Abstract
Cervical cancer (CC) is the leading cause of cancer death among female South Africans (SA). Improved access to reproductive health services following multi-ethnic democracy in 1994, HIV epidemic, and the initiation of CC population-based screening in early 2000’s have influenced the epidemiology of CC in SA. We therefore evaluated the trends in CC age-standardized incidence (ASIR) (1994 - 2009) and mortality rates (ASMR) (2004 - 2012) using data from the South African National Cancer Registry and the Statistics South Africa, respectively. Five-year relative survival rates and average percent change (AAPC) stratified by ethnicity and age-groups was determined. The average annual CC cases and mortalities were 4,694 (75,099 cases/16years) and 2,789 (25,101 deaths/9years) respectively. The ASIR was 22.1/100,000 in 1994 and 23.3/100,000 in 2009, with an average annual decline in incidence of 0.9% per annum (AAPC = -0.9%, P-value<0.001). The ASMR decreased slightly by 0.6% per annum from 13.9/100,000 in 2004 to 13.1/100,000 in 2012 (AAPC = -0.6%, P-value < 0.001). In 2012, ASMR was 5.8-fold higher in Blacks than in Whites. The 5-year survival rates were higher in Whites and Indians/Asians (60-80%) than in Blacks and Coloureds (40-50%). The incidence rate increased (AAPC range: 1.1% to 3.1%, P-value<0.001) among young women (25-34 years) from 2000 to 2009. Despite interventions, there were minimal changes in overall epidemiology of CC in SA but there were increased CC rates among young women and ethnic disparities in CC burden. A review of the CC national policy and directed CC prevention and treatment are required to positively impact the burden of CC in SA.
Keywords: Incidence, Mortality, Survival, Cervical Cancer, South Africa, Temporal trends, Trends, ethnicity
Introduction
Cervical cancer (CC) is a largely preventable disease, yet the global incidence is high with approximately 528,000 cases reported in 2012(1). More than 80% of these cases are in low and middle income countries (LMICs) (1). Globally, CC is the fourth leading cause of cancer deaths in women, but it is the leading cause of female cancer deaths in Sub-Saharan Africa (SSA)(1). In 2012, the estimated age-standardized incidence rate (ASIR) in the Eastern, Southern, Middle and Western African regions were 42.7, 31.5, 30.6 and 29.3 cases per 100,000 women, respectively compared to 4.4, 5.5, and 6.6 per 100,000 women in Western Asia, Australia/New Zealand and Northern American regions respectively (1). These disparities have been attributed to differences in the scales of CC prevention programs, that include sexual and reproductive health education, vaccination campaigns, access to CC screening, integration of health systems and management of Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) (1,2).
The Human Papilloma Virus (HPV) is a necessary cause of CC (2). Most HPV infections are sexually transmitted and the virus is usually spontaneously cleared by the body. However, about 2% of infections by ‘high risk’ (or oncogenic) strain of HPV persists in the cervix to cause premalignant and malignant lesions (1-3). In South Africa (SA), the common high risk HPV (hrHPV) for CC includes HPV 16,18,35,45,33 and 52 (in order of prevalence)(2). SA has the largest population of people living with HIV and HIV infection has been found to increase the rate of HPV infection and its persistence (2-5). Other common co-factors or predisposing factors for HPV infection and persistence includes smoking, early sexual debut, multiparity and multiple sexual partnership (2,3,6,7).
SA is an upper middle-income, multi-ethnic country. In 2015, the proportions of females in the four main population groups were 80.4% for Blacks, 8.9% for Coloureds (mixed ancestry), 8.3% for Whites and 2.4% for Indians/Asians (8). This classification of population groups is historical and reflects how data is collected in SA by Statistics South Africa (Stats SA) and the South African National Cancer Registry (SANCR). In the South African context, prevalence of CC risk factors and some HPV predisposing factors such as HIV/AIDS, smoking, risky sexual and reproductive behaviors, and socio-economic differences are associated with ethnicity (5,8-11). There are also ethnic differences with respect to accessibility to SA’s dual health system with most Black healthcare seekers utilizing the public health facilities and the majority of White healthcare users utilizing the private health facilities (10).
South Africa is currently undergoing an epidemiological and health transition characterized by a high burden of hypertension, type 2 diabetes mellitus, road traffic accidents and violence-related deaths, HIV/AIDS, other infections and under-nutrition (10,12). Following the dawn of multi-ethnic democracy in 1994, access to reproductive health services improved, especially among the previously marginalised ethnic groups (10). In 2000, the SA government initiated a CC population-based screening program (13) and in 2014, a national human papilloma virus (HPV) vaccination program for public school girls (≥ 9 years old) was implemented (14). In 2004, the SA government rolled-out a nation-wide anti-retroviral therapy (ART) program and the country currently has the largest global ART program (15). There has also been ongoing scaling-up of tobacco control programs since 1994 (5,11,13).
The SANCR was established in 1986 and is currently the only source of national data on cancer incidence (16). The registry publishes annual national cancer incidence, stratified by gender, population and age-group (16-18). On the other hand, Stats SA is responsible for collecting and publishing national vital statistics, including cancer mortality (16). Despite the available information from these data repositories, very little has been reported on the national trends of CC burden in the country. To appropriately evaluate the impact of CC interventions, a comprehensive understanding of the trends in CC burden over time is warranted. Hence, we evaluated the temporal trends in CC incidence, mortality and survival by ethnicity and age group in South Africa from 1994 to 2012.
Methods
We conducted a trend analysis of secondary data of CC incidence (1994 - 2009) and mortality (2004 - 2012), using data from SANCR and Stats SA respectively. At the time of commencement of the study, cancer incidence data from SANCR was not available beyond 2009 and cancer mortality data for Stats SA was only available until 2012.
Data Sources
The methodologies of the SANCR (16-18) and Stats SA (8,19) have been previously described but are summarised below.
South African National Cancer Registry (SANCR)
The SANCR receives information on pathologically confirmed cancer cases from all private and government laboratories in SA (17,18). The registry receives an average of 80,000 cancer cases per annum (16). The information received on each cancer case includes patient’s name, date and age at diagnosis, place and province of diagnosis, ethnicity/population group, name of the reporting laboratory and the tumour topography and morphology (17,18). Cancer cases are coded according to the International Classification of Diseases for Oncology Version 3 (ICD-O-3) and CC cases were coded as: ICD-O-3: Code C53. Hot-deck imputation techniques were used to account for missing data on population group (17,20).
Statistics South Africa (Stats SA)
Stats SA mortality data are derived from death notifications reported to the Department of Home Affairs (8). Causes of death were coded using the International Classification of Diseases, Tenth Revision (ICD-10) (9). The underlying cause of death with code ICD-10, C53 was used to obtain data on CC mortality. Mid-year population estimates of females (≥15 years) were obtained from Stats SA from 1994 to 2012.
Ethics
Ethical approval was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (Clearance certificate number: M141163). Permission to use the datasets was obtained from SANCR and Stats SA.
Statistical Analysis
Descriptive statistics of the socio-demographic and histological characterises of the CC cases and deaths were conducted. Analysis of Variance (ANOVA) was conducted to determine the relationship of age across the four ethnic groups (Post-Hoc Bonferroni test was also conducted). Pearson’s Chi Square test was conducted to determine association between ethnicity and the other categorical socio-demographic variables. Unknown age and ethnic group were not adjusted for in the analysis. Statistical significance level was set at P-value < 0.05.
