Abstract
During breast/chest wall and regional nodal irradiation (RNI), standard 3D conformal techniques can fail to meet the dosimetric constraints for the heart and ipsilateral lung. VMAT can improve the dosimetric sparing of the heart and lungs. However the unnecessary increase in dose to the organs in the supraclavicular region as a result of using VMAT can be avoided. In this work we investigate potential dosimetric advantages of combining 3D with VMAT to improve sparing of these organs. Ten breast cancer patients requiring radiation therapy to the breast/chest wall and RNI including the IMNs, and who did not have a viable 3D conformal plan were chosen for the study. Each patient was planned with VMAT and with a combination of 3D for the supraclavicular region and VMAT for the breast/chest wall followed by a dosimetric comparison. Prescription dose was 50.4 Gy in 28 fractions. For similar coverage to the PTV and IMNs, doses to the esophagus and cord were reduced by 17.8 Gy and 15.5 Gy while mean dose to the thyroid and larynx were also reduced by 16.5 Gy and 11.7 Gy respectively. Maximum brachial plexus dose was the same in both techniques. The ipsilateral lung V20Gy increased by 3.1% but was still < 30%. No significant differences were noted in doses to the heart, total lung and contralateral breast. However V5Gy to the contralateral lung was reduced by 8.5% with the combined plan. Using 3D conformal planning for the supraclavicular region and VMAT over the breast/chest wall improves sparing of the esophagus, cord, thyroid and larynx while reducing low dose exposure to the contralateral lung and does not compromise doses to the heart, ipsilateral lung and total lung.
Introduction
The role of regional nodal irradiation (RNI) during postmastectomy radiotherapy (PMRT) to the chest wall reduces locoregional recurrence distant metastases and improves overall survival [1, 2]. With the recent publication of two randomized trials that showed improvement in disease-free survival in 1–3 node-positive breast cancer patients receiving whole breast irradiation (WBI) after breast conserving surgery (BCS) along with RNI is going to increase the likelihood of inclusion of the internal mammary nodes (IMNs) in the treatment volume [3, 4]. Depending on the anatomy, standard 3D conformal planning techniques may not suffice to produce a treatment plan that is able to adequately spare the heart and the ipsilateral lung while irradiating the target volume that includes the IMNs. Partially wide tangents (PWT) and photon/electron (P/E) matching are commonly used 3D conformal techniques; with the former being the most appropriate balance between target coverage and normal tissue sparing [5,6] although the P/E technique can be useful in certain situations when depth of the lung included in the tangential field is > 3 cm [7].
Irrespective of the technique, the requirement to irradiate the IMNs increases exposure to the nearby critical organs especially the heart and the ipsilateral lung. For certain unfavorable anatomies, VMAT has been shown to be useful in reducing dose to the heart and lungs while generating more conformal isodose distributions [8]. However, there is also an increase in low dose exposure to non-target tissue. Specifically, organs in the supraclavicular region can be spared from this unnecessary exposure. At our institution, we have used VMAT to treat patients who are unable to have a viable 3D conformal plan. However, patients are seen to develop symptoms of grade 2–3 esophagitis within the first 2 weeks of treatment. 3D conformal planning over the supraclavicular region typically consists of an oblique field that does not enter/exit through structures medial to the supraclavicular node. Therefore a combination of 3D conformal planning over the supraclavicular region and VMAT over the breast/chest wall is likely to reduce unnecessary exposure to organs in this region without compromising the advantage of VMAT in improved sparing of the heart and lungs over standard 3D conformal planning. In this work, we report on a dosimetric comparison of VMAT versus a combination of 3D and VMAT for breast cancer cases requiring radiation to the breast/chest wall and RNI including the IMNs.
