Summary
While traditional drug discovery continues to be an important platform for the search of new antibiotics, alternative approaches should also be pursued to complement these efforts. We herein designed a class of molecules that decorate bacterial cell surfaces with the goal of re-engaging components of the immune system towards Escherichia coli and Pseudomonas aeruginosa. More specifically, conjugates were assembled using polymyxin B (an antibiotic that inherently attaches to the surface of Gram-negative pathogens) and antigenic epitopes that recruit antibodies found in human serum. We established that the spacer length played a significant role in hapten display within the bacterial cell surface, a result that was confirmed both experimentally and via molecular dynamics simulations. Most importantly, we demonstrated the specific killing of bacteria by our agent in the presence of human serum. By enlisting the immune system, these agents have the potential to pave the way for a potent antimicrobial modality.
In Brief

Feigman et al. describe a mode of re-engaging components of the immune system to target Gram-negative bacteria for destruction. By modifying polymyxin B to include antibody recruiting epitopes, bacterial cell surfaces were decorated with agents that triggered antibody binding and cell killing.
Introduction
Bacterial cell walls are the primary targets of several classes of potent antibiotics due to their critical role in regulating cellular growth and division (Koch, 2003). During active stages of infection, bacterial cell walls come in direct contact with their human hosts, a feature that can be exploited by the immune system in detecting and eradicating these pathogens (Kieser and Kagan, 2017; Royet, et al., 2011). We have recently demonstrated that synthetic cell wall building blocks can metabolically label the surface of Gram-positive bacteria with antigenic epitopes (Fura, et al., 2016; Fura and Pires, 2015; Fura, et al., 2014; Fura, et al., 2017). In turn, grafting of haptens onto bacterial cell surfaces triggered the recruitment of endogenous antibodies (pools of existing antibodies in humans). We have now turned our attention to targeting Gram-negative pathogens, which are considered high priority because of reduced treatment options (Fischbach and Walsh, 2009; Nikaido, 1994). In this work, we describe a strategy aimed at tagging Gram-negative bacteria for destruction via small molecule conjugates that specifically home to bacterial cell surfaces.
The human immune system has powerful mechanisms in place to prevent the entry and colonization of most pathogens (Finlay and Hancock, 2004; Hancock, et al., 2012). Once bacterial pathogens escape detection they often extensively colonize the patient, which can result in severe symptoms and even death in the absence of medical intervention. Our main goal is to modulate the immune response in a manner that reverses disease progression and eliminates bacterial pathogens from the system. Today, there is mounting evidence showing that engineered immune responses to diseased tissues can dramatically reverse disease progression (Killock, 2014; Masihi, 2001; Rosenberg, et al., 2004; Topalian, et al., 2012). As an example, cancer immunotherapy has emerged as a breakthrough in treating multiple types of cancer (Topalian, et al., 2015). The use of small molecules to graft immunogenic epitopes onto target cells is particularly attractive because of its versatility and close mimicry to native immune responses. Such designs have been successfully applied against cancer cells (Murelli, et al., 2009; Owen, et al., 2007), including advanced clinical candidates developed by Low (Amato, et al., 2014; Amato, et al., 2013; Lu and Low, 2002; Lu, et al., 2005), and other pathogens (Bertozzi and Bednarski, 1992; Kobertz, et al., 1996; Metallo, et al., 2003; Parker, et al., 2009).
Progress in cancer immunotherapy has been achieved despite similarities between cancer and patient cells, which can lead to off-target toxicity. In contrast, there are distinct differences in cell size, shape, and composition between bacterial and human cells. We reasoned that these physiological differences could provide a larger window for selectively targeting bacterial pathogens. The cell envelope of Gram-negative bacteria is composed of an inner membrane, periplasm, and outer membrane (OM) (Figure 1A). The OM displays an unusual asymmetry in which phospholipids populate the inner leaflet and lipopolysaccharides (LPS) make up the outer leaflet (Brade, et al., 1999; Kamio and Nikaido, 1976; Silipo, et al., 2010; Smit, et al., 1975; Wang and Quinn, 2010; Yuriy and Miguel, 2011). Lipid A, an essential anchor of LPS to the OM, is composed of a phosphorylated diglucosamine unit connected to lipid chains (Figure 1B) (Morrison and Jacobs, 1976). The natural product antibiotics polymyxin B (PMB) and polymyxin E (colistin) are among the few small molecules that associate with lipid A with high affinity and specificity (Figure 1C). PMB and colistin are proposed to impart their antibacterial activity by binding to lipid A and destabilizing the OM layer – although the exact mechanism has yet to be fully elucidated (Sahalan and Dixon, 2008; Schindler and Teuber, 1975). Polymyxins are considered true last-resort antibiotics for the treatment of multidrug-resistant Gram-negative infections that fail to respond to any other antibiotic (Zavascki, et al., 2007). For these reasons, there is a resurging interest in the development of next-generation PMB derivatives (Brown and Dawson, 2017; Falagas and Kasiakou, 2005; Gallardo-Godoy, et al., 2016).
