Abstract
Purpose
The vocalizations of young infants often sound nasalized, suggesting that the velopharynx is open during the 1st few months of life. Whereas acoustic and perceptual studies seemed to support the idea that the velopharynx closes for vocalization by about 4 months of age, an aeromechanical study contradicted this (Thom, Hoit, Hixon, & Smith, 2006). Thus, the current large-scale investigation was undertaken to determine when the velopharynx closes for speech production by following infants during their first 2 years of life.
Method
This longitudinal study used nasal ram pressure to determine the status of the velopharynx (open or closed) during spontaneous speech production in 92 participants (46 male, 46 female) studied monthly from age 4 to 24 months.
Results
The velopharynx was closed during at least 90% of the utterances by 19 months, though there was substantial variability across participants. When considered by sound category, the velopharynx was closed from most to least often during production of oral obstruents, approximants, vowels (only), and glottal obstruents. No sex effects were observed.
Conclusion
Velopharyngeal closure for spontaneous speech production can be considered complete by 19 months, but closure occurs earlier for speech sounds with higher oral pressure demands.
The velopharynx and associated structures undergo substantial modification during the first 2 years of life. They increase in size, but perhaps more importantly, their relative sizes, configurations, and spatial relationships change during development. Accordingly, the function of the velopharynx also changes as infants grow older.
It has long been known that the velopharynx is open during the birth cry (Bosma, Truby, & Lind, 1965) and closed during speech production by 3 years of age (Thompson & Hixon, 1979), though the specific timing of quick velopharyngeal events continues to modify over the next few years (Leeper, Tissington, & Munhall, 1998; Zajac, 2000; Zajac & Hackett, 2002). What has not been known is the time course of velopharyngeal closure during infancy and the youngest age at which it can be expected to be fully closed for speech production.
The velopharynx and surrounding oral and pharyngeal structures change rapidly during early development. Relevant examples of such change include the following: (a) The larynx descends and the pharynx lengthens, causing separation of the velum and epiglottis; (b) the hard and soft palates grow rapidly and at different rates; (c) the spatial relation among key velopharyngeal muscles changes; and (d) the nasopharyngeal tonsil hypertrophies.
The velum and epiglottis separate by about 4 to 6 months of age (Sasaki, Levine, Laitman, & Crelin, 1977) as the larynx moves from the level of the first to the third cervical vertebra. This is accompanied by rapid growth of the pharynx in the vertical dimension from about 4 cm in the newborn pharynx (Crelin, 1973) to approximately 12 cm in the adult (Sasaki et al., 1977). The rate of laryngeal descent is accelerated during the first 2 years of life, with pharyngeal length increasing by up to 2 cm during this period, followed by a more gradual lengthening (Vorperian et al., 2005, 2009). In contrast, the anteroposterior dimension of the pharynx changes little from infancy to adulthood (Berland, 1963; Handelman & Osborne, 1976; King, 1952; Tourne, 1991; Vorperian et al., 2009).
The most rapid growth of the hard and soft palates occurs during infancy, with more gradual growth thereafter. The length of the hard palate reaches 84% of its adult size by age 2 years (Vorperian et al., 2005, 2009). The soft palate, in contrast, appears to follow a somatic growth curve through adolescence with no dramatic changes in growth rate during this period, reaching only 69% of its adult length by 2 years.
The spatial relation of the palate to its extrinsic muscles changes during the first year of life (Fletcher, 1973). Because the palate occupies a relatively high position within the infant pharynx, the levator veli palatini muscles are oriented to serve primarily as palatal tensors. As the palate descends, the orientation of these muscles changes so that they begin to function as palatal elevators, which continues to be their function into adulthood.
The nasopharyngeal tonsil enlarges during early childhood, plateaus between the ages of 2 and 14 years, and atrophies rapidly after 15 years of age (Capitanio & Kirkpatrick, 1970; Johannesson, 1968). This tonsil (sometimes called the adenoids when enlarged) has been shown to be identifiable in only 18% of infants less than 3 months of age, 75% of infants at 4 months, and 100% of infants at 5 months or older (Jaw, Sheu, Liu, & Lin, 1999).
These changes in size, configuration, and spatial relationships of the velopharynx and associated structures, especially in light of the concurrent rapid increase in expressive language, point to the probability that velopharyngeal function for speech production also changes during development. There is, in fact, evidence from acoustic, perceptual, and aeromechanical studies for such change; however, the evidence regarding its time course is somewhat contradictory.
Acoustic and perceptual studies suggest that the velopharynx is open during nondistress (noncry) vocalizations in the very young infant and closed once the infant reaches approximately 4 months of age. An acoustic study by Kent and Murray (1982) revealed that nondistress vocalizations of 3-month-old infants were lower in overall amplitude and contained an additional low frequency energy component when compared with those of 6- and 9-month-old infants. Given that both of these acoustic features are associated with nasalization (open velopharynx) in adults (Fant, 1960; Stevens, 2000), this finding seemed to support the idea that the velopharynx closed sometime between 3 and 6 months of age. A similar conclusion was reached by Oller (1986) who observed lower overall energy and somewhat indistinct formants in the vocalizations of 2- and 3-month-old infants and the emergence of a distinct second formant above 2000 Hz sometime between 4 and 6 months of age. Further support came from an auditory–perceptual study of 4- to 24-month-old infants in which the perceived nasality of their vocalizations was found to decline after the age of 4 months (Hsu, Fogel, & Cooper, 2000). Thus, all evidence pointed to the idea that the velopharynx closes for vocalization sometime between 4 and 6 months of age.
Somewhat surprisingly, the findings of an investigation by Thom, Hoit, Hixon, and Smith (2006) called this evidence into question. Thom et al. used an aeromechanical approach to monitor the status of the velopharynx that involved sensing pressure within the nares to determine, moment by moment, when the velopharynx is open and when it is closed. Their study included six infants whom they studied monthly from the age of 2 to 6 months. Although there was substantial variability across the six infants, the group results indicated that the average frequency of velopharyngeal closure increased with age but was far from complete (approximately 60%) by the age of 6 months. This small, yet revealing, study provided the impetus for pursuing this line of research on a larger scale.
Thus, the purpose of the current investigation was to determine the age at which the velopharynx closes for speech production by studying a large number of typically developing infants longitudinally over a nearly 2-year period. A second purpose was to determine if sound class (and associated oral pressure demands) influences velopharyngeal closure in very young children.
Method
This project was approved by the Institutional Review Board at the University of Arizona. Written consent was obtained from the caregiver (usually a parent) at the initial study visit. Caregivers were present during all sessions and were provided with monetary compensation.