Materials and methods
Patient selection
The Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai is the ethics and Institutional Review Board (IRB) committee that approved this study. 10 breast cancer patients (5 left sided and 5 right sided) were retrospectively chosen for the study. Since this was a retrospective study, the need for informed consent was waived. These patients required RNI including the IMNs, however due to unfavorable anatomy, standard 3D techniques such as PWT or P/E were found to irradiate too much heart and/or the ipsilateral lung. The first 6 out of these 10 patients were planned and treated with VMAT alone. However, 2 of these patients (~33%) were found to develop grade 2–3 esophagitis within completion of 30%-50% of their treatment. It was hence decided to plan and treat the next 4 patients using a combination of 3D conformal planning over the supraclavicular region and VMAT over the breast/chest wall to better spare the esophagus as well as other structures medial to the supraclavicular node. Patients in this study had stage II-IV breast cancer. Two patients had undergone a bilateral mastectomy with one patient receiving bilateral tissue expanders. Five patients had unilateral mastectomy and the remaining were intact breast. 6 patients who were treated using VMAT alone were retrospectively planned with a combination of VMAT and 3D conformal planning and 4 patients who were treated with the combination were retrospectively planned with VMAT alone. Patients were simulated in the supine position on an angle board with both arms raised and head turned away to the contralateral side. CT scans were obtained at 3 mm intervals extending from the chin to the upper abdomen during free breathing.
Target delineation
The clinical target volume (CTV) consisted of the chest wall/breast tissue, axillary level I, II, III, supraclavicular and IMNs and were done as per published guidelines [9]. The planning target volume (PTV) was formed by adding a 5 mm margin to the CTV in all directions and included the skin in the chest wall region for mastectomy cases. This margin was provided to account for respiratory motion and setup errors. For intact breast cases, the first 5 mm of the skin was excluded from the PTV. Critical organs contoured were the heart, ipsilateral lung, contralateral lung, total lung and contralateral breast/implant where applicable. The energy used for all the plans was 6 MV. Dose prescribed was 50.4 Gy in 28 fractions. Dosimetric constraints are outlined in Table 1.
Table 1. Dose constraints.
| Structure | Parameter | Constraint |
|---|---|---|
| PTV | D95 (%) V95 (%) D05 (%) |
≥ 95 ≥ 95 ≤ 115 |
| IMN | D95 (%) | ≥ 90 |
| Ipsilateral lung | V20Gy (%) | ≤ 30 |
| Heart | Mean (Gy) | ≤ 8 |
VMAT planning
VMAT plans were generated using 2 coplanar arcs. To decide upon the geometry of these arcs, first, the gantry angle at which the projected separation of the PTV in the beam’s eye view (BEV) was found to be the largest was chosen. Since treatment volumes tend to be large and due to limitations of MLC leaf travel, on certain LINACS as being 15 cm, coverage of the PTV had to be split into two arcs. These arcs overlapped at the isocenter by 2 cm and were within a 200° range. The collimator angle was kept at 0°. Details regarding the field arrangement have been published elsewhere [8]. All treatment plans were performed with the Eclipse treatment planning system version 13.6 (Varian Medical Systems, Palo Alto, USA). The anisotropic analytical algorithm (AAA) was used for dose calculation. The optimization algorithm used was the Progressive Resolution Optimizer (PRO). During optimization, priority was given to cover 95% of the IMNs with at least 90% of the prescription dose (i.e 45 Gy) or more while achieving D95, V95 ≥ 95% and D5 ≤ 115% for the PTV, followed by mean heart dose (MHD), ipsilateral lung V20 Gy and dose to the contralateral lung and breast/implant.
VMAT and 3D conformal planning
The target volume superior to the supraclavicular junction was planned using a single off-cord oblique supraclavicular field with appropriate blocking of the cord, esophagus and other structures medially while covering the supraclavicular node. The humeral head was blocked laterally. The energy used was 6 MV and the prescription depth was chosen accordingly such that nodal areas within the supraclavicular field were covered by at least 45 Gy. VMAT planning was used to cover the PTV inferior to the junction over the heart and the ipsilateral lung. Matching of the superior and inferior plans was accomplished using the single isocenter half beam block technique [10, 11] with asymmetric jaw. The collimator angle was kept at 0° for both the superior and inferior plans so that to prevent beam divergence into each other. The PTV for optimization purposes in this VMAT plan (PTV_OPT) was defined such that the superior-most slice was 6 mm inferior to the matchline/supraclavicular junction. This was done to avoid hotspots at the junction while planning with VMAT. On the other hand to avoid areas of underdosing at the junction, the slices of the PTV in between the level of the isocenter and the superior-most slices of the PTV_OPT were constrained in the optimizer to receive at least 45 Gy. This dose was determined iteratively such that the hotspot at the junction was limited to ≤ 105%. The VMAT plan was optimized such that priority was given to cover 95% of the IMNs with at least 90% of the prescription dose or more while achieving D95 and V95 of the combined plan to be ≥ 95% and the D5 at ≤ 115% for the PTV followed by mean heart dose (MHD), V20Gy of the ipsilateral lung and dose to the contralateral lung and breast/implant.