The annual crude incidence (1994-2009) and mortality (2004-2009) rates (CIR, CMR) were calculated for five-year age groups among women ≥15 years with the appropriate mid-year population estimates (21). Age-standardized incidence (ASIR) and mortality (ASMR) rates were calculated using the direct method and the 1964 SEGI world standard population (1,21). The Mortality Rate : Incidence Rate (MR:IR) ratio was calculated by dividing the CMR by the CIR to estimate a standard, population-based measure of fatality (22). Five-year relative survival rates were calculated using the formula: 1-(MR:IR) (22) and are expressed as percentages. Annual survival rates were calculated for years in which incidence and mortality data were available (2004 - 2009).
Linear regression models were fitted to estimate the average annual percent change (AAPC) (95% confidence interval) in ASIRs and ASMRs. ln(rate) was the outcome variable while calendar year was the explanatory variable, using the equation below (23):
Thus,
Where β = coefficient of the calendar year, e = 2.7 and C = constant (intercept)
Based on the visual inspection of the graphs of the incidence and mortality trends, partitioned AAPC for incidence and mortality trends were computed for the periods: 1994-1998; 1998-2001; 2001-2009 and 2004-2008; 2008-2012, respectively. The variance–covariance matrix estimate (vce (robust)) option of linear regression was used to obviate any deviation from the linear regression assumptions. Calculations and analyses were conducted using Excel (Microsoft 2010) and Stata version 13 (Statacorp, College Station, TX) statistical software.
Results
Over the 16-year study period (1994-2009), 75,099 incident CC cases were reported and the average CC cases per annum were 4,694 (Supl Table 1). The majority of cases (90%) were diagnosed in public sector laboratories and squamous cell carcinoma (SCC) was the most common histological type (79.9%) (Table 1). Seventy-nine percent of all CC cases occurred among Black women and the least (1.4%) occurred in Indian/Asian women (Fig 1A, Table 1). The mean age for CC diagnosis (all population groups) was 52.3 (±13.7) years and almost half (43.1%) of the cases occurred in women of reproductive age (15-49 years) (Table 1). The mean age at diagnosis of CC was 50.9 years (±14.), 51.1(±13.1), 52.5(±13.7) and 53.0 (±12.8) among White, Coloured, Black and Indian/Asian ethnic groups respectively. (Table 2). One-third of CC cases among the White population were diagnosed at a private laboratory while other ethnic groups had significantly lower percentage of CC cases that were diagnosed from private laboratories (Table 2).
Table 1.
Demographic and histological characteristics of cervical cancer cases (1994-2009) and deaths (2004-2012) in South Africa
| Characteristics | Cervical cancer cases N= 75,099 N, (%) | Cervical cancer deaths N=25,101 N, (%) |
|---|---|---|
| Population group | ||
| Blacks | 59,333 (79.0) | 17,422 (69.4) |
| Coloureds | 5,548 (7.4) | 1,771 (7.1) |
| Whites | 6,062 (8.1) | 724 (2.9) |
| Indians/ Asians | 1,016 (1.4) | 218 (0.9) |
| Missing | 3,140 (4.2) | 4,966 (19.8) |
| Age (mean±SD) | 52.3 (±13.7) | 56.14 (±14.7) |
| <20 | 87 (0.1) | 14 (0.1) |
| 20-29 | 2,136 (2.8) | 515 (2.1) |
| 30-39 | 11,442 (15.2) | 2,775 (11.1) |
| 40-49 | 18,717 (24.9) | 5,647 (22.5) |
| 50-59 | 17,264 (23.0) | 6,117 (24.4) |
| 60-69 | 13,546 (18.0) | 4,936 (19.7) |
| 70-79 | 6,677 (8.9) | 3,492 (13.9) |
| 80 & above | 2,049 (2.7) | 1,565 (6.2) |
| Missing | 3,181 (4.2) | 40 (0.2) |
| Histologic types | ||
| SCC | 60,053 (79.9) | - |
| ADC | 7,300 (9.7) | - |
| Others | 7,746 ()10.3 | - |
| Cervical cancer site | - | |
| Endocervix | 1,611 (2.2) | - |
| Exocervix | 238 (0.3) | - |
| Overlapping | 121 (0.2) | - |
| Cervix Uteri | 73,129 (97.4) | - |
| Type of laboratory | ||
| Government | 67,564 (90.0) | - |
| Private | 7,535 (10.0) | - |
| Marital status | ||
| Never married | - | 9,088 (36.2) |
| Married | - | 5,153 (20.5) |
| Widowed | - | 1,954 (7.8) |
| Divorced | - | 2,349 (9.4) |
| Missing | - | 6,557 (26.1) |
| Smoking status | ||
| Yes | - | 1,771 (7.1) |
| No | - | 9,661 (38.5) |
| Missing | - | 13,669 (54.5) |
| Highest Education | ||
| No education | - | 3,175 (12.7) |
| Primary school | - | 4,406 (17.6) |
| High school | - | 3,926 (15.6) |
| Tertiary | - | 219 (0.9) |
| Missing | - | 13,375 (53.3) |
SD: Standard deviation
Figure 1.
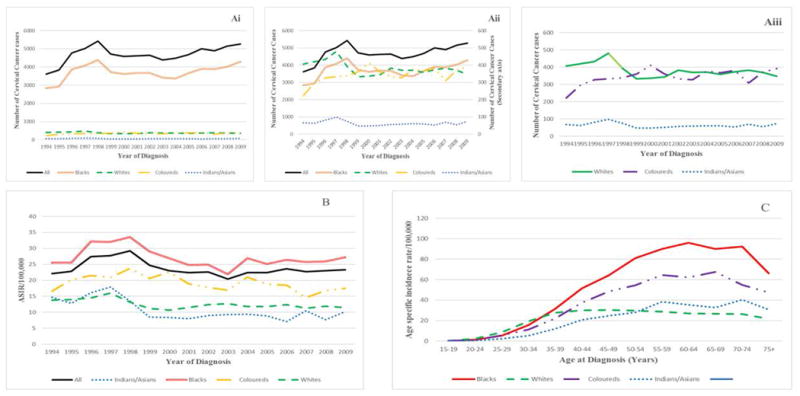
Cervical cancer incidence in South African women, by ethnic group (1994-2009) (Ai), (Aii), (Aiii) Annual numbers of cervical cancer cases (B) Annual age-standardized incidence rates (ASIR) and (C) Age-specific incidence rates for the study period (Note that secondary axis in Fig Aii applies to other ethnic groups other than Blacks)
Table 2.
