Abstract
Introduction:
Microbial involvement in colorectal cancer (CRC) is now well established. Short Chain Fatty Acids (SCFA) is the main products of anaerobic microbial fermentation in the large intestine and affects colonic health. SCFA mainly produced as microbial metabolites, acetate, propionate, and butyrate acids. Several in vitro studies showed that butyrate induce expression of heat shock protein (HSP) 70 that has function in the beginning of apoptosis.
Aim:
The aim of this study was investigating the differences level SCFA and HSP 70 expression in CRC compared with non-CRC patients.
Material and methods:
The study consists of 14 patients diagnosed with CRC and 14 non-CRC patients. Stool sample were analyzed for SCFA (acetate, propionate, and butyrate acids) with gas chromatography and the result is given as μg/mL and the protein expression of HSP70 was determined by immunohistochemistry (IHC) and haematoxylin-eosin staining to determine the morphological changes in colon tissue.
Results:
We found that CRC patients had lower level of acetate, propionate and butyrate acids than non-CRC. Whereas in CRC patients, the mean concentration of acetate was 8,55 μg/mL, propionate was 5,61 μg/mL and butyrate acids were 3,79 μg/mL respectively (all P < 0.05). And among the samples of patients with colorectal cancer was obtained the highest expression of HSP-70.
Conclusions:
Short chain fatty acids were indirectly contributed in the role of pathogenesis in CRC despite another factor could affect for this disease.
Keywords: Colorectal Cancer, Heat Shock Proteins 70, Microbiotas, Short-Chain Fatty Acids
1. INTRODUCTION
Colorectal cancer (CRC), been familiar as large bowel cancer, is the most general forms of cancer and listed as the third most often cause of cancer mortality in the world (1). Latest reports from the World Health Organization (WHO) indicated that the case of colorectal cancer is incredibly increasing at any countries in Asia, e.g.: China, Japan, Korea and Singapore (2). Whereas based on epidemiological report during 1996-1999 from Pathology Anatomy Division of Medical Faculty, Indonesia University, wrote the colorectal cancer patients with age under 40 years are around 36.26% (3). Research of epidemiology and experiments has shown that dietary fibre plays an important role of a healthy diet and may affect early symptoms at the carcinogenic process of CRC (4).
Short Chain Fatty Acids (SCFA) which consist of acetate, propionate and butyrate are final products of fermented dietary fibers produced by the anaerobic intestinal microbiota, have been indicated to put out multiple beneficial effects on mammalian energy metabolism (5). The level of SCFA found at fecal samples proved having a correlation with some diseases like IBD, irritable bowel syndrome (IBS), cardiovascular disease (CVD), diarrhea and cancer (6). Thus, some increasing at SCFA production and delivery of SCFA, especially butyrate to the distal colon will cause a protective effect (7). Heat shock protein (HSP) 70 is one of the most preserved and inducible proteins familiarly known recently with 60% phylogenetic identically between microbes and mammals. Several vitro researches indicated that butyrate produce expression of heat shock protein (HSP) 70 that has function in the beginning of apoptosis (8).
2. AIM
The aim of this present study was to investigate whether there are the differences level SCFA and HSP 70 in CRC compared with non-CRC patients.
3. MATERIAL AND METHODS
Participants and sample collection
The study consists of fourteen subjects with CRC and non-CRC was from in the Gastroenterology-Hepatology Department at Dr. Zainoel Abidin General Teaching Hospital Banda Aceh, Indonesia. The patients were selected based on the following inclusion criteria: 1) Patients aged 18 years or over; 2) Indonesian citizen that proved by identity cards; 3) Patients with colorectal cancer confirmed by pathological examination; 4) Patients instead of CRC; and 5) Patients who are able to cooperate in the study. None of the patients had active antibiotic treatment or within the month prior to the colonoscopy, yogurt consumption or laxative medicine for the last five weeks and none were treated with chemotherapy and/or radiotherapy in the previous six months. One stool sample was collected from each participant, where stool samples will be labeled and stored at -20°C freezer service by the researchers. The study was approved by the Ethical Review Committee of Medical Faculty, Syiah Kuala University, Banda Aceh, Indonesia.
Gas chromatography analysis of faecal SCFA concentration
Stool samples were analyzed for SCFA concentration with gas chromatography (GC) as described from a previous method (4, 8). The amounts of acetate, propionate and butyrate acids have been reported as μg/ml and %.
