Abstract
Purpose
Research suggests that abnormal levels of intrinsic laryngeal muscle (ILM) contraction is a potential causal factor in stress-induced voice disorders. This study seeks to characterize the ILM stress response in a cohort of vocally healthy women.
Method
The authors used an unblinded, nonrandomized, repeated-measures design. Forty vocally healthy female adults were subjected to a stressful speech preparation task. Measurements of heart rate, blood pressure, trapezius muscle (positive control) activation, and tibialis muscle (negative control) activation were obtained from 37 participants before and during stressor exposure, in a nonvoice and nonspeaking task paradigm, to confirm physiological stress response compared to baseline. Fine wire electromyography of the ILMs (posterior cricoarytenoid, thyroarytenoid/lateral cricoarytenoid muscle complex, and cricothyroid) was performed simultaneously so that the activity of these muscles could be measured prior to and during stressor exposure.
Results
The protocol successfully elicited the typical and expected physiological stress responses. Findings supported the hypothesis that, in some individuals, the ILMs significantly increase in activity during stress reactions compared to baseline, as do the control muscles.
Conclusions
This study characterizes ILM responses to psychological stress in vocally healthy participants. Some of the female adults in this study appeared to be “laryngeal stress responders,” as evidenced by increased activity of the ILMs during a silent (i.e., nonvocal, nonspeech) speech preparation task that they considered to be stressful.
Stress affects the voice. This position is held as widespread clinical consensus, and a good deal of anecdotal, clinical, and research-based evidence supports it (Dietrich & Verdolini Abbott, 2012; Hecker, Stevens, von Bismarck, & Williams, 1968; Laukka et al., 2008; Van Lierde, Van Heule, De Ley, Mertens, & Claeys, 2009). However, the extent to which stress affects the intrinsic laryngeal muscles (ILMs), which are essential contributors to the “source” of the final vocal product, has been sparsely investigated. This issue is highly relevant to clinical and investigative voice specialists, as ILM hyperfunction is alleged to underlie a variety of common voice disorders, such as primary muscle tension dysphonia and vocal nodules (Altman, Atkinson, & Lazarus, 2005; Froeschels, 1952; Morrison, Nichol, & Rammage, 1986; Morrison & Rammage, 1993; Oates & Winkworth, 2008; Rubin, Blake, & Mathieson, 2007), and even vocal fold edema, general “vocal fold thickening,” vocal fold polyps, and contact ulcers (Hillman, Holmberg, Perkell, Walsh, & Vaughan, 1989).
“Stress” is a broad term that refers to somatic or psychological “tension” stemming from factors that disrupt one's equilibrium (Lovallo, 1997). Conditions or experiences that cause stress and that possibly tax an organism's ability to adapt are referred to as “stressors.” Stressors can be physical, biological, or psychological and may exist acutely or chronically (Dickerson & Kemeny, 2004).
We previously showed that in the face of a physical stressor, a task designed to agonize the sympathetic nervous system, the ILMs exhibited a statistically significant increase in activation (Helou, Wang, Ashmore, Rosen, & Abbott, 2013). Specifically, in previous work, the cold pressor task was implemented to trigger an autonomic (i.e., “fight or flight”) response in eight adult vocally healthy female participants. The task involves submerging the participant's hand and lower arm into ice cold water. The cold pressor method is commonly used in experimental stress studies because it rapidly increases cardiac output and peripheral vasoconstriction as part of the autonomic response (Lovallo, 1975). Each of the eight participants in this study exhibited an increased level of activation in several ILMs as measured via fine wire laryngeal electromyography (EMG).
Despite the lack of empirical evidence for increased ILM activity as a response to psychological stress, some theoretical proposals have been made to explain the putative phenomenon. One model of particular interest is the Psychobiological Framework of Voice Disorders proposed by Dietrich and Verdolini Abbott (2008), which submits that laryngeal muscle tension arises from the concurrent and interactive engagement of neuroendocrine, autonomic nervous, and somatic motor systems. Dietrich and Verdolini Abbott (2008) advance that when psychological conditions align with somatic stress responses in a chronically unhealthy fashion, the resulting laryngeal environment can lead to the development of one specific voice disorder, muscle tension dysphonia.
The Psychobiological Framework of Voice Disorders seems a reasonable framework for investigations into the pathogenesis of not only muscle tension dysphonia but also other disorders of voice. However, because muscle tension dysphonia is not the only voice disorder proposed to be associated with increased ILM activity and because very little is known about ILM response to stress in individuals without impaired voice, the present investigation focuses specifically on ILM activity rather than any one specific voice impairment. This study will directly examine the assumption that the ILMs respond to stress with increased activation. Furthermore, the nature of the ILM response to a psychological stressor will be characterized. It is hypothesized that ILM activity will increase during psychological stressor exposure and resolve once the stressor is removed.
Method
Forty vocally healthy female adults between the ages of 18 and 30 years were recruited from the Pittsburgh metropolitan region. Female adults were selected for this study to minimize heterogeneity in physiological stress responses. Participants were paid upon completion of the study protocol. The University of Pittsburgh Institutional Review Board approved the study (Protocol 012110063).
Stages I and II—Web and Live Screening
The following exclusionary criteria by self-report were documented via web-based survey: below 18 or above 30 years of age; frequent or high level of comfort with public speaking; pregnant; current lower or upper respiratory illness or seasonal allergies with respiratory manifestation; known allergy to local anesthetic medications such as lidocaine; history of voice disorders, difficulty in breathing or known respiratory disorders, neck or throat surgery; autonomic dysfunction or dysautonomia; clinically diagnosed or suspected psychological disorders; asthma; and blood clotting or coagulation disorders. Participants reported their height and weight, and those with body mass index at or above 31 (i.e., obese individuals) were excluded from participation because (a) obesity might impact respiration (of interest in the study) and (b) excessive fatty tissue in the neck might make it difficult to identify landmarks for laryngeal hook wire electrode placement. Participants eligible for face-to-face screening were subsequently assessed clinically at the University of Pittsburgh Voice Center to establish (a) tolerance of laryngeal palpation and manipulation and (b) normal laryngeal structure and physiology as judged by a fellowship-trained laryngologist based on flexible transnasal laryngoscopy.
Experimental Stages
All research questions were investigated using a single-subject experimental design with multiple subjects. The direction, magnitude, and time course of ILM activity during stressor exposure were compared to baseline and poststressor recovery phases.
Data collection and experimental procedures occurred on a separate day from the screening day, as follows. The experiment took place in a quiet clinical procedure room at the University of Pittsburgh Voice Center and lasted for 90–120 min. All unnecessary electronic equipment and lights were turned off and unplugged to minimize ambient noise. Ambient temperature was maintained at 73.88°F on average (0.43°F average fluctuations within single experimental sessions, range 0°–2.8°, SD = 0.45°).
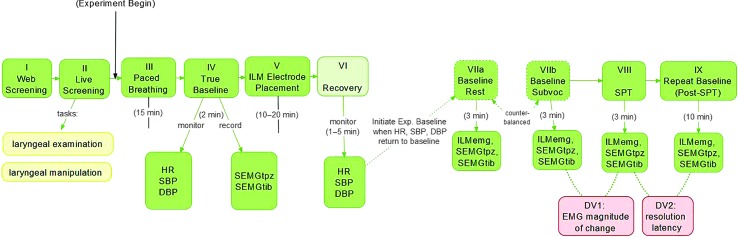
For the following description of the experimental session, refer to Figure 1. It is important to note that this study involved three specific elements of intentional deception, one that occurred during informed consent and another two that occurred on the experimental day. All instances of intentional deception were related to the speech preparation task (SPT; Stage VIII; see Figure 1) and were institutional review board–approved; they will be described subsequently.
Figure 1.
Schematic of screening and experimental conditions. HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; SEMGtpz = surface electromyography of trapezius muscle; SEMGtib = surface electromyography of tibialis muscle; Exp. = experiment; ILMemg = intrinsic laryngeal muscle electromyography; SPT = speech preparation task; DV1 = dependent variable 1; DV2 = dependent variable 2.
On the experimental day, participants were seated in an examination chair at a semiupright angle (120°), which was required to accommodate the equipment. The participant's head was fully supported by a head rest, and her legs were fully supported by a leg rest. Participants were fitted with (a) left arm cuff for intraexperimental measurement of average heart rate (HR) and arterial systolic and diastolic blood pressure (SBP and DBP, respectively) using an ambulatory blood and HR monitor (Omron Digital Blood Pressure Monitor, HEM 907-XL), (b) surface EMG (SEMG) electrode positioned on the upper portion of the left upper neck over the trapezius muscle as a positive control site, 1 (c) SEMG electrode positioned on the left anterior leg over the tibialis muscle as a negative control site, 2 (d) Piezo Crystal respiratory effort transducers situated on the chest wall over Ribs 5–8 to measure respiratory rate (Grass Technologies, Astro-Med, Inc.), and (e) ground and reference electrodes on the right olecranon (bony protrusion of the elbow) and the right earlobe, respectively. All surface and ground electrodes were 20 mm bipolar Ag/AgCl surface electrodes (Grass Technologies, Astro-Med, Inc.).
Immediately following the fitting of this equipment, participants were presented with a series of 100-mm visual analog scales. They were instructed to mark the point corresponding with their perceived stress and anxiety, where the leftmost point of the line (i.e., rating of zero) represented “not stressed/anxious at all” and the rightmost point of the line (i.e., rating of 100) represented “more stressed/anxious than ever.” Participants were asked to rate their self-perceived stress and anxiety levels in the same manner immediately following each subsequent stage shown in the experimental flowchart, excluding Stage VI (Recovery) because it was essentially the same as the subsequent task into which it flowed (ILM Baseline, Stage VII) without announcement by the investigator. To minimize the cognitive burden on participants, they were allowed to define the terms “stress” and “anxiety” using their own internal constructs for each term (i.e., the terms were not defined by the investigators). In the course of the study, no participants asked for a definition or clarification regarding either term.
Stage III—Paced Breathing
Next, participants underwent a paced breathing task according to published methods (Egizio, Eddy, Robinson, & Jennings, 2011). This procedure was implemented for later controlling for the confounding effects of respiratory rate and tidal volume on respiratory sinus arrhythmia (RSA) values, as part of another experimental question embedded in this study. Specifically, because respiratory rate and tidal volume directly influence RSA values, they must be accounted for in order to understand the degree to which the RSA signal is truly reflecting what it is purported to reflect, that is, parasympathetic outflow to the sinoatrial node. These methods and underlying rationale are described in a partner paper and will not be described in detail herein. Briefly, participants were asked to match their inspiratory and expiratory patterns to an audiovisual cue that paced their breathing at 8, 10.5, 13, and 18 breaths/min. Each pacing condition lasted for 2 min and was interspersed by a 2-min unpaced (i.e., spontaneous) breathing condition.
Stage IV—True Baseline
Next, the participant remained at rest for 180 s while exposed to emotionally neutral video stimuli (Van Emden, 2011) on a computer screen. Participants were instructed “to lie here quietly while keeping your body still and focusing on the video,” and at Minutes 1 and 2, participants were gently reminded to “keep focusing on the video.” This condition was used to obtain True Baseline values as follows: (a) electrocardiogram (ECG) and SEMG signals were continuously recorded and stored for later analysis, as described shortly, and (b) HR, SBP, and DBP values were automatically calculated and manually recorded by a research assistant approximately every 30 s during all tasks so that the experimenter could monitor cardiovascular return to baseline throughout the study. This assistant also monitored to ensure that participants attended to the neutral stimuli for the duration of the task; no intervention was required to redirect any participant's attention to the task during this condition.