Demographic and histological characteristics of cervical cancer cases and deaths by ethnicity in South Africa (1994-2012)
| Characteristics | Blacks N (%) | Coloureds N (%) | Indians/Asians N (%) | Whites N (%) | P-value |
|---|---|---|---|---|---|
| Demographic and histological characteristics of cervical cancer cases by ethnicity in South Africa (1994-2009) | |||||
| Age (Mean±SD) years | 52.5(±13.7) | 51.1(±13.1) | 53.0 (±12.8) | 50.9 (±14.7) | <0.001 |
| <20 | 72 (0.13) | 4 (0.07) | 0 (0.0) | 7 (0.12) | <0.001 |
| 20-29 | 1,600 (2.8) | 169 (3.2) | 23 (2.4) | 260 (4.4) | |
| 30-39 | 8,829 (15.6) | 858 (16.0) | 117 (12.1) | 1,193(20.3) | |
| 40-49 | 14,600(25.7) | 1,597(29.7) | 264 (27.2) | 1,546(26.3) | |
| 50-59 | 13,654(24.1) | 1,353(25.2) | 274 (28.2) | 1,261(21.5) | |
| 60-69 | 10,988(19.4) | 907 (16.9) | 180 (18.5) | 869(14.8) | |
| 70-79 | 5,399 (9.5) | 366 (6.8) | 91(9.4) | 519 (8.8) | |
| 80 & above | 1,591 (2.8) | 119 (2.2) | 22 (2.3) | 223 (3.8) | |
| Histologic types | |||||
| SCC | 47,493(80.0) | 4,642(83.7) | 735 (72.3) | 4,633(76.4) | <0.001 |
| ADC | 5,442(9.2) | 564(10.2) | 121(11.9) | 884(14,6) | |
| Others | 6,398 (10.8) | 342 (6.2) | 160 (15.7) | 545 (9.0) | |
| Cervical cancer site | |||||
| Endocervix | 1,131 (1.9) | 143 (2.6) | 21 (2.1) | 218 (3.60) | <0.001 |
| Exocervix | 142 (0.2) | 48 (0.9) | 2 (0.2) | 25 (0.4) | |
| Overlapping | 25 (0.04) | 76 (1.4) | 1(0.1) | 18 (0.3) | |
| Cervix Uteri | 58,035 (97.8) | 5,281 (95.2) | 992(97.6) | 5,801(95.7) | |
| Type of laboratory | |||||
| Government (Public) | 54,788(92.3) | 5,179(93.4) | 845 (83.2) | 4,073(67.2) | <0.001 |
| Private | 4,545 (7.7) | 369 (6.7) | 171 (16.8) | 1,989(32.8) | |
| Demographic characteristics of cervical cancer deaths by ethnicity in South Africa (2004-2012) | |||||
| Age (Mean±SD) | 56.1(±14.8) | 55.1(±13.4) | 58.3(±12.3) | 58.7(±15.8) | <0.001 |
| <20 | 22(0.13) | 1(0.06 | 0(0.00) | 0(0.00 | <0.001 |
| 20-29 | 358(2.1) | 31(1.8) | 0(0.0) | 14(1.9) | |
| 30-39 | 2,003(11.5) | 173(9.8) | 17(7.8) | 53(7.3) | |
| 40-49 | 3,909(22.5) | 434(24.5) | 39(17.9 | 165(22.8) | |
| 50-59 | 4,095(23.5) | 504(28.5) | 61(28.0) | 179(24.7) | |
| 60-69 | 3,480(20.0) | 356(20.1) | 54(24.8) | 113(15.6) | |
| 70-79 | 2,465(14.2) | 200(11.3) | 37(17.0) | 117(16.2) | |
| 80&above | 1,074(6.2) | 72(4.1) | 10(4.6) | 83(11.5) | |
| !Survival Period of mean age (years) | 3.6 | 4.0 | 5.3 | 7.8 | |
| Marital Status | |||||
| Never married | 6,690(51.4) | 558(41.2) | 27(15.2) | 101(18.1) | <0.001 |
| Married | 3,497(26.9) | 445(32.9) | 62(34.8) | 226(40.6) | |
| Widowed | 1,371(10.5) | 136(10.0) | 37(20.8) | 83(14.9) | |
| Divorced | 1,449(11.1) | 215(15.9) | 52(29.2) | 147(26.4) | |
| Smoking status | |||||
| Yes | 1,044(12.0) | 346(52.8) | 16(11.1) | 113(33.9) | <0.001 |
| No | 7,641(88.0) | 309(47.2) | 128(88.9) | 220(66.1) | |
| Highest Educational attainment | |||||
| No education | 2,561(29.2) | 109(18.1) | 14(10.6) | 12(3.6) | <0.001 |
| primary school | 3,325(37.9) | 263(43.8) | 52(39.4) | 22(6.6) | |
| high school | 2,743(31.2) | 225(37.4) | 63 (47.7) | 268 (80.5) | |
| Tertiary | 151(1.7) | 4(0.67) | 3(2.3) | 31(9.3) | |
SD: Standard deviation. ! mean age at death –mean age at diagnosis
Incidence trends
Figure 1B shows that although there was an overall 0.9% per annum average decrease in ASIR from 1994 to 2009 (AAPC= -0.9%, 95%CI: -0.91 to -0.88; P-value < 0.001), the ASIR increased by 7.6% per annum from 1994 (22.1/100,000 women) to 1998 (29.2/100,000), (AAPC = 7.6%, 95%CI: 7.60 to 7.62; P-value < 0.001) and subsequently decreased by 8.5% per annum until 2001 (22.4/100,000) (AAPC= -8.5%; 95% CI: -8.53 to -8.45; P-value < 0.001). Slight increase in CC rates was noted thereafter (2001-2009) (AAPC= 0.9%, 95%CI: 0.93 to 0.95; P-value < 0.001). Compared to the annual ASIR, annual crude incidence rates (CIR) were higher and of similar patterns (Supl Table 1).
The highest ASIR was observed among Black women (27.2 per 100,000) in 2009 and was 1.6-, 2.4- and 2.7-fold higher than in Coloured (17.5/100,000), White (11.5/100,000) and Indian/Asian (10.2/100,000) women respectively. While CC incidence increased among Black (AAPC=1.3%, 95% CI: 1.28 to 1.31; P-value < 0.001) and Indian/Asian women (AAPC=1.1%, 95% CI: 0.77 to 1.42) from 2001 to 2009, it decreased among Coloured (AAPC=-1.5%, 95% CI: -1.57 to -1.41; P-value < 0.001) and White (AAPC=-0.55%, 95% CI: -0.60 to 0.50; P-value < 0.001) women (Figure 1B).
From 2000 to 2009, the highest increase in AAPC for CC incidence was observed in women ≥75 years (AAPC=6.8%, 95% CI: 6.71 to 6.92; P-value <0.001), followed by those between 25 - 49 years (AAPC range: 1.1% to 3.1 %,). In the same period, the greatest decrease in incidence was observed among teenagers (15-19 years) (AAPC = -12.3 %, 95% CI: -15.89 to – 8.50; P-value <0.001) and lesser decreases were observed in women aged 50-64 years (AAPC range: -1.04% to -3.0 %,). The incidence rates among women aged 20-24 and 65-74 years were relatively stable (Table 3).
Table 3.
Age-specific trends in age standardized cervical cancer incidence (2000-2009) and mortality rates (2004–2012) in South African women
| Age group (years) | Incidence (2000 and 2009) | Mortality (2004-2012) | ||||
|---|---|---|---|---|---|---|
| AAPC (%) | 95%CI | P-value | AAPC (%) | 95%CI | P-value | |
| 15 – 19 | - 12.3 | -15.89 - -8.50 | <0.001 | 1.2 | -7.23 - 10.38 | 0.776 |
| 20 -24 | 0.02 | -0.45 - 0.49 | 0.937 | -4.5 | -5.76 - -3.23 | <0.001 |
| 25 – 29 | 2.6 | 2.35 - 2.94 | <0.001 | 4.1 | 3.75 - 4.40 | <0.001 |
| 30 – 34 | 2.8 | 2.69 - 2.89 | <0.001 | 6.3 | 6.00 - 6.68 | <0.001 |
| 35 – 39 | 2.9 | 2.85 - 2.98 | <0.001 | 0.9 | 0.75 - 1.07 | <0.001 |
| 40 – 44 | 3.1 | 2.99 - 3.12 | <0.001 | 0.7 | 0.65 - 0.84 | <0.001 |
| 45 – 49 | 1.1 | 1.03 - 1.10 | <0.001 | 0.7 | 0.60 - 0.71 | <0.001 |
| 50 – 54 | -1.2 | -1.26 - -1.19 | <0.001 | -1.0 | -1.09 - -0.98 | <0.001 |
| 55 – 59 | -1.0 | -1.08 - -1.00 | <0.001 | -1.5 | -1.5 - -1.41 | <0.001 |
| 60 – 64 | -3.0 | -3.03 - -2.96 | <0.001 | -1.5 | -1.55 - -1.35 | <0.001 |
| 65 – 69 | -0.4 | -0.47 - -0.38 | <0.001 | 0.6 | 0.51 - 0.65 | <0.001 |
| 70 – 74 | 0.3 | 0.17 - 0.41 | <0.001 | 0.7 | 0.61 - 0.76 | <0.001 |
| 75 and above | 6.8 | 6.71 - 6.92 | <0.001 | 0.7 | 0.64 - 0.76 | <0.001 |
CI: Confidence interval
The cumulative age-specific incidence rates over the study period increased with increasing age in all population groups (Figure 1C). Black women had the highest rates in all age groups, except in the 25-34 year age-group where White women had higher rates. The rates among Whites subsequently declined gradually. Age-specific incidence rates sharply declined among the elderly (beyond 65 years) in all population groups.
