Summary
Background:
In sub-Saharan Africa, migrants typically have higher HIV prevalence than non-migrants; however, whether HIV acquisition precedes or follows migration is generally unknown. Here, we assessed risk of HIV following migration.
Methodology:
We assessed the association between migration and HIV acquisition using prospective data from HIV-negative participants aged 15–49 enrolled in an open population-based cohort in Rakai, Uganda between 1999 and 2015. Individuals were classified as recent in-migrants if they moved into study communities within the prior two years, non-recent migrants (>2 years in community), or permanent residents with no migration history. Poisson regression was used to estimate incidence rate ratios (IRR) of HIV associated with residence status with adjustment for demographics, sexual behaviors, and time.
Results:
15,187 HIV-negative individuals were followed for 89,292 person-years of whom 29% (n=4451) were ever in-migrants. There were 841 incident HIV events, including 243 (29%) among inmigrants. Relative to permanent residents, HIV incidence was significantly increased among recent inmigrants among women (1.92/100 pys in recent migrants vs. 0.93/100 pys in permanent residents; adjIRR=1.74, 95%CI: 1.12–2.71) and men (1.52/100 pys vs 0.84/100 pys; adjIRR=1.74, 95%CI: 1.12–2.71), but was not among non-recent in-migrants. HIV incidence declined over time with scale-up of combination HIV prevention among permanent residents and non-recent in-migrants, but was unchanged among recent in-migrants.
Conclusion:
The first two years after migration are associated with increased risk of HIV acquisition. Findings highlight the need for prevention programs focused on migrants to reduce HIV incidence in sub-Saharan Africa.
Introduction
Migration has long been recognized as a driver of HIV spread in sub-Saharan Africa, particularly in the early phases of the epidemic as it disseminated from Central Africa.1,2 Numerous reports since then have shown that HIV prevalence is higher among migrant populations relative to longer-term resident populations continent-wide.1,3–5 For example, a study conducted in Uganda in the early 1990s, found HIV prevalence was 16% among individuals had who migrated in the prior three years compared to only 6% among non-migrants.5 In a more recent study conducted in Tanzania, odds of HIV-infection were found to be more than two-fold higher among migrants compared to non-migrants.4 While these and other cross-sectional studies have established a positive link between migration and an individual’s HIV status, little is known about the pathways linking them.1 In particular, it is unclear if HIV acquisition is a cause and or a consequence of migration. Establishing such causal mechanisms may help target HIV prevention and treatment resources more effectively.
There are three primary hypotheses potentially explaining the higher HIV prevalence in migrants compared to their non-migrating peers. The first is that migrants are inherently higher risk persons and that regardless of them migrating they have a higher likelihood of acquiring HIV (hypothesis 1).6 A prior study from Rakai showed HIV-negative out-migrant youth had higher levels of HIV risk behaviors before migrating, including increased multiple and concurrent sexual partners, compared to youth who did not migrate.7 Another hypothesis is that individuals are more likely to migrate after becoming HIV-positive (hypothesis 2). Indeed, data from a retrospective cohort study in Malawi showed significantly greater odds of migration among HIV positive individuals compared to HIV-negative persons.8 The third hypothesis is that individuals who migrate are at higher risk as a consequence of migrating which destabilizes personal social networks and affects an individual’s HIV risk environment (hypothesis 3).9 For example, a South African study of individuals who migrated and then returned to their home communities showed these circular migrants had significantly higher HIV incidence compared to individuals who spent no time outside their home community.10 However, it is unknown when these migrants acquired HIV in relation to the migration process or whether the findings extend to persons who permanently leave their home communities.
The reasons for higher HIV prevalence in African migrants are poorly understood in part because mobile populations are difficult to enroll and follow-up. Additionally, definitions of migration vary widely with some studies only assessing migration across international borders and others focusing solely on internal movement of persons within countries and districts.1,11 The Rakai Community Cohort (RCCS), an open population-based census and cohort of HIV incidence in Rakai District, Uganda, captures in-migration and out-migration in study communities regardless of source or destination location, offering a rare opportunity to prospectively study the association between HIV and migration. Here, we test the third of the three hypotheses explaining increased HIV burden among migrants. Specifically, we assess whether initially HIV-negative individuals are at higher HIV risk following in-migration using longitudinal data from the RCCS collected between 1999 and 2015. We hypothesize that in-migrants, regardless of gender, are at higher risk of HIV acquisition compared to permanent residents, with no history of migration but that this elevated HIV risk among migrants has decreased over calendar time with the scale-up of combination HIV prevention programs in Uganda.
Methods
Study population
The Rakai Community Cohort Study (RCCS) is a population-based HIV incidence cohort in Rakai District, Uganda. As previously described, household censuses are conducted at approximately 18-month intervals to collect demographic data on all residents and to record all births, deaths and in- and out-migrations since the prior survey, with no age truncation12. The RCCS surveys, which occur shortly after the census (~2 weeks), collects detailed sociodemographic and behavioral data as well as blood samples for HIV testing on all residents aged 15–49. Individuals who migrate into study communities (i.e. in-migrants) and individuals who migrate out of study communities (i.e. out-migrants) between surveys are documented in the census, and information on source, destination communities, and reason for migration is obtained via survey or interview with family members. At the start of each survey, the RCCS opens enrollment to all new in-migrants, newly age-eligible participants, and any prior non-participants resident in the community.