Stage V—ILM Electrode Placement
Next, in preparation for ILM EMG electrode placement, a fellowship-trained laryngologist (CAR, second author) administered a superficial injection of 1–2 cc of 1% lidocaine subcutaneously over the cricothyroid (CT) membrane. Then, using audiovisual guidance via a clinical EMG system (TECA Synergy 4.3, Oxford Instruments), hook wire electrodes were inserted into the right posterior cricoarytenoid (PCA), bilateral thyroarytenoid/lateral cricoarytenoid (TA/LCA) muscle complex, 3 and bilateral CT—thus three muscle groups of interest and five individual EMG channels—according to previously published methods (Munin, Rosen, & Zullo, 2003). Construction of the hook wire electrodes has been described elsewhere (Helou et al., 2013). Laryngeal rotation (approximately 15°–20°) was required for PCA electrode placement. After one electrode is seated in a PCA muscle, rotating the larynx again to place a contralateral electrode would risk dislocation of the first electrode and may cause pain. Thus, PCA electrodes were placed unilaterally for all participants and to accommodate the physician's handedness on the right side.
Accuracy of electrode placement was verified with the hook wire electrodes still coupled to the clinical EMG system. This was done both visually and auditorily, using three consecutive sniff (PCA), valsalva (TA/LCA), and pitch glide (CT) tasks to verify placement for each muscle group. For each of these tasks, participants were only required to produce a behavior that yielded audiovisual EMG signals that could be clearly appreciated by the laryngologist (CAR) and principal investigator (LBH); if any behavior yielded questionably viable signals, the participants were asked to duplicate it at a greater magnitude (e.g., stronger Valsalva, higher pitch glide). The only exception to this approach was when a participant reported great discomfort with the EMG needle placement, in which case that and/or other muscle EMG placements might have been abandoned. Once electrode placement was verified for each muscle, (a) the micrograbbers coupling the electrodes to the clinical EMG system were removed, (b) the midportion of the hook wire electrodes were secured to the subject's neck with tape, and (c) the distal end of the wires were reattached to the micrograbbers, which were then coupled to two bridged 16-channel g.USB Biosignal Amplifiers and A/D Converters (Guger Technologies).
Verification tasks were repeated and recorded at that time to allow a posteriori determination that uncoupling and recoupling the electrodes to the equipment did not disrupt electrode placement (see section below titled “Verifying Electrode Placement”). Verification of placement of the control sites were performed similarly—once during initial placement and then again during the recording epoch following ILM verification tasks—for the control sites, using shoulder elevation (upper trapezius) and foot dorsiflexion (anterior tibialis) tasks. All ECG, hook wire EMG, and SEMG signals were digitized using a sampling rate of 9,600 samples/s/channel using g.USBamp Biosignal Amplifiers and BCI 2000 acquisition software (Albany), and no online filtering was applied during data acquisition. Recordings were saved to files on a laptop computer. Following placement of ILM and control site electrodes and placement verification procedures, HR and blood pressure were monitored during recovery from ILM electrode placement (Stage VI; see Figure 1) until values returned to baseline per standard research protocol (Christenfeld, Glynn, & Gerin, 2000).
Stages VIIa and VIIb—Baseline Rest and Baseline Subvocalization
When HR, SBP, and DBP return to baseline was verified, two experimental baseline tasks were completed, with the task order counterbalanced for all participants. These two baseline tasks are part of a separate experiment embedded in this larger experiment. One task was the Baseline Rest task, during which participants remained at rest while observing emotionally neutral audiovisual stimuli as described previously for Stage IV (True Baseline). Halfway through the 3-min task period, participants were gently encouraged by the investigator to “keep focusing on the video while keeping your body still and quiet.”
The other of the two tasks was the Baseline Subvocalization (Baseline Subvoc) task, during which participants engaged in a nonstressful, nonverbal activity that involved a nontrivial level of linguistic processing and thus might theoretically involve subvocalization at the level of the larynx (Aarons, 1971). This task was designed to be as parallel as possible to the experimental stressor (SPT), without actually causing stress or risking attenuation of the stress response triggered by the experimental stressor (e.g., due to practice effects).
During the Baseline Subvoc condition, the participants were prompted to “imagine a small group of people with whom you are very comfortable and at ease, and to imagine that you are talking with these people about your dream job.” Participants were presented with short, bulleted, written prompts to imagine themselves describing (a) what their dream job entails, (b) the “who, what, where, and when” of this dream job, and (c) what they will accomplish in this dream job. Halfway through the 3-min task period, participants were gently encouraged by the investigator to “keep imagining that you are talking about your dream job, while keeping your body still and quiet.” Otherwise, the investigator did not directly address or pointedly observe the participant in an attempt to avoid stress induction. It is this task that serves as the referent baseline condition in this study.
Stage VIII—SPT
Immediately following the baseline epochs, participants were told to present a speech before a web-based audience of four confederates and were given 3 min to prepare for the speech. Participants were told they would be both observed and videotaped during speech preparation and presentation. Recall that this experimental condition was fully unanticipated by all participants, in accordance with the purposeful element of intentional deception. This task lasted for 3 min while ECG, ILM EMG, and SEMG data were continuously sampled. Further details about the SPT and the intentional deception related to it are detailed below.
Stage IX—Repeat Baseline
Immediately following the SPT, participants were informed of the intentional deception related to the SPT (i.e., debriefed) and instructed to relax for the next 15 min before terminating their participation in the experiment. Repeat baseline data from ECG, ILM EMG, and SEMG channels (Stage IX; see Figure 1) were collected while the participant rested for 10 min. As during the Baseline Rest condition, participants were provided emotionally neutral audiovisual stimuli and were instructed to attend to them (e.g., Van Emden, 2011). Participants were instructed to rest, relax, and remain as motionless as possible during this phase. Immediately following this task, as also occurred after every previous task, participants were presented with the visual analog scale on which they rated the degree of stress and anxiety that they felt during the task just completed.
Finally, all participants ended the experimental session with three repeated trials each of sniff, valsalva, and pitch glides for final verification of accurate placement of the hook wire ILM electrodes. Electrodes substantially compromised or lost during the experiment were identified based on these tasks, and data from these electrodes were excluded from analysis, as described next. All experimental equipment was removed from the participant, thus concluding the experimental session.
SPT
During informed consent, participants were told that they would simply engage in “different speech and nonspeech tasks”; they were not informed that they would be exposed to a psychological stressor. This was the first instance of intentional deception in this study. In actuality, participants were exposed to the SPT (Stage VIII), which was modeled on the Trier Social Stress Test (Kirschbaum, Pirke, & Hellhammer, 1993). The Trier Social Stress Test is a well-established experimental protocol to induce moderate psychosocial stress, and it yields significant increases in cardiovascular parameters and subjective stress ratings (Kirschbaum et al., 1993). In this study, during the SPT, prerecorded videos were shown to subjects who were led to believe the videos were actual people interacting in real time from their own office. The confederates were sex-balanced because the effects of panel sex composition in the Trier Social Stress Test is known to influence the physiological stress responses in women (Duchesne, Tessera, Dedovic, Engert, & Pruessner, 2012). In the videos, two confederates (sex-balanced) adopted attentive but neutral expressions for the duration of the task (Kirschbaum et al., 1993). An additional two confederates (sex-balanced) intermittently expressed somewhat more nonaccepting and critical facial expressions (i.e., nonsmiling, impatient, “stone-faced”) during the task (Gruenewald, Kemeny, Aziz, & Fahey, 2004). The confederates were arranged on the screen in four quadrants, with the order of presentation as follows from top left, clockwise to bottom left: Man 1 (neutral expression), Woman 1 (critical expression), Man 2 (critical expression), Woman 2 (neutral expression).
When the SPT was first introduced to the participants (immediately following the second baseline condition, Stage VIIa/b), the investigator appeared to be speaking via a headset to someone on the laptop (e.g., “Thanks for taking the time to do this,” “Can you hear me clearly?,” etc.). The laptop screen was then made visible to the participant, such that they could see four people on the screen (as described in previous paragraph) who were ostensibly observing the participant. Participants were told that (a) these people were professionals who specialize in nonverbal communication; (b) the professionals had Skyped in from their offices to observe the participant during this task; (c) the professionals would be evaluating and rating participants' speech preparation behaviors, speech performance, and ability to communicate ideas successfully in a social situation; (d) the participants would be video-recorded during the SPT; and (e) the video might be selected for later review by a class of undergraduates learning to score speech preparation and presentation behaviors.
Once participants indicated general understanding of the SPT, they were asked to prepare a speech that would mimic elements of a job interview. They needed to prepare the speech nonverbally (“in your head”) for 3 min. The speech needed to address three specific issues that they would be expected to recall without props, and participants were told that they would be evaluated by the four observers on the following subtasks: (a) present three of your best and worst characteristics, (b) use math to make a case for how much this job is worth to you and how much you expect to be paid (factor in how much you have spent to date on education, travel and living expenses, and any other relevant financial details), and (c) describe your goals for the future in the form of a “5-year plan.” At this point, the active phase of the SPT task commenced, and recordings of cardiovascular, EMG, and respiratory signals began. One research assistant played the role of videographer and trained a camera directly on the participant while approximately 2 ft in front of her, and the remaining two members of the research party sat quietly to the side of the participant approximately 3 ft away, maintaining a neutral expression throughout the task.
At the end of the silent preparation phase, participants were debriefed. The rationale for the intentional deception involved in the SPT was as follows. First, we sought to minimize anticipatory stress, particularly in baseline recording epochs. Second, two potent elements of stressors that influence the magnitude of the perceived and physiological stress response are predictability and controllability (Dickerson & Kemeny, 2004). By using intentional deception, both elements were manipulated in this study in an attempt to maximize the acute psychological and physiological stress response during the SPT epoch. Finally, the true nature of the stressor was purposefully exaggerated during the SPT epoch. Participants were ultimately relieved to learn that (a) they were not and would not be evaluated or observed by any individuals outside those in the room with them during the investigations (i.e., neither the four confederates nor selected undergraduates), (b) they were not videotaped, and (c) they were not required to deliver their prepared speech.
Data Reduction and Analysis
Data reduction and analysis was performed using MATLAB 7.8.0 r2009a (MathWorks, Inc.). A 20-Hz high-pass filter was applied to all EMG channels in order to remove drift and offset. Notch filters (4 Hz) of 60, 120, and 180 Hz were applied to all data channels, and all EMG data channels were full-wave rectified and low-pass filtered for analysis. Electrocardiogram (EKG) and respiratory channels were filtered as above and down-sampled to 1000 Hz.