Participants
One hundred eight healthy infants were enrolled in the study. Most infants began participating at age 4 months and were studied monthly (± 1 week) until either they stopped participating or they reached 24 months. In most cases, infants stopped participating when they began to reject the nasal cannula (as demonstrated by pulling it off, crying, or both). Other reasons for stopping participation were that the infant had missed more than two consecutive sessions or other scheduling difficulties arose. Data from 16 of the 108 infants were excluded from the final data set for one or more of the following, sometimes overlapping, reasons: (a) 13 infants were never able to tolerate the nasal cannula or stopped vocalizing when wearing it; (b) five infants exhibited delayed language development or other developmental delays; (c) two parents decided to stop participating; and (d) one family moved away. Of the 92 remaining participants, 46 were male and 46 were female.
All infants met a set of initial criteria: (a) they were from unremarkable full-term pregnancies and deliveries with no known neural or structural abnormalities; (b) they had passed the newborn hearing screening; (c) they were from first-language American English–speaking families; and (d) they were healthy on the day of testing. During subsequent sessions, the infants met the following additional criteria: (a) they exhibited communication and language skills that were typical for their age as measured by The Rossetti Infant–Toddler Language Scale (Rossetti, 2006; 4 through 24 months) and the MacArthur Communicative Development Inventory (CDI; Fenson et al., 1993) Words and Gestures subtest (8 through 16 months) and the Words and Sentences subtest (17 through 24 months); and (b) they obtained an age equivalence score that was expected for their age on the Preambulatory Motor Skills and Behaviors, Gross Motor Skills and Behaviors, and Fine Motor Skills and Behaviors subtests of the Brigance Diagnostic Inventory of Early Development–Second Edition (Brigance, 2004; 4 through 21 months; note that only parent report was used, not direct observation of test items). Occasionally, an infant scored below the expected range on a communication/language or motor measure, but then met or exceeded expectations in subsequent sessions. Data from these infants were included in all analyses.
Data Collection
Data collection was conducted in the University of Arizona Speech Acoustics and Physiology Laboratory in an area that was decorated with child-friendly pictures and contained toys, blankets, and other accessories. Infants were brought to the laboratory by a caregiver (parent, in most cases) and, often, were accompanied by other family members. Each data collection session lasted an hour or less, depending on the infant's mood, verbal output, and whether or not a feeding break was needed. Data collection consisted of the recording of nasal ram pressure during play and other communicative interactions and the administration of communication/language and motor inventory measures (to ensure that participants continued to meet the criterion of being typically developing).
Nasal ram pressure data were obtained using a nasal cannula, such as that shown in Figure 1. The cannula was placed in the anterior nares and was connected on the other end to a sensitive pressure transducer (Validyne ± 2 cmH2O), and the resultant signal was amplified and recorded on a computer using LabChart software (LabChart, 2017). Although infants often “fussed” when the cannula was first placed, they almost always appeared to ignore it after a minute or two. An investigator monitored the nasal ram pressure signal during data collection. Occasionally, the cannula needed to be removed and cleared of mucous (or replaced if the mucous could not be cleared). Speech/vocalization was sensed with a small microphone that was taped to the forehead or shoulder, and the participant's face was video-taped with a webcam. The audio and video signals were recorded simultaneously with the nasal ram pressure signal, and all three were stored as a single LabChart data file.
Figure 1.

Twelve-month-old participant wearing a nasal cannula.
Nasal ram pressure, acoustic, and video signals were recorded while an investigator and/or family members interacted with the participant. These interactions and the physical setup generally differed with the age of the participant. A 4-month-old infant was usually studied in a semirecumbent position with the communication partner leaning over him or her while verbalizing and presenting toys. Once an infant was able to sit up unsupported, sessions were usually conducted with the infant seated on a floor mat and surrounded by toys and books (as shown in Figure 1). The investigators and other communication partners also sat on the mat and played with the infant/toddler. Older toddlers sometimes sat in a chair at a small table with coloring materials or other age-appropriate toys with a communication partner or two sitting across the table.
Data Analysis
Nasal Pressure Data
All the data recorded during a given session were reviewed to determine which utterances met criteria for analysis. An utterance was operationally defined as a sound, syllable, or series of syllables produced on a breath group (an expiration containing vocalization or speech; Lynch, Oller, Steffens, & Buder, 1995; Thom et al., 2006). Although there were no direct measures of breathing, it was possible to determine with relative certainty the boundaries of a breath group by viewing the nasal ram pressure waveform (inspirations were often accompanied by negative pressures, unless all inspiration was directed through the mouth), listening to the audio recordings (inspirations could often be heard), and watching the video recording (inspirations were sometimes detectable as a small movement of the chest wall). To be included in the analysis, utterances needed to meet the following criteria.
(a) The utterance was judged to be a nondistress utterance produced in a communicative context. Other types of distress (cries, windups, and whimpers) and nonspeech (raspberries and laughs) utterances were not included in this study.
(b) The nasal pressure signal was free of artifacts that suggested an invalid signal. The most common problem was a mucous plug that had not been cleared during data collection.
(c) The utterance was audible. At times, a family member or investigator spoke simultaneously, and the participant's utterance could not be heard well enough to transcribe.
The nasal ram pressure signal was analyzed for utterances that met these criteria. This pressure signal reflects a local ram pressure sensed at the outlets of the nasal cannula. It is directly related to the local airflow within the outlets and is relatively insensitive to most head and body movements. The signal-to-noise ratio of the nasal ram pressure measuring system was determined to be 71.4 dB (by calculating the mean of a series of peak pressures, dividing by the mean noise pressure, and converting to decibels). Although the nasal ram pressure signal is sensitive to local airflow (and pressure change), it is not necessarily related to the mass airflow through the nose. Thus, the signal was not calibrated because the relative magnitude of the pressure was not judged to provide meaningful information regarding velopharyngeal status. What was judged to be meaningful was the sign of the nasal ram pressure relative to atmospheric pressure. The nasal ram pressure signal was interpreted as follows: (a) negative pressure indicated inspiratory flow through the nose and an open velopharynx; (b) positive pressure indicated expiratory flow out the nose and an open velopharynx; and (c) zero (atmospheric) pressure indicated no airflow through the nose and indicated either breath-holding or a closed velopharynx.