Communities for the Rakai Community Cohort Study originally were selected as part of a community-randomized trial on treatment of sexually transmitted infections for HIV prevention as previously described.13 We used RCCS data from 30 communities which were continuously surveyed between April 6, 1999 and January 30, 2015 including a total of eleven surveys for this study. Over the analysis period, study participation rates ranged from 59–65% of the eligible censused population and 74–98% of the eligible population present at time of survey with lower participation rates among men, persons less than 20 years-old, and residents in trading communities.14
This study was independently reviewed and approved by the Ugandan (Ugandan Virus Research Institute Security and Ethics Committee; Protocol GC/127/13/01/16) and US (Western Institutional Review Board; Protocol 20031318) institutional review boards. All participants provided written informed consent at baseline and follow-up visits.
Combination HIV prevention scale-up in Rakai, District
Antiretroviral therapy (ART) became available in Rakai in 2004. All HIV-positive individuals were referred to care and offered CD4 testing and those who were ART-eligible were offered ART with CD4 and viral load monitoring per WHO criteria, which changed over the course of the study.15 As of 2013, HIV-positive persons were ART eligible if their CD4 cell count was less than or equal to 500 cells/mm3 or at time of diagnosis if pregnant, in a serodiscordant relationship, or identified as a commercial sex worker or fisherfolk.15 By 2015, prevalence of self-reported ART use among all HIV-positive participants was 60%.14
With PEPFAR funding, the Rakai Health Sciences Program has provided free voluntary medical male circumcision (VMMC) to males thirteen years and older per WHO recommendations. Between 1999 and 2016, population prevalence of male circumcision increased from 15 to 59%.14
Laboratory methods
Participants provided finger stick blood for rapid HIV testing and venous blood was collected. Prior to 2011, HIV tests were performed with a laboratory-based ELISA test with confirmation by Western blot. Subsequent tests were performed with a validated parallel three test rapid HIV testing algorithm.16 Discordant results were confirmed by two enzyme immunoassays, EIA (Vironostika HIV-1, BioMerieux, and Recombigen, Cambridge Biotech). Where the EIA results were discordant, Western blots (GS HIV-1 Western Blot, Bio-Rad Laboratories, Redmond, WA, USA, BioMerieux-Vitek) or PCR were used for confirmation of discordant EIAs and all seroconverters.17
Procedures
We defined in-migrants as participants who had moved into an RCCS community at any point during the analysis period with intention to stay. We then estimated duration of residence in a community as the difference between an in-migrant’s self-reported date of arrival to the mid-point of the interval between sequential study visits (i.e., the average length of time spent in that community during a visit interval at risk). Using these data, we first classified participants as either permanent residents with no migration history or in-migrants. Migrants were then further stratified by their length of residence in the community (0–1 years, 1–2 years, 2–3 years, 4–5 years, and greater than five years) as a time-varying variable. Permanent residents with no history of migration were defined as the referent group.
Other covariates included age, education, marital status religion, occupation and male circumcision status. Sexual behaviors included having partners outside the community, number of sexual partners in past year, number of lifetime sexual partners, having non-marital sexual partners, consistent condom use with a non-marital partner, and alcohol use with sex.
The primary outcome was incident HIV infection defined as a first HIV-positive test result preceded by an HIV-seronegative test at the prior visit allowing for only one missed visit. HIV acquisition was assumed to occur at the mid-point of the visit interval.
To assess changes in the risk of HIV acquisition with scale up of combination HIV prevention, (CHP) we constructed a categorical variable of three calendar periods corresponding to phases in the roll-out of ART and VMMC programs. In the earliest calendar period (1994–2004), there was no ART or VMMC programs in Rakai (1999–2004). The second calendar period (2004–2011) corresponded to early CHP scale-up with modest coverage of ART and VMMC. The most recent calendar period included mature CHP programmatic efforts. Median community-level ART and VMMC coverage in the second of the three calendar periods (2004–2011) was 20% and 31%, respectively. In the most recent calendar period (2011–2015), median community-level ART coverage was 62% and male circumcision coverage was 56%.
Statistical analysis
Baseline and sexual behavior characteristics of in-migrants and permanent residents were compared using proportions for categorical and binary variables and median and interquartile ranges (IQR) for continuous variables. Statistical differences were assessed using chi-square tests for categorical and binary variables and Wilcoxon rank sum tests for continuous variables.
The unit of analysis was person-intervals of follow-up. Poisson regression models with generalized estimating equations were used to estimate incidence rate ratios (IRR) of HIV acquisition and 95% confidence interval (95% CI) among in-migrants by duration of residence relative to permanent residents. In multivariate analyses, we first adjusted for demographics and calendar time only and then included high risk sexual behaviors in subsequent analyses. This two-stage approach was taken because sexual behaviors could conceivably operate as either mediators or confounders of the association between migration and HIV acquisition. Analyses were also stratified by age, gender and by CHP calendar period. A sensitivity analysis was performed to account for potential bias due to selective participation and loss to follow up using inverse probability weighting (Appendix p 1).14,18 Lastly, HIV incidence was estimated by place of origin, distance travelled, and reason for migrating in a sub-analysis including only inmigrants. Analyses were performed using STATA (version 14; College Station, Texas) and the R statistical software package (version 3.3.3).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. OO and MKG had full access to all the data and had final responsibility for submission of publication.
