Abstract
Objective
Ovarian cancers comprise several histologically distinct tumour groups with widely different prognosis. We aimed to describe the worldwide distribution of ovarian cancer histology and to understand what role this may play in international variation in survival.
Methods
The CONCORD programme is the largest population-based study of global trends in cancer survival. Data on 681,759 women diagnosed during 1995-2009 with cancer of the ovary, fallopian tube, peritoneum and retroperitonum in 51 countries were included. We categorised ovarian tumours into six histological groups, and explored the worldwide distribution of histology.
Results
During 2005-2009, type II epithelial tumours were the most common. The proportion was much higher in Oceania (73.1%), North America (73.0%) and Europe (72.6%) than in Central and South America (65.7%) and Asia (56.1%). By contrast, type I epithelial tumours were more common in Asia (32.5%), compared with only 19.4% in North America. From 1995 to 2009, the proportion of type II epithelial tumours increased from 68.6% to 71.1%, while the proportion of type I epithelial tumours fell from 23.8% to 21.2%. The proportions of germ cell tumours, sex cord-stromal tumours, other specific non-epithelial tumours and tumours of non-specific morphology all remained stable over time.
Conclusions
The distribution of ovarian cancer histology varies widely worldwide. Type I epithelial, germ cell and sex cord-stromal tumours are generally associated with higher survival than type II tumours, so the proportion of these tumours may influence survival estimates for all ovarian cancers combined. The distribution of histological groups should be considered when comparing survival between countries and regions.
Introduction
Of all gynaecological malignancies, ovarian cancer causes the second highest number of deaths worldwide, accounting for over 151,000 deaths annually(1). Symptoms, such as persistent abdominal pain, bloating or decreased appetite, are vague(2). Most women present with advanced-stage disease(3) and five-year survival is around 30-40%(4). Ovarian cancer is not a single disease(2, 5), but includes several histological subtypes that have widely different prognosis(6, 7).
Ovarian cancer has been divided into epithelial and non-epithelial groups for many years, but recent work has enabled finer subdivision of epithelial ovarian cancers into different groups according to a combination of morphological and clinical characteristics(6–10). Type I epithelial tumours include low-grade serous, endometrioid, clear cell, mucinous and transitional cell (Brenner) carcinomas. They often present at an early stage, may arise from borderline ovarian tumours or endometriosis and typically have a good prognosis. Type II epithelial tumours comprise high-grade serous carcinoma, undifferentiated carcinomas and malignant mixed mesodermal tumours. They account for around 75% of epithelial ovarian cancers, typically present at an advanced stage and have a poor prognosis(6, 7, 9). Each histological group has distinct molecular pathways that influence chemosensitivity, the pattern of metastasis and the probability of survival(9, 11).
The pathogenesis of ovarian cancer is not fully understood. Recent evidence, particularly from prophylactic oophorectomies in women at a high risk of ovarian cancer because of BRCA gene mutations, suggests that the most common subtype, high-grade serous carcinoma, originates either in the fallopian tube or on the surface of the ovary. Therefore, fallopian tube carcinoma has more recently been included in a broader definition of ovarian cancer(7). Primary peritoneal carcinoma is also managed in the same way as advanced-stage epithelial ovarian cancer(6, 12).
International comparisons of cancer incidence, mortality and survival are crucial to inform and plan health policy and cancer control programmes. Low survival has been a stimulus for cancer plans and strategies in many countries, such as the United Kingdom and Denmark(3). Comparisons of lung cancer survival have routinely been divided into small-cell and non-small cell subtypes due to the different prognosis and behaviour of these tumours. Ovarian cancer is arguably an even more heterogeneous disease than lung cancer, and histology should thus be considered in the interpretation of international variation in ovarian cancer survival. Type I epithelial tumours are generally associated with higher survival than type II tumours, so the proportion of type I epithelial tumours may influence survival estimates for all ovarian cancers combined. Differences in the distribution of histology may thus contribute to international variations in survival from all ovarian cancers combined, in addition to international differences in stage at diagnosis and treatment.
The CONCORD-2 study on the global surveillance of cancer survival has shown the extent to which ovarian cancer survival varies worldwide(4). However, it remains unclear how much of the variation in ovarian cancer survival could be attributed to international variation in the histological groups, in particular the distribution of type I and type II epithelial tumours. Using population-based data from the CONCORD-2 study, we have examined the international distribution of ovarian cancer histology. Our aims were to describe the worldwide variation of ovarian cancer histological groups, and then to discuss whether this variation may influence international comparisons of population-based cancer survival.
Methods
The CONCORD-2 study(4) collected information for over 779,000 adult women (aged 15-99 years) in 61 countries who were diagnosed during the 15-year period 1995-2009 with a cancer of the ovary, fallopian tube, uterine ligaments and adnexa, other specific and unspecified female genital organs, peritoneum or retroperitoneum (International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) topography codes C56.9, C57.0-C57.4, C57.7-C57.9, C48.0-C48.2)(13). The CONCORD-2 protocol, the ethical approvals and the quality control procedures have been described(4).
We defined six histological groups based on previous literature(14) and clinical advice [Table 1]. Clear cell, endometrioid, mucinous, squamous and transitional cell carcinomas were grouped as type I epithelial tumours, and serous carcinoma, mixed epithelial and stromal carcinoma and undifferentiated and other epithelial carcinoma were grouped as type II epithelial tumours.
Table 1. Ovarian cancer histological groups and subtypesa.
a No information on grade was available, therefore all endometrioid tumours were classified as type I epithelial.
b No information on grade was available, therefore all serous tumours were classified as type II epithelial
c Borderline tumours (ICD-O-3 codes: 8442, 8444, 8451, 8462, 8463, 8472, 8473) were excluded from the analysis of distribution of histological subtypes (see text).
| Histological group | Histological subtype | ICD-O-3 morphology code |
|---|---|---|
| Type I epithelial | Clear cell carcinoma | 8005, 8310, 8443, 9110 |
| Endometrioid carcinomab | 8380, 8382-8383, 8560, 8570 | |
| Mucinous carcinoma | 8470-8471, 8480-8482, 8490 | |
| Squamous carcinoma | 8051-8084 | |
| Transitional cell or Brenner carcinoma | 8120-8131, 9000 | |
| Type II epithelial | Serous carcinomac | 8050, 8441, 8450, 8460-8461 |
| Mixed epithelial-stromal carcinoma | 8313, 8323, 8381, 8930-8991, 9010-9030 | |
| Undifferentiated or other epithelial | 8010-8015, 8020-8046, 8090-8110, 8140-8231, 8246-8300, 8311-8312, 8314-8322, 8324-8325, 8336-8337, 8341-8375, 8384-8440, 8452-8454, 8500-8551, 8561-8562, 8571-8589 | |
| Germ cell | Germ cell | 8240-8245, 8330-8335, 8340, 9060-9105, 9380-523 |
| Sex cord-stromal | Sex cord-stromal | 8590-8671, 8810 |
| Other specific non-epithelial | Other specific non-epithelial | 8680-8806, 8811-8921, 9040-9055, 9120-9373, 9530-9589 |
| Non-specific | Non-specific | 8000-8004 |
Ovarian cystadenomas were reclassified in ICD-O-3 from invasive (behaviour code of 3) to borderline (behaviour code of 0 or 1), but some registries coded tumours of borderline behaviour as invasive despite the changes from ICD-O-2 to ICD-O-3. Borderline tumours were excluded from the analysis of the distribution. Morphology codes for haematological malignancies were also excluded from analysis.