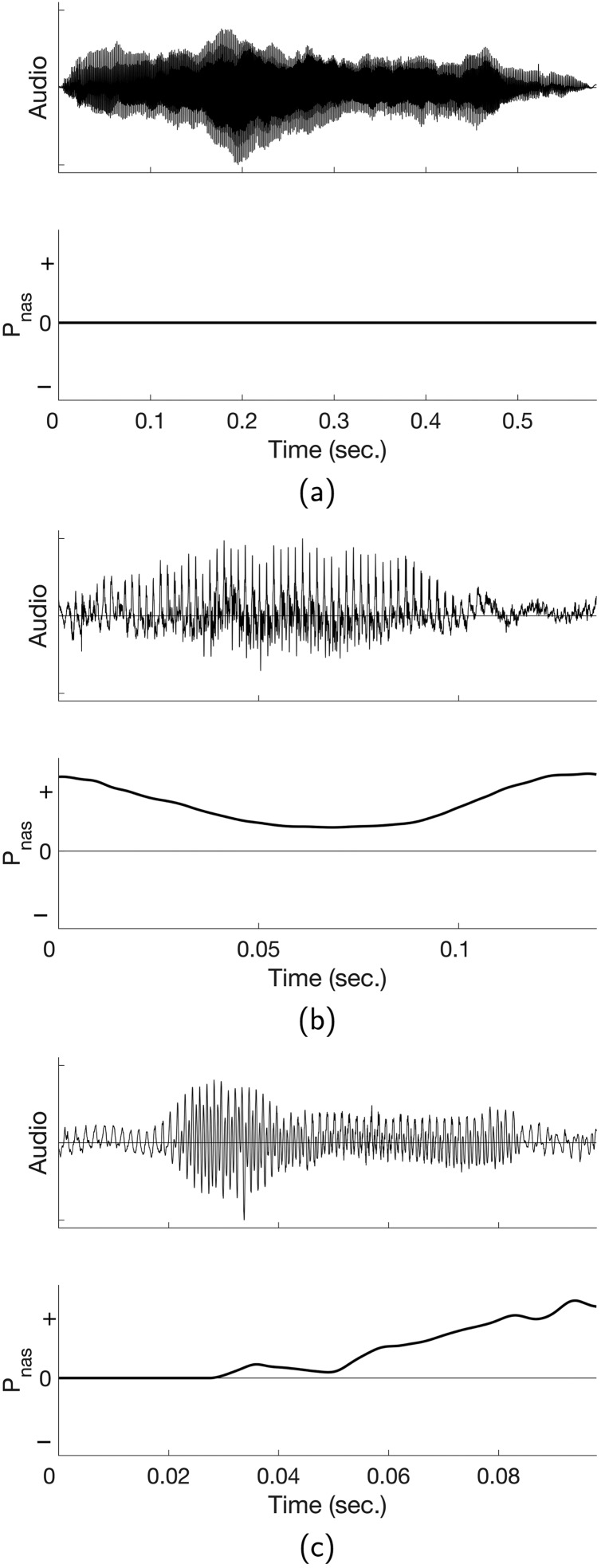
As operationally defined above, all utterances were produced on expiration; thus, only positive and zero nasal ram pressures were included in the analysis. Utterances were coded as being produced with a velopharynx that was (a) closed throughout the utterance (zero pressure throughout), (b) closed during part of the utterance (zero pressure during part of the utterance and positive pressure during part of the utterance), and (c) open throughout the utterance (positive pressure throughout the utterance). Figure 2 provides examples of all three of these utterance types produced by the same infant.
Figure 2.
Examples of utterances produced by one male participant. The top tracing in each panel is the acoustic speech signal, and the bottom tracing is the nasal ram pressure signal (uncalibrated). Zero on the pressure scale is atmospheric pressure; above 0 is positive pressure, and below 0 is negative pressure. The top panel (a) shows an utterance (transcribed as /wæ/) with pressure at 0 indicating a closed velopharynx. The middle panel (b) contains an utterance (transcribed as /wɑ/) with positive pressure throughout the utterance, indicating an open velopharynx. The bottom panel (c) shows an utterance (transcribed as /bɑbɑ/) with a combination of 0 and positive pressure, indicating that the velopharynx was closed part of the time and open part of the time.
Utterance Transcription and Categorization
Utterances that met criteria were transcribed using broad phonetic transcription from the audio and video recordings by trained graduate students. To determine if sound category (and the accompanying differences in physiological demands) had an effect on velopharyngeal closure, utterances were classified as (a) vowels (alone), (b) approximant consonants (liquids and glides, accompanied by vowels), (c) oral obstruent consonants (oral stops, fricatives, and affricates, accompanied by vowels), and (d) glottal obstruent consonants (glottal stops and glottal fricatives, accompanied by vowels). Glottal obstruents were considered in a separate category because the physiological demands for velopharyngeal closure were judged to be much lower for glottal obstruents when compared with obstruents that are produced in the oral airway.
Final Data Set
The final data set comprised data from the 92 participants, all of whom had participated in a minimum of four consecutive recording sessions. For a participant's data to be included in the final data set for a particular phonetic category, his or her data needed to include at least six utterances that met criteria for analysis (described above). Table 1 lists the number of boys and girls at each age level whose data are represented in the final data set. The number of participants decreased over the course of the second year as several of them dropped out of the study when they grew older (usually because they no longer tolerated the cannula).
Table 1.
The number of participants (male and female) who produced usable data at each month.
| Age (months) | No. of boys | No. of girls | Total |
|---|---|---|---|
| 4 | 35 | 40 | 75 |
| 5 | 40 | 40 | 80 |
| 6 | 44 | 40 | 84 |
| 7 | 41 | 40 | 81 |
| 8 | 41 | 38 | 79 |
| 9 | 39 | 33 | 72 |
| 10 | 32 | 26 | 58 |
| 11 | 30 | 25 | 55 |
| 12 | 25 | 23 | 48 |
| 13 | 26 | 18 | 44 |
| 14 | 18 | 14 | 32 |
| 15 | 16 | 13 | 29 |
| 16 | 18 | 11 | 29 |
| 17 | 15 | 9 | 24 |
| 18 | 13 | 8 | 21 |
| 19 | 12 | 7 | 19 |
| 20 | 12 | 6 | 18 |
| 21 | 11 | 6 | 17 |
| 22 | 8 | 6 | 14 |
| 23 | 3 | 5 | 8 |
| 24 | 5 | 5 | 10 |
Statistical Analyses
To determine if the male and female data needed to be treated separately, t tests were used to test for sex-related differences in the percentage of utterances produced with a closed velopharynx for any month. A conservative alpha level (.01) was used to adjust for inflation of power due to multiple tests.
Both descriptive and inferential statistical analyses were performed on the data. Descriptive statistical analysis consisted of calculations of group means of percentage of utterances produced with a closed velopharynx throughout the utterance and during part of the utterance across age (in months). These calculations were done for the entire data set and for the individual sound categories of vowels (alone), approximant consonants, oral obstruent consonants, and glottal obstruent consonants.