Mortality trends
Over the 9-year study period (2004 - 2012), 25,101 CC mortalities were recorded and the average number of deaths per annum was 2,789. The majority of deaths (69.4%) occurred among Black women (Figure 2A, Table 1). The mean age at CC death was 56.1(±14.7) years which was about 4 years after the mean incidence of 52.3 (±13.7) years. About one-third (35.7%) of CC mortalities occurred in women of the reproductive age group (15 - 49 years) (Table 1). Complete case analysis of mortality data indicated that 7.1% were smokers while of those whose educational status was known, 64% had primary education or lower. The highest and least mean age at CC death occurred among Whites and Coloureds respectively (Mean age at death of Whites, Indians/Asians, Blacks, Coloureds were: 58.7(±15.8), 58.3(±12.3), 56.1(±14.8), 55.1 (±13.4)). Table 2. White women had the longest survival period of 7.8 years between mean CC diagnosis and mean CC death. Table 2. About half (52.8%) and one-third (33.9%) of Coloureds and Whites who died of CC were smokers while a small proportion of Blacks (12.0%) and Indians/Asians (11.1%) were smokers. Table 2
Figure 2.
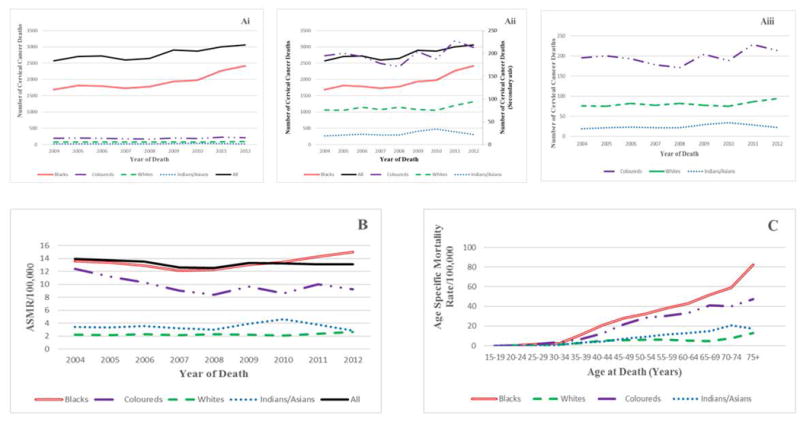
Cervical cancer mortality in South African women, by ethic group (2004-2012) (A) (Ai), (Aii), (Aiii). Annual numbers of cervical cancer deaths, (B) Annual age-standardized mortality rates and (C) Age-specific mortality rates. (Note that secondary axis on Fig 2Aii applies to other ethnic groups other than Blacks)
The overall (all population groups) ASMR over the 9-year study period slightly decreased from 2004 (13.9/100,000) to 2012 (13.1/100,000) (AAPC = -0.6% 95% CI: -0.60 to -0.58; P<0.001) (Figure 2B, Supl Table 1). We observed two phases in the mortality trends: There was an initial average decline of 3% per annum in mortality rates from 2004 to 2008 (AAPC = -3.0%, 95% CI: -3.04 to -3.55; P<0.001) and a subsequent slight increase of 0.8% per annum from 2008 to 2012 (AAPC = 0.8%; 95% CI: 0.76 to 0.81; P<0.001) (Figure 2B, Supl Table 1). The annual crude mortality rates were higher but of similar pattern as the annual ASMR throughout the study period (Supl Table 1). In 2012, the ASMR in Black women (15.0/100,000) was about 1.6, 5.2 and 5.8 fold higher compared to Coloured (9.2/100,000), Indian/Asian (2.9/100,000) and White women (2.6/100,000) respectively. An average annual increase in ASMR among Indian/Asian (AAPC=1.1%, 95% CI: 0.3 to 1.8; P<0.001), White (AAPC=1.4%, 95% CI: 1.4 to 1.5; P<0.001) and Black women (AAPC = 1.5%, 95% CI: 1.4 to 1.7; P<0.001) was observed between 2004 and 2012 while there was a decrease in CC mortality among Coloured women (AAPC= -2.9%, 95% CI: -2.7 to -3.0; P<0.001) (Figure 2B).
Table 3 illustrates that women in the younger age group (25-34 years) had the highest average annual increase in mortality rates (AAPC range: 4.1% to 6.3%) from 2004 to 2012. However, significant declines in the annual mortality rates were observed among women in the age groups 20-24 and 50-64 years (AAPC range: -4.5% to -1.5%).
Over the 9-year study period, the cumulative age-specific mortality rates increased with increasing age in all population groups (Figure 2C). A steep rise in mortality rates was observed from 30 years among all the population groups and the rates were lowest among White and highest in Black women. The rates in White women showed a steady decline from 40 to 69 years. There was a steep rise in mortality rates among the elderly in all population groups except among Indian/Asian women (Figure 2C).
Survival trends
The overall 5-year relative survival rates from 2004 to 2009 were poor (37.9-45.7%). Although, the Indians/Asians had a point value in 2006 where the survival rate was 49.3%, White (80.0–82.1%) and Indian/Asian (60.8-69.5%) women had relatively high 5-year relative survival rates during the period of study compared to Coloured (37.7-50.0%) and Black women (48.5-52.2%). An overall increase in 5-year relative survival rate of 13.2% was observed between 2004 and 2009, driven by gains among the Coloured (8.8%) and Black ethnic groups (7.6%). However for the same period a decline in survival rate was observed among Indian/Asian (-3.2%) and White women (-1.7%). Figure 3
Figure 3.
