Abstract
Purpose
The aim of this study was to examine the effects of frozen and mixed-consistency boluses on the swallowing physiology of younger and older adults. We also aimed to quantify factors that lead to increased variability in swallowing outcomes (i.e., age, sex, bolus type).
Method
Forty-one healthy adults (18–85 years old) swallowed 5 blocks of 5 different boluses: 10-ml ultrathin liquid, a teaspoon of iced barium, a teaspoon of room-temperature pudding, a teaspoon of frozen pudding, and ultrathin barium with chocolate chips. All data were recorded with videofluoroscopy and underwent detailed timing kinematic measurements.
Results
Neither barium ice nor frozen pudding sped up swallow responses. Many healthy adults initiated swallowing with the bolus as deep as the pyriform sinuses. Swallowing temporal kinematics for ultrathin liquid consistencies are most different from all others tested, requiring the best possible physiological swallowing performance in younger and older healthy individuals (i.e., faster reaction times, longer durations) compared with other bolus types tested. In each measure, older adults had significantly longer durations compared with the younger adults. More variability in swallowing kinematics were seen with age and laryngeal vestibule kinematics.
Conclusion
This study provides important contributions to the literature by clarifying normal variability within a wide range of swallowing behaviors and by providing normative data from which to compare disordered populations.
Dysphagia is commonly characterized by the inability to swallow a wide range of bolus types without significant aspiration or residue. Consequently, when a patient demonstrates disordered swallowing, clinicians often recommend bolus modifications that eliminate the most problematic bolus types (Curran & Groher, 1990; Leder, Judson, Sliwinski, & Madson, 2013). However, the underlying pathophysiology that is responsible for difficulty with certain bolus properties sometimes remains unknown and uninvestigated in clinical settings. This is because, in the clinical realm, there is an urgency to provide effective care to patients, so clinical practice often outpaces best evidence from scientific studies because patients require management immediately (Langmore, 1995; Langmore & Pisegna, 2015; Rosenbek, 1995). Still, assumptions in the clinical realm can drive practice patterns in dysphagia management in either appropriate or inappropriate ways. Therefore, evidence is needed to address the underlying reasons some bolus types might challenge swallowing physiology differently than others.
Frozen Boluses
Among healthy individuals, studies support the theory that the motor plan for a swallow is influenced by sensory input provided by the bolus, likely during the oral preparatory phase before swallow onset (Humbert, Lokhande, Christopherson, German, & Stone, 2012; Steele & Miller, 2010). Still, several unknowns remain regarding the relationship between bolus properties and the resulting swallowing physiology, including the variability in response to bolus consistencies within and between persons. For instance, patients on a nil per os (NPO or nothing by mouth) status are deemed to be at a high risk for aspiration pneumonia, malnutrition, and/or dehydration, yet clinicians commonly recommend intake of a frozen bolus (typically ice chips) for NPO patients (Carnaby & Harenberg, 2013). Heimlich (1983) stated that ice provides a substance that can be sensed in the mouth yet, if aspirated, can cause little harm. It has also been suggested that ingesting ice initiates oral feedings and, over time, helps patients to regain a normal diet (Heimlich, 1983). Anecdotally, ice might be recommended to NPO patients to encourage swallowing (prevent disuse atrophy), to prevent dry mouth, and to increase hydration. In addition, an iced bolus might increase thermal sensory input during a swallow to promote a more timely or robust swallow response. In addition, frozen boluses might enter the pharynx in smaller volumes and slower speeds because they are believed to become mixed consistencies during oral processing, thus increasing swallowing safety if swallowing reaction times (SRTs) are prolonged. Many of these assumptions about frozen boluses have not been vetted in the research literature. Similarly, as discussed below, the effectiveness of thermal stimulation (cold) and mixed bolus consistencies is not consistent across research studies.
Mixed Bolus Consistencies
Bolus consistency has also been found to alter swallowing physiology (Lee, Kim, Kim, & Lee, 2012; Mishellany, Woda, Labas, & Peyron, 2006; Saitoh et al., 2007). Mixed-consistency foods (e.g., cold cereal) are thought to pose an increased risk of aspiration in dysphagic individuals with impaired airway protection. With mixed consistencies, the solid portion of a mixed bolus is being manipulated orally, whereas the liquid might spill into the pharynx before the swallow is initiated. This spilling phenomenon is thought to occur if the tongue–palate seal is open, leading to early arrival of food (which needed chewing) into the hypopharynx (Okada et al., 2007; Palmer, Hiiemae, Matsuo, & Haishima, 2007). Opening of the tongue–palate seal might allow the bolus to spill into the pharynx before volitional initiation of the swallow, thereby increasing the risk of aspiration and possibly even acute obstruction of the airway. However, these findings were challenged by Lee et al. (2012), where 29 dysphagic patients were not at a higher risk of penetration or aspiration with a mixed consistency (cooked rice + thin liquid barium) compared with thin liquid.
Variability in Swallowing Function
Many of the discrepancies in the studies of the swallow response to cold stimuli and to mixed consistencies might be due to inherent variability in swallowing function within and across healthy and disordered populations that was not directly quantified in the results. Rosenbek, Roecker, Wood, and Robbins (1996) highlighted the variability across patients in the thermal–tactile stimulation study and its potential meaning for understanding swallowing mechanics. Healthy adults are also quite variable as swallowing demands change (Molfenter & Steele, 2011, 2012a, 2012b). Several motor control theories argue that the central nervous system can produce the same movement successfully in countless different ways (muscle patterns, kinematics; Brunner et al., 2011; Brunner & Hoole, 2012; Latash & Anson, 2006; Scholz & Schoner, 2014). Thus, variability can be good when a movement is successful. In swallowing, variability is vital to ensure that several appropriate movement plans exist so they can be applied to the manifold of possible swallowing demands (bolus type, body positions, swallowing command, and bolus delivery mode; Daniels, Schroeder, DeGeorge, Corey, & Rosenbek, 2007; Kahrilas, Lin, Chen, & Logemann, 1996; Kahrilas & Logemann, 1993; Logemann et al., 1992; Molfenter & Steele, 2012b, 2014; Nagy et al., 2013). Bad variability, however, might contribute to movement outcomes that are less successful, as seen with healthy aging (Hsu, Chou, & Woollacott, 2013; Rastatter, McGuire, Bushong, & Loposky, 1987). Many have concluded that the risk of dysphagia increases with age because of declines in strength and pressure reserve of muscles and oral sensation (Crow & Ship, 1996; Humbert & Robbins, 2008; Nicosia et al., 2000; Tracy et al., 1989). Thus, variability is a critical factor that should be considered in studies that focus on bolus characteristics and swallowing physiology; however, the extent to which this variability occurs in normal swallowing in different age groups and bolus types is unclear.
The primary goal of this article was to examine the effects of frozen and mixed-consistency boluses on the swallowing physiology of younger and older adults using videofluoroscopy. The barium boluses that were tested under videofluoroscopy include (a) barium ice, (b) ultrathin liquid, (c) room-temperature pudding, (d) frozen pudding, and (e) ultrathin liquid barium with chocolate chips. Given the aforementioned studies' concern over swallow onset times relative to the bolus flow of frozen or mixed consistencies, our primary research question was: Does cold temperature, mixed consistency, or the combination of both modify SRT (also known as swallow response time, swallow delay time, or stage transition duration)? We hypothesized that cold temperature of the same consistency (i.e., room-temperature vs. frozen pudding) would not impact SRTs because most healthy individuals may already swallow with shorter latencies compared with individuals with dysphagia; thus, a cold bolus would not hasten the swallow further (ceiling effect). However, we expected that mixed consistencies (i.e., ice, thin liquid barium plus chocolate chips) would lead to longer delays in SRTs compared with boluses with only one consistency. The rationale for this hypothesis is that mixed consistencies (solid and thin consistency) likely involve oral preparation to break down the solid bolus while thinner liquids enter the pharynx, as previously described by Saitoh et al. (2007) and Palmer et al. (2007). Although ice chips are thought to lead to a robust swallow response because of the cold temperature, we hypothesized that its mixed consistency (partially melting from solid to thin) would lead to early entry of the thin bolus into the pharynx; thus, SRTs would be more influenced by mixed consistency behavior rather than perceived cold stimuli effects. Our secondary goal was to quantify factors that lead to increased variability in swallowing outcomes (i.e., age, sex, bolus type). We expected that older adults would have greater variability in swallowing behaviors, as shown in other sensorimotor systems (Kwon, Baweja, & Christou, 2011; Enoka et al., 2003). The outcomes of this study are important contributions to the literature because they clarify normal variability within a wide range of swallowing behaviors and can serve as normative data from which to compare disordered populations.
