Abstract
Deep brain stimulation (DBS) is an effective therapy for Parkinson’s disease (PD) patients experiencing motor fluctuations, medication resistant tremor, and/or dyskinesia. Currently, the subthalamic nucleus and the globus pallidus internus are the two most widely used targets, with individual advantages and disadvantages influencing patient selection. Potential DBS patients are selected using the few existing guidelines and the available DBS literature, and many centers employ an interdisciplinary team review of the individual’s risk-benefit profile. Programmed settings vary based on institution or physician specific protocols designed to maximize benefits and limit adverse effects. Expectations should be realistic and clearly defined during the evaluation process, and each bothersome symptom should be addressed in the context of building the risk-benefit profile. Current DBS research is focused on improved symptom control, the development of newer technologies, and the improved efficiency of stimulation delivery. Techniques deliver stimulation in a more personalized way, and methods of adaptive DBS such as closed loop approaches are already on the horizon.
Keywords: Parkinson’s disease, Deep Brain Stimulation, Closed-loop
Introduction
Deep brain stimulation (DBS) has been shown to be an effective therapy for patients with Parkinson’s disease (PD) who have motor fluctuations, medication resistant tremor, and/or troublesome dyskinesia.1,2 DBS has been established as an adjunctive treatment to address the cardinal PD motor symptoms (i.e. tremor, rigidity, and bradykinesia) and, for some patients, medication reduction can be achieved.1,2 The exact mechanisms of action for DBS applied in the most commonly used PD targets, the globus pallidus internus (GPi) and the subthalamic nucleus (STN), remain unknown. However recent evidence has shown that DBS may provide modulation of abnormal neuronal signals that are propagated through nodes of a complex neural network.1
Target and patient selection are of great importance to the overall surgical process and efficacy of the procedure. Extensive preoperative discussions with surgical candidates regarding which specific troublesome symptoms may or may not respond to neuromodulation can set the foundation for a discussion of reasonable expectations. Postoperatively, target-specific expert programming by experienced clinicians can facilitate maximum benefit while minimizing side effects. In the long term, a plan for continued efficacy monitoring, stimulation-induced side effects, and hardware malfunctions is important in the care of patients with DBS. This article will briefly review the historical and clinical aspects of DBS therapy, the indications for treatment, DBS target selection, as well as long term programming strategies across different brain targets. Finally, we will review the future horizons of DBS therapy including new hardware and current research on novel paradigms and interventions.
DBS in PD: The era prior to DBS
The history of innovations impacting modern DBS in neurological and psychiatric disorders dates as far back as the late 1940’s, when Spiegel and Wycis described the stereotactic apparatus which, after adaptation to the human brain, allowed surgeons to target specific structures within the brain using tridimensional coordinates.3,4 In the 1950’s, pallidotomy became a popular surgical PD treatment, and intraoperative macrostimulation was initially used as part of localization of the globus pallidus target. According to reports drawn from the 1960’s, surgeons were able to suppress parkinsonian symptoms temporarily with pallidal macrostimulation during the intraoperative localization procedure.4. In the mid-1960’s, multiple electrodes were implanted as part of the process for identification of the ideal pallidotomy location, and it was observed by several surgeons that the electrodes could remain implanted for days to weeks.4 With the introduction of levodopa as a treatment for PD in 1968, pallidotomy became less commonly used in PD, and was largely restricted to severe or refractory cases, particularly for patients presenting with tremors as the dominant feature.4–6
The idea of using electrical stimulation as a modality for chronic therapy took shape in the 1970’s, when thalamic electrodes were implanted and multiple sessions of high and low frequency stimulation were applied prior to a final surgical lesion.4 Around the same time, Medtronic trademarked the term “deep brain stimulation,” which was evolving in Europe as a treatment for chronic pain.4 The first reports of therapeutic DBS for movement disorders including PD occurred in the early 1980’s, when chronic stimulation of thalamic and subthalamic areas was observed to effectively suppress parkinsonian symptoms. Finally, in 1987, Dr. Alim Benabid and colleagues reported the first series of tremor and PD patients undergoing bilateral chronic thalamic stimulation, and this set the stage for a new era of DBS targeted at alleviating tremor and some of the other symptoms of PD.7,8
Deep brain stimulation in PD: two targets, one neural network
Following Benabid et al. (1987), various groups across the globe started evaluating the clinical effectiveness and efficacy of DBS in PD by targeting the globus pallidus internus (GPi) and subthalamic nucleus (STN). Both structures are nodes of the fronto-basal-ganglia-thalamo-cortical networks that are involved in cognitive, behavioral and motor function. Abnormal signaling within this network has been closely associated with the motor and non-motor features of PD.9
The STN is part of the indirect and hyper-direct pathways, and the GPi is the main outflow nucleus of the basal ganglia system (Figure 1).10 Following the first large scale DBS outcomes reported by Pollack and Benabid in 1993,11 the STN gained considerable early popularity as the preferred DBS target for PD. The rational for STN stimulation was largely driven by the hypothesis from DeLong and colleagues regarding hyper-excitability within the circuitry and changes in rate and pattern of neuromodulation between key nodes in the network,12 as demonstrated in their seminal paper in Science showing that lesioning the STN in a primate alleviated MPTP-induced parkinsonism.12 Concomitantly, and following the previous successes of pallidotomy in addressing PD cardinal motor symptoms as well as dyskinesia, Siegfried et al. (1994) reported chronic bilateral GPi DBS as a potential alternative approach to ameliorate human PD symptoms.13 Subsequent randomized and observational studies comparing conventional pallidotomy and DBS targeting either the STN 14 or GPi 15 collectively revealed a reduction in Unified Parkinson’s Disease Rating Scale (UPDRS) OFF scores, an improvement in dyskinesia, suppression of tremor, and in some cases, medication reduction. Due to the increased risk of significant speech, swallowing, and cognitive deficits associated with lesioning surgery,14,15 DBS gradually became the procedure of choice for PD patients refractory to medical management.