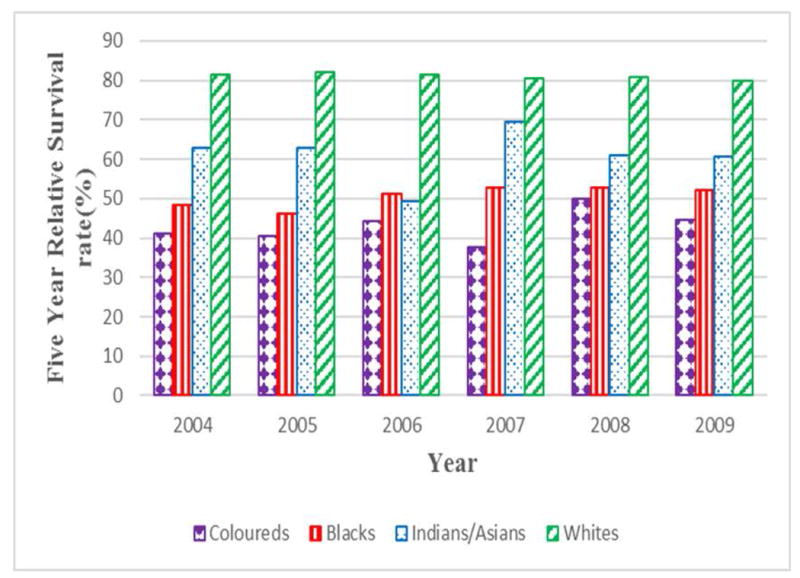
Annual five-year relative survival rates (1-(MR:IR)) of cervical cancer by population group in South African women (2004-2009)
Discussion
This is the first study to report and compare temporal trends in national CC incidence, mortality and survival by ethnicity and age group in SA after the commencement of multi-ethnic democracy in 1994 and during the ART era (2004 - 2012).
Summary of main findings
We reported that approximately 5,000 new CC cases and 3,000 deaths occurred annually in SA from 1994 to 2012. There was a slight increase in CC ASIR (0.9% per annum) from 2001 to 2009. The CC ASMR declined by 3% per annum between 2004 and 2008 but subsequently increased by 0.8% per annum from 2008 to 2012. Ethnic differences in the epidemiology of CC were observed. Black and Coloured women had higher incidence and mortality rates and lower 5-year relative survival rates compared to Indian/Asian and White women. Women of the reproductive age (15-49 years) accounted for almost half of CC cases and about a third of mortalities. Also, young women had increased incidence (25-49 years) and mortality (25-34 years) trends.
Incidence trends
A sharp increase in CC incidence (7.6% per annum) between 1994 and 1998 was observed. This could be attributed to the commencement of multi-ethnic democracy in 1994 that reduced barriers to reproductive health services, especially among the Black population, who contributed about four-fifths of the total CC incidence. This may have led to increased diagnosis and reporting of CC to SANCR (10,20,24).
The incidence rates of CC also increased by 0.9% per annum from 2001 to 2009, though at a much slower rate compared to the 1994-1998 period (0.9% per annum Vs 7.9% per annum). The slight increase in incidence from 2001 to 2009 mirrored the complex interactions between HIV prevalence, ART coverage and CC preventive programs. SA’s HIV prevalence rate increased by about 32% from 2002 (15.1%) to 2012 (19.9%), among individuals who were older than 24 years, because increased nationwide coverage of ART in the country led to improved longevity of People living with HIV/AIDS (PLWHA) (5,19). Thus, the increased CC rates from 2001 to 2009 could be related to increased HIV prevalence in the country. Since PLWHA that were on ART are encouraged to screen more frequently, the rate of diagnosis of early staged CC cases could have increased during the ART era (25). Dryden-Peterson and co-workers also reported increased trends in CC incidence after ART expansion in Botswana (26), but Uganda and Zimbabwe did not have similar patterns as SA, possibly because of low ART coverage (27,28).
Although the advent of ART dramatically reduced the incidence of other AIDS-defining cancers like non-Hodgkin’s lymphoma and Kaposi sarcoma, the impact of ART on the incidence of CC remains unclear (6,29). Several studies have demonstrated that ART reduces HPV persistence, increases the rate of regression of pre-cancerous lesions and stimulates immune reconstitution, thereby reducing the risk of CC (6,7). Although ART reduced early deaths from opportunistic infections, nonetheless PLWHA that were on ART still have chronic latent defective immunity that predispose them to CC at older age (26,30,31). Interestingly, emerging in-vitro evidence shows that some anti-retroviral drugs (especially protease inhibitors) have therapeutic anti-CC effect at the onco-genetic level (32). Nevertheless, current empirical evidence calls for concerted CC surveillance among PLWHA (including those on ART).
Of note, in 2000, SA introduced a population-based CC screening program (13). The initial paradoxical increase in incidence of early staged CC after the commencement of a population based screening program (10,33) seems to have occurred in SA since there was a slight increase in CC of about 0.9% per annum from 2001 to 2009. Nonetheless, the subsequent decline in CC incidence rate that is traditionally associated with an effective screening program is not yet observed in SA. This may be attributed to a number of reasons. The CC screening program is not widely established in SA, and the targeted 70% screening coverage rate for maximal efficacy was not achieved in most provinces (10,34). The low coverage rate was associated with poor awareness among the targeted population coupled with inadequate health personnel and infrastructure (10,34). Furthermore, the prevalence of HIV infection is higher in SA than in high income countries that have well established CC screening programs(2,6). This may also explain the lag in the impact of current CC screening in the country.
Mortality trends
The overall ASMR slightly declined by 0.6% per annum from 2004 to 2012. This is in contrast to the upward trends reported in other SSA countries (35,36). Two phases in mortality trends were observed in this study. The first phase was a decline at 3% per annum from 2004 to 2008 and the second phase was an increase of 0.8% per annum from 2008 to 2012. Such patterns previously occurred in some high income countries because of sporadic shifts in risk factors of CC and screening practices (37,38). However, the reason for the pattern in SA appears to differ.
The reduction in CC mortality from 2004 to 2008 in SA may be related to the nation-wide roll-out of ART in SA in 2004, since CC is an AIDS defining illness which was the leading competing risk of premature death in SA prior to the roll-out of ART (8,15,19,39). Furthermore, ART improves outcome of CC in HIV positive patients. Hence, initiation of ART is a standard of care in HIV positive ART naïve CC patients. Thus, increased access to ART among HIV positive CC women who would have died from complications of HIV engendered improved quality of life and longevity (19,39).
The slight increase in CC mortality from 2008 to 2012 could be due to an improvement in reporting and recording of cancer mortalities in the country (40). We however speculate that the increased CC mortality between 2008 and 2012, after increased ART coverage can be partly attributed to the improved longevity among PLWHA that were on ART that unmasked the background impact of epidemiological and health transition among them. Thus, associated cardiovascular and other risks of death may supervene (10,12,19,24,39). Nevertheless, the overall downward CC mortality trend between 2004 and 2012 in SA may suggest some improvement in oncology care after the apartheid era, as the democratic government made efforts to reduce the inequity related to healthcare access in the country.
Ethnic disparity in trends
Findings from this study indicate significant differences in the CC burden by ethnicity. Indian/Asian and White women in SA had relatively low incidence and mortality rates, with higher survival rates compared to Black and Coloured women. However, the survival rates in Blacks and Coloured women were still higher than in other LMICs (28,41,42). The ethnic disparity of CC trends in SA contrasts with the trends in the United States of America (USA) where rates in all ethnic groups declined considerably during a similar time period as our study (43).
The low incidence and mortality rates observed among Indian/Asian women could be attributed to the less risky sexual behaviours and low HIV and smoking prevalence among them (5,20,44,45). Higher socio economic status, greater access to healthcare facilities (especially screening facilities), low parity and low HIV prevalence could have accounted for the low incidence and mortality rates in White women (5,44,45). In contrast, Blacks and Coloureds had higher incidence and mortality rates because they had: relatively poor access to healthcare and CC screening, low socio economic status and high prevalence of HIV, tobacco smoking and teenage pregnancy (5,10,44,45). While other ethnic groups had slightly increased CC mortality rate from 2004 to 2012, a 3% decline in CC mortality rate occurred among the Coloured women during the same period. Since HIV prevalence and smoking rates were very high among the Coloured women, the roll-out of ART in 2004, coupled with the public health interventions on tobacco control in the country may partly explain the significantly reduced trends among the Coloured women (5,9). However, our study highlights the need for further investigation of drivers of ethnic disparity in CC trends in the country.
