Abstract
Purpose
Previous behavioral studies have found deficits in lexical–semantic abilities in children with specific language impairment (SLI), including reduced depth and breadth of word knowledge. This study explored the neural correlates of early emerging familiar word processing in preschoolers with SLI and typical development.
Method
Fifteen preschoolers with typical development and 15 preschoolers with SLI were presented with pictures followed after a brief delay by an auditory label that did or did not match. Event-related brain potentials were time locked to the onset of the auditory labels. Children provided verbal judgments of whether the label matched the picture.
Results
There were no group differences in the accuracy of identifying when pictures and labels matched or mismatched. Event-related brain potential data revealed that mismatch trials elicited a robust N400 in both groups, with no group differences in mean amplitude or peak latency. However, the typically developing group demonstrated a more robust late positive component, elicited by mismatch trials.
Conclusions
These initial findings indicate that lexical–semantic access of early acquired words, indexed by the N400, does not differ between preschoolers with SLI and typical development when highly familiar words are presented in isolation. However, the typically developing group demonstrated a more mature profile of postlexical reanalysis and integration, indexed by an emerging late positive component. The findings lay the necessary groundwork for better understanding processing of newly learned words in children with SLI.
Children with specific language impairment (SLI) have hallmark deficits in morphosyntax (Leonard et al., 1992; Rice & Wexler, 1996). However, weaknesses in lexical–semantic knowledge also have been noted, with reduced breadth and depth of word knowledge (Kan & Windsor, 2010; Sheng & McGregor, 2010). Given that word knowledge is a strong predictor of reading abilities and academic success (Catts, Fey, Tomblin, & Zhang, 2002; Ouellette, 2006; Quinn, Wagner, Petscher, & Lopez, 2015), it is important to form a more complete understanding of word processing abilities in children with SLI. The current study aimed to examine the neural correlates of word processing in preschoolers with SLI and typically developing (TD) preschoolers.
SLI is a neurodevelopmental, multifactorial language disorder that affects approximately 7% of the preschool-age population (Tomblin et al., 1997). It is characterized by language deficits that cannot be explained by neurological insult, hearing impairment, or intellectual disability (Leonard, 2014). Although language deficits are the primary impairment observed in this group, other nonlinguistic deficits often co-occur, such as reduced processing speed, deficits in working memory, and executive function deficits (Miller, Kail, Leonard, & Tomblin, 2001; Montgomery & Evans, 2009; Pauls & Archibald, 2016). As such, the label SLI has been debated (Reilly et al., 2014). At present, the term most frequently used to refer to this group in the research literature is children with SLI (Bishop, 2014; Leonard, 2014). However, based on a recent consensus study on terminology, the term developmental language disorder has emerged as an alternative that avoids the connotation that these children are free of weaknesses outside of language (Bishop, Snowling, Thompson, Greenhalgh, & CATALISE-2 consortium, 2017).
Word Processing
Children with SLI have been reported to experience word-finding difficulties (McGregor, Friedman, Reilly, & Newman, 2002) and to be slower to name pictures (Kail, Hale, Leonard, & Nippold, 1984; Lahey & Edwards, 1999; Leonard, Nippold, Kail, & Hale, 1983). Additionally, children with SLI experience difficulties in suppressing competing words during word processing tasks (McMurray, Samelson, Lee, & Tomblin, 2010). The storage-elaboration hypothesis postulates that children with SLI not only have smaller lexicons relative to their chronological age-mate peers, but the words that are known are stored with insufficient detail (Kail & Leonard, 1986; Kail et al., 1984; Leonard et al., 1983). Leonard et al. suggest that children with SLI have difficulties encoding and storing rich information about words and, as a result, have lexical–semantic networks that are more similar to younger TD children rather than their age-mate peers. Therefore, difficulties in storing and elaborating lexical–semantic information result in sparse lexical–semantic networks with weak links, which may cause children with SLI to have superficial lexical–semantic representations and to experience difficulties in word processing and word recall (Leonard, 2014).
Despite this, in many studies, children with SLI perform within normal limits on receptive vocabulary assessments, like the Peabody Picture Vocabulary Test (Dunn & Dunn, 2007; Gray, Plante, Vance, & Henrichsen, 1999; Haebig, Kaushanskaya, & Ellis Weismer, 2015; cf., Rice, Buhr, & Oetting, 1992). As such, it has been suggested that some standardized assessments may be less sensitive to lexical–semantic weaknesses in children (Gray et al., 1999). It has also been noted that conflicts in lexical–semantic processing can resolve quickly; thus, tasks that use offline measures to examine processing abilities may be less sensitive to subtle differences that children with SLI experience (Borovsky, Burns, Elman, & Evans, 2013). Offline measures also provide less information about real-time lexical–semantic activation within the lexical network (Pizzioli & Schelstraete, 2011). Therefore, online measures of word processing can complement behavioral studies and potentially provide a more nuanced understanding of word processing in children with SLI. Neural indices provide one such type of online processing information.
Neural Indices of Word Processing
Event-related brain potentials (ERPs) reflect synchronized neural activity from populations of neurons in response to a stimulus, such as a flash of light, or to a cognitive process, such as lexical access (Luck, 2014). In the current study, we focus on two ERP components associated with word processing: the N400 and the late positive component (LPC).
The N400 component is linked to the processing of meaning and is often thought to index lexical–semantic access and the semantic fit of an item within a certain context (Kutas & Federmeier, 2011). After a semantic violation, such as the presentation of a semantically anomalous word (e.g., “Peanut butter and mountain”), the N400 is observed with an increase in negative polarity that peaks between 200 ms and 600 ms. The N400 has been elicited in young children and adults (Friedrich & Friederici, 2005; Mills, Coffey-Corina, & Neville, 1993; Silva-Pereyra, Rivera-Gaxiola, & Kuhl, 2005). Over development, the N400 becomes more focal over central–parietal electrodes. In addition, with development, the mean amplitude decreases, and the N400 component peaks earlier (Hahne, Eckstein, & Friederici, 2004; Holcomb, Coffey, & Neville, 1992). Changes in the N400 within an individual also can emerge with stimuli repetition. Adult studies have revealed that the N400 mean amplitude reduces and the duration shortens with repetition of incongruent stimuli (Batterink & Neville, 2011; Besson, Kutas, & Petten, 1992). Despite this reduction in mean amplitude, the N400 is still apparent in semantic tasks when stimuli are presented in nonsequential repetitions (Renoult, Brodeur, & Debruille, 2010; Renoult & Debruille, 2009).
