Abstract
BACKGROUND
Frameless, non-isocentric irradiation of an extended segment of the trigeminal nerve introduces new concepts in stereotactic radiosurgery for medically resistant trigeminal neuralgia (TN).
OBJECTIVE
To report the results of the largest single-center experience about image-guided robotic radiosurgery for TN.
METHODS
A cohort of 138 patients treated with CyberKnife® (Accuray Incorporated, Sunnyvale, California) radiosurgery with a minimum follow-up of 36 mo were recruited. Pain relief, medications, sensory disturbances, rate and time of pain recurrence were prospectively analyzed.
RESULTS
Median follow-up was 52.4 mo; median dose 75 Gy; median target length 5.7-mm; median target volume 40 mm³; median prescription dose 60 Gy (80% isodose line). Actuarial pain control rate (Barrow Neurological Institute [BNI] class I-IIIa) at 6, 12, 24, and 36 mo were 93.5%, 85.8%, 79.7%, and 76%, respectively. Overall, 33 patients (24%) required a second treatment.
Overall, 18.1% developed sensory disturbances after 16.4 ± 8.7 mo. One patient (0.7%) developed BNI grade IV dysfunction; 6 (4.3%) developed BNI grade III (somewhat bothersome) hypoesthesia after retreatment; BNI grade II (not bothersome) hypoesthesia was reported by 18 patients (11 after retreatment). Shorter nerve length (<6 mm vs 6 mm), smaller nerve volume (<30 mm3 vs >30 mm3), and lower prescription dose (<58 vs >58 Gy) were associated with treatment failure (P = .01, P = .02, P = .03, respectively). Re-irradiation independently predicted sensory disturbance (P < .001).
CONCLUSION
Targeting a 6-mm segment of the trigeminal nerve with a prescribed dose of 60 Gy appears safe and effective. Persistent pain control was achieved in most patients with acceptable risk of sensory complications, which were typically found after re-irradiation.
Keywords: Trigeminal neuralgia, Pain, Stereotactic radiosurgery, Robotic, Image-guided, CyberKnife
ABBREVIATIONS
- BNI
Barrow Neurological Insti- tute
- MVD
microvascular decompression
- NRS
nu- merical rating scale
- SRS
stereotactic radiosurgery
- TN
Trigeminal neuralgia
- TTE
total treatment error
Trigeminal neuralgia (TN) is a severe condition requiring long-term medical treatment. Nonetheless, up to 10% of patients suffer major adverse drug-related events,1,2 and some sort of surgical treatment must be offered to these patients. Ablative procedures aim to interrupt the trigeminal nociceptive pathways either by a percutaneous lesion of the Gasserian ganglion or by stereotactic irradiation of the trigeminal nerve. On the other hand, microvascular decompression (MVD)3 aims to resolve the putative cause of the TN by direct, intracranial removal of a neurovascular conflict.4,5
Stereotactic radiosurgery (SRS) is a proven method to treat TN.6 A remarkable body of experience is available in the use of Gamma Knife® (Elekta AB, Stockholm, Sweden) radiosurgery consisting of a retrogasserian rhizotomy obtained through high doses delivered using a single isocenter of 4 mm.7-15 CyberKnife® image-guided robotic radiosurgery (Accuray Inc,
Sunnyvale, California) is a frameless radiosurgery technique.16-18,28,30,31 In a limited number of series, CyberKnife radiosurgery proved to be an effective and minimally invasive modality to perform retrogasserian rhizotomy. Because of the non-isocentric geometry of CyberKnife radiation delivery, it provides the possibility of homogeneous irradiation of an extended segment of the trigeminal nerve, thereby introducing new options for the radiosurgical treatment of TN.19–21 Available data are limited by either the numbers of patients or a relatively short follow-up17,22,23 or multicentric and heterogeneous experiences.18,24
Objectives
Here, we report the results obtained in the largest series of patients treated in a single center with fixed treatment parameters and followed up longer than 36 mo. The safety and effectiveness of CyberKnife radiosurgery for TN were analyzed in a homogenous and prospectively followed cohort. Optimal dose and volume (length of the nerve treated) were analyzed in relation to pain response, complications, and recurrence of symptoms to identify and recommend best practice.
METHODS
Setting and Study Design
Patients presenting with medically intractable TN between November 2010 and March 2014, the treated, were included in this study. Data were prospectively analyzed. All patients were followed up, according to our prospective protocol, for more than 3 yr.
Participants
Patients fulfilling the criteria of the International Headache Society (2003)25 for TN who received frameless radiosurgery for medically resistant pain were included in this study. The Local Ethics Committee approved this study and all patients signed a written consent.
Variables
The type of trigeminal pain was evaluated according to the classification proposed by Eller et al.26 Pain was classified as typical (TN1), described as sharp, shooting, electrical shock-like, with pain-free intervals between the attacks. It was classified as atypical (TN2) in patients describing pain as an aching, throbbing, or burning for more than 50% of the time and constant in nature (constant background pain being the most significant attribute).