Next, to determine whether laryngeal muscle change was statistically significant across conditions, within-subject analysis of amounts and amplitudes of muscle activation per unit/time via interrupted time-series analysis (ITSA) were performed using the ARIMA Model 2 in MATLAB. ITSA is intended to identify whether an event (e.g., Repeat Baseline task) is associated with the time-series pattern present in observations prior to the event (e.g., Baseline Rest). ITSA essentially estimates the amount of autocorrelated data in each set of data, subtracts the autocorrelated data from the raw data, and performs a t test on the remaining nonautocorrelated data (Crosbie, 1993). This repeated-measures approach was selected on the basis that we sampled muscle activity repeatedly throughout the experimental session from each participant; those signals might be expected to be highly autocorrelated given the fact that they were obtained from the same region of a given muscle, in the same person on the same day. Thus, removing the autocorrelated activity in this fashion was an attempt at statistical rigor in an experimental model that does not feasibly allow us to perform statistically independent trials of the experimental tasks. Rolling windows shifted in 5-s increments. Individual ITSAs were performed comparing (a) Baseline Rest epoch to the Baseline Subvoc epoch and (b) and Baseline Subvoc epoch to the SPT epoch. These repeated ITSA analyses yielded a p value for each muscle (each of the ILMs, the upper trapezius, and the anterior tibialis muscle) for each participant. Then, to estimate the strength of the muscle response to the psychological stressor, effect sizes were calculated for individual muscles for each participant, and descriptive statistics of the effect sizes (average, SD, minimum, maximum) were obtained. Conceptually, this approach is similar to a meta-analysis and provides information regarding the magnitude of the total effect size of the phenomenon of interest (i.e., muscular response to the speech preparation stressor).
ITSA was also used to calculate resolution latency. In essence, resolution latency was represented by the time required for the Repeat Baseline signal to show no statistically significant difference from the Baseline Rest condition. This variable was calculated for each of the ILMs and the two control sites. To calculate resolution latency, within-subject analysis of amounts and amplitudes of muscle activation per unit/time via ITSA was performed using the ARIMA Model 2 in MATLAB. ITSA is intended to identify whether an event (e.g., Repeat Baseline task) is associated with the time-series pattern present in observations prior to the event (e.g., Baseline Rest). ITSA essentially estimates the amount of autocorrelated data in each set of data, subtracts the autocorrelated data from the raw data, and performs a t test on the remaining nonautocorrelated data (Crosbie, 1993). ITSA was performed to compare the entire Baseline Rest signal to 30-s rolling windows of Repeat Baseline data. Rolling windows shifted in 5-s increments. The time point at which three subsequent windows of Repeat Baseline data exhibited a nonstatistically significant value as compared to the Baseline Rest condition (i.e., ILM/trapezius/tibialis activity returns to baseline) was deemed the resolution latency. All dependent variable values were obtained using custom MATLAB scripts.
In addition, cardiovascular data were exported from MATLAB, and statistical analyses were performed using SPSS 21.0. General descriptive statistics and repeated-measures analyses of variance were calculated for participant demographics, cardiovascular values by experimental stage, and perceived stress and anxiety by experimental stage.
Verifying Electrode Placement
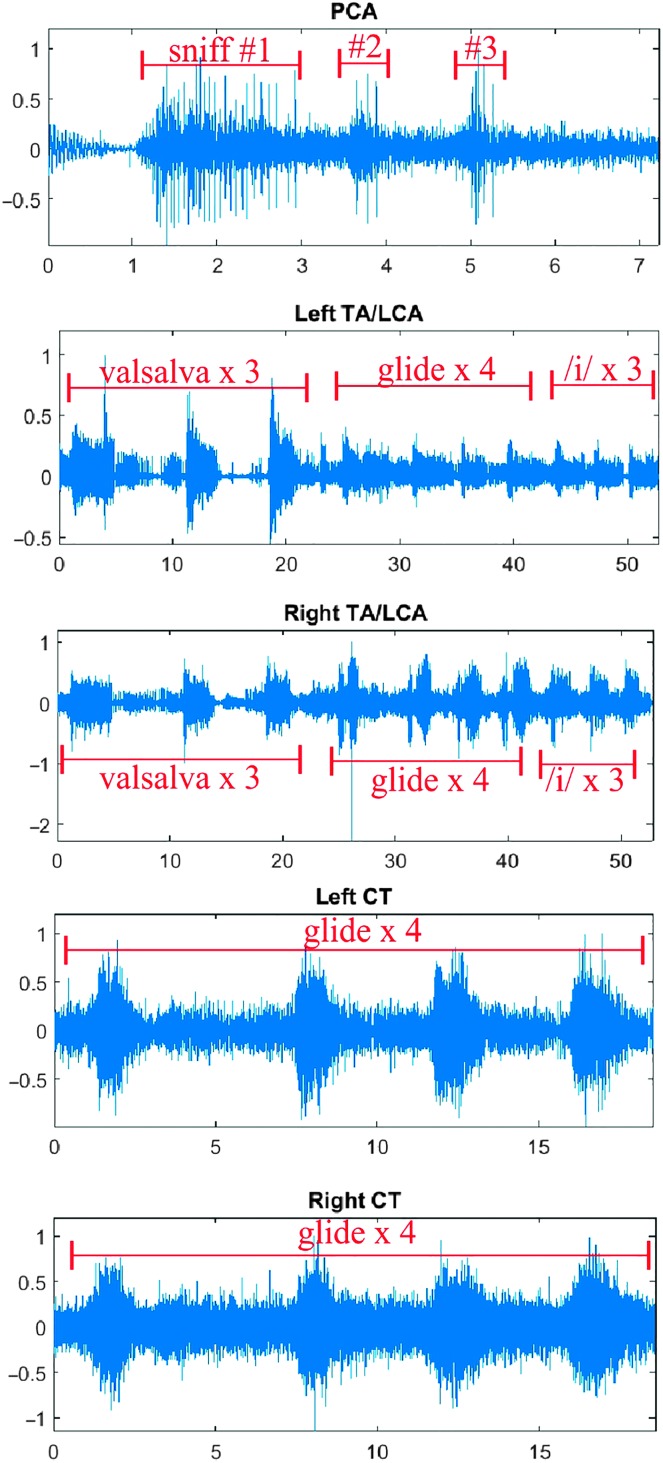
EMG waveforms corresponding with pre- and postexperimental laryngeal electrode placement verification tasks (PCA, sniff; TA/LCA, valsalva; CT, pitch glide) were visually assessed a posteriori by the first author. Pre- and postexperimental verification files were individually prepared, and all channels were inspected. If no clear muscle activity was evident during the time in which the verification task was recorded, data from that channel were excluded from all analyses. Figure 2 shows the ILM voluntary contraction tasks by channel for one participant. Based on the voluntary contraction trials for this particular subject (Subject 49), all channels were judged to have viable signals.
Figure 2.
Example of voluntary contraction tasks for one participant. Time is on x-axis, and normalized electromyography activity is on y-axis. Activity for each muscle during a corresponding voluntary contraction task is indicated in red. Note that thyroarytenoid/lateral cricoarytenoid (TA/LCA) activity is evident during upward pitch glides, although that task is specifically intended to highlight cricothyroid (CT) activity. The upward pitch glide tokens seen in the TA/LCA tracings are the same as those seen in the CT tracings. PCA = posterior cricoarytenoid.
The same level of methodological rigor was not necessary for the trapezius and tibialis EMG channels, as accurate electrode placement is substantially easier to verify during the experiment compared to ILM electrode placement. Trapezius and tibialis EMG waveforms were reviewed visually by the first author, and all exhibited clear activation during the appropriate/corresponding voluntary contraction task.
Results
Seventy-eight potential participants completed the web-based screening survey, and 50 potential participants attended the face-to-face screening. Forty participants satisfied all of the inclusion criteria and participated in the study. Of those 40 participants, one could not tolerate the placement of the fine wire electrodes and was dismissed from the study after that experimental stage. Data from two participants were corrupted for unknown reasons and hence could not be analyzed. Thus, complete data sets for 37 individuals are presented herein.
Racial/ethnic information for this cohort is as follows: non-Hispanic (n = 36), Hispanic (n = 1), White/Caucasian (n = 28), Black/African American (n = 5), Asian (n = 4). Table 1 shows age and body mass index values for all participants whose data were included in analysis. Data from one participant exceeding the body mass index criterion were included in the study; during the screening, she was just below the Obese Class I threshold, but by the time she participated in the experiment, her weight had increased. This difference was not realized or confirmed by the investigators until after the participant had completed the experiment, and her data are included herein.
Table 1.
Anthropometric indices.
| Measurement | M (SD) | Range (min–max) |
|---|---|---|
| Age (years) | 23.09 (3.10) | 19–30 |
| Height (in.) | 64.78 (2.59) | 60.5–71.0 |
| Weight (lb) | 139.19 (23.43) | 99–185 |
| Body mass index (kg/m2) | 23.27 (3.34) | 18.60–31.75 |
| Cutoff points/ranges | n (%) | |
| Body mass index classification a | ||
| Underweight | < 18.50 | 0 |
| Normal | 18.50–24.99 | 24 (67) |
| Overweight | 25.00–29.99 | 11 (31) |
| Obese class I | 30.00–34.99 | 1 (3) |
| Obese class II | 35.00–39.99 | 0 |
| Obese class III | ≥ 40.00 | 0 |
According to World Health Organization (2000) classification criteria.
As previously described, raw EMG waveforms recorded during voluntary contraction tasks were reviewed a posteriori to confirm that the signals stood out against the noise of that channel. This step preceded all descriptive and statistical analyses. Data from channels that did not exhibit clear increases in muscle activity in the time frame in which the voluntary contraction task was performed were excluded from further analysis. Table 2 provides information regarding which channels/muscles were deemed viable based on voluntary contraction signals collected intermittently during the experiment.
Table 2.
Viable intrinsic laryngeal muscle electromyography signals at each phase of the experiment.
| Subject ID | At time of placement |
Pre |
Post |
Final |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCA | LTA | RTA | LCT | RCT | PCA | LTA | RTA | LCT | RCT | PCA | LTA | RTA | LCT | RCT | PCA | LTA | RTA | LCT | RCT | |
| 01 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 02 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 03 | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| 04 | + | + | + | + | + | − | + | − | + | + | − | + | − | + | + | − | + | − | + | + |
| 09 | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + |
| 10 | + | + | + | + | + | − | − | − | + | + | − | − | − | + | + | − | − | − | + | + |
| 11 | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| 12 | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + |
| 17 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 18 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| 19 | + | + | + | + | + | − | + | + | − | + | − | + | + | − | − | − | + | + | − | + |
| 20 | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − |
| 22 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 24 | + | + | + | + | − | − | + | + | + | − | − | + | + | + | − | − | + | + | + | − |
| 26 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| 28 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 29 | + | + | + | + | + | + | − | + | − | + | + | − | + | − | + | + | − | + | − | + |
| 34 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 35 | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| 31 | + | + | − | + | − | − | + | − | + | − | − | + | − | + | − | − | + | − | + | − |
| 32 | − | + | + | + | − | − | + | + | + | − | − | + | + | + | − | − | + | + | + | − |
| 36 | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − |
| 39 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 48 | + | + | + | − | − | + | + | + | − | − | + | + | + | − | − | + | − | − | − | − |
| 49 | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 50 | − | + | + | + | + | − | + | + | − | + | − | + | + | + | + | − | + | + | + | + |
| 52 | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + |
| 55 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + |
| 57 | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + | + |
| 60 | + | + | − | + | − | + | + | − | + | − | + | + | − | + | − | + | + | − | + | − |
| 66 | + | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | − | + |
| 71 | + | + | + | + | + | + | + | + | + | − | − | + | + | + | + | − | + | + | + | − |
| 72 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 74 | − | + | + | + | + | − | + | + | − | − | − | + | + | − | − | − | + | + | − | − |
| 75 | + | + | + | − | − | + | + | + | − | − | + | + | + | − | − | + | + | + | − | − |
| 76 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 78 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| No. of missing | 4 | 0 | 2 | 3 | 8 | 11 | 2 | 6 | 8 | 10 | 12 | 3 | 7 | 9 | 10 | 14 | 3 | 9 | 7 | 10 |
| No. of obtained | 33 | 37 | 35 | 34 | 29 | 26 | 35 | 31 | 29 | 27 | 25 | 35 | 30 | 30 | 27 | 23 | 34 | 28 | 30 | 27 |
| % obtained | 89 | 100 | 95 | 92 | 78 | 70 | 95 | 84 | 78 | 73 | 68 | 95 | 81 | 78 | 73 | 62 | 92 | 76 | 81 | 73 |
Note. Cells with a negative (−) sign are indicative of “lost” channels at each time point in the study, whereas cells with a positive (+) sign are indicative of viable channels. PCA = posterior cricoarytenoid; LTA = left thyroarytenoid/lateral cricoarytenoid complex; RTA = right thyroarytenoid/lateral cricoarytenoid complex; LCT = left cricothyroid; RCT = right cricothyroid.
