Abstract
Background
We evaluated whether routine biannual sexually transmitted disease (STD) testing coupled with brief risk-reduction counseling reduces STD incidence and high-risk behaviors.
Methods
The SUN study is a prospective observational HIV cohort study conducted in 4 US cities. At enrollment and every 6 months thereafter, participants completed a behavioral survey and were screened for STDs, and if diagnosed, were treated. Medical providers conducted brief risk-reduction counseling with all patients. Among men who have sex with men (MSM), we examined trends in STD incidence and rates of self-reported risk behaviors before and after exposure to the risk-reduction intervention. The “preintervention” visit was the study visit that was at least 6 months after enrollment STD screening and treatment and at which the participant was first exposed to the intervention. The “postintervention” visit was 12 months later.
Results
Among 216 MSM with complete STD and behavioral data, median age was 44.5 years; 77% were non-Hispanic white; 83% were on highly active antiretroviral treatment; 84% had an HIV RNA level <400 copies/mL and the median CD4 (cluster of differentiation 4) count was 511 cells/mm3. Twelve months after first exposure to the risk-reduction intervention, STD incidence declined from 8.8% to 4.2% (P = 0.041). Rates of unprotected receptive or insertive anal intercourse with HIV-positive partners increased (19% to 25%, P = 0.024), but did not change with HIV-negative partners or partners of unknown HIV status (24% to 22%, P = 0.590).
Conclusions
STD incidence declined significantly among HIV-infected MSM after implementing frequent, routine STD testing coupled with risk-reduction counseling. These findings support adoption of routine STD screening and risk-reduction counseling for HIV-infected MSM.
Men who have sex with men (MSM) have historically been at high risk for acquiring sexually transmitted diseases (STDs), including HIV infection.1 Recently, in the United States and Europe, rates of syphilis, gonorrhea, and chlamydia have increased among MSM with a concomitant rise in rates of unsafe sex behaviors.2,3 The Centers for Disease Control and Prevention (CDC) STD treatment guidelines recommend that sexually active MSM receive routine STD/HIV risk assessment and prevention counseling to reduce the risk of STD and HIV transmission, and be screened annually for HIV infection, syphilis, gonorrhea, and chlamydia. MSM who have multiple or anonymous partners, who have sex in conjunction with alcohol and drug use or whose sex partners participate in these activities should be screened more frequently (i.e., at 3- to 6-month intervals).4 Despite these guidelines, some providers may not offer routine screening and counseling due to time constraints or to personal discomfort discussing homosexual behavior, thereby missing opportunities to diagnose and treat asymptomatic STDs.5,6 Interventions are needed to improve STD prevention counseling and screening among MSM in clinical settings.
Recent research has demonstrated that a brief, structured, prevention intervention called “Partnership for Health” delivered by HIV health care providers during routine medical visits reduced self-reported sexual transmission risk behaviors in HIV-infected patients.7 Between 2005 and 2007, we evaluated whether routine biannual STD testing coupled with the Partnership for Health intervention reduced the incidence of STDs and high-risk behaviors among HIV-infected MSM in care who enrolled in the study to understand the natural history of HIV and AIDS in the era of effective therapy (the SUN Study).
METHODS
Study Population
SUN Study
The SUN study is a prospective, observational cohort study funded by the CDC that monitors the clinical course of HIV-infected individuals treated with highly active antiretroviral treatment (HAART) at 7 HIV-specialty clinics in 4 US cities: St. Louis, MO; Providence, RI; Minneapolis, MN; and Denver, CO. Seven hundred HIV-infected patients were enrolled between March 1, 2004 and June 30, 2006. The study’s design and methods have been described previously.8 Participants were generally healthy HIV-infected adults receiving routine outpatient care and were either treatment naïve, or had been treated with HAART without previous exposure to mono or dual therapy. Patient data, including sociodemographic characteristics, all diagnoses and treatments (including dosage and duration of all medications), and all clinical laboratory data were abstracted from medical charts and entered into an electronic database (Clinical Practice Analyst; Cerner Corporation, Vienna, VA) by trained staff.