Data were available for 793,098 women for analysis [supplementary Figure 1]. Women diagnosed with borderline tumours, haematological malignancies or whose records included invalid ICD-O-3 codes (codes not included in either ICD-O-2 or ICD-O-3) were excluded (n=13,073). Of the remaining 780,025 women, 90.6% (706,807) had tumours that were coded by the registry as having been morphologically verified, while 7.5% (58,682) were coded as not morphologically verified and 1.9% (14,536) were coded as unknown whether morphologically verified or not. For tumours coded as morphologically verified, 705,997 (99.9%) had a valid ICD-O-3 morphology code, but no morphology code was available for 810 (0.1%), and these tumours were excluded. Tumours coded as not morphologically verified were primarily tumours of unknown morphology (30,287, 51.6% of non-morphologically verified tumours); these tumours were excluded. We excluded a further 18,200 non-morphologically verified tumours with non-specific morphology. We included the remaining 10,195 tumours that had been coded as not having been morphologically verified, because a specific ICD-O-3 morphology code was nevertheless available, implying that morphological verification had in fact been performed. Tumours for which it was unknown whether morphological verification had been performed or not were evenly distributed across specific (n=5,017), non-specific (n=4,798) and unknown morphology (n=4,721). Of these tumours, we excluded non-specific and unknown tumours. We included the remaining 5,017 tumours coded as unknown whether morphologically verified, because a specific morphology was also recorded, again implying that morphological verification had been completed.
In total, 721,209 women (98.3% with specific ICD-O-3 morphology codes and 1.7% with non-specific codes) were available for analysis after the first round of exclusions.
We examined the distribution of ovarian cancer histology for all countries in any calendar period (1995-1999, 2000-2004 and 2005-2009) for which data were available for at least 100 women. Registries from which the survival estimates in the main CONCORD-2 analysis were considered less reliable(4) were also excluded, because the results from this analysis will be used to inform the results of survival analyses of ovarian cancer. Survival estimates were flagged as less reliable if a higher than usual proportion of patients was excluded from analyses because the cancer was registered only through a death certificate, or the date of last vital status was not known. The focus of this analysis was the distribution of specific histological groups, so women diagnosed in Sweden had to be excluded, because 97.5% of tumours were coded by the registry as undifferentiated or other epithelial carcinoma or as non-specific histology (ICD-O-3 codes 8000-8004). After all exclusions, 681,759 women (86.0% of the total number for whose data were available for analysis) were included in the analysis of the histological distribution (192,080 in 1995-1999; 240,397 in 2000-2004; 249,282 in 2005-2009) [supplementary Table 1].
Results
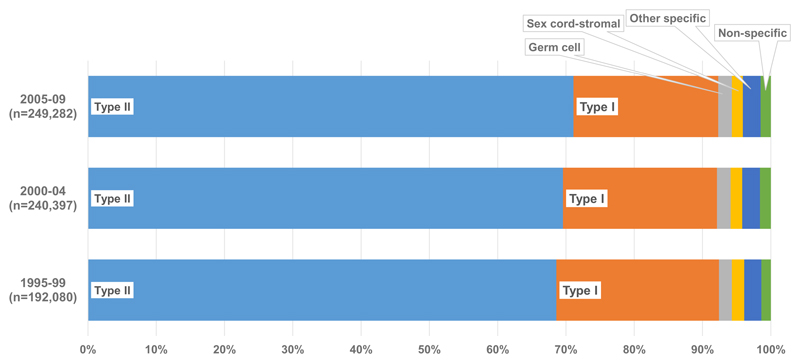
Type II epithelial tumours were the most common histology worldwide (476,461; 69.9%), followed by type I epithelial (152,874; 22.4%) [Figure 1]. Germ cell, sex cord-stromal, other specific non-epithelial and non-specific tumours were all rare and they only comprised 8% of tumours worldwide; the distribution of these groups remained relatively stable over the 15-year period 1995 to 2009. The proportion of type II epithelial tumours increased slightly from 68.6% to 71.1% from 1995 to 2009, and there was a corresponding decrease in type I epithelial tumours (from 23.8% to 21.2%: supplementary Table 1).
Figure 1. Worldwide distribution of ovarian cancera histology (%): 51 countries, 1995-2009.
a Malignancies of the ovary (ICD-O-3 C56.9), fallopian tube, uterine ligaments and adnexa, and other and unspecified female genital organs (C57.0-C57.4, C57.7-C57.9), and peritoneum and retroperitoneum (C48.0-C48.2). Endometrioid tumours are classified as type I epithelial (see text).
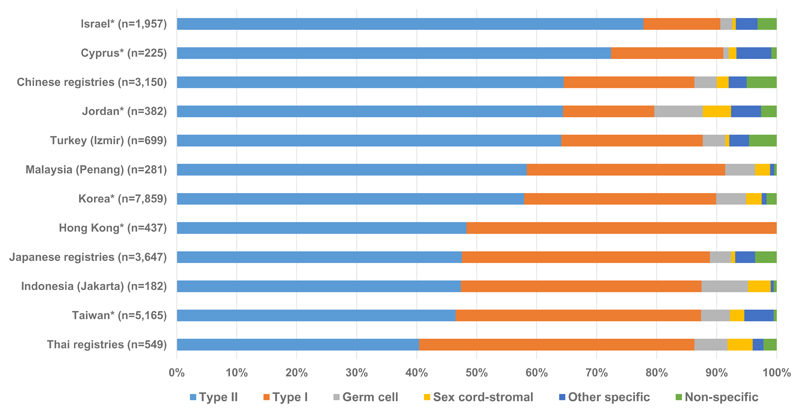
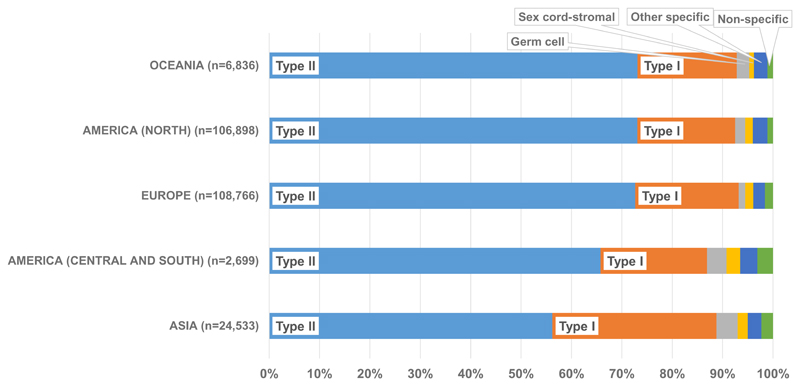
During 2005-2009, type II epithelial was the most common group in all continents, although the proportion was much higher in Oceania (73.1%), North America (73.0%) and Europe (72.6%) than in Central and South America (65.7%) and Asia (56.1%) [Table 2]. The range at the national level, however, was much wider. The highest proportion of type II tumours was in Latvia (78.9%), with the lowest proportion in Thailand (40.4%) [supplementary Table 4]. There was little between-country variation in the proportion of type II tumours in Central and South America, North America and Oceania. However, the proportion varied widely in Asia, where the proportion of type II tumours was lower than that of type I epithelial tumours in Hong Kong and Thailand [Figure 3]. There was also variation in the proportion of type II tumours in Europe, where they accounted for over 70% of tumours in 15 countries, 60% in 11 countries and only 50.2% in Russia [supplementary Table 4]. The distribution of type II epithelial subtypes (serous, undifferentiated and other epithelial and mixed epithelial and stromal carcinoma) also varied by country, continent and calendar period [supplementary Table 2, supplementary Table 3 and supplementary Table 5].