A post-N400 ERP component also has been associated with semantic violations (i.e., LPC). In adults, the LPC typically occurs between 500 ms and 900 ms after a semantic violation, but later in children (Sabisch, Hahne, Glass, von Suchodoletz, & Friederici, 2006; Weber-Fox, Hampton Wray, & Arnold, 2013). Previous studies have proposed several processes that may be associated with the LPC, including effortful postlexical integration of verbal meaning following a semantic violation (Batterink & Neville, 2011; Van Petten & Luka, 2012; Weber, Hahne, Friedrich, & Friederici, 2004; Weber-Fox et al., 2013). It is suggested that, when a semantically implausible violation has occurred, individuals may require additional resources for extended processing or semantic reanalysis (DeLong, Quante, & Kutas, 2014; Kolk, Chwilla, Van Herten, & Oor, 2003; see also Kuperberg, 2007). The LPC is thought to increase in mean amplitude and decrease in latency with age and increased language proficiency (Juottonen, Revonsuo, & Lang, 1996; Licht, Kok, Bakker, & Bouma, 1986). In addition, within a person, the LPC has been found to increase in amplitude with nonsequentially repeated stimuli (Renoult et al., 2010).
ERP studies examining word processing in children with SLI have yielded mixed findings. Archibald and Joanisse (2012) presented 8-year-old children with SLI and typical development with pictures and auditory labels. They found that picture–word mismatches elicited a similar N400 for both groups. Similarly, Malins et al. (2013) also examined picture–word processing in 8- to 12-year-old children and found no differences between children with SLI and typical development in the N400s that were elicited from picture–word mismatches (however, some differences were found in conditions that manipulated the degree of phonological overlap with the picture). In contrast, Cummings and Ceponiene (2010) found that children with SLI between 7 and 15 years of age demonstrated delayed peak latencies of the N400 component after a picture–word mismatch, relative to their peers. Lastly, Kornilov et al. (2015) found that picture–word mismatches elicited N400 components that were similar in amplitude between 7- to 15-year-old children with SLI and typical development when measured in the early N400 window (Kornilov, Magnuson, Rakhlin, Landi, & Grigorenko, 2015). However, the SLI group had an attenuated N400 during the late N400 window (Kornilov et al., 2015). Many of these studies used words from a variety of word classes that emerge at different points in development; therefore, the different methodologies across the studies may have taxed the language system to different degrees and led to mixed findings. Additionally, these studies have only examined lexical–semantic processing in school-age children and adolescents with SLI. Given the fast rate of language development that occurs within the first 5 years of life and given that impairments in language development become apparent in the preschool years, it is important to examine word processing skills in preschool children with SLI.
Thus far, only one study has examined semantic processing in preschool children with SLI (Pijnacker et al., 2017). Pijnacker et al. presented auditory recordings of sentences with canonical and anomalous sentence-final nouns. The anomalous nouns elicited an N400 during both the early and late windows in the TD preschoolers. In contrast, the N400 condition effect only emerged during the later time window in the preschoolers with SLI. Additionally, the N400 was more broadly distributed in the SLI group, relative to the posteriorly focal N400 that was observed in the TD preschoolers. No study has examined the dynamics of lexical–semantic activation in preschoolers with SLI when processing words in isolation. Such a task would provide important information about lexical–semantic processing in preschool children with SLI because word-level tasks reduce other processing demands that are related to sentence-level constraints.
The Current Study
The aim of the current study was to examine word processing using ERPs as a means to measure online processing and behavioral judgments to lay the groundwork for future novel word learning tasks. Limitations in word-learning abilities in young children with SLI constrain the number of words that can be taught in word-learning experiments. Therefore, when using an ERP paradigm to examine newly taught words, it is necessary to have repeated presentations of stimuli in order to have a sufficient number of trials to analyze. Given that previous findings have noted that stimuli repetition can affect ERP components, the current study sought to examine the N400 and LPC during word processing of early emerging words that were presented multiple times in a nonsequential order. This is a necessary first step for future studies that wish to examine the neural correlates associated with processing newly taught words. We predicted that behavioral accuracy would be high for both groups given that the current study examined early emerging words. We also predicted that preschool children would demonstrate an N400 and possibly an emerging LPC elicited by a picture–word mismatch, even with stimuli repeated (nonconsecutively) during the task. Additionally, when examining lexical–semantic processing of early emerging words that were presented at the word level, we predicted that preschoolers with SLI would have similar neural profiles to their TD peers.
Method
Participants
Participants included 15 TD children and 15 children with SLI, who were matched on chronological age (M TD = 4;11, SD TD = 7 months; M SLI = 4;11, SD SLI = 4 months; t[28] = 0.10, p = .92), gender (χ2 = 0.13, p = .72), and maternal years of education (U = 93.5, p = .39). This study was approved by the institutional review board. All participants provided verbal assent and a parent provided informed written consent.
Children completed a battery of standardized assessments. All children scored within or above 1 SD of the mean on nonverbal cognitive tests—the Primary Test of Nonverbal Intelligence (Ehrler & McGhee, 2008) or the Kaufman Assessment Battery for Children–Second Edition (Kaufmann & Kaufman, 2004). However, as is characteristic of children with SLI more generally (Gallinat & Spaulding, 2014), the children with SLI in the current study had significantly lower nonverbal cognitive scores, despite being within 1 SD of the mean (see Table 1).
Table 1.
Participant characteristics.
| Variable | TD, n = 15 (8 boys, 7 girls) |
SLI, n = 15 (7 boys, 8 girls) |
Group comparisons | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Chronological age (years; months) | 4;11 | 0;4 | 4;11 | 0;7 | p = .92 |
| Receptive vocabulary (PPVT-4 standard score) | 119.80 | 10.80 | 105.27 | 10.70 | p < .001 |
| Expressive vocabulary (EVT-2 standard score) a | 115.93 | 11.00 | 100.45 | 9.09 | p < .001 |
| Expressive grammar (SPELT-P 2 standard score) | 118.13 | 8.80 | 80.27 | 8.07 | p < .001 |
| Nonverbal cognition (K-ABC2 or P-TONI standard score) | 115.93 | 11.42 | 107.13 | 11.19 | p = .04 |
Note. TD = typically developing; SLI = specific language impairment; PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; EVT-2 = Expressive Vocabulary Test–Second Edition; SPELT-P 2 = Structured Photographic Expressive Language Test–Preschool 2; K-ABC2 = Kaufman Assessment Battery for Children–Second Edition; P-TONI = Primary Test of Nonverbal Intelligence.