Additional data were collected through biannually scheduled study visits, which included physical examination, comprehensive testing for STDs, and an audio computer-assisted self-interview (ACASI) that collected behavioral risk data and other health-related information including use of tobacco, alcohol, and recreational drugs. Men were categorized as MSM if at the study enrollment ACASI they reported any history of sex with a man. The study protocol was approved and has been reviewed annually by the CDC and each participating site’s institutional review board.
Testing for STDs
At study enrollment (i.e., baseline) and every 6 months thereafter, all male participants were screened for Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections, using the APTIMA COMBO 2 nucleic acid amplification assay (Gen-Probe, Inc; San Diego, CA) with both oropharyngeal and rectal swabs. These tests were conducted centrally at CDC. Genitourinary GC and CT testing of urine specimens were performed using the Food and Drug Administration-approved commercial method, at each site’s local clinical laboratory. Sera were tested for syphilis at each site using nonspecific assays (e.g., Venereal Disease Research Laboratory test, rapid plasma reagin) with reflex confirmation assays (e.g., MHA-TP [microhemagglutination assay], FTA-ABS [fluorescent treponemal antibody absorption test]) if positive. Titers of nontreponemal tests were examined to determine syphilis incidence. Symptom data related to oropharyngeal, rectal, and genitourinary infection with GC and CT were collected at the time of specimen collection on specimen collection forms. All diagnosed STDs were treated as per the standard of care.
Intervention: Brief Risk-Reduction Counseling by Providers
Medical providers conducted brief risk-reduction counseling at each clinical visit with all patients in their practices, including SUN participants. Prevention messages were framed to emphasize the negative health consequences of unsafe sex and thereby instill motivation to engage in safer sex to protect patients’ and their partners’ health. Messages were presented to patients in written form and then reinforced by provider counseling during the patient’s medical examination and at every subsequent clinical encounter. The intervention materials consisted of posters, brochures, and flyers with additional information for patients, and pocket guides and training videos for providers. All clinic staff (including support staff) received a 4-hour group training before implementing the intervention. The training was conducted by an experienced 2-person team and consisted of lecture, audio-visual, small-group activities, and role-playing exercises to simulate patient-provider interactions. The prevention intervention was introduced after clinics achieved at least 80% of their SUN Study enrollment goal and therefore adequate baseline data on STD incidence and reported risky sexual behavior had been collected. Because local study sites enrolled participants at different rates, the introduction of the prevention intervention at different clinics was staggered. A booster training session, which offered additional training and a chance to ask questions and clarify procedures, was conducted at each clinic 1 month after the intervention began.
Statistical Analysis
We examined trends in STD incidence among the cohort’s MSM as well as their self-reported sexual risk behaviors at visits before and after exposure to the Partnership for Health intervention. The “preintervention” visit was the first scheduled study visit at which the participant was exposed to the intervention and that was at least 6 months after baseline STD screening at the time of SUN study enrollment. The “postintervention” visit was the scheduled study visit 12 months after the preintervention visit. An incident STD was defined as a new STD diagnosis (i.e., no history of that STD or evidence of successful treatment such as a subsequent negative test result) at the preintervention and postintervention study visit. ACASI data from the preintervention and postintervention visits were examined to describe behavior change. We focused on unprotected insertive and receptive anal intercourse in the past 6 months with partners perceived to be HIV-negative, HIV-positive, or of unknown serostatus status. The χ2 or Fisher exact test was used to compare categorical variables and Student t test or the Kruskal-Wallis test was used to compare continuous variables in univariate analysis. All statistical analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
Among the 425 MSM enrolled in the SUN study, 216 (51%) had complete STD and behavioral data collected at both the preintervention and postintervention visits and were included in this analysis. The average time interval per individual between the SUN enrollment visit and the preintervention visit was 21.1 (range: 3.3–25.5) months. Of the 209 MSM who were excluded, 124 (59%) did not have a preintervention visit, 60 (29%) did not have a postintervention visit, and 25 (12%) had incomplete testing results. At the preintervention visit, median age was 44.5 years (interquartile range [IQR], 38–50 years), 77% were of non-Hispanic white race/ethnicity, the median cluster of differentiation 4 (CD4) cell count was 511 cells/mm3 (IQR, 352–685 cells/mm3), the median nadir CD4 cell count was 202 cells/mm3 (IQR, 99.5–321 cells/mm3), 84% had undetectable plasma HIV RNA (<400 copies/mL), 83% were currently prescribed HAART, and 75% were currently sexually active (anal or vaginal intercourse or oral sex) (Table 1). The 216 MSM included in the analysis were not significantly different from 209 MSM excluded from the analysis in terms of age, race, and high-risk sexual and drug using behaviors; however, the 216 MSM included in the analysis were more likely to use methamphetamine (11% vs. 5%, P = 0.03). During the 12-month study period, only 3% of the participants who were sexually active at the preintervention visit reported no sexual activity. Among the 25% of MSM who were not sexually active at the preintervention visit, 28 (53%) reported becoming sexually active during the 12-month study period. Participants’ sexual risk and drug use behaviors are described in the Table 1.