Dosimetric evaluation
Dose volume histograms (DVH) were generated and the dosimetric parameters collected for plans for each patient were compared. Statistical analysis was performed in MATLAB using the Wilcoxon signed-rank test at a significance level of ≤ 0.05. This test is a non-parametric hypothesis test used when comparing two related samples and does not assume the population to be normally distributed.
Results
Dosimetric comparison of critical organs namely the spinal cord, brachial plexus, esophagus, thyroid and larynx between VMAT versus VMAT + 3D is shown in Table 2, while comparison of PTV and IMN coverage, doses to the heart, ipsilateral lung, total lung, contralateral lung and contralateral breast/implant are shown in Table 3.
Table 2. Comparison of critical organ dose namely the spinal cord, brachial plexus, esophagus, thyroid and larynx for VMAT versus VMAT + 3D.
| Structure | Parameter | VMAT | VMAT +3D | P value |
|---|---|---|---|---|
| Cord | Maximum (Gy) | 28.0±1.9 | 12.5±2.0 | <0.01 |
| Brachial Plexus | Maximum (Gy) | 56.4±1.1 | 55.2±1.5 | NS |
| Esophagus | Maximum (Gy) | 45.0±2.2 | 27.2±4.5 | 0.03 |
| Mean (Gy) | 9.0±0.6 | 6.3±0.5 | <0.01 | |
| Thyroid | Mean (Gy) | 30.1±2.9 | 13.6±2.9 | <0.01 |
Table 3. Dosimetric comparison of PTV coverage, IMN coverage and critical organ doses namely the heart, ipsilateral lung, total lung, contralateral lung and contralateral breast for VMAT versus VMAT + 3D.
| Structure | Parameter | VMAT | VMAT+3D | P value |
|---|---|---|---|---|
| PTV | D95 (%) | 97.0±0.6 | 96.4±0.4 | NS |
| V95 (%) | 96.3±0.6 | 96.1±0.3 | NS | |
| D5 (%) | 113.2±1.2 | 113.3±0.9 | NS | |
| IMN | D95 (%) | 97.8±1.3 | 96.3±1.1 | NS |
| Heart | Mean (Gy) | 6.5±1.5 | 5.5±0.6 | NS |
| V25Gy (%) | 0.7±0.3 | 0.6±0.3 | NS | |
| V15Gy (%) | 4.0±1.4 | 3.9±1.4 | NS | |
| V5Gy (%) | 39.0±9 | 40.2±8.3 | NS | |
| Ipsilateral Lung | Mean (Gy) V20Gy (%) V10Gy (%) V5Gy (%) |
15.3±0.3 24.7±0.7 46.9±1.7 84.3±3.1 |
16.4±0.5 27.8±1.2 49.2±1.6 84.9±2.8 |
0.01 0.02 NS NS |
| Contralateral | Mean (Gy) | 3.6±0.4 | 3.2±0.3 | 0.02 |
| Lung | V10Gy (%) | 4.8±1.7 | 2.7±1.2 | NS |
| V5Gy (%) | 24.4±3.5 | 15.9±3.2 | 0.04 | |
| Contralateral Breast |
Mean (Gy) |
4.2±0.2 | 4.1±0.2 | NS |
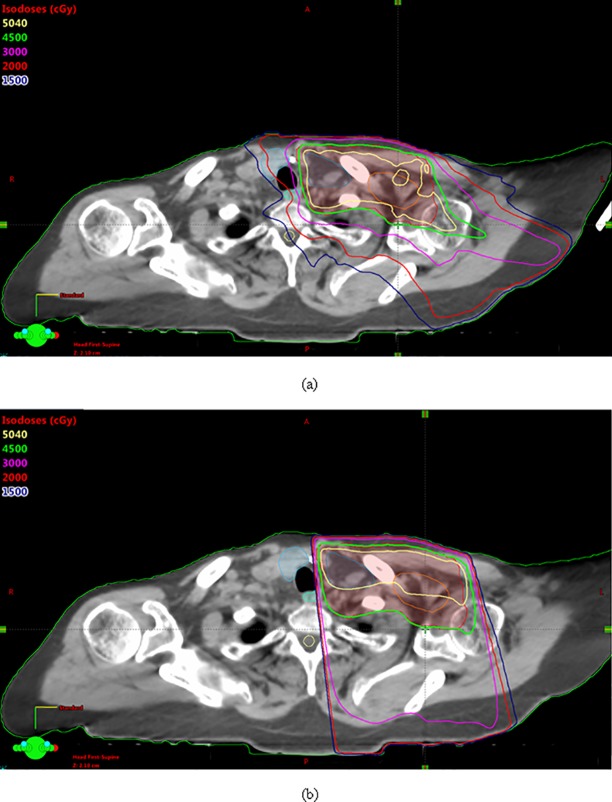
Dose distribution using only VMAT has been shown in Fig 1. Two partial coplanar arcs, one rotating in the clockwise and the other in the counterclockwise direction to cover the PTV are used for this plan. The arcs overlap at the isocenter by 2 cm and are 200° in range. The collimator angle used is 0°. Dose distribution using a combination of VMAT over the breast/chest wall region and 3D conformal planning with an off-cord oblique to cover the supraclavicular area is shown in Fig 2. Fig 3 shows a comparison of the dose distribution