Histopathological Analysis and Immunohistochemistry
Observations for colorectal cancer were conducted by making the histology slide. To ensure the representativeness of each area selected in the paraffin blocks for immunohistochemical analysis, two samples were collected from different sections of the same block. A biopsy from patients was fixated on 10% formalin solution, followed by organ cutting and dehydration using serial alcohol (70%, 90%, Absolute), each for 60 minutes. The sample was cleared with xylol for 30 minutes. Then it was continued with impregnation, embedding and sectioning of paraffin blocks. The slide was stained using Haematoxylin-Eosin. Observation was conducted using electronic microscope BX-53 (Olympus). Histology classification was determined based on Lanza et al. (9), which classified the appearance as follow: Category one: Negative for neoplasia (+) , Category two: Indefinite for neoplasia (++), Category three: Mucosal low grade neoplasia, low grade adenoma, low grade dysplasia (+++) , Category four: Mucosal high grade neoplasia, high grade adenoma/dysplasia, non-invasive carcinoma, suspicious for invasive carcinoma, intramucosal carcinoma, intramucosal carcinoma (++++), Category five: sub mucosal invasion by carcinoma (+++++).
The expression of HSP70 on the slides was examined and scored with colour proportion by Leake Scoring (10), that direct count of the proportion of stain, stain intensity plus a measure of intensity of stain with two different pathologists.
Statistical analysis
The exact chi-square test and Student t-test were used for the comparisons between the groups, and nonparametric statistics was used in addition for variables without normal distribution. P-values < 0.05 were judged as statistically significant.
4. RESULTS
Total 28 participants were included in this study. Table 1 shows the characteristics of participants. There were 10 males and 4 females in the CRC group, and 9 males and 5 female in non-CRC group.
Table 1. Characteristic of participants in this study.
| Variable | Colorectal cancer (% or SD) | Non – colorectal cancer (% or SD) |
|---|---|---|
| Gender Male Female |
10 (72%) 4 (28%) |
9 (64%) 5 (36%) |
| Age (years, mean) | 53.8 ± 13.3 | 50.0 ± 17.6 |
| BMI (kg/m2, mean) | 20.21 ± 2.65 | 23.6 ± 1.91 |
| Hemoglobin (g/dl, mean) | 10.6 ± 2.1 | 12.3 ± 1.2 |
| Albumin (g/dl, mean) | 3.24 ± 0.71 | 3.91 ±0.53 |
| Colonoscopy | ||
| Ca Rectum | 11 (79%) | |
| Ca Colon Descending | 3 (21%) | |
| Colitis Infection | 10 (72%) | |
| Colitis Infection + Hemorrhoid Interna | 4 (28%) |
The means (±SD) was 53.8± 13.3 years for CRC and 50.0 ± 17.6 years for non-CRC. Haemoglobin, BMI and albumin in the CRC group was lower than non-CRC group. Percentage of cancers based on location: rectum 79% and colon descending 21%.
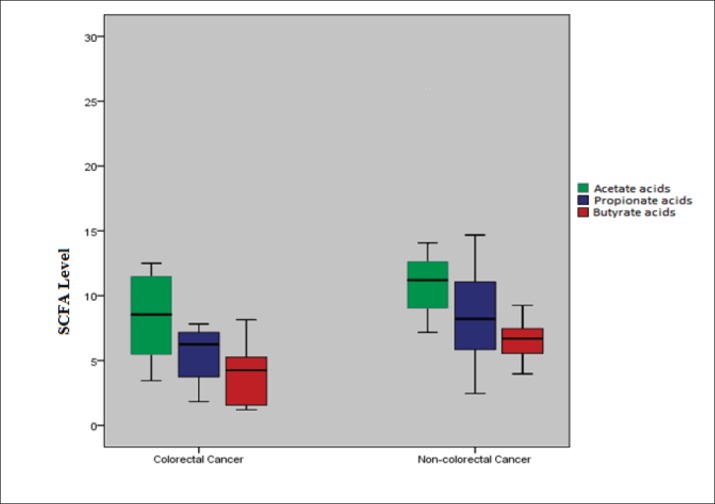
The mean fecal concentrations of acetate, propionate dan butyrate were significantly lower in patients with CRC compared non-CRC. The results revealed that the mean concentration of acetate 8,55 μg/mL, propionate 5,61 μg/mL and butyrate acids 3,79 μg/mL respectively (all P < 0.05) (Table 2 and Figure 1).
Table 2. Fecal short-chain fatty acids in subjects with and without colorectal cancer. The results are given as mean values with SD.
| Variable | Colorectal cancer patients (N=14) | Non-colorectal cancer patients (N=14) | P-value |
|---|---|---|---|
| Acetate Acids | 8.55 ± 3.06 | 11.78 ± 4.61 | 0.038 |
| Propionate Acids | 5.61 ± 1.95 | 8.61 ± 3.40 | 0.008 |
| Butyrate Acids | 3.79 ± 2.04 | 6.81 ± 2.59 | 0.002 |
Figure 1. Comparisons of fecal short chain fatty acids level in the colorectal cancer group and non-colorectal cancer.