Scores for four children with SLI were not available.
Hearing was screened at 20 dB through headphones at 500, 1000, 2000, and 4000 Hz (American Speech-Language-Hearing Association, 1997). All children passed each frequency in at least one ear. Handedness was assessed using an abbreviated handedness assessment (Edinburg Handedness Inventory; Oldfield, 1971). All children were right-handed except one child with SLI who was left-handed and one TD child who was ambidextrous.
We classified children as having SLI according to scores on measures that have been found to have good sensitivity and specificity for identifying SLI. Children with SLI earned a standard score of 87 or below on the Structured Photographic Expressive Language Test–Preschool 2 (Dawson et al., 2005). This cutoff score was empirically determined to be the cutoff point yielding high sensitivity and specificity for this age group (Greenslade, Plante, & Vance, 2009). Three children with SLI scored above this cutoff score but were retained because their developmental sentence score (Lee, 1974), derived from a language sample, was below the 10th percentile. We ensured that all children recruited for the TD group scored above 87 on the Structured Photographic Expressive Language Test–Preschool 2.
Although not used as selection criteria, receptive and expressive vocabulary tests were administered for descriptive purposes, given the focus of the study. These were the Peabody Picture Vocabulary Test–Fourth Edition (Dunn & Dunn, 2007) and the Expressive Vocabulary Test–Second Edition (Williams, 2007), respectively. The children with typical development performed within or above 1 SD from the mean on these measures. As can been seen in Table 1, the SLI group demonstrated the common pattern seen in earlier studies of lexical abilities in SLI, as noted in the Introduction. The children with SLI scored significantly lower than the TD group on the Peabody Picture Vocabulary Test–Fourth Edition and Expressive Vocabulary Test–Second Edition, yet almost all of their scores fell within 1 SD of the normative sample.
Experimental Task
Children participated in a word processing task involving six early emerging real words (baby, cookie, dog, flower, spoon, truck) with corresponding and easy-to-identify pictures. Each word has been reported to be known (i.e., understood) by 75% of children within the Wordbank database by at least 18 months of age and to be produced by 75% of children by at least 24 months of age (Frank, Braginsky, Yurovsky, & Marchman, 2017). The task followed a match–mismatch paradigm. During match trials, a picture was displayed on a screen, and an auditory recording of the correct label for the picture was played (e.g., picture: dog, label: “dog”) via sound field. In mismatch trials, the label did not match the picture (e.g., picture: dog, label: “spoon”). At the end of each trial, children judged whether or not the picture and label matched. Each word and picture was presented 10 times: five in the match condition and five in the mismatch condition. Match and mismatch trials were pseudorandomized.
Visual task stimuli consisted of two-dimensional pictures that depicted a prototypical image for each of the six familiar words. Each familiar word corresponded to only one of the two-dimensional pictures. The images were approximately 13–14.5 cm wide and 10.5 cm tall and were presented on a 47.5-cm monitor that was 164 cm in front of the child. Auditory stimuli were recorded in isolation by a young female adult with midwestern American English dialect. Sound stimuli were normalized to have an amplitude of approximately 65–70 dB using PRAAT software (Boersma & Weenink, 2006). The sound stimuli ranged in duration between 683 ms and 967 ms.
Children first completed five practice trials during which different familiar pictures (e.g., lion and boat) appeared on the screen and a matching or mismatching label was presented. The examiner explained to the child that he or she would see a picture and hear a name and that the child's job was to tell the examiner if the name matched the picture (i.e., “yes/no”). Children were provided feedback on their judgments of whether or not the picture and label matched. Following the practice trials, the children completed 60 test trials (30 match condition, 30 mismatch condition) presented in a pseudorandomized order, without examiner feedback.
Electroencephalographic Recordings
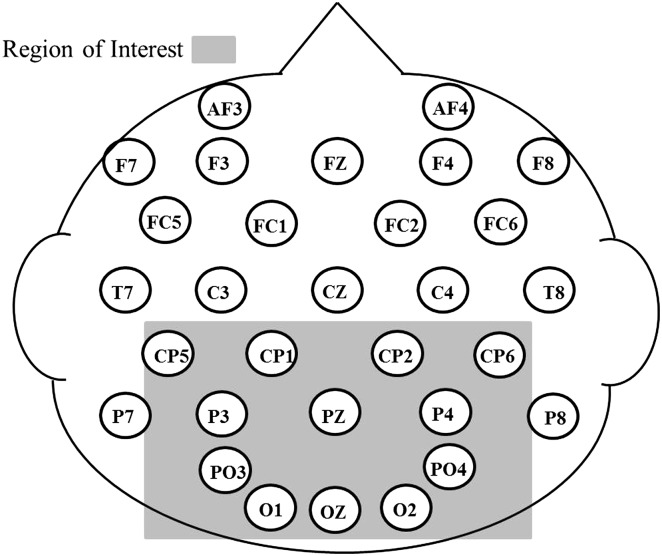
In addition to collecting behavioral data, electroencephalographic data were recorded during the task. Prior to the familiar word experiment, children sat and watched a child movie of choice while an examiner placed the appropriately sized elastic cap on the child (ActiveTwo head cap, Version 7.0 AD conversion in 24 bit, Cortech Solutions). A second experimenter sat with the child and conversed with him or her about the movie while the capping examiner applied gel to each location and attached the corresponding electrode. The 32 electrodes were positioned over homologous hemisphere locations according to the International 10–10 system (Jurcak, Tsuzuki, & Dan, 2007). Locations were lateral sites F7/F8, FC5/FC6, T7/T8, CP5/CP6, P7/P8; midlateral sites FP1/FP2, AF3/AF4, FC1/FC2, F3/F4, CP1/CP2, P3/P4, PO3/PO4, O1/O2; and midline sites FZ, CZ, PZ, OZ. Electrodes placed over the left and right outer canthi recorded horizontal eye movements. Vertical eye movement was monitored through recordings from electrodes placed over the left inferior and superior orbital ridge. The continuous electroencephalogram (EEG) data were recorded using the Biosemi ActiveTwo® system and bandpass filtered between 0.1 Hz and 100 Hz.
