Abstract
Background
Effective HIV prevention programs rely on accurate estimates of the per-act risk of HIV acquisition from sexual and parenteral exposures. We updated the previous risk estimates of HIV acquisition from parenteral, vertical, and sexual exposures, and assessed the modifying effects of factors including condom use, male circumcision, and antiretroviral therapy.
Methods
We conducted literature searches to identify new studies reporting data regarding per-act HIV transmission risk and modifying factors. Of the 7339 abstracts potentially related to per-act HIV transmission risk, three meta-analyses provided pooled per-act transmission risk probabilities and two studies provided data on modifying factors. Of the 8119 abstracts related to modifying factors, 15 relevant articles, including three meta-analyses, were included. We used fixed-effects inverse-variance models on the logarithmic scale to obtain updated estimates of certain transmission risks using data from primary studies, and employed Poisson regression to calculate relative risks with exact 95% confidence intervals for certain modifying factors.
Results
Risk of HIV transmission was greatest for blood transfusion, followed by vertical exposure, sexual exposures, and other parenteral exposures. Sexual exposure risks ranged from low for oral sex to 138 infections per 10 000 exposures for receptive anal intercourse. Estimated risks of HIV acquisition from sexual exposure were attenuated by 99.2% with the dual use of condoms and antiretroviral treatment of the HIV-infected partner.
Conclusion
The risk of HIV acquisition varied widely, and the estimates for receptive anal intercourse increased compared with previous estimates. The risk associated with sexual intercourse was reduced most substantially by the combined use of condoms and antiretroviral treatment of HIV-infected partners.
Keywords: HIV, per-act, prevention, risk, transmission
Introduction
Accurate estimates of per-act HIV transmission risk from various exposures are necessary for individuals and public health programs to prevent infection. When the Centers for Disease Control and Prevention (CDC) last produced estimates in 2005 [1], many per-act transmission probabilities for sexual exposures [2,3] relied heavily on estimates derived from a single study of heterosexual couples [4]. Since 2005, new data have been reported from cohort studies of heterosexuals and of MSM, and new systematic reviews and meta-analyses of certain transmission risks have been published. Additionally, the published literature quantifying the effects of modifying factors known to either increase or decrease transmission risk has expanded substantially. Thus, we have updated our estimates of per-act HIV transmission risks from an infected source to an HIV-uninfected person for parenteral, vertical, and sexual exposures. These transmission estimates may not reflect true infectivity and may obscure important differences associated with factors that may modify transmission risk. Therefore, we have also summarized the relative effects of factors that modify per-act transmission risks, such as condom use and antiretroviral therapy, and have examined their individual and combined effects on per-act infectivity for high-risk sexual exposures.
Methods
Literature search and review
We conducted a five-step process of literature search and review. First, we established what was already known, starting with a series of recent systematic reviews and meta-analyses that were identified through a comprehensive literature review conducted for a related project that also examined per-act HIV transmission risk and provided estimates of pooled per-act HIV transmission probabilities for blood transfusion [5], parenteral exposures [5], receptive anal intercourse [6], receptive penile–vaginal intercourse [7], insertive penile–vaginal intercourse [7], and mother-to-child transmission [8]. Each of these peer-reviewed studies included a comprehensive literature review and employed accepted and robust meta-analytic methods. We then reviewed the 2011 British Pre-exposure Prophylaxis Guidelines [9], which provided a summary table of per-act HIV transmission risks using estimated medians and ranges based largely on the results of the meta-analyses noted above.
Second, we conducted a literature search to identify data published after the publications noted above. We searched for human studies published in English language only between 1 January 2008 and 22 February 2012 within the following databases: Medline (Ovid), Embase (Ovid), CINAHL (EbscoHost), Web of Science, Global Health, and the Cochrane Library. We used the following search string: [‘HIV’ or ‘HIV infections’ or ‘human immunodeficiency virus’ or ‘AIDS’] and [’disease transmission’ or ‘infectious/infectivity/infectiousness’ or ‘transmissibility’ or ‘contact/contacts/per-contact’ or ‘per-act’] and [’sexual’ or ‘heterosexual’ or ‘homosexual’ or ‘coital’ or ‘intercourse’ or ‘anal’ or ‘oral’ or ‘blood transfusion’ or ‘needle-sharing’ or ‘needle stick’ or ‘perinatal’ or ‘mother to child’]. We highlighted data from developed regions to more closely reflect the US epidemic; this strategy was consistent with that used for the relevant meta-analyses, which did not pool data from developed and developing countries due to heterogeneity among studies, except for the per-act HIV-transmission risk from parenteral exposures, which is less geographically dependent. We used the results of this literature search to ensure that the above-mentioned meta-analyses were up to date. For the exposures for which there were no recent reviews or meta-analyses, we reviewed the literature cited in CDC’s last summary [1] and the 2011 British Pre-exposure Prophylaxis Guidelines [9]. We also contacted subject matter experts to ascertain whether other studies or unpublished data of which we were unaware existed.
Third, we reviewed the resulting abstracts to identify articles that mentioned HIV transmission or any type of transmission risk estimate, or described models that were used to generate these estimates, both among serodiscordant couples and MSM. Fourth, we reviewed the text and bibliographies of all those publications that met these criteria to identify additional sources of transmission-risk data. We synthesized the information from these first four steps to generate updated per-act transmission risk estimates. We favored pooled estimates with 95% confidence intervals (CIs) reported from the meta-analyses that either used fixed-effects models or that used random-effects models that adjusted for the heterogeneity between studies, because such models provide more robust transmission risk estimates than simple medians and ranges.
Lastly, we conducted a literature search of human studies in PubMed to identify articles about factors known to modify sexual HIV transmission risk published between 1 January 2008 and 13 May 2013. We used the following search strings: ‘HIV transmission’ and each of the following separately: ‘genital ulcer disease’, ‘circumcision’, ‘condom use’, ‘pre-exposure prophylaxis’, ‘acute HIV infection’, ‘acute stage of disease’, ‘viral load’, ‘treatment’, ‘early antiretroviral therapy’.