Results
Between 1999 and 2013, the mean participation rate of all census eligible persons (n=53,933), including HIV-positive persons and those away for work or school, was 62% (n=103,013 study visits contributed/167,043 census-eligible visits). Participation rates were similar between individuals who had migrated into study communities since the prior survey and residents (61% vs. 63%). Among 26,995 HIV-negative persons who participated in the RCCS survey, 15,187 (56.3%) contributed one or more follow-up visits and were included in our final analysis: 55% (n=8326) were women and 29% (n=4451) were ever in-migrants. In-migrants were predominately female (69%, n=3,055). Overall, participants contributed 66,956 person-visits over 89,292 person-years and 841 incident HIV events were identified.
Reasons for in-migration are shown in Supplementary Table 1 (appendix p 2). Among female in-migrants, movement to live with a friend/relative, a new marriage/consensual relationship, and work were the most common responses. In contrast, men most commonly reported migrating for work (or to start a new household. More than half of all migrations were in-migrants moving from communities elsewhere in Rakai District, accounting for 57% (n=795/1396) and 67% (n=2033/3055) of migrations among men and women, respectively. The most common places of origin outside Rakai District included the neighboring Masaka District and the capital city, Kampala.
Baseline demographics and sexual behaviors
Demographic characteristics at baseline are shown in Table 1. The median age was 23 years for female in-migrants and 22 years for permanent resident women, while in-migrant men were older than permanent resident men (26 vs. 22 years). Among women, 58% (n=1768/3055) of in-migrants were in a monogamous marriage compared to 41% (2178/5271) of permanent residents (p<0.0001). In-migrant men were also more likely to be married (45%, n=634/1396) than permanent resident men (36%, 1974/5465) (p<0.0001). The majority of participants regardless of migration status had a primary education; however, a higher proportion of in-migrants had technical or university education.
Table 1:
Demographic characteristics of in-migrants and residents at baseline in the RCCS, 1999–2015
| Women (n= 8,326) | Men (n = 6,861) | |||||
|---|---|---|---|---|---|---|
| In-migrants N=3055 (37%) |
Residents N=5271 (63%) |
p value | In-migrants N=1396 (20%) |
Residents N=5465 (80%) |
P value | |
| Median Age (IQR) | 23 (18–31) | 22 (19–27) | 0.02 | 26 (21–31) | 22 (17–30) | <0.0001 |
| Age category | ||||||
| 15–19 | 870 (28.5%) | 1736 (32.9%) | 230 (16.5%) | 2186 (40%) | ||
| 20–24 | 1061 (34.7%) | 1178 (22.3%) | 378 (27.1%) | 1050 (19.2%) | ||
| 25–29 | 605 (19.8%) | 881 (16.7%) | <0.0001 | 353 (25.3%) | 841 (15.3%) | <0.0001 |
| 30–34 | 255 (8.3%) | 496 (9.4%) | 191 (13.7%) | 586 (10.7%) | ||
| 35–39 | 139 (4.5%) | 405 (7.7%) | 122 (8.7%) | 368 (6.7%) | ||
| 40 or Older | 125 (4.1%) | 576 (10.9%) | 122 (8.7%) | 434 (7.9%) | ||
| Marital status | ||||||
| Monogamous | 1768 (57.9%) | 2178 (41.3%) | 634 (45.4%) | 1974 (36.1%) | ||
| Never Married | 511 (16.7%) | 1792 (34.0%) | 562 (40.3%) | 2941 (53.8%) | ||
| Polygamous | 373 (12.2%) | 606 (11.5%) | <0.0001 | 106 (7.6%) | 325 (5.9%) | <0.0001 |
| Previously Married | 401 (13.1%) | 692 (13.1%) | 92 (6.6%) | 212 (3.9%) | ||
| Data Missing | 2 (<1%) | 3 (<1%) | 2 (<1%) | 13 (<1%) | ||
| Educational status | ||||||
| Primary | 692 (55.4%) | 3158 (59.9%) | 739 (52.9%) | 3472 (63.5%) | ||
| Secondary | 895 (29.3%) | 1526 (28.9%) | <0.0001 | 342 (24.5%) | 1414 (25.9%) | <0.0001 |
| Technical/University | 313 (10.2%) | 266 (5.0%) | 231 (16.5%) | 334 (6.1%) | ||
| None | 138 (4.5%) | 289 (5.5%) | 60 (4.3%) | 159 (2.9%) | ||
| Data Missing | 17 (<1%) | 32 (<1%) | 24 (1.7%) | 86 (1.6%) | ||
| Religion | ||||||
| Catholic | 1902 (62.3%) | 3503 (66.5%) | 806 (57.7%) | 3619 (66.2%) | ||
| Muslim | 403 (13.2%) | 687 (13.0%) | 164 (11.7%) | 704 (12.9%) | ||
| Protestant | 565 (18.5%) | 879 (16.7%) | <0.0001 | 312 (22.3%) | 895 (16.4%) | <0.0001 |
| Saved/Pentecostal | 145 (4.7%) | 125 (2.4%) | 73 (5.2%) | 126 (2.3%) | ||
| None/Other | 23 (0.7%) | 45 (0.8%) | 17 (1.2%) | 35 (0.6%) | ||
| Data Missing | 17 (<1%) | 32 (<1) | 24 (1.7%) | 86 (1.6%) | ||
| Occupation | ||||||
| Agriculture | 1271 (41.6%) | 2573 (48.8%) | 332 (23.8%) | 1484 (27.1%) | ||
| Administrative/Teaching | 488 (16.0%) | 1312 (24.9%) | 369 (26.4%) | 1889 (34.6%) | ||
| Bar | 62 (2.0%) | 79 (1.5%) | <0.0001 | 6 (0.4%) | 16 (0.3%) | <0.0001 |
| Trading | 377 (12.3%) | 453 (8.5%) | 212 (15.2%) | 744 (13.6%) | ||
| Other | 857 (28.0%) | 854 (16.2%) | 477 (34.2%) | 1332 (24.4%) | ||
| Male Circumcision | ||||||
| No | 1048 (75.1%) | 4438 (81.2%) | ||||
| Yes | 346 (24.8%) | 1012 (18.5%) | <0.0001 | |||
| Data Missing | 2 (<1%) | 16 (<1%) | ||||
Data are number (%). Some percentages do not add up to 100 because of rounding
Selected sexual behaviors of in-migrants and permanent residents stratified by gender are shown in Table 2. In-migrants of both genders generally had higher levels of HIV-related risk behaviors. Among those who reported non-marital partnerships, in-migrants of both genders were less likely to consistently use condoms and were more likely to report having sexual partners who resided outside their community. A greater proportion of in-migrants also reported more sexual partners in the previous year and over their lifetime compared to permanent residents.