ERP Measures
The EEG data were processed using EEGLAB and ERPLAB (Lopez-Calderon & Luck, 2010), which are MATLAB© toolboxes (MathWorks, Natick, MA, USA). During data processing, the electrical recordings were referenced to the average of the electrodes on the left and right mastoids. The EEG signals were down-sampled at a rate of 256 Hz and were bandpass filtered from 0.1 to 30 Hz with a 12-dB roll-off to remove high-frequency noise and to minimize offsets and drift. Eye artifact was removed through independent component analysis (EEGLAB). Independent component analysis identifies independent sources of EEG signals and yields components that represent patterns from the EEG signal. Components that represent artifact, such as blinks, horizontal eye movements, and voltage drifts, were identified by two independent, trained research assistants. Discrepancies were resolved by a third research assistant. The data were epoched from 200 ms prior to the onset of the label to 2000 ms poststimulus to allow for averaging and ERP component measures. Epochs were baseline corrected from −200 ms to the onset of the label (0 ms). All the EEG channels underwent automatic voltage-dependent thresholds to remove any trials that still contained artifact. Each participant contributed at least 14 artifact-free correct trials within each condition. The average number of correct artifact-free trials within the match condition was 26.4 trials for the TD group and 23.3 trials for the SLI group, and the average number of correct artifact-free trials for the mismatch condition for the TD group was 27.1 trials and 23.5 trials for the SLI group. Finally, the EEG epochs from correct artifact-free trials were averaged within task conditions for each individual, and analyses were conducted to examine the N400 and LPC ERP components. We chose to examine the ERP components of interest from correct trials only in order to constrain the interpretation of the results yielded from analyses comparing the N400 and LPC between the two groups. Because all of the participants performed with high accuracy, the majority of artifact-free trials were utilized.
Temporal windows for measuring the N400 and the LPC were selected after the grand averages were examined for each group. The windows were centered around the regions of maximal activity, which aligned with windows that were used in previous studies examining language processing in children (Neville, Coffey, Holcomb, & Tallal, 1993; Pijnacker et al., 2017; Sabisch et al., 2006; Usler & Weber-Fox, 2015). In the current data set, the same temporal windows were appropriate for each group's grand averages. As a second step in the window-selecting procedure, we examined each individual's peak latency of the N400 window to ensure that the window captured the component of interest. The mean amplitudes and the peak latencies of the N400 were measured within the temporal window of 300–800 ms, and the mean amplitudes of the LPC were measured within the temporal window of 1200–1700 ms. We measured the N400 and the LPC from a specified region of interest (CP1, CP2, CP5, CP6, P3, Pz, P4, PO3, PO4, O1, Oz, O2), which aligned with regions of interest used in previous studies examining word processing in children with SLI (Haebig, Weber, Leonard, Deevy, & Tomblin, 2017; Pijnacker et al., 2017; Sabisch et al., 2006). See Figure 1.
Figure 1.
Head map including the region of interest for the current study.
Analysis Procedure
In order to control for response bias, A′ scores served as the dependent variable for the behavioral measure (Grier, 1971; Rice, Wexler, & Redmond, 1999). Briefly, A′ scores serve as a measure of the proportion of correct responses in a two-alternative, forced-choice task. The A′ value consists of scores from a control condition and an experimental condition (e.g., correct sentences and sentences with syntactic violations). The formula was A′ = 0.5 + (y − x) (1 + y − x) / 4y (1 − x), where y represents correct identifications (hits) and x represents incorrect identifications (false alarms; Linebarger, Schwartz, & Saffran, 1983). An A′ value of 1.00 represents perfect discrimination of correct and incorrect picture-label pairs. An A′ value of .50 indicates chance performance. A t test compared the group performance on the behavioral measure.
The ERP data were analyzed using a series of repeated-measures analysis of variance. The omnibus models included condition (match vs. mismatch), electrodes, group, and interactions across the predictor variables. When there was more than 1 degree of freedom in the numerator, the Huynh–Feldt adjusted p value was used to determine significance (Hays, 1994). We report main effect findings for group, condition, and interactions involving both group and condition.
Results
Behavioral Performance
Both groups had high accuracy in judging match and mismatch trials (M TD = 97.27%, SD TD = 2.90%; M SLI = 95.33%, SD SLI = 4.95%). Similarly, A′ scores were well above chance (A′ M TD = 0.942, SD TD = 0.062; A′ M SLI = 0.902, SD SLI = 0.096). The groups did not significantly differ in behavioral judgments in the word processing task, t(28) = 1.35, p = .19.
ERP Patterns Elicited by Word Processing
N400
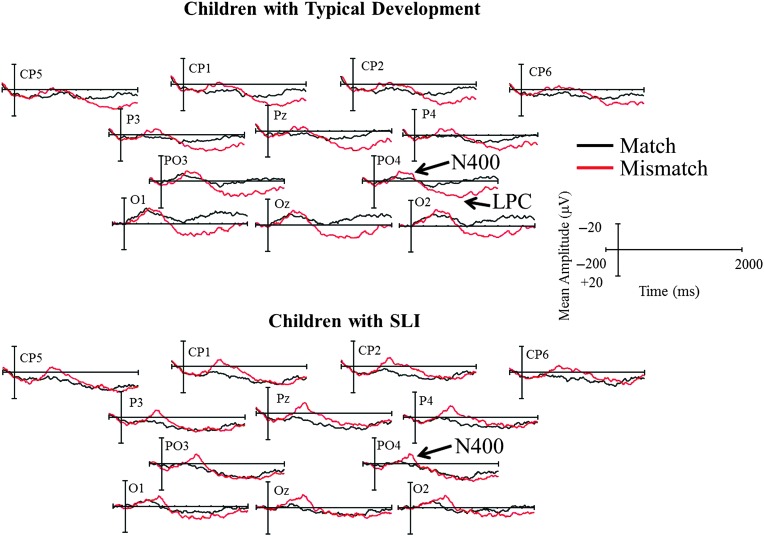
The mean amplitude of the N400 for electrode sites within our region of interest served as the dependent variables in a repeated-measures analysis of variance. There was a main effect of condition, F(1, 28) = 15.21, p = .001, ηp 2 = .352, indicating that mismatch trials elicited an increased N400 amplitude relative to match trials. There was no effect of group, F(1, 28) = 0.57, p = .46. Additionally, there was no interaction of group by condition, F(1, 28) = 1.87, p = .18, or group by condition by electrodes, F(11, 308) = 0.54, p = .76. We present the waveforms for each group in Figure 2.
Figure 2.