Descriptive Findings
Assessment of Physiological Stress Response by Experimental Stage
To assess whether physiologically and statistically meaningful stress responses could be detected across experimental stages, cardiovascular data (HR, SBP, DBP) were analyzed using a repeated-measures analysis of variance using a within-subject factor of Study Stage (True Baseline, Recovery, Baseline Rest, Baseline Subvoc, SPT, and Baseline Repeat; see Table 3). Mauchly's test indicated that the assumption of sphericity had been violated for SBP, X 2(2) = 55.247, p < .001, DBP, X 2(2) = 53.133, p < .001, and HR, X 2(2) = 185.082, p < .001; therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = .593, .670 and .307, respectively). A statistically significant main effect of Study Stage was observed for each dependent variable: SBP, F(2.967,106.81) = 77.82, p < .001, ηp 2 = .684, DBP, F(3.35,120.65) = 54.35, p < .001, ηp 2 = .602, and HR, F(1.54,55.29) = 99.32, p < .001, ηp 2 = .734.
Table 3.
Mean (SD) cardiovascular values by stage of experiment.
| Physiological measure | IV True Baseline | VI Recovery | VIIa Baseline Rest | VIIb Baseline Subvocalization | VIII SPT | IX Repeat Baseline |
|---|---|---|---|---|---|---|
| Heart rate | 63.82 (9.61) | 64.99 (9.61) | 65.01 (8.46) | 66.31 (9.11) | 86.07 (17.45) | 66.07 (8.94) |
| Systolic blood pressure | 104.32 (7.52) | 106.44 (7.88) | 104.45 (7.48) | 105.69 (8.12) | 118.02 (10.26) | 104.07 (8.163) |
| Diastolic blood pressure | 63.56 (6.40) | 65.80 (6.86) | 62.26 (7.59) | 64.33 (6.88) | 73.01 (8.99) | 60.77 (7.52) |
Note. SPT = speech preparation task.
Post hoc pair-wise comparisons using a least significant difference test showed that all three cardiovascular variables—SBP, DBP, and HR—were significantly higher during the speech preparation stressor (Stage VIII) compared to the other experimental stages. To summarize, physiological evidence of a stress response was shown in the direction and at times intended.
Perceived Stress and Anxiety by Experimental Stage
Self-reported Stress and Anxiety data were analyzed using a repeated-measures analysis of variance using a within-subject factor of Study Stage (Equipment Setup, Paced Breathing, True Baseline, ILM Electrode Placement, Baseline Rest, Baseline Subvoc, SPT, and Repeat Baseline). Mauchly's test indicated that the assumption of sphericity had been violated for both Stress, X 2(2) = 136.6, p < .001, and Anxiety, X 2(2) = 128.4, p < .001; therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = .561 and .516, respectively). A statistically significant main effect of Study Stage was observed for self-reported Stress, F(3.9, 149.2) = 42.829, p < .001, ηp 2 = .529, as well as for self-reported Anxiety, F(3.6,137) = 51.031, p < .001, ηp 2 = .573. Table 4 provides mean (SD) values for Stress and Anxiety at each stage in the experiment. In short, the SPT elicited levels of reported stress and anxiety that were comparable to placement of the fine wire electrodes into ILMs.
Table 4.
Mean (SD) self-reported stress and anxiety values by stage of experiment.
| Self-reported measure | Setup | III Paced Breathing | IV True Baseline | V Electrode Placement | VIIa Baseline Rest | VIIb Baseline Subvocalization | VIII SPT | IX Repeat Baseline |
|---|---|---|---|---|---|---|---|---|
| Stress | 8 (8) | 11 (12) | 4 (6) | 39 (25) | 10 (15) | 10 (10) | 39 (22) | 8 (12) |
| Anxiety | 10 (11) | 11 (11) | 5 (7) | 45 (26) | 10 (15) | 10 (11) | 41 (24) | 11 (19) |
Note. SPT = speech preparation task.
Post hoc pair-wise comparisons using a least significant difference test confirmed that self-reported Stress was significantly higher during two stages—ILM electrode placement (Stage V) and SPT (Stage VIII)—compared to all other experimental stages, which are as follows: Equipment Setup, Paced Breathing (Stage III), True Baseline (Stage IV), Baseline Rest (Stage VIIa), Baseline Subvoc (Stage VIIb), and Repeat Baseline (Stage IX). In addition, participants reported significantly more stress during the Paced Breathing task as compared to the True Baseline that immediately followed that task. Finally, participants self-reported significantly more stress during the True Baseline as compared to the Baseline Subvoc condition; neither of those conditions differed significantly from the Baseline Rest condition.
The same post hoc pair-wise comparisons were also examined for the construct of Anxiety. The findings mirrored those of self-reported Stress in that ILM Electrode Placement (Stage V) and the SPT (Stage VIII) elicited significantly higher Anxiety than all other stages of the experiment. Both the Equipment Setup and the Paced Breathing stages elicited significantly higher levels of self-reported Anxiety than did the True Baseline stage.
Respiratory Changes
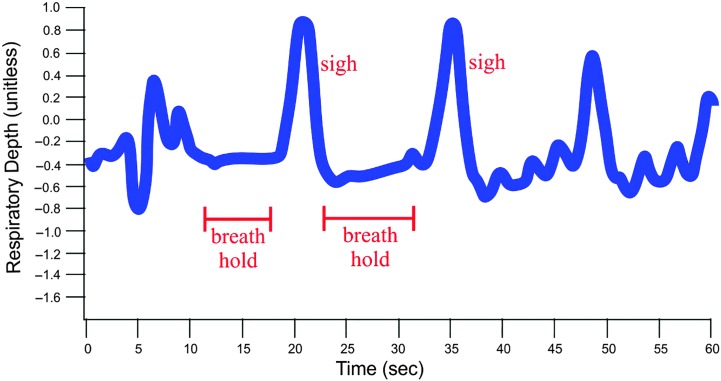
On average from baseline to stressor, the respiratory rate increased by 5 breaths/min. However, not all participants had a marked increase in respiratory rate during the stressor as compared to baseline; five participants even exhibited diminished respiratory rate. Breath-holding was one respiratory behavior observed during the SPT. As an example of this observation, Figure 3 shows the respiratory band signal for one participant as a green tracing (the red tracing is the electrocardiographic signal). It is evident that this particular subject held her breath for several seconds during the stressor. Specifically, note the apneic periods from approximately 11–18 s and 24–31 s on the y-axis.
Figure 3.
Tracing of a respiratory band signal showing breath-holding in one participant (EMG 60). EMG = electromyography.
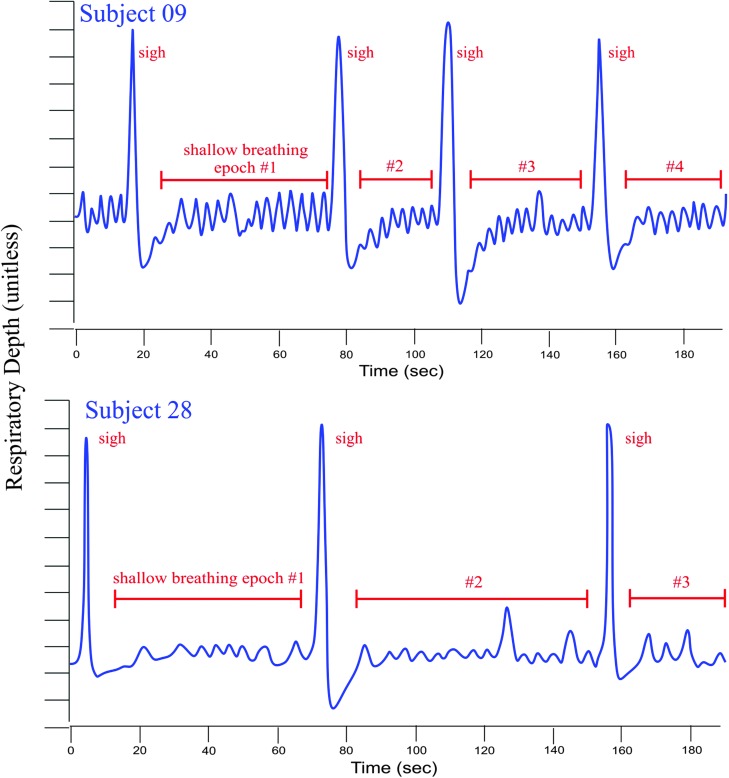
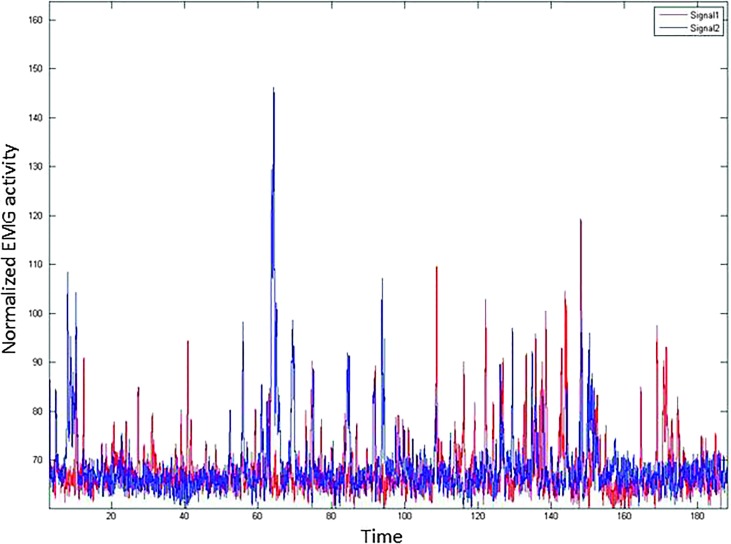
Another behavior commonly observed during the SPT was sighing, which typically refers to large, intermittent exhalations against a general backdrop of shallow breathing. Figure 4 illustrates two examples of sighing during the SPT epoch, each from a different participant. Note that, in the lower image, brief periods of near breath-holding follow each sigh. It is plausible that the statistically significant changes in laryngeal muscle activity were driven by some of these more nuanced respiratory behaviors—for example, TA/LCA activity increase in association with periods of breath-holding and PCA activity increase with each sigh—even if the respiratory rate per se is not driving the differences in laryngeal muscle activity.