Table 2. Distribution of histological groups by continent and calendar period of diagnosisa.
a Borderline tumours (ICD-O-3 codes: 8442, 8444, 8451, 8462, 8463, 8472, 8473) were excluded from the analysis of distribution of morphological subtypes (see text).
b No information on grade was available, therefore all endometrioid tumours were classified as type I epithelial.
c No information on grade was available, therefore all serous tumours were classified as type II epithelial.
d Morphologically verified tumours with ICD-O-3 morphology codes 8000-8004. Only countries with at least 100 women in any given time period were included. All tumours with a specific ICD-O-3 morphology code were included.
| Total | Type I epithelialb | Type II epithelialc | Germ cell | Sex cord-stromal | Other specific non-epithelial | Non-specificd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
|
AMERICA (CENTRAL AND SOUTH) | |||||||||||||
| 1995-99 | 1,113 | 220 | 19.8 | 720 | 64.7 | 50 | 4.5 | 30 | 2.7 | 61 | 5.5 | 32 | 2.9 |
| 2000-04 | 3,278 | 587 | 17.9 | 2,209 | 67.4 | 124 | 3.8 | 90 | 2.7 | 131 | 4.0 | 137 | 4.2 |
| 2005-09 | 2,699 | 570 | 21.1 | 1,772 | 65.7 | 106 | 3.9 | 74 | 2.7 | 92 | 3.4 | 85 | 3.1 |
|
AMERICA (NORTH) | |||||||||||||
| 1995-99 | 87,459 | 20,783 | 23.8 | 60,433 | 69.1 | 1,591 | 1.8 | 1,360 | 1.6 | 2,413 | 2.8 | 879 | 1.0 |
| 2000-04 | 101,774 | 22,007 | 21.6 | 72,480 | 71.2 | 1,907 | 1.9 | 1,454 | 1.4 | 2,734 | 2.7 | 1,192 | 1.2 |
| 2005-09 | 106,898 | 20,710 | 19.4 | 78,075 | 73.0 | 2,167 | 2.0 | 1,656 | 1.5 | 3,079 | 2.9 | 1,211 | 1.1 |
|
ASIA | |||||||||||||
| 1995-99 | 12,920 | 4,324 | 33.5 | 6,775 | 52.4 | 770 | 6.0 | 268 | 2.1 | 361 | 2.8 | 422 | 3.3 |
| 2000-04 | 19,312 | 6,588 | 34.1 | 10,242 | 53.0 | 940 | 4.9 | 422 | 2.2 | 609 | 3.2 | 511 | 2.6 |
| 2005-09 | 24,533 | 7,979 | 32.5 | 13,775 | 56.1 | 1,042 | 4.2 | 497 | 2.0 | 667 | 2.7 | 573 | 2.3 |
|
EUROPE | |||||||||||||
| 1995-99 | 84,056 | 18,887 | 22.5 | 59,229 | 70.5 | 1,149 | 1.4 | 1,633 | 1.9 | 1,897 | 2.3 | 1,261 | 1.5 |
| 2000-04 | 108,891 | 23,625 | 21.7 | 77,376 | 71.1 | 1,537 | 1.4 | 1,965 | 1.8 | 2,526 | 2.3 | 1,862 | 1.7 |
| 2005-09 | 108,766 | 22,313 | 20.5 | 78,957 | 72.6 | 1,453 | 1.3 | 1,784 | 1.6 | 2,491 | 2.3 | 1,768 | 1.6 |
|
OCEANIA | |||||||||||||
| 1995-99 | 6,532 | 1,556 | 23.8 | 4,546 | 69.6 | 133 | 2.0 | 89 | 1.4 | 156 | 2.4 | 52 | 0.8 |
| 2000-04 | 7,142 | 1,466 | 20.5 | 5,201 | 72.8 | 180 | 2.5 | 66 | 0.9 | 182 | 2.5 | 47 | 0.7 |
| 2005-09 | 6,386 | 1,259 | 19.7 | 4,671 | 73.1 | 160 | 2.5 | 56 | 0.9 | 170 | 2.7 | 70 | 1.1 |
Figure 3. Histological groups of ovarian cancera by country (Asia), 2005-09.
a Malignancies of the ovary (ICD-O-3 C56.9), fallopian tube, uterine ligaments and adnexa, and other and unspecified female genital organs (C57.0-C57.4, C57.7-C57.9), and peritoneum and retroperitoneum (C48.0-C48.2). Endometrioid tumours are classified as type I epithelial (see text). *Data with 100% coverage of the national population.
Type I epithelial tumours were the second most common group for all continents during 2005-2009, but the range was wide. The highest proportion was seen in Asia (32.5%), while North America showed the lowest proportion (19.4%) [Table 2]. The proportion was similar in all countries in Central and South America, North America and Oceania [supplementary Table 4]. In Europe, however, there was wider variation, the proportion ranging from 11.3% in Latvia to 28.7% in Finland [supplementary Table 4]. The variation was even wider for countries in Asia, with the lowest proportion in Israel (12.8%) and the highest in Hong Kong (51.7%) [Figure 3]. The distribution of specific type I epithelial subtypes (clear cell, endometrioid, mucinous, squamous and transitional cell (Brenner)) also varied over time and differed by country and continent [supplementary Table 2, supplementary Table 3 and supplementary Table 5].
Germ cell tumours were uncommon everywhere; the proportion in Asia (4.2%) was the highest in any continent, over three times the proportion seen in Europe (1.3%) [Table 2]. The proportion was similar for all countries in Europe (1.3%), North America (2.0%) and Oceania (2.5%). However, there was wide variation between countries in Central and South America and Asia. In Central and South America, the lowest proportion (1.6%) was seen in Cuba, and the highest (7.8%) in Ecuador [supplementary Table 4]. Among Asian countries, the variation was wider, with the lowest proportion in Cyprus (0.9%), and the highest in Jordan (8.1%) [Figure 3].
Sex cord-stromal tumours were even more uncommon than germ cell tumours. The proportion also varied widely between countries in Asia, Central and South America and Europe. The proportion was similar for all countries in North America (1.5%) and Oceania (0.9%) [Table 2, supplementary Table 4]. The widest between-country variation was seen in Europe, with only 0.3% of tumours diagnosed as sex cord-stromal in Denmark, but 11.4% in Russia [supplementary Table 4]. In Central and South America, the proportion ranged from 1.6% in Brazil and Puerto Rico to 4.5% in Cuba. The lowest proportion in Asia was in Israel (0.6%), while the highest proportion was in Jordan (4.7%) [Figure 3].
The highest proportion of other specific non-epithelial tumours (3.4%) was in Central and South America. The proportion was generally less than 5% in all countries, and between-country variation within each continent was small. The widest variation in the proportions was seen in Asia (0.5% in Indonesia and 5.8% in Cyprus) and Europe (0.6% in Croatia and 5.9% in Iceland) [supplementary Table 4].