Waveforms of mismatch trials (depicted in red) relative to match trials (depicted in black), averaged within groups. LPC = late positive component; SLI = specific language impairment.
In addition to examining the mean amplitude, we compared the N400 peak latency across conditions and groups. There was a main effect of condition, F(1, 28) = 6.90, p = .01, ηp 2 = .198, indicating that peak latencies during mismatch trials were later than match trials. However, there was no main effect of group, F(1, 28) = 0.07, p = .80. There also were no interactions involving both group and condition, ps > .25.
LPC
We also examined the mean amplitude of the LPC. There was a main effect of condition, F(1, 28) = 5.94, p = .02, ηp 2 = .175; however, there also was a significant interaction of group by condition, F(1, 28) = 4.76, p = .04, ηp 2 = .145, indicating that mismatch trials elicited a larger LPC in the TD group. There was no main effect of group, F(1, 28) = 0.41, p = .53. Descriptively, six of the 15 (40%) children with SLI had positive mean amplitude difference values in the LPC window, whereas 10 of the 15 (66.67%) TD children had positive values, including two children with very large amplitude differences.
Discussion
The current study provided an initial step in examining the neural activity mediating processing of early acquired words in preschool children with SLI and typical development. As anticipated, children attained high levels of accurate behavioral judgments in assessing picture–word presentations of early emerging words; there were no group differences. Additionally, there were no group differences in the N400 component. However, the TD group demonstrated an emerging LPC for mismatch trials, indexing a more mature profile of word processing relative to the children with SLI.
Given the focus of the current study, it is important to note that children with SLI often have smaller vocabulary sizes relative to their age-match peers (as also observed in our participants). Despite this, most children with SLI score within 1 SD of the mean on standardized assessments of vocabulary, which was also observed in the current study. Although this may seem contradictory, previous studies have suggested that traditional vocabulary assessments, such as the Peabody Picture Vocabulary Test, do not have adequate diagnostic sensitivity and specificity (Gray et al., 1999; Spaulding, Hosmer, & Schechtman, 2013). Instead, different measures may be more sensitive to the lexical–semantic deficits that children with SLI experience. The current study provides a preliminary step in examining online lexical–semantic processing abilities by specifically examining early acquired words.
Although our children with SLI had significantly smaller vocabulary sizes, their behavioral accuracy was high and comparable to those of their TD peers when judging early emerging words. This feature ensured similar behavioral proficiency for preschoolers with SLI and typical development, which allowed us to compare the online neural correlates of familiar word processing for trials to which children responded correctly. It is possible that differences may have emerged had we increased the complexity of the task by testing later-acquired words (Cummings & Ceponiene, 2010). Furthermore, differences most likely would have been identified if the linguistic complexity had been increased through the use of auditory sentence stimuli (Sabisch et al., 2006; Weber-Fox, Leonard, Hampton Wray, & Tomblin, 2010). However, the current study sought to focus on lexical–semantic processing abilities independent of the confound that weak syntactic processing skills could introduce. Furthermore, it is possible that a different behavioral and/or neural profile could be seen for children with SLI who have markedly impaired lexical–semantic knowledge. Future studies will need to specifically examine this to develop a more comprehensive picture of lexical–semantic processing abilities in children with SLI who fall on the lowest end of the language continuum.
In addition to constraining our stimuli to the early acquired words presented in isolation, we narrowed our study to an earlier point in development relative to previous studies. Therefore, this study provides needed data to develop a more comprehensive understanding of word processing in children with SLI across development. This aim was especially important given that patterns of language difficulties have been found to shift with maturation (Hesketh & Conti-Ramsden, 2013). Furthermore, it is important to examine word processing in preschool-age children because word knowledge is a strong predictor of reading abilities and academic success (Conti-Ramsden, Durkin, Simkin, & Knox, 2009; Duff, Tomblin, & Catts, 2015). Additionally, the developmental stage that we selected in this study was clinically relevant given the large language gains that children make during the first 5 years of their life. Lastly, we chose to examine 4- and 5-year-olds because SLI is most often diagnosed during this point in development (Leonard, 2014).
Beyond our behavioral measure, the current study also collected ERP data as a means to measure online processing of lexical–semantic information and to enhance our understanding of the neural correlates of word processing. This aim is particularly important given that previous studies have demonstrated that children with neurodevelopmental disorders may have similar behavioral performance on a task; however, the neural processes underlying the behavior may differ from TD children (Karmiloff-Smith, 2009). In the current study, there were no differences in the mean amplitude or peak latency of the N400 between preschoolers with SLI and typical development. The current findings are similar to those of Archibald and Joanisse (2012) and Malins et al. (2013). However, our findings differ from those of Cummings and Ceponiene (2010) and Kornilov et al. (2015), who reported smaller amplitudes of the N400 and/or delayed N400 components in response to picture–word mismatch trials in 7- to 15-year-old children with SLI. The mixed findings within the literature may be attributed to the different stimuli (e.g., nouns and verbs) and methods that were used across the studies. It will be important for future studies to separately analyze word processing within word classes to determine whether or not word processing differs across different word classes and if such patterns differ between TD children and children with SLI.
Although we did not find differences in the N400 component between groups, there were differences in the LPC. The TD children demonstrated a condition effect during the LPC window, with a late positive polarity shift during the mismatch trials. In contrast, a clear condition effect during the LPC window was not observed in the children with SLI. The N400 and the LPC are associated with semantic processing; however, it has been suggested that the components index different aspects of semantic processing. Juottonen et al. (1996) suggest that the N400 may more heavily index automatic semantic processing (though see Kutas & Federmeier, 2011 for a more thorough discussion of automatic and controlled semantic processing indexed by the N400). The LPC, on the other hand, may index more controlled/postlexical processing associated with conflict monitoring of semantic plausibility and postlexical reanalysis and integration (DeLong et al., 2014; Kutas, DeLong, & Smith, 2011; Van Petten & Luka, 2012). Juottonen et al. (1996) have suggested that the attention-demanding processes associated with the LPC may only be possible after children reach a more proficient level of semantic memory processing skills.