Method
Participants
The local institutional review board approved all procedures in this study. Forty-one healthy adults participated in the study (M = 55.3 years old, range = 18–85 years old; age and sex distributions in Table 1). Participants were stratified into two groups: younger healthy adults (N = 20; 12 women and eight men, M age = 36.1 years, age range = 18–58 years) and older healthy adults (N = 21; 11 women and 10 men, mean age = 73.5 years, age range = 61–85 years). Six of the older participants reported having dentures and wore them during the study procedures. Older participants were 60 years or greater because age-related changes in swallowing physiology (presbyphagia) become more apparent after the age of 60 years (Robbins, Levine, Wood, Roecker, & Luschei, 1995). In the patient screening process, all participants denied having previous or current speech or swallowing impairments. This included no history of surgery or treatment to oropharyngeal structures involved in swallowing as well as cervical spinal impairments. All participants denied having head or neck cancer, neurological or psychological disorders, or any condition that could impact the structures involved in swallowing. Before participation, written informed consent was provided, and each individual reported a negative history of swallowing, speech and voice disorders, neurological disease, and oropharyngeal surgeries that could impact swallowing.
Table 1.
Age and sex distribution.
| Age (years) | Group | M/F |
|---|---|---|
| 18 | Younger | F |
| 19 | Younger | F |
| 20 | Younger | F |
| 21 | Younger | F |
| 22 | Younger | F |
| 22 | Younger | M |
| 23 | Younger | F |
| 23 | Younger | F |
| 26 | Younger | F |
| 32 | Younger | F |
| 33 | Younger | F |
| 35 | Younger | F |
| 37 | Younger | M |
| 47 | Younger | M |
| 51 | Younger | M |
| 52 | Younger | M |
| 52 | Younger | M |
| 53 | Younger | M |
| 55 | Younger | M |
| 56 | Younger | F |
| 58 | Younger | F |
| 61 | Older | F |
| 67 | Older | M |
| 68 | Older | F |
| 68 | Older | F |
| 70 | Older | F |
| 70 | Older | F |
| 70 | Older | M |
| 70 | Older | M |
| 71 | Older | M |
| 72 | Older | M |
| 72 | Older | M |
| 75 | Older | F |
| 75 | Older | F |
| 75 | Older | M |
| 77 | Older | M |
| 77 | Older | F |
| 79 | Older | F |
| 79 | Older | F |
| 79 | Older | M |
| 84 | Older | F |
| 85 | Older | M |
Note. F = female; M = male.
Study Design
Participants swallowed five trials of five different Varibar barium bolus types (25 total swallows), including (a) 10-ml room-temperature ultrathin liquid barium by straw, which is closer in consistency to water (51 g of Varibar thin liquid barium powder and 240-ml water; T. A. Fink & Ross, 2009; Steele, Molfenter, Peladeau-Pigeon, & Stokely, 2013); (b) a teaspoon of barium ice chips (frozen ultrathin liquid barium); (c) a heaping teaspoon of room-temperature barium pudding; (d) a heaping teaspoon of frozen barium pudding; and (e) a teaspoon of room-temperature thin liquid barium with mini chocolate chips (mixed consistency). Although participants obtained ultrathin liquid into the oral cavity via straw, they were asked to hold the bolus until cued; thus, this task might not be defined as typical straw drinking. In addition, 10 ml was selected for ultrathin because it is similar to the sip volume obtained among healthy adults (Jones & Work, 1961). The order for each participant was randomized by blocks of the same swallow type (i.e., five thin liquid, five pudding, five ice chips). The investigators measured bolus volumes using widely practiced clinical approaches (i.e., heaping teaspoon as opposed to exact bolus weight) so that the outcomes of this study can be more readily generalized to the clinical domain. To minimize radiation exposure time to healthy participants, all swallows were cued to synchronize recordings with oral preparation and swallowing behaviors.
Videofluoroscopy
All swallows were captured with continuous videofluoroscopy using a Siemens Axiom Sireskop SD in real time (30 pulses per second), acquired in the lateral plane. The field of view included the oral cavity, velum, pharynx, hyoid bone, larynx, upper esophageal sphincter (UES), cervical esophagus, and cervical vertebrae. Each swallow trial was randomly assigned a four-digit number to facilitate blinded analyses. The images were collected with TIMS Medical imaging equipment. Those files were converted to .mov format and analyzed on a computer using QuickTime Version 7, which displays a frame-by-frame counter that was used to identify each kinematic event noted below.
Kinematic Measures
Means and standard deviations were derived to determine effects across our study questions. Nine measures were used to investigate several bolus flow and kinematic events during swallowing, which are discussed below.
Primary Outcome Measure
SRT also known as stage transition duration and swallow response time: interval between the bolus entry into the pharynx (the first frame that the bolus head passes the ramus of the mandible) and the onset of anterior–superior hyoid excursion (hyoid burst).
Secondary Outcome Measures
These measures were taken to more fully understand whether swallowing kinematics (postinitiation) were further influenced by frozen or mixed-consistency bolus types.
Duration to maximum hyoid elevation: interval between hyoid burst and the first frame when the hyoid reaches its highest elevation point (y-axis).
Duration of laryngeal vestibule closure (dLVC): interval between the first frame of laryngeal vestibule closure (LVC) and the first frame of laryngeal vestibule reopening during the swallow.
LVC reaction time (bolus; LVCrt-B): interval between bolus entry into the pharynx (the first frame that the bolus head passes the ramus of the mandible) and the first frame of LVC.
LVCrt (hyoid; LVCrt-H): interval between hyoid burst and the first frame of LVC.
Duration of UES opening (dUESO): interval between the first frame of UES opening and the first frame of UES closure.
UES opening reaction time (UESOrt; bolus): interval between bolus entry into the pharynx (the first frame that the bolus head passes the ramus of the mandible) and the first frame of UES opening.
UESOrt (hyoid; UESOrt-H): interval between hyoid burst and the first frame of UES opening.
Pharyngeal transit time (PTT): interval between bolus entry into the pharynx (the first frame that the bolus head passes the ramus of the mandible) and the first frame that the bolus tail passes through the UES.
Location of Bolus Head
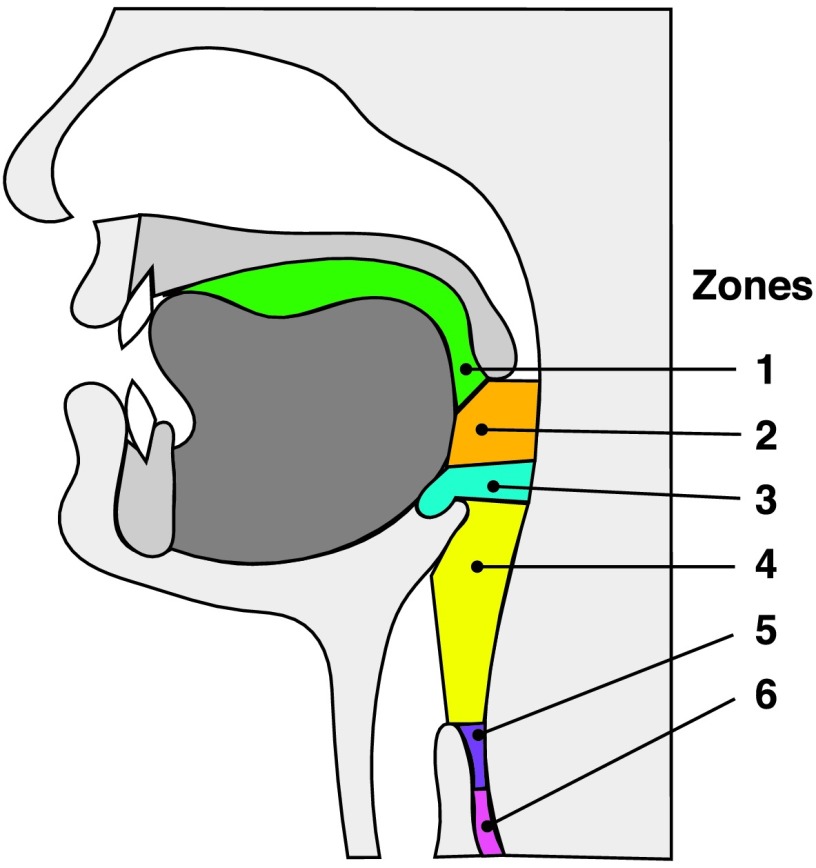
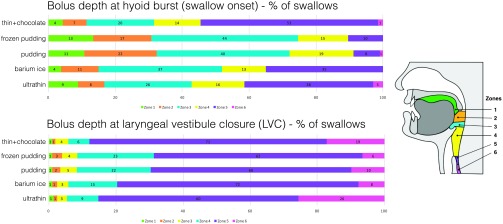
Swallowing safety can be impacted by the bolus position at critical periods during the swallow (Steele & Cichero, 2014). We considered the time of hyoid burst (swallow onset) and the time of LVC to be important events for swallowing safety. Thus, we also derived the location of the leading edge of the bolus head (six different zones) at the time of hyoid burst and LVC. The visual depth of the bolus is a more clinically relevant way to assess swallow onset than traditional kinematic measures described above (i.e., such as LVCrt and SRT) and is an alternate way to assess SRTs relative to bolus position (Martin-Harris, Brodsky, Michel, Lee, & Walters, 2007). The six zones, depicted in Figure 1, included the following:
Figure 1.