Figure 4.
Tracings of respiratory band signals showing examples of sighing for two participants (EMG 09 and EMG 28). EMG = electromyography.
Primary Outcomes
Activity Change in Muscles of Interest From Baseline to Stressor Exposure
ITSA values were examined to determine the number of viable channels that exhibited statistically significant changes from Baseline Subvoc during the SPT. Table 5 provides this information. On the whole, all statistically significant changes were in the increased direction. Approximately two thirds of all participants showed a statistically significant increase in PCA, left TA/LCA, right TA/LCA, and trapezius muscle activity from Baseline Subvoc to the SPT condition. Fewer participants showed statistically significant increases in the tibialis (59%), left CT (50%), and right CT (32%) muscle activity.
Table 5.
Number of viable channels with significant change from Baseline Subvocalization (BL Subvoc) stage to speech preparation task (SPT).
| Variable analyzed | PCA | Left TA/LCA | Right TA/LCA | Left CT | Right CT | TPZ | TIB |
|---|---|---|---|---|---|---|---|
| No. of viable channels from 37 subjects | 23 | 33 | 30 | 30 | 28 | 37 | 37 |
| No. of subjects with significant change (BL Subvoc vs. SPT) | 16 | 21 | 18 | 15 | 9 | 24 | 23 |
| No. of (%) significant increase in activation (vs. decrease) | 16 (69) | 21 (64) | 18 (60) | 15 (50) | 9 (32) | 24 (65) | 22 (59) |
Note. PCA = posterior cricoarytenoid; TA/LCA = thyroarytenoid/lateral cricoarytenoid complex; CT = cricothyroid; TPZ = trapezius; TIB = tibialis.
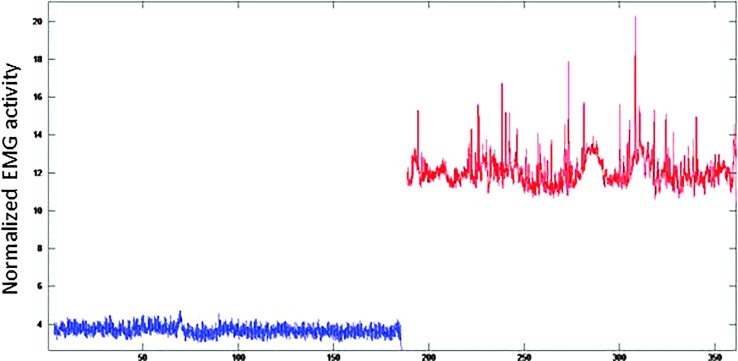
Although a good deal of variability in muscle response was qualitatively observed upon inspection of the EMG waveforms, a common pattern of response was increased baseline level of activation as well as increased “spikes” of activation. Figure 5 shows representative waveforms for one participant's TA muscle activity during the 3-min Baseline Subvoc period (blue waveform) as compared to the 3-min SPT (red waveform). In this image, the increased baseline and increased “spikes” can be appreciated.
Figure 5.
Representative example of change in thyroarytenoid/lateral cricoarytenoid muscle activity from 3-min baseline (blue signal) to 3-min stressor (red signal). EMG = electromyography.
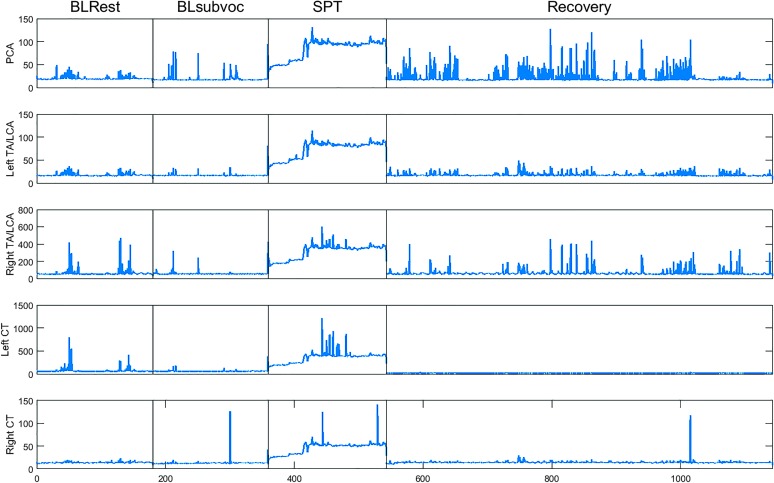
In addition, Figure 6 shows activity for each of these laryngeal muscles across the key experimental conditions. Note that both baseline conditions are generally comparable in terms of muscle activity, which then ramps up to more elevated levels during the SPT and returns to a lower baseline level of activity during the recovery phase.
Figure 6.
Representative example of laryngeal muscle activity across key conditions for one participant. Baseline Rest (BLRest), Baseline Subvocalization (BLsubvoc), and speech preparation task (SPT) epochs lasted 3 min each, and the Repeat Baseline (i.e., Recovery) epoch lasted 10 min. PCA = posterior cricoarytenoid; TA/LCA = thyroarytenoid/lateral cricoarytenoid complex; CT = cricothyroid.
To further characterize the change in muscle activity from Baseline Subvoc to SPT in the present cohort, effect sizes were examined as a function of statistical significance based on ITSA. Table 6 provides the descriptive statistics (mean, minimum, maximum, and SD of effect sizes) for all the muscles deemed to have viable signals. These data are further separated within Table 6 by statistical significance versus nonsignificance. The effect size values were calculated according to Cohen (1992) by measuring the distance between the two means (i.e., Baseline Subvoc and SPT) and dividing by the pooled standard deviations for the samples. Note that the effect sizes for ILMs were generally greater than for either of the control sites.
Table 6.
Effect sizes for all viable muscles.
| Descriptive statistics | PCA | Left TA/LCA | Right TA/LCA | Left CT | Right CT | TPZ | TIB |
|---|---|---|---|---|---|---|---|
| Effect sizes for muscles with statistically significant changes | |||||||
| n = 16 | n = 21 | n = 18 | n = 15 | n = 9 | n = 24 | n = 23 | |
| Mean | 3.84 | 4.55 | 5.15 | 3.80 | 4.20 | 0.98 | 2.70 |
| Min | 0.03 | 0.10 | 0.90 | 0.33 | 0.27 | 0.04 | 0.03 |
| Max | 11.85 | 15.59 | 18.00 | 8.26 | 24.88 | 3.19 | 13.37 |
| SD | 3.57 | 4.38 | 4.39 | 2.60 | 8.01 | 0.93 | 3.60 |
| Effect sizes for muscles with nonsignificant changes | |||||||
| n = 7 | n = 14 | n = 12 | n = 15 | n = 18 | n = 13 | n = 14 | |
| Mean | 0.39 | 0.21 | 0.85 | 1.19 | 0.92 | 0.32 | 0.40 |
| Min | −0.66 | −1.64 | −0.33 | 0.02 | −1.05 | 0.001 | 0.005 |
| Max | 1.55 | 1.57 | 6.48 | 4.91 | 2.71 | 1.16 | 0.97 |
| SD | 0.69 | 0.77 | 1.84 | 1.34 | 0.93 | 0.36 | 0.31 |
Note. PCA = posterior cricoarytenoid; TA/LCA = thyroarytenoid/lateral cricoarytenoid complex; CT = cricothyroid; TPZ = trapezius; TIB = tibialis.
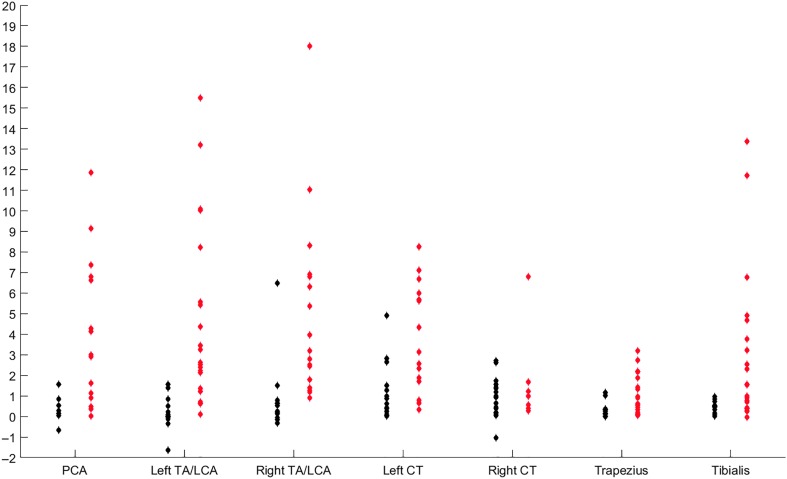
These effect size data are shown in Figure 7 for each muscle to illustrate the nature of the data spread. One outlying data point—an effect size of 24.88 for the right CT muscle in Subjection 12—was omitted from this graph to avoid further condensing the lower effect sizes.
Figure 7.
Effect size data from Baseline Subvocalization to speech preparation task for all participants. Data in red represent participants with statistically significant changes across conditions, and those in black showed no statistically significant change. PCA = posterior cricoarytenoid; TA/LCA = thyroarytenoid/lateral cricoarytenoid complex; CT = cricothyroid.
Finally, Table 7 provides the following information by subject: statistical significance of muscle activity change from Baseline Subvoc to SPT (based on ITSA calculations), change in HR (beats/min, HRΔ) from Baseline Subvoc to SPT, change in SBP and DBP (mm Hg, SBPΔ and DBPΔ, respectively), and change in respiratory rate from Baseline Subvoc to SPT. For each muscle, statistically significant and nonsignificant ITSA findings are represented by “SIG” and “NO,” respectively, and are color coded to assist with review. All difference (Δ) scores were calculated by subtracting the Baseline Subvoc values from the SPT values. Missing data are represented by “—.”
Table 7.