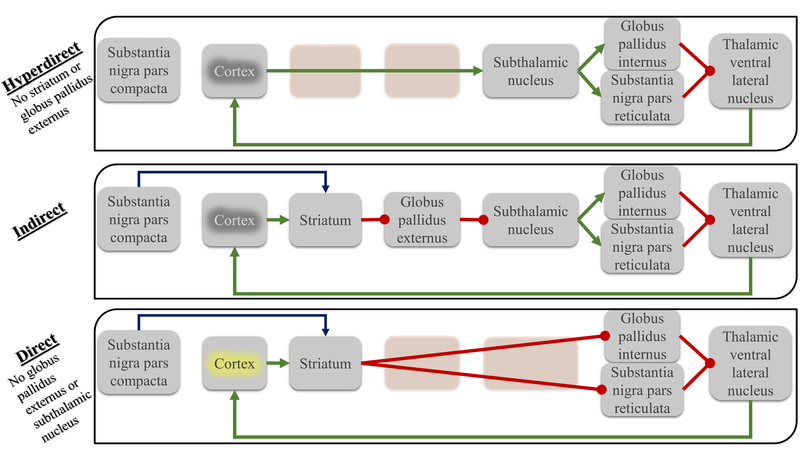
Figure 1 |.
Comparison of the proposed fronto-basal ganglia-thalamic-cortical circuits. Three circuits are described: hyperdirect, indirect and direct. The direct circuit is associated with increased excitation of the motor cortex and a resultant facilitation of movement while the indirect circuits induce an opposite effect. The hyperdirect pathway is hypothesized to widely inhibit the cortex allowing direct pathway activity to release a selected motor program. PD is thought to be associated with an imbalance of activity of these circuits, characterized by a decrease in the direct pathway circuit activity and an increase in the hyperdirect and indirect pathways. Green arrows indicate a glutamatergic (excitatory) synaptic connections, red point arrows indicate GABAergic (inhibitory) synaptic connections and the blue arrows indicate dopaminergic input from the substantia nigra to the striatum. Yellow and gray highlighting of the cortex indicates excitation and inhibition, respectively.
As experience with both STN and GPI DBS has accumulated over time, important differences between outcomes has led to an “STN versus GPi” debate regarding which is the optimal target in patients with PD.16 Table 1 summarizes the critical findings from the largest studies published to date. In 2001 the Deep Brain Stimulation for Parkinson’s Disease Study Group reported the first blinded non-randomized study of 96 bilateral STN and 38 bilateral GPi DBS patients.17 The study concluded that bilateral DBS of either target was associated with significant symptomatic improvement in PD, and that neither GPi nor STN DBS was clearly superior; based on these results, both targets subsequently gained FDA approval in 2002.
Table 1.
Main outcomes of principal clinical trials comparing STN and GPi
| Study | Year | Outcomes |
|---|---|---|
| DBS for PD Study Group17 STN vs GPi |
2001 | STN and GPi DBS have similar and significant improvements in motor function, ADLs, total “on” time and reduction in dyskinesias at both 3 and 6 months. |
| Rodriguez-Oroz et al.18 STN vs GPi |
2005 | STN and GPi DBS have similar and significant improvement in motor function, ADLs and total “on” time at 3 to 4 years. There was a 35% reduction of daily levodopa dosing in the STN group only. Adverse events, such as cognitive decline, speech difficulty, instability, gait disorders and depression were more common in the STN group. |
| COMPARE / Okun et al23 STN vs GPi |
2009 | STN and GPi DBS showed no significant difference in mood and cognitive outcomes at 7 months. STN and GPi DBS showed similar motor improvements. STN DBS may lead to worsenign of verbal fluency when compared to GPi. |
| Moro et al.19 STN vs GPi |
2010 | STN and GPi DBS showed significant, sustained improvement in motor function, ADLs and dyskinesias at 5 to 6 years. STN DBS required lower levodopa dosages at 5 to 6 years. GPi showed no such reduction. STN DBS was associated with more frequent adverse events at 5 to 6 years. |
| Follett et al (VA Study)22 STN vs GPi |
2010 | STN and GPi DBS showed no significant difference in motor function and QoL at 2 years. STN DBS required lower levodopa doses when compared to GPi. STN and GPi DBS showed no significant difference in adverse events of surgery. |
| NSTAPs study / Oderkerken et al.20 STN vs GPi |
2013 | STN and GPi DBS showed no significant difference in disability, cognitive, mood or behavioral symptoms at 1 year. STN DBS showed larger improvements in off-drug motor scores than GPi. |
| NSTAPs study / Oderkerken et al.21 STN vs GPi |
2016 | STN DBS showed significantly more improvement in off-drug motor scores than GPi at 3 years. STN DBS required lower levodopa doses when compared to GPi at 3 years. STN and GPi DBS showed no significant differences in adverse events (including cognition, mood and behavior). |
Follow-up of this cohort at four18 and six19 years confirmed sustained clinical benefit without troublesome dyskinesias in both STN and GPi groups. However, only STN DBS provided a significant reduction in dopaminergic medications over time,18,19 though this was counterbalanced by a higher occurrence of side effects including cognitive dysfunction, depression, as well as speech and gait impairment in the STN patients.18,19 Subsequent results in Europe20,21 and the United States22,23 would add to the collective evidence supporting DBS therapy. The first randomized STN vs. GPi study was the unilaterally implanted NIH COMPARE cohort by Okun at al.23, randomizing 45 PD patients from a single center to either side. There were no statistically significant differences in motor efficacy, nor in the occurrence of cognitive or psychiatric side effects captured by the scales collected. This was the first study to show that the most common cognitive side effect resulting from DBS (decreased verbal fluency) was mainly a surgical and not a stimulation induced issue.23 A larger randomized trial published a year later included six different U.S. based Veterans Affairs centers and studied a cohort of 299 patients randomized to either bilateral GPi or STN DBS.22 The findings replicated the unilateral NIH COMPARE cohort and showed a good clinical response in both targets.22 STN DBS patients experienced a significantly larger medication reduction, but also had a higher rate of psychiatric and cognitive side effects in long-term follow up of the cohort.22 Most recently in the Netherlands, a randomized trial of 128 patients implanted in either the STN or GPi reported similar motor benefits in both targets. The authors found a slightly higher reduction in UPDRS motor scores and total dopaminergic reduction in STN DBS at 120 and 3 years21, suggesting a slight advantage for STN DBS; however, direct comparisons to previous trials is limited by differences in primary outcome variables.