Table 2:
Sexual risk behaviors at baseline among in-migrants and long-term residents in the RCCS, 1999–2015
| Women (n=8326) | Men (n= 6861) | |||||
|---|---|---|---|---|---|---|
|
In-migrants No. (%) |
Residents No. (%) |
p. value |
In-migrants No. (%) |
Residents No. (%) |
p. value | |
| Total | 3055 (37) | 5271 (63) | 1396 (20) | 5465 (80) | ||
| Non-marital partnership | ||||||
| No | 2123 (69.5%) | 3627 (68.8%) | 585 (41.9%) | 2710 (49.6%) | < 0.0001 | |
| Yes | 932 (30.5%) | 1644 (31.2%) | 0.52 | 811 (58.1%) | 2755 (50.4%) | |
| Sex with a partner residing outside the community in the past year | ||||||
| Not sexually active in past year | 242 (7.9%) | 1253 (23.8%) | 219 (15.7%) | 1630 (29.8%) | ||
| No | 2097 (68.6%) | 3166 (60.1%) | 621(44.5%) | 2359 (43.2%) | ||
| Yes | 714 (23.4%) | 849 (16.1%) | <0.0001 | 556 (39.8%) | 1474 (27.0%) | <0.0001 |
| Data missing | 2 (<1%) | 3 (<1%) | − | 2 (<1%) | ||
| Consistent condom use with non-marital partner(s) | ||||||
| No | 706 (75.7%) | 1180 (71.8%) | 0.03 | 1546 (60.6%) | 7155 (58.0%) | 0.01 |
| Yes | 226 (24.2%) | 464 (28.2%) | 1003 (39.3%) | 5178 (42.0%) | ||
| Number of lifetime sexual partner | ||||||
| 1 | 655 (21.4%) | 1409 (26.7%) | 125 (8.9%) | 563 (10.3%) | ||
| 2–3 | 1711 (56.0%) | 2250 (42.7%) | 324 (23.2%) | 1162 (21.3%) | ||
| 3–5 | 451 (14.8%) | 616 (11.7%) | 275 (19.7%) | 864 (15.8%) | ||
| Greater than 5 | 138 (4.5%) | 201 (3.8%) | <0.0001 | 359 (25.7%) | 1073 (19.6%) | <0.0001 |
| Can’t remember/Unknown | 22 (0.7%) | 61 (1.2%) | 215 (15.4%) | 650 (11.9%) | ||
| Not sexually active | 71 (2.3%) | 708 (13.4%) | 96 (6.9%) | 1131 (20.7%) | ||
| Data Missing | 7 (<1%) | 26 (<1%) | 2 (<1%) | 22 (<1%) | ||
| Number of different sexual partners in the past year | ||||||
| 1 | 2485 (81.3%) | 3807 (72.2%) | 621 (44.5%) | 2149 (39.3%) | ||
| 2–3 | 313 (10.2%) | 208 (3.9%) | 484 (34.7%) | 1444 (26.4%) | ||
| 3–5 | 4 (0.1%) | 3 (0.06%) | <0.0001 | 46 (3.3%) | 148 (2.7%) | <0.0001 |
| Greater than 5/Can’t Remember | 2 (0.07%) | 2 (0.04%) | 28 (2.0%) | 102 (1.9%) | ||
| No sex in past year/Not sexually active | 249 (8.1%) | 1248 (23.7%) | 217 (15.5%) | 1622 (29.7%) | ||
| Data Missing | 2 (<1%) | 3 (<1%) | − | 2 (<1%) | ||
| Alcohol with sex1 | ||||||
| No | 2325 (77.9%) | 3430 (75.2%) | 918 (70.6) | 2900 (66.9%) | ||
| Yes | 654 (21.9%) | 1127 (24.7%) | 0.02 | 376 (28.9) | 1424 (32.9%) | 0.01 |
| Data Missing | 5 (<1%) | 6 (<1%) | 6 (<1) | 10 (<1%) | ||
Some percentages do not add up to 100 because of rounding.
Analysis restricted to participants who reported sexual activity.