Zones used to determine bolus depth at the time of hyoid burst and laryngeal vestibule closure.
Oral cavity (anterior/superior to the ramus of the mandible)
Between the ramus of the mandible and valleculae
Within the valleculae
Between valleculae and pyriform sinuses (adjacent to airway)
Pyriform sinuses
Inferior to the UES (esophagus)
Penetration–Aspiration Scores
The Penetration–Aspiration Scale (PAS; Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996) was used to score the occurrence of penetration or aspiration. The PAS is a widely used severity measure for bolus entry into the airway. Scores of 1 and 2 are considered normal (Allen, White, Leonard, & Belafsky, 2010; Daggett, Logemann, Rademaker, & Pauloski, 2006; Robbins, Coyle, Rosenbek, Roecker, & Wood, 1999). Scores of 3–5 indicate that the material has entered the airway, remains above the vocal folds (penetration), or has passed below the vocal folds (score of 6–8 = aspiration). The percentage of each PAS score (1–8) was derived for each of the five bolus types.
Statistical Analyses
For statistical analysis of kinematic timing variables, the mean and standard deviation of each of the 10 variables were calculated for each individual-consistency (bolus type) combination, so that the unit of analysis was by individual response to consistency. Mean and standard deviation were then analyzed separately using a general linear model approach for each variable, with age, consistency, sex, and all interactions included. This approach tested for both changes in central tendency and changes in variation of each variable. When the standard deviation was derived and included in the statistical model, it accounted for variability among the five trials within a bolus type condition rather than only the means of a bolus type across participants.
When the consistency main effect was significant, pairwise Tukey's honestly significant difference tests were used to determine which pairs of consistencies differed. Results are reported graphically using least squares means. This analysis was conducted with SYSTAT.
For statistical analysis of ordinal data for leading head of the bolus in various zones, first, in a manner analogous to the other variables, the modes of both bolus head at the time of hyoid burst and at the time of LVC were derived for each individual/consistency combination to reduce pseudoreplication. To test for an effect of the factors on the two ordinal variables, ordered logistic regression was used, with all three fixed factors and all interactions, as parameters, in a manner analogous to the analysis with linear models for the timing kinematic variables. An ordered logistic regression is the best for ordinal data, as it both accounts for the discontinuity of the data (the reason linear methods cannot be used) and incorporates the information contained in the fact that the dependent variable is ordered (making it preferable to a standard multinomial logistic regression). The best-fit ordered logistic regression was calculated to test the hypothesis that the parameters of the model (the fixed effects and their interactions) were different from zero as a means of identifying which factors were important for the model. This analysis was conducted with R.
The raters (S. K. and A. V.) had at least 2 years of experience with swallowing kinematic analysis. Ratings were completed while blinded to the bolus type and age whenever possible. However, it should be noted that certain consistencies can likely be differentiated subjectively on videofluoroscopy because of speed of flow (i.e., pudding vs. ultrathin). Similarly, the shape of the cervical vertebrae and condition of dentition can also lead to subjective differentiation between the younger and older cohorts. To test reliability of the kinematic measurements, interrater (20% of data) and intrarater (10% of data) reliability analyses were conducted by randomly selecting swallows from the complete data set.
Results
One thousand fifteen swallows were analyzed. Ten swallows were eliminated when the videofluoroscopic image was too dark or when the participant swallowed before the onset of videofluoroscopic recording. All subjects completed the study without adverse effects. Interrater and intrarater reliabilities were good to excellent (Table 2). Bolus type differences discussed below represent the mean of the five boluses within a bolus type condition (i.e., ultrathin Boluses 1–5).
Table 2.
Reliability table for analyses of kinematics and zones.
| Outcome Variable | Intrarater | Lower bound | Upper bound | Significance | Interrater | Lower bound | Upper bound | Significance |
|---|---|---|---|---|---|---|---|---|
| Kinematics | ||||||||
| SRT | .97 | .95 | .98 | p ≤ .001 | .97 | .95 | .98 | p ≤ .001 |
| dLVC | .92 | .88 | .94 | p ≤ .001 | .92 | .88 | .94 | p ≤ .001 |
| LVCrt-B | .97 | .95 | .98 | p ≤ .001 | .97 | .95 | .98 | p ≤ .001 |
| LVCrt-H | .70 | .58 | .79 | p ≤ .001 | .80 | .73 | .83 | p ≤ .001 |
| dtMHE | .97 | .95 | .98 | p ≤ .001 | .70 | .58 | .73 | p ≤ .001 |
| UESOrt-B | .97 | .96 | .98 | p ≤ .001 | .97 | .96 | .98 | p ≤ .001 |
| UESOrt-H | .94 | .91 | .96 | p ≤ .001 | .94 | .91 | .96 | p ≤ .001 |
| PTT | .97 | .96 | .98 | p ≤ .001 | .97 | .96 | .98 | p ≤ .001 |
| dUESO | .99 | 1.00 | 1.00 | p ≤ .001 | .84 | .79 | .87 | p ≤ .001 |
| Zones | ||||||||
| Bolus position at hyoid | .90 | .85 | .93 | p ≤ .001 | .93 | .91 | .95 | p ≤ .001 |
| Bolus position at LVC | .74 | .63 | .82 | p ≤ .001 | .89 | .85 | .91 | p ≤ .001 |
Note. Confidence intervals (95% lower and upper bounds) and statistical significance (p values) are also shown. SRT = swallowing response time; dLVC = duration of laryngeal vestibule closure; LVC = laryngeal vestibule closure; LVCrt-B = laryngeal vestibule closure reaction time (bolus); LVCrt-H = laryngeal vestibule closure reaction time (hyoid); dtMHE = duration to maximum hyoid elevation; UESOrt-B = upper esophageal sphincter opening reaction time (bolus); UESOrt-H = upper esophageal sphincter opening reaction time (hyoid); PTT = pharyngeal transit time; dUESO = duration of upper esophageal opening.
Kinematic Measure Outcomes
Does Cold Temperature, Mixed Consistency, or the Combination of Both Modify SRT (Primary Question)?
We hypothesized that cold temperatures would not impact SRTs, but mixed consistencies (i.e., ice, thin liquid barium plus chocolate chips) would lead to longer delays in SRTs compared with boluses with only one consistency. SRT had significant fixed effects for consistency (p < .004; F ratio = 4.1). Pairwise comparisons revealed that ultrathin liquids (212.1 ms) had shorter SRTs compared with barium ice chips (1101 ms; p < .007), frozen pudding (1070 ms; p < .01), and the thin liquid with chocolate chips mix (1064.1 ms; p < .011) but were not different than room-temperature pudding (827 ms; p = .839; Tables 3 –5, Figure 2).
Table 3.
Fixed effects for consistency, age, sex, and interactions.