Figure 1.
Association of PMB nonapeptide (PMBN) with lipid A. (A) Cartoon representation of the surface architecture of Gram-negative bacteria. (B) Structure of lipid A within LPS. (C) Basic unit of PMBN, where R represents the variations within the polymyxin family of antibiotics. (D) General representation of polymyxin-hapten conjugates tagging the surface of Gram-negative bacteria followed by a specific immune response (A simplified LPS is shown for clarity).
By exploiting surface exposed features (e.g., lipid A) unique to Gram-negative bacteria, we hypothesized that heterobifunctional agents composed of PMB would graft haptens onto bacterial cell surfaces that engage components of the immune system (e.g., antibodies and primary immune cells) (Figure 1D). PMB (and the related antibiotics) have two principal design features: (1) a cationic cyclic peptide that binds to lipid A on bacterial OMs and (2) an aliphatic fatty acid chain to anchor and disrupt the OM (Wu and Hancock, 1999). The use of surface homing agents that inherently possess antimicrobial activity represents a significant advance due to the potential synergism between the direct bactericidal activity of the homing beacon and the engagement of immune system. Combined, our agents target pathogenic bacteria in two distinct ways to generate promising lead immunotherapeutic agents for advanced in vivo testing.
Results and Discussion
Initially, we identified a fragment of PMB known as PMB nonapeptide (PMBN) (Figure 1C, where R=H) as a potential targeting unit to decorate the surface of Gram-negative bacteria. PMBN is devoid of the membrane-disrupting fatty acid tail, but retains the cyclic hexapeptide that is responsible for association to lipid A (Vaara and Viljanen, 1985; Viljanen and Vaara, 1984). The smaller PMBN fragment has attenuated antimicrobial activity, which was useful in isolating the surface-homing features of our synthetic agents. Deprotected PMBN was generated by papain-mediated removal of the acyl chain along with the N-terminal residue from the parent PMB (Chihara, 1973). Next, the tetra-Boc protected PMNB was produced using the reagent Boc-ON, which yielded an unprotected N-terminus amino group and all the amino sidechains Boc-protected (O’Dowd, et al., 2007). This common Boc-protected PMBN building block was used for the synthesis of all subsequent PMB derivatives.
We initially modified PMBN with a fluorescein moiety at the N-terminus by reacting the free amino group on PMBN with fluorescein isothiocyanate (FITC) followed by the deprotection of the sidechain Boc groups (PF). The goal was to synthesize a PMBN derivative that could be used to establish the initial working parameters for cell surface tagging. To quantify surface labeling of live bacteria, Escherichia coli (E. coli) cells were incubated with increasing concentrations of PF and cellular fluorescence was measured by flow cytometry (Figure 2A). Our results showed that cellular fluorescence levels increased with increasing concentrations of PF, reaching levels 86-fold over background by 40 µM. The same cells were analyzed by confocal microscopy to delineate the cellular organization of the fluorescence signal (Figure 2B). Labeling was observed primarily on the outer periphery, which is also the site of LPS within the OM. Moreover, cellular fluorescence levels were found to be relatively stable; cells retained 76% of fluorescence levels after 2 hours following an initial washing step, which highlights the avidity of PMBN to the cell surface (Figure S1). Our results are consistent with previous reports of fluorescently-labeled PMBN that used dansyl as the fluorescent handle (Moore, et al., 1986; Tsubery, et al., 2000). Most importantly, these results confirmed that PMBN conjugates effectively label the surface of Gram-negative bacteria.
Figure 2.
Fluorescent derivatives of PMB. (A) E. coli cells were incubated overnight with the stated concentrations of PF (structure shown in the inset) and analyzed using flow cytometry. Data are represented as mean + SD (n =3). (B) Confocal microscopy images of E. coli K12 treated with PF (40 µM) for 30 min, washed three times with 1× PBS, and analyzed. Scale bar = 2 µm.