Although some studies have suggested that STN may be the preferred target for PD patients with problematic tremor,24,25 there have been no studies directly comparing STN and GPi DBS that have been specifically focused on tremor. Large multicenter trials have actually reported similar motor outcomes in all the cardinal symptoms inclusive of tremors for both GPi and STN.18,20–23,26–30 STN DBS does, however, allow for a more significant reduction of dopaminergic medication compared to GPi DBS, a finding that has been replicated by several large multicenter trials.18,19,22,29,31 On the other hand, this reduction is often instituted to avoid troublesome dyskinesias,32,33 which may limit flexibility in levodopa dosing later in the disease course. GPi DBS has been shown to be a better suppressor of dyskinesias, and to allow more flexibility in adjusting medications short- and long-term.33
Across most comparative studies, STN DBS has been associated with a greater incidence of stimulation-induced side effects, neuropsychiatric issues, and cognitive side effects in the post-operative period,18,23,29,34–43 although a few recent studies have found no differences in depression or cognitive dysfunction between STN and GPi DBS.21,44 Although less extensively studied, other side effects that have been reported at a higher incidence in STN DBS include dysphagia,36,41,45 speech abnormalities20,26,46 as well as gait and balance dysfunction.41,47 In addition to basal ganglia structures targeted by DBS, lead depth and placement accuracy have also been shown to influence side effect profiles. The COMPARE cohort demonstrated that when patients underwent cognitive testing after being randomized to activation of an optimal DBS contact on the DBS lead versus a more ventral contact (deeper), the latter condition was associated with a greater occurrence of mood and cognitive side effects in both GPi and STN targets,23 thus suggesting that optimal lead location could play an important role in outcomes. The 2005 paper on DBS failures48 further emphasized this point, revealing that half of all DBS failures were due to suboptimal lead placement.
Patient Screening and Individualized Target Selection
The studies described do not support a “one size fits all” approach to target selection, but rather a choice of DBS target that is individualized for each patient. This process begins with careful patient selection and (where possible) a multi-disciplinary evaluation.49,50 A thorough history and clinical evaluation will ensure that previous attempts at optimal medical management were adequate, and that the symptoms targeted are those most likely to improve with DBS. Typically, a disease course of at least five years of progressive levodopa-responsive parkinsonism with at least a 30% improvement in UPDRS with dopaminergic therapy can help to identify those patients likely to benefit from DBS 51 and to avoid operating on patients with atypical parkinsonism (a notable exception is levodopa-resistant PD tremor, which can still respond to DBS therapy)48,52,53. The EarlyStim study has identified that earlier use of DBS systems (within 3 years of motor fluctuations) is associated with benefit compared to best medical therapy54,55. The FDA has indeed changed its recommendation for DBS from advanced PD to patients with motor complications not properly controlled by best medical therapy and of at least 4-year duration.54 However, early DBS implantation will bring some challenges in terms of recognizing earlier stages of atypical parkinsonian syndromes which can overlap with PD and may only unmask themselves later in the disease course, leading to reservations regarding formal recommendations for widespread DBS implantation in parkinsonian patients at earlier stages.56,57
It is critical to set patient expectations in concrete terms of potential benefits and risks. Common misconceptions observed in practice include the notion that DBS will 1) be a replacement to all medications (e.g. becoming drug free); 2) delay disease progression; and 3) ultimately ameliorate all PD symptoms including those that do not respond to levodopa (e.g. gait, balance, voice, swallowing, and cognition). Table 2 summarizes examples of PD symptoms that commonly do or do not respond to DBS.
Table 2.
Response of PD symptoms to DBS
| Symptoms that typically respond to DBS | Symptoms that may respond to DBS | Symptoms that do not respond to DBS77 |
|---|---|---|
| Bradykinesias | Camptocormia*78 | Autonomic Dysfunction |
| Rigidity | Pisa Syndrome*79 | Dysphagia |
| Tremor | Fatigue* | Dysphonia |
| Dystonia | Inner restlessness* | Cognition or Verbal Fluency |
| Dyskinesia | Depression | Postural Instability |
| Total “On” Time | Freezing of Gait* | |
| Motor fluctuations |
Response is conditional to levodopa response
DBS has in almost all cases failed to replace medication therapy, and it has not been shown to slow disease progression. In cases with aggressive medication reduction, apathy and other symptoms have emerged.58 Additionally, non-motor symptoms may become a main source of disability and are, in many cases, unchanged by DBS, though there have been some minor non-motor improvements reported post-DBS.59
Once disabling symptoms that are potentially DBS responsive are identified, patients should be evaluated by an experienced multidisciplinary team. Teams vary in their composition, but typically include a movement disorders neurologist, a neurosurgeon, rehabilitation services (physical, occupation, speech and swallow therapies), a psychiatrist, a neuropsychologist and, if possible, a social worker (for pre- and post-operative care planning).52,60 The potential surgical candidates can then be discussed and the input from all specialists used to establish a risk-benefit profile prior to deciding on a brain target and approach for DBS surgery (unilateral vs. bilateral; simultaneous versus staged, etc.) Higuchi et al. recently evaluated 166 patients undergoing a multidisciplinary DBS screening approach, and patients were stratified into major, minor, or no concerns for DBS surgery.60 Patients with major concerns had higher rates of post-operative hospitalization (89% on major versus 33% on minor and 3% on no concerns), and worse quality of life scores at 12-months post-surgery.60
The results from the interdisciplinary evaluation inform the decisions for final candidacy including bilateral versus unilateral surgery, implantation of simultaneous DBS leads versus staging them over time (e.g. one lead at a time), and for target selection. Surgical decisions should consider the patient’s impression of the most troublesome or disabling symptoms and preference for avoiding adverse events.52 Motor fluctuations coupled with a strong desire for medication reduction without significant dyskinesias might support the use of STN DBS, while a known underlying psychiatric or cognitive problem or severe dyskinesias may favor GPi as the optimal DBS target.