Radiosurgical Technique
Immobilization and Imaging
Patients were treated with SRS using a CyberKnife G4 model (Accuray Inc). Before the treatment, the patient lay supine on the treatment couch and a custom-fitted thermoplastic mask (Orfit® Industries America, New York, New York) was molded. For all patients, a thin-slice contrast-enhanced computed tomography (CT) scanning and T1-weiged and constructive interference in steady state (CISS) magnetic resonance imaging (MRI) were performed (see Appendix, Supplemental Digital Content for details).
Target and Dose Selection
CT and MRI datasets were coregistered and the quality of the coregistration was visually checked using multiple views and transparency tools of the treatment planning system (MultiPlan®, Accuray Inc). The Gasser ganglion and the retrogasserian portion of the trigeminal nerve on the MRI and a bony canal on the edge of the petrous bone, clearly marking the ostium of Meckel's cave, were identified. This point could be immediately and constantly identifiable on 0.75 mm thin CT slices in axial view and then checked on the sagittal and coronal view using a crosshair. Shifting the imaging from the CT to the MRI, the pars triangularis was pointed out. An elongated target, 6 mm long and including the lateral margins of the nerve, was drawn on 2 or 3 slices depending on the nerve thickness. The brainstem, the mesial temporal lobe, the acoustic and facial nerves, the cochlea, and semicircular canals were specifically delineated as critical structures to minimize the radiation dose with an inverse planning algorithm. Other critical volumes, including the eyes, lenses, optic nerves, whole brain, and skin were also delineated for dose calculations. Furthermore, 2 tuning structures were delineated at 3-mm and 10-mm distances to restrict the isodose distribution outside the target within a precise distance.
A non-isocentric beams distribution was chosen; the maximum dose was 75 Gy. The maximum point dose to the brainstem and medial temporal lobe were set at 15 Gy and 36 Gy, respectively. Eight Gy was the maximum dose allowed to the cranial nerves, whereas 4 Gy was the limit to the middle ear. The outermost tuning structure (10 mm outside the target) had a dose limit of 15 Gy.
Once the calculation was completed, we verified that a 6-mm segment of the trigeminal nerve was included in the 80% isodose line (60 Gy). The maximum volume eventually included in the 80% isodose line was determined by individual anatomy, the length of nerve and relative dose received by the brainstem and mesial temporal lobe. For shorter nerves, we moved the target forward, toward the Gasserian ganglion, but always within the root. If this was not sufficient to obey the dose limit to the brainstem, we forced the inverse plan by allowing a smaller portion of the nerve to be included within the 60 Gy isodose line. Overall, the planning process required 45 to 90 min.
Data Sources
Clinical and treatment data were prospectively collected in a digital archive. Follow-up information was obtained by outpatient clinical evaluation or telephone interviews at defined time intervals.
Bias
To avoid inconsistent interpretation, clinical results were evaluated according to numerical scales and a score was assigned at each patient at different time points.
Study Size
In this cohort study, all patients fulfilling inclusion criteria who reached at least 36 mo follow-up were included.
Assessment of Outcome
The end-points analyzed were: (i) effects on pain scores, (ii) effects on medication, (iii) latency to pain reduction, (iv) occurrence of sensory disturbance, and (v) rate and time of pain recurrence.
Quantitative Variables
Pain level was scored using the Barrow Neurological Institute scale (BNI; class I: no trigeminal pain, no medication; II: occasional pain, not requiring medication; IIIa: no pain, continued medication; IIIb: controlled with medication; IV: some pain, not adequately controlled with medication; V: severe pain, no pain relief)27. For hypoesthesia evaluation, we used the BNI facial hypoesthesia scale (class I: no facial numbness; II: mild facial numbness, not bothersome; III: facial numbness, somewhat bothersome; IV: facial numbness, very bothersome)27.
We also recorded any possible trigeminal motor deficits. All patients were interviewed and a specific question was posed about a possible weakness or pain during mastication. Throughout outpatient assessment, the strength and the tone of masseter muscles against light resistance were evaluated. The jaw jerk reflex and the presence of fasciculation of chewing muscles were assessed as well.
Statistical Methods
For evaluation of outcomes such as initial pain cessation and recurrence, time to event was estimated by using the Kaplan–Meier method. Contingency tables (Fisher's exact test) were used to compare categorical variables in univariate analysis. A multivariate logistic regression analysis was then performed to identify predictive factors among the collected variables. Multivariate analysis was performed using the multiple logistic regression method. Variables showing statistical significance in the univariate analysis were transformed into binary variables to be used in the logistic regression model. To perform analyses, STATCALC 7.1.1 software (AcaStat, Poinciana, Florida) was used. Values of P < .05 were considered statistically significant.
RESULTS
Participants
In the considered period, a cohort of 138 patients presenting with medically intractable TN were recruited. An ipsilateral neurovascular conflict was disclosed in 63% of those patients. These patients were provided with all relevant information about the different surgical treatments and in particular about the suitability of MVD for their case. They were eventually deemed candidates for radiosurgical treatment because they were considered at risk for major surgery or for a resolute personal preference. Table 1 summarizes our preoperative demographic and clinical data.
Table 1.