Study selection
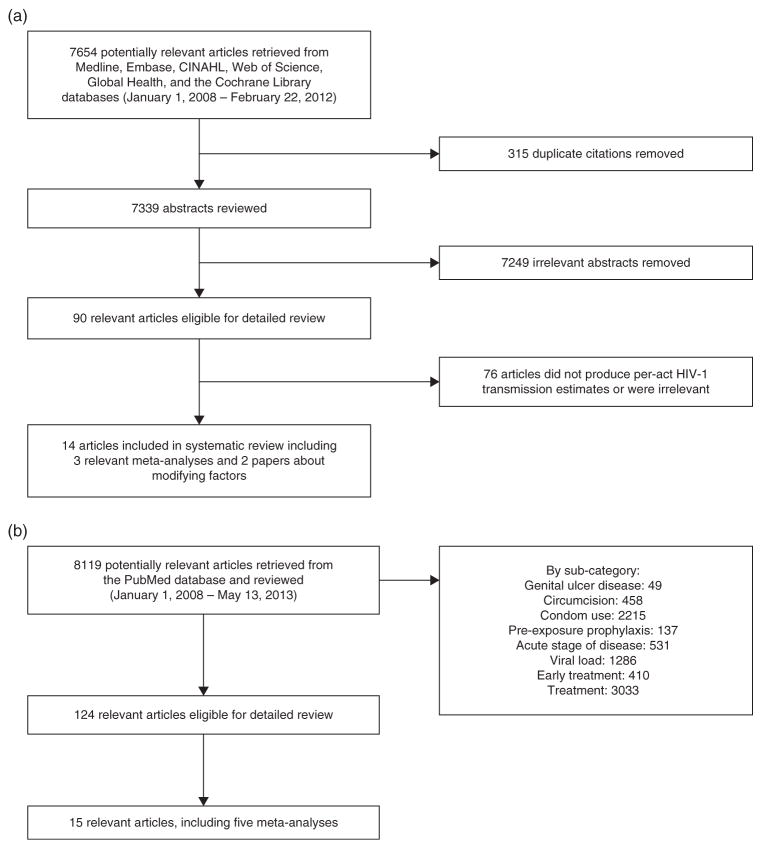
Inclusion criteria were randomized controlled trials or observational studies that examine per-act HIV transmission risk or the effect of modifying factors on HIV transmission risk, meta-analytic studies that provided pooled estimates of per-act HIV transmission risk or the effect of modifying factors on HIV transmission risk. Studies without statistically robust methods to ensure reproducibility and precision were excluded. Figure 1a details the study selection procedure for the summary of transmission-risk estimates; 7654 abstracts were reviewed, from which 14 articles were identified, including three relevant meta-analyses and two papers about modifying factors. The literature search for papers about factors known to modify sexual transmission risk produced 8119 abstracts, from which 15 articles were identified, including 5 meta-analyses (Fig. 1b).
Fig. 1.
(a) Selection of studies regarding per-act HIV-1 transmission probabilities. (b) Selection of studies regarding factors that modify HIV transmission risk.
Statistical methods
On the basis of the results of our literature search and the studies that we examined, we determined that recently published meta-analyses provided up-to-date summary estimates of transmission risks for all but the following exposures: needle-sharing injection drug use, receptive anal intercourse, insertive anal intercourse, receptive oral sex, and insertive oral sex. For needle-sharing injection drug use, we re-evaluated three published studies [10–12] and adopted the most statistically robust estimate that was applicable to the US epidemic. The meta-analysis for receptive anal intercourse did not include relevant data from one recently published study [13]. For receptive anal intercourse, we found four published sources [3,13–15], and for insertive anal intercourse, we found two published sources [13,14]. For each of these two estimates, we combined the results of the available studies using a fixed-effects inverse-variance model on the logarithmic scale in order to obtain updated estimates of these transmission risks. Specifically, we first transformed the reported point estimates and 95% CIs to the logarithmic scale, estimated the standard errors from the width of each 95% CI (divided by 2*1.96), calculated the weighted mean of these point estimates and the accompanying asymptotic normal 95% CI using the inverse of the estimated variances (i.e. the squared standard errors) as weights, and finally back-transformed the weighted mean and its 95% CI to the original scale using exponentiation. We also calculated Cochran’s Q test for heterogeneity. For oral sex, where no transmissions were observed out of a large number of acts, we calculated Clopper–Pearson exact binomial 95% CIs.
We also determined that meta-analyses published between 2005 and 2012 provided acceptable summary estimates of relative risks for various factors that modify sexual HIV transmission risk, except for pre-exposure prophylaxis among heterosexuals and for condom use. For pre-exposure prophylaxis among heterosexuals, we combined the number of events and person-time data from two studies [16,17] and then employed Poisson regression to calculate the estimated relative risk with an exact 95% CI. For condom use, we used the result of a meta-analysis [18] and then employed Poisson regression with the reported data to calculate an exact 95% CI. All regressions were performed in SAS software, version 9.3 (SAS Institute, Inc., Cary, North Carolina, USA).
To estimate the reduction in sexual HIV transmission risk in three scenarios – when the HIV-uninfected insertive partner used condoms, when the HIV-infected partner was treated with antiretrovirals, and when both were used together – we multiplied the original transmission risks by the relative risk of that factor. To estimate the 95% CIs for the reduced transmission risks, we first transformed the reported 95% CIs for the transmission risks and the risk reductions to the logarithmic scale and then estimated the standard errors from the width of each 95% CI (divided by 2*1.96). We next computed the variance of the logarithm of the reduced transmission risk and the accompanying asymptotic normal 95% CI, and finally back-transformed the 95% CI to the original scale using exponentiation. This calculation assumed that the covariances between the transmission risks from sexual intercourse and the relative reductions due to the modifying factors were zero, to a first-order approximation.
Internal and external review
The results presented here were vetted with CDC scientists as the project progressed. This internal iterative process included a critical review of the study design and statistical approach of each peer-reviewed publication upon which our new estimates relied as well as of our decision to present summary estimates from published meta-analyses. Our preliminary new estimates were critically reviewed by subject matter experts external to CDC (see Acknowledgments section), each of whom signed a nondisclosure agreement to ensure confidentiality.
Results
Summary of HIV transmission risk estimates
The estimated per-act HIV transmission risk (all expressed as per 10 000 exposures) was greatest for blood transfusion [9250 (95% CI 8900–9610)], followed by mother-to-child transmission [2260 (95% CI 1700–2900)], receptive anal intercourse [138 (95% CI 102–186)], needle-sharing injection drug use [63 (95% CI 41–92)], and percutaneous needle stick injuries [23 (95% CI 0–46)] [5,6,8,10]. Risk for other sexual exposures were 4 (95% CI 1–14) for insertive penile–vaginal intercourse, 8 (95% CI 6–11) for receptive penile–vaginal intercourse, and 11 (95% CI 4–28) for insertive anal intercourse [7,13,14] (Table 1). The transmission risk for receptive and insertive oral sex is quite low (95% CI 0–4) [19].