The high survival rates among Indian/Asian and White women may be because they presented to hospital with early stage disease that is amenable to curative treatment (28,41). The modest increase in the survival rates in the general population, more importantly among Coloreds and Blacks may suggest some relatively improved access to CC down staging procedures, oncological care in public healthcare facilities and possibly ART coverage. At the population level, it appears that there was a poor average CC survival period of four years from mean age of diagnosis of 52.3years to mean age at death of 56.1 years. However, this observation should be interpreted with caution as ecological fallacy, sub-population variables and the limitations of the data may play varying roles in this conclusion
Differential ethnic hrHPV prevalence can influence differences in ethnic CC rates (2,3). However, the researchers could find no previous study on ethnic prevalence of hrHPV in SA. Emerging evidence suggest that a contributory factor to observed ethnic disparity of CC burden is genetic polymorphism (46). Such genetic information can aid the design of a targeted, cost effective and risk-based CC preventive (or screening) programs among vulnerable ethnic groups (46). Nevertheless, the impact of such genetic information may not supersede the current evidence-based preventive interventions and concerted efforts aimed at reducing ethnic socio-economic and healthcare inequity, that is presently believed to be the driver of ethnic disparity of CC evolution (10,19,43,47).
In addition to evaluating the incidence and mortality rates, our study utilized the complement of the ratio of mortality to incidence rate to estimate survival rates and obtained results that were plausible and comparable to rates from other LMICs and high income countries that used the traditional methods of survival estimation (33,41,42,48). This approach provides a useful proxy estimation of population-level 5-year relative survival rate of CC, especially in countries without robust follow-up and cancer related data linkage (22).
Temporal trends for age at diagnosis and death
CC incidence and mortality were hitherto rare in women younger than 35 years (1,13,25,28). However, this study illustrated that about half of all the reported cases and a third of the mortalities occurred in women of the reproductive age (15-49 years). The increasing rates of CC at young ages (25-49 years) in SA may be associated with the high prevalence of HIV in this age group, poor health care access (including screening), decreasing age at sexual debut and prevalent teenage pregnancy (38,49). However, initiation of ART in 2004 could have accounted for a reduction in mortality rates among women aged 20-24 (AAPC= -4.5%), since HIV/AIDS is a major driver of CC in young women. This study highlights the need to fully incorporate CC prevention programs into maternal and child health initiatives, since a considerable proportion of CC patients were women of the reproductive age (31). In addition, programs that ensure linkages and cooperation between CC preventive programs and institutions that engage young women (such as immunisation programs, educational institutions, banks, hotels and markets) should be encouraged. This will improve coverage of CC preventive efforts among the target population (31).
We observed that White women had the lowest mean age at CC diagnosis (50.9 years) and the highest mean age at death (58.7 years). Furthermore, the age specific incidence rates was slightly higher among Whites between 25 and 34 year age-group and it subsequently declined in older age group. The foregoing may suggest that White women had more opportunities for CC screening and diagnosis at early age of between 25 and 34 years as compared to the other ethnic groups(45). The difference in mean age at diagnosis and age at death was highest (about 7.8years) among the White population which may be a reflection of early diagnosis and improved survival among them. Although, some CC diagnoses also occurred among young Black women on account of HIV prevalence, most Black women were still diagnosed at later age (and probably at advanced disease stage) possibly because of poor screening practice among them (34,45). Thus, Blacks had low difference in mean age at diagnosis and mean at death (3.6 years). Similarly, the age specific incidence rates in Black and Coloured women were high until age 65 years. This is comparable to reports among Black women in Harare, Zimbabwe during the same study period (27). The sharp decline in the age specific incidence rates beyond 65 years might be due to poor ascertainment since histological diagnosis may be difficult to obtain in the elderly (50). As expected, age-specific mortality rates increased steeply in the elderly (26).
Limitations
Since SANCR is a passive pathology-based registry, CC patients without histological diagnoses are not reported to the registry (27,42,50). Furthermore, under-reporting of diagnosed CC cases to SANCR and CC deaths to Stats SA may also have occurred (16). (about 20% of SA female deaths were not reported circa 2004-2007)(40). The majority of Blacks and Coloureds utilised public laboratories while Private Health facilities are mainly accessed by the Whites and some Indians/Asians, because of differences in socio-economic status (Table 2) (16). While all public laboratories report cancer cases to SANCR, private laboratories may not have adequately reported cancer cases to SANCR from 2005 to 2007 because of concern about patients’ confidentiality. This may cause underreporting of cancer incidence among Whites and Indians/Asians during that period(16). However, this suspicion may not have affected CC incidence as our study did not reveal a sharp dip in incidence rates among Whites and Indian/Asians from 2005 to 2007. Furthermore, a higher proportion of unreported cancer deaths may arise among the Black population as more deaths may occur outside the healthcare facility. However, it is mandatory for all deaths (both within and outside the hospital) to be reported in SA(8)
Nevertheless, there has been marked improvement in the rate of reporting of cancer cases and deaths to SANCR and Stats SA in recent years (9,16). Thus, our study reported the minimal CC incidence and mortality rates in SA which were still unacceptably high. The study was unable to relate survival rates with cancer stage because such information was not available from either the SANCR or Stats SA. Despite these limitations, these data remain an important primary source to understand and create innovative strategies, interventions and policies to alleviate CC burden in SA.
Conclusion
In summary, we demonstrate that despite preventive strategies and interventions, there was a slight increase in CC incidence between 2001 and 2009 (which mirrors increased ART coverage) and a slight decrease in CC mortality between 2004 and 2012 in SA. A significant ethnic disparity in CC burden still exists and CC incidence and mortality rates increased among young women (25-34 years) during the study period. There is therefore a need for a review of current CC preventive policies and control programs in the country.
Supplementary Material
Novelty and Impact of the study.
This study suggest that there was no meaningful change in overall and racial disparity in CC rates over the last two decades (1994 – 2012) in South Africa (SA), despite efforts to reduce racial health inequity and initiation of population based screening in the country. The increased coverage of ART program in SA appears to increase CC incidence but caused reduction in CC mortality. For the first time in Africa, the complement of mortality to incidence ratio was estimated as proxy of CC survival.
Acknowledgments
The authors are thankful to the Stats SA and SANCR for making data available for this study. Additionally, we thank late Dr Danuta Kielkowski, Prof Freddy Sitas, and Dr Segun Bello for their significant inputs in this work. This research is part of post-graduate student work, supported by University of the Witwatersrand, South Africa.
Funding:
Funding was provided by the South African Medical Research Council by a grant awarded to the Wits Common Epithelial Cancer Research Centre. Dr Gbenga Olorunfemi acknowledges the sponsorship of the Medical Education Partnership in Nigeria (MEPIN/Fortgarty/ NIH Award number R24TW008878) for a fellowship award (Seed 02 award). Also, the School of Public Health, University of the Witwatersrand awarded Dr Gbenga Olorunfemi a travel grant to attend the Public Health Association of South Africa (PHASA) conference in Durban, 2015 to present the preliminary report of this study. The funders have no role in the study design, conclusions and decision to publish.