DBS programming in PD
Following lead and pulse generator (IPG) implantation, patients will usually undergo initial programming in the outpatient neurology or neurosurgery clinic. The purpose of the initial visit is to identify the therapeutic and side effect thresholds for each electrode on the lead (monopolar survey). The clinician programmer usually sets a fixed pulse width and frequency, and then the voltage is gradually increased in a monopolar configuration until benefit and/or side effect is reached at each sequential electrode. Picillo et al. recently cited rigidity as a quick and consistent responder to effective stimulation, and thus rigidity may be useful during threshold mapping and setting DBS devices.61 There are, however, other symptoms such as tremor and occurrence of side effects that are useful during programming. Following the identification of clinical thresholds for benefits and side effects, currently available devices allow clinicians to program multiple patient-programmable group settings. Patients are encouraged to try these group settings systematically until their subsequent follow-up visit. The information from this threshold mapping and setting trials can be used to guide further DBS programming adjustments, which typically takes 3–6 months to find the optimal IPG settings.
We have found in our practice that it is important to examine all of the motor and cognitive symptoms relevant to an individual patient during DBS programming sessions to maximize benefit and minimize side effects. Continued medication adjustments and screening for symptoms such as falls, apathy, and depression can help to reduce adverse events such as suicide or neuropsychiatric decline due to over-aggressive medication reduction.
When PD patients do not respond to DBS: The need for troubleshooting
Troubleshooting the patient with a suboptimal response to DBS should be systematic and comprehensive. Possible explanations for a suboptimal response to DBS include erroneous diagnosis, inappropriate patient selection, hardware failure, suboptimal programming, inadequate medical management, delayed lead migration, and suboptimal placement. Suboptimal patient selection, including inaccurate diagnosis, accounts for approximately 10% of the suboptimal DBS responses.62 Often clues suggesting atypical parkinsonism, such as a poor response to levodopa, rapid progression, and early postural instability and gait impairment, were overlooked during the patient selection process. These features can be overlooked by inexperienced clinicians or if DBS is performed early in the disease course.
Tertiary care DBS centers frequently encounter suboptimally placed DBS leads and lead migration, the latter of which can occur due to cap failure (e.g. failure to secure the lead) or inadvertent traction during IPG replacement. Cortical atrophy may also play a role in lead shifts over time.63 Three dimensional reconstruction and measurement of DBS lead location using neuroimaging can be helpful in uncovering such issues as the cause of poor response and/or side effects. Occasionally, revisiting a monopolar survey might yield an alternative programming strategy. If attempts to reprogram the device fail or suboptimal lead location is excessive, lead revision or implantation of an additional rescue DBS lead should be considered. A common error in the management of patients who lose clinical benefit after a period of good response to DBS is exhaustive programming trials. In actuality, device settings will typically need to change very little once an optimal programming setting is achieved. Once settings are optimized, long-term management of implanted patients is typically focused on monitoring battery life and addressing issues related to the medication regimen and non-motor symptoms of the disease.
Future of DBS in PD: Technologies and research
The clinical use of DBS as an adjunctive therapy for PD is now in its third decade, and DBS is now considered the standard of care for patients with motor complications and dyskinesias, or those refractory to medical management. Researchers and clinicians have reached a reasonable level of comfort in developing institutional guidelines, however the field lacks standardization in patient selection and targeting, surgical techniques, and device programming. Current limitations in lead technology limit control of stimulation-induced side effects. Rechargeable devices capable of reducing long-term morbidity from surgical IPG replacements have been slowly improving in technology and usability. There is also a drive to develop wireless stimulation systems, further decreasing the surgical morbidity.64 More advanced power solutions will be required as remote monitoring becomes available to avoid battery drain. These factors have encouraged researchers to seek hardware and software improvements.
Traditionally, IPG systems operated using a constant voltage. Newer systems are using constant current. The constant current systems adjust the voltage of stimulation with changes in tissue impedance, allowing a more constant tissue stimulation.65
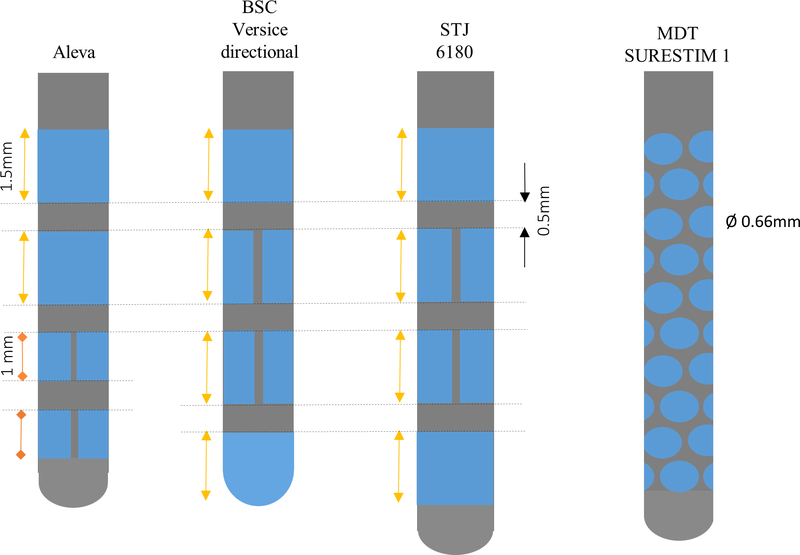
The avoidance of stimulation-induced side effects is another important area of DBS research. These side effects occur when current spills into undesired tissues/pathways such as the internal capsule as a result of a suboptimal placement of the lead.54,66 To avoid side effects, different programming techniques can be used such as using bipolar configuration or interleaving settings, though often with limited success or worsening of symptom control.54 Newer DBS leads have been introduced that are capable of current shaping or steering.1,67 These new DBS leads provide directional stimulation rather than the traditional elliptical-shaped field (Figure 2), widening the therapeutic threshold.68 Some of the leads shape the field of stimulation through multiple independent current control (MICC) of their different contacts as well as steer it through segmented electrodes. The Versice system from Boston Scientific55,66 uses a MICC system, and the St. Jude Infinity system, recently approved in the USA, can utilize a segmented lead. Other systems, through the use of multiple small segmented elliptical electrodes (such as the Sapiens system), allow current steering by directing the current in all of the quadrants.54 This new technology will facilitate shaping the stimulation into the area of interest within a structure and/or fiber bundle and away from undesired areas where stimulation results in untoward effects. Current steering technologies have been aimed at changing the size of the stimulation field and also by using segmented contacts to point stimulation toward beneficial regions and away from suboptimal areas (e.g. internal capsule). Contarino et al68 in a questionnaire survey of DBS providers in the Netherlands identified that an average of 21% of patients might benefit from using steering technology, as they were limited by side effects of dysarthria and muscle contractions. Interestingly, they reported that a higher proportion of patients with GPi and thalamic leads could benefit from steering compared to STN leads (respectively, 48% and 39% versus 13%),68 however many experts would consider STN-related side effects a very attractive use for this technology.