Table 1.
Estimated per-act probability of acquiring HIV from an infected source, by exposure route.
| Exposure route | Risk per 10 000 exposures to an infected source | 95% Confidence interval | Reference(s) |
|---|---|---|---|
| Parenteral exposure | |||
| Blood transfusion | 9250 | (8900–9610) | [5] |
| Needle-sharing injection drug use | 63b | (41–92) | [12] |
| Percutaneous needle stick | 23 | (0–46) | [5] |
| Sexual exposurea | |||
| Receptive anal intercourse | 138c | (102–186) | [3,13–15] |
| Insertive anal intercourse | 11d | (4–28) | [13,14] |
| Receptive penile–vaginal intercourse | 8e | (6–11) | [7] |
| Insertive penile–vaginal intercourse | 4e | (1–14) | [7] |
| Receptive oral sex | Lowf | (0–4) | [14,19] |
| Insertive oral sex | Lowf | (0–4) | [19] |
| Vertical transmission | |||
| Mother-to-child transmission | 2260g | (1700–2900) | [8] |
Factors that may increase the risk of HIV transmission include sexually transmitted diseases, acute and late-stage HIV infection, and high viral load. Factors that may decrease the risk include condom use, male circumcision, antiretroviral treatment, and pre-exposure prophylaxis.
Estimate of risk of transmission from sexual exposure to an HIV-infected partner and assumes no condom use.
A pooled estimate was not calculated due to the heterogeneity of the studies (different study designs and HIV subtype made the data difficult to combine) for injection drug use [10–12]; therefore, we present the most robust and applicable estimate to the US epidemic of five estimates from three studies.
A similar pooled estimate [140 per 10 000 exposures, 95% confidence interval (CI) 20–250] was calculated using a random-effects model [6]. Jin et al. [13] reported an estimated per-contact probability of HIV transmission for unprotected receptive anal intercourse (URAI) of 143 per 10 000 exposures (95% CI 48–285) with ejaculation inside the rectum, and of 65 per 10 000 exposures (95% CI 15–153) with withdrawal prior to ejaculation. Regardless of when ejaculation occurred, the estimated per-contact probability of HIV transmission for URAI was 91 per 10 000 exposures (95% CI 41–207) (James Jansson, personal communication). By comparison, two other large prospective studies that did not distinguish when ejaculation occurred reported similar results. Vittinghoff et al. [14] reported an estimate of 82 per 10 000 exposures (95% CI 24–276) and a recent study by Scott et al. [20] reported an estimate of 73 per 10 000 exposures (95% CI 45–98).
The US study [14] may underestimate transmission risk because partners of unknown HIV status were also included without attempting to estimate the HIV prevalence among these partners (i.e. assumed all persons with unknown HIV status were infected). A recent study by Scott et al. [20] reported an estimated per-contact probability of HIV transmission for unprotected insertive anal intercourse (UIAI) of 22 per 10 000 exposures (95% CI 5–39).
These estimates represent the asymptomatic phase of HIV infection and do not account for various factors that can affect infectivity. Pooled estimates from low-income countries were generated despite substantial heterogeneity existing across studies. The difference in per-act transmission attributable to receptive and insertive penile–vaginal intercourse is attenuated when adjusted for cofactors in meta-regression models suggesting that infectivity is similar for receptive and insertive penile–vaginal intercourse [7].
Risk is considered to be low relative to the other sexual exposures, but it is not zero. The Clopper–Pearson exact binomial 95% CIs are based on observing no events out of 8965 receptive oral sex acts; the sample size was not large enough to generate a more precise point estimate.
With antiretroviral use, there was a 67.4% relative reduction in risk of HIV transmission from 22.6 to 7.6%. These results were not combined with studies conducted in developing countries because substantial heterogeneity existed across studies [68].
Blood transfusion
We obtained our updated estimate for the per-act risk of HIV transmission from exposure to a contaminated blood product from a meta-analysis [5], which used a fixed-effects model with data limited to six studies where the blood donations were known to be contaminated with HIV [21–26]. This meta-analysis included updated results from the Transfusion Safety Study [24]; earlier results from this study were used to derive the previous CDC estimate [27]. This meta-analysis pooled data from developed and developing countries because there was no heterogeneity of findings among studies.
Needle-sharing injection drug use
We identified three studies [10–12] that provided estimates of the per-act risk of HIV transmission from injection drug use with a contaminated needle. One study [10] estimated this risk as 67 per 10 000 exposures (without a CI) using differential equation models and a small sample of data from a US needle exchange program. Two other studies [11,12] provided overall and subtype B and E-specific estimates using robust semi-parametric statistical methods and data from a cohort of injection drug users in Bangkok, Thailand. We adopted their subtype B-specific estimate of 63 per 10 000 exposures (95% CI 41–92 per 10 000) as the best estimate of this risk for the current US epidemic.
Percutaneous needle stick
The estimates for per-act transmission risk for percutaneous needle stick were more reliable than per-act transmission risk for injecting drugs, primarily because the infection status of the index case for a percutaneous needle stick was generally known and the number of exposures quantifiable. The meta-analysis [5] that provided the new estimate included data from 21 published studies [28–53], the majority of which reported no transmissions [29–38,40–42,50]. There was no evidence of heterogeneity of findings among studies, and the overall estimate was calculated using a fixed-effects model. An analysis of a subset of studies from this meta-analysis that included only estimates from studies with no other reported risk factor for HIV transmission produced per-act transmission risk estimates that did not differ significantly from the overall estimate [5].
Receptive anal intercourse
MSM account for the majority (60–70%) of prevalent and incident HIV infections in the United States; most infections are transmitted through unprotected receptive anal intercourse (URAI). The previous CDC estimate of the per-act risk of transmission from URAI was extrapolated from data on heterosexual couples and was assumed to be approximately five times that of receptive penile–vaginal intercourse, or 50 transmissions per 10 000 exposures [2,4]. A 2010 meta-analysis [6] provided an estimate of this risk based on data from published studies at that time [3,14,15,54]; however, we have updated this estimate to include relevant new data [13] and have excluded data [54] that were not a point estimate but a risk relative to the risk of receptive penile–vaginal intercourse.