Non-specific tumours generally accounted for 3% or less of ovarian tumours in all countries. The highest proportion was recorded in Russia (17.7%), much higher than the next highest proportion (Malta, 6.3%). The lowest proportions of non-specific tumours were seen in the Netherlands and Slovenia (0.1%) [supplementary Table 4].
Discussion
This is the largest study of the distribution of ovarian cancer histology. It is based on individual patient records from 218 population-based cancer registries in 51 countries. Data were available for 681,759 women, including 249,282 diagnosed between 2005 and 2009. Type II epithelial tumours were the most common histological group in each continent, but the distribution of histological groups varied greatly worldwide. The distribution was similar in Europe, North America and Oceania, while there was a much higher proportion of type I epithelial tumours seen in Asia and Central and South America.
Previous studies of the histological subtypes of ovarian cancer have focused on epithelial tumours, and they have generally been limited to a small number of countries. One meta-analysis included data for 98,099 women from 41 studies published between 1992 and 2012, only 12 of which used data from population-based registries(15). The results were similar to those found in this study, with type II epithelial tumours more common than type I epithelial tumours. The distribution of subtypes between countries included in the meta-analysis was heterogeneous.
Some of the variations in the distribution of ovarian cancer histology may be explained by ethnicity. A higher proportion of type II epithelial tumours diagnosed between 2005 and 2009 was reported in Israel (77.8%) than in most other countries. This may be attributable to the fact that a high percentage of the population in Israel is of Jewish ancestry, in whom BRCA1 and BRCA2 gene mutations are more common than in other populations. Serous tumours, which are classified as type II epithelial, are the most common histological subtype among women with BRCA1 and BRCA2 mutations(16).
The proportions of type I and type II epithelial tumours were markedly different between the US and Japan. In Japan, 41.3% of tumours were type I epithelial and 47.5% were type II epithelial, compared to 19.0% and 73.2% in the US [supplementary Table 4]. The lower proportion of serous tumours in Japan and other East Asian countries is due in part to the higher proportion of clear cell cancers [supplementary Table 5]. These differences are most probably due to the higher incidence of endometriosis, a potential pre-cursor of clear cell and endometrioid tumours(17), in East Asian women(18).
The proportion of mucinous tumours varied, ranging from over 10% in most Asian countries to 5-6% in most North American, European and Oceanian countries. The higher proportion in Japan is not clearly explained. Many tumours classified as mucinous may in fact be metastatic to the ovary from the gastrointestinal tract, including the stomach, which has a high incidence in Asia(19, 20). The reduction in the worldwide proportion of mucinous ovarian cancer from 9.2% to 6.8% between 1995-1999 and 2005-2009 [supplementary Table 5] may be partially attributable to more accurate immunohistochemical and imaging assessment, which allows for the exclusion of primary mucinous tumours from a different primary site, particularly those of the gastrointestinal tract. It can otherwise be difficult to differentiate a true primary mucinous ovarian cancer from mucinous tumours that are metastatic to the ovary(21).
Germ cell and sex cord-stromal tumours of the ovary should be considered separately in survival analysis, because they typically have higher survival than epithelial ovarian cancers. The proportion of germ cell tumours was less than 3% in most countries, but in some Asian and Central and South American countries, the proportions were much higher (5-8%). These differences are important, because the incidence of germ cell tumours is highest among young women and survival is usually very high, even with the tumour is diagnosed at an advanced stage, if optimal treatment is achievable(22). The higher proportion of germ cell tumours in Asia and Central and South America may therefore be due to the younger age profile of populations in these regions. The proportion of sex cord-stromal tumours was less than 2% in most countries, but much higher in some European countries. These differences are also important in the comparison of survival from ovarian cancers combined, because survival is much higher for sex cord-stromal tumours than for epithelial ovarian cancers(23).
Variation in the distribution of histological groups of ovarian cancer may impact international comparisons of survival from all ovarian cancers combined if countries with more favourable histological distributions, where more tumours are classified as type I epithelial, germ cell or sex cord-stromal, are compared to survival in countries with higher proportions of type II epithelial tumours. In the main CONCORD-2 analysis(4), age-standardised 5-year survival from all ovarian tumours combined was higher in some East Asian countries than in Europe, North America and Oceania. In Hong Kong, 5-year survival was 52.9% for women diagnosed from 2005 to 2009, much higher than the highest level of survival in Europe (Finland: 44.9%), North America (US: 40.9%) and Oceania (Australia: 37.5%)(4). The proportion of type I epithelial tumours in Hong Kong (51.7%) was the highest among the 51 countries, and Hong Kong was one of only two countries where type I epithelial tumours were more common than type II epithelial tumours. Thus, the higher survival for all ovarian cancers combined in Hong Kong may be partially explained by the more favourable distribution of histology. A favourable distribution was also seen in Ecuador, with one of the highest proportions of germ cell tumours (7.8%), and age-standardised 5-year survival was 47.0% for all tumours combined(4).
For many areas of the world, data from population-based cancer registries are still insufficient to allow meaningful comparisons of ovarian cancer histology.. Lack of accurate cancer registration in many areas, and the high proportion of non-specific morphology in many countries, still limits worldwide comparison of survival by histology.
During 2005-2009, the highest proportion of tumours of non-specific morphology was seen in Russia (17.7%), which may explain the low proportion of type II epithelial tumours in the country, because many non-specific tumours will be diagnosed at an advanced stage [supplementary Table 4]. In order to classify a tumour as a specific subtype, such as serous or endometrioid, a tissue biopsy or surgical resection is required; thus, histology may not be correctly classified into a specific subtype if the disease is diagnosed at an advanced stage. In Central and South America, the largest registry (Puerto Rico) provided data only for 684 women, of which 24.3% were recorded as having been diagnosed with undifferentiated or other epithelial carcinoma. The accuracy of morphology data is also reliant upon data transmission to the cancer registries and recording of morphology codes, so the distribution of subtypes may be affected by registry procedures and the classifications in use. For example, in Sweden, only 324 of 12,969 (2.5%) women with ovarian cancer were reported as being diagnosed with a specific morphology, compared with 6,311 of 7,322 women (86.2%) in Finland. Previous reports on ovarian cancer in Sweden showed over 98% specific morphology codes(24). Additionally, the distribution for Hong Kong included only epithelial tumours, because other ovarian cancer subtypes were not submitted. While Sweden was excluded from these analyses, Hong Kong was included because comparison of the most common subtypes, type I and type II epithelial, was still achievable.
Variation between pathologists in the classification of ovarian tumours into specific histological subtypes may affect the distribution of subtypes within a country, and thus, comparisons of the distributions of subtypes between countries. Various studies conducted from 1984 to 1994 of the reproducibility of the World Health Organization’s 1973 histological classification of ovarian tumours(25) showed only moderate levels of reproducibility(26). The WHO classification for ovarian tumours was updated in 1999(27), 2003(28) and 2014(2). Because tumours diagnosed from 1995 to 2009 were included in the analysis, pathologists could have used either the 1973, 1999 or 2003 criteria to assign a histological subtype to a tumour included in the study. The definitions of the various histological subtypes do not change drastically over time from 1973 to 2003, so the edition used by the pathologist is not necessarily relevant. However, the definitions of the subtypes are general and the 2003 criteria did not include changes or criteria that could improve reproducibility; thus, observer variation remains an issue(26).