The percentage of utterances produced with a closed velopharynx (throughout the utterance) in the four sound categories (vowels, approximants, oral obstruents, glottal obstruents) was calculated for each child by month. A regression was fit across time for each child with at least six data points (6 months of usable data) and four utterances within a given sound category at each data point to estimate each child's progress. The initial analyses used both a linear and quadratic term to account for any curvilinear effects. Because none of the quadratic terms were statistically significant, the regressions were recalculated with a linear term only. The linear regression weights were then averaged across children to obtain an estimate of the average monthly change in percentage of utterances produced with a closed velopharynx throughout the utterance. These means were then tested for significance (H0: B1 = 0).
Differences in velopharyngeal closure across the four sound categories (vowels, approximants, oral obstruents, and glottal obstruents) were tested using a Friedman test on repeated measures of ranks (Lehmann, 1975) to determine whether the rank orders of the percentage of utterances produced with the velopharynx closed throughout or closed during part differed among the four sound categories. This was followed by Wilcoxon signed-ranks tests for each pair (vowels vs. approximants, vowels vs. oral obstruents, etc.), using a Bonferroni-corrected alpha level (.05/6 = .0083).
Intercoder reliability was determined by reanalyzing 20% of the sessions (180 sessions, randomly selected across participants) for velopharyngeal status (closed throughout, closed during part, or open during the utterance). Intracoder reliability was based on 60 sessions. Mean intercoder reliability was 92.7% (SD = 5.9), and mean intracoder reliability was 97.7% (SD = 3.1).
Results
Potential sex differences in the percentage of utterances produced with a closed velopharynx were examined at each age level (4 through 24 months). Only one significant sex-related difference was found (at 21 months), wherein boys had a significantly higher percentage of utterances produced with a closed velopharynx than girls. Because this was the only instance of a significant difference and it pertained to a month in which only a small number of participants were represented (11 boys, 6 girls), the combined data set was used for the remainder of the analyses.
Results of the linear regression analyses are shown in Table 2, along with the number of participants (N) included in the analyses for all of the utterance productions combined (total) and for each sound category (vowels, approximants, oral obstruents, and glottal obstruents). The b1 values represent the average of the monthly change estimates across children in percentage of utterances produced with a closed velopharynx throughout the study. For example, the value of 2.69 in the row labeled Total Productions is the average regression weight across all 78 children. This can be interpreted as meaning that, on average, a child increased his or her total closed productions by about 2.7% each month over the course of the study. The t value represents the test statistic for H0: B1 = 0. All of the values were positive, indicating an increase in the percentage across time. This positive change was statistically significant for all utterance categories (indicated by asterisks in the table).
Table 2.
Results of linear regression analyses for all utterances combined (total) and for each sound category.
| Utterance type | M(b1) | t | Pr(t) | N |
|---|---|---|---|---|
| Total productions | 2.69 | 7.11 | < .0001* | 78 |
| Vowels | 1.77 | 4.10 | .0001* | 76 |
| Approximants | 1.70 | 3.02 | .0043* | 43 |
| Oral obstruents | 2.91 | 5.51 | < .0001* | 51 |
| Glottal obstruents | 1.68 | 3.65 | .0005* | 69 |
p < .0083.
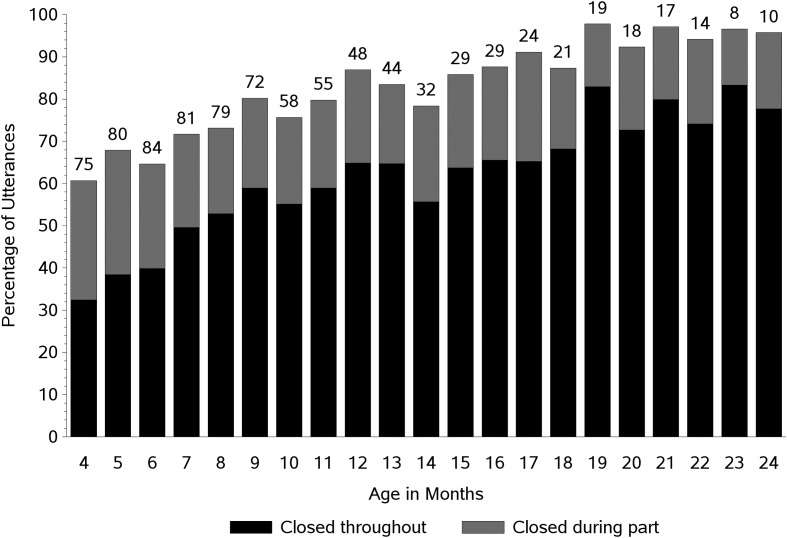
Descriptive results for the entire data set (all sound categories combined) are shown in Figure 3. Each bar represents the mean percentage of utterances produced with a closed velopharynx throughout the utterance (dark portion of bars) and during part of the utterance (light portion of bars) for each age level tested (shown in months). The numbers above the bars indicate the number of participants represented at each month. The percentage of utterances in which the velopharynx was closed at least part of the time ranged from about 60% at 4 months to about 96% at 19 months, with a generally increasing trajectory across age up to 19 months. Most of this increase was accounted for by the percentage of utterances in which the velopharynx was closed throughout (dark portion of bars) and ranged from about 32% at 4 months to about 82% at 23 months. The regression analysis showed a statistically significant age-related increase in utterances produced with a closed velopharynx throughout (see Table 2; dark portion of bars in Figure 3). As stated previously, the increase averaged 2.7% per month.
Figure 3.
The mean percentage of utterances produced with a closed velopharynx throughout the utterance (dark portion of bars) and with a closed velopharynx during part of the utterance (light portion of bars) across age (in months). The numbers above the bars indicate the number of participants who produced usable data at each age level.