For the updated estimate, we identified four studies that estimated the per-act transmission risk for URAI using binomial or Bernoulli models, three from the pre-HAARTera [3,14,15] and one from the HAARTera [13]: a cross-sectional study in Boston that recruited 329 MSM, representing 155 sexual partnerships, from 1984 to 1987 [15]; The European Study on Heterosexual Transmission of HIV, which recruited 499 HIV-infected persons and their regular heterosexual partners in nine European countries from 1987 to 1992 [3]; The Collaborative HIV Seroincidence Study (CHSS), which followed a prospective cohort of 2189 HIV-negative high-risk homosexual and bisexual men in San Francisco, Denver, and Chicago from 1992 to 1994 and excluded participants who reported any injection drug use from the analysis [14]; and The Health in Men (HIM) study, which followed a prospective cohort of 1427 HIV-negative MSM in Sydney, Australia from 2001 to 2007 [13].
Using a variety of modeling assumptions and expert opinion, the Boston study [15] presented a range of plausible values of 50–300 per 10 000 exposures for the URAI transmission risk (midpoint 175 per 10 000). The European Study on Heterosexual Transmission of HIV [3] estimated a transmission risk for URAI of 138 per 10 000 exposures (standard error 102) during the period between initial infection and late-stage disease (i.e. AIDS), from which we calculated a 95% CI of 32–588 per 10 000 using a logarithmic transformation. The CHSS [14] estimated a transmission risk for URAI of 82 per 10 000 exposures (95% CI 24–276 per 10 000). Note that this study may underestimate risk because it did not distinguish between URAI with and without ejaculation. The HIM study [13] estimated a transmission risk for URAI of 143 per 10 000 exposures (95% CI 49–285 per 10 000). Using these data, we computed an updated estimate of the transmission risk for URAI of 138 per 10 000 exposures (95% CI 102–186 per 10 000).
Insertive anal intercourse
As for URAI, the previous CDC transmission risk estimate for unprotected insertive anal intercourse (UIAI) was extrapolated from data on heterosexual couples. Specifically, this risk was assumed to be approximately 1.3 times that of insertive penile–vaginal intercourse, and thus 6.5 transmissions per 10 000 exposures [2,3]. We identified two studies that estimated the transmission risk for UIAI among MSM, one from the pre-HAARTera – the CHSS [14], and one from the HAARTera – the HIM study [13].
The CHSS used a Bernoulli model to estimate a transmission risk of 6 per 10 000 exposures (95% CI 2–19 per 10 000) for UIAI with an HIV-positive or serostatus-unknown partner. There were too few contacts with known HIV-positive partners to provide stable estimates of this risk for HIV-positive partners alone. The HIM study used a Bernoulli model to estimate a transmission risk of 16 per 10 000 exposures (95% CI 5–31 per 10 000) for UIAI with an HIV-positive partner. This study also provided separate estimates for UIAI transmission risk by circumcision status: for circumcised participants, this risk was 11 per 10 000 exposures (95% CI 2–24 per 10 000) and for uncircumcised participants, this risk was 62 per 10 000 exposures (95% CI 7–168 per 10 000). We computed an updated estimate of the transmission risk for UIAI of 11 per 10 000 exposures (95% CI 4–28 per 10 000).
Receptive and insertive penile–vaginal intercourse
Our updated estimates for penile–vaginal intercourse were obtained from meta-analyses of 10 studies that used random-effects models of homogenous data to evaluate heterosexual risk of HIV infection among persons in high-income countries [7]. Data from low-income countries were too heterogeneous to be combined with high-income country data. The risk estimate for receptive penile–vaginal intercourse was obtained from a meta-analysis of all 10 studies [55–64], whereas the estimate for insertive penile–vaginal intercourse was obtained from a meta-analysis of three estimates from two of these 10 studies [55,60]. Of the 10 total studies, nine were conducted in the pre-HAART era, eliminating a major effect of antiretroviral use on the estimates. These new estimates are slightly lower than the previous CDC estimates, which fall within the new estimates’ CIs. Like the previous CDC estimates, the updated receptive penile–vaginal intercourse risk estimate is twice as high as that for insertive penile–vaginal intercourse.
Receptive and insertive oral sex
The previous CDC estimates for per-act transmission risk associated with receptive and insertive oral sex were extrapolated from estimates of per-act penile–vaginal intercourse transmission risk (oral sex is 1/10 times as risky as vaginal sex) [2]. Two studies have provided per-act estimates based on prospective comprehensive collection of sexual behaviors including oral sex [14,19]. A 1992–1994 US MSM cohort study [14] provided an estimate for receptive oral sex equal to our updated estimate for per-act transmission from insertive penile–vaginal intercourse (4 per 10 000 exposures), which seems improbable because the oropharynx is considerably less susceptible to HIV infection than the cervico-vaginal environment or penis by virtue of the oropharynx’s thicker epithelial layer, low number of CD4+ lymphocytes, and the presence of antiviral antibodies and various endogenous factors that inhibit HIV transmission [65]. A 10-year Spanish study conducted from 1990 to 2000 among serodiscordant heterosexual couples [19] observed no transmissions due to receptive oral sex among 8965 acts. We used data from this study to estimate 95% CIs for receptive and insertive oral sex transmission risk (95% CI 0–4). A study among lesbians also observed no transmissions due to oral sex [66]. A meta-analysis to establish the per-act transmission risk for oral sex could not be conducted because data were from three disparate sources [67]. Furthermore, estimating per-act transmission risk for low-risk acts, such as oral sex, is often confounded by the complex patterns of sexual exposure where higher-risk exposures occur during the same sexual encounter. Given these general limitations and the individual limitations of the previous estimates, we believe that although HIV transmission via oral sex is biologically plausible, we are unable to provide a precise numeric estimate.
Mother-to-child transmission
Our estimate for mother-to-child transmission of 2260 per 10 000 exposures (95% CI 1700–2900 per 10 000) was based on the transmission risk observed in the placebo arm of a randomized, double-blind, placebo-controlled clinical trial, of the safety and efficacy of zidovudine to reduce mother-to-child HIV transmission [8]. We did not combine these results with those from developing countries because substantial heterogeneity existed across studies [68].