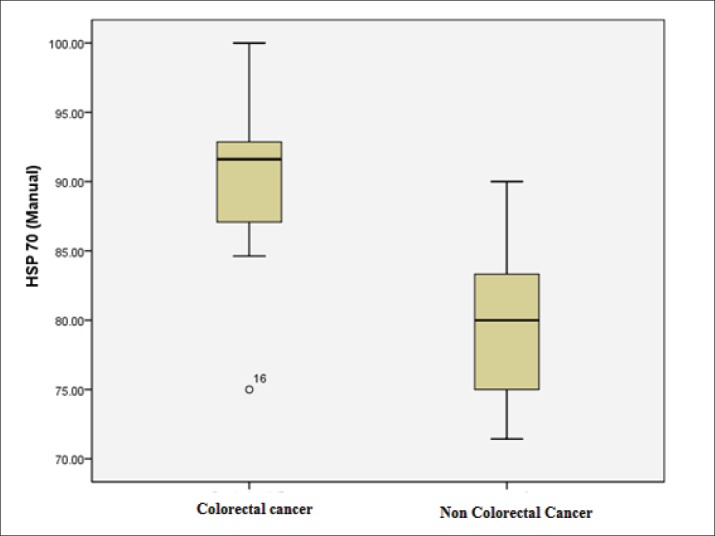
The level of HSP 70 was detected to be significantly higher in the CRC group compared with non-CRC group (89.73±5.55 vs 79.51± 4.90, respectively; P < 0.001) (Table 3, Figure 2).
Table 3. Between-group comparison of HSP-70 level in CRC compared non-CRC group * T test independent.
| Group | N | Mean 95% CI | SD | Min-Max | p* |
|---|---|---|---|---|---|
| CRC | 14 | 89.73 86.77-92.69 | 5.55 | 75.00-100.00 | <0.001 |
| Non-CRC | 14 | 79.51 76.90-82.12 | 4.90 | 71.43-90.00 | |
Figure 2. Box plot graph of expression of HSP70 in colorectal cancer patients and non-colorectal cancer patients. This study showed the highest expression of HSP70 in CRC than non-CRC patients.
5. DISCUSSION
The growing evidence shows that in intestinal microbial community influence the important part in the pathogenesis of the progression CRC. The microbial community has caused the fate of dietary fiber and its SCFA products.. The present study demonstrated that the plasma level of SCFA, acetate, propionate, and butyrate acids were decreased significantly in CRC patients with P < 0.05. In addition, among a sample of patients with colorectal cancer was obtained the highest expression of HSP-70.
The microbiota is expected to produce path physiological reactions like the activation of mucosal immune system, continuously intestinal permeability, activation of pathways sensoric and modulation of the enteric motility (10). SCFA are produced by the microbiota through the process of fermentation ingestible poly saccharides and proteins, and have been presented as the connection between microbes and the host. Among gut microbiota, several distinct bacterial community live at specific ratio of steady condition. The environment factors, lifestyle, disease and infections has caused the change compositions of bacterial communities (11, 12). The studies of epidemic have indicated the decreasing of butyrate producing gut bacteria, like those belongs to genus Roseburia and Lachnospiraceae, individual feces of colon cancer’s patients compared with healthy donors (13). The others of our study suggest that the appearance of Bifidobacterium as one of the indicators of detections for colorectal cancer (14).
There is a specific relation between SCFA levels and microbiota’s composition, the high luminal colonic PH (5.5–6.5 in proximal colon, meanwhile the highest fermentation level PH 6.5–7.0 located at the distal colon) and block the growth of Gram-negative Enterobacteriaceae including Salmonella spp and Escherichia coli. Specifically, butyrate has been reported to protect against the growth of colitis and colorectal cancer (15). In our study, we found that decreased significantly of SCFA level in CRC compared non-CRC patients.
There are various studies about fecal levels of butyrate from colonic neoplasia patients. Vernia et.al. comparing to 20 colorectal cancer’s patients, 8 colon polyps patients is no significant difference. However, at rectal cancer’s patients are shown a bit lower level of propionate and butyrate than the other with more proximal cancer (16). Hence, the molecular analysis report of SCFA effect in colonic tumors genesis might slightly explain these seemingly controversial observations. The researches made in recent years to understand basic physiology of SCFA should be developed in future to understand better about SCFA clinical role (10, 17).
