. https://doi.org/10.1016/j.heares.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. A., Turner C. W., & Behrens A. (2005). Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. The Journal of the Acoustical Society of America, 118(2), 1111–1121. [DOI] [PubMed] [Google Scholar]
- Hopkins K., & Moore B. C. (2011). The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. The Journal of the Acoustical Society of America, 130(1), 334–349. https://doi.org/10.1121/1.3585848 [DOI] [PubMed] [Google Scholar]
- Humes L. E., & Dubno J. R. (2010). Factors affecting speech understanding in older adults. Aging Auditory System, 34, 211–257. https://doi.org/10.1007/978-1-4419-0993-0_8 [Google Scholar]
- Humes L. E., Dubno J. R., Gordon-Salant S., Lister J. J., Cacace A. T., Cruickshanks K. J., … Wingfield A. (2012). Central presbycusis: A review and evaluation of the evidence. Journal of the American Academy of Audiology, 23(8), 635–666. https://doi.org/10.3766/jaaa.23.8.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski N., Depireux D. A., & Shamma S. A. (1996). Analysis of dynamic spectra in ferret primary auditory cortex.1. Characteristics of single-unit responses to moving ripple spectra. Journal of Neurophysiology, 76(5), 3503–3523. [DOI] [PubMed] [Google Scholar]
- Leek M. R., Dorman M. F., & Summerfield Q. (1987). Minimum spectral contrast for vowel identification by normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 81(1), 148–154. https://doi.org/10.1121/1.395024 [DOI] [PubMed] [Google Scholar]
- Lentz J. J. (2006). Spectral-peak selection in spectral-shape discrimination by normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 120(2), 945–956. [DOI] [PubMed] [Google Scholar]
- Lentz J. J., & Leek M. R. (2002). Decision strategies of hearing-impaired listeners in spectral shape discrimination. The Journal of the Acoustical Society of America, 111(3), 1389–1398. [DOI] [PubMed] [Google Scholar]
- Lentz J. J., & Leek M. R. (2003). Spectral shape discrimination by hearing-impaired and normal-hearing listeners. The Journal of the Acoustical Society of America, 113(3), 1604–1616. [DOI] [PubMed] [Google Scholar]
- Leshowitz B., & Lindstrom R. (1977). Measurement of nonlinearities in listeners with sensorineural hearing loss. In Evans E. F. & Wilson J. P. (Eds.), Psychophysics and physiology of hearing (pp. 283–292). London, England: Academic. [Google Scholar]
- Levitt H. (1971). Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America, 49(2), 467–477. https://doi.org/10.1121/1.1912375 [PubMed] [Google Scholar]
- Liu C., & Eddins D. A. (2008). Effects of spectral modulation filtering on vowel identification. The Journal of the Acoustical Society of America, 124(3), 1704–1715. https://doi.org/10.1121/1.2956468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutman M. E., Gatehouse S., & Worthington A. G. (1991). Frequency resolution as a function of hearing threshold level and age. The Journal of the Acoustical Society of America, 89(1), 320–328. [DOI] [PubMed] [Google Scholar]
- Martin E. S., & Pickett J. M. (1971). Sensorineural hearing loss and upward spread of masking-reply. Journal of Speech and Hearing Research, 14(1), 223–224. https://doi.org/10.1044/jshr.1401.223 [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., & Glasberg B. R. (1987). Formulas describing frequency-selectivity as a function of frequency and level, and their use in calculating excitation patterns. Hearing Research, 28(2–3), 209–225. https://doi.org/10.1016/0378-5955(87)90050-5 [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., & Glasberg B. R. (2004). A revised model of loudness perception applied to cochlear hearing loss. Hearing Research, 188(1–2), 70–88. https://doi.org/10.1016/S0378-5955(03)00347-2 [DOI] [PubMed] [Google Scholar]
- Nejime Y., & Moore B. C. (1997). Simulation of the effect of threshold elevation and loudness recruitment combined with reduced frequency selectivity on the intelligibility of speech in noise. The Journal of the Acoustical Society of America, 102(1), 603–615. [DOI] [PubMed] [Google Scholar]