HIV incidence by migration status
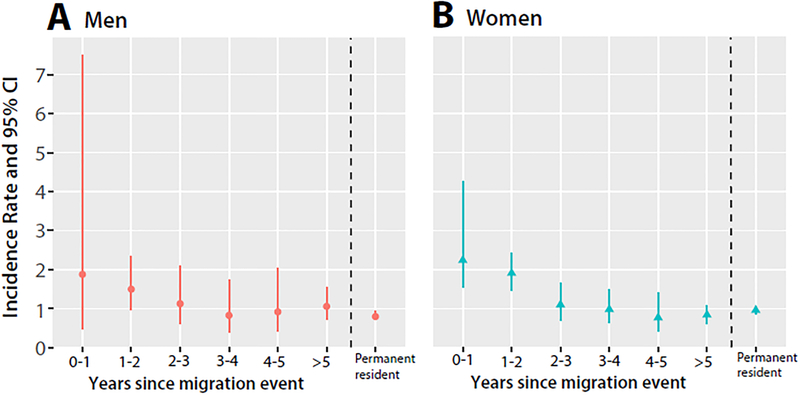
There were 172 HIV incident events among ever in-migrant women accounting for 35% of all new female infections (n=487), and of these 41% (n=70/172) occurred within the first two years following migration. HIV incidence among recent in-migrant women during the first and second year after in-migration was higher than in permanent resident women. However, after two years of living in the community, HIV incidence among longer-term in-migrants was similar to that of permanent residents (Figure 1 and supplementary table 2; appendix p 3). As shown in Table 3, during the first two years after in-migration HIV incidence among in-migrant women was 1.92/100 py, compared to 0.93/100 py among resident women (adjIRRdem+ sex=2.35, 95%CI 1.61–3.42).
Figure 1. HIV incidence by year since migration among in- migrants and permanent residents, stratified by gender.
HIV incidence was elevated among in-migrant men (A) and women (B) in the first two years following the migration event and declined thereafter.
Table 3:
HIV incidence and crude and adjusted HIV incidence rate ratios by migrant and resident status among women and men
| Resident Status | No of events/ py* at risk |
Incidence rate per 100 py (95%CI) |
Crude IRR (95% CI) |
Demographics only adjIRR (95%) |
Demographics+sexual behaviors adjIRR (95% CI) |
|---|---|---|---|---|---|
| Women (N=8,326) | |||||
| Permanent residents | 315/33730 | 0.93 (0.84, 1.04) | Ref. | Ref. | Ref. |
| In-migrants (time since arrival) | |||||
| 0–2 years | 70/3646 | 1.92 (1.52, 2.43) | 2.04 (1.58, 2.63) | 1.75 (1.33, 2.33) | 2.35 (1.61, 3.42) |
| > 2 years | 102/11703 | 0.87 (0.72, 1.06) | 0.93 (0.75, 1.16) | 0.94 (0.74, 1.19) | 0.99 (0.69, 1.42) |
| Men (N= 6,861) | |||||
| Permanent residents | 283/33849 | 0.84 (0.74, 0.94) | Ref. | Ref. | Ref. |
| In-migrants (time since arrival) | |||||
| 0–2 years | 21/1384 | 1.52 (0.99, 2.33) | 1.81 (1.17, 2.81) | 1.74 (1.12, 2.71) | 1.89 (1.12, 3.19) |
| > 2 years | 50/4980 | 1.00 (0.76, 1.32) | 1.20 (0.89, 1.62) | 1.28 (0.94, 1.74) | 1.25 (0.84, 1.87) |
| Women and Men (N=15,187) | |||||
| Permanent residents | 598/67579 | 0.88 (0.82, 0.96) | Ref. | Ref. | Ref. |
| In-migrants (time since arrival) | |||||
| 0–2 years | 91/5031 | 1.81 (1.47, 2.22) | 2.03 (1.64, 2.53) | 1.76 (1.40, 2.23) | 2.10 (1.56, 2.83) |
| > 2 years | 152/16682 | 0.91 (0.78, 1.07) | 1.02 (0.86, 1.23) | 1.00 (0.83, 1.21) | 1.03 (0.79, 1.35) |
IRR=incidence rate ratio; adjIRR=adjusted incidence rate ratio.
person-years;
no migration history. Model adjusted for demographics included the following variables in addition to migration history: age in years, marital status, education, religion, time period, occupation and ma le circumcision; Models adjusted for sexual behaviors included sex with partners residing outside community in the past year, number of different sexual partners in the past year, number of lifetime sexual partner, number of non-marital partners, consistent condom use with a non-marital partner and alcohol use with sex; Bolded estimates are statistically significant at p < 0.05
We identified 71 incident HIV events among in-migrant men which accounted for 20% of all male HIV incident cases (n=354). HIV incidence in the first and second years following male migration was higher than in permanent resident males (Figure 1 and supplementary table 2; appendix p 3). Over the first two years following migration, male recent in-migrant HIV incidence was 1.52/100py compared to 0.84/100 py in permanent resident men (adjIRRdem+ sex= 1.89, 95% CI: 1.56–3.19). Beyond two years after migration, HIV incidence was comparable to that of male permanent residents. Sensitivity analyses with inverse probability weights did not change these inferences among men or women (Supplementary table 3; appendix p 4).
HIV risk among in-migrants and permanent residents was also stratified by ten-year age groups (15–24, 25–34, 35 years and older; Supplementary table 4; appendix p 5). Among women, there was higher HIV incidence among recent in-migrants compared to permanent residents in all age groups. However, this disparity was concentrated in the two older age groups and increased with age. There was no evidence for effect modification by age on the association between migration and HIV incidence among men.