Studies of immunohistochemical biomarkers and molecular genetic features for certain histological subtypes may allow for more reproducible diagnoses. TP53 mutations are found in 80% of women diagnosed with high-grade serous carcinoma, while KRAS, BRAF and ERBB2 mutations are more common in women with low-grade serous carcinoma. Mutations of CTNNB1, PTEN, PIK3CA are common in endometrioid tumours and KRAS mutations can be found in 50% of mucinous tumours. For clear cell carcinoma, mutations or ARID1A and PIK3CA are common(2, 6, 7, 9). With this knowledge and the updated WHO classification of 2014, reproducibility of the histological typing of ovarian cancers should improve.
In order to classify serous tumours appropriately into histological groups, knowledge of the tumour grade is important. However, data on tumour grade are not routinely collected by cancer registries. For ovarian cancer, most serous carcinomas are high-grade, and will have been correctly classified in our analysis as type II epithelial, but a small proportion are low-grade, and should have been classified as type I epithelial(6, 7, 9, 10, 29, 30). Because the proportion of low-grade serous tumours is small(2), the effect of any misclassification on the distribution of histology is expected to be minimal. The distinction between high-grade and low-grade serous carcinoma is important, because they have a distinct pathogenesis and are thought to be different diseases(6, 7). Low-grade serous carcinoma is more common in younger women, and is thought to arise from borderline serous tumours. In contrast, high-grade serous carcinoma is more common in older women, is thought to arise from tubal disease and typically exhibits p53 mutation(6, 7, 31). Similarly, endometrioid tumours are classified as either low- or high-grade, and classification into type I or type II epithelial has previously depended on tumour grade(7). Most endometrioid ovarian tumours will be low-grade(2), and some pathologists have argued that high-grade endometrioid tumours may not exist(7, 10). Distinguishing between high-grade endometrioid and high-grade serous tumours is difficult, and when distinction between endometrioid and serous tumours is unclear, most high-grade tumours may be classified as high-grade serous, because this subtype is more common than high-grade endometrioid(7, 10). Following an update in 2016 of the original definitions of type I and type II epithelial tumours, all endometrioid tumours would now be categorised as type I, regardless of tumour grade(6). Future analyses of ovarian cancer survival should, if possible, incorporate a distinction between high- and low-grade serous carcinoma, to reflect the current understanding of ovarian cancer pathogenesis and behaviour, and to classify serous carcinomas appropriately into type I and type II epithelial tumours.
Carcinoma, NOS (ICD-O-3 morphology code 8010), large cell carcinoma, NOS (8012) and adenocarcinoma, NOS (8140) were categorised as undifferentiated and other epithelial tumours and grouped broadly as type II epithelial. There may also be some misclassification of these tumours, because these morphology codes are not specific codes, so classification into type I or type II is difficult. However, carcinoma (NOS), large cell carcinoma (NOS) and adenocarcinoma (NOS) are treated clinically as if they were high-grade serous carcinomas, which are classified as type II. Therefore, we decided to categorise these tumours as type II epithelial. They comprise 20.9% of tumours included in the analysis.
Only morphologically verified tumours, or those with specific morphologies that implied morphological verification, were included in the analysis. This restriction may affect the distribution of histological subtypes, because the histology of advanced-stage tumours that are not fully investigated may be coded as non-specific or unknown. If more advanced-stage tumours are not morphologically verified and therefore excluded from analysis, the distribution of histological groups may appear more favourable than it actually is.
This worldwide study of ovarian cancer histology has identified striking variations in histological distribution, using data from population-based cancer registries in 51 countries. The two main histological groups of ovarian cancer have different prognosis, primarily due to differences in the distribution of stage, sensitivity to chemotherapy and response to surgical resection. International comparisons of ovarian cancer survival should take histology into account, to help identify whether the distribution of histology contributes to international differences in ovarian cancer survival, which is typically reported for all histological groups combined. To understand further the impact on survival, we are examining international differences in ovarian cancer survival by histological group. Registration of both the histology and the grade of ovarian cancers is important to help categorise these tumours more accurately into histological group, especially type I and type II epithelial. Increased support for the development of high-quality population-based cancer registries in low-income countries will also help improve international comparisons of ovarian cancer survival.
Supplementary Material
Figure 2. Histological groups of ovarian cancera: distribution by continent, 2005-09.
a Malignancies of the ovary (ICD-O-3 C56.9), fallopian tube, uterine ligaments and adnexa, and other and unspecified female genital organs (C57.0-C57.4, C57.7-C57.9), and peritoneum and retroperitoneum (C48.0-C48.2). Endometrioid tumours are classified as type I epithelial (see text).
Acknowledgements
We would like to thank Mr. John Butler for proposing the idea for the manuscript. This work was funded by the Canadian Partnership Against Cancer, Cancer Focus Northern Ireland, Cancer Institute New South Wales, Cancer Research UK (C1336/A16148), US Centers for Disease Control and Prevention (CDC; 12FED03123, ACO12036), Swiss Re, Swiss Research foundation, Swiss Cancer League, and the University of Kentucky (3049024672-12-568).
CONCORD Working Group
Africa—Algeria: S Bouzbid (Registre du Cancer d'Annaba); M Hamdi-Chérif*, Z Zaidi (Registre du Cancer de Sétif); Gambia: E Bah, R Swaminathan (National Cancer Registry); Lesotho: SH Nortje, DC Stefan (Children’s Haematology Oncology Clinics - Lesotho); Libya: MM El Mistiri (Benghazi Cancer Registry); Mali: S Bayo, B Malle (Kankou Moussa University); Mauritius: SS Manraj, R Sewpaul-Sungkur (Mauritius Cancer Registry); Nigeria: A Fabowale, OJ Ogunbiyi* (Ibadan Cancer Registry); South Africa: D Bradshaw, NIM Somdyala (Eastern Cape Province Cancer Registry); Sudan: M Abdel-Rahman (University of Khartoum); Tunisia: L Jaidane, M Mokni (Registre du Cancer du Centre Tunisien).
America (Central and South)—Argentina: I Kumcher, F Moreno (National Childhood Cancer Registry – National Cancer Institute); MS González, EA Laura (Registro Regional de Tumores del Sur de la Provincia de Buenos Aires); SB Espinola, GH Calabrano (Registro Poblacional de Tumores de la Provincia del Chubut); B Carballo Quintero, R Fita (Registro Provincial de Tumores de Córdoba); DA Garcilazo, PL Giacciani (Entre Rios Cancer Registry); MC Diumenjo, WD Laspada (Registro Provincial de Tumores de Mendoza); MA Green, MF Lanza (Registro de Cáncer de Santa Fe); SG Ibañez (Cancer Registry of Tierra del Fuego Province); Brazil: CA Lima, E Lobo de Oliveira (Registro de Câncer de Base Populacional de Aracaju); C Daniel, C Scandiuzzi (Cancer Registry of Distrito Federal); PCF De Souza, CD Melo (Registro de Câncer de Base Populacional de Cuiabá); K Del Pino, C Laporte (Registro de Curitiba); MP Curado, JC de Oliveira (Registro de Goiânia); CLA Veneziano, DB Veneziano (Registro de Câncer de Base Populacional de Jahu); TS Alexandre, AS Verdugo (Registro de Câncer de São Paulo); G Azevedo e Silva* (University of Rio de Janeiro); Chile: JC Galaz, JA Moya (Registro Poblacional de Cáncer Region de Antofagasta); DA Herrmann, S Vargas (Registro Poblacional Region de Los Rios); Colombia: VM Herrera, CJ Uribe (Registro Poblacional de Cáncer Area Metropolitana de Bucaramanga); LE Bravo (Cali Cancer Registry); NE Arias-Ortiz (Registro Poblacional de Cáncer de Manizales); DM Jurado, MC Yépez (Registro Poblacional de Cáncer del Municipio de Pasto); Cuba: YH Galán, P Torres (Registro Nacional de Cáncer de Cuba); Ecuador: F Martínez-Reyes, ML Pérez-Meza (Cuenca Tumor Registry); L Jaramillo, R Quinto (Guayaquil Cancer Registry); P Cueva, JG Yépez (Quito Cancer Registry); Puerto Rico: CR Torres-Cintrón, G Tortolero-Luna (Puerto Rico Central Cancer Registry); Uruguay: R Alonso, E Barrios (Registro Nacional de Cáncer).