Summary cofactors that modify per-act transmission risk for sexual exposures
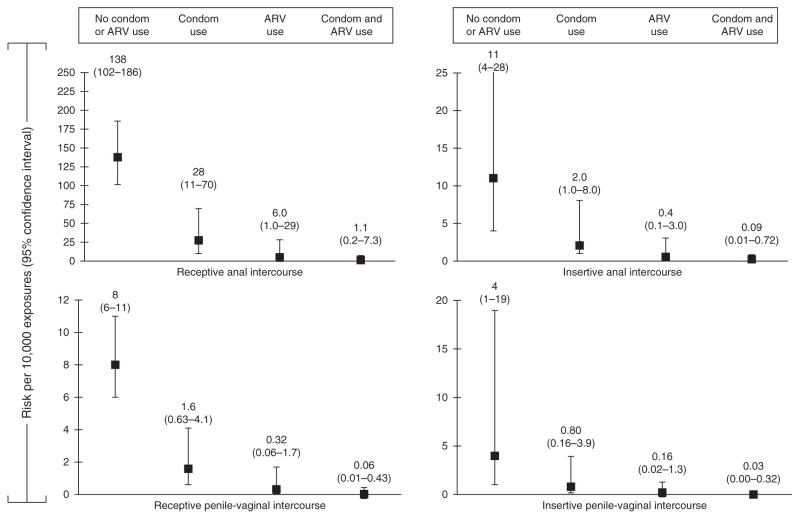
Table 2 summarizes data regarding cofactors that modify transmission risk for sexual exposures. Factors that increase transmission risk are high viral load [69], genital ulcer disease [70], and acute and late-stage disease [70,71], whereas factors that decrease risk are use of antiretrovirals for treatment [72,73], pre-exposure prophylaxis [16,17,74], male condom use [18], and male circumcision [75–80]. We further depicted the effect of antiretroviral treatment and condom use on HIV transmission due to anal and vaginal intercourse in Fig. 2. We estimate that used together, antiretroviral treatment and condom use could reduce HIV transmission by up to 99.2% (Fig. 2).
Table 2.
Relative risks of factors that increase or decrease per-act HIV transmission risk for sexual exposures.
| Cofactor | Relative risk | 95% Confidence interval | References |
|---|---|---|---|
| Factors that increase transmission probability | |||
| High plasma viral load (log10 copies/ml) | 2.89 | (2.19, 3.82) | [69] |
| Genital ulcer diseasea | 2.65 | (1.35, 5.19) | [69] |
| Acute versus asymptomatic stage of disease | 7.25b | (3.05, 17.3) | [70] |
| Late versus asymptomatic stage of disease | 5.81b | (3.00, 11.4) | [70] |
| Factors that decrease transmission probability | |||
| Use of antiretrovirals by HIV-infected partner | |||
| Early versus delayed treatment | 0.04c | (0.01, 0.27) | [72] |
| Received treatment versus no treatment | 0.08 | (0.00, 0.57) | [73] |
| Pre-exposure prophylaxis of HIV-uninfected partner | |||
| Among heterosexual couples | 0.29d | (0.17, 0.47) | [16,17] |
| Among MSM | 0.56 | (0.37, 0.85) | [74] |
| Among injection drug users | 0.52 | (0.28, 0.90) | [75] |
| Condom use | 0.20e | (0.08, 0.47) | [18] |
| Male circumcision (heterosexual partners) | |||
| HIV-uninfected partner is male | 0.50f | (0.34, 0.72) | [76] |
| HIV-uninfected partner is female | 0.80 | (0.53, 1.36) | [77] |
| Male circumcision (MSM) | |||
| Insertive partner is HIV-uninfected | 0.27g | (0.17, 0.44) | [78] |
| Receptive partner is HIV-uninfected | 1.20g | (0.63, 2.29) | [78] |
Characteristic of the HIV-uninfected partner; therefore relative risk reflects the increased risk of acquisition of HIV infection from an infected partner
Hazard of transmission that accounts for duration of infectiousness was calculated using these data: hazard of transmission per person-year for early versus asymptomatic stage of disease is 2.76 [95% confidence interval (CI) 1.31–5.09] and for late versus asymptomatic stage is 0.76 (95% CI 0.41–1.28); thus 26 and seven times more infectious, respectively [71].
The reported hazard ratio was used to approximate the relative risk.
For this estimate, we combined the number of events and person-time data from the tenofovir and emtricitabine (TDF-FTC) and placebo arms from two studies of pre-exposure prophylaxis [16,17] and then employed Poisson regression to calculate the estimated relative risk with an exact 95% CI.
This review indicates that consistent use of condoms results in 80% reduction in HIV incidence. Consistent use is defined as using a condom for all acts of penetrative vaginal intercourse. Because the studies used in this review did not report on the ‘correctness’ of use, namely whether condoms were used correctly and perfectly for each and every act of intercourse, effectiveness and not efficacy is estimated. Effectiveness was estimated from two separate incidence estimates: one minus the ratio of incidence among always users to the incidence among never users.
This review combined the survival estimates from three trials [78–80] at 12 months and also at 21 or 24 months in a meta-analysis using the random-effects model. The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% CI of 0.34–0.72, and 0.46 at 21 or 24 months (95% CI 0.34–0.62).
The reported odds ratios were used to approximate the relative risks.
Fig. 2.
Per-act HIV-1 transmission risk of anal and vaginal intercourse and the modifying effects of antiretroviral treatment for the HIV-infected partner and condom use on the per-act HIV transmission risk estimates.
Discussion
We estimate that the current per-act risk of HIV transmission via sexual exposures ranges from 4 per 10 000 exposures for insertive penile–vaginal intercourse to 138 for receptive anal intercourse. Our updated estimates for both receptive and insertive anal intercourse are substantially higher than previously reported (increased 1.8 and 0.7-fold, respectively); however, the previous estimates fall within our updated CIs for these exposures. Additionally, the per-act risk for all sexual exposures could be substantially attenuated through the use of condoms and of antiretrovirals. Understanding the effects of modifying factors when estimating per-act transmission risk can better inform an individual’s personal risk and HIV-prevention efforts.
The published literature regarding per-act HIV transmission risk from sexual exposures is estimated using observational studies and has many, often unavoidable, limitations. Ideal estimates would be calculated from serodiscordant partners for whom all sex acts and their context were recorded prospectively. In reality, most estimates have relied on longitudinal or cross-sectional studies of individuals using population-based HIV prevalence estimates. Retrospective studies may be subject to recall bias. Key variables that would permit more precise estimations are often missing, such as the HIV status of all sexual partners. Most persons do not practice one type of sex act to the exclusion of others with a partner during a single encounter (e.g. oral sex and vaginal sex). The broad and often overlapping CIs for many of these updated per-act sexual transmission risk estimates reflect the imprecision imposed by these limitations, in light of which our estimates should be interpreted cautiously. Furthermore, we have used estimates of efficacy (for treatment) and effectiveness (for condom use) somewhat interchangeably to demonstrate risk reduction in Fig. 2, thus, overestimating the effect of treatment.