TABLE 1.
Characteristics of Men Who Have Sex With Men (MSM) in the SUN Study Who Were Exposed to the Partnership for Health Intervention, 2005–2007
| Characteristics at Preintervention Visit | Total (N = 216) |
|---|---|
| Median age, yr (IQR) | 44.5 (38–50) |
| Race/ethnicity (n, %) | |
| Non-Hispanic white | 167 (77) |
| Non-Hispanic black | 24 (11) |
| Hispanic | 23 (11) |
| Other | 2 (1) |
| Median CD4 count, cells/mm3 (IQR) | 511 (352–685) |
| Nadir CD4 count, cells/mm3 (IQR) | 202 (99.5–321) |
| Undetectable viral load (n, %) | 182 (84) |
| Using HAART (n, %) | 179 (83) |
| Sexually active (n, %) | 163 (75) |
| Any unprotected vaginal or anal intercourse (n, %) | 78 (36) |
| Unprotected anal intercourse (n, %) | |
| Receptive | 60 (28) |
| Insertive | 53 (25) |
| Unprotected anal intercourse with HIV-positive partners (n, %) | |
| Receptive | 30 (14) |
| Insertive | 34 (16) |
| Unprotected anal intercourse with HIV-negative or unknown status partners (n, %) | |
| Receptive | 40 (19) |
| Insertive | 31 (14) |
| One STD (n, %) | 17 (8) |
| More than 1 STD (n, %) | 2 (12) |
| Drug use in previous 6 mo (n, %) | |
| Any drug use other than marijuana | 81 (38) |
| Methamphetamine | 16 (7) |
| Inhaled nitrites | 67 (31) |
| Erectile dysfunction medication | 46 (21) |
| Alcohol use (n, %) | |
| At least one drink in past 30 d | 169 (78) |
| >2 drinks per day | 123 (58) |
| >4 drinks per week | 82 (38) |
| Never had more than 5 drinks on occasion in the past 30 d | 147 (69) |
| Self-reported depression (n, %) | 103 (48) |
STDs examined in this analysis: rectal chlamydia, oral chlamydia, oral gonorrhea, rectal gonorrhea, and syphilis.
IQR indicates interquartile range; STD, sexually transmitted disease; HAART, highly active antiretroviral therapy; CD4, cluster of differentiation 4.