Figure 2 |.
Representation of directional DBS electrode lead designs. BSC, Boston Scientific Neuromodulation; STJ, St. Jude Medical; MDT, Medtronic.
Recent research has focused on software modifications (updated firmware) that may facilitate delivery of different pulses in different pulse shapes, potentially enhancing outcomes.69 Akbar et al.69 investigated several novel stimulation strategies including square biphasic pulses (with active depolarization and repolarization phases). This approach provided statistically significant improvements in UPDRS scores. Updates to the firmware may be an important horizon for DBS therapy.
In additional to its utility as a treatment modality, DBS provides PD researchers with a window into the functioning basal ganglia-thalamo-cortical network in humans, and increases our understanding of the underlying pathophysiological mechanisms of the disease and the behavior of neural populations within a complex network. Basal ganglia neuronal oscillations play a variety of roles in cognitive, emotional, and motor function, and represent synchronized neuronal activity propagated throughout functional circuits. In PD, aberrant coupling between the phase of activity in the beta band and the amplitude of activity in the gamma band has been reported, and is thought to be associated with at least some of cortical dysfunction that gives rise to the symptoms of the disease.70 Additionally, abnormal beta band activity and its excessive phase-amplitude coupling to broad-band gamma activity both appear to respond to neuromodulation, making them exciting potential electrical biomarkers of the disease.1,71–73
The identification of electrical biomarkers is critical for the development of adaptive closed-loop approaches to DBS therapy in PD. Current generation DBS devices are programmed in a “set it and forget” manner, in which settings are programmed by providers at clinical visits and remain constant until the next clinical visit. In this paradigm, patients are programmed with specific settings that provide stimulation at a constant voltage without any feedback. The identification of potential electrical biomarkers of abnormal network activity has opened the possibility for devices to deliver “responsive” or “smart” stimulation, in which the parameters of stimulation self-adjust as needed in real time based on brain activity. This technique has been referred to as closed-loop or adaptive DBS (Figure 3). Closed-loop DBS is capable of delivering therapy that can be customized to individual patient and pathology status, and may provide more effective therapeutic benefit with less side effects and increased battery longevity. Currently only manual on/off or simple cycling modes are available to patients to adjust stimulation parameters based on their activities or to compensate for DBS-related side effects (e.g. dysphagia, speech changes). Realization of closed-loop DBS could dramatically increase the degree to which stimulation can be individualized in future patients with PD.
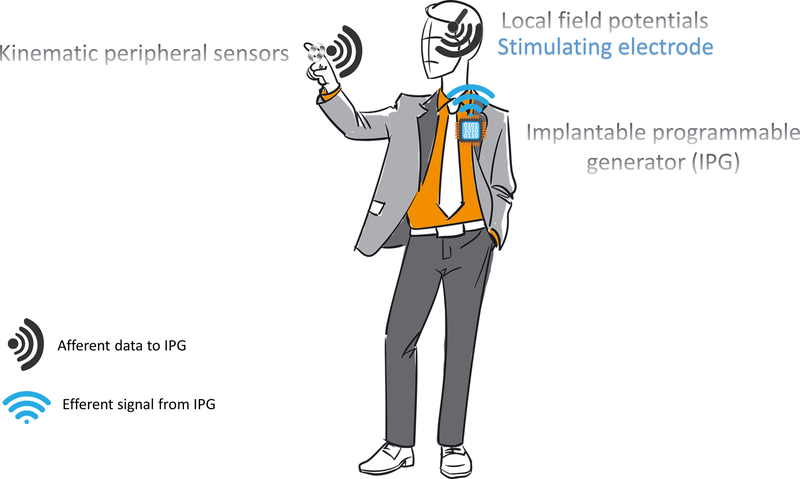
Figure 3 |.
Cartoon of a closed-loop DBS system. Signals are transmitted from peripheral sources (such as accelerometers) and central sources (such as the local field potential measured by the implanted electrode) toward the implanted programmable generator (IPG). The IPG analyzes the data and adaptively responds by altering the stimulation settings.
Early experiments in developing a closed loop paradigm have focused on sensing or recording from electrodes not being actively used for stimulation or from electrocorticography (additional motor cortical strips), or from the use of peripheral sensors including surface electromyography electrodes and/or accelerometers. A variety of research groups are pursuing closed loop DBS to target specific symptoms such as resting tremor71 and medication-induced dyskinesias.74 Our group at the University of Florida is currently investigating a closed-loop system for freezing of gait (utilizing leads placed in both the GPi and peduncolopontine nucleus (PPN), analyzing the physiological relationship between these two structures and a potential responsive mechanism of stimulation for this refractory symptom). Others are exploring closed loop DBS for dyskinesia and tremor.75 Though the breadth and variety of disabling motor and non-motor features may ultimately limit the usefulness of a closed loop approach, promising findings are already being reported.76
The future goals of DBS therapy should include the treatment of symptoms that are disabling yet not currently effectively treated using current medical and surgical approaches. Levodopa-resistant signs and symptoms such as gait and postural instability, dysarthria, and cognitive and affective dysfunction are of particular relevance. Advances in stimulation delivery through directional current steering, novel patterns of stimulation, and adaptive or closed loop systems may provide a mechanism of addressing current limitations in treatment. More research will be needed to expand our understanding of the biological changes in the brain resulting from both the disease and the effects of chronic electrical stimulation. Finally, we will need to consider the costs required infrastructure of providing more and more patients with potentially expensive and complicated new therapeutic options. A multi-disciplinary approach that includes physicians, advanced care providers, and technology support staff will pave the way to the coming bionic PD generation.