Muscle, cardiovascular, respiratory, and stress reactivity data by subject.
| Subject ID | PCA | Left TA/LCA | Right TA/LCA | Left CT | Right CT | TPZ | TIB | HRΔ | SBPΔ | DBPΔ | RRΔ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | NO | NO | NO | NO | SIG | SIG | NO | 11.92 | 10.50 | 3.92 | −1.44 |
| 02 | SIG | SIG | NO | SIG | NO | SIG | SIG | 24.93 | 15.00 | 12.93 | 5.10 |
| 03 | — | NO | SIG | SIG | NO | SIG | SIG | 14.27 | 18.60 | 10.40 | 3.78 |
| 04 | — | NO | NO | SIG | NO | SIG | SIG | 26.25 | 18.95 | 13.00 | 4.16 |
| 09 | SIG | NO | — | — | NO | SIG | SIG | 24.83 | 13.17 | −0.58 | 0.19 |
| 10 | — | — | NO | NO | NO | NO | NO | 30.65 | 21.95 | 16.45 | — |
| 11 | — | NO | SIG | SIG | NO | NO | NO | 19.27 | 15.47 | 14.40 | 3.00 |
| 12 | SIG | SIG | — | NO | SIG | SIG | SIG | 24.80 | 12.00 | 4.20 | 9.33 |
| 17 | SIG | SIG | NO | SIG | NO | SIG | SIG | 6.58 | 12.33 | 3.83 | 5.79 |
| 18 | SIG | SIG | — | SIG | SIG | SIG | SIG | 48.67 | 14.73 | 18.53 | 6.35 |
| 19 | — | NO | NO | — | SIG | NO | NO | 7.67 | 12.33 | 9.58 | 3.73 |
| 20 | NO | NO | NO | NO | — | NO | NO | 45.33 | 27.27 | 1.13 | — |
| 22 | NO | SIG | NO | NO | NO | SIG | NO | 16.33 | 10.40 | 14.67 | −3.81 |
| 24 | — | NO | NO | NO | — | NO | NO | 17.20 | 6.80 | 7.07 | 15.62 |
| 26 | SIG | SIG | SIG | NO | NO | NO | NO | 22.92 | 6.50 | 12.42 | 2.91 |
| 28 | SIG | SIG | SIG | SIG | NO | SIG | SIG | 22.87 | 14.53 | 10.87 | 3.67 |
| 29 | SIG | — | SIG | — | SIG | SIG | SIG | 47.67 | 20.80 | 14.20 | 3.43 |
| 31 | — | NO | — | NO | — | SIG | NO | 16.25 | 21.17 | 15.17 | 0.27 |
| 32 | — | SIG | NO | NO | — | SIG | SIG | 13.87 | 8.53 | 7.33 | — |
| 34 | SIG | NO | SIG | SIG | NO | SIG | SIG | 7.50 | −0.67 | 7.75 | 10.05 |
| 35 | — | SIG | SIG | NO | NO | NO | NO | 11.67 | 8.33 | 1.67 | 4.29 |
| 36 | SIG | NO | SIG | NO | — | NO | SIG | 8.87 | 10.13 | 11.40 | 8.23 |
| 39 | SIG | SIG | SIG | NO | SIG | NO | SIG | 23.87 | 15.87 | 8.73 | — |
| 48 | SIG | SIG | — | — | — | SIG | SIG | 28.93 | 22.73 | 14.20 | 14.61 |
| 49 | SIG | SIG | SIG | SIG | SIG | SIG | SIG | 20.25 | 4.92 | 7.92 | 9.92 |
| 50 | — | SIG | NO | SIG | SIG | SIG | SIG | 8.20 | 7.33 | 4.20 | 4.56 |
| 52 | NO | NO | — | SIG | NO | NO | NO | 22.40 | 20.55 | 15.90 | 16.48 |
| 55 | SIG | SIG | SIG | NO | NO | SIG | SIG | 13.25 | 6.58 | 4.42 | 18.72 |
| 58 | NO | NO | — | NO | NO | NO | NO | 18.75 | 23.58 | 25.00 | 10.27 |
| 60 | SIG | SIG | — | SIG | — | SIG | SIG | 37.95 | 8.20 | 3.85 | −5.84 |
| 66 | SIG | SIG | SIG | — | NO | SIG | SIG | 31.00 | 20.93 | 15.80 | 8.00 |
| 71 | — | SIG | SIG | SIG | — | SIG | SIG | 7.27 | 2.13 | 3.73 | 5.39 |
| 72 | SIG | SIG | SIG | NO | SIG | SIG | SIG | 39.95 | 13.85 | 2.55 | 10.45 |
| 74 | — | SIG | NO | — | — | NO | NO | 36.40 | 8.33 | 2.27 | 3.85 |
| 75 | SIG | SIG | SIG | — | — | SIG | SIG | 16.50 | 5.75 | 5.75 | 2.99 |
| 76 | SIG | SIG | SIG | SIG | NO | SIG | SIG | 14.27 | 29.67 | 21.80 | 6.69 |
| 78 | NO | NO | NO | SIG | NO | NO | NO | 34.60 | 17.53 | 3.27 | 11.59 |
Note. PCA = posterior cricoarytenoid; TA/LCA = thyroarytenoid/lateral cricoarytenoid complex; CT = cricothyroid; TPZ = trapezius; TIB = tibialis; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; RR = respiratory rate; NO = not significant; SIG = significant; — indicates missing data/channels.
Resolution Latency
Resolution Latency was calculated as planned and found to have extremely poor distribution. Specifically, for all muscles in most participants, activity had returned to baseline by the very beginning of the 10-min Basline Repeat task. In fact, for any given muscle including the positive and negative control muscles, only one participant took longer than the first 30 s of the Baseline Repeat task to return to her baseline level of muscle activity. Visual inspection of waveforms during each task confirmed that the ITSA output was accurate. Figure 8 shows an example of a “typical” participant's (Subject 60, TA/LCA muscle) waveforms comparing Baseline Rest (red signal) and (the first 3 min of) the Repeat Baseline (blue signal) epochs. Note that both signals are comparable in terms of average amplitude and relative size and number of spikes throughout the sample. From the end of the SPT stressor to the beginning of the Baseline Repeat task, approximately 3–5 min passed while the participant was debriefed by the investigator, the second set of voluntary contraction tasks was performed, and the staging for the Baseline Repeat task was prepared. Presumably it was during this period, when no physiological recording was conducted, that muscles resumed their baseline activity levels. Because of the poor distribution of this dependent variable, Resolution Latency was not further explored.
Figure 8.
Representative data sample of Baseline Rest (red) and Repeat Baseline (blue) signals, which showed no statistically significant difference via interrupted time-series analysis. Both signals, lasting just over 3 min, generally exhibit the same average activity level in terms of overall amplitude and individual spike amplitude. EMG = electromyography.
Discussion
Primary Outcomes
As anticipated, the SPT stressor elicited strong cardiovascular responses in this cohort. All participants responded to the SPT task with a statistically significant increase in HR compared to values collected during their True Baseline. The average magnitude of the change in HR—about 22 beats/min—was just slightly lower than the 26 beats/min increase observed by Kirschbaum et al. (1993) in their laboratory investigation of the Trier Social Stress Test, on which the psychological stressor in this study was based. The fact that the methods in this study approximated nearly the same magnitude of HR response as the original Trier Social Stress Test is notable, because this study involved no actual delivery of a speech, which was the element of the Trier Social Stress Test that involved the fastest HR across participants. Furthermore, participants are typically instructed to stand as part of the Trier Social Stress Test, which would also increase the cardiovascular response to the task, yet participants in this study remained in a semireclined position for the duration of the experiment. Despite the semireclined posture, blood pressure measures also reflected the desired physiological stress response. With the exception of two cases in which a negligible increase was observed in SBP (n = 1) and DBP (n = 1), each of these cardiovascular measures also increased from baseline during the SPT stressor. It should be noted that, in these two cardiovascular “nonresponders,” a significant increase was observed in the laryngeal muscles from baseline to SPT stressor. For the entire cohort, SBP increased about 12 mm Hg from True Baseline to SPT, and DBP increased about 10 mm Hg. The magnitude of these cardiovascular changes was medium to large based on the interpretation of effect sizes according to Cohen (1992). Overall, the cardiovascular responses observed were consistent with “classic” stress responses as seen, for example, in increased force and pace of cardiac contraction and skeletal muscle vasodilation (Herd, 1991).
In addition to these cardiovascular indicators that the SPT was successful in eliciting a stress response, participants consistently reported increased Stress and Anxiety during the SPT. All but two participants reported an increase in self-perceived Stress and Anxiety as measured by an undifferentiated visual analog scale, and as a group, these increases were found to be statistically significant. Interestingly, despite reporting less Stress and Anxiety during the SPT compared to Baseline Rest, these two participants did exhibit clinically significant increases in HR (16 and 25 beats/min), SBP (21 and 12 mm Hg), and DBP (15 and 4 mm Hg) that are consistent with physiological stress responses. In fact, the perceived Stress and Anxiety during the SPT was, on average, about as much as was experienced during the insertion of needles in the neck. According to Cohen's interpretation of effect sizes (Cohen, 1992), a medium effect size was observed for both variables.
Characterizing the Muscular Response to SPT
As anticipated, given the proposed causal model wherein psychological stress leads to increased laryngeal muscle activity, the SPT task elicited statistically significant changes in muscle activity (compared to Baseline Subvoc) for many individuals, in one or more of the five ILMs tested (PCA, left and right TA/LCA muscle complex, and left and right CT). Approximately two thirds of subjects exhibited increased “responsiveness” in the PCA and bilateral TA/LCA muscles. These findings are debatably consistent with those of Dietrich and Verdolini Abbott (2008, 2012), who reported increases in both submental and infrahyoid muscle activity during a public speech stressor but who did not see statistically significant increases in activity of these muscles during preparation for that stressor, a condition that was more comparable (than their stressful speech task) to the stressor in this study. It is plausible that in “front-loading” the stressful elements of the SPT task in our nonverbal condition, we successfully approximated the level of stress elicited by Dietrich and Verdolini Abbott in their spoken stressor condition, which was our intent. It is also possible that the extrinsic laryngeal muscles (i.e., submental complex and infrahyoid muscles) are less sensitive to stress than the ILMs (i.e., PCA and TA/LCA), such that extralaryngeal stress responses are only evident when speech is involved. To definitively make this claim, though, we would have to present data regarding extralaryngeal muscle activity during our stressor. Future investigations might seek to clarify the extent to which intra- and extralaryngeal musculature show complimentary stress responses.
Also in contrast to the findings of Dietrich and Verdolini Abbott (2012), statistically significant changes were seen in activity of the positive and negative control muscles (upper trapezius and anterior tibialis, respectively) for approximately two thirds of all participants. The overwhelming majority of these changes—all but one subject whose tibialis muscle activity decreased during the stressor—involved increased muscle activity (as opposed to decreased muscle activity) from baseline to SPT stressor. Further discussion of this finding is forthcoming.
Although most participants in this study exhibited increased activity in the PCA and TA/LCA muscle(s), only one third to one half (depending on side) of participants showed increased activity in the CT muscle, that is, the right CT muscle activity increased in only 32% of participants, as compared to 50% for the left CT muscle. It is entirely possible that these findings are representative of the actual situation.
We acknowledge two technical reasons why the right CT placements might have been suboptimal. First, hook wire electrodes were always placed in the same order: PCA, left TA/LCA, right TA/LCA, left CT, and right CT. Although the participants in this study tolerated the needle-guided hook wire electrode placement quite well, it is a decidedly unpleasant experience. For hook wire electrode placement, any time an electrode is guided too far afield of the target muscle, the needle must be removed, the electrode discarded, and a new needle–electrode set must be reintroduced. By the time the electrodes were introduced to the CT muscles, many participants were reaching a limit on their tolerance for the procedure, and we were highly sensitive to their comfort. Thus, we were more likely to accept a suboptimal placement in the CT muscles as compared to the PCA and TA/LCA complex. Second, from a technical perspective, optimal needle localization of electrodes in the right CT muscle was simply more challenging for our right-handed laryngologist.
Some of the discussion about incongruent CT activity can be extended to incongruent findings across any other bilaterally sampled muscles (i.e., PCA and TA/LCA). For instance, if an electrode is placed suboptimally, for example, such that it is situated closer to the point of attachment rather than seated firmly in the belly of a muscle, then the signals might be insufficient to rise above the ambient biological noise. In particular, given the absence of overt muscle activation such as that in a voice or speech task, true increases in laryngeal muscle activity might easily be missed if the electrode placement is less than perfect. Thus, it remains to be known if the asymmetrical activation of muscles observed in this study (e.g., in right- vs. left-sampled muscles) is a true phenomenon or an error product stemming from the known technical limitations of hook wire EMG.