Changes in HIV risk by migration status with combination HIV prevention scale-up
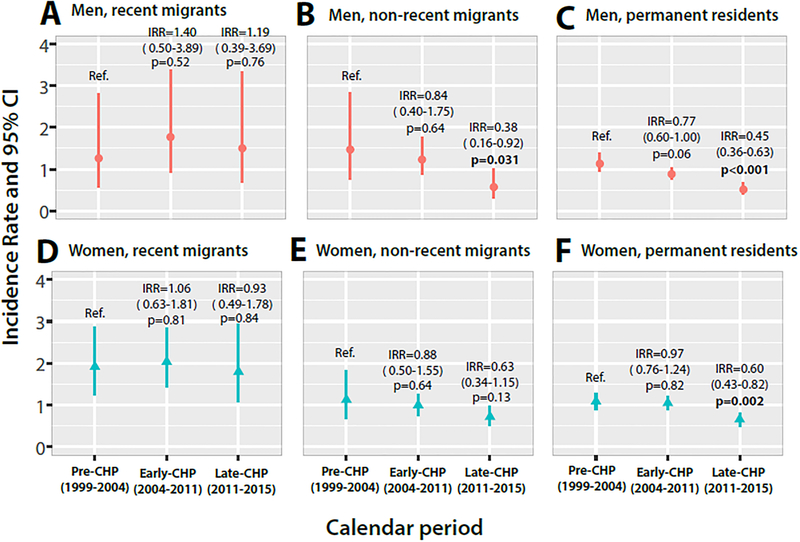
To assess the impact of combination HIV prevention scale-up on HIV incidence among in-migrants and permanent residents, we conducted a stratified analysis by calendar period (Figure 2, Supplementary table 5; appendix p 6). HIV incidence substantially declined in permanent residents and non-recent migrants with greater coverage of ART and VMMC, but incidence remained persistently high among recent in-migrant men and women. Prior to combination prevention scale-up (pre-CHP; 1999–2004), HIV incidence was 1.89/100 py among recent in-migrant women and 1.26/100 py among recent in-migrant men compared to 1.06/100 py among permanent resident women (adjIRRdem=1.63, 95%CI: 1.01–2.62) and 1.13/100 py among permanent resident men (adjIRRdem=1.17; 95%CI: 0.52–2.64). In the most recent calendar period with mature ART and VMMC programs (2011–2015), HIV incidence was 1.77/100 py in recent in-migrant women and 1.50/100 py in recent in-migrant men compared to 0.63/100 py in resident women (adjIRRdem=2.15; 95%CI: 1.15–4.00) and 0.51/100 py in men (adjIRRdem=2.47; 1.06–5.75). After adjustment, HIV incidence declined by 32% in resident women (adjIRRdem0.68; 95%CI: 0.47–0.94) and 47% in resident men (adjIRRdem=0.53; 95%CI: 0.37–0.76) between the pre-CHP period and mature CHP scale-up. There were no significant changes in HIV incidence among recent in-migrants of either gender.
Figure 2. HIV incidence among recent in-migrants (≤ 2 years), non-recent in-migrants (>2 years), and permanent residents stratified by gender and combination HIV prevention (CHP) scale-up calendar period.
HIV incidence declined in permanent resident and non-recent in-migrant women and men, but not in recent in-migrants with CHP scale-up.
Risk of HIV among migrant sub-groups
HIV incidence rates by reason for in-migration, place of origin, and distance traveled, stratified by gender are shown in Supplementary table 6 (appendix p 7). HIV incidence was highest among women moving to start a new household and men who were newly married. We observed no trends in HIV risk by distance traveled or place of origin.
Discussion
In this longitudinal population-based study, we find that in-migrants higher levels of sexual risk behaviors and HIV incidence following migration to a new community compared to permanent residents with no history of migration. We also show that this elevated HIV incidence among in-migrants is concentrated during the first two years following migration. Despite significant HIV incidence declines in residents with scale-up of ART and VMMC programs, HIV incidence has remained persistently high among recent migrants. To our knowledge, this is the first study to use longitudinal population-based data to assess the duration of time during which migrants are at risk for HIV following migration or risk before and during combination HIV prevention scale-up in sub-Saharan Africa.
We observed significantly higher levels of high risk sexual behaviors among in-migrants compared to residents, which is consistent with previous studies showing migrants typically have higher HIV risk profiles.3,11,19 Both male and female in-migrants reported more sexual activity, greater proportions of lifetime sexual partners, non-marital partnerships and sex with partners outside the community compared to permanent residents. They were also less likely to report consistent condom use with a non-marital partner. An earlier study from Rakai found that sex with extra-community partners was associated with substantially increased risk among women.17
In-migrants also had significantly higher HIV risk compared to permanent residents but only during the first two years following migration. Similar findings were reported in a cross-sectional study of HIV prevalence in Tanzania which found that the odds of HIV infection were significantly higher among recent migrants but not non-recent migrants as compared to residents.4 This higher HIV risk among migrants soon after moving may be due in part to higher levels of sexual risk behaviors during the migration process; however, we found migration remained associated with increased HIV acquisition even after adjustment for sexual behaviors suggesting causal pathways independent of individual-level behaviors. The immediate period after migration is associated with instability, detachment from family, friends and previous community and less constrained by social norms governing risk behaviors. Migrant women may be vulnerable to sexual exploitation and male migrant workers (e.g., fishermen) may congregate in high risk environments with exposure to commercial sex and alcohol.20,21 Surprisingly, the excess HIV incidence observed among migrant women was largely concentrated in women over 25 years, whereas young African women 15–24 years are at highest risk for HIV in sub-Saharan Africa overall.22 We did not observe a relationship between distance traveled and HIV risk unlike a prior study of circular (i.e. return) migration in KwaZulu-Natal, South Africa which found higher risk with greater distances migrated. 10
Our results, taken together with prior research from Rakai and elsewhere in sub-Saharan Africa, provide support for each of the three hypotheses potentially explaining the higher HIV prevalence observed among migrants. In this study, we find that HIV-negative migrants have higher levels of risky sexual behaviors (hypothesis 1). Earlier studies from Uganda and Malawi also show that HIV-positive individuals are more likely to migrate compared to HIV-negative persons (hypothesis 2).8,23 Furthermore, we find that migrants are more likely to acquire HIV within the first two years following migration compared to residents with no migration history, suggesting that HIV is a consequence of migration, at least in some cases (hypothesis 3).