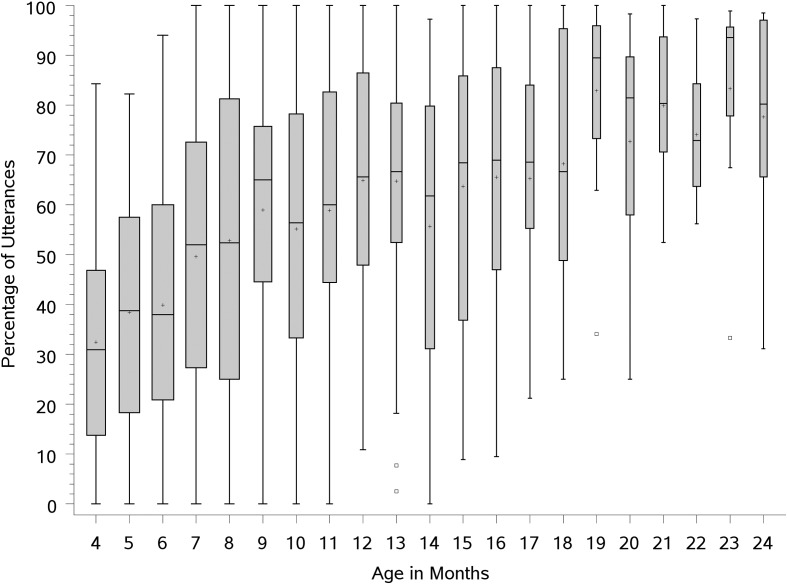
Between-participant variability was also examined and found to be substantial, as is evident in the box plot provided in Figure 4. The height of the box is based on the interquartile range, which represents the distance between the 25th and 75th percentiles in the data. The median is shown as a line within the box and the mean as a plus symbol (+). The width of the bars reflects the number of utterances analyzed at each month, so thicker boxes represent larger samples. Variability appears lower from 19 months on; however, this could be because the means approach 100% in this older age range or because there is a small number of infants included in the sample at those ages.
Figure 4.
Box plot of all utterances produced with the velopharynx closed at least part of the time across age (in months). The box width varies with the number of participants represented at each age.
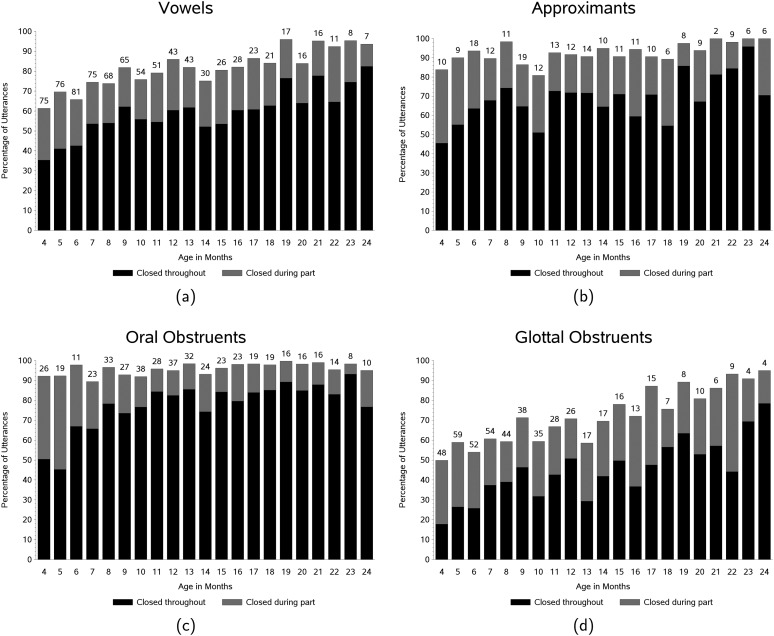
Figure 5 contains descriptive results for the four sound categories: (a) vowel (alone), (b) approximants, (c) oral obstruents, and (d) glottal obstruents. Results for vowel (alone) productions (Figure 5a) show that the percentage of velopharyngeal closure and its change across age was similar to that for the overall data set (shown in Figure 3). This reflects the fact that most of the utterances, particularly in the early months, were primarily vowel productions. The regression analysis for vowels was statistically significant (see Table 2). The estimated monthly increase represented by the dark portion of the bars was about 1.8% per month.
Figure 5.
Descriptive results for the four sound categories: (a) vowels (alone), (b) approximants, (c) oral obstruents, and (d) glottal obstruents. The bars represent the mean percentage produced with a closed velopharynx throughout the utterance (dark portion of bars) and with a closed velopharynx during part of the utterance (light portion of bars) across age (in months). The numbers above the bars indicate the number of participants who produced usable vowel data at each age level.
Approximants (accompanied by vowels; Figure 5b) were produced with a closed velopharynx more often than were vowels (alone). The percentage of productions that were closed at least part of the time ranged from approximately 80% to 100%, with only a slight developmental trend characterized by a lower percentage of velopharyngeal closure in the 4- to 10-month age range compared with the 19- to 24-month age range. The regression analysis showed a significant effect for approximants with an expected increase of about 1.7% per month (dark portion of bars).
The percentage of utterances containing oral obstruents (accompanied by vowels; Figure 5c) were produced with a closed velopharynx at least 90% of the time across age. A developmental increase can be seen from 4 to 11 months for utterances in which the velopharynx was closed throughout (dark portion of bars), with a greater proportion being closed from age 11 months on compared with earlier months. Regression analysis revealed a linear effect for oral obstruents that were closed throughout utterances (dark portion of the bars). The mean regression coefficient differed significantly from 0 (see Table 2) with an estimated increase of 2.9% per month.
Results for glottal obstruents (accompanied by vowels; Figure 5d) show a strong developmental trend from 4 months, when only 50% were produced with a closed velopharynx at least part of the time, to 24 months, when 95% were produced with a closed velopharynx at least part of the time. The regression for glottal obstruents was statistically significant (see Table 2; dark portion of bars), with an average increase of about 1.7% per month.
The variability for vowels (alone), approximants, and glottal obstruents was similar to the variability for all sound categories combined (see Figure 4). The variability for oral obstruents was lower than the other sound categories because the percentage of utterances produced with a closed velopharynx was so high.
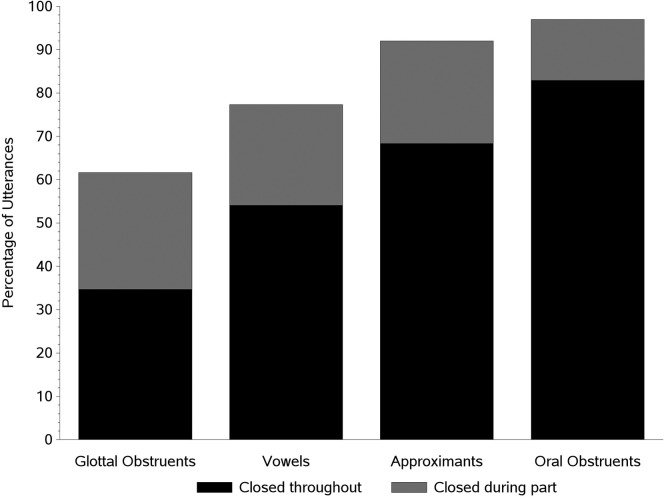
Figure 6 shows the mean percentage of utterances produced with a closed velopharynx during all or part of the utterance (all ages combined) for the four sound categories: vowels (alone), approximants, oral obstruents, and glottal obstruents. Whereas the percentage produced with a closed velopharynx during part of the utterance (light portion of bars) is relatively similar across the sound categories (except for oral obstruents), the percentage produced with a closed velopharynx throughout the utterance (dark portion of bars) shows clear differences across sound categories. The sound categories were ranked for each child from 1 to 4 and then tested for differences using the Friedman test for repeated ranks. The test statistic is given as Q and is distributed as χ2 with 3 degrees of freedom. The test was statistically significant, Q(3) = 156.06, p < .0001, and follow-up Wilcoxon signed-ranks tests revealed that all six pairwise comparisons differed significantly (p < .0001). This indicates that the percentage produced with a closed velopharynx throughout the utterance was significantly smaller for glottal obstruents than for the other three categories, significantly smaller for vowels than for approximants and oral obstruents, and significantly smaller for approximants than oral obstruents.