Abbreviations
- AAPC
Average annual percent change
- ADC
Adenocarcinoma
- ART
Anti-retroviral Therapy
- ASIR
Age standardized incidence rate
- ASMR
Age standardized mortality rate
- CC
Cervical cancer
- CIR
Crude Incidence Rate
- CMR
Crude Mortality Rate
- hrHPV
High risk Human Papilloma Virus
- HIV/AIDS
Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome
- HPV
Human Papilloma Virus
- ICD-O3
International Classification of Diseases for Oncology Version 3
- ICD-10
International Classification of Diseases, Tenth revision
- LMIC
Low and middle income country
- PLWHA
People living with HIV/AIDS
- SA
South Africa
- SANCR
South African National Cancer Registry
- SCC
Squamous cell carcinoma
- SSA
Sub Saharan African countries
- Stats SA
Statistics South Africa
- VCE (robust)
variance–covariance matrix estimate (robust)
Footnotes
The abstract from this study was presented at the African Cancer Congress organized by the African Organization for Research and Training in Cancer (AORTIC) at Kigali, Rwanda (7-10 November, 2017).
Conflict of Interest: None declared
References
- 1.Ferlay J, Soerjomataram I, I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer [Internet] 2014;136(5):E359–86. doi: 10.1002/ijc.29210. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25220842%5Cnhttp://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [DOI] [PubMed] [Google Scholar]
- 2.Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer [Internet] 2014;134(6):1389–98. doi: 10.1002/ijc.28425. Available from: http://doi.wiley.com/10.1002/ijc.28425. [DOI] [PubMed] [Google Scholar]
- 3.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX de SS. Human Papillomavirus and Related Diseases Report [Internet] ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre) 2017 Available from: http://www.hpvcentre.net/statistics/reports/ZAF.pdf.
- 4.National Department of Health Republic of South Africa. White paper. Pretoria: 2015. National Health Insurance for South Africa [Internet] pp. 4–101. Available from: http://www.gov.za/sites/www.gov.za/files/39506_gon1230.pdf. [Google Scholar]
- 5.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. HSRC Press. Cape Town: HSRC Press; 2014. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012; p. 194. [Google Scholar]
- 6.Firnhaber C, Westreich D, Schulze D, Williams S, Siminya M, Michelow P, et al. Highly active antiretroviral therapy and cervical dysplasia in HIV-positive women in South Africa. J Int AIDS Soc. 2012;15(2):2–7. doi: 10.7448/IAS.15.2.17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezechi OC, Pettersson KO, Okolo CA, Ujah IAO, Ostergren PO. The association between HIV infection, antiretroviral therapy and cervical squamous intraepithelial lesions in South Western Nigerian women. [2015 Dec 13];PLoS One [Internet] 2014 Jan 8;9(5):e97150. doi: 10.1371/journal.pone.0097150. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statistics South Africa. South Africa. Mid-year population estimates 2015. 2015 [Google Scholar]
- 9.Sitas F, Egger S, Bradshaw D, Groenewald P, Laubscher R, Kielkowski D, et al. Differences among the coloured, white, black, and other South African populations in smoking-attributed mortality at ages 35-74 years: a case-control study of 481,640 deaths. [2015 Dec 11];Lancet [Internet] 2013 382(9893):685–93. doi: 10.1016/S0140-6736(13)61610-4. Available from: http://www.sciencedirect.com/science/article/pii/S0140673613616104. [DOI] [PubMed] [Google Scholar]
- 10.Cooper D, Morroni C, Orner P, Moodley J, Harries J, Cullingworth L, et al. Ten years of democracy in South Africa: Documenting transformation in reproductive health policy and status. Reprod Health Matters. 2004;12(24):70–85. doi: 10.1016/s0968-8080(04)24143-x. [DOI] [PubMed] [Google Scholar]
- 11.Reddy P, James S, Sewpaul R, Yach D, Resnicow K, Sifunda S, et al. A decade of tobacco control: The South African case of politics, health policy, health promotion and behaviour change. South African Med J [Internet] 2013;103(12):835–40. doi: 10.7196/samj.6910. Available from: http://www.samj.org.za/index.php/samj/article/view/6910. [DOI] [PubMed] [Google Scholar]
- 12.Pisa PT, Pedro TM, Kahn K, Tollman SM, Pettifor JM, Norris SA. Nutrient patterns and their association with socio-demographic, lifestyle factors and obesity risk in rural South African adolescents. Nutrients. 2015;7(5):3464–82. doi: 10.3390/nu7053464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Department of Health Republic of South Africa. National Guidelines for Cervical Cancer Screening Programme. 2002 [Google Scholar]
- 14.Tathiah N, Naidoo M, Moodley I. Human papillomavirus (HPV) vaccination of adolescents in the South African private health sector: Lessons from the HPV demonstration project in KwaZulu-Natal. South African Med J [Internet] 2015;105(11):954. doi: 10.7196/samj.2015.v105i11.10135. Available from: http://www.samj.org.za/index.php/samj/article/view/10135. [DOI] [PubMed] [Google Scholar]
- 15.Simelela NP, Venter WDF. A brief history of South Africa’s response to AIDS. South African Med J [Internet] 2014;104(3):249. doi: 10.7196/samj.7700. Available from: http://samj.org.za/index.php/samj/article/view/7700. [DOI] [PubMed] [Google Scholar]
- 16.Singh E, Underwood JM, Nattey C, Babb C, Sengayi M, Kellett P. South African National Cancer Registry: Effect of withheld data from private health systems on cancer incidence estimates. South African Med J [Internet] 2015;105(2):107. doi: 10.7196/SAMJ.8858. Available from: http://www.samj.org.za/index.php/samj/article/view/8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdmann F, Kielkowski D, Schonfeld SJ, Kellett P, Stanulla M, Dickens C, et al. Childhood cancer incidence patterns by race, sex and age for 2000-2006: A report from the South African National Cancer Registry. Int J Cancer. 2015;136(11):2628–39. doi: 10.1002/ijc.29308. [DOI] [PubMed] [Google Scholar]
- 18.Schonfeld SJ, Erdmann F, Wiggill T, Singh E, Kellett P, Babb C, et al. Hematologic malignancies in South Africa 2000-2006: analysis of data reported to the National Cancer Registry. Cancer Med [Internet] 2016;5(4):278–378. doi: 10.1002/cam4.597. Available from: http://doi.wiley.com/10.1002/cam4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Heal [Internet] 2016;4(9):e642–53. doi: 10.1016/S2214-109X(16)30113-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2214109X16301139. [DOI] [PubMed] [Google Scholar]
- 20.Singh E, Joffe M, Cubasch H, Ruff P, Norris SA, Pisa PT. Breast cancer trends differ by ethnicity: a report from the South African National Cancer Registry (1994–2009) Eur J Public Health [Internet] 2016;27(1):173–8. doi: 10.1093/eurpub/ckw191. Available from: http://eurpub.oxfordjournals.org/lookup/doi/10.1093/eurpub/ckw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva IS. WHO. Lyon: IARC; 1999. Cancer Epidemiology: Principles and Methods [Internet] pp. 1–441. Available from: https://www.iarc.fr/en/publications/pdfs-online/epi/cancerepi/CancerEpi.pdf. [Google Scholar]
- 22.Vostakolaei FA, Karim-kos HE, Janssen-heijnen MLG, Visser O. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health. 2010;21(5):573–7. doi: 10.1093/eurpub/ckq120. [DOI] [PubMed] [Google Scholar]
- 23.Shamseddine A, Saleh A, Charafeddine M, Seoud M, Mukherji D, Temraz S, et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003-2008 and projections until 2018. Popul Health Metr [Internet] 2014;12(1):4. doi: 10.1186/1478-7954-12-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24593777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn K. Population health in South Africa: dynamics over the past two decades. J Public Health Policy [Internet] 2011;32(Suppl 1 (S1)):S30–6. doi: 10.1057/jphp.2011.27. Available from: [DOI] [PubMed] [Google Scholar]