in the supraclavicular region in the axial plane between VMAT alone versus VMAT + 3D. The plan was done such that at least 45 Gy covered the supraclavicular PTV and nodes. Higher volumes of the esophagus, thyroid and the spinal cord are being covered by isodose lines lower than this prescription dose, namely 30 Gy, 20 Gy and 15 Gy in the VMAT plan. However in the combination plan, where a single off-cord oblique is used, lower volumes of these structures are exposed to these doses.
Fig 1.
Dose distribution in the axial (a), coronal (c) and sagittal (d) planes using only VMAT to cover the PTV, while (b) shows the field arrangement.
Fig 2.
Dose distribution in the axial (a), coronal (c) and sagittal (d) planes using a combination of VMAT over the breast/chest wall region and 3D conformal planning with an off-cord oblique to cover the supraclavicular area, while (b) shows the field arrangement.
Fig 3.
Dose distribution in the axial plan for the VMAT only plan (a) and the combination of VMAT + 3D (b).
PTV coverage
Differences in D95 and V95 for the PTV as well as D95 for the IMN were insignificant as were the differences in D5 for the PTV.
Critical organs in the supraclavicular region
Using the combination of VMAT and 3D, the maximum dose to the esophagus was significantly reduced by 17.8 Gy and that to the cord by 15.5 Gy over using VMAT alone. Mean esophagus, thyroid and larynx doses were also reduced by 2.7 Gy, 16.5 Gy and 11.7 Gy respectively. No difference was noted in maximum dose to the brachial plexus.
Heart
The mean heart dose (MHD) was lower with the combination of VMAT and 3D conformal planning by 1 Gy, however this was not statistically significant. Significant differences were also not noted in the V25Gy or in the volumes of the heart covered by lower doses such as V15Gy and V5Gy between the two techniques.
Ipsilateral lung
The ipsilateral lung V20Gy was increased with the combination of VMAT and 3D by 3.1% and the mean ipsilateral lung dose was also slightly raised by almost 1 Gy compared to using VMAT alone. These differences were found to be statistically significant. Volumes of the ipsilateral lung covered by lower doses namely V10Gy and V5Gy showed no significant difference between the two planning techniques.
Total lung
The difference in mean total lung dose for combination of VMAT and 3D versus VMAT alone was < 0.5 Gy and that in the V20Gy was < 1.5%. However, no significant differences in volumes of the total lung covered by lower doses namely V10Gy and V5Gy were noted.
Contralateral lung
The difference in mean contralateral lung dose was < 0.5 Gy between the two techniques. However, the V5Gy to the contralateral lung was reduced by 8.5 Gy on using a combination of VMAT and 3D, a result found to be statistically significant.