Magnitude of Change in Laryngeal Muscle Activity Cannot Be Explained by Respiratory Rate
The activity of the PCA and TA/LCA muscles increased significantly from Baseline Subvoc to SPT in two thirds of the participants studied. Moreover, these two muscles were largely congruent in their responses. That is, if the PCA activity increased, so did the TA/LCA activity, and if the PCA was nonresponsive to the stressor, then the TA/LCA tended to also be nonresponsive. The CT, on the other hand, was nonresponsive during stressor exposure in about half of participants, and its activity was only congruent with the activity of the other two muscles about half of the time.
One tempting explanation for the increased activity of these laryngeal muscles during the SPT is that these muscles were simply responding to accommodate the increased respiratory rate observed during the stressor. After all, on average from baseline to stressor, the respiratory rate increased by 5 breaths/min. However, simply considering the average change in respiratory rate is not the most appropriate approach to understanding the respiratory behaviors associated with the SPT in this study. Although the average increase was 5 breaths/min, some participants' respiratory rate increased more than 10 breaths/min from baseline to SPT. Hillel (2001) reported increased activity of the PCA, TA, and CT muscles during “fast breathing” as compared to “slow breathing.”
To summarize Hillel's study, it was shown that during “slow breathing” in a cohort of healthy participants, “mild activity” was observed in the PCA of two out of eight subjects, and “very mild” activity was observed in three of eight subjects' CT muscles. This muscle activity was observed only during inspiration, and no muscle activity was observed during expiration in slow breathing. The TA muscle was not active at all during slow breathing. During “fast” breathing, the PCA was active during both inspiration and expiration for all subjects (n = 8), the TA was active during inspiration in nine of 10 subjects and silent during expiration for all subjects, and the CT was active during inspiration in all subjects (n = 9) and less vigorous but still active during expiration in four of nine subjects (Hillel, 2001).
An important limitation prevents interpretation of Hillel's (2001) findings relative to the findings in this study. That is, the actual rate of breathing in Hillel's “slow” and “fast” conditions was not noted. Three images provided as figures in that manuscript included a scale marker for the x-axis of the EMG signals. Using the scale markers in these images to calculate breathing rate, it appears that the “slow” breathing occurred at approximately 17 breaths/min and “fast” breathing occurred at approximately 48 breaths/min. Neither the average nor the range of respiratory rate for these conditions was given. Importantly, no one in this study breathed as fast as 48 breaths/min. In fact, the fastest breathing rate during the SPT was about 20 breaths less than Hillel's apparent “fast” rate at 28.72 breaths/min.
If the changes in laryngeal muscle activity in this study were solely due to their role in valving for ventilatory purposes, it would stand to reason that those breathing within the “slow” range during the SPT might not exhibit significantly increased laryngeal muscle activation, as reported by Hillel. Recall that, in Hillel's study, the “slow breathing” condition was associated with very little muscle activation at all, even in the PCA, and this judgment was made by visual assessment of the EMG waveform. In fact, by Hillel's apparent criteria, “slow breathing” was very common during the SPT in this study, as 14 out of 32 of our participants breathed at ≤ 18 breaths/min during this condition. Nonetheless, these particular participants generally exhibited statistically significant increases in activity of the PCA (7/10) and at least one of the TA/LCA (11/14) and CT (7/11) muscles. In fact, the laryngeal muscles studied globally increased in activity even for the participants whose respiratory rate decreased during SPT compared to baseline. Moreover, for all of our participants, if increased laryngeal muscle activity was a direct function of respiratory rate, then Hillel's findings would lead us to expect more overall involvement of the CT muscle across participants. Hillel observed increased engagement of the CT muscle during both inspiration and expiration from slow to fast breathing, whereas none of the TA muscles were engaged during expiration in either task. However, in our study, the TA/LCA muscle was more active in more participants during the SPT as compared to the CT muscle. Thus, it appears that the increase of laryngeal muscle activity in this study cannot justifiably be attributed solely to changes in respiration rate.
Building on the observation that the laryngeal muscle activity changes from baseline to stressor do not appear to be cleanly linked to respiration rate changes, it should be noted that exploratory correlational analyses of respiratory rate and magnitude of change in muscle activity did not show statistically significant relationships for all muscles. If the role of the laryngeal muscles in pulmonary valving is to be considered a causal reason for their increased activity from baseline to stressor, it is critical that analyses include more than simply examining average changes in respiratory rate.
Recovery Latency in Laryngeal Muscles
Regardless of the magnitude of the stress response in the laryngeal muscles studied, it appears that it does not last longer than a few minutes, at least in a healthy cohort such as the one in this study. Nearly all muscles studied for all participants had returned to their approximate Baseline Rest values by the time the Repeat Baseline task commenced. Preliminary data suggested that, in most subjects, laryngeal muscle activity following exposure to the cold pressor task (a physical stressor) remained elevated for at least 3 min after the stressor ceased (Helou et al., 2013). This elevation in laryngeal muscle activity remained even after HR had returned to normal. It cannot be ruled out that a recovery latency of 1–5 min did actually occur in this study, as in our preliminary study (Helou et al., 2013). However, it is a limitation of this study that laryngeal muscle activity was not recorded during the several minutes of debriefing subsequent to the SPT, which would have allowed observation of how and when muscle activity returned to baseline.
Nonetheless, given the fact that the women in this study had no apparent history of voice disorders or psychological disorders, it is not surprising that they would recover quickly and fully following cessation of the stressor. The resolution latency measures may have been less homogeneous if measured in a cohort of voice-disordered patients (e.g., patients with muscle tension dysphonia), as observed in studies comparing patients with chronic trapezius myalgia to controls (Sjörs, Larsson, Dahlman, Falkmer, & Gerdle, 2009). A final consideration is that the experimental act of debriefing might have facilitated more rapid recovery than what would have been observed in a real-world situation and as compared to in the previous study involving the cold pressor task.
Control Muscles' Response to SPT
The activity of both the positive control muscle (upper trapezius) and the negative control muscle (anterior tibialis) generally increased from Baseline Subvoc to SPT. The increased activity of the trapezius muscle was anticipated as it is a well-known “stress responder” (Stephenson & Maluf, 2010; Westgaard, 1999), but the increased anterior tibialis activity was somewhat more surprising. Not only was the total number of “responders” in the present cohort comparable for the two muscles, but the anterior tibialis muscle activity had a greater overall effect size than that of the upper trapezius.
Nevertheless, it is well within reason to anticipate skeletal muscle responses to stress throughout the body. Anderson and colleagues demonstrated evidence of increased sympathetic efferent nerve activity to the leg during mental stress and that the sympathetic outflow to the leg was substantially greater than that to the arm, in which sympathetic outflow peaked after the stressor (Anderson, Wallin, & Mark, 1987). Especially germane to our findings in the anterior tibialis muscle, some investigators have found that, in anxious subjects, muscles high in activity at baseline remained high and exhibited relatively little change during psychological stress, whereas muscles that were low in activity at baseline increased to a greater extent during the stressor (Balshan, 1962; Hoehn-Saric, McLeod, & Zimmerli, 1989). This response was in accordance with the law of initial values (Wilder, 1962) and has been observed in other physiological domains than EMG (e.g., see Hoehn-Saric & McLeod, 2000). Because the EMG values in this study were not normalized to maximal contraction, we cannot investigate further to determine if a similar phenomenon existed in our data set.
A few additional explanations for the unanticipated findings regarding anterior tibialis activity are possible. The most likely reason regards the role of posture in each muscle's response. In the study by Dietrich that used the anterior tibialis muscle as a negative control site (Dietrich & Verdolini Abbott, 2008, 2012), participants sat in a wheelchair with their feet positioned in foot rests throughout the experiment. The downward bearing of participants' leg weight and gravity likely served to minimize anterior tibialis muscle activity. Conversely, in this study, participants' legs were elevated and supported by the extended exam chair, so that the legs were generally horizontal to the floor with a slight bend in the knee allowed for participant comfort. Relative to Dietrich's study conditions, this study allowed for more free movement of the leg. It should also be noted that, apart from systematic reminders by the investigator to “keep your body still and quiet” (these reminders occurred regularly at the beginning and the midpoint of each baseline and stressor condition), participants were not specifically asked or advised to keep their legs still, and some did exhibit shaking of the foot (a natural secondary coping mechanism for some individuals) during the SPT condition. Conversely, movements of the head and neck region were not noted with the same magnitude or frequency.
Also related to the proposal that posture might have contributed to the discrepant findings between upper trapezius and anterior tibialis activity, the reclined position of the subjects may have attenuated trapezius response to stress. For the present experiment, participants were generally reclined at a 120°–140° seated angle. Other investigators have shown that the response of the upper trapezius muscle to physical and mental stress is decreased by lying in the supine position (McCann, Wootten, Kadaba, & Bigliani, 1993; Rubini, Paoli, & Parmagnani, 2012), elbow support and/or arm suspension (Schüldt, 1988), and neck and head support (e.g., Yoo, Lee, Jung, & Yang, 2011). Another study that aimed to assess the ability of cervical traction to treat trapezius myalgia conceded inconclusive findings, because once the patients complaining of shoulder pain were put in the supine position to receive the treatment, the electrical activity of the trapezius was “completely silenced” and thus could not be improved upon with treatment (Jette, Falkel, & Trombly, 1985).
As with the laryngeal muscles, the resolution latency of the two control muscles indicated that their recovery was apparently rapid and complete. Nilsen, Sand, Stovner, Leistad, and Westgaard (2007) implemented a reaction time task in healthy participants and found statistically significant increase in trapezius muscle activity, although the effect did not last once the task ceased. Relatively rapid recovery of the trapezius muscle following a stressor was also observed in a group of controls compared to a cohort with chronic trapezius myalgia (Sjörs et al., 2009). These findings are consistent with our proposal that, in a healthy cohort, resolution latency may show little variation across individuals.
Conclusions
This study provides direct experimental evidence to support the hypothesis that the ILMs are responsive to stress in some individuals. These findings appear to support a perspective that increased laryngeal muscular activity might be closely linked to stress when stress is present. Furthermore, because both adductor and abductor muscles were often coactivated during the stressor, it remains to be seen if the increased laryngeal muscle activity would result in (a) an adducted “sphincterlike” form, (b) an abducted “splinted” form, or (c) no apparent change in glottal configuration. Such various distinctions have been made by other investigators. All laryngeal muscle activity returned to normal within 3 min of stressor removal, showing that laryngeal stress responses occur and resolve quickly in vocally healthy women. Finally, this study shows that some vocally healthy female adults might very well be classified as “laryngeal responders” in the face of a stressor, whereas others are not similarly vulnerable. Future studies should include a cohort of individuals with voice disorders, a vocalization and/or speech condition, and a laryngeal visualization to determine the “final product” of laryngeal muscle coactivation.
Acknowledgments
This study was completed at the University of Pittsburgh in partial fulfillment of requirements for the first author's doctoral dissertation. This study was partially supported by the School of Health and Rehabilitation Science Doctoral Award (Helou), a research grant from the Voice Foundation (Helou), the University of Pittsburgh Medical Center Rehabilitation Institute (Wang), and the National Institutes of Health (Grants 3R01NS050256-05S1 [Wang], 8KL2TR000146 [Wang], and R01 DC008567 [Verdolini Abbott]). The authors wish to acknowledge the efforts of Adrianna Shembel and Catherine Bean for their assistance with data collection and Neil Szuminsky for his assistance with fine wire electrode design and construction.