The absence of a difference between match and mismatch trial waveforms during the LPC window likely indicates that some children with SLI have a less mature pattern of postlexical reanalysis and integration when distinguishing words that match and do not match a picture. It is possible that the difference in the LPC may align with Locke's (1993, 1994) theory of a neuromaturational lag in children with SLI. The late positivity observed for match and mismatch conditions in the SLI group also may index less efficient word processing abilities stemming from a less mature lexical–semantic network with weaker links between words (i.e., storage-elaboration hypothesis; Kail et al., 1984; Leonard et al., 1983). Additional work is needed to extend our preliminary examination of the LPC in preschool children with SLI in order to better understand postlexical integration and how the LPC is associated with behavioral measures of lexical–semantic processing during these early years. Also, it will be important to link future work examining the LPC to theoretical accounts of lexical–semantic processing.
In addition to presenting needed information about lexical–semantic processing in young children with SLI, the current study provides the necessary groundwork to support future word-learning studies that incorporate ERP data. In order to design an appropriate word-learning study that avoids floor effects, the number of words that are taught must be limited. This then necessitates that corresponding ERP tasks have a high degree of repetition because an adequate number of trials are needed for improving the signal-to-noise ratio, such that ERP components can be discerned and more accurately represented (Luck, 2014). Given that previous studies have demonstrated that ERP components are influenced by the degree of stimulus repetition in adults (Besson & Kutas, 1993; Besson et al., 1992; Renoult et al., 2010), it will be important to document ERP components that have been elicited in tasks that incorporate repeated stimulus presentation in children. The current data provide much-needed preliminary evidence that clear N400 effects can be obtained from preschool children when stimuli are presented in a lexical–semantic task that incorporates nonsequential repetitions (i.e., 10 presentations of each word). Additionally, the LPC also can be elicited in such a task in children. This information will support experimental design decisions in future word-learning studies.
Study Limitations and Future Directions
The current study was the first to examine word processing abilities in preschool children with SLI when early emerging words were presented in isolation. Although this is a meaningful contribution, our study has limitations. Most notably, our study would have benefited from larger sample sizes within each group. Including additional children in each group could have strengthened our ability to examine individual differences and to develop a greater understanding of the distribution of the LPC at this age. Additionally, it would be informative to include both a chronological age-matched group and a vocabulary-matched group of TD children to better understand word processing in young children with SLI. Furthermore, as a first step in examining ERP components associated with word-level lexical–semantic processing, our study would have benefited from the inclusion of a larger set of early emerging words. The current study limited the set of words that were included, and therefore, the task included multiple repetitions of each word. This decision was purposeful; we wanted to demonstrate that a robust N400 component can be elicited despite multiple presentations of picture–word mismatches. Nonetheless, our field would benefit from a separate study that is designed to carefully examine whether ERP components change as the frequency of stimulus presentation changes.
Conclusion
The current study demonstrates that the neural correlates of lexical–semantic access, indexed by the N400, do not differ between preschoolers with SLI and typical development, when examining early acquired words presented at the word level. Conversely, differences in the neural indices of postlexical reanalysis and integration, indexed by the LPC, indicate that preschoolers with SLI may have a less mature neural profile of lexical processing despite high behavioral accuracy. Lastly, the current study demonstrates that ERP components indexing word processing can be elicited in semantic tasks that incorporate nonsequential repetition of task stimuli in 4- and 5-year-old children.
Acknowledgments
This study was supported by the National Institute on Deafness and Other Communication Disorders (R01-DC014708 and T32-DC00030 granted to L. Leonard). The authors thank the families that participated in this study. Also, the authors thank Jen Schumaker, Katie Gerwin, Connor Slavich, and Gina Catania for help with data collection and data processing.
Funding Statement
This study was supported by the National Institute on Deafness and Other Communication Disorders (R01-DC014708 and T32-DC00030 granted to L. Leonard).
References
- American Speech-Language-Hearing Association. (1997). Guidelines for audiologic screening. Retrieved from http://www.asha.org/policy
- Archibald L. M. D., & Joanisse M. F. (2012). Atypical neural responses to phonological detail in children with developmental language impairments. Developmental Cognitive Neuroscience, 2, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L., & Neville H. (2011). Implicit and explicit mechanisms of word learning in a narrative context: An event-related potential study. Journal of Cognitive Neuroscience, 23, 3181–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M., & Kutas M. (1993). The many facets of repetition: A cued-recall and event-related potential analysis of repeated words in same versus different sentence contexts. Journal of Experimental Psychology: Learning, Memory, and Cognition, 19, 1115–1133. [DOI] [PubMed] [Google Scholar]
- Besson M., Kutas M., & Petten C. Van (1992). An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience, 4, 132–149. [DOI] [PubMed] [Google Scholar]
- Bishop D. V. M. (2014). Ten questions about terminology for children with unexplained language problems. International Journal of Language & Communication Disorders, 49(4), 381–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. V. M., Snowling M. J., Thompson P. A., Greenhalgh T., & CATALISE-2 consortium. (2017). Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. The Journal of Child Psychology and Psychiatry, 58, 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma M., & Weenink D. (2006). PRAAT: Doing phonetics by computer. Amsterdam, Netherlands: University of Amsterdam; Retrieved from http://www.praat.org [Google Scholar]
- Borovsky A., Burns E., Elman J. L., & Evans J. L. (2013). Lexical activation during sentence comprehension in adolescents with history of specific language impairment. Journal of Communication Disorders, 46, 413–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts H. W., Fey M. E., Tomblin J. B., & Zhang X. (2002). A longitudinal investigation of reading outcomes in children with language impairments. Journal of Speech, Language, and Hearing Research, 45, 1142–1157. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G., Durkin K., Simkin Z., & Knox E. (2009). Specific language impairment and school outcomes. I: Identifying and explaining variability at the end of compulsory education. International Journal of Language & Communication Disorders, 44, 15–35. [DOI] [PubMed] [Google Scholar]