Contralateral breast
Mean contralateral breast dose was 4.2 Gy for VMAT and 4.1 Gy for combination of VMAT and 3D and was not significantly different between the two techniques.
Discussion
The choice of the appropriate planning technique when treating the IMNs strongly depends on the patient’s anatomy [5–8]. The PWT and P/E techniques are commonly used standard 3D conformal planning techniques, with the former considered to be a good balance of target coverage and dose to the heart and ipsilateral lung [6]. When a 3D conformal planning technique is able to meet the desired dosimetric constraints, is a preferable treatment planning and delivery technique because it uses fewer gantry angles and avoids increase in low dose to the patient. Each of the cases in our study had either a mean heart dose (MHD) ≥ 10 Gy and/or the ipsilateral lung V20 Gy ≥ 35% when using 3D conformal planning. It is understood that radiation to the heart is unavoidable for left-sided cases and inclusion of the IMNs only further increases this exposure and likelihood of developing ischemic heart disease in long term. The rate of a major coronary event (MCE) increases linearly with MHD at the rate of 7.4% per Gy [12]. Similarly, exposure to the ipsilateral lung cannot be ignored.
Studies in patients who received RT to the IMNs while maintaining the ipsilateral lung V20Gy < 30% showed ~6% grade 1 and 2 radiation pneumonitis (RP) and no incidence of grade 3 and 4 RP [13–15]. However, for patients in whom the ipsilateral lung V20Gy was increased to around 35%, the grade 1 and 2 complications were higher at 23% and 11.5% respectively. With 3D conformal planning, the concern tends to be the rather larger volumes of heart and ipsilateral lung covered by higher doses. Due to the use of multiple arcs/projections with VMAT, doses 30 Gy and higher are better conformed to the target volume while being carved around the heart and the ipsilateral lung [8]. This reduces volume of the heart and ipsilateral lung receiving 30 Gy or more compared with standard techniques, thus improving their sparing and reducing the risk of MCE and RP after RT. Due to increased use in projection angles for beam delivery, there is increase in low dose exposure with VMAT, which is unavoidable over the heart and ipsilateral lung. However, increase in exposure to other organs by the supraclavicular region such as the esophagus, spinal cord, larynx and thyroid is unnecessary. At our institution we noted that some patients started developing symptomatic esophagitis within 2 weeks of treatment. One way to mitigate this was to keep the standard 3D conformal planning technique to treat the supraclavicular region using an off-cord oblique field while utilizing VMAT only over the heart and ipsilateral lung where it was required to improve sparing. This helped reduce the mean and/or maximum dose to the esophagus, spinal cord, larynx and thyroid significantly without compromising sparing of the heart and lung achieved with VMAT inferior to the supraclavicular junction. Although the ipsilateral lung V20Gy was ≤ 30% in each case, on average it was slightly increased when 3D conformal planning was used to plan the supraclavicular region. This is because when planning using VMAT in this region, the isodose lines 20 Gy or higher are more conformal around the target volume, thereby sparing the lung as opposed to a single field which irradiates the entire lung in the field with at least 20 Gy.
The correlation between volume of heart covered by low dose such as 1 to 2 Gy from radiation therapy for breast cancer and the incidence of heart disease has been investigated in the literature [16]. Results indicate that there are no perfusion defects or cardiac function defects as a result of this low dose exposure. No worsening of these defects within a 1 year of exposure has been found either. In our study, with VMAT, we were able to reduce the MHD on average from 10 Gy with 3D conformal planning to ≤ 6.5 Gy using VMAT or a combination of VMAT for the breast/chest wall and 3D for the supraclavicular region, reducing the risk of MCE from radiotherapy in the long term for the patient. However, it is still desirable to keep the low dose exposure to the heart as low as possible while maintaining adequate coverage to the target because of the absence of long term follow-up data from treatments with VMAT for breast cancer. In addition to the heart, low dose to the lung also needs to be minimized. Studies indicate that total dose, dose per fraction and volume of irradiated lung influence the amount of lung morbidity that is caused from RT [17, 18]. In patients who received concurrent chemotherapy for esophageal cancer, the V5Gy of the contralateral lung was noted to be an important predictor of radiation pneumonitis (RP) [19]. Contralateral lung V5Gy ≥ 58% was associated with symptomatic RP ≥ grade 2. In our study, no significant differences were seen in low dose to the ipsilateral lung and total lung between VMAT versus combination of VMAT and 3D. Although the contralateral lung V5Gy was much lower in our study with both the techniques, it was still lower with the combination plan by almost 9%. This improved sparing of the contralateral lung is expected because of the absence of entrance and exit dose from VMAT arcs into the contralateral side in the supraclavicular area that is covered with a single oblique field.