STD Incidence
Twelve months after first exposure to the Prevention for Health intervention, overall STD incidence declined from 8.8% to 4.2% (P = 0.041) and incidence of anorectal STDs declined from 6.9% to 2.8% (P = 0.039) (Fig. 1). We examined STD incidence at all study visits before the initiation of the intervention and at 6 months after intervention onset but did not see any significant declines. Among a small subset of participants (n = 92) who attended two 6-monthly study visits during the preintervention phase, STD incidence rates were likewise not significantly different between these visits and at 6 months after the intervention (10% vs. 8% vs. 8%, P = 0.405), but had declined to 2% (P = 0.011) at 12 months postintervention. Of the 19 MSM who had an STD at the preintervention visit, 17 (89%) remained free of STDs at the postintervention visit 12 months later. Of the 197 MSM who did not have an STD at the preintervention visit, 7 (3.5%) were diagnosed with an STD at the postintervention visit. Two men (1%) had an STD at both the preintervention and postintervention visits. Of the 28 STDs identified during the analysis period, only 3 (11%) were symptomatic.
Figure 1.
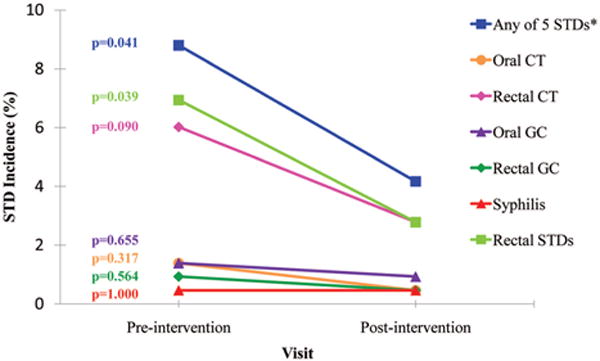
A, Change in STD incidence from pre- to postintervention among men who have sex with men in the SUN study, 2005–2007. *Any 5 STDs refers to any of the 5 STDs examined in this analysis: rectal CT, oral CT, oral GC, rectal GC, and syphilis. STD indicates sexually transmitted diseases; CT, chlamydia; GC, gonorrhea.
Rates of Reported Risk Behaviors
Prevalence of any unprotected vaginal or anal intercourse remained constant (36% to 38%, P = 0.602) from pre-to postintervention visits. Notably, the rates of unprotected receptive or insertive anal intercourse with HIV-positive partners increased (19% to 25%, P = 0.024) but did not change with HIV-negative partners or with partners of unknown HIV status (24% to 22%, P = 0.590). Examined separately, the self-reported rates of unprotected receptive and insertive anal intercourse with HIV-negative partners remained stable—10% to 8% (P = 0.549) and 6% to 6%, (P = 1.000), respectively. The rate of unprotected receptive and insertive anal intercourse with HIV unknown status partners also remained constant: (11% to 11%, P = 0.842) and (12% to 13%, P = 0.808), respectively. The rate both of unprotected receptive and insertive anal intercourse with HIV-positive partners demonstrated a modest increase of 14% to 18%, (P = 0.106) and 16% to 19%, (P = 0.178), respectively.
There were no significant differences in the rates of risky sexual, alcohol use, and drug use behaviors reported by MSM at the preintervention visit compared with the postintervention visit 12 months later by STD status. Of 9 men diagnosed with an STD at the postintervention visit, 6 (67%) reported no unprotected (i.e., without a condom) vaginal or anal intercourse in the previous 6 months, and only 1 man with an STD at the postintervention visit reported an increased number of sex partners. Among the 17 men who had an STD at the preintervention visit but remained STD-free at the postintervention visit, rates of unprotected anal intercourse modestly declined, specifically unprotected receptive anal intercourse (Fig. 2).
Figure 2.
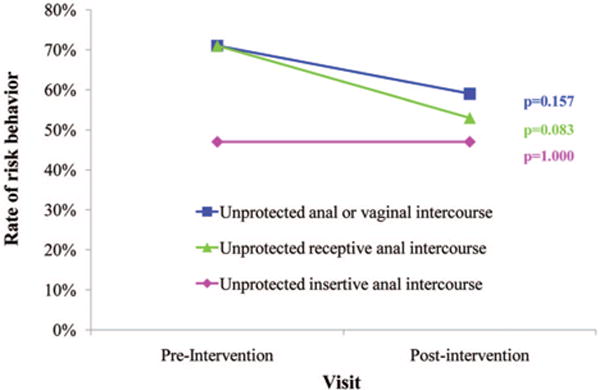
Change in rates of sexual risk behaviors among men who have sex with men who had an STD at the preintervention visit but not at the postintervention visit (n 17), SUN study, 2005–2007.