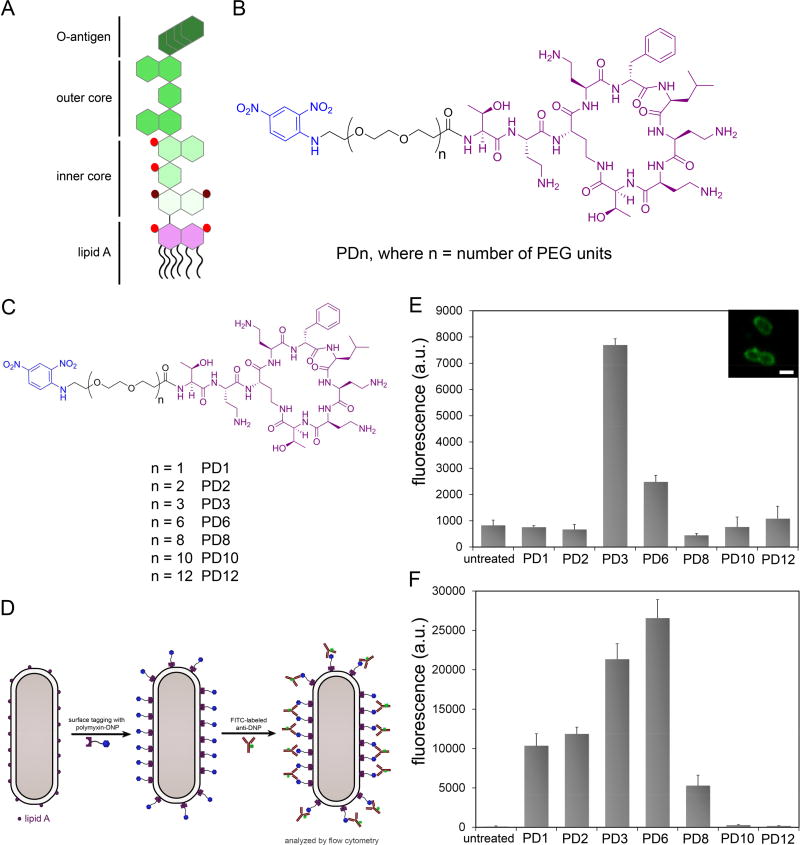
Next, we set out to build PMBN derivatives containing hapten units to direct the recruitment of antibodies. Given the location of lipid A within LPS, it was important to consider the display of haptens within the surface of Gram-negative bacteria. The inner core, outer core, and O-antigen segments extend away from lipid A units within LPS, which can potentially obscure binding of small molecules and antibodies (Figure 3A). We reasoned that the spacer connecting the hapten and PMBN together would play a determinant role in the availability of haptens to interact with antibodies from the extracellular space. Flexible and polar polyethylene glycol (PEG) spacers of defined lengths were used to empirically select the most appropriate spacer (Figure 3B). 2,4-dinitrophenol (DNP) was chosen as the hapten due to the high abundance of endogenous anti-DNP antibodies in human serum (Jakobsche, et al., 2013; Lu, et al., 2005; Sheridan, et al., 2014). Critically, the use of DNP haptens for the recruitment of endogenous antibodies potentially eliminates the need for pre-immunization.
Figure 3.
(A) Cartoon representation of a basic LPS unit. The purple segment represents lipid A and the green segments represent the various sugar units attached to lipid A. Red spheres represent phosphorylation sites. Maroon spheres represent carboxylate groups. (B) Chemical structure of the series of PMBN modified with DNP units. Each member of this series varies in the number of PEG units in the tether connecting DNP to PMBN. (C) Chemical structures of the PDn series. (D) Cartoon representation of the assay used to determine antibody recruitment to the surface of E. coli cells based on the PDn series of agents. E. coli WT (E) and K12 (F) were incubated for 2 h with 40 µM of PDn followed by incubation with FITC-conjugated anti-DNP antibodies and analyzed using flow cytometry. Data are represented as mean + SD (n =3). Inset, confocal microscopy imaging of E. coli treated with PF3.
With the first series of PDn (n represents the number of diethyleneoxy units, Figure 3C) agents in hand, we set out to determine their ability to induce bacterial opsonization by anti-DNP antibodies. Antibody recruitment was assessed by treating E. coli (WT with intact O-antigen) with each individual agent, followed by an incubation period with fluorescently labeled anti-DNP antibodies, and antibody recruitment was measured by flow cytometry (Figure 3D). Fluorescence levels were expected to reflect the level of anti-DNP antibodies associated with bacterial cell surfaces. Satisfyingly, treatment of E. coli WT with PDn agents resulted in high levels of anti-DNP recruitment (Figure 3E). From our results, it is evident that there is a significant spacer length dependence. We hypothesize that PD3 may represent a balance in spacer length by optimizing both permeation into the LPS matrix and availability of DNP epitopes. A similar molecule to PD3 was synthesized in which a fluorescein handle was installed in place of DNP (PF3). Based on the confocal microscopy analysis (Figure 3E), it is evident that despite the inclusion of the PEG spacer the modified PMBN fragment was able to label bacterial cell surfaces similar to PF.