In conclusion, we have updated the 2005 CDC per-act HIV transmission risks for major exposures. We have also summarized the effects of various cofactors that modify the per-act risk of sexual exposures permitting improved estimation of individual and population-based risk. To the extent possible, future studies of sexual per-act transmission risk should carefully consider these transmission factors, which vary in prevalence and are critical to accurate risk assessment.
Acknowledgments
We would like to thank our external review panel for their thoughtful comments and critical appraisal. Panel members included (in alphabetical order): Rebecca Baggaley, PhD, Imperial College, London; Marie-Claude Boily, PhD, Imperial College, London; Susan Buchbinder, MD, University of California, San Francisco, California; Myron Cohen, MD, University of North Carolina, Chapel Hill, North Carolina; Don DesJarlais, PhD, Beth Israel Medical Center, New York, New York; Julie Fox, PhD, NHS/Kings College, London; Samuel Friedman, PhD, Institute for AIDS Research National Development and Research Institutes, Inc. Director, Interdisciplinary Theoretical Synthesis Core Center for Drug Use and HIV Research, New York, New York; Andrew Grulich, MD, University of New South Wales, Sydney; James P. Hughes, MD, University of Washington, Seattle, Washington; Kimberly A. Powers, PhD, University of North Carolina, Chapel Hill, North Carolina; and Eric Vittinghoff, MD, University of California, San Francisco, California.
We would also like to thank our CDC colleagues: Charles LeBaron, MD; Steven Nesheim, MD; Gary Marks, PhD; Charles Rose, PhD; Stephanie Sansom, PhD; and Dawn Smith, MD for their thoughtful input and insight.
Footnotes
Authors roles and responsibilities: P.P. critically reviewed all abstracts and journal articles for inclusion in the summary; led the internal to CDC and external to CDC review process with subject matter experts; worked closely with the statistician to calculate new estimates; outlined, wrote, edited, and critically reviewed the manuscript.
C.B.B. critically reviewed major journal articles included in the summary with Dr Patel; worked with Dr Patel to calculate new estimates; and critically reviewed and edited the manuscript.
J.T.B. critically reviewed and edited the manuscript.
A. Lasry critically reviewed several journal articles with P.P. and reviewed and edited the manuscript.
A. Lansky reviewed and provided comments on the manuscript.
J.M. conceived the concept for paper; supervised manuscript development; and critically reviewed and edited the manuscript.
Disclaimer: The findings and conclusions from this review are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
There are no conflicts of interest.
References
- 1.CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. Morb Mortal Wkly Rep. 2005;54(RR–2):1–20. [PubMed] [Google Scholar]
- 2.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29:38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Leynaert B, Downs AM, De Vincenzi I European Study Group on Heterosexual Transmission of HIV. Heterosexual transmission of HIV: variability of infectivity throughout the course of infection. Am J Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 4.European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. Br Med J. 1992;304:809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parental exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20:805–812. doi: 10.1097/01.aids.0000218543.46963.6d. [DOI] [PubMed] [Google Scholar]
- 6.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 9.Benn P, Fischer M, Kulasegaram R BASSH, PEPSE Guidelines Writing Group Clinical Effectiveness Group. UK guideline for the use of post-exposure prophylaxis for HIV following sexual exposure (2011) Int J STD AIDS. 2011;22:695–708. doi: 10.1258/ijsa.2011.171011. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr. 1992;5:1116–1118. [PubMed] [Google Scholar]
- 11.Hudgens MG, Longini IM, Jr, Choopanya K, Vanichseni S, Kitayaporn D, Mastro TD, et al. Estimating the transmission probability of human immunodeficiency virus in injecting drug users in Thailand. J R Stat Soc Ser C Appl Statist. 2001;50:1–14. [Google Scholar]
- 12.Hudgens MG, Longini IM, Jr, Vanichseni S, Hu DJ, Kitayaporn D, Mock PA, et al. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol. 2002;155:159–168. doi: 10.1093/aje/155.2.159. [DOI] [PubMed] [Google Scholar]
- 13.Jin F, Jansson J, Law M, Prestage GP, Zablotska I, Imrie JCG, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vittinghoff V, Douglas J, Judson F, McKiman D, MacQueen K, Buchbinder S. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 15.DeGruttola V, Seage GR, 3rd, Mayer KH, Horsburgh CR., Jr Infectiousness of HIV between male homosexual partners. J Clin Epidemiol. 1989;42:849–856. doi: 10.1016/0895-4356(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 16.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. TDF2 Study Group. Antiretroviral pre-exposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 18.Weller SC, Davis-Beaty K. Condom effectiveness in reducing heterosexual HIV transmission (Review) Cochrane Database Syst Rev. 2002:CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 19.delRomano J, Marincovich B, Castilla J, García S, Campo J, Hernando V, Rodríguez C. Evaluating the risk of HIV transmission through unprotected orogenital sex. AIDS. 2002;16:1296–1297. doi: 10.1097/00002030-200206140-00017. [DOI] [PubMed] [Google Scholar]
- 20.Scott HM, Vittinghoff E, Irvin R, Sachdev D, Liu A, Gurwith M, Buchbinder SP. Age, race/ethnicity, and behavioral risk factors associated with per contact risk of HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2014;65:115–121. doi: 10.1097/QAI.0b013e3182a98bae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward JW, Bush TJ, Perkins HA, Lieb LE, Allen JR, Goldfinger D, et al. The natural history of transfusion-associated infection with human immunodeficiency virus. Factors influencing the rate of progression to disease. N Engl J Med. 1989;321:947–952. doi: 10.1056/NEJM198910053211406. [DOI] [PubMed] [Google Scholar]
- 22.Berglund O, Beckman S, Grillner L, Jansson B, Lidbrink P, Karlsson A, et al. HIV transmission by blood transfusion in Stockholm 1979–1985: nearly uniform transmission from infected donors. AIDS. 1988;2:51–54. [PubMed] [Google Scholar]