Preoperative Characteristics of Patients
| Age/Sex | Mean 57.8 yr79F/59M |
| Type of neuralgia | TN1 (typical) 124 (89.8%)TN2 (atypical) 14 (11.2%) |
| Pathology | Multiple sclerosis 6 (4.3%)Neurovascular conflict 87 (63%) |
| Pain distribution | |
| Right | 62% |
| Left | 38% |
| V1 | – |
| V2 | 33 (23.9%) |
| V3 | 29 (21%) |
| V1-V2 | 12 (8.7%) |
| V2-V3 | 51 (37%) |
| V1-V2-V3 | 13 (9.4%) |
| Average time from onset | 4.3 yr |
| BNI pain score | |
| IIIb | – |
| IV | 27 (19.6%) |
| V | 111 (80.4%) |
| NRS score | |
| 10 | 51 (37%) |
| 9 | 44 (31.9%) |
| 8 | 22 (15.9%) |
| 7 | 20 (14.5%) |
| 6 | 1 (0.7%) |
| Previous treatments | Percutaneous rhizotomy 12 (8.6%)MVD 2 (1.4%) |
Abbreviations: NRS = numerical rating scale; BNI = Barrow Neurological Institute; MVD = microvascular decompression
Descriptive Data
Preoperatively, all patients had severe pain with numerical rating scale (NRS) score of >6 (median score 9) and were in BNI class IV (33%) or V (67%). Six patients (4.3%) had multiple sclerosis. A neurovascular conflict was visible on MRI in 87/138 patients (63%). Twelve patients had undergone previous rhizotomy (SRS or percutaneous techniques). Two patients had undergone MVD (1.4%).
Pain was classified as TN1 in 124/138 patients (89.8%). Pain was referred to the right side in 62% and to left side in 38% of the patients. Sixty-three percent had V3 involvement, 70% had V2 involvement, and 18% had V1 involvement. All patients had taken medications for an average of 4.3 yr (range, 11 mo-17 yr) before treatment.
Main Results
Radiosurgical Accuracy
Monthly tests of the 6D Skull Tracking mode demonstrated a median total treatment error (TTE) of 0.30 ± 0.12 mm. Median vertical deviation was measured as 0.4 mm; median horizontal deviation was 0.2 mm. Thus, even in the presence of planned displacements of target position, the system delivered the sub-millimetric accuracy necessary for the treatment of TN.
Target and Treatment Data
All treatments were performed in a single fraction using a non-isocentric technique. The median prescription isodose line was 80% (mean 82.9% ± 3.2) accounting for a mean radiation dose of 59.3 ± 2.7 Gy (median 60 Gy; Figure 1). The isodose incorporated a segment of the trigeminal root with a median length of 5.7 mm (mean 5.7 ± 1.6 mm). The median nerve volume included in the prescription isodose was 40.6 mm3 (mean 29 ± 14 mm3). In 16% of patients (22/138 pts), because of a short cisternal segment of the trigeminal nerve, the final target turned out to be as short as 4 mm with a median volume of 28 mm3.
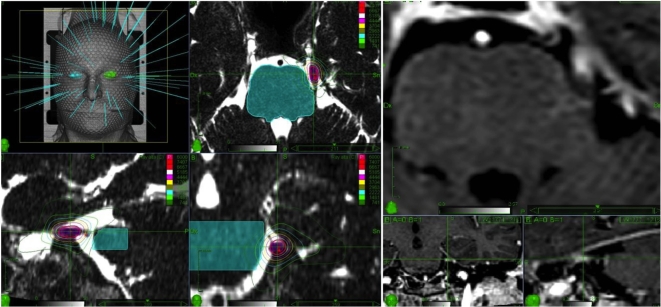
Figure 1.
Left. Frameless radiosurgery treatment plan for TN. A 6-mm (30-40 mm3) retrogasserian section of the trigeminal nerve was targeted, excluding the root entry zone. The brainstem was kept outside the 20% (15 Gy) isodose line. Cranial nerve VIII, the cochlea, and the inner ear received doses far below respective tolerance limits. Right. Trigeminal nerve focal contrast enhancement, 12 mo after treatment, witnessing the precision of dose delivery along the nerve.
The median maximum dose was 75 Gy (range, 65-75 Gy). The median new conformation index was 1.9 (mean 2 ± 0.62); the median homogeneity index was 1.25. Median number of beams was 105 (range, 90-110); median number of nodes was 87 (range, 85-90). Treatment time, including patient set up, ranged from 45 to 55 min with beam on time ranging from 15 to 21 min.
Pain Control
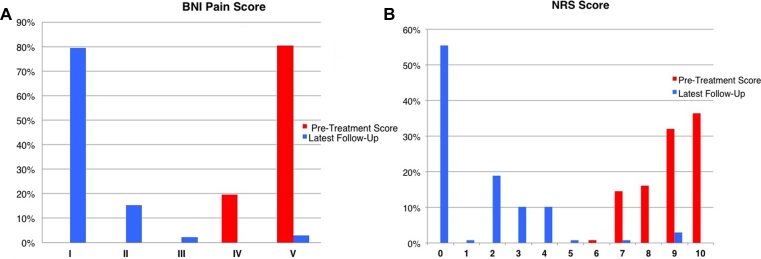
The median follow-up was 52.4 mo (range, 36-79 mo). After a median 3 wk (range, 1-6 wk), significant pain relief (a decrease in NRS score of >5) was achieved in 129 of 138 (93.5%) patients (Figure 2). One hundred twenty-nine patients (93.5%) were able to decrease the dose of medications intended to control pain throughout follow-up and finally ceased pain medication. One hundred nine patients (78.9%) were completely pain and medication free (BNI pain class I) within 6 mo after treatment.