Acknowledgements:
Drs. Almeida, Gunduz, Martinez-Ramirez, Deeb, Spears, Opri, and Molina declare no conflict of interest. Dr. Hess receives grant support from the University of Florida Clinical and Translational Research Institute, which is supported in part by NIH award KL2 TR001429. He has served as a research committee member for the Michael J. Fox Foundation and as a speaker for the National Parkinson Foundation, the Parkinson’s Disease Foundation, and the Davis Phinney Foundation. Dr. Hess has participated in CME and educational activities on movement disorders sponsored by Allergan, Ipsen, Mertz Pharmaceuticals, Peerview Online, and QuantiaMD. Dr. Okun serves as a consultant for the National Parkinson Foundation and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation and is an associate editor for New England Journal of Medicine Journal Watch Neurology. His DBS research is supported by R01 NR014852. He has previously received honoraria, but in the past >60 months has received no support from industry. The University of Florida receives grants from Medtronic, AbbVie, Allergan, and ANS/St. Jude, and Dr. Okun has no financial interest in these grants. He has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and by Vanderbilt University.
Abbreviations:
- DBS
Deep Brain Stimulation
- FDA
Food and Drug Administration
- GPi
Globus pallidus interna
- IPG
Implantable pulse generator
- MICC
Multiple independent current control
- NIH
National Institutes of Health
- PD
Parkinson’s disease
- PPN
Pedunculopontine nucleus
- STN
Subthalamic nucleus
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- 1.Martinez-Ramirez D, Hu W, Bona AR, Okun MS, Wagle Shukla A. Update on deep brain stimulation in Parkinson’s disease. Transl Neurodegener. 2015;4:12. doi: 10.1186/s40035-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronstein JM, Tagliati M, Alterman RL, et al. Deep Brain Stimulation for Parkinson Disease. Arch Neurol. 2011;68(2):165–171. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gildenberg PL. Spiegel and Wycis – The Early Years. Stereotact Funct Neurosurg. 2001;77(1–4):11–16. doi: 10.1159/000064587. [DOI] [PubMed] [Google Scholar]
- 4.Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010;29(2):E1. doi: 10.3171/2010.4.FOCUS10106. [DOI] [PubMed] [Google Scholar]
- 5.Schwalb JM, Hamani C. The history and future of deep brain stimulation. Neurotherapeutics. 2008;5(1):3–13. doi: 10.1016/j.nurt.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70(2):163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- 7.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1–6):344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 8.Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson’s disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien). 1993;58:39–44. [DOI] [PubMed] [Google Scholar]
- 9.Pötter-Nerger M, Volkmann J. Deep brain stimulation for gait and postural symptoms in Parkinson’s disease. Mov Disord. 2013;28(11):1609–1615. doi: 10.1002/mds.25677. [DOI] [PubMed] [Google Scholar]
- 10.DeLong MR, Wichmann T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol. 2015;72(11):1354–1360. doi: 10.1001/jamaneurol.2015.2397. [DOI] [PubMed] [Google Scholar]
- 11.Pollak P, Benabid AL, Gross C, et al. [Effects of the stimulation of the subthalamic nucleus in Parkinson disease]. Rev Neurol (Paris). 1993;149(3):175–176. http://www.ncbi.nlm.nih.gov/pubmed/8235208. [PubMed] [Google Scholar]
- 12.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249(4975):1436–1438. http://www.ncbi.nlm.nih.gov/pubmed/2402638. [DOI] [PubMed] [Google Scholar]
- 13.Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35(6):1126–9– 30 http://www.ncbi.nlm.nih.gov/pubmed/7885558. [DOI] [PubMed] [Google Scholar]
- 14.Esselink RAJ, De Bie RMA, De Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: A randomized trial. Neurology. 2004;62(2):201–207. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L38167125\nhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=00283878&id=doi:&atitle=Unilateral+pallidotomy+versus+bilateral+subthalamic+nucleus+stimulation+in+PD:+A+randomized+trial. [DOI] [PubMed] [Google Scholar]
- 15.Blomstedt P, Hariz GM, Hariz MI. Pallidotomy versus pallidal stimulation. Park Relat Disord. 2006;12(5):296–301. doi: 10.1016/j.parkreldis.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Xu F, Ma W, Huang Y, Qiu Z, Sun L. Deep brain stimulation of pallidal versus subthalamic for patients with Parkinson’s disease: a meta-analysis of controlled clinical trials. Neuropsychiatr Dis Treat. 2016;12:1435–1444. doi: 10.2147/NDT.S105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 19.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25(5):578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 20.Odekerken VJJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 21.Odekerken VJJVJJ Boel JA, Schmand BABA, et al. GPi vs STN deep brain stimulation for Parkinson disease. Neurology. 2016;86(8):755–761. doi: 10.1212/WNL.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 22.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 23.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond A, Shahed J, Jankovic J. The effects of subthalamic nucleus deep brain stimulation on parkinsonian tremor. J Neurol Sci. 2007;260(1–2):199–203. doi: 10.1016/j.jns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Blahak C, Wöhrle JC, Capelle HH, et al. Tremor reduction by subthalamic nucleus stimulation and medication in advanced Parkinson’s disease. J Neurol. 2007;254(2):169–178. doi: 10.1007/s00415-006-0305-x. [DOI] [PubMed] [Google Scholar]
- 26.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Li W, Tan C, et al. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg. 2014;121(3):709–718. doi: 10.3171/2014.4.JNS131711. [DOI] [PubMed] [Google Scholar]