| Main effects | |||
|---|---|---|---|
| Temporal measure | Factor | F ratio | p value |
| Swallowing reaction time | Consistency | 4.055 | .004 |
| Swallowing reaction time | Sex | 5.972 | .012 |
| Swallowing reaction time | Age | 6.514 | .015 |
| Swallowing reaction time | Consistency × Sex | 0.607 | .658 |
| Swallowing reaction time | Consistency × Age | 1.622 | .171 |
| Swallowing reaction time | Sex × Age | 0.057 | .811 |
| Swallowing reaction time | Consistency × Age × Sex | 0.167 | .955 |
| Duration of laryngeal vestibule closure | Consistency | 4.303 | .002 |
| Duration of laryngeal vestibule closure | Sex | 1.485 | .225 |
| Duration of laryngeal vestibule closure | Age | 8.171 | .005 |
| Duration of laryngeal vestibule closure | Consistency × Sex | 1.006 | .406 |
| Duration of laryngeal vestibule closure | Consistency × Age | 0.595 | .666 |
| Duration of laryngeal vestibule closure | Sex × Age | 2.068 | .152 |
| Duration of laryngeal vestibule closure | Consistency × Age × Sex | 0.219 | .927 |
| Laryngeal vestibule closure reaction time (bolus) | Consistency | 4.215 | .003 |
| Laryngeal vestibule closure reaction time (bolus) | Sex | 7.360 | .007 |
| Laryngeal vestibule closure reaction time (bolus) | Age | 6.938 | .009 |
| Laryngeal vestibule closure reaction time (bolus) | Consistency × Sex | 0.630 | .642 |
| Laryngeal vestibule closure reaction time (bolus) | Consistency × Age | 1.644 | .165 |
| Laryngeal vestibule closure reaction time (bolus) | Sex × Age | 0.130 | .718 |
| Laryngeal vestibule closure reaction time (bolus) | Consistency × Age × Sex | 0.192 | .942 |
| Laryngeal vestibule closure reaction time (hyoid) | Consistency | 1.172 | .324 |
| Laryngeal vestibule closure reaction time (hyoid) | Sex | 6.683 | .011 |
| Laryngeal vestibule closure reaction time (hyoid) | Age | 9.701 | .002 |
| Laryngeal vestibule closure reaction time (hyoid) | Consistency × Sex | 0.146 | .964 |
| Laryngeal vestibule closure reaction time (hyoid) | Consistency × Age | 0.147 | .964 |
| Laryngeal vestibule closure reaction time (hyoid) | Sex × Age | 4.271 | .040 |
| Laryngeal vestibule closure reaction time (hyoid) | Consistency × Age × Sex | 0.842 | .500 |
| Duration to maximum hyoid elevation | Consistency | 0.362 | .836 |
| Duration to maximum hyoid elevation | Sex | 3.571 | .060 |
| Duration to maximum hyoid elevation | Age | 49.985 | .001 |
| Duration to maximum hyoid elevation | Consistency × Sex | 0.133 | .970 |
| Duration to maximum hyoid elevation | Consistency × Age | 0.362 | .835 |
| Duration to maximum hyoid elevation | Sex × Age | 0.302 | .583 |
| Duration to maximum hyoid elevation | Consistency × Age × Sex | 1.095 | .360 |
| UES opening reaction time (bolus) | Consistency | 4.555 | .002 |
| UES opening reaction time (bolus) | Sex | 6.932 | .009 |
| UES opening reaction time (bolus) | Age | 7.203 | .008 |
| UES opening reaction time (bolus) | Consistency × Sex | 0.634 | .639 |
| UES opening reaction time (bolus) | Consistency × Age | 1.615 | .172 |
| UES opening reaction time (bolus) | Sex × Age | 0.092 | .762 |
| UES opening reaction time (bolus) | Consistency × Age × Sex | 0.165 | .956 |
| UES opening reaction time (hyoid) | Consistency | 11.361 | .001 |
| UES opening reaction time (hyoid) | Sex | 2.520 | .114 |
| UES opening reaction time (hyoid) | Age | 26.657 | .001 |
| UES opening reaction time (hyoid) | Consistency × Sex | 0.328 | .859 |
| UES opening reaction time (hyoid) | Consistency × Age | 0.096 | .984 |
| UES opening reaction time (hyoid) | Sex × Age | 2.021 | .157 |
| UES opening reaction time (hyoid) | Consistency × Age × Sex | 0.607 | .658 |
| Pharyngeal transit time | Consistency | 3.719 | .006 |
| Pharyngeal transit time | Sex | 6.389 | .012 |
| Pharyngeal transit time | Age | 8.165 | .005 |
| Pharyngeal transit time | Consistency × Sex | 0.649 | .628 |
| Pharyngeal transit time | Consistency × Age | 1.688 | .155 |
| Pharyngeal transit time | Sex × Age | 0.182 | .670 |
| Pharyngeal transit time | Consistency × Age × Sex | 0.177 | .950 |
| Duration of UES opening | Consistency | 8.520 | .001 |
| Duration of UES opening | Sex | 11.979 | .001 |
| Duration of UES opening | Age | 12.987 | .001 |
| Duration of UES opening | Consistency × Sex | 0.032 | .998 |
| Duration of UES opening | Consistency × Age | 0.270 | .897 |
| Duration of UES opening | Sex × Age | 0.563 | .454 |
| Durations of UES opening | Consistency × Age × Sex | 0.173 | .952 |
| Bolus head location at hyoid burst | Consistency | 13.750 | .469 |
| Bolus head location at hyoid burst | Sex | 0.441 | .831 |
| Bolus head location at hyoid burst | Age | 2.594 | .474 |
| Bolus head location at hyoid burst | Consistency × Sex | 1.095 | .659 |
| Bolus head location at hyoid burst | Consistency × Age | 0.660 | .680 |
| Bolus head location at hyoid burst | Sex × Age | 1.337 | .228 |
| Bolus head location at hyoid burst | Consistency × Age × Sex | 1.253 | .298 |
| Bolus head at LVC | Consistency | 3.697 | .567 |
| Bolus head at LVC | Sex | 0.691 | .056 |
| Bolus head at LVC | Age | 2.447 | .360 |
| Bolus head at LVC | Consistency × Sex | 0.560 | .193 |
| Bolus head at LVC | Consistency × Age | 0.398 | .682 |
| Bolus head at LVC | Sex × Age | 1.338 | .025 |
| Bolus head at LVC | Consistency × Age × Sex | 0.524 | .153 |
Note. Bolded text indicates statistically significant outcomes. UES = upper esophageal sphincter; LVC = laryngeal vestibule closure.
Table 4.
Significant pairwise comparisons for consistency comparisons including 95% confidence intervals.
| Swallowing reaction time | p value | 95% Confidence interval |
|
|---|---|---|---|
| Lower | Upper | ||
| Ultrathin vs. barium ice | .007 | −48.514 | −5.380 |
| Ultrathin vs. pudding | .134 | −40.230 | 2.903 |
| Ultrathin vs. frozen pudding | .010 | −47.568 | −4.435 |
| Ultrathin vs. thin + chocolate | .011 | −47.386 | −4.252 |
| Barium ice vs. pudding | .839 | −13.283 | 29.850 |
| Barium ice vs. frozen pudding | 1.000 | −20.621 | 22.512 |
| Barium ice vs. thin + chocolate | 1.000 | −20.438 | 22.695 |
| Pudding vs. frozen pudding | .890 | −28.904 | 14.229 |
| Pudding vs. thin + chocolate | .899 | −28.722 | 14.411 |
| Frozen pudding vs. thin + chocolate | 1.000 | −21.384 | 21.749 |
| Duration of laryngeal vestibule closure | |||
| Ultrathin vs. barium ice | .021 | 0.341 | 6.041 |
| Ultrathin vs. pudding | .087 | −0.192 | 5.508 |
| Ultrathin vs. frozen pudding | .051 | 0.024 | 5.724 |
| Ultrathin vs. thin + chocolate | .001 | 1.307 | 7.007 |
| Barium ice vs. pudding | .987 | −3.383 | 2.317 |
| Barium ice vs. frozen pudding | .998 | −3.167 | 2.533 |
| Barium ice vs. thin + chocolate | .892 | −1.884 | 3.816 |
| Pudding vs. frozen pudding | 1.000 | −2.633 | 3.066 |
| Pudding vs. thin + chocolate | .615 | −1.351 | 4.349 |
| Frozen pudding vs. thin + chocolate | .743 | −1.567 | 4.133 |
| Laryngeal vestibule closure reaction time (bolus) | |||
| Ultrathin vs. barium ice | .005 | −49.164 | −6.155 |
| Ultrathin vs. pudding | .119 | −40.545 | 2.464 |
| Ultrathin vs. frozen pudding | .008 | −48.129 | −5.120 |
| Ultrathin vs. thin + chocolate | .011 | −47.227 | −4.218 |
| Barium ice vs. pudding | .816 | −12.885 | 30.124 |
| Barium ice vs. frozen pudding | 1.000 | −20.469 | 22.540 |
| Barium ice vs. thin + chocolate | .999 | −19.567 | 23.442 |
| Pudding vs. frozen pudding | .877 | −29.089 | 13.920 |
| Pudding vs. thin + chocolate | .919 | −28.187 | 14.822 |
| Frozen pudding vs. thin + chocolate | 1.000 | −20.603 | 22.406 |
| Laryngeal vestibule closure reaction time (hyoid) | |||
| Ultrathin vs. barium ice | .001 | 1.146 | 3.847 |
| Ultrathin vs. pudding | .001 | 0.585 | 3.287 |
| Ultrathin vs. frozen pudding | .001 | 1.070 | 3.771 |
| Ultrathin vs. thin + chocolate | .001 | 0.832 | 3.533 |
| Barium ice vs. pudding | .796 | −1.911 | 0.790 |
| Barium ice vs. frozen pudding | 1.000 | −1.427 | 1.275 |
| Barium ice vs. thin + chocolate | .971 | −1.665 | 1.037 |
| Pudding vs. frozen pudding | .870 | −0.866 | 1.836 |
| Pudding vs. thin + chocolate | .988 | −1.104 | 1.597 |
| Frozen pudding vs. thin + chocolate | .990 | −1.589 | 1.112 |
| Duration to maximum hyoid elevation | |||
| Ultrathin vs. barium ice | .003 | −50.186 | −7.194 |
| Ultrathin vs. pudding | .082 | −41.739 | 1.253 |
| Ultrathin vs. frozen pudding | .004 | −49.531 | −6.539 |
| Ultrathin vs. thin + chocolate | .008 | −47.897 | −4.905 |
| Barium ice vs. pudding | .827 | −13.049 | 29.943 |
| Barium ice vs. frozen pudding | 1.000 | −20.841 | 22.151 |
| Barium ice vs. thin + chocolate | .999 | −19.207 | 23.785 |
| Pudding vs. frozen pudding | .865 | −29.288 | 13.704 |
| Pudding vs. thin + chocolate | .939 | −27.654 | 15.338 |
| Frozen pudding vs. thin + chocolate | 1.000 | −19.862 | 23.130 |
| UES opening reaction time (bolus) | |||
| Ultrathin vs. barium ice | .003 | −50.186 | −7.194 |
| Ultrathin vs. pudding | .082 | −41.739 | 1.253 |
| Ultrathin vs. frozen pudding | .004 | −49.531 | −6.539 |
| Ultrathin vs. thin + chocolate | .008 | −47.897 | −4.905 |
| Barium ice vs. pudding | .827 | −13.049 | 29.943 |
| Barium ice vs. frozen pudding | 1.000 | −20.841 | 22.151 |
| Barium ice vs. thin + chocolate | .999 | −19.207 | 23.785 |
| Pudding vs. frozen pudding | .865 | −29.288 | 13.704 |
| Pudding vs. thin + chocolate | .939 | −27.654 | 15.338 |
| Frozen pudding vs. thin + chocolate | 1.000 | −19.862 | 23.130 |
| UES opening reaction time (hyoid) | |||
| Ultrathin vs. barium ice | .001 | −2.702 | −0.765 |
| Ultrathin vs. pudding | .001 | −2.539 | −0.601 |
| Ultrathin vs. frozen pudding | .001 | −2.993 | −1.055 |
| Ultrathin vs. thin + chocolate | .501 | −1.542 | 0.396 |
| Barium ice vs. pudding | .991 | −0.805 | 1.132 |
| Barium ice vs. frozen pudding | .928 | −1.260 | 0.678 |
| Barium ice vs. thin + chocolate | .011 | 0.192 | 2.130 |
| Pudding vs. frozen pudding | .713 | −1.423 | 0.515 |
| Pudding vs. thin + chocolate | .044 | 0.029 | 1.966 |
| Frozen pudding vs. thin + chocolate | .001 | 0.483 | 2.420 |
| Pharyngeal transit time | |||
| Ultrathin vs. barium ice | .011 | −47.758 | −4.359 |
| Ultrathin vs. pudding | .159 | −39.857 | 3.542 |
| Ultrathin vs. frozen pudding | .012 | −47.460 | −4.062 |
| Ultrathin vs. thin + chocolate | .024 | −45.649 | −2.251 |
| Barium ice vs. pudding | .864 | −13.798 | 29.600 |
| Barium ice vs. frozen pudding | 1.000 | −21.402 | 21.996 |
| Barium ice vs. thin + chocolate | .999 | −19.591 | 23.807 |
| Pudding vs. frozen pudding | .879 | −29.303 | 14.095 |
| Pudding vs. thin + chocolate | .952 | −27.492 | 15.906 |
| Frozen pudding vs. thin + chocolate | .999 | −19.888 | 23.510 |
Note. Bolded text indicates statistically significant outcomes. UES = upper esophageal sphincter.