Figure 6.
The percentage of utterances produced with velopharyngeal closure throughout the utterance (dark portion of bars) and during part of the utterance (light portion of bars) by sound category, with data from all age levels combined.
Discussion
In the 92 infants followed longitudinally in this study, velopharyngeal closure for oral sound production generally increased from 4 months to 19 months of age and then leveled off. Velopharyngeal closure occurred most frequently for oral obstruents and least frequently for glottal obstruents across all ages. The discussion below considers what is now known about the development of velopharyngeal closure, the influence of sound category, other sources of variability, why the present findings appear to contradict earlier acoustic and perceptual evidence, and what directions this line of research might take in the future.
Development of Velopharyngeal Closure
Consideration of the overall data set (Figure 3) led to the conclusion that velopharyngeal closure for oral sound production is complete by 19 months of age with no consistent change up through 24 months when the study stopped, a finding that held for both boys and girls. In this context, “complete” does not mean that the velopharynx was closed 100% of the time but, rather, that there was no additional increase in velopharyngeal closure as the participants grew older. A more precise way to characterize the results of this study is to say that, by 19 months, the velopharynx was closed during at least part of the utterance approximately 90% of the time and during the entire utterance approximately 75% of the time.
Although available evidence indicates that there might be a further developmental increase in the frequency of velopharyngeal closure between 2 and 3 years of age, that evidence is not entirely convincing when applied to spontaneous speech production. The evidence comes primarily from Thompson and Hixon (1979) who reported that velopharyngeal closure was complete (100% of the time) by 3 years; however, there is a critical difference in the nature of the speaking tasks used by Thompson and Hixon that makes it difficult to compare their results to those of this study. Whereas this study elicited spontaneous speech through natural play and interaction, the earlier study constrained speech to sustained sound productions, syllable repetitions, and vowel–consonant–vowel productions within a carrier phrase, all elicited through imitation. Also, it is relevant to point out that the earlier study included only one or two 3-year-old toddlers 1 making it impossible to assess the interparticipant variability, something that was a hallmark of the present data. Perhaps, a more precise conclusion for this study is that velopharyngeal closure for spontaneous speech production is essentially complete at 19 months.
The developmental trend seen in the present data may be explained, at least in part, by the anatomical and physiological changes that take place during infancy and early childhood, such as those described in the introduction. Nevertheless, it is important to note that, at 4 months, and even as early as 2 months (Thom et al., 2006), infants occasionally close the velopharynx for oral sound production. This begs the question: if infants are capable of closing the velopharynx but do not, what variables drive the developmental change in velopharyngeal closure over these first months of life? One answer seems to lie in the strong influence exerted by the phonetic nature of the speech being produced.
Influence of Sound Category
The power of phonetic influences on velopharyngeal closure is clearly depicted in Figure 5, which shows the developmental data from each of the four sound classes, and Figure 6, which combines data from the four sound classes for all ages studied. These figures reveal that oral obstruents were most frequently produced with a closed velopharynx, followed by approximants, vowels, and glottal obstruents. Approximately 97% of the utterances containing oral obstruents were produced with a closed velopharynx during at least part of the utterance, and about 83% were produced with a closed velopharynx throughout the utterance. These observations for oral obstruents are very similar to those recently reported by Eshghi, Vallino, Baylis, Preisser, and Zajac (2017), who used a nasal ram pressure approach to study nine typically developing toddlers at 12, 14, and 18 months of age.
This sound-related pattern is best explained by differences in oral pressure demands. That is, sounds with the greatest oral pressure demands—oral obstruents (sometimes called high-pressure consonants)—are the sounds most often produced with velopharyngeal closure. It is impressive to see that these sounds were produced with a closed velopharynx at least 90% of the time, on average, starting at the youngest age studied (4 months) and continuing throughout development. In contrast, sounds with the lowest oral pressure demands—glottal obstruents, which can be produced with an open velopharynx with little or no negative perceptual consequence—were the sounds least often produced with velopharyngeal closure. Thus, it appears that the prevalence of “pressure” consonants is a strong controlling variable for whether or not an infant or toddler closes the velopharynx.
Other Sources of Variability
Despite the strong and statistically significant developmental and sound-related trends identified in the present data set, its variability was a particularly salient characteristic. For example, velopharyngeal closure could range as widely as from 0% of utterances for one participant to 100% of utterances for another participant of the same age (see Figure 4). A search for other sources of variability led to examination of data collected as part of the participant qualification procedures for the study.
Two potential sources of variability were language level and general motor skill level. These variables were tested by regressing the slopes for the percentage of utterances produced with a closed velopharynx (see Table 2) against slopes obtained for vocabulary comprehension, vocabulary production, and total gestures on the basis of scores from the MacArthur CDI (Fenson et al., 1993; only Months 8 through 16 were included in this analysis), and fine motor skills and gross motor skills on the basis of scores from the Brigance Diagnostic Inventory of Early Development–Second Edition (Brigance, 2004). The expectation was that, if a relationship existed, a positive change in language or motor skill should relate to a positive change in the percentage of utterances produced with a closed velopharynx.
For the language measures, results of these analyses were not statistically significant, indicating no apparent relationship between velopharygeal closure and language level. Nip, Green, and Marx (2011), who studied orofacial kinematics in infants 9 to 21 months of age, also reported no significant relationship between speech production movements and scores on the MacArthur CDI (Fenson et al., 1993). Nevertheless, they did find a significant relationship when using scores from the Batelle Developmental Inventory–Second Edition (Newborg, 2005). Thus, it is possible that a relationship might be found between velopharyngeal closure and language level if different language measures were used or if the age range was expanded.