Figure 2.
Results of treatment in terms of control of pain as measured by the BNI pain intensity scale A and the NRS B. Abbreviations: NRS = numerical rating scale. BNI = Barrow Neurological Institute.
Twenty-four of 129 (18.6%) pain-free patients experienced recurrent pain within 3 yr from the treatment. Peak of recurrent pain was reached at 12 mo after the first procedure (12 patients), while another 7 recurred at 18 mo and 5 at 24 mo (Figure 3). Therefore, the actuarial portion of pain-free patients (BNI score of I-IIIa) was 93.5% at 6 mo, 85.8% at 12 mo, 79.7% at 18 mo, and 76% at 24 mo. The percentage remained stable the later follow-ups. Figure 3 shows the rate of pain control after treatment.
Figure 3.
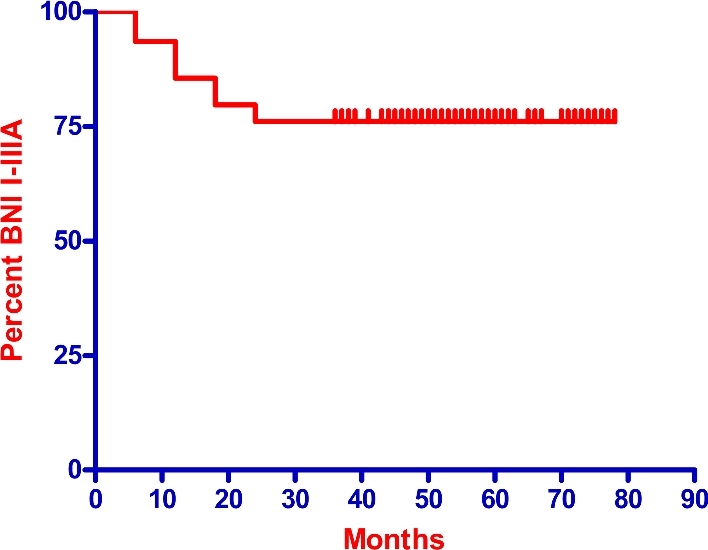
Actuarial rate of pain relief after the first radiosurgery treatment.
Salvage Therapy
Nine patients (6.5%) who failed to achieve pain control after a minimum of 6 mo underwent a second CyberKnife (Accuray Inc) treatment. Twenty-four of 129 pain-free patients (18.6%) experienced recurrent pain within 3 yr from the treatment and underwent retreatment. Overall, retreatments were performed in 33 patients to the same target, 6 mm of the retrogasserian to mid-cisternal segment of the trigeminal nerve, and the same dose/volume characteristics: 60 Gy median prescription dose and 75 Gy median maximum dose. All 33 patients achieved pain control (BNI pain scores I-IIIa) at the last follow-up. Nevertheless, re-irradiation alone was effective in 20 out of 33 patients (61%). Six patients required radiofrequency rhizotomy; 7 underwent MVD with partial rhizotomy.
Sensory Dysfunction and Other Complications
Six patients developed a BNI numbness scale grade III (somewhat bothersome) hypoesthesia, exclusively following re-irradiation. This corresponds to 4.3% of the entire cohort of 138 patients and 18.2% of the 33 patients who received a second treatment. One further patient (0.7%) developed BNI numbness scale grade IV hypoesthesia after the first treatment. This patient had a pontine myelopathy, visible on T2-weighted MRI. The treatment plan of this patient was carefully reviewed. We concluded that a portion of the brainstem received a dose higher than that intended as a consequence of a very short cisternal segment. As a result, the brainstem received a high point dose despite the volume receiving 10 Gy and that receiving 12 Gy corresponded to 1.5% and 1% of the volume.
Mild, non-bothersome sensory disturbances, ie BNI numbness scale grade II, were reported by 18 patients (7 after the first and 11 after retreatment), with an overall rate of 18.1% (25 of 138 patients) developing some sensory disturbance, 5% of which were bothersome, after an average of 16.4 ± 8.7 mo.
No further complications, such as temporal lobe radionecrosis, anesthesia dolorosa, lockjaw, weakness of the mandible, diplopia, dry-eye syndrome, keratitis, or hearing loss were reported in this present series.
Other Analyses
Factors Affecting Outcome
Univariate analysis showed that a shorter nerve length (<6 mm vs 6 mm, P = .01), smaller nerve volume (<30 mm3 vs >30 mm3, P = .02), and lower prescription dose (<58 vs >58 Gy, P = .03) were associated with treatment failure. None of the variables considered above, however, retained statistical significance in the multivariate analysis.