- Ouda L., Profant O., & Syka J. (2015). Age-related changes in the central auditory system. Cell and Tissue Research, 361(1), 337–358. https://doi.org/10.1007/s00441-014-2107-2 [DOI] [PubMed] [Google Scholar]
- Ozmeral E. J., Eddins A. C., Frisina D. R., & Eddins D. A. (2016). Large cross-sectional study of presbycusis reveals rapid progressive decline in auditory temporal acuity. Neurobiology of Aging, 43, 72–78. https://doi.org/10.1016/j.neurobiolaging.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauler M., Schuknecht H. F., & White J. A. (1988). Atrophy of the stria vascularis as a cause of sensorineural hearing-loss. Laryngoscope, 98(7), 754–759. [DOI] [PubMed] [Google Scholar]
- Peters R. W., & Moore B. C. (1992). Auditory filter shapes at low center frequencies in young and elderly hearing-impaired subjects. The Journal of the Acoustical Society of America, 91(1), 256–266. [DOI] [PubMed] [Google Scholar]
- Qian J., & Eddins D. A. (2008). The role of spectral modulation cues in virtual sound localization. The Journal of the Acoustical Society of America, 123(1), 302–314. https://doi.org/10.1121/1.2804698 [DOI] [PubMed] [Google Scholar]
- Saoji A. A., & Eddins D. A. (2007). Spectral modulation masking patterns reveal tuning to spectral envelope frequency. The Journal of the Acoustical Society of America, 122(2), 1004–1013. https://doi.org/10.1121/1.2751267 [DOI] [PubMed] [Google Scholar]
- Saoji A. A., Litvak L., Spahr A. J., & Eddins D. A. (2009). Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. The Journal of the Acoustical Society of America, 126(3), 955–958. https://doi.org/10.1121/1.3179670 [DOI] [PubMed] [Google Scholar]
- Schmiedt R. A., Mills J. H., & Boettcher F. A. (1996). Age-related loss of activity of auditory-nerve fibers. Journal of Neurophysiology, 76(4), 2799–2803. [DOI] [PubMed] [Google Scholar]
- Schulte B. A., & Schmiedt R. A. (1992). Lateral Wall Na, K-Atpase and endocochlear potentials decline with age in quiet-reared gerbils. Hearing Research, 61(1–2), 35–46. https://doi.org/10.1016/0378-5955(92)90034-K [DOI] [PubMed] [Google Scholar]
- Shamma S. A., Fleshman J. W., Wiser P. R., & Versnel H. (1993). Organization of response areas in ferret primary auditory-cortex. Journal of Neurophysiology, 69(2), 367–383. [DOI] [PubMed] [Google Scholar]
- Shrivastav M. N., Humes L. E., & Kewley-Port D. (2006). Individual differences in auditory discrimination of spectral shape and speech-identification performance among elderly listeners. The Journal of the Acoustical Society of America, 119(2), 1131–1142. [DOI] [PubMed] [Google Scholar]
- Summers V., & Leek M. R. (1994). The internal representation of spectral contrast in hearing-impaired listeners. The Journal of the Acoustical Society of America, 95(6), 3518–3528. [DOI] [PubMed] [Google Scholar]
- Supin A. Y., Popov V. V., Milekhina O. N., & Tarakanov M. B. (1999). Ripple depth and density resolution of rippled noise. The Journal of the Acoustical Society of America, 106(5), 2800–2804. https://doi.org/10.1121/1.428105 [DOI] [PubMed] [Google Scholar]
- Vanveen T. M., & Houtgast T. (1985). Spectral sharpness and vowel dissimilarity. The Journal of the Acoustical Society of America, 77(2), 628–634. https://doi.org/10.1121/1.391880 [DOI] [PubMed] [Google Scholar]
- Viemeister N. F. (1979). Temporal modulation transfer functions based upon modulation thresholds. The Journal of the Acoustical Society of America, 66(5), 1364–1380. [DOI] [PubMed] [Google Scholar]
- Viemeister N. F., & Wakefield G. H. (1991). Temporal integration and multiple looks. The Journal of the Acoustical Society of America, 90(2), 858–865. https://doi.org/10.1121/1.401953 [DOI] [PubMed] [Google Scholar]
- Wightman F. L., McGee T., & Kramer M. (1977). Factors influencing frequency selectivity in normal and hearing impaired listeners. In Evans E. F. & Wilson J. P. (Eds.), Psychophysics and physiology of hearing (pp. 295–308). London, England: Academic. [Google Scholar]