Contrary to our initial hypothesis and of critical importance to ongoing HIV control efforts, we found that HIV incidence in recent migrants remained persistently higher than in permanent residents or non-recent migrants of both sexes, despite substantial scale-up of CHP services. These results suggest that prevention programs have had minimal impact in recently migrating populations. This may be because services in destination locations fail to reach recent migrants upon arrival or because migrants are dissociated from treatment and HIV prevention services at their place of origin. Migrants might also maintain sexual relationships with outside partners in areas with lower service coverage or initiate relationships with riskier partners less likely to use combination HIV prevention and treatment services. While we found no significant differences in circumcision levels among migrant men in this study, a prior analysis of the HIV care cascade conducted from 2013 to 2015 in Rakai found that HIV positive inmigrants were significantly less likely to be enrolled in HIV care and on ART.24 Assortative sexual mixing within the migrant community, which has lower levels of viral suppression, could explain some of the increased risk of HIV acquisition among migrants.
From the broader public heath perspective, our findings imply that identifying recent migrants and effectively linking them to prevention and treatment services in a timely manner is important for HIV control. Pre-exposure prophylaxis (PrEP) may be particularly important for HIV-negative migrants; however, PrEP is not yet widely available in sub-Saharan Africa. Programmatic tools to rapidly identify migrants and link them to HIV services are also lacking. Application of mobile technology to access hard-to-reach mobile population may be an effective strategy.25 Traveling community health workers have been utilized in some settings to increase retention of HIV-positive persons in treatment and care and may be effective for engaging migrant populations irrespective of HIV status.26 Cultural or language barriers and social dislocation facing migrants in their new communities, as well as participatory approaches including migrant populations, should be considered during planning and implementation of services.27
There are limitations to this study. Demographic and sexual behavior data were self-reported and may be subject to measurement error or desirability bias. Lack of study participation and loss to follow-up may have biased results; however, sensitivity analysis using inverse probability weights to account for selective participation did not affect our inferences. While we adjusted for demographics and sexual behaviors, we cannot rule out the possibility of residual confounding due to unmeasured or poorly measured confounding variables. Given there is a short period following infection that antibodies to HIV are not detected in the blood, there is also a possibility that a small number of participants may have been infected prior to migration. However, of the 243 incident cases among in-migrants, only two occurred in persons who had arrived in the prior month. We did not assess circular migration or identify migrants who moved in and out of study communities between censuses, so our results might not apply to all migrant populations. Results from this study may not be generalizable to other settings; although, we know of no unique social or cultural practices related to migration in Rakai and RCCS demographic and behavioral data are similar to results from other surveys conducted in south-central Uganda.28 Migration patterns in Rakai are also similar to other rural African settings in so much that migration is pervasive and more common among women.7,20 Furthermore, results from PEPFAR’s Population-based HIV Impact Assessments survey in Uganda and other countries suggest that scale-up of combination HIV prevention is occurring continent-wide.29
In conclusion, we find that the first two years following migration are associated with substantially increased HIV risk and that HIV incidence remains high among migrant populations compared to residents despite scale-up of combination HIV prevention programs. Active surveillance, to identify migrant populations and to engage them in prevention and treatment programs is urgently needed to reduce the burden of HIV in sub-Saharan Africa.
Research in context
Evidence before this study
We searched PubMed for studies on HIV among migrants in sub-Saharan Africa published up to August 22, 2017. Key search terms included “HIV”, “human mobility”, “migration”, and “migrant”. No language limitations were set. Since the early 1990s, numerous studies have reported on higher HIV sexual risk behaviors among migrants. However, only two studies, conducted in Malawi and South Africa, have assessed HIV incidence among migrants. Neither of these studies assessed the duration of time that individuals were at increased risk for infection following migration or changes in HIV risk among migrants over calendar time with the scale-up of combination HIV prevention programs.
Added value of this study
This study is the first prospective study to provide evidence that there is increased risk of HIV acquisition among sub-Saharan African migrants in the first two years following migration relative to permanent residents with no migration history and non-recent migrants regardless of gender. Additionally, we show that despite scale up of prevention interventions in destination locations, HIV incidence has not declined among recent migrants in contrast to trends of declining HIV incidence observed among permanent residents and non-recent migrants.
Implications of all the available evidence
The available evidence suggests the need for better understanding of HIV transmission dynamics among migrant populations in sub-Saharan Africa and for early identification and linkage of recent migrants to appropriately tailored HIV prevention programs.
Supplementary Material
Acknowledgements
Presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, February 13–17, 2017. Supported by the National Institute of Mental Health (R01MH107275), the National Institute of Allergy and Infectious Diseases (R01AI110324, U01AI100031, U01AI075115, R01AI110324, R01AI102939, R01AI128779, K01AI125086–01), the National Institute of Child Health and Development (RO1HD070769, R01HD050180), the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases, the World Bank, the Doris Duke Charitable Foundation, the Bill & Melinda Gates Foundation (#08113, 22006.02), the Johns Hopkins University Center for AIDS Research (P30AI094189), and the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention (NU2GGH000817). We also appreciate data management support provided in part by the Office of Cyberinfrastructure and Computational Biology (OCICB) at the National Institute for Allergy and Infectious Diseases. The findings and conclusions in this report are those of the authors and do not represent the official position of the funding agencies.