Retreatment was the only factor associated with the development of post-treatment sensory dysfunction (P < .001). Re-irradiation retained significance at the multivariate logistic regression as an independent predictor of bothersome numbness (P < .0001; Odds Ratio = 40.8, 95% confidence interval = 4.8-343.3) and of sensory dysfunctions of all categories (P < .001; Odds Ratio = 4.6, 95% confidence interval = 1.9-11-2). Table 2 summarizes the statistical data.
Table 2.
Factors Affecting Outcome
| Univariate | Multivariate | |
|---|---|---|
| Pain | ||
| Sex | – | – |
| Age | – | – |
| Side | – | – |
| TN1/TN2 | – | – |
| Target length | P = .01 | – |
| Target volume | P = .02 | – |
| Target dose | P = .03 | – |
| Sensory dysfunction | ||
| Sex | – | – |
| Age | – | – |
| Side | – | – |
| TN1/TN2 | – | – |
| Target length | – | – |
| Target volume | – | – |
| Target dose | – | – |
| Re-treatment | P < .0001 | P < .001 |
TN1/TN2: pain characteristics according to the Eller7 classification.
DISCUSSION
Key Results
This series represents the largest cohort to date reported treated with the frameless, non-isocentric radiosurgery technique for primary TN consistently performed by a single surgeon (P.R.) using definite treatment constraints. Furthermore, results were obtained from patients with follow-up longer than 3 yr. According to these data, 76% of patients benefited from the treatment, achieving stable pain control (BNI pain class I-IIIa) 3 yr after the first treatment. Failing patients were salvaged by re-treatment.
These results are consistent with those reported by the 2 largest studies on Gamma Knife surgery (Elekta AB).8,15 The Marseille experience illustrated the results of 497 patients with primary TN after 1 yr of follow-up. Of these patients, 91% were pain-free within a median time of 10 d. Pain recurred in 34.4% of patients.15 In their report on 448 patients, Marshall et al8 described satisfactory pain control in 86% and recurrence in 28% after 3 mo of follow-up.
On the other hand, 18% of patients reported sensory disturbances, which is also in line with the literature. Regis et al28 have recently updated their original study of 497 patients, reporting the long-term results of this large group of patients. The rate of new sensory disturbances was 20.4%.28 Furthermore, in our series, only 5% of patients had bothersome hypoesthesia and all these patients but one had received multiple treatments. Therefore, the risk of bothersome sensory deficit after a single treatment can be calculated as less than 1% in treatments performed using a retrogasserian target and specific dose/volume constraints.
Interpretation
The use of frameless and non-isocentric SRS for the treatment of TN was first reported by Romanelli et al17 at Stanford, in a study that was the first clinical demonstration of the submillimetric accuracy of image-guided radiosurgery. Almost immediate pain relief (within days) was found in this first cohort of patients following the delivery of a prescribed dose ranging from 65 to 70 Gy to a nerve segment up to 11 mm. The irradiation of such a long nerve segment, however, caused a high rate of bothersome numbness that developed over time in these patients and prompted a reduction of dose and length of the nerve treated.
Indeed, the treatment planning for CyberKnife (Accuray Inc) radiosurgical retrogasserian rhizotomy introduces a number of novel, yet highly critical treatment planning variables, in particular the necessity to identify a favorable proportion between the radiation dose and the target volume of trigeminal nerve. It has not been easy to determine an optimal dose range for CyberKnife treatments. In 2008, Villavicencio et al18 published data from a multicenter study illustrating the results of 95 patients who underwent CyberKnife radiosurgery. This heterogeneous study included patients treated with widely different modalities (isocentric and non-isocentric) as well as doses and treatment volumes. The median dose used was 75 Gy. Certain variables were predictive of stable pain relief over pain recurrence including the median maximum dose (77.5 vs 65 Gy), median minimum dose (64 vs 52 Gy), and median nerve length treated (4 mm vs 6 mm). After 2 yr, 50% of the population had excellent results, but 47% suffered new facial numbness.18 An update from the Stanford series reported on 46 patients receiving a treatment delivered over a 6-mm segment of the nerve, with a mean marginal prescription dose of 58.3 Gy and a mean maximal dose of 73.5 Gy.16 Symptoms disappeared completely in 39 patients (85%). After a mean follow-up period of 14.7 mo, patient-reported outcomes that were excellent in 33 patients (72%), good in 11 patients (24%), and poor in 2 patients (4%). Ipsilateral bothersome facial numbness (grade III on the BNI numbness scale) was reported in 7 patients (15%).16
The above studies show that CyberKnife radiosurgery (Accuray Inc) to an elongated segment of the trigeminal nerve is associated with a very high rate of pain control. Actually, the distinct advantage of a non-isocentric technique is the ability to define the target volume based on the individual patient's anatomy. During treatment planning, we worked on the size and the position of the prescription isodose line (averaging 60 Gy). We set the prescription isodose size to embrace a portion of the nerve of 6 mm. The length and the anatomic characteristics of the nerve and the relative distance from the brainstem, however, influenced the final target volume. Eventually, a marginal dose averaging 60 Gy delivered to a segment of the retrogasserian root averaging 5.7 mm in length provided a satisfactory therapeutic ratio with stable pain control and only 5% suffering bothersome hypoesthesia.