America (North)—Canada: C Nikiforuk, L Shack (Alberta Cancer Registry); AJ Coldman, RR Woods (British Columbia Cancer Registry); G Noonan, D Turner* (Manitoba Cancer Registry); E Kumar, B Zhang (New Brunswick Provincial Cancer Registry); FR McCrate, S Ryan (Newfoundland and Labrador Cancer Registry); H Hannah (Northwest Territories Cancer Registry); RAD Dewar, M MacIntyre (Nova Scotia Surveillance and Epidemiology Unit); A Lalany, M Ruta (Nunavut Department of Health and Social Services); L Marrett, DE Nishri* (Ontario Cancer Registry); C McClure, KA Vriends (Prince Edward Island Cancer Registry); C Bertrand, R Louchini (Registre Québécois du Cancer); KI Robb, H Stuart-Panko (Saskatchewan Cancer Registry); S Demers, S Wright (Yukon Government); USA: JT George, X Shen (Alabama Statewide Cancer Registry); JT Brockhouse, DK O'Brien (Alaska Cancer Registry); KC Ward (Georgia Comprehensive Cancer Registry; Metropolitan Atlanta Registry); L Almon (Metropolitan Atlanta Registry); J Bates (California State Cancer Registry); R Rycroft (Colorado Central Cancer Registry); L Mueller, C Phillips (Connecticut Tumor Registry); H Brown, B Cromartie (Delaware Cancer Registry); AG Schwartz, F Vigneau (Metropolitan Detroit Cancer Surveillance System); JA MacKinnon, B Wohler (Florida Cancer Data System); AR Bayakly (Georgia Comprehensive Cancer Registry); CA Clarke, SL Glaser (Greater Bay Area Cancer Registry); D West (Cancer Registry of Greater California); MD Green, BY Hernandez (Hawaii Tumor Registry); CJ Johnson, D Jozwik (Cancer Data Registry of Idaho); ME Charlton, CF Lynch (State Health Registry of Iowa); B Huang, TC Tucker* (Kentucky Cancer Registry); D Deapen, L Liu (Los Angeles Cancer Surveillance Program); MC Hsieh, XC Wu (Louisiana Tumor Registry); K Stern (Maryland Cancer Registry); ST Gershman, RC Knowlton (Massachusetts Cancer Registry); J Alverson, GE Copeland (Michigan State Cancer Surveillance Program); DB Rogers (Mississippi Cancer Registry); D Lemons, LL Williamson (Montana Central Tumor Registry); M Hood (Nebraska Cancer Registry); GM Hosain, JR Rees (New Hampshire State Cancer Registry); KS Pawlish, A Stroup (New Jersey State Cancer Registry); C Key, C Wiggins (New Mexico Tumor Registry); AR Kahn, MJ Schymura (New York State Cancer Registry); G Leung, C Rao (North Carolina Central Cancer Registry); L Giljahn, B Warther (Ohio Cancer Incidence Surveillance System); A Pate (Oklahoma Central Cancer Registry); M Patil, SS Schubert (Oregon State Cancer Registry); JJ Rubertone, SJ Slack (Pennsylvania Cancer Registry); JP Fulton, DL Rousseau (Rhode Island Cancer Registry); TA Janes, SM Schwartz (Seattle Cancer Surveillance System); SW Bolick, DM Hurley (South Carolina Central Cancer Registry); J Richards, MA Whiteside (Tennessee Cancer Registry); LM Nogueira (Texas Cancer Registry); K Herget, C Sweeney (Utah Cancer Registry); J Martin, S Wang (Virginia Cancer Registry); DG Harrelson, MB Keitheri Cheteri (Washington State Cancer Registry); S Farley, AG Hudson (West Virginia Cancer Registry); R Borchers, L Stephenson (Wisconsin Department of Health Services); JR Espinoza (Wyoming Cancer Surveillance Program); HK Weir* (Centers for Disease Control and Prevention); BK Edwards* (National Cancer Institute).
Asia—China: N Wang, L Yang (Beijing Cancer Registry); JS Chen (Changle City Cancer Registry); GH Song (Cixian Cancer Registry); XP Gu (Dafeng County Center for Disease Control and Prevention); P Zhang (Dalian Centers for Disease Prevention and Control); HM Ge (Donghai County Center for Disease Prevention and Control); DL Zhao (Feicheng County); JH Zhang (Ganyu Center for Disease Prevention and Control); FD Zhu (Guanyun Cancer Registry); JG Tang (Haimen Cancer Registry); Y Shen (Haining City Cancer Registry); J Wang (Jianhu Cancer Registry); QL Li (Jiashan County Cancer Registry); XP Yang (Jintan Cancer Registry); J Dong, W Li (Lianyungang Center for Disease Prevention and Control); LP Cheng (Henan Province Central Cancer Registry); JG Chen (Qidong County Cancer Registry); QH Huang (Sihui Cancer Registry); SQ Huang (Taixing Cancer Registry); GP Guo (Cancer Institute of Yangzhong City); K Wei (Zhongshan City Cancer Registry); WQ Chen*, H Zeng (National Central Cancer Registry China); Cyprus: AV Demetriou, P Pavlou (Cyprus Cancer Registry); Hong Kong: WK Mang, KC Ngan (Hong Kong Cancer Registry); India: R Swaminathan (Chennai Cancer Registry); AC Kataki, M Krishnatreya (Guwahati Cancer Registry); PA Jayalekshmi, P Sebastian (Karunagappally Cancer Registry); SD Sapkota, Y Verma (Population Based Cancer Registry, Sikkim); A Nandakumar* (National Centre for Disease Informatics and Research; National Cancer Registry Programme); Indonesia: E Suzanna (Jakarta Cancer Registry); Israel: L Keinan-Boker, BG Silverman (Israel National Cancer Registry); Japan: H Ito, H Nakagawa (Aichi Cancer Registry); M Hattori, Y Kaizaki (Fukui Cancer Registry); H Sugiyama, M Utada (Hiroshima Prefecture Cancer Registry); K Katayama, H Narimatsu (Kanagawa Cancer Registry); S Kanemura (Miyagi Prefectural Cancer Registry); T Koike (Niigata Prefecture Cancer Registry); I Miyashiro (Osaka Cancer Registry); M Yoshii (Saga Prefectural Cancer Registry); I Oki (Tochigi Prefectural Cancer Registry); A Shibata (Yamagata Cancer Registry); T Matsuda* (National Cancer Center); Jordan: O Nimri (Jordan National Cancer Registry); Malaysia: A Ab Manan, N Bhoo Pathy (Penang Cancer Registry); Mongolia: O Chimedsuren, S Tuvshingerel (Cancer Registry of Mongolia); Qatar: AHM Al Khater, MM El Mistiri (Qatar Cancer Registry); Saudi Arabia: H Al-Eid (Saudi National Cancer Registry); South Korea: KW Jung, YJ Won (Korea Central Cancer Registry); Taiwan: CJ Chiang, MS Lai (Taiwan Cancer Registry); Thailand: K Suwanrungruang, S Wiangnon (Khon Kaen Provincial Registry); K Daoprasert, D Pongnikorn (Lampang Cancer Registry); SL Geater, H Sriplung (Songkhla Cancer Registry); Turkey: S Eser, CI Yakut (Izmir Cancer Registry).