Maximum dose to the brachial plexus showed no difference between the two techniques. This was because part of the brachial plexus was within the PTV below the supraclavicular junction covered by VMAT. Dose to the contralateral breast was not compromised on using the combination plan because most of the contralateral breast tissue in the superior-inferior direction was located by the region covered by VMAT.
Conclusions
In situations when 3D conformal planning is unable to meet dosimetric constraints for the heart and ipsilateral lung, VMAT can help meet the dose constraints for these structures. A combination of VMAT over the breast/chest wall and 3D conformal planning over the supraclavicular region can further help reduce unnecessary exposure to the esophagus, cord, thyroid and larynx without compromising on the dosimetric advantage already attained with the VMAT plan.
Supporting information
VMAT VERSUS VMAT+3D.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366: 2087–2106. 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 2.McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014; 383(9935): 2127–2135. 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015; 373(4): 307–316. 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015; 373(4): 317–327. 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 5.Marks LB, Hebert ME, Bentel G, Spencer D, Sherouse G, Prosnitz L, et al. To treat or not to treat the internal mammary nodes: A possible compromise. Int J Radiat Oncol Biol Phys. 1994; 29(4): 903–909. [DOI] [PubMed] [Google Scholar]
- 6.Pierce LJ, Butler JB, Martel MK, Normolle DP, Koelling T, Marsh RB, et al. Postmastectomy radiotherapy of the chest wall: Dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys. 2002; 52(5): 1220–1230. [DOI] [PubMed] [Google Scholar]
- 7.Kong FM, Klein EE, Bradley JD, Mansur BD, Taylor ME, Perez CA, et al. The impact of central lung distance, maximal heart distance, and radiation technique on the volumetric dose of the lung and heart for intact breast radiation. Int J Radiat Oncol Biol Phys. 2002; 54(3): 963–971. [DOI] [PubMed] [Google Scholar]
- 8.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010; 76(1): 287–295. 10.1016/j.ijrobp.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 9.Radiation Therapy Oncology Group. RTOG Breast Cancer Contouring Atlas. https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx
- 10.Li JG, Liu C, Kim S, Amdur RJ, Palta JR. Matching IMRT fields with static photon field in treatment of head-and-neck cancer. Med Dosim. 2005; 30(3): 135–138. 10.1016/j.meddos.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 11.Amdur RJ, Liu C, Li J, Mendenhall W, Hinerman R. Matching intensity-modulated radiation therapy to an anterior low neck field. Int J Radiat Oncol Biol Phys. 2007; 69(2): S46–S48. [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013; 368(11): 987–998. 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 13.Goldman UB, Anderson M, Wennberg B, Lind P. Radiation pneumonitis and pulmonary function with lung dose-volume constraints in breast cancer irradiation. Journal of Radiotherapy in Practice. 2014; 13: 211–217. 10.1017/S1460396913000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind PARM, Wennberg B, Gagliardi G, Fornander T. Pulmonary complications following different radiotherapy techniques for breast cancer, and the association to irradiated lung volume and dose. Breast Cancer Research and Treatment. 2001; 68: 199–210. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol. 1985; 4(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 16.Chung E, Corbett JR, Moran JM, Griffith KA, Marsh RB, Feng M, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys. 2013; 85(4): 959–964. 10.1016/j.ijrobp.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overgaard M, Bentzen SM, Christensen JJ, Madsen EH. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol. 1987; 9(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol. 1985; 4(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 19.Asakura H, Hashimoto T, Zenda S, Harada H, Hirakawa K, Mizumoto M, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol 2010; 95(2): 240–244. 10.1016/j.radonc.2010.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VMAT VERSUS VMAT+3D.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.