Outside the length of the nerve, we would indeed emphasize the importance of the volume of the nerve receiving the prescription dose. A volume >30 mm3 was associated with a higher probability of pain control.
On the other hand, caution must be paid to the doses delivered to the brainstem. For treatment planning, we set a dose constraint to the brainstem of 15 Gy. This seems to be lower than most reports of Gamma Knife treatment (Elekta AB) of TN.7-9,14,29–34
In the series by Massager et al,9 a dose of 90 Gy was delivered to a target 5 to 8 mm from the brainstem, and the treatment was planned such that a dose of 13 to 15 Gy was received by less than 1 mm3 and 10 to 12 Gy by less than 10 mm3 of the brainstem.9 In the series in which the root entry zone was targeted, the dose to the brainstem varied. Dosing could be summarized as follows: 21 to 28 Gy for a maximum dose of 70 Gy, 24 to 40 Gy for a maximum dose of 80 Gy, and 18 to 57.6 Gy for a maximum dose of 90 Gy.7,8,14,29–34 Actually, the incidence of numbness increases with dose escalation to the brainstem. At a maximum dose of 90 Gy, with the brainstem along either the 30% or 50% isodose line, the rate of grade 4 to 5 numbness was 10% and 17%, respectively.34 We set a lower limit considering that the dose to the brainstem is 1 major cause of sensory disturbances. Nonetheless, 1 patient in our series reported BNI grade IV hypoesthesia after the first treatment. In that patient, due to a short cisternal segment of the nerve, the brainstem received a high point dose despite the volume receiving 10 Gy and that receiving 12 Gy corresponded to 1.5% (0.2 cc) and 1% (0.14 cc). Therefore, we recommend a thorough review of dose-volume histogram of the brainstem to avoid suboptimal dose distributions.
There are some technical aspects of image-guided robotic radiosurgery that deserve also to be mentioned. With the inverse planning technique used by the CyberKnife (Accuray Inc) treatment planning, all brain structures have to be contoured35-37. All dose-volume histograms were evaluated as the risk of high doses to sensitive structures, even to those that were distant from the target, was elevated because of the non-isocentric beam distribution. In particular, the medial-most portion of the temporal lobe could receive doses as high as 45 Gy after the initial planning which then were reduced to <15 Gy/1 mm3 to avoid late mediotemporal radionecrosis.
One of the main criticisms of the use of CyberKnife (Accuray Inc) for TN was the necessity to plan on CT cisternography due to a potentially unsafe MR-CT fusion in the earlier versions of the system. CT cisternography has been abandoned after the preliminary Stanford experience because later versions of MultiPlan-TPS (Accuray Inc) provided a rather accurate CT-MR fusion capability. Also, bony landmarks, indicating the entrance of the trigeminal nerve root into Meckel's cave, are easily recognizable directly on a bone CT scan. The identification of these points greatly supports a precise co-registration of the CT with MR sequence.
SRS represents a good option for patients who are candidates for retrogasserian rhizotomy. Other techniques seem to have worse results in terms of long-term pain control. Recently Alvarez-Pinzon et al38 compared percutaneous retrogasserian balloon compression and Gamma Knife radiosurgery (Elekta AB) in 202 patients with multiple sclerosis. Fewer complications and superior long-term relief were associated with radiosurgery. Similarly, radiosurgery demonstrated fewer complications than percutaneous retrogasserian glycerol rhizotomy in these patients.39
Limitations
Our follow-up length is in line with that of principal studies describing the efficacy of SRS in TN,7–15 but a very long term follow-up would be desirable for a definitive evaluation of the technique. Actually, good to excellent mid-term outcomes have been reported, but long-term results can be less satisfactory with up to 60% of patients with some episode of pain recurrence15,28,40. This is particularly important to obtain a relevant comparison with MVD. Actually, a neurovascular conflict structure was detectable on MR of 63% of patients of this series. These patients would be candidates for MVD, but radiosurgical treatment was selected for different reasons, including: patient preference, age, performance status, and other contraindication for major surgical treatments. All patients were informed of the short and long-term benefits and risks of both procedures based on our current knowledge.
CONCLUSION
MVD has proven to provide higher rates of control pain at short and long term and longer pain-free intervals as compared with SRS41,42. Nonetheless, in patients who are not candidates for MVD, SRS may represent the first treatment option for classical TN, while other invasive rhizotomy techniques should be reserved for patients with acute, intractable pain requiring immediate postoperative relief. Using our constraints for dose, volume of the nerve, and dose to the brainstem, the incidence of bothersome hypoesthesia was low, whereas a durable pain control was achieved in 76% of patients. The rarity of bothersome complications and the fact that frameless radiosurgery represents the less invasive technique for the surgical treatment of TN provide a particularly favorable profile for this technique as compared with other non-medicinal treatments.