- 28.Sako W, Miyazaki Y, Izumi Y, Kaji R. Which target is best for patients with Parkinson’s disease? A meta-analysis of pallidal and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2014;85(9):982–986. doi: 10.1136/jnnp-2013-306090. [DOI] [PubMed] [Google Scholar]
- 29.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs Subthalamic Nucleus Deep Brain Stimulation in Parkinson Disease. Arch Neurol. 2016;62. [DOI] [PubMed] [Google Scholar]
- 30.Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79(1):55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portman AT, van Laar T, Staal MJ, Rutgers AWF, Journee HL, Leenders KL. Chronic stimulation of the subthalamic nucleus increases daily on-time without dyskinesia in advanced Parkinson’s disease. Park Relat Disord. 2006;12(3):143–148. doi: 10.1016/j.parkreldis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Oyama G, Foote KD, Jacobson CE, et al. GPi and STN deep brain stimulation can suppress dyskinesia in Parkinson’s disease. Park Relat Disord. 2012;18(7):814–818. doi: 10.1016/j.parkreldis.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Combs HL, Folley BS, Berry DTR, et al. Cognition and Depression Following Deep Brain Stimulation of the Subthalamic Nucleus and Globus Pallidus Pars Internus in Parkinson’s Disease: A Meta-Analysis. Neuropsychol Rev. 2015;25(4):439–454. doi: 10.1007/s11065-015-9302-0. [DOI] [PubMed] [Google Scholar]
- 35.Dietz J, Noecker AM, McIntyre CC, et al. Stimulation region within the globus pallidus does not affect verbal fluency performance. Brain Stimul. 2013;6(3):248–253. doi: 10.1016/j.brs.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gervais-Bernard H, Xie-Brustolin J, Mertens P, et al. Bilateral subthalamic nucleus stimulation in advanced Parkinson’s disease: Five year follow-up. J Neurol. 2009;256(2):225–233. doi: 10.1007/s00415-009-0076-2. [DOI] [PubMed] [Google Scholar]
- 37.York MK, Dulay M, Macias a, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):789–795. doi: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- 38.Rothlind JC, Cockshott RW, Starr PA, Marks Jr WJ. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13(1):68–79. doi: 10.1017/S1355617707070105. [DOI] [PubMed] [Google Scholar]
- 39.HMM S, JD S, Koning-Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology. 2006;66(12):1830–1836 7p. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=106149330&lang=nl&site=ehost-live&scope=site. [DOI] [PubMed] [Google Scholar]
- 40.Kalteis K, Standhardt H, Kryspin-Exner I, Brücke T, Volc D, Alesch F. Influence of bilateral Stn-stimulation on psychiatric symptoms and psychosocial functioning in patients with Parkinson’s disease. J Neural Transm. 2006;113(9):1191–1206. doi: 10.1007/s00702-005-0399-9. [DOI] [PubMed] [Google Scholar]
- 41.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: Summary and meta-analysis of outcomes. Mov Disord. 2006;21(S14):S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 42.Erola T, Heikkinen ER, Haapaniemi T, Tuominen J, Juolasmaa A, Myllylä VV. Efficacy of bilateral subthalamic nucleus (STN) stimulation in Parkinson’s disease. Acta Neurochir (Wien). 2006;148(4):389–393. doi: 10.1007/s00701-005-0662-8. [DOI] [PubMed] [Google Scholar]
- 43.Visser-Vandewalle V, van der Linden C, Temel Y, et al. Long-term effects of bilateral subthalamic nucleus stimulation in advanced Parkinson disease: a four year follow-up study. Parkinsonism Relat Disord. 2005;11(3):157–165. doi: 10.1016/j.parkreldis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Massano J, Bronstein JM. Writeclick: Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2016;86(16):1563–1564. doi: 10.1212/01.wnl.0000482821.31709.31. [DOI] [PubMed] [Google Scholar]
- 45.Troche MS, Brandimore AE, Foote KD, et al. Swallowing outcomes following unilateral STN vs. GPi surgery: a retrospective analysis. Dysphagia. 2014;29(4):425–431. doi: 10.1007/s00455-014-9522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkmann J, Albanese A, Kulisevsky J, et al. Long-term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson’s disease. Mov Disord. 2009;24(8):1154–1161. doi: 10.1002/mds.22496. [DOI] [PubMed] [Google Scholar]
- 47.van Nuenen BFL, Esselink RAJ, Munneke M, Speelman JD, van Laar T, Bloem BR. Postoperative gait deterioration after bilateral subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord. 2008;23(16):2404–2406. doi: 10.1002/mds.21986. [DOI] [PubMed] [Google Scholar]
- 48.Okun MS, Tagliati M, Pourfar M, et al. Management of Referred Deep Brain Stimulation Failures. Arch Neurol. 2005;62:1250–1255. [DOI] [PubMed] [Google Scholar]
- 49.Williams NR, Foote KD, Okun MS. Subthalamic Nucleus Versus Globus Pallidus Internus Deep Brain Stimulation: Translating the Rematch Into Clinical Practice. Mov Disord Clin Pract. 2014;1(January):24–35. doi: 10.1002/mdc3.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Marck MA, Munneke M, Mulleners W, et al. Integrated multidisciplinary care in Parkinson’s disease: A non-randomised, controlled trial (IMPACT). Lancet Neurol. 2013;12(10):947–956. doi: 10.1016/S1474-4422(13)70196-0. [DOI] [PubMed] [Google Scholar]
- 51.Morishita T, Rahman M, Foote KD, et al. DBS candidates that fall short on a levodopa challenge test: alternative and important indications. Neurologist. 2011;17(5):263–268. doi: 10.1097/NRL.0b013e31822d1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Ramirez D, Okun MS. Rationale and clinical pearls for primary care doctors referring patients for deep brain stimulation. Gerontology. 2014;60(1):38–48. doi: 10.1159/000354880. [DOI] [PubMed] [Google Scholar]