DISCUSSION
In this cohort of HIV-infected MSM receiving consistent outpatient care, we observed a significant reduction in STD incidence after implementing routine biannual STD testing coupled with a brief risk-reduction counseling from medical providers. Our findings are consistent with other studies that have shown that prevention messages from patients’ primary provider may be effective in reducing the incidence of STDs.9,10 Incorporating prevention counseling into each medical visit could reinforce earlier messages, which may improve the effectiveness of the intervention over time.11 Regular discussions with patients about risks of STDs may also prompt health care providers to screen for STDs (per CDC guidelines) and improve detection rates, particularly of asymptomatic cases. Because frequent STD testing, as implemented in our study, can increase rates of STD diagnosis by improved detection of asymptomatic cases, the decline in STD incidence in our study is noteworthy. Thus, routine STD testing even in the absence of symptoms may be worthwhile in at-risk populations.
Although high-risk behaviors did not decline overall, some MSM appeared to adopt serosorting12 as a risk-reduction strategy. Recent reports suggest that the practice of serosorting among MSM is increasing and may be contributing to the stabilization of HIV incidence.13,14 However, a consequence of serosorting is the potential acquisition of bacterial, viral, or parasitic STDs.12 These data support the need for prevention-for-positives interventions for MSM in clinical settings to ensure personal choices are fully informed by complete knowledge of the residual risks associated with various risk-reduction strategies (e.g., strategic positioning, which is a practice in which partners position themselves [as either insertive or receptive] during anal sex in a manner that they believe reduces risk of transmitting HIV).15 The importance of consistent and correct condom use to decrease patients’ own risk of acquiring STDs should be emphasized.
In this study, we cannot determine the extent to which the reduction in STD incidence stemmed from routine risk-reduction counseling alone, routine STD screening alone, to a combination of the 2. At least one modeling study has suggested that increased frequency of testing (i.e., every 3 months) may mitigate syphilis epidemics among certain subpopulations of MSM, such as those who have not been previously tested, who engage in group sex, or who have a large number of partners (>10 per year).16 We hypothesize that both risk-reduction counseling and biannual STD screening likely complemented each other and contributed to the decline in STD incidence and that as a matter of clinical practice the 2 interventions should be coupled. We note that within a small subset of participants who attended two 6-monthly study visits during the preintervention phase during which they were screened and treated for STDs, STD incidence was unchanged before the intervention but had declined at 12 months postintervention. This observation suggests the behavioral intervention added independently to the objective outcome of decreased STD rates. Furthermore, we noted that 3% of the analytic sample that were sexually active at study entry were not sexually active later on in the study and among the 25% of the sample who were not sexually active at the preintervention visit, over half became sexually active during the 12-month study period. This suggests that sexual activity is dynamic and that frequent exposure to the intervention, even among persons who previously stated they were abstinent, as well as STD screening may be warranted. The optimal frequency of STD screening for this population remains unknown, and studies are needed to evaluate the effectiveness, feasibility, and cost-benefit of various strategies.
Our study had limitations. As noted, we could not tease out the individual effect of risk-reduction counseling versus frequent STD testing on the decline in STD incidence. Also, the disconnect between STD incidence and behavior change may be because there was a lagged effect of the intervention or that very recent changes in behavior may not have been picked up with the 6-month recall. Fluctuations in the incidence of STDs circulating within the local MSM population could not be assessed or controlled for. We could not determine whether acceptability of the “Partnership for Health” intervention differed among providers thereby affecting their delivery of risk-reduction counseling. These findings may not be generalizable to all HIV-infected MSM in care, given that over one-third of the HIV-infected MSM in the United States are black and only 11% of the men in our sample were black.17 The cost of regular STD screening of multiple anatomical sites and the added time, however modest, to deliver brief counseling to patients may be barriers to broad implementation of interventions modeled after this effort.