We thank the cohort participants and the many staff and investigators who have made this study possible over these many years.
Funding: National Institute of Mental Health, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Development, the National Institute for Allergy and Infectious Diseases Division of Intramural Research, National Institutes of Health; the Bill & Melinda Gates Foundation; and the Johns Hopkins University Center for AIDS Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests.
References
- 1.Deane KD, Parkhurst JO, Johnston D. Linking migration, mobility and HIV. Trop Med Int Health. 2010;15(12):1458–1463. [DOI] [PubMed] [Google Scholar]
- 2.Mann JM, Francis H, Quinn T, et al. Surveillance for AIDS in a Central African city. Kinshasa, Zaire. JAMA. 1986;255(23):3255–3259. [PubMed] [Google Scholar]
- 3.Kishamawe C, Vissers DC, Urassa M, et al. Mobility and HIV in Tanzanian couples: Both mobile persons and their partners show increased risk. AIDS. 2006;20(4):601–608. [DOI] [PubMed] [Google Scholar]
- 4.Mmbaga EJ, Leyna GH, Hussain A, Mnyika KS, Sam NE, Klepp K. The role of in-migrants in the increasing rural HIV-1 epidemic: Results from a village population survey in the Kilimanjaro region of Tanzania. International Journal of Infectious Diseases. 2008;12(5):519–525. [DOI] [PubMed] [Google Scholar]
- 5.Nunn AJ, Wagner HU, Kamali A, Kengeya-Kayondo JF, Mulder DW. Migration and HIV-1 seroprevalence in a rural Ugandan population. AIDS. 1995;9(5):503–506. [PubMed] [Google Scholar]
- 6.Petersen W A general typology of migration. Am Sociol Rev. 1958;23(3):256–266. [Google Scholar]
- 7.Schuyler AC, Edelstein ZR, Mathur S, et al. Mobility among youth in Rakai, Uganda: Trends, characteristics, and associations with behavioural risk factors for HIV. Glob Public Health. 2015:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anglewicz P, VanLandingham M, Manda-Taylor L, Kohler HP. Migration and HIV infection in Malawi. AIDS. 2016;30(13):2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soskolne V Social networks, social capital, and HIV risks among migrants In: Population mobility and infectious disease. Boston, MA: Springer US; 2007:55–72. 10.1007/978-0-387-49711-2_4.10.1007/978-0-387-49711-2_4. [DOI] [Google Scholar]
- 10.Dobra A, Barnighausen T, Vandormael A, Tanser F. Space-time migration patterns and risk of HIV acquisition in rural South Africa. AIDS. 2017;31(1):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagarde E, Schim van der Loeff M, Enel C, et al. Mobility and the spread of human immunodeficiency virus into rural areas of West Africa. Int J Epidemiol. 2003;32(5):744–752. [DOI] [PubMed] [Google Scholar]
- 12.Chang LW, Grabowski MK, Ssekubugu R, et al. Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: An observational epidemiological study. Lancet HIV. 2016;3(8):e388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in uganda: A randomised community trial. rakai project study group. Lancet. 1999;353(9152):525–535. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med. 2017;377(22):2154–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uganda Ministry of Health. Addendum to the national antiretroviral treatment guidelines. 2013.
- 16.Galiwango RM, Musoke R, Lubyayi L, et al. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. J Virol Methods. 2013;192(1–2):25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabowski MK, Lessler J, Redd AD, et al. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: Evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med. 2014;11(3):e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lydie N, Robinson NJ, Ferry B, et al. Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. J Acquir Immune Defic Syndr. 2004;35(1):67–74. [DOI] [PubMed] [Google Scholar]
- 20.Camlin CS, Kwena ZA, Dworkin SL, Cohen CR, Bukusi EA. “She mixes her business”: HIV transmission and acquisition risks among female migrants in western kenya. Soc Sci Med. 2014;102:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubega M, Nakyaanjo N, Nansubuga S, et al. Risk denial and socio-economic factors related to high HIV transmission in a fishing community in Rakai, Uganda: A qualitative study. PLoS One. 2015;10(8):e0132740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2013. 2013.
- 23.Sully E, Reniers G, Kasamba I. HIV, marital dissolution and migration: A longitudinal analysis of differential risk of migration by sero-status in rural Uganda. 2011. [Google Scholar]
- 24.Billioux VG, Chang LW, Reynolds SJ, et al. Human immunodeficiency virus care cascade among sub-populations in Rakai, Uganda: An observational study. J Int AIDS Soc. 2017;20(1):21590. doi: 10.7448/17582652.2017.1333701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell ML. Interventions to increase antiretroviral adherence in sub-Saharan Africa: A systematic review of evaluation studies. Lancet Infect Dis. 2011;11(12):942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: A qualitative study. PLoS Med. 2013;10(1):e1001369; discussion e1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS. 2015;10(6):430–438. [DOI] [PubMed] [Google Scholar]
- 28.Uganda Bureau of Statistics (UBOS) and ICF International Inc. Uganda demographic and health survey 2011. 2012.
- 29.PHIA. Population-based HIV impact assessments. http://phia.icap.columbia.edu/resources/. Updated 2017. Accessed 10/19, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.