For the motor measures, results were not significant for the fine motor measure, but they were significant for the gross motor measure. Specifically, the regression for change in percentage closure in total utterances (dependent variable) showed significant effects for gross motor skills: b(linear) = 0.31, t(67) = 2.16, p = .0344; b(quadratic) = 5.98, t(67) = 2.08, p = .0412. This suggests that a positive change in percentage of utterances produced with a closed velopharynx is related to a positive increase in gross motor performance.
This significant positive correlation between velopharyngeal closure and gross motor skill is consistent with the view that there is a relationship between speech motor development and general motor development. The apparent yoking of speech development to motor milestones has been recognized for a long time (Lenneberg, 1967). Recent work has documented speech-to-motor links, such as the onsets of rhythmic arm movements and reduplicated babble, the beginning of unsupported sitting and an increase in number of syllables per breath group, and the use of recognitory actions (such as bringing a telephone up to an ear) and the production of first words (Iverson, 2010). Thus, the present findings provide further support for the link between speech development and overall motor development.
Aeromechanical Versus Acoustic/Perceptual Evidence
The findings of this study—that velopharyngeal closure is not complete until 19 months of age—are in stark contrast to the findings of earlier acoustic and auditory–perceptual studies that provided evidence that velopharyngeal closure occurs around the age of 4 to 6 months (Hsu et al., 2000; Kent & Murray, 1982; Oller, 1986). Although it is not obvious why acoustic/perceptual studies have rendered such different conclusions than the present aeromechanical study, this discrepancy raises some interesting questions. For example, do the acoustic features associated with nasalization in infant vocalizations differ from those of adult speech? Similarly, do the perceptual cues associated with nasalization differ between infants and adults?
Because the vocal tract of an infant is so different from the adult vocal tract, the act of opening the velopharynx may not have the same acoustic consequences as it does in an adult. That is, nasalization in the infant may not result in the reductions in amplitude or frequency of the spectrum that are observed in nasalized adult speech. It is conceivable that changes in the effective location of nasal coupling to the main vocal tract occur when the epiglottis and velum begin to separate (Sasaki et al., 1977), the soft palate grows rapidly (Vorperian et al., 2005, 2009), and the adenoids appear (Jaw et al., 1999) between 4 and 6 months of age, which, in turn, may change the frequency range at which nasalization affects the spectrum. Determining the acoustic consequences of velopharyngeal coupling in an infant may require using a model of the speech production system to sort out what is due to nasalization versus what is linked to developmental changes in vocal tract shape.
Regarding the perception of nasalization, there is preliminary evidence that the perceptual cues associated with nasalization may differ between infants and adults or, at least, that listeners are unreliable in determining velopharyngeal status (open or closed) when listening to infants' utterances. In an unpublished pilot study conducted by Le (2011), eight listeners (four experienced, four inexperienced) were asked to judge utterances produced by six infants (two boys and four girls from the present participant sample) at ages 5 and 6 months. Because nasal ram pressure was recorded simultaneously from these infants, the velopharyngeal status (open or closed) was known for each of the utterances. For one task, the listeners were presented with pairs of vocalizations from the same infant, one that was produced with an open velopharynx and the other that was produced with a closed velopharynx. The listeners were asked to select the one that was “more nasal” with the choices being “Sample 1,” “Sample 2,” or “no difference.” The results showed that the listeners performed at chance level or below. A second task was performed only by the experienced listeners, all of whom were certified speech-language pathologists. These listeners were presented with single utterances and asked to identify if each utterance was produced with an “open” or “closed” velopharynx. Their accuracy ranged from 49% to 71% (M = 60%). Thus, it appears that even experienced listeners are not able to use the acoustic signal to identify velopharyngeal status in infants much above chance level. Similar concerns have been raised about the reliability of ratings of nasality for adult talkers (Whitehill, Lee, & Chun, 2002; Zraick & Liss, 2000).
Future Directions
This study has laid the groundwork for at least two key future research directions. The first involves further exploration of the relation of spoken language development to velopharyngeal closure. The second relates to the identification of acoustic features associated with velopharyngeal status in infants and toddlers.
It has been suggested that the acquisition of motor skills allows infants the opportunity to practice behaviors that are relevant to language development (Iverson, 2010), although the nature of the relation is complex and multifaceted. One interesting approach to exploring this relation would be to examine whether there is a correlation between rapid changes in a child's phonetic inventory and the frequency of utterances produced with a closed velopharynx. Another approach might be to explore whether the frequency of velopharyngeal closure differs for the production of real words compared with sound sequences that have no apparent meaning.
The second future direction relates to the identification of acoustic correlates of an open velopharynx in infants and young children, given that there is a strong possibility that they are not the same as those of adults. Data from this study offer a unique opportunity to examine the influence of velopharyngeal status (open vs. closed) on the acoustic signal. Although previous studies have described acoustic features that were assumed to reflect an open velopharynx in infants and children, none of these studies has provided confirmation that the velopharynx was actually open. Because both acoustic and aeromechanical data have been recorded simultaneously in this study, there is an opportunity to identify acoustic correlates of an open velopharynx with a greater degree of certainty than has been possible in previous research on infants and very young children.
Acknowledgments
This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders R01 DC010140, awarded to Jeannette D. Hoit. The authors want to thank all the graduate students who contributed to this research, especially Caety Chong, Keegan Gallagher, Alyssa Heeman, Sarah (Wikert) Kaskie, Amy Lougher, Amanda (Moody) Morse, Kristin Rumery, Christine (Dawson) Williams, Morgan Wilson, Amanda (Van Vianen) Woodmansee, and Olivia Vinikoor. The authors are grateful to Patricia Jones for her invaluable statistical consultation and analyses and to David Zajac and Mary Alt for their advice on certain aspects of the protocol. Most of all, the authors want to express their gratitude to the late Thomas J. Hixon who was a key player at the inception of this study and who inspired the authors to pursue this line of research.
Funding Statement
This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders R01 DC010140, awarded to Jeannette D. Hoit.
Footnote
The exact number of participants at each age level is not specified in the Thompson and Hixon (1979) article.