Europe—Austria: M Hackl (Austrian National Cancer Registry); H Mühlböck, W Oberaigner (Tyrol Cancer Registry); Belarus: AA Zborovskaya (Belarus Childhood Cancer Subregistry); OV Aleinikova (Belarusian Research Center for Pediatric Oncology, Hematology and Immunology); Belgium: K Henau, L Van Eycken (Belgian Cancer Registry); Bulgaria: N Dimitrova, Z Valerianova (Bulgarian National Cancer Registry); Croatia: M Šekerija (Croatian National Cancer Registry); Czech Republic: M Zvolský (Czech National Cancer Registry); Denmark: G Engholm, H Storm* (Danish Cancer Society); Estonia: K Innos, M Mägi (Estonian Cancer Registry); Finland: N Malila, K Seppä (Cancer Society of Finland); France: J Jégu, M Velten (Bas-Rhin General Cancer Registry); E Cornet, X Troussard (Registre Régional des Hémopathies Malignes de Basse Normandie); AM Bouvier, J Faivre (Burgundy Digestive Cancer Registry); AV Guizard (Calvados General Cancer Registry); V Bouvier, G Launoy (Calvados Digestive Cancer Registry); P Arveux (Côte-d'Or Gynaecologic Cancer Registry); M Maynadié, M Mounier (Côte-d'Or Haematopoietic Malignancies Registry); E Fournier, AS Woronoff (Doubs and Belfort Territory General Cancer Registry); M Daoulas (Finistère Cancer Registry); J Clavel (National Registry of Childhood Haematopoietic Malignancies); S Le Guyader-Peyrou, A Monnereau (Gironde Haematopoietic Malignancies Registry); B Trétarre (Hérault General Cancer Registry); M Colonna (Isère General Cancer Registry); A Cowppli-Bony, F Molinié (Loire-Atlantique-Vendée Cancer Registry); S Bara, D Degré (Manche General Cancer Registry); O Ganry, B Lapôtre-Ledoux (Somme General Cancer Registry); P Grosclaude (Tarn General Cancer Registry); J Estève (Hospices Civils de Lyon); F Bray*, M Piñeros* (International Agency for Research on Cancer); F Sassi (Organisation for Economic Co-operation and Development); Germany: R Stabenow (Common Cancer Registry of the Federal States); A Eberle (Bremen Cancer Registry); C Erb, A Nennecke (Hamburg Cancer Registry); J Kieschke, E Sirri (Epidemiological Cancer Registry of Lower Saxony); H Kajueter (North Rhine Westphalia Cancer Registry); K Emrich, SR Zeissig (Rhineland Palatinate Cancer Registry); B Holleczek (Saarland Cancer Registry); N Eisemann, A Katalinic (Schleswig-Holstein Cancer Registry); H Brenner (German Cancer Research Center); Gibraltar: RA Asquez, V Kumar (Gibraltar Cancer Registry); Iceland: EJ Ólafsdóttir, L Tryggvadóttir (Icelandic Cancer Registry); Ireland: H Comber, PM Walsh (National Cancer Registry); H Sundseth* (European Institute of Women’s Health); Italy: E Devigili, G Mazzoleni (Registro Tumori Alto Adige); A Giacomin (Registro Tumori Biella); F Bella, M Castaing (Integrated Cancer Registry of Catania-Messina-Siracusa-Enna); A Sutera (Registro Tumori Catanzaro); G Gola (Registro Tumori della Provincia di Como); S Ferretti (Registro Tumori della Provincia di Ferrara); D Serraino, A Zucchetto (Registro Tumori del Friuli Venezia Giulia); R Lillini, M Vercelli (Registro Tumori Regione Liguria); S Busco, F Pannozzo (Registro Tumori della Provincia di Latina); S Vitarelli (Registro Tumori della Provincia di Macerata); P Ricci (Registro Tumori Mantova); C Pascucci (Registro Tumori Marche Childhood); M Autelitano (Registro Tumori Milano); C Cirilli, M Federico (Registro Tumori della Provincia di Modena); M Fusco, MF Vitale (Registro Tumori della ASL Napoli 3 sud); M Usala (Nuoro Cancer Registry); R Cusimano, W Mazzucco (Registro Tumori di Palermo e Provincia); M Michiara, P Sgargi (Registro Tumori della Provincia di Parma); MM Maule, C Sacerdote (Piedmont Childhood Cancer Registry); R Tumino (Registro Tumori della Provincia di Ragusa); E Di Felice, M Vicentini (Registro Tumori Reggio Emilia); F Falcini (Registro Tumori della Romagna); L Cremone (Registro Tumori Salerno); M Budroni, R Cesaraccio (Registro Tumori della Provincia di Sassari); ML Contrino, F Tisano (Registro Tumori Siracusa); AC Fanetti, S Maspero (Registro Tumori della Provincia di Sondrio); G Candela, T Scuderi (Registro Tumori Trapani); MA Gentilini, S Piffer (Registro Tumori Trento); S Rosso, L Sacchetto (Registro Tumori Piemonte Città di Torino); A Caldarella (Registro Tumori della Regione Toscana); F La Rosa, F Stracci (Registro Tumori Umbro di Popolazione); P Contiero, G Tagliabue (Registro Tumori Lombardia, Provincia di Varese); AP Dei Tos, M Zorzi (Registro Tumori Veneto); R Zanetti* (International Association of Cancer Registries); P Baili, F Berrino*, G Gatta, M Sant* (National Cancer Institute); R Capocaccia*, R De Angelis (National Centre for Epidemiology); Latvia: E Liepina, A Maurina (Latvian Cancer Registry); Lithuania: G Smailyte (Lithuanian Cancer Registry); Malta: D Agius, N Calleja (Malta National Cancer Registry); Netherlands: S Siesling, O Visser (Comprehensive Cancer Centre of the Netherlands); Norway: S Larønningen, B Møller (The Cancer Registry of Norway); Poland: A Dyzmann-Sroka, M Trojanowski (Greater Poland Cancer Registry); S Góźdż, R Mężyk (Cancer Registry of Kielce); M Grądalska-Lampart, AU Radziszewska (Podkarpackie Cancer Registry); JA Didkowska, U Wojciechowska (National Cancer Registry); J Błaszczyk, K Kępska (Lower Silesian Cancer Registry); M Bielska-Lasota, K Kwiatkowska (National Institute of Public Health - NIH); Portugal: G Forjaz, RA Rego (Registo Oncológico Regional dos Açores); J Bastos, MA Silva (Registo Oncológico Regional do Centro); L Antunes, MJ Bento (Registo Oncológico Regional do Norte); A Mayer-da-Silva, A Miranda (Registo Oncólogico Regional do Sul); Romania: D Coza, AI Todescu (Cancer Institute I. Chiricuta); Russian Federation: MY Valkov (Arkhangelsk Regional Cancer Registry); Slovakia: J Adamcik, C Safaei Diba (National Cancer Registry of Slovakia); Slovenia: M Primic-Žakelj, T Žagar (Cancer Registry of Republic of Slovenia); J Stare (University of Ljubljana); Spain: E Almar, A Mateos (Registro de Cáncer de Albacete); JR Quirós (Registro de Tumores del Principado de Asturias); J Bidaurrazaga, N Larrañaga (Basque Country Cancer Registry); JM Díaz García, AI Marcos (Registro de Cáncer de Cuenca); R Marcos-Gragera, ML Vilardell Gil (Registre de Càncer de Girona); E Molina, MJ Sánchez (Registro de Cáncer de Granada); P Franch Sureda, M Ramos Montserrat (Mallorca Cancer Registry); MD Chirlaque, C Navarro (Murcia Cancer Registry); EE Ardanaz, CC Moreno-Iribas (Registro de Cáncer de Navarra); R Fernández-Delgado, R Peris-Bonet (Registro Español de Tumores Infantiles (RETI-SEHOP)); J Galceran (Tarragona Cancer Registry); Sweden: S Khan, M Lambe (Swedish Cancer Registry); Switzerland: B Camey (Registre Fribourgeois des Tumeurs); C Bouchardy, M Usel (Geneva Cancer Registry); SM Ess (Cancer Registry Grisons and Glarus); C Herrmann (Cancer Registry Grisons and Glarus; Cancer Registry of St Gallen-Appenzell); JL Bulliard, M Maspoli-Conconi (Registre Neuchâtelois des Tumeurs); H Frick (Cancer Registry of St Gallen-Appenzell); CE Kuehni, M Schindler (Swiss Childhood Cancer Registry); A Bordoni, A Spitale (Registro Tumori Cantone Ticino); A Chiolero, I Konzelmann (Registre Valaisan des Tumeurs); SI Dehler, KL Matthes (Krebsregister der Kantone Zürich und Zug); United Kingdom: J Rashbass, C Stiller* (Public Health England); D Fitzpatrick, A Gavin (Northern Ireland Cancer Registry); F Bannon (Queens University, Belfast); RJ Black, DH Brewster (Scottish Cancer Registry); DW Huws, C White (Welsh Cancer Intelligence & Surveillance Unit); P Finan (Leeds General Infirmary); C Allemani*, A Bonaventure, H Carreira, MP Coleman*, V Di Carlo, R Harewood, K Liu, M Matz, L Montel, M Nikšić, B Rachet*, N Sanz, D Spika (London School of Hygiene & Tropical Medicine); R Stephens* (National Cancer Research Institute, London); M Peake (University of Leicester).
Oceania—Australia: E Chalker, L Newman (Australian Capital Territory Cancer Registry); D Baker, MJ Soeberg (NSW Cancer Registry); J Aitken, C Scott (Queensland Cancer Registry); BC Stokes, A Venn (Tasmanian Cancer Registry); H Farrugia, GG Giles (Victorian Cancer Registry); T Threlfall (Western Australian Cancer Registry); D Currow*, H You (Cancer Institute NSW); New Zealand: J Hendrix, C Lewis (New Zealand Cancer Registry).
*CONCORD Steering Committee
Footnotes
Conflict of interest
The authors declare there are no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [cited 2015 18 May]. [Internet] [Google Scholar]
- 2.Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Geneva: WHO; 2014. [Google Scholar]
- 3.Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecologic Oncology. 2012;127:75–82. doi: 10.1016/j.ygyno.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) The Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor H. Malignant and semi-malignant tumours of the ovary. Surg Gynecol Obsts. 1929(48):204–30. [Google Scholar]
- 6.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186(4):733–47. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCluggage WG. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61(2):152–63. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 9.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43(5):420–32. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19(5):961–8. doi: 10.1158/1078-0432.CCR-12-2243. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®) National Cancer Institute at the National Institutes of Health; 2015. [updated 21/08/2015; cited 2016 21/04/2016]. Health Professionals Version:[Available from: http://www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdq. [Google Scholar]
- 13.Fritz AG, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, et al., editors. International Classification of Diseases for Oncology (ICD-O) 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Trent Cancer Registry National Cancer Intelligence Network. Overview of ovarian cancer in England: incidence, mortality and survival. London: Trent Cancer Registry; 2012. [Google Scholar]
- 15.Sung PL, Chang YH, Chao KC, Chuang CM, Task Force on Systematic R, Meta-analysis of Ovarian C Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol. 2014;133(2):147–54. doi: 10.1016/j.ygyno.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Moslehi R, Chu W, Karlan B, Fishman D, Risch H, Fields A, et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet. 2000;66(4):1259–72. doi: 10.1086/302853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Mang M, Wang Y, Wang L, Klein R, Kong B, et al. Tubal origin of ovarian endometriosis and clear cell and endometrioid carcinoma. Am J Cancer Res. 2015;5(3):869–79. [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202(6):514–21. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer. 2008;18(2):209–14. doi: 10.1111/j.1525-1438.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 20.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–90. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, El-Bahrawy MA. Expression profile of mucins in ovarian mucinous tumors: distinguishing primary ovarian from metastatic tumors. Int J Gynecol Pathol. 2014;33(2):166–75. doi: 10.1097/PGP.0b013e318288b384. [DOI] [PubMed] [Google Scholar]
- 22.Mangili G, Sigismondi C, Gadducci A, Cormio G, Scollo P, Tateo S, et al. Outcome and risk factors for recurrence in malignant ovarian germ cell tumors: a MITO-9 retrospective study. Int J Gynecol Cancer. 2011;21(8):1414–21. doi: 10.1097/IGC.0b013e3182236582. [DOI] [PubMed] [Google Scholar]
- 23.Holscher G, Anthuber C, Bastert G, Burges A, Mayr D, Oberlechner E, et al. Improvement of survival in sex cord stromal tumors - an observational study with more than 25 years follow-up. Acta Obstet Gynecol Scand. 2009;88(4):440–8. doi: 10.1080/00016340902741208. [DOI] [PubMed] [Google Scholar]
- 24.Oberaigner W, Minicozzi P, Bielska-Lasota M, Allemani C, De Angelis R, Mangone L, et al. Survival for ovarian cancer in Europe: the across-country variation did not shrink in the past decade. Acta Oncologica. 2012;51(4):441–53. doi: 10.3109/0284186X.2011.653437. [DOI] [PubMed] [Google Scholar]
- 25.Servov S, Scully R, Sobin LH. Histological typing of ovarian tumours. Geneva: World Health Organization; 1973. [Google Scholar]
- 26.Clarke B, Gilks B. Ovarian carcinoma: recent developments in classification of tumour histological subtype. Canadian Journal of Pathology. 2011:33–42. [Google Scholar]
- 27.Scully R, Sobin LH. Histological typing of ovarian tumours. 2nd ed. Geneva: World Health Organization; 1999. [Google Scholar]
- 28.Tavassoli FA, D P, editors. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. [Google Scholar]
- 29.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):x111–7. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 30.Seidman JD, Horkayne-Szakaly I, Cosin JA, Ryu HS, Haiba M, Boice CR, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103(2):703–8. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Vang R, Shih Ie M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267–82. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.