Table 5.
Means and standard deviation for each kinematic measure across all bolus consistencies.
| All | Ultrathin liquid | Barium ice chips | Room-temperature pudding | Frozen pudding | Ultrathin + chocolate |
|---|---|---|---|---|---|
| Swallowing reaction time | 212.12 (±579.54) | 1101.31 (±1590.06) | 827.97 (±1448.47) | 1070.12 (±1410.51) | 1064.09 (±2791.52) |
| Duration of laryngeal vestibule closure | 603.70 (±272.53) | 498.43 (±137.28) | 516.02 (±123.23) | 508.86 (±123.32) | 466.52 (±131.45) |
| Laryngeal vestibule closure reaction time (bolus) | 307.07 (±576.79) | 1219.81 (±1601.71) | 935.39 (±1453.58) | 1185.66 (±1421.71) | 1155.89 (±2800.52) |
| Laryngeal vestibule closure reaction time (hyoid) | 95.27 (±53.7) | 118.50 (±40.5) | 107.42 (±37.4) | 115.566 (±45.6) | 91.806 (±51.5) |
| Duration to maximum hyoid elevation | 115.698 (±43.6) | 99.957 (±43.1) | 107.58 (±44.8) | 109.824 (±44.5) | 109.296 (±49.2) |
| UES opening reaction time (bolus) | 333.30 (±583.34) | 1280.04 (±1599.48) | 1001.29 (±1442.60) | 1258.42 (±1406.15) | 1204.50 (±2796.99) |
| UES opening reaction time (hyoid) | 121.51 (±75.63) | 178.73 (±73.64) | 173.35 (±74.57) | 188.33 (±87.59) | 140.42 (±62.63) |
| Pharyngeal transit time | 789.62 (±603.58) | 1649.54 (±1612.95) | 1388.81 (±1460.10) | 1639.74 (±1419.95) | 1579.97 (±2809.85) |
| Duration of UES opening | 429.53 (±87.44) | 347.13 (±80.32) | 365.64 (±83.01) | 349.64 (±85.76) | 357.52 (±85.50) |
Note. UES = upper esophageal sphincter.
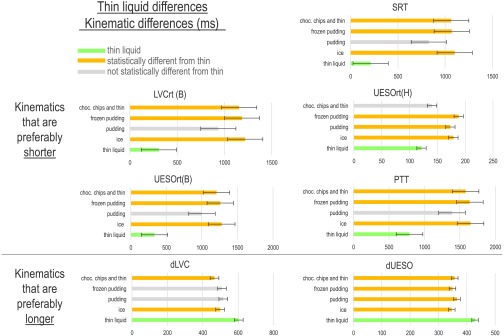
Figure 2.
Statistically significant consistency differences for each temporal measure. Error bars indicate standard error. SRT = swallowing reaction time; LVCrt = laryngeal vestibule closure reaction time; UESOrt(H) = upper esophageal opening reaction time (hyoid); UESOrt(B) = upper esophageal opening reaction time (bolus); PTT = pharyngeal transit time; dLVC = duration of laryngeal vestibule closure; dUESO = duration of upper esophageal opening.
In addition to SRT, fixed effects revealed that every other kinematic measure included in this study, except duration to maximum hyoid elevation and LVCrt relative to hyoid burst (LVCrt-H), was different when bolus types were compared (see Table 4). In particular, and similar to SRT, the ultrathin liquid bolus was most different from other bolus types. These include longer dLVCs for ultrathin liquid (604 ms) compared with barium ice chips (498 ms; p = .021) and compared with the thin liquid with chocolate chips mix (467 ms; p = .001). LVCrt (relative to the bolus head reaching the ramus of the mandible) was shorter for ultrathin liquid (307 ms) compared with barium ice chips (1220 ms; p < .005), frozen pudding (1186 ms; p < .008), and the thin liquid with chocolate chips mix (1156 ms; p < .011). UESOrt (relative to the bolus head reaching the ramus of the mandible) was also shorter for ultrathin liquid (333 ms) compared with barium ice chips (1280 ms; p < .003), frozen pudding (1258 ms; p < .004), and the thin liquid with chocolate chips mix (1205 ms; p < .008). UESOrt (relative to hyoid burst) was also shorter for ultrathin liquid (121.5 ms) compared with barium ice chips (178.7 ms; p < .001), frozen pudding (188.3 ms; p < .001), and room-temperature pudding (178.3 ms; p < .001). For PTT, ultrathin liquid was fastest (790 ms) compared with barium ice chips (1650 ms; p < .011), frozen pudding (1640 ms; p < .012), and the thin liquid with chocolate chips mix (1580 ms; p < .024). dUESO followed a similar trend, where ultrathin liquid was most different. In particular, ultrathin liquid swallows had the longest dUESOs (430 ms) compared with all other bolus types (p < .001; barium ice chips: 347 ms, room-temperature pudding: 366 ms, frozen pudding: 350 ms, thin liquid with chocolate chips: 357 ms).
The only other significant kinematic outcome that was not driven primarily by ultrathin liquids includes UESOrt (relative to hyoid burst onset). The thin liquid with chocolate chips mix had a faster UESOrt relative to hyoid burst (140 ms) than barium ice chips (p = .011), room-temperature pudding (p = .044), and frozen pudding (p = .001). All means and standard deviation can be found in Table 5.
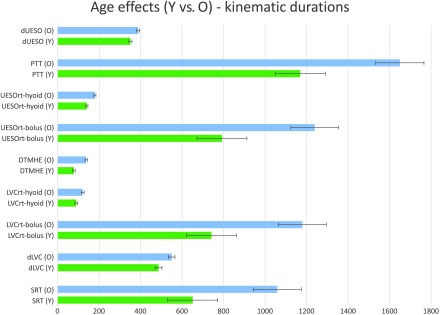
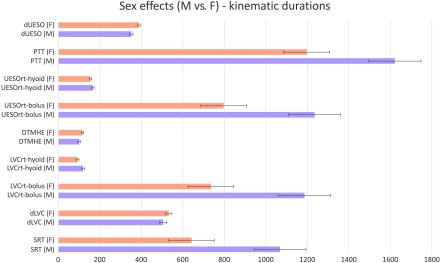
Age and Sex Effects
Age impacted every kinematic measure that was tested (all main effects, p ≤ .015). In each measure, older adults had significantly longer durations compared with the younger adults (Figure 3). Sex impacted six of eight kinematic measures (all main effects, p ≤ .012), including SRT, LVCrt-H, LVCrt-B, UESOrt (bolus), PTT, and dUESO. In each of these six measures, except dUESO, males had longer durations than females (Figure 4). A significant Sex × Age interaction was found for only LVCrt-H (p = .04; Table 3).
Figure 3.