Disclosures
Dr Romanelli and Dr Conti served as Scientific Consultants for Accuray Inc. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
COMMENT
This article reports a range of standard outcome measures for a sizable cohort of patients with idiopathic trigeminal neuralgia. The overall conclusion is that CyberKnife treatment (Accuray Inc) was safe and effective for this condition. Although the subtle technical differences between the novel method of radiosurgical rhizotomy presented here and the more traditional Gamma Knife (Elekta AB) methods are notable, more study, and even a head to head comparison, is needed to determine if such differences ultimately correlate with clinical benefits.
For me, even more important that the specific clinical results being reported in this series, is the fact that stereotactic targeting of the trigeminal nerve was done exclusively through image-guidance, ie a stereotactic frame was not utilized for either targeting or head immobilization. The seeming success of this technical approach reported here flies in the face of the all too common “belief” that image-guidance is not sufficiently accurate to target the trigeminal nerve, or for that matter, other functional brain circuits. Therefore, this article provides important corroboration that the “prejudice” against the accuracy of image-guided targeting is a false construct. Yet again the data suggests that the accuracy of image-guided targeting and delivery of radiosurgery is every bit on par with frame-based methods.
John R. Adler, Jr
Stanford, California
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. BMJ. 2007;334(7586):201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord. 2010;3(2):107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mclaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(1):1-8. [DOI] [PubMed] [Google Scholar]
- 4. Zakrzewska JM. Treatment for trigeminal neuralgia. Controlled long term study of all surgical options is being planned. BMJ. 1997;314(7079):520. [PMC free article] [PubMed] [Google Scholar]
- 5. Zakrzewska JM, Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev. 2011(9):CD007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971;137(4948331):311-314. [PubMed] [Google Scholar]
- 7. Kondziolka D, Zorro O, Lobato-Polo J et al. . Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010;112(4):758-765. [DOI] [PubMed] [Google Scholar]
- 8. Marshall K, Chan MD, Mccoy TP et al. . Predictive variables for the successful treatment of trigeminal neuralgia with gamma knife radiosurgery. Neurosurgery. 2012;70(3):566-573. [DOI] [PubMed] [Google Scholar]
- 9. Massager N, Lorenzoni J, Devriendt D, Desmedt F, Brotchi J, Levivier M. Gamma knife surgery for idiopathic trigeminal neuralgia performed using a far-anterior cisternal target and a high dose of radiation. J Neurosurg. 2004;100(4):597-605. [DOI] [PubMed] [Google Scholar]
- 10. Pollock BE. Fitting radiosurgery into the trigeminal neuralgia management puzzle. World Neurosurgery. 2010;74(4-5):448-450. [DOI] [PubMed] [Google Scholar]
- 11. Regis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille-Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006;104(6):913-924. [DOI] [PubMed] [Google Scholar]
- 12. Riesenburger RI, Hwang SW, Schirmer CM et al. . Outcomes following single-treatment Gamma Knife surgery for trigeminal neuralgia with a minimum 3-year follow-up. J Neurosurg. 2010;112(4):766-771. [DOI] [PubMed] [Google Scholar]
- 13. Sheehan J, Pan H, Stroila M, Steiner L. Gamma knife surgery for trigeminal neuralgia: outcomes and prognostic factors. J Neurosurg. 2005;102(3):434-441. [DOI] [PubMed] [Google Scholar]
- 14. Tawk RG, Duffy-Fronckowiak M, Scott BE et al. . Stereotactic gamma knife surgery for trigeminal neuralgia: detailed analysis of treatment response. J Neurosurg. 2005;102(3):442-449. [DOI] [PubMed] [Google Scholar]
- 15. Tuleasca C, Carron R, Resseguier N. Patterns of pain-free response in 497 cases of classic trigeminal neuralgia treated with Gamma Knife surgery and followed up for least 1 year. J Neurosurg. 2012;117(Suppl):181-188. [DOI] [PubMed] [Google Scholar]
- 16. Adler JR, Bower R, Gupta G et al. . Nonisocentric radiosurgical rhizotomy for trigeminal neuralgia. Neurosurgery. 2009;64(suppl_2):A84-A90. [DOI] [PubMed] [Google Scholar]
- 17. Romanelli P, Heit G, Chang SD, Martin D, Pham C, Adler J. Cyberknife radiosurgery for trigeminal neuralgia. Stereotact Funct Neurosurg. 2003;81(1-4):105-109. [DOI] [PubMed] [Google Scholar]
- 18. Villavicencio AT, Lim M, Burneikiene S et al. . Cyberknife radiosurgery for trigeminal neuralgia treatment: a preliminary multicenter experience. Neurosurgery. 2008;62(3):647-655. [DOI] [PubMed] [Google Scholar]
- 19. Romanelli P, Schaal DW, Adler JR. Image-guided radiosurgical ablation of intra- and extra-cranial lesions. Technol Cancer Res Treat. 2006;5(4):421-428. [DOI] [PubMed] [Google Scholar]