References
- Berland O. (1963). The bony nasopharynx. Acta Odontologica Scandinavia, 21(Suppl. 35), 1–137. [PubMed] [Google Scholar]
- Bosma J., Truby H., & Lind J. (1965). Cry motions of the newborn infant. Acta Paediatrica Scandinavica, 54(s163), 60–92. [Google Scholar]
- Brigance A. (2004). Brigance diagnostic inventories: Inventory of early development II. North Billareca, MA: Curriculum Associates Inc. [Google Scholar]
- Capitanio M., & Kirkpatrick J. (1970). Nasopharyngeal lymphoid tissue. Radiology, 96, 389–391. [DOI] [PubMed] [Google Scholar]
- Crelin E. (1973). Functional anatomy of the newborn. New Haven, CT: Yale University Press. [Google Scholar]
- Eshghi M., Vallino L. D., Baylis A. L., Preisser J. S., & Zajac D. J. (2017). Velopharyngeal status of stop consonants and vowels produced by young children with and without repaired cleft palate at 12, 14, and 18 months of age. Journal of Speech, Language, and Hearing Research, 60, 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant G. (1960). Acoustic theory of speech production. The Hague, Netherlands: Mouton. [Google Scholar]
- Fenson L., Dale P., Reznick J., Thal D., Bates E., Hartung J., … Reilly J. (1993). MacArthur Communicative Development Inventories: User's Guide and Technical Manual. San Diego, CA: Singular. [Google Scholar]
- Fletcher S. (1973). Maturation of the speech mechanism. Folia Phoniatrica, 25, 161–172. [DOI] [PubMed] [Google Scholar]
- Handelman C., & Osborne G. (1976). Growth of the nasopharynx and adenoid development from one to eighteen years. Angle Orthodontist, 46, 243–259. [DOI] [PubMed] [Google Scholar]
- Hsu H.-C., Fogel A., & Cooper R. (2000). Infant vocal development during the first 6 months: Speech quality and melodic complexity. Infant and Child Development, 9, 1–16. [Google Scholar]
- Iverson J. M. (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37(2), 229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaw T., Sheu R., Liu G., & Lin W. (1999). Development of adenoids: A study by measurement with MR images. Kaohsiung Journal of Medical Sciences, 15, 12–18. [PubMed] [Google Scholar]
- Johannesson S. (1968). Reontgenologic investigation of the nasopharyngeal tonsil in children of different ages. Acta Radiologica (Diagnosis) Stockholm, 7, 299–304. [DOI] [PubMed] [Google Scholar]
- Kent R., & Murray A. (1982). Acoustic features of infant vocalic utterances at 3, 6, and 9 months. The Journal of the Acoustical Society of America, 72, 353–365. [DOI] [PubMed] [Google Scholar]
- King E. (1952). A roentgenographic study of pharyngeal growth. Angle Orthodontist, 22, 23–37. [Google Scholar]
- LabChart (Version 8.1.9) [Computer software]. (2017). Colorado Springs, CO: ADInstruments. [Google Scholar]
- Le G. N. (2011, August). Auditory–perceptual judgement of velopharyngeal status and nasality in infant vocalizations. Paper presented at the Annual Ronald E. McNair Scholars Symposium, Berkeley, CA. [Google Scholar]
- Leeper H., Tissington M., & Munhall K. (1998). Temporal aspects of velopharyngeal function in children. The Cleft Palate–Craniofacial Journal, 35, 215–221. [DOI] [PubMed] [Google Scholar]
- Lehmann E. L. (1975). Nonparametrics: Statistical methods based on ranks. Oakland, CA: Holden-Day, Inc. [Google Scholar]
- Lenneberg E. H. (1967). The biological foundations of language. Hospital Practice, 2(12), 59–67. [Google Scholar]
- Lynch M. P., Oller D. K., Steffens M. L., & Buder E. H. (1995). Phrasing in prelinguistic vocalizations. Developmental Psychobiology, 28, 3–23. [DOI] [PubMed] [Google Scholar]
- Newborg J. (2005). Batelle Developmental Inventory–Second Edition. Itasca, IL: Riverside Publishing. [Google Scholar]
- Nip I. S. B., Green J. R., & Marx D. B. (2011). The co-emergence of cognition, language, and speech motor control in early development: A longitudinal correlational study. Journal of Communication Disorders, 44, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller D. (1986). Metaphonology and infant vocalizations. In Lindblom B. & Zetterstrom R. (Eds.), Precursors of early speech (pp. 21–35). New York, NY: Stockholm Press. [Google Scholar]
- Rossetti L. M. (2006). The Rossetti Infant–Toddler Language Scale: A measure of communication and interaction. East Moline, IL: LinguiSystems. [Google Scholar]
- Sasaki C., Levine P., Laitman J., & Crelin E. (1977). Postnatal descent of the epiglottis in man. Archives of Otolaryngology, 103, 169–171. [DOI] [PubMed] [Google Scholar]
- Stevens K. N. (2000). Acoustic phonetics (Vol. 30). Cambridge, MA: MIT Press. [Google Scholar]
- Thom S., Hoit J., Hixon T., & Smith A. (2006). Velopharyngeal development in infants. The Cleft Palate–Craniofacial Journal, 43, 539–546. [DOI] [PubMed] [Google Scholar]
- Thompson A., & Hixon T. (1979). Nasal air flow during normal speech production. Cleft Palate Journal, 16, 412–420. [PubMed] [Google Scholar]
- Tourne L. (1991). Growth of the pharynx and its physiologic implications. American Journal of Orthodontics and Dentofacial Orthopedics, 99, 129–139. [DOI] [PubMed] [Google Scholar]
- Vorperian H., Kent R., Lindstrom M., Kalina C., Gentry L., & Yandell B. (2005). Development of vocal tract length during childhood: A magnetic resonance imaging study. The Journal of the Acoustical Society of America, 117, 338–350. [DOI] [PubMed] [Google Scholar]
- Vorperian H. K., Wang S., Chung M. K., Schimek E. M., Durtschi R. B., Kent R. D., … Gentry L. R. (2009). Anatomic development of the oral and pharyngeal portions of the vocal tract: An imaging study. The Journal of the Acoustical Society of America, 125(3), 1666–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehill T., Lee A. S. Y., & Chun J. C. (2002). Direct magnitude estimation and interval scaling of hypernasality. Journal of Speech, Language, and Hearing Research, 45, 80–88. [DOI] [PubMed] [Google Scholar]
- Zajac D. (2000). Pressure-flow characteristics of /m/ and /p/ production in speakers without cleft palate: Developmental findings. The Cleft Palate–Craniofacial Journal, 37, 468–477. [DOI] [PubMed] [Google Scholar]
- Zajac D., & Hackett A. (2002). Temporal characteristics of aerodynamic segments in the speech of children and adults. The Cleft Palate–Craniofacial Journal, 39, 432–438. [DOI] [PubMed] [Google Scholar]
- Zraick R., & Liss J. (2000). A comparison of equal-appearing interval scaling and direct magnitude estimation of nasal voice quality. Journal of Speech, Language, and Hearing Research, 43, 979–988. [DOI] [PubMed] [Google Scholar]