- 53.Okun MS, Foote KD. Parkinson’s disease DBS: what, when, who and why? The time has come to tailor DBS targets. Expert Rev Neurother. 2010;10(12):1847–1857. doi: 10.1586/ern.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verhagen Metman L, Pal G, Slavin K. Surgical Treatment of Parkinson’s Disease. Curr Treat Options Neurol. 2016;18(11):49. doi: 10.1007/s11940-016-0432-3. [DOI] [PubMed] [Google Scholar]
- 55.Volkmann J, Chabardes S, Steinke GK, Carcieri S. 375 DIRECT DBS: A Prospective, Multicenter Clinical Trial With Blinding for a Directional Deep Brain Stimulation Lead. Neurosurgery. 2016;63 Suppl 1(1):211–212. doi: 10.1227/01.neu.0000489863.00935.ea. [DOI] [Google Scholar]
- 56.Deuschl G, Schüpbach M, Knudsen K, et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson’s disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord. 2013;19(1):56–61. doi: 10.1016/j.parkreldis.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Mestre TA, Espay AJ, Marras C, Eckman MH, Pollak P, Lang AE. Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease-the EARLYSTIM trial: early is not always better. Mov Disord. 2014;29(14):1751–1756. doi: 10.1002/mds.26024. [DOI] [PubMed] [Google Scholar]
- 58.Thobois S, Ardouin C, Lhommée E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: Predictors and underlying mesolimbic denervation. Brain. 2010;133(4):1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 59.Zibetti M, Torre E, Cinquepalmi A, et al. Motor and nonmotor symptom follow-up in Parkinsonian patients after deep brain stimulation of the subthalamic nucleus. Eur Neurol. 2007;58(4):218–223. doi: 10.1159/000107943. [DOI] [PubMed] [Google Scholar]
- 60.Higuchi MA, Martinez-Ramirez D, Morita H, et al. Interdisciplinary Parkinson’s disease deep brain stimulation screening and the relationship to unintended hospitalizations and quality of life. PLoS One. 2016;11(5):1–13. doi: 10.1371/journal.pone.0153785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms . Brain Stimul. 2016;9(3):425–437. doi: 10.1016/j.brs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Farris S, Giroux M. Retrospective review of factors leading to dissatisfaction with subthalamic nucleus deep brain stimulation during long-term management. Surg Neurol Int. 2013;4:69. doi: 10.4103/2152-7806.112612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Ramirez D, Morishita T, Zeilman PR, Peng-Chen Z, Foote KD, Okun MS. Atrophy and other potential factors affecting long term deep brain stimulation response: a case series. PLoS One. 2014;9(10):e111561. doi: 10.1371/journal.pone.0111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ughratdar I, Samuel M, Ashkan K. Technological Advances in Deep Brain Stimulation . J Parkinsons Dis. 2015;5(3):483–496. doi: 10.3233/JPD-150579. [DOI] [PubMed] [Google Scholar]
- 65.Alonso F, Latorre M, Göransson N, Zsigmond P, Wårdell K. Investigation into Deep Brain Stimulation Lead Designs: A Patient-Specific Simulation Study. Brain Sci. 2016;6(3):39. doi: 10.3390/brainsci6030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord. 2016;31(8):1240–1243. doi: 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hariz M, Blomstedt P, Zrinzo L. Future of brain stimulation: New targets, new indications, new technology. Mov Disord. 2013;28(13):1784–1792. doi: 10.1002/mds.25665. [DOI] [PubMed] [Google Scholar]
- 68.Contarino MF, Brinke TR Ten, Mosch A, et al. How Many Patients would Benefit from Steering Technology for Deep Brain Stimulation? Brain Stimul. 2016;9(1):144–145. doi: 10.1016/j.brs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Akbar U, Raike RS, Hack N, et al. Randomized, Blinded Pilot Testing of Nonconventional Stimulation Patterns and Shapes in Parkinson’s Disease and Essential Tremor: Evidence for Further Evaluating Narrow and Biphasic Pulses. Neuromodulation. 2016;2015. doi: 10.1111/ner.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(12):4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215(1):20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Bour LJ, Lourens MAJ, Verhagen R, et al. Directional Recording of Subthalamic Spectral Power Densities in Parkinson’s Disease and the Effect of Steering Deep Brain Stimulation. Brain Stimul. 2015. doi: 10.1016/j.brs.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 73.De Jesus S, Almeida L, Peng-Chen Z, Okun MS, Hess CW. Novel targets and stimulation paradigms for deep brain stimulation. Expert Rev Neurother. 2015;15(9):1067–1080. doi: 10.1586/14737175.2015.1083421. [DOI] [PubMed] [Google Scholar]
- 74.Swann NC, de Hemptinne C, Miocinovic S, et al. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci. 2016;36(24):6445–6458. doi: 10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deeb W, Giordano JJ, Rossi PJ, et al. Proceedings of the Fourth Annual Deep Brain Stimulation Think Tank: A Review of Emerging Issues and Technologies. Front Integr Neurosci. 2016;10(November). doi: 10.3389/fnint.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2015:jnnp-2015–310972. doi: 10.1136/jnnp-2015-310972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashkan K, Samuel M, Reddy P, Ray Chaudhuri K. The impact of deep brain stimulation on the nonmotor symptoms of Parkinson’s disease. J Neural Transm. 2013;120(4):639–642. doi: 10.1007/s00702-012-0912-x. [DOI] [PubMed] [Google Scholar]
- 78.Chieng LO, Madhavan K, Wang MY. Deep brain stimulation as a treatment for Parkinson’s disease related camptocormia. J Clin Neurosci. 2015;22(10):1555–1561. doi: 10.1016/j.jocn.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 79.Contralateral W, Stimulation P. Improvement of Pisa Syndrome With Contralateral Pedunculopontine Stimulation Legend to the Video Transdermal Rotigotine in the Treatment of Aromatic L-Amino Acid Decarboxylase Deficiency. 2013;28(4):555–556. doi: 10.1002/mds.25301. [DOI] [Google Scholar]