- 23.Colebunders R, Ryder R, Francis H, Nekwei W, Bahwe Y, Lebughe I, et al. Seroconversion rate, mortality, and clinical manifestations associated with the receipt of a human immunodeficiency virus-infected blood transfusion in Kinshasa, Zaire. J Infect Dis. 1991;164:450–456. doi: 10.1093/infdis/164.3.450. [DOI] [PubMed] [Google Scholar]
- 24.Donegan E, Lee TH, Operskalski EA, Shaw GM, Kleinman SH, Busch MP, et al. Transfusion transmission of retroviruses: human T-lymphotrophic virus types I and II compared with human immunodeficiency virus type 1. Transfusion. 1994;34:478–483. doi: 10.1046/j.1537-2995.1994.34694295061.x. [DOI] [PubMed] [Google Scholar]
- 25.Busch MP, Operskalski EA, Mosley JW, Lee TH, Henrard D, Herman S, et al. Factors influencing human immunodeficiency virus type 1 transmission by blood transfusion. Transfusion Safety Study Group. J Infect Dis. 1996;174:26–33. doi: 10.1093/infdis/174.1.26. [DOI] [PubMed] [Google Scholar]
- 26.Moore A, Herrera G, Nyamongo J, Lackritz E, Granade T, Nahlen B, et al. Estimated risk of HIV transmission by blood transfusion in Kenya. Lancet. 2001;358:657–660. doi: 10.1016/S0140-6736(01)05783-X. [DOI] [PubMed] [Google Scholar]
- 27.Donegan E, Stuart M, Niland JC, Sacks HS, Azen SP, Dietrich SL, et al. Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations. Ann Intern Med. 1990;113:733–739. doi: 10.7326/0003-4819-113-10-733. [DOI] [PubMed] [Google Scholar]
- 28.Weiss SH, Saxinger WC, Rechtman C, Grieco MH, Nadler J, Holman S, et al. HTLV-III infection among health care workers: association with needle-stick injuries. J Am Med Assoc. 1985;254:2089–2093. [PubMed] [Google Scholar]
- 29.Moss A, Osmond D, Bacchetti P, Gerberding J, Levy J, Carlson J, Casavant C. Risk of seroconversion for acquired immunodeficiency syndrome (AIDS) in San Francisco health workers. J Occup Med. 1986;28:821–824. doi: 10.1097/00043764-198609000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Kuhls TL, Viker S, Parris NB, Garakian A, Sullivan-Bolyai J, Cherry JD. Occupational risk of HIV, HBV and HSV-2 infections in health care personnel caring for AIDS patients. Am J Public Health. 1987;77:1306–1309. doi: 10.2105/ajph.77.10.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wormser GP, Joline C, Sivak SL, Arlin ZA. Human immunodeficiency virus infections: considerations for health care workers. Bull NY Acad Med. 1988;64:203–215. [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández E, Puyuelo T, Gatell JM, Barrera JM, Pumarola T. Occupational risk of infection by human immunodeficiency virus. Article in Spanish. Med Clin (Barc) 1988;90:767–768. [PubMed] [Google Scholar]
- 33.Weiss SH, Goedert JJ, Gartner S, Popovic M, Waters D, Markham P, et al. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988;239:68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- 34.Strickler AC. Occupational exposure to HIV infection among health-care workers at the Toronto General Hospital. Can Dis Week Rep. 1988;14:141–146. [PubMed] [Google Scholar]
- 35.Jorbeck H, Steinkeller E. 41 cases of accidental infection with HIV-positive blood (in Swedish) Lakartidningen. 1988;85:3044–3045. [PubMed] [Google Scholar]
- 36.Jorbeck H, Marland M, Steinkeller E. Accidental exposures to HIV-positive blood among health-care workers in 2 Swedish hospitals. Fifth International Conference on AIDS; Montreal, Quebec, Canada. 1989; [Abstract A517] [Google Scholar]
- 37.Rogers PL, Lane HC, Henderson DK, Parrillo J, Masur H. Admission of AIDS patients to a medical intensive care unit: causes and outcome. Crit Care Med. 1989;17:113–117. doi: 10.1097/00003246-198902000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Puro V, Ranchino M, Profili F. Occupational exposures to blood and risk of HIV transmission in a general hospital (1986–88) Eur J Epidemiol. 1990;6:67–70. doi: 10.1007/BF00155552. [DOI] [PubMed] [Google Scholar]
- 39.Henderson DK, Fahey BJ, Willy M, Schmitt JM, Carey K, Koziol DE, et al. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures. A prospective evaluation. Ann Intern Med. 1990;113:740–746. doi: 10.7326/0003-4819-113-10-740. [DOI] [PubMed] [Google Scholar]
- 40.McCormick RD, Meisch MG, Ircink FG, Maki DG. Epidemiology of hospital sharps injuries: a 14-year prospective study in the pre-AIDS and AIDS eras. Am J Med. 1991;91:301S–307S. doi: 10.1016/0002-9343(91)90386-c. [DOI] [PubMed] [Google Scholar]
- 41.National surveillance of occupational exposure to the human immunodeficiency virus (HIV) Can Commun Dis Rep. 1992;18:103–104. [PubMed] [Google Scholar]
- 42.Cavalcante NJ, Abreu ES, Fernandes ME, Richtmann R, Piovesana MN, Yamada FT, Carvalho ES. Risk of healthcare professionals acquiring HIV infection in Latin America. AIDS Care. 1991;3:311–316. doi: 10.1080/09540129108253078. [DOI] [PubMed] [Google Scholar]
- 43.Cavalcante NJ, Richtmann R, Abreu ES, Ramalho MO, Piovesana MN, Carvalho ES. Changing of notifications of accidents with HIV infective materials, among healthcare professionals (HCP) at Emilio Ribas Institute, Sao Paolo, Brazil. IX International Conference on AIDS; Berlin. June 1993; [abstract PO-C18-3030] [Google Scholar]
- 44.Ippolito G, Puro V, De Carli G. The risk of occupational human immunodeficiency virus infection in healthcare workers. Italian Multicenter Study. The Italian Study Group on Occupational Risk of HIV Infection. Arch Intern Med. 1993;153:1451–1458. [PubMed] [Google Scholar]
- 45.Ippolito G, De Carli G, Puro V, Petrosillo N (SIROH) SIROdH. Risk of occupational HIV and HCV infection after occupational exposure. XI International Conference on AIDS; Vancouver. July 1996; [abstract TuC123] [Google Scholar]
- 46.Tokars JI, Marcus R, Culver DH, Schable CA, McKibben PS, Bandea CI, Bell DM. Surveillance of HIV infection and zidovudine use among healthcare workers after occupational exposure to HIV-infected blood. The CDC Cooperative Needlestick Surveillance Group. Ann Intern Med. 1993;118:913–919. doi: 10.7326/0003-4819-118-12-199306150-00001. [DOI] [PubMed] [Google Scholar]