HSP 70 is the most commonly studied of among other HSP and has many important protection functions. Stress would speed up the synthesis of HSP 70, which influence the death cell process of intestinal mucosal cells. Further connection between the gut commensal microbiota and HSP in vivo have been presented in growing pigs (18, 19). Zhang et al. reported that transcription factor of HSPs family such as HSP-60, HSP-70 and HSP- 90α were elevated significantly on colorectal cancer patient compare to para-cancerous tissue (20). Other previous study also showed increasing of HSP-70 on esophageal carcinoma and colorectal cancer (21). In our study, measurements of HSP-70 expression showed a significantly higher concentration in cancer patients compare to non-colorectal cancer patients.
The results of the present study are limited by the relatively low number of participants and a larger study population would provide enhanced statistical reliability. In addition, we did not study for identifies bacteria as far as we know, SCFA are the main products of anaerobic microbial fermentation. Another limitation is the fact that we cannot eliminate environment factors such as diet and everything related to microbiota.
6. CONCLUSIONS
In conclusion, the results of the present study show that the decreasing of SCFA concentrations is an important role in the pathophysiology of CRC. With the data from this study, the level of HSP-70 in patients with colorectal cancer was higher than non-colorectal cancer group.
Authors’ contributions:
All the authors were involved during the investigation process in all stages of this study including a primary data collection, analysis and the documentation of the collection.
Conflict of interest:
none declared.
REFERENCES
- 1.Gao R, Gao Z, Huang L, Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;(301):757–69. doi: 10.1007/s10096-016-2881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layton MC, Army M, Hospital C, Benning F. Colorectal Cancer Screening and Surveillance. Am Fam Physician. 2015;91(2):93–100. [PubMed] [Google Scholar]
- 3.Sudoyo AW, Hernowo B, Krisnuhoni E, Reksodiputro AH, Hardjodisastro D. Colorectal cancer among young native Indonesians: A clinicopathological and molecular assessment on microsatellite instability. Med J Indone. 2010;19(4):245–51. [Google Scholar]
- 4.Chen H, Yu Y, Wang J, Lin Y, Kong X, Yang C, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013:1044–52. 1–4. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 5.Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D, Bakker BM. The role of short-chain fatty acids in the interplay between diet , gut microbiota , and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faujani NH, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. The Open Biochemistry Journal. 2010;4:53–58. doi: 10.2174/1874091X01004010053. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonc P, Martel F. Regulation of colonic epithelial butyrate transport: Focus on colorectal cancer. Porto Biomedical Journal. 2016;1(3):83–91. doi: 10.1016/j.pbj.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatraman A, Ramakrishna BS, Shaji R V, Kumar NSN, Pulimood A, Patra S, et al. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am J Physiol - Gastroint Liver Physiol. 2003;285(1):G177–184. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- 9.Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. 2016 Colorectal Cancer: Global view Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501–518. doi: 10.3748/wjg.v22.i2.501. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, et al. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol. 2000;53(8):634–5. doi: 10.1136/jcp.53.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivaprakasam S, Prasad PD, Singh N. Benefits of Short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacology and Therapeutics. 2016:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf F, Ilyas S. Damanik HAR. Microbiota Composition, HSP70 and Caspase-3 Expression as Marker for Colorectal Cancer Patients in Aceh. Acta Medica Indonesiana. 2016;48(4):289–299. [PubMed] [Google Scholar]
- 15.Raskov H, Burcharth J, Pommergaard HS. Linking Gut Microbiota to Colorectal Cancer. J Cancer. 2017;8(17):3378–3395. doi: 10.7150/jca.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernia P, Cittadini M. Short chain fatty acids and colorectal cancer. Eur J Clin Nutr. 1995;49:18–20. [PubMed] [Google Scholar]
- 17.McNabney SM, Henagan TM. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients. 2017;9(12):1348. doi: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6(2):41–51. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Yin Y, Zhang Y, Wu G. Roles of heat-shock protein 70 in protecting againts intestinal mucosal damaged. Frontiers in Bioscienes. 2013;18:356–65. doi: 10.2741/4106. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W-L, Gao X, HAn J, Wang G, Yue L. Expressions of heat schock protein (HSP) family HSP 60, 70 and 90a in colorectal cancer tissues and their correlations to pathohistological characteristics. Chin J Cancer. 2009;28(6):1–7. [PubMed] [Google Scholar]
- 21.Black JD, Rezvani K. Heat Shock Protein 70s as Potential Molecular Targets for Colon Cancer Therapeutics. Curr Med Chem. 2016;23(28):3171–3188. doi: 10.2174/0929867323666160627105033. [DOI] [PubMed] [Google Scholar]