In conclusion, more effective prevention programs directed towards HIV-infected MSM are needed to curtail the HIV epidemic. The observed 50% reduction in incident STDs among HIV-infected MSM in care support adoption of routine STD screening coupled with brief and repeated risk-reduction counseling for this and other at-risk populations in the outpatient setting.
Acknowledgments
The authors thank all the SUN study participants.
Supported by Centers for Disease Control and Prevention contract numbers: 200–2002–00610, 200–2002–00611, 200–2002–00612, 200–2002–00613, 200–2007–23633, 200–2007–23634, 200–2007–23635, and 200–2007–23636.
Footnotes
These data were presented in part to the XVIII International AIDS Conference, July 18–23, 2010, Vienna, Austria.
The investigation followed the guidelines of the U.S. Department of Health and Human Services regarding protection of human subjects. The study protocol was approved and renewed annually by each participating institutions’ ethical review board. All study participants provided written, informed consent.
The findings and conclusions from this review are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflicts of interest: T.O. has served as a consultant or on an advisory board for the following companies: Gilead, Bristol Myers Squibb, Glaxo-Smith-Kline, Tibotec, Merck, and Monogram Sciences.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2.Mayer KH, Klausner JD, Hunter HH. Intersecting epidemics and educable moments: Sexually transmitted disease risk assessment and screening in men who have sex with men. Sex Transm Dis. 2001;28:464–467. doi: 10.1097/00007435-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Klausner JD, Wong W. Sexually transmitted diseases in men who have sex with men: a clinical review. Curr Infect Dis Rep. 2003;5:135–144. doi: 10.1007/s11908-003-0050-6. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Sexually Transmitted Disease Guidelines, 2010. MMWR. 2010;59(No. RR-12):1–114. [Google Scholar]
- 5.Hoover KM, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37:771–776. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 6.Tao G, Irwin KL, Kassler WJ. Missed opportunities to assess sexually transmitted diseases in US adults during routine medical checkups. Am J Prev Med. 2000;18:109–114. doi: 10.1016/s0749-3797(99)00139-7. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JL, Milam J, McCutchan A, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: a multi-clinic assessment. AIDS. 2004;18:1179–1186. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]
- 8.Vellozzi C, Brooks JT, Bush TJ, et al. The Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN Study) Am J Epidemiol. 2009;169:642–652. doi: 10.1093/aje/kwn361. [DOI] [PubMed] [Google Scholar]
- 9.Myers JJ, Shade SB, Rose CD, et al. Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: Results from the health resources and services administration (HRSA)’s special projects of national significance initiative. AIDS Behav. 2010;14:483–492. doi: 10.1007/s10461-010-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitlock EP, Orleans T, Pender N, et al. Evaluating primary care behavioral counseling interventions. Am J Prev Med. 2002;22:267–284. doi: 10.1016/s0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 11.The Healthy Living Project Team. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV. J Acquir Immun Defic Syndr. 2007;44:213–221. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 12.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: Is it safe? J Acquir Immune Syndr. 2008;49:212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 13.Truong HHM, Kellogg T, Klausner JD, et al. Increases in sexually transmitted infections and sexual risk behavior without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: A suggestion of HIV serosorting? Sex Transm Infect. 2006;82:461–466. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepaz N, Marks G, Liau A, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: A meta-analysis. AIDS. 2009;23:1617–1629. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 15.Marks G, Millet GA, Bingham T, et al. Prevalence and protective value of serosorting and strategic positioning among black and Latino men who have sex with men. Sex Transm Dis. 2010;37:325–327. doi: 10.1097/OLQ.0b013e3181c95dac. [DOI] [PubMed] [Google Scholar]
- 16.Gray RT, Hoare A, Prestage GP, et al. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis. 2010;37:298–305. doi: 10.1097/OLQ.0b013e3181ca3c0a. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Subpopulation estimates from the HIV incidence surveillance system—–United States 2006. MMWR. 2008;57:985–989. [PubMed] [Google Scholar]