Funding Statement
This study was completed at the University of Pittsburgh in partial fulfillment of requirements for the first author's doctoral dissertation. This study was partially supported by the School of Health and Rehabilitation Science Doctoral Award (Helou), a research grant from the Voice Foundation (Helou), the University of Pittsburgh Medical Center Rehabilitation Institute (Wang), and the National Institutes of Health (Grants 3R01NS050256-05S1 [Wang], 8KL2TR000146 [Wang], and R01 DC008567 [Verdolini Abbott]).
Footnotes
The trapezius muscle increases activation in the face of a stressor and is considered to be a highly stress-responsive muscle (Johansson & Sojka, 1991; Lundberg et al., 2002).
The anterior tibialis was specifically selected as a negative control point because it is not “classically” considered a stress responsive muscle, it is not functionally related to any of the tasks used in the present study, and others have successfully used it as a negative control in methodologically and theoretically similar research paradigms (Dietrich, 2008; Dietrich & Verdolini Abbott, 2012).
The thyroarytenoid and lateral cricoarytenoid are anatomically fused muscles (Zemlin, 1998) that activate for the same functional tasks. As such, the muscles are difficult to differentiate even in anatomical dissection and impossible to truly disambiguate via voluntary contraction tasks or based on external landmarks. Electrode insertion in this study was sufficiently lateral that insertion of electrodes into the thyrovocalis muscle was highly improbable.
References
- Aarons L. (1971). Subvocalization: Aural and EMG feedback in reading. Perceptual and Motor Skills, 33(1), 271–306. [DOI] [PubMed] [Google Scholar]
- Altman K. W., Atkinson C., & Lazarus C. (2005). Current and emerging concepts in muscle tension dysphonia: A 30-month review. Journal of Voice, 19(2), 261–267. https://doi.org/10.1016/j.jvoice.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Anderson E. A., Wallin B. G., & Mark A. L. (1987). Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension, 9(6 Pt. 2), III114–III119. [DOI] [PubMed] [Google Scholar]
- Balshan I. D. (1962). Muscle tension and personality in women. Archives of General Psychiatry, 7, 436–448. [DOI] [PubMed] [Google Scholar]
- Christenfeld N., Glynn L. M., & Gerin W. (2000). On the reliable assessment of cardiovascular recovery: An application of curve-fitting techniques. Psychophysiology, 37(4), 543–550. [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Crosbie J. (1993). Interrupted time-series analysis with brief single-subject data. Journal of Consulting and Clinical Psychology, 61(6), 966–974. [DOI] [PubMed] [Google Scholar]
- Dickerson S. S., & Kemeny M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. https://doi.org/10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dietrich M. (2008). The effects of stress reactivity on extralaryngeal muscle tension in vocally normal participants as a function of personality (Doctoral dissertation). University of Pittsburgh, Pittsburgh, PA. [Google Scholar]
- Dietrich M., & Verdolini Abbott K. (2008). Psychobiological framework of stress and voice: A psychobiological framework for studying psychological stress and its relation to voice disorders. In Izdebski K. (Ed.), Emotions in the human voice (Vol. II). San Diego, CA: Plural Publishing. [Google Scholar]
- Dietrich M., & Verdolini Abbott K. (2012). Vocal function in introverts and extraverts during a psychological stress reactivity protocol. Journal of Speech, Language, and Hearing Research, 55, 973–987. https://doi.org/10.1044/1092-4388(2011/10-0344) [DOI] [PubMed] [Google Scholar]
- Duchesne A., Tessera E., Dedovic K., Engert V., & Pruessner J. C. (2012). Effects of panel sex composition on the physiological stress responses to psychosocial stress in healthy young men and women. Biological Psychology, 89(1), 99–106. https://doi.org/10.1016/j.biopsycho.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Egizio V. B., Eddy M., Robinson M., & Jennings J. R. (2011). Efficient and cost-effective estimation of the influence of respiratory variables on respiratory sinus arrhythmia. Psychophysiology, 48(4), 488–494. https://doi.org/10.1111/j.1469-8986.2010.01086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeschels E. (1952). Chewing method as therapy; a discussion with some philosophical conclusions. A.M.A. Archives of Otolaryngology, 56(4), 427–434. [DOI] [PubMed] [Google Scholar]
- Gruenewald T. L., Kemeny M. E., Aziz N., & Fahey J. L. (2004). Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine, 66(6), 915–924. https://doi.org/10.1097/01.psy.0000143639.61693.ef [DOI] [PubMed] [Google Scholar]
- Hecker M. H., Stevens K. N., von Bismarck G., & Williams C. E. (1968). Manifestations of task-induced stress in the acoustic speech signal. The Journal of the Acoustical Society of America, 44(4), 993–1001. [DOI] [PubMed] [Google Scholar]
- Helou L. B., Wang W., Ashmore R. C., Rosen C. A., & Abbott K. V. (2013). Intrinsic laryngeal muscle activity in response to autonomic nervous system activation. The Laryngoscope, 123, 2756–2765. https://doi.org/10.1002/lary.24109 [DOI] [PubMed] [Google Scholar]
- Herd J. A. (1991). Cardiovascular response to stress. Physiological Reviews, 71(1), 305–330. [DOI] [PubMed] [Google Scholar]
- Hillel A. D. (2001). The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. The Laryngoscope, 111(4 Pt. 2, Suppl. 97), 1–47. https://doi.org/10.1097/00005537-200104001-00001 [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Holmberg E. B., Perkell J. S., Walsh M., & Vaughan C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech and Hearing Research, 32(2), 373–392. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R., & McLeod D. R. (2000). Anxiety and arousal: Physiological changes and their perception. Journal of Affective Disorders, 61(3), 217–224. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R., McLeod D. R., & Zimmerli W. D. (1989). Somatic manifestations in women with generalized anxiety disorder. Psychophysiological responses to psychological stress. Archives of General Psychiatry, 46(12), 1113–1119. [DOI] [PubMed] [Google Scholar]
- Jette D. U., Falkel J. E., & Trombly C. (1985). Effect of intermittent, supine cervical traction on the myoelectric activity of the upper trapezius muscle in subjects with neck pain. Physical Therapy, 65(8), 1173–1176. [DOI] [PubMed] [Google Scholar]
- Johansson H., & Sojka P. (1991). Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: A hypothesis. Medical Hypotheses, 35(3), 196–203. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K. M., & Hellhammer D. H. (1993). The “Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Laukka P., Linnman C., Åhs F., Pissiota A., Frans Ö., Faria V., … Furmark T. (2008). In a nervous voice: Acoustic analysis and perception of anxiety in social phobics' speech. Journal of Nonverbal Behavior, 32(4), 195–214. https://doi.org/10.1007/s10919-008-0055-9 [Google Scholar]
- Lovallo W. (1975). The cold pressor test and autonomic function: A review and integration. Psychophysiology, 12(3), 268–282. [DOI] [PubMed] [Google Scholar]
- Lovallo W. (1997). Stress & health: Biological and psychological interactions. London, United Kingdom: Sage. [Google Scholar]
- Lundberg U., Forsman M., Zachau G., Eklof M., Palmerud G., Melin B., & Kadefors R. (2002). Effects of experimentally induced mental and physical stress on motor unit recruitment in the trapezius muscle. Work and Stress, 16(2), 166–178. [Google Scholar]
- McCann P. D., Wootten M. E., Kadaba M. P., & Bigliani L. U. (1993). A kinematic and electromyographic study of shoulder rehabilitation exercises. Clinical Orthopaedics and Related Research, (288), 179–188. [PubMed] [Google Scholar]
- Morrison M., Nichol H., & Rammage L. (1986). Diagnostic criteria in functional dysphonia. Laryngoscope, 96(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Morrison M., & Rammage L. (1993). Muscle misuse voice disorders: Description and classification. Acta Oto-Laryngologica, 113(3), 428–434. [DOI] [PubMed] [Google Scholar]
- Munin M. C., Rosen C. A., & Zullo T. (2003). Utility of laryngeal electromyography in predicting recovery after vocal fold paralysis. Archives of Physical Medicine and Rehabilitation, 84(8), 1150–1153. https://doi.org/10.1016/S0003-9993(03)00146-1 [DOI] [PubMed] [Google Scholar]
- Nilsen K. B., Sand T., Stovner L. J., Leistad R. B., & Westgaard R. H. (2007). Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculoskeletal Disorders, 8, 81 https://doi.org/10.1186/1471-2474-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J., & Winkworth A. (2008). Current knowledge, controversies and future directions in hyperfunctional voice disorders. International Journal of Speech-Language Pathology, 10(4), 267–277. https://doi.org/10.1080/17549500802140153 [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Blake E., & Mathieson L. (2007). Musculoskeletal patterns in patients with voice disorders. Journal of Voice, 21(4), 477–484. https://doi.org/10.1016/j.jvoice.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Rubini A., Paoli A., & Parmagnani A. (2012). Body metabolic rate and electromyographic activities of antigravitational muscles in supine and standing postures. European Journal of Applied Physiology, 112(6), 2045–2050. https://doi.org/10.1007/s00421-011-2180-0 [DOI] [PubMed] [Google Scholar]
- Schüldt K. (1988). On neck muscle activity and load reduction in sitting postures. An electromyographic and biomechanical study with applications in ergonomics and rehabilitation. Scandinavian Journal of Rehabilitation Medicine. Supplement, 19, 1–49. [PubMed] [Google Scholar]
- Sjörs A., Larsson B., Dahlman J., Falkmer T., & Gerdle B. (2009). Physiological responses to low-force work and psychosocial stress in women with chronic trapezius myalgia. BMC Musculoskeletal Disorders, 10, 63 https://doi.org/10.1186/1471-2474-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. L., & Maluf K. S. (2010). Discharge behaviors of trapezius motor units during exposure to low and high levels of acute psychosocial stress. Journal of Clinical Neurophysiology, 27(1), 52–61. https://doi.org/10.1097/WNP.0b013e3181cb81d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Emden R. (2011). Neutral stimulus video-waves [Audio-video]. Retrieved from http://vimeo.com/19900030
- Van Lierde K., Van Heule S., De Ley S., Mertens E., & Claeys S. (2009). Effect of psychological stress on female vocal quality. A multiparameter approach. Folia Phoniatrica et Logopaedica, 61(2), 105–111. [DOI] [PubMed] [Google Scholar]
- Westgaard R. H. (1999). Muscle activity as a releasing factor for pain in the shoulder and neck. Cephalalgia, 19(Suppl. 25), 1–8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894). Geneva, Switzerland: Author. [PubMed] [Google Scholar]
- Wilder J. (1962). Basimetric approach (law of initial value) to biological rhythms. Annals of the New York Academy of Sciences, 98, 1211–1220. [DOI] [PubMed] [Google Scholar]
- Yoo I.-G., Lee J., Jung M.-Y., & Yang N.-Y. (2011). Neck and shoulder muscle activation in farm workers performing simulated orchard work with and without neck support. Work, 40(4), 385–391. https://doi.org/10.3233/WOR-2011-1250 [DOI] [PubMed] [Google Scholar]
- Zemlin W. (1998). Speech and hearing science: Anatomy and physiology (4th ed.). Needham Heights, MA: Allyn & Bacon. [Google Scholar]