- 20. Romanelli P, Adler JR. Technology Insight: image-guided robotic radiosurgery—a new approach for noninvasive ablation of spinal lesions. Nat Clin Prac Oncol. 2008;5(7):405-414. [DOI] [PubMed] [Google Scholar]
- 21. Romanelli P, Schweikard A, Schlaefer A, Adler J. Computer aided robotic radiosurgery. Comput Aided Surg. 2006;11(4):161-174. [DOI] [PubMed] [Google Scholar]
- 22. Lim M, Cotrutz C, Romanelli P et al. . Stereotactic radiosurgery using CT cisternography and non-isocentric planning for the treatment of trigeminal neuralgia. Comput Aided Surg. 2006;11(1):11-20. [DOI] [PubMed] [Google Scholar]
- 23. Conti A, Pontoriero A, Iati G et al. . Frameless stereotactic radiosurgery for the treatment of multiple sclerosis-related trigeminal neuralgia. World Neurosurgery. 2017:103:702-712. [DOI] [PubMed] [Google Scholar]
- 24. Lim M, Villavicencio A, Burneikiene S et al. . 817 Multicenter clinical evaluation of CyberKnife radiosurgery for idiopathic trigeminal neuralgia. Neurosurgery. 2005;57(2):401. [DOI] [PubMed] [Google Scholar]
- 25. Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004;63(3):427-435. [DOI] [PubMed] [Google Scholar]
- 26. Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18(5):E3. [DOI] [PubMed] [Google Scholar]
- 27. Rogers CLeland, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013-1019. [DOI] [PubMed] [Google Scholar]
- 28. Regis J, Tuleasca C, Resseguier N et al. . Long-term safety and efficacy of Gamma Knife surgery in classical trigeminal neuralgia: a 497-patient historical cohort study. J Neurosurg. 2016;124(4):1079-1087. [DOI] [PubMed] [Google Scholar]
- 29. Fountas KN, Smith JR, Lee GP, Jenkins PD, Cantrell RR, Sheils WC. Gamma Knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: long-term outcome and complications. Neurosurg Focus. 2007;23(6):E8. [DOI] [PubMed] [Google Scholar]
- 30. Jawahar A, Wadhwa R, Berk C. Assessment of pain control, quality of life, and predictors of success after gamma knife surgery for the treatment of trigeminal neuralgia. Neurosurg Focus. 2005;18(5):E8. [DOI] [PubMed] [Google Scholar]
- 31. Maesawa S, Salame C, Flickinger JC, Pirris S, Kondziolka D, Lunsford LD. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94(1):14-20. [DOI] [PubMed] [Google Scholar]
- 32. Park S, Hwang S. Outcomes of gamma knife radiosurgery for trigeminal neuralgia after a minimum 3-year follow-up. J Clin Neurosci. 2011;18(5):645-648. [DOI] [PubMed] [Google Scholar]
- 33. Pollock BE, Phuong LK, Foote RL, Stafford SL, Gorman DA. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001;49(1):58-62. [DOI] [PubMed] [Google Scholar]
- 34. Smith ZA, Gorgulho AA, Bezrukiy N et al. . Dedicated linear accelerator radiosurgery for trigeminal neuralgia: a single-center experience in 179 patients with varied dose prescriptions and treatment plans. Int J Radiat Oncol Biol Phys. 2011;81(1):225-231. [DOI] [PubMed] [Google Scholar]
- 35. Conti A, Pontoriero A, Arpa D et al. . Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent glioma. Acta Neurochir. 2012;154(2):203-209. [DOI] [PubMed] [Google Scholar]
- 36. Conti A, Pontoriero A, Ricciardi GK et al. . Integration of functional neuroimaging in CyberKnife radiosurgery: feasibility and dosimetric results. Neurosurg Focus. 2013;34(4):E5. [DOI] [PubMed] [Google Scholar]
- 37. Conti A, Pontoriero A, Salamone I et al. . Protecting venous structures during radiosurgery for parasagittal meningiomas. Neurosurg Focus. 2009;27(5):E11. [DOI] [PubMed] [Google Scholar]
- 38. Alvarez-Pinzon AM, Wolf AL, Swedberg HN. Comparison of percutaneous retrograsserian balloon compression and gamma knife radiosurgery for the treatment of trigeminal neuralgia in multiple sclerosis: a clinical research study article. World Neurosurg. 2016. [DOI] [PubMed] [Google Scholar]
- 39. Mathieu D, Effendi K, Blanchard J, Seguin M. Comparative study of Gamma Knife surgery and percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia in patients with multiple sclerosis. J Neurosurg. 2012;117(Suppl):175-180. [DOI] [PubMed] [Google Scholar]
- 40. Regis J, Tuleasca C, Resseguier N et al. . The very long-term outcome of radiosurgery for classical trigeminal neuralgia. Stereotact Funct Neurosurg. 2016;94(1):24-32. [DOI] [PubMed] [Google Scholar]
- 41. Gubian A, Rosahl SK. Meta-analysis on safety and efficacy of microsurgical and radiosurgical treatment of trigeminal neuralgia World Neurosurgery. 2017;103:757-767. [DOI] [PubMed] [Google Scholar]
- 42. Wang DD, Raygor KP, Cage TA. Prospective comparison of long-term pain relief rates after first-time microvascular decompression and stereotactic radiosurgery for trigeminal neuralgia [published online ahead of print] J Neurosurg. 2017:1-10. doi: 10.3171/2016.9.JNS16149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.