- 47.Cardo D, Tokars J, Marcus R, McKibben P, Culver D, Ciesielski C, Bell D Cooperative Needlestick Group. Zidovudine (AZT) use after occupational HIV exposure: toxicity and failures reported to CDC; an update [Abstract]. Infect Control Hosp Epidemiol; Conference on Prevention of Transmission of Bloodborne Pathogens in Surgery and Obstetrics; Atlanta. 1994. p. 342. [Google Scholar]
- 48.Communidad de Madrid CdS. Registro de inoculaciones accidentales (VIH) en personal sanitario de la CAM. Vigilancia Epidemiologica del SIDA/VIH. 1993;12:65–72. [Google Scholar]
- 49.de Juanes JR, Lago E, Davila F, Aragon AJ, Arrazola P, Garcia Codes A, et al. Injuries with exposure to blood in surgeons of a university hospital, over a year period. Infect Control Hosp Epidemiol; Abstract Conference on prevention of transmission of bloodborne pathogens in surgery and obstetrics; Atlanta. 1994. p. 343. [Google Scholar]
- 50.Nelsing S, Nielsen TL, Nielsen JO. Occupational exposure to human immunodeficiency virus among healthcare workers in a Danish hospital. J Infect Dis. 1994;169:478. doi: 10.1093/infdis/169.2.478. [DOI] [PubMed] [Google Scholar]
- 51.Gerberding JL. Incidence and prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and cyto-megalovirus among health care personnel at risk for blood exposure: final report from a longitudinal study. J Infect Dis. 1994;170:1410–1417. doi: 10.1093/infdis/170.6.1410. [DOI] [PubMed] [Google Scholar]
- 52.Jost J, Iten A, Meylan P, Columbo C, Maziero A. Mise a jour sur les expositions au VIH en milieu medical. Mesures generales, chimioprophylaxie, declaration. Bull Office Fed Sante Pub. 1997;7:5–12. [Google Scholar]
- 53.PHLS. Occupational exposures associated with HIV infection in healthcare workers: update to Sept 97. PHLS AIDS and STD Centre & Scottish Centre for Infection and Environmental Health; [Google Scholar]
- 54.Halperin DT, Shiboski SC, Palefsky SC, Padian NS. High level of HIV-1 infection from anal intercourse: a neglected risk factor in heterosexual AIDS prevention. Int Conf AIDS; 7–12 July 2002; p. 14. [abstract ThPeC7438] [Google Scholar]
- 55.Kim HC, Raska K, III, Clemow L, Eisele J, Maits L, Saidi P, Raska K., Jr Human immunodeficiency virus infection in sexually active wives of infected hemophilic men. Am J Med. 1988;85:472–476. doi: 10.1016/s0002-9343(88)80080-9. [DOI] [PubMed] [Google Scholar]
- 56.Longini IM, Scott Clark W, Haber M, Horsburgh R., Jr . The stages of HIV infection: waiting times and infection transmission probabilities. In: Castillo-Chavez C, editor. Mathematical and statistical approaches to AIDS epidemiology. Berlin: Springer-Verlag; 1989. pp. 111–137. [Google Scholar]
- 57.Brooks Jackson J, Kwok SY, Hopsicker JS, Sannerud KJ, Sninsky JJ, Edson R, Ballour HH., Jr Absence of HIV-1 infection in antibody-negative sexual partners of HIV-1 infected hemophiliacs. Transfusion. 1989;29:265–267. doi: 10.1046/j.1537-2995.1989.29389162735.x. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence DN, Jason JM, Holman RC, Heine P, Evatt BL. Sex practice correlates of human immunodeficiency virus transmission and acquired immunodeficiency syndrome incidence in heterosexual partners and offspring of U.S. hemophilic men. Am J Hematol. 1989;30:68–76. doi: 10.1002/ajh.2830300204. [DOI] [PubMed] [Google Scholar]
- 59.Kim MY, Lagakos SW. Estimating the infectivity of HIV from partner studies. Ann Epidemiol. 1990;1:117–128. doi: 10.1016/1047-2797(90)90003-b. [DOI] [PubMed] [Google Scholar]
- 60.Downs AM, De Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. European Study Group in Heterosexual Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:388–395. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 61.Marincovich B, Castilla J, del Romero J, García S, Hernando V, Raposo M, Rodríguez C. Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex Transm Infect. 2003;79:160–162. doi: 10.1136/sti.79.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 63.Shiboski SC, Padian NS. Epidemiologic evidence for time variation in HIV infectivity. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:527–535. doi: 10.1097/00042560-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 64.Saracco A, Veglia F, Lazzarin A. Risk of HIV-1 transmission in heterosexual stable and random couples. The Italian Partner Study. J Biol Regul Homeost Agents. 1997;11:3–6. [PubMed] [Google Scholar]
- 65.Campo J, Perea MA, del Romano J, Cano J, Hernando V, Bascones A. Oral transmission of HIV, reality or fiction? An update. Oral Dis. 2006;12:219–228. doi: 10.1111/j.1601-0825.2005.01187.x. [DOI] [PubMed] [Google Scholar]
- 66.Raiteri R, Baussano I, Giobbia M, Fora R, Sinicco A. Lesbian sex and risk of HIV transmission. AIDS. 1998;12:450–451. [PubMed] [Google Scholar]
- 67.Baggaley RF, White RG, Boily MC. Systematic review of orogenital HIV-1 transmission probabilities. Int J Epidemiol. 2008;37:1255–1265. doi: 10.1093/ije/dyn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Anti-retrovirals for reducing the risk of mother-to-child transmission of HIV infection (Review) Cochrane Database of Systematic Reviews. 2011:CD003510. doi: 10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 69.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Partners in Prevention HSV/HIV Transmission Study Team. Determinants of per-coital HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 71.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 72.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donnell D, Baeten JM, Klarie J, Thomas KK, Stevens W, Cohen CR, et al. Partners in Prevention HSV/HIV Transmission Study Team. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men (Review) Cochrane Database Syst Rev. doi: 10.1002/14651858.CD003362.pub2. 2009CD003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss HA, Hankins CA, Dickson K. Male circumcision and risk of HIV infection in women: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:669–677. doi: 10.1016/S1473-3099(09)70235-X. [DOI] [PubMed] [Google Scholar]
- 77.Wiysonge CS, Kongnyuy EJ, Shey M, Muula AS, Navti OB, Akl EA, Lo YR. Male circumcision for prevention of homosexual acquisition of HIV in men (Review) Cochrane Database Syst Rev. 2011:CD007496. doi: 10.1002/14651858.CD007496.pub2. [DOI] [PubMed] [Google Scholar]
- 78.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 80.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomized trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]