Age effects for younger (Y) and older (O) healthy participants for each kinematic measure. Error bars indicate standard error. dUESO = duration of upper esophageal opening; PTT = pharyngeal transit time; UESOrt = upper esophageal opening reaction time; DTMHE = duration to maximum hyoid elevation; LVCrt = laryngeal vestibule closure reaction time; dLVC = duration of laryngeal vestibule closure; SRT = swallowing reaction time.
Figure 4.
Sex effects for female (F) and male (M) healthy participants for each kinematic measure. Error bars indicate standard error. dUESO = duration of upper esophageal opening; PTT = pharyngeal transit time; UESOrt = upper esophageal opening reaction time; DTMHE = duration to maximum hyoid elevation; LVCrt = laryngeal vestibule closure reaction time; dLVC = duration of laryngeal vestibule closure; SRT = swallowing reaction time.
Location of Bolus Head Outcomes
A significant Sex × Age interaction was found (p = .025), but none survived the pairwise comparisons (Figure 5, Table 3).
Figure 5.
Percentage of swallows across the six bolus depth zones at the time of hyoid burst (swallow onset) and laryngeal vestibule closure.
Variability Findings
Age
When examining the effect of age on swallow variability, there were several findings. Multiple swallow durations were noted to be more variable in the older participants' swallows as compared with those of the younger participants. dLVC showed a significantly higher standard deviation for older adults as compared with younger adults (M = 14.36, p = .005). This is indicative of greater variability seen in this measure for older adults' swallows. LVCrt relative to the bolus (LVCrt-B) had significantly greater variability in older versus younger participants (M = 13.19, p = .003), as did UESOrt-H (M = 5.16, p = .028). In addition, a significant age effect was found for location of bolus head at LVC, characterized by greater variability in older adults (M = 1.73, p = .006; Table 3).
Sex and Consistency
When looking at the effect of sex on swallow variability, there was only one significant finding. Females were found to be more variable for measures of bolus head at LVC (M = 1.60, p = .008). With consistency, there was a significant effect found for ultrathin liquid boluses. When compared with room-temperature pudding consistencies, ultrathin liquid was found to result in greater variability for dLVC measures (M = 4.26, p = .048).
Penetration–Aspiration Scores
It has been reported that penetration–aspiration (PA) scores of 1 and 2 are considered normal for healthy participants (Martin-Harris et al., 2007; Robbins et al., 1999; Rosenbek, Robbins, et al., 1996). Across all ultrathin liquid swallows, a PA score of 1 was recorded for 88% of swallows, a score of 2 was reported for 10% of swallows, and a PA score of 3 or 4 was each reported for 1% of swallows. For the barium ice chip swallows, a PA score of 1 was assigned for 93% of swallows, a score of 2 was reported for 6% of swallows, and a PA score of 4 was recorded for 1% of swallows. Across all room-temperature and frozen pudding trials, a PA score of 1 was reported for 100% of swallows. For “thin liquid with chocolate chips” trials, a PA score of 1 was assigned to 88% of swallows, a score of 2 was recorded for 8% of swallows, a PA score of 3 was reported for 4% of swallows, and less than 1% of swallows were assigned a PA score of 8. The PAS of 8 (material entered the airway and passed below the vocal folds, and no effort was made to eject) was only noted for one swallow during the “thin liquid with chocolate chips” trial.
Discussion
The primary outcome of this study is that swallowing temporal kinematics for thin liquid consistencies are most different from all others tested. Specifically, our data indicate that thin liquids require the best possible physiological swallowing performance in younger and older healthy individuals (i.e., faster reaction times, longer durations) than other bolus types tested (Figure 2). Thus, our study provides one reasonable physiological rationale for why thin liquids are more challenging to swallow safely. These outcomes are significant because thin liquids often lead to more frequent aspiration events than thicker boluses among individuals with swallowing impairments (dysphagia; Steele et al., 2015). If thin liquids require the most optimal timing in healthy individuals, it could explain why thin liquids also have the greatest likelihood for failure in disordered swallowing. As a next step, thin liquid swallowing physiology is important to directly test with detailed kinematics in patients, because elimination of thin liquids is a common diet recommendation but can also lead to dehydration and poor quality of life (Crary, Carnaby, Shabbir, Miller, & Silliman, 2016; Swan, Speyer, Heijnen, Wagg, & Cordier, 2015). Of course, the nature of swallowing impairments can dictate which bolus types are more or less difficult to swallow efficiently and without airway compromise, which cannot be addressed within the scope of this article.
Effects of Temperature and Mixed Consistencies
The cold boluses tested in this study did not modify normal swallowing timing in our participants and failed to induce faster swallow initiation. To date, no conclusive data have been published to evaluate the normal swallowing kinematics of ice chips, which could serve as a comparison for patients with dysphagia. Although clinicians recommend ice chips based on clinical evaluations, they are typically recommending a consistency that is not directly tested under videofluoroscopy. Therefore, the justification of this recommendation remains unclear in the literature. Other cold stimuli options include thermal–tactile stimulation. Thermal–tactile stimulation consists of applying cold contact to the base of the anterior faucial arches to stimulate the oral cavity before the introduction of a bolus to trigger the swallowing reflex more rapidly (Lazzara, Lazzarus, & Logemann, 1986). Rosenbek, Roecker, et al. (1996) revealed that cold thermal application to the anterior faucial arches preceding a swallow leads to short-term effects characterized by shorter total swallow duration and stage transition duration (SRT) in 22 dysphagic stroke patients. However, faster stage transition duration was reported in healthy adults by Kaatzke-McDonald, Post, and Davis (1996) and Selcuk, Uysal, Aydogdu, Akyuz, and Ertekin (2007), whereas others have reported limited or no effects with cold stimuli on swallowing physiology in patients and healthy adults (Ali, Laundl, Wallace, deCarle, & Cook, 1996; Bove, Mansson, & Eliasson, 1998; Shaker et al., 1994). Overall, the effects of cold stimuli (bolus or nonbolus stimuli) on swallowing remain inconsistent in deglutition research.
Both the barium ice chips and the thin barium with chocolate chips were mixed consistencies in this study. Regarding the thin barium with chocolate chips, two behaviors were observed: (a) swallow the thin liquid and then chew the mini chocolate chips or (b) chew the mini chocolate chips and then swallow the whole mixed bolus. It was more common for subjects to chew the mini chocolate chips and then swallow the masticated bolus. With both barium ice chips and the thin barium with chocolate chips, participants often allowed thin liquids to flow into the valleculae or pyriform sinuses while masticating the solid part of the bolus, where it would aggregate until all contents where eventually swallowed, similar to previous studies (Palmer et al., 2007). Despite this variable behavior, we did not find significantly greater variability with either mixed consistency compared with the other three bolus types that were not mixed. Instead, we found that the thin liquid with chocolate chips mix had faster UESOrt relative to hyoid burst (UESOrt-H) compared with barium ice chips, room-temperature pudding, and frozen pudding. On the other hand, UESOrt-H was not different in the thin liquid with chocolate chips mix compared with the ultrathin liquid (both faster than barium ice and frozen and room-temperature pudding). The thin barium with chocolate chips bolus may have had a faster UESOrt relative to hyoid burst because it provided sensations of fast-moving fluid combined with the texture of the chocolate chips, which may have resulted in better awareness about the position of the bolus to plan the most appropriate UES response (Kendall, McKenzie, Leonard, Goncalves, & Walker, 2000).
Timing Measures Versus Zones
We included the measure of bolus head depth at the onset of two important swallowing events: hyoid burst and LVC. Bolus depth at hyoid burst is often measured clinically, but bolus depth at LVC might be unique to this study (Martin-Harris et al., 2007). No significant differences were found across consistency, age, or sex. However, the temporal kinematic measures in this study that consider swallowing initiation (SRT) and LVCrt-B both had significant consistency, age, and sex effects. This contrast highlights two important concepts. First, the kinematic measures, although not used clinically because of time and training deficits, might be more sensitive to critical swallowing physiological events that impact swallowing safety. This is likely because temporal measures consider two distinct time points of different kinematic events. Second, Martin-Harris et al. (2007) have already shown that bolus head position as low as the pyriform sinuses relative to swallow onset is both normal and varied across healthy adults, but with significant differences with age (Martin-Harris et al., 2007). The Martin-Harris et al. study may have found significant differences because only a 5-ml thin liquid bolus was tested across fewer oropharyngeal zones in a larger sample (N = 76).
Effects of Age
Our outcomes support findings from existing published literature that have already illustrated how swallowing function declines with age. According to Ney, Weiss, Kind, and Robbins (2009), this increased risk in older adults is due to two factors: presbyphagia and increases in diseases and disorders that also lead to dysphagia (Ney et al., 2009). However, as stated before, the specific deficits within aging swallowing function need to be examined. It is well understood that muscle strength and movement accuracy decline over the life span. Enoka et al. (2003) found that healthy older adults demonstrate greater force fluctuations with submaximal contractions (Enoka et al., 2003). The increase in force fluctuations negatively affect accuracy of goal-directed movements. Other age-related deficits in swallow function have been noted, including a recent report that, with normal aging, pharyngeal wall thickness decreases while pharyngeal lumen area increases (Molfenter et al., 2015).