- Cummings A., & Ceponiene R. (2010). Verbal and nonverbal semantic processing in children with developmental language impairment. Neuropsychologia, 48, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Stout C., Eyer J. A., Tattersall P., Fonkalsrud J., & Croley K. (2005). Structured Phonotactic Expressive Language Test–Preschool 2. DeKalb, IL: Janelle. [Google Scholar]
- DeLong K. A., Quante L., & Kutas M. (2014). Predictability, plausibility, and two late ERP positivities during written sentence comprehension. Neuropsychologia, 61, 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff D., Tomblin J. B., & Catts H. W. (2015). The influence of reading on vocabulary growth: A case for a Matthew effect. Journal of Speech, Language, and Hearing Research, 58, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. M., & Dunn D. M. (2007). Peabody Picture Vocabulary Test–Fourth Edition. Bloomington, MN: NCS Pearson. [Google Scholar]
- Ehrler D. J., & McGhee R. L. (2008). Primary Test of Nonverbal Intelligence. Austin, TX: Pro-Ed. [Google Scholar]
- Frank M. C., Braginsky M., Yurovsky D., & Marchman V. A. (2017). Wordbank: An open repository for developmental vocabulary data. Journal of Child Language, 44, 677–694. [DOI] [PubMed] [Google Scholar]
- Friedrich M., & Friederici A. D. (2005). Lexical priming and semantic integration reflected in the event-related potential of 14-month-olds. NeuroReport, 16, 653–656. [DOI] [PubMed] [Google Scholar]
- Gallinat E., & Spaulding T. J. (2014). Differences in the performance of children with specific language impairment and their typically developing peers on nonverbal cognitive tests: A meta-analysis. Journal of Speech, Language, and Hearing Research, 57, 1363–1382. [DOI] [PubMed] [Google Scholar]
- Gray S., Plante E., Vance R., & Henrichsen M. (1999). The diagnostic accuracy of four vocabulary tests administered to preschool-age children. Language, Speech, and Hearing Services in Schools, 30, 196–207. [DOI] [PubMed] [Google Scholar]
- Greenslade K. J., Plante E., & Vance R. (2009). The diagnostic accuracy and construct validity of the Structured Photographic Expressive Language Test–Preschool: Second Edition. Language, Speech, and Hearing Services in Schools, 40, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier J. B. (1971). Nonparametric indexes for sensitivity and bias: Computing formulas. Psychological Bulletin, 75, 424–429. [DOI] [PubMed] [Google Scholar]
- Haebig E., Kaushanskaya M., & Ellis Weismer S. (2015). Lexical processing in school-age children with autism spectrum disorder and children with specific language impairment: The role of semantics. Journal of Autism and Developmental Disorders, 45, 4109–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haebig E., Weber C., Leonard L. B., Deevy P., & Tomblin J. B. (2017). Neural patterns elicited by sentence processing uniquely characterize typical development, SLI recovery, and SLI persistence. Journal of Neurodevelopmental Disorders, 9, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne A., Eckstein K., & Friederici A. D. (2004). Brain signatures of syntactic and semantic processes during children's language development. Journal of Cognitive Neuroscience, 16, 1302–1318. [DOI] [PubMed] [Google Scholar]
- Hays W. L. (1994). Inferences about population means. Statistics, 5, 311–342. [Google Scholar]
- Hesketh A., & Conti-Ramsden G. (2013). Memory and language in middle childhood in individuals with a history of specific language impairment. PLoS ONE, 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb P. J., Coffey S. A., & Neville H. (1992). Visual and auditory sentence processing: A developmental analysis using event-related brain potentials. Developmental Neuropsychology, 8, 203–241. [Google Scholar]
- Juottonen K., Revonsuo A., & Lang H. (1996). Dissimilar age influences on two ERP waveforms (LPC and N400) reflecting semantic context effect. Cognitive Brain Research, 4, 99–107. [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., & Dan I. (2007). 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage, 34, 1600–1611. [DOI] [PubMed] [Google Scholar]
- Kail R., & Leonard L. B. (1986). Word-finding abilities in language-impaired children. ASHA Monographs, 25, 1–39. [PubMed] [Google Scholar]
- Kail R. V., Hale C. A., Leonard L. B., & Nippold M. A. (1984). Lexical storage and retrieval in language-impaired children. Applied Psycholinguistics, 5, 37–49. [Google Scholar]
- Kan P. F., & Windsor J. (2010). Word learning in children with primary language impairment: A meta-analysis. Journal of Speech, Language, and Hearing Research, 53, 739–757. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. (2009). Nativism versus neuroconstructivism: Rethinking the study of developmental disorders. Developmental Psychology, 45, 56–63. [DOI] [PubMed] [Google Scholar]
- Kaufmann A., & Kaufman N. L. (2004). Kaufman Assessment Battery for Children. Circle Pines, MN: AGS. [Google Scholar]
- Kolk H. H. J., Chwilla D. J., Van Herten M., & Oor P. J. W. (2003). Structure and limited capacity in verbal working memory: A study with event-related potentials. Brain and Language, 85, 1–36. [DOI] [PubMed] [Google Scholar]
- Kornilov S. A., Magnuson J. S., Rakhlin N., Landi N., & Grigorenko E. L. (2015). Lexical processing deficits in children with developmental language disorder: An event-related potentials study. Development and Psychopathology, 27, 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G. R. (2007). Neural mechanisms of language comprehension: Challenges to syntax. Brain Research, 1146, 23–49. [DOI] [PubMed] [Google Scholar]
- Kutas M., DeLong K. A., & Smith N. J. (2011). A look around at what lies ahead: Prediction and predictability in language processing. In Bar M. (Ed.), Predictions in the brain: Using our past to generate a future (pp. 190–207). New York, NY: Oxford University Press. [Google Scholar]
- Kutas M., & Federmeier K. D. (2011). Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62, 621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey M., & Edwards J. (1999). Naming errors of children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 130–153. [DOI] [PubMed] [Google Scholar]
- Lee L. (1974). Developmental sentence analysis. Evanston, IL: Northwestern University Press. [Google Scholar]
- Leonard L. B. (2014). Children with specific language impairment (2nd ed.). Cambridge, MA: MIT Press. [Google Scholar]
- Leonard L. B., Bortolini U., Caselli M. C., McGregor K. K., Taylor P., & Leonard B. (1992). Morphological deficits in children with specific language impairment: The status of features in the underlying grammar. Language Acquisition, 2, 151–179. [Google Scholar]
- Leonard L. B., Nippold M. A., Kail R. V., & Hale C. A. (1983). Picture naming in language-impaired children. Journal of Speech and Hearing Research, 26, 609–615. [DOI] [PubMed] [Google Scholar]
- Licht R., Kok A., Bakker D. J., & Bouma A. (1986). Hemispheric distribution of ERP components and word naming in preschool children. Brain and Language, 27, 101–116. [DOI] [PubMed] [Google Scholar]