- 25.World Health Organisation. WHO Library Cataloguing-in-Publication Data. Geneva: World Health Organisation (WHO); 2014. Comprehensive Cervical Cancer Control [Internet] p. 364. Available from: http://www.who.int/reproductivehealth/publications/cancers/cervical-cancer-guide/en/ [Google Scholar]
- 26.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, Seage GR, Suneja G, Kayembe MKA, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One. 2015;10(8):1–13. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991-2010. Int J Cancer. 2013;133(3):721–9. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 28.Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991-2010. Int J Cancer [Internet] 2014;135(2):432–9. doi: 10.1002/ijc.28661. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24615279. [DOI] [PubMed] [Google Scholar]
- 29.Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy (Review) Oncol Rep. 2007;17(5):1121–6. [PubMed] [Google Scholar]
- 30.Abraham AG, Strickler HD, Jing Y, Gange SJ, Timothy R, Moore RD, et al. Invasive cervical cancer among HIV-infected women: A North American multi-cohort collaboration prospective study. 2014;62(4):405–13. doi: 10.1097/QAI.0b013e31828177d7. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3633634/pdf/nihms433254.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huchko MJ, Maloba M, Nakalembe M, Cohen CR. The time has come to make cervical cancer prevention an essential part of comprehensive sexual and reproductive health services for HIV-positive women in low-income countries. J Int AIDS Soc. 2015;18(Suppl 5):1–5. doi: 10.7448/IAS.18.6.20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandiera E, Todeschini P, Romani C, Zanotti L, Erba E, Colmegna B, et al. The HIV-protease inhibitor saquinavir reduces proliferation, invasion and clonogenicity in cervical cancer cell lines. Oncol Lett [Internet] 2016;12:2493–500. doi: 10.3892/ol.2016.5008. Available from: http://www.spandidos-publications.com/10.3892/ol.2016.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz N, Bravo Ocaña LE. Epidemiology of cervical cancer in Colombia. Colomb Med. 2012;43(4):298–304. [PMC free article] [PubMed] [Google Scholar]
- 34.Massyn N, Day C, Padarath A, Barron P ER, editors. District Health Barometer 2013/2014 [Internet] Durban: Health System Trust; 2014. p. 635. Available from: http://www.health-e.org.za/wp-content/uploads/2014/10/DHB_2013-14.pdf. [Google Scholar]
- 35.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. [2015 Dec 12];Lancet Oncol [Internet] 2008 9(7):683–92. doi: 10.1016/S1470-2045(08)70175-X. Available from: http://www.sciencedirect.com/science/article/pii/S147020450870175X. [DOI] [PubMed] [Google Scholar]
- 36.Kalima Mulele, Lishimpi Kennedy, Meza Jane L, Watanabe-Galloway Shinobu, Msadabwe Susan C, Mwaba Catherine K, Shibemba Aaron L, Banda L, Wood Charles, Chamberlain Robert M, Soliman AS. Observed and Expected Incidence of Cervical Cancer in Lusaka and the Southern and Western Provinces of Zambia, 2007 - 2012. Int J Gynecol Cancer. 2015;25(1):98–105. doi: 10.1097/IGC.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Semenciw R, Probert A, Mao Y. Cervical cancer in Canada: Changing patterns in incidence and mortality. [2015 Dec 16];Int J Gynecol Cancer [Internet] 2001 11(1):24–31. doi: 10.1046/j.1525-1438.2001.011001024.x. Available from: http://onlinelibrary.wiley.com/doi/10.1046/j.1525-1438.2001.011001024.x/full. [DOI] [PubMed] [Google Scholar]
- 38.Cervantes-Amat M, López-Abente G, Aragonés N, Pollán M, Pastor-Barriuso R, Pérez-Gómez B. The end of the decline in cervical cancer mortality in Spain: trends across the period 1981-2012. BMC Cancer [Internet] 2015;15(1):287. doi: 10.1186/s12885-015-1306-x. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84928320166&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartorius B, Kahn K, Collinson MA, Sartorius K, Tollman SM. Dying in their prime: Determinants and space-time risk of adult mortality in rural South Africa. Geospat Health. 2013;7(2):237–49. doi: 10.4081/gh.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joubert J, Rao C, Bradshaw D, Vos T, Lopez AD. Evaluating the Quality of National Mortality Statistics from Civil Registration in South Africa, 1997-2007. PLoS One [Internet] 2013;8(5) doi: 10.1371/journal.pone.0064592. Available from: http://journals.plos.org/plosone/article/asset?id=10.1371/journal.pone.0064592.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantelhardt EJ, Moelle U, Begoihn M, Addissie A, Trocchi P, Yonas B, et al. Cervical cancer in Ethiopia: survival of 1,059 patients who received oncologic therapy. Oncologist [Internet] 2014;19(7):727–34. doi: 10.1634/theoncologist.2013-0326. Available from: http://theoncologist.alphamedpress.org/content/19/7/727.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sankaranarayanan R. IARC Sci Publ. 162. 2011. Cancer survival in Africa, Asia, the Caribbean and Central America. Introduction; pp. 1–5. [PubMed] [Google Scholar]
- 43.Adegoke O, Kulasingam S, Virnig B. Cervical Cancer Trends in the United States: A 35-Year Population-Based Analysis. J Women’s Heal. 2012;21(10):1031–7. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jemal A, Center MM, DeSantis C, Ward EM. Global Patterns of Cancer Incidence and Mortality Rates and Trends. Cancer Epidemiol Biomarkers Prev [Internet] 2010;19(8):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. Available from: http://cebp.aacrjournals.org/cgi/doi/10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 45.Bailie RS, Selivey CE, Bourne DBD. Trends in cervical cancer mortality in South Africa. Int J Epidemiol [Internet] 1996;25(3):488–93. doi: 10.1093/ije/25.3.488. Available from: http://ije.oxfordjournals.org/content/25/3/488.long. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Zhang L, Tian C, Yang L, Wang Z. Genetic variants and risk of cervical cancer: epidemiological evidence, meta-analysis and research review. BJOG. 2014;121(6):664–74. doi: 10.1111/1471-0528.12638. [DOI] [PubMed] [Google Scholar]
- 47.Hébert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer [Internet] 2009;115(11):2539–52. doi: 10.1002/cncr.24270. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2688832&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mascarello KC, Zondonade E, Amorim MHC. Survival analysis of women with cervical cancer treated at a referral hospital for oncology in Espírito Santo State, Brazil, 2000-2005 Análise da sobrevida de mulheres com câncer do colo do útero atendidas em hospital de referência para oncologia no Esp. Cad Saúde Pública, Rio Janeiro. 2013;29(4):823–31. [PubMed] [Google Scholar]
- 49.Patel A, Galaal K, Burnley C, Faulkner K, Martin-Hirsch P, Bland MJ, et al. Cervical cancer incidence in young women: a historical and geographic controlled UK regional population study. Br J Cancer [Internet] 2012;106(11):1753–9. doi: 10.1038/bjc.2012.148. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3364121&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce Campbell CM, Curado MP, Harlow SD, Soliman AS. Variation of cervical cancer incidence in Latin America and the Caribbean. Rev Panam Salud Publica [Internet] 2012;31(6):492–8. doi: 10.1590/s1020-49892012000600007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22858816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