Furthermore, oral sensation, including oral perception of viscosity, declines with age (Calhoun, Gibson, Hartley, Minton, & Hokanson, 1992; Smith, Logemann, Burghardt, Zecker, & Rademaker, 2006). A combination of these motor and sensory deficits that impact bulbar innervated structures likely explains the significant age-related increase in duration across every temporal measure included in this study. However, swallowing likely remains functional in the healthy older population, because while all reaction times increased (not preferable, but normal), durations of key events such as laryngeal closure and UES opening also increased, providing balanced change overall.
Variability
Greater variability was observed in the healthy older adults, likely because of all the reasons previously discussed. Furthermore, not all older individuals will demonstrate the same age-related changes at the same time (if at all; Christou, 2011).
Laryngeal vestibule kinematics were also more frequently variable. Laryngeal vestibule kinematics are complicated because they occur passively because of forces applied from other structures (tongue, bolus, and pharyngeal movements impact epiglottic inversion) as well as direct involvement of neuromuscular innervation for hyoaryngeal elevation and for arytenoid adduction and anterior pivoting (Ekberg, 1982; Ekberg & Sigurjonsson, 1982; B. R. Fink, 1974; B. R. Fink & Demarest, 1978; B. R. Fink, Martin, & Rohrmann, 1979; Logemann et al., 1992; Pearson, Hindson, Langmore, & Zumwalt, 2013; Pearson, Taylor, Blair, & Martin-Harris, 2016). This complicated mechanism likely enables several fail-safes, meaning that, if one component is impaired or perturbed, other components might compensate to ensure adequate airway protection (Humbert, Christopherson, & Lokhande, 2015). On the other hand, because LVC is influenced by several structures (larynx, hyoid, tongue, pharynx) as well as bolus differences, its behavior might be more susceptible to variability than other swallowing kinematics. Given evidence that the central nervous system can plan and execute the same movement successfully in countless different ways (muscle patterns, kinematics), LVC variability in a healthy population is likely beneficial and might be better termed “flexibility” (Brunner et al., 2011; Brunner & Hoole, 2012; Latash & Anson, 2006; Scholz & Schoner, 2014). Thus, researchers should be cognizant of LVC flexibility when reporting no significant effects in swallowing experiments and provide individual kinematic data whenever possible. Likewise, clinicians should consider that behaviors that are “out of the range of normal” might not be necessarily impaired. This will foster a deeper understanding of LVC, which is the first line of defense in airway protection.
Limitations and Conclusions
These outcomes are limited to healthy adults but can serve as a normal reference point for comparison with patient studies. As with all videofluoroscopy studies, barium swallowing in an experimental environment cannot be directly generalized to swallowing during mealtimes. In addition, the room-temperature ultrathin barium had a specific volume, whereas the boluses presented by teaspoon were not precisely measured, which might have impacted kinematics that are more responsive to volume. These findings warrant physiological examination of similar bolus types in individuals with swallowing impairments, especially frozen and mixed consistencies, to further justify common diet recommendations (i.e., NPO except ice, thickened liquids) in clinical settings. Furthermore, analysis of hyolaryngeal displacement is needed for comparisons made in this study, given previous reports of significant differences and variability in these outcomes as well (Molfenter & Steele, 2011). This study provides a plausible physiological basis (based on kinematic analysis) for why certain types of boluses, notably thin liquids, are more difficult to handle safely and perhaps pose a greater risk of aspiration. In addition, we have provided some of the first data on the swallowing timing parameters associated with swallowing frozen boluses. Still, it should be noted that several critical swallowing events could not be measured with videofluoroscopy (i.e., tongue, pharynx) and that, given the complexity of swallowing, dysphagia should not be viewed through a simplified lens that can be fully objectively understood using only the methods or perspective of this investigation. In addition to temporal kinematics, we have added knowledge to the literature regarding variability in temporal swallowing events by bolus type, sex, and age.
Acknowledgments
This work was supported by the National Institutes of Health R01 DC014285 (PI: Humbert).
Funding Statement
This work was supported by the National Institutes of Health R01 DC014285 (PI: Humbert).
References
- Ali G. N., Laundl T. M., Wallace K. L., deCarle D. J., & Cook I. J. (1996). Influence of cold stimulation on the normal pharyngeal swallow response. Dysphagia, 11(1), 2–8. [DOI] [PubMed] [Google Scholar]
- Allen J. E., White C. J., Leonard R. J., & Belafsky P. C. (2010). Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngology—Head and Neck Surgery, 142(2), 208–213. [DOI] [PubMed] [Google Scholar]
- Bove M., Mansson I., & Eliasson I. (1998). Thermal oral–pharyngeal stimulation and elicitation of swallowing. Acta Oto-laryngologica, 118(5), 728–731. [DOI] [PubMed] [Google Scholar]
- Brunner J., Ghosh S., Hoole P., Matthies M., Tiede M., & Perkell J. (2011). The influence of auditory acuity on acoustic variability and the use of motor equivalence during adaptation to a perturbation. Journal of Speech, Language, and Hearing Research, 54(3), 727–739. [DOI] [PubMed] [Google Scholar]
- Brunner J., & Hoole P. (2012). Motor equivalent strategies in the production of German /integral/ under perturbation. Language and Speech, 55(Pt 4), 457–476. [DOI] [PubMed] [Google Scholar]
- Calhoun K. H., Gibson B., Hartley L., Minton J., & Hokanson J. A. (1992). Age-related changes in oral sensation. Laryngoscope, 102(2), 109–116. [DOI] [PubMed] [Google Scholar]
- Carnaby G. D., & Harenberg L. (2013). What is “usual care” in dysphagia rehabilitation: A survey of USA dysphagia practice patterns. Dysphagia, 28(4), 567–574. [DOI] [PubMed] [Google Scholar]
- Christou E. A. (2011). Aging and variability of voluntary contractions. Exercise and Sport Sciences Reviews, 39(2), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary M. A., Carnaby G. D., Shabbir Y., Miller L., & Silliman S. (2016). Clinical variables associated with hydration status in acute ischemic stroke patients with dysphagia. Dysphagia, 31(1), 60–65. [DOI] [PubMed] [Google Scholar]
- Crow H. C., & Ship J. A. (1996). Tongue strength and endurance in different aged individuals. The Journals of Gerontolology: Series A, Biological Sciences and Medical Sciences, 51(5), M247–M250. [DOI] [PubMed] [Google Scholar]
- Curran J., & Groher M. E. (1990). Development and dissemination of an aspiration risk reduction diet. Dysphagia, 5(1), 6–12. [DOI] [PubMed] [Google Scholar]
- Daggett A., Logemann J., Rademaker A., & Pauloski B. (2006). Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia, 21(4), 270–274. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Schroeder M. F., DeGeorge P. C., Corey D. M., & Rosenbek J. C. (2007). Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology, 16(2), 140–147. [DOI] [PubMed] [Google Scholar]
- Ekberg O. (1982). Closure of the laryngeal vestibule during deglutition. Acta Oto-laryngologica, 93(1–2), 123–129. [DOI] [PubMed] [Google Scholar]
- Ekberg O., & Sigurjonsson S. (1982). Movement of epiglottis during deglutition. Gastrointestinal Radiology, 7, 101–107. [DOI] [PubMed] [Google Scholar]
- Enoka R. M., Christou E. A., Hunter S. K., Kornatz K. W., Semmler J. G., Taylor A. M., & Tracy B. L. (2003). Mechanisms that contribute to differences in motor performance between young and old adults. Journal of Electromyography and Kinesiology, 13(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Fink B. R. (1974). Folding mechanism of the human larynx. Acta Oto-laryngologica, 78(1–2), 124–128. [DOI] [PubMed] [Google Scholar]
- Fink B. R., & Demarest R. J. (1978). Laryngeal biomechanics. New York, NY: Raven Press. [Google Scholar]
- Fink B. R., Martin R. W., & Rohrmann C. A. (1979). Biomechanics of the human epiglottis. Acta Oto-laryngologica, 87, 554–559. [DOI] [PubMed] [Google Scholar]
- Fink T. A., & Ross J. B. (2009). Are we testing a true thin liquid? Dysphagia, 24(3), 285–289. [DOI] [PubMed] [Google Scholar]
- Heimlich H. J. (1983). Rehabilitation of swallowing after stroke. The Annals of Otology, Rhinology & Laryngology, 92(4, Pt. 1), 357–359. [DOI] [PubMed] [Google Scholar]