- Linebarger M. C., Schwartz M. F., & Saffran E. M. (1983). Sensitivity to grammatical structure in so-called agrammatic aphasics. Cognition, 13, 361–392. [DOI] [PubMed] [Google Scholar]
- Locke J. L. (1993). The child's path to spoken language. Cambridge, MA: Harvard University Press. [Google Scholar]
- Locke J. L. (1994). Gradual emergence of developmental language disorders. Journal of Speech, Language, and Hearing Research, 37(3), 608–616. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J., & Luck S. J. (2010). ERPLAB Toolbox (1.1.0). Retrieved from http://erpinfo.org/erplab [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. J. (2014). An introduction to the event-related potential technique (2nd ed.). Boston, MA: MIT Press. [Google Scholar]
- Malins J. G., Desroches A. S., Robertson E. K., Newman R. L., Archibald L. M. D., & Joanisse M. F. (2013). ERPs reveal the temporal dynamics of auditory word recognition in specific language impairment. Developmental Cognitive Neuroscience, 5, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor K. K., Friedman R. M., Reilly R. M., & Newman R. M. (2002). Semantic representation and naming in young children. Journal of Speech, Language, and Hearing Research, 45, 332–346. [DOI] [PubMed] [Google Scholar]
- McMurray B., Samelson V. M., Lee S. H., & Tomblin J. B. (2010). Individual differences in online spoken word recognition: Implications for SLI. Cognitive Psychology, 60, 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Kail R., Leonard L. B., & Tomblin J. B. (2001). Speed of processing in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 44, 416–433. [DOI] [PubMed] [Google Scholar]
- Mills D. L., Coffey-Corina S. A., & Neville H. J. (1993). Language acquisition and cerebral specialization in 20-month-old infants. Journal of Cognitive Neuroscience, 5, 317–334. [DOI] [PubMed] [Google Scholar]
- Montgomery J. W., & Evans J. L. (2009). Complex sentence comprehension and working memory in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 52, 269–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville H., Coffey S. A., Holcomb P. J., & Tallal P. (1993). The neurobiology of sensory and language processing in language-impaired children. Journal of Cognitive Neuroscience, 5, 235–253. [DOI] [PubMed] [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Ouellette G. P. (2006). What's meaning got to do with it: The role of vocabulary in word reading and reading comprehension. Journal of Educational Psychology, 98, 554–566. [Google Scholar]
- Pauls L. J., & Archibald L. M. D. (2016). Executive functions in children with specific language impairment: A meta-analysis. Journal of Speech, Language, and Hearing Research, 59, 1074–1086. [DOI] [PubMed] [Google Scholar]
- Pijnacker J., Davids N., Van Weerdenburg M., Verhoeven L., Knoors H., & Van Alphen P. (2017). Semantic processing of sentences in preschoolers with specific language impairment: Evidence from the N400 effect. Journal of Speech, Language, and Hearing Research, 60, 627–639. [DOI] [PubMed] [Google Scholar]
- Pizzioli F., & Schelstraete M.-A. (2011). Lexico-semantic processing in children with specific language impairment: The overactivation hypothesis. Journal of Communication Disorders, 44, 75–90. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., Wagner R. K., Petscher Y., & Lopez D. (2015). Developmental relations between vocabulary knowledge and reading comprehension: A latent change score modeling study. Child Development, 86, 159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S., Tomblin B., Law J., McKean C., Mensah F. K., Morgan A., … Wake M. (2014). Specific language impairment: A convenient label for whom? International Journal of Language & Communication Disorders, 49, 416–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L., Brodeur M. B., & Debruille J. B. (2010). Semantic processing of highly repeated concepts presented in single-word trials: Electrophysiological and behavioral correlates. Biological Psychology, 84, 206–220. [DOI] [PubMed] [Google Scholar]
- Renoult L., & Debruille J. B. (2009). N400-like potentials and reactiontimes index semantic relations between highly repeated individual words. Journal of Cognitive Neuroscience, 23, 905–922. [DOI] [PubMed] [Google Scholar]
- Rice M. L., Buhr J., & Oetting J. B. (1992). Specific-language-impaired children's quick incidental learning of words: The effect of a pause. Journal of Speech and Hearing Research, 35, 1040–1048. [DOI] [PubMed] [Google Scholar]
- Rice M. L., & Wexler K. (1996). Toward tense as a clinical marker of specific language impairment in English-speaking children. Journal of Speech and Hearing Research, 39, 1239–1257. [DOI] [PubMed] [Google Scholar]
- Rice M. L., Wexler K., & Redmond S. M. (1999). Grammaticality judgments of extended optional infinitive grammar: Evidence from English-speaking children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 943–961. [DOI] [PubMed] [Google Scholar]
- Sabisch B., Hahne A., Glass E., von Suchodoletz W., & Friederici A. D. (2006). Lexical-semantic processes in children with specific language impairment. NeuroReport, 17, 1511–1514. [DOI] [PubMed] [Google Scholar]
- Sheng L., & McGregor K. K. (2010). Lexical-semantic organization in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 53, 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Pereyra J., Rivera-Gaxiola M., & Kuhl P. K. (2005). An event-related brain potential study of sentence comprehension in preschoolers: Semantic and morphosyntactic processing. Cognitive Brain Research, 23, 247–258. [DOI] [PubMed] [Google Scholar]
- Spaulding T. J., Hosmer S., & Schechtman C. (2013). Investigating the interchangeability and diagnostic utility of the PPVT-III and PPVT-IV for children with and without SLI. International Journal of Speech-Language Pathology, 15, 453–462. [DOI] [PubMed] [Google Scholar]
- Tomblin J. B., Records N. L., Buckwalter P., Zhang X., Smith E., & O'Brien M. (1997). Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research, 40, 1245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usler E., & Weber-Fox C. (2015). Neurodevelopment for syntactic processing distinguishes childhood stuttering recovery versus persistence. Journal of Neurodevelopmental Disorders, 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C., & Luka B. J. (2012). Prediction during language comprehension: Benefits, costs, and ERP components. International Journal of Psychophysiology, 83, 176–190. [DOI] [PubMed] [Google Scholar]
- Weber C., Hahne A., Friedrich M., & Friederici A. D. (2004). Discrimination of word stress in early infant perception: Electrophysiological evidence. Cognitive Brain Research, 18, 149–161. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Hampton Wray A., & Arnold H. (2013). Early childhood stuttering and electrophysiological indices of language processing. Journal of Fluency Disorders, 38, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C., Leonard L. B., Hampton Wray A., & Tomblin J. B. (2010). Electrophysiological correlates of rapid auditory and linguistic processing in adolescents with specific language impairment. Brain and Language, 115, 162–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. T. (2007). Expressive Vocabulary Test–Second Edition. Minneapolis, MN: Pearson Assessments. [Google Scholar]