- Hsu W. L., Chou L. S., & Woollacott M. (2013). Age-related changes in joint coordination during balance recovery. Age, 35(4), 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I. A., Christopherson H., & Lokhande A. (2015). Surface electrical stimulation perturbation context determines the presence of error reduction in swallowing hyolaryngeal kinematics. American Journal of Speech-Language Pathology, 24(1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I. A., Lokhande A., Christopherson H., German R. Z., & Stone A. (2012). Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral versus pharyngeal sensory processing. Journal of Applied Physiology, 112(10), 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I. A., & Robbins J. (2008). Dysphagia in the elderly [review]. Physical Medicine and Rehabilitation Clinics of North America, 19(4), 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. V., & Work C. E. (1961). Volume of a swallow. American Journal of Diseases of Children, 102, 427. [DOI] [PubMed] [Google Scholar]
- Kaatzke-McDonald M. N., Post E., & Davis P. J. (1996). The effects of cold, touch, and chemical stimulation of the anterior faucial pillar on human swallowing. Dysphagia, 11(3), 198–206. [DOI] [PubMed] [Google Scholar]
- Kahrilas P. J., Lin S., Chen J., & Logemann J. A. (1996). Oropharyngeal accommodation to swallow volume. Gastroenterology, 111(2), 297–306. [DOI] [PubMed] [Google Scholar]
- Kahrilas P. J., & Logemann J. A. (1993). Volume accommodation during swallowing. Dysphagia, 8(3), 259–265. [DOI] [PubMed] [Google Scholar]
- Kendall K. A., McKenzie S., Leonard R. J., Goncalves M. I., & Walker A. (2000). Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia, 15(2), 74–83. [DOI] [PubMed] [Google Scholar]
- Kwon M., Baweja H. S., & Christou E. A. (2011). Age-associated differences in positional variability are greater with the lower limb. Journal of Motor Behavior, 43, 357–360. [DOI] [PubMed] [Google Scholar]
- Langmore S. E. (1995). Efficacy of behavioral treatment for oropharyngeal dysphagia. Dysphagia, 10(4), 259–262. [DOI] [PubMed] [Google Scholar]
- Langmore S. E., & Pisegna J. M. (2015). Efficacy of exercises to rehabilitate dysphagia: A critique of the literature. International Journal of Speech-Language Pathology, 17(3), 222–229. [DOI] [PubMed] [Google Scholar]
- Latash M. L., & Anson J. G. (2006). Synergies in health and disease: Relations to adaptive changes in motor coordination. Physical Therapy, 86(8), 1151–1160. [PubMed] [Google Scholar]
- Lazzara G., Lazzarus C., & Logemann J. A. (1986). Impact of thermal stimulation on the triggering of the swallow reflex. Dysphagia, 1, 73–77. [Google Scholar]
- Leder S. B., Judson B. L., Sliwinski E., & Madson L. (2013). Promoting safe swallowing when puree is swallowed without aspiration but thin liquid is aspirated: Nectar is enough. Dysphagia, 28(1), 58–62. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Kim W. H., Kim E. J., & Lee J. K. (2012). Is swallowing of all mixed consistencies dangerous for penetration–aspiration? American Journal of Physical Medicine and Rehabilitation, 91(3), 187–192. [DOI] [PubMed] [Google Scholar]
- Logemann J. A., Kahrilas P. J., Cheng J., Pauloski B. R., Gibbons P. J., Rademaker A. W., & Lin S. (1992). Closure mechanisms of laryngeal vestibule during swallow. American Journal of Physiology, 262(2, Pt. 1), G338–G344. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Lee F. S., & Walters B. (2007). Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. Journal of Speech, Language, and Hearing Research, 50(3), 585–594. [DOI] [PubMed] [Google Scholar]
- Mishellany A., Woda A., Labas R., & Peyron M. A. (2006). The challenge of mastication: Preparing a bolus suitable for deglutition. Dysphagia, 21(2), 87–94. [DOI] [PubMed] [Google Scholar]
- Molfenter S. M., Amin M. R., Branski R. C., Brumm J. D., Hagiwara M., Roof S. A., & Lazarus C. L. (2015). Age-related changes in pharyngeal lumen size: A retrospective MRI analysis. Dysphagia, 30(3), 321–327. [DOI] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2011). Physiological variability in the deglutition literature: Hyoid and laryngeal kinematics. Dysphagia, 26(1), 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2012a). Temporal variability in the deglutition literature. Dysphagia, 27(2), 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2012b). Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia, 28(2), 226–233. [DOI] [PubMed] [Google Scholar]
- Molfenter S. M., & Steele C. M. (2014). Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. Journal of Speech, Language, and Hearing Research, 57(3), 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Leigh C., Hori S. F., Molfenter S. M., Shariff T., & Steele C. M. (2013). Timing differences between cued and noncued swallows in healthy young adults. Dysphagia, 28(3), 428–434. [DOI] [PubMed] [Google Scholar]
- Ney D. M., Weiss J. M., Kind A. J., & Robbins J. (2009). Senescent swallowing: Impact, strategies, and interventions. Nutrition in Clinical Practice, 24(3), 395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia M. A., Hind J. A., Roecker E. B., Carnes M., Doyle J., Dengel G. A., & Robbins J. (2000). Age effects on the temporal evolution of isometric and swallowing pressure. The Journals of Gerontolology: Series A, Biological Sciences and Medical Sciences, 55(11), M634–M640. [DOI] [PubMed] [Google Scholar]
- Okada S., Saitoh E., Palmer J. B., Matsuo K., Yokoyama M., Shigeta R., & Baba M. (2007). What is the chin-down posture? A questionnaire survey of speech language pathologists in Japan and the United States. Dysphagia, 22(3), 204–209. [DOI] [PubMed] [Google Scholar]
- Palmer J. B., Hiiemae K. M., Matsuo K., & Haishima H. (2007). Volitional control of food transport and bolus formation during feeding. Physiology and Behavior, 91(1), 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. G. Jr., Hindson D. F., Langmore S. E., & Zumwalt A. C. (2013). Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. International Journal of Radiation Oncology, Biology, Physics, 85(3), 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. G. Jr., Taylor B. K., Blair J., & Martin-Harris B. (2016). Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope, 126(8), 1854–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastatter M. P., McGuire R. A., Bushong L., & Loposky M. (1987). Speech-motor equivalence in aging subjects. Perceptual and Motor Skills, 64(2), 635–638. [DOI] [PubMed] [Google Scholar]
- Robbins J., Coyle J., Rosenbek J., Roecker E., & Wood J. (1999). Differentiation of normal and abnormal airway protection during swallowing using the penetration–aspiration scale. Dysphagia, 14(4), 228–232. [DOI] [PubMed] [Google Scholar]
- Robbins J., Levine R., Wood J., Roecker E. B., & Luschei E. (1995). Age effects on lingual pressure generation as a risk factor for dysphagia. The Journals of Gerontolology: Series A, Biological Sciences and Medical Sciences, 50(5), M257–M262. [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C. (1995). Efficacy in dysphagia. Dysphagia, 10(4), 263–267. [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., & Wood J. L. (1996). A penetration–aspiration scale. Dysphagia, 11(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C., Roecker E. B., Wood J. L., & Robbins J. (1996). Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia, 11(4), 225–233. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Shibata S., Matsuo K., Baba M., Fujii W., & Palmer J. B. (2007). Chewing and food consistency: Effects on bolus transport and swallow initiation. Dysphagia, 22(2), 100–107. [DOI] [PubMed] [Google Scholar]
- Scholz J. P., & Schoner G. (2014). Use of the uncontrolled manifold (UCM) approach to understand motor variability, motor equivalence, and self-motion. Advances in Experimental Medicine and Biology, 826, 91–100. [DOI] [PubMed] [Google Scholar]
- Selcuk B., Uysal H., Aydogdu I., Akyuz M., & Ertekin C. (2007). Effect of temperature on electrophysiological parameters of swallowing. Journal of Rehabilitation Research and Development, 44(3), 373–380. [DOI] [PubMed] [Google Scholar]
- Shaker R., Ren J., Zamir Z., Sarna A., Liu J., & Sui Z. (1994). Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology, 107(2), 396–402. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Logemann J. A., Burghardt W. R., Zecker S. G., & Rademaker A. W. (2006). Oral and oropharyngeal perceptions of fluid viscosity across the age span. Dysphagia, 21(4), 209–217. [DOI] [PubMed] [Google Scholar]
- Steele C. M., Alsanei W. A., Ayanikalath S., Barbon C. E., Chen J., Cichero J. A., … Wang H. (2015). The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia, 30(1), 2–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., & Cichero J. A. (2014). Physiological factors related to aspiration risk: A systematic review. Dysphagia, 29(3), 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., & Miller A. J. (2010). Sensory input pathways and mechanisms in swallowing: A review. Dysphagia, 25(4), 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Molfenter S. M., Peladeau-Pigeon M., & Stokely S. (2013). Challenges in preparing contrast media for videofluoroscopy. Dysphagia, 28(3), 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan K., Speyer R., Heijnen B. J., Wagg B., & Cordier R. (2015). Living with oropharyngeal dysphagia: Effects of bolus modification on health-related quality of life—A systematic review. Quality of Life Research, 24(10), 2447–2456. [DOI] [PubMed] [Google Scholar]
- Tracy J. F., Logemann J. A., Kahrilas P. J., Jacob P., Kobara M., & Krugler C. (1989). Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia, 4(2), 90–94. [DOI] [PubMed] [Google Scholar]