The role of O-antigens within LPS was investigated next by performing a similar antibody recruitment assay using E. coli (K12), which lacks O-antigen segments. As expected, there was a general increase in fluorescence levels across the entire PDn series relative to untreated bacteria. In contrast to E. coli WT, treatment of K12 cells with the smaller sized PEG units (PD1, PD2, PD3, and PD6) resulted in significant recruitment levels over background. These results suggest that accessibility and hapten display control opsonization of tagged cells (Figure 3F). A similar rationale based on LPS structure was previously attributed to the binding of anti-porin antibodies to E. coli.(Bentley and Klebba, 1988) Interestingly, there was a shift in the most efficient opsonization inducer towards PD6. In an attempt to disentangle the contribution of surface binding and DNP availability, the two types of E. coli were treated with the fluorescein-modified PF3 and PF6 (Figure S2). While PF3 labeled both strains equally well, the larger PF6 labeled E. coli K12 cells nearly 50% better than E. coli WT. These results are supportive of permeability through the LPS being a factor in determining labeling efficiency.
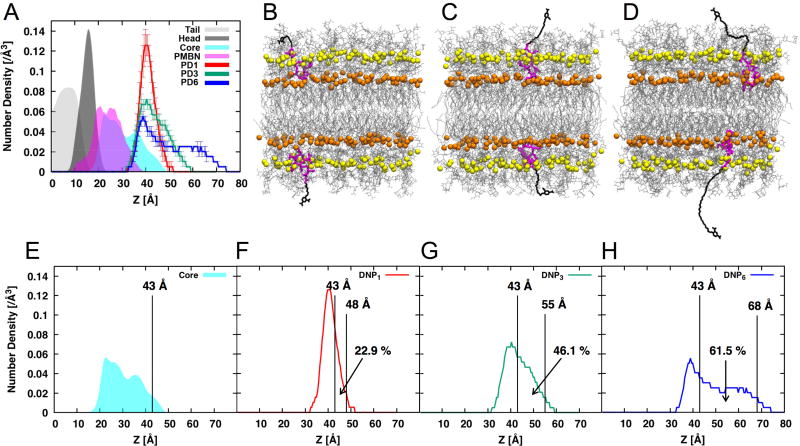
To explore the influences of PEG spacer length on the DNP distribution and availability on E. coli K12 cell surfaces, we performed molecular dynamics (MD) simulations of three different PDn (PD1, PD3, and PD6) agents in K12 LPS bilayers. The E. coli K12 LPS modeling, its assembly to a bilayer, and simulation protocols followed the CHARMM-GUI LPS Modeler (Jo, et al., 2017; Jo, et al., 2015) and Membrane Builder step-by-step protocol (Jo, et al., 2008; Jo, et al., 2009; Wu, et al., 2014), which were successfully generalized and applied in the previous LPS simulation (Lee, et al., 2017; Patel, et al., 2016; Wu, et al., 2013) (see Methods for the details). Figure 4A shows the heavy-atom number density profile along the z-axis (i.e., the bilayer normal) for PDn moieties (PMBN and DNP) and LPS components (lipid A tail, lipid A head, and K12 core). The positively charged PMBN of PDn stays in between the phosphorylated glucosamine residues of lipid A and the K12 inner core containing the carboxyl (in Kdo) and phosphate (in Hep) groups. Interestingly, DNP in PD1 was most fluctuating in the outer region of the K12 outer core and the PEG spacer clearly does not provide sufficient length for DNP to be well displayed to anti-DNP antibodies (Figure 4B; Movie S1), although it can be exposed to the region within the space in between outer core sugars. As the PEG spacer length increases, MD simulations clearly showed that DNP epitopes became more exposed to the bulk medium and thus showed broader distribution above the K12 core for anti-DNP antibody recruitment (Figure 5C and D; Movie S2 and S3 for PD3 and PD6, respectively). When the 90% confidence interval was used to measure solvent-exposed DNP epitopes above the K12 core (i.e., above z = 43 Å in Figure 4E), the populations of solvent-exposed DNP epitopes are 22.9% (PD1), 46.1% (PD3), and 61.5% (PD6) (Figure 4F–H). When normalized by PD6, these exposed populations correspond to a ratio of 0.37:0.75:1, which is well correlated with a ratio of 0.39:0.80:1 based on the fluorescence levels of PD1, PD3, and PD6 in the E. coli K12 cell (Figure 3F; normalized by the PD6 value).
Figure 4.
(A) Density profiles of system components along the bilayer normal (the z-axis): lipid A carbon tail (gray), lipid A head (dark gray), K12 core (cyan), PMBN (magenta), and DNP in PD1 (red), PD3 (green), and PD6 (blue). The bilayer center is located at z = 0 and the profiles are averaged over three replicas and also symmetrized. All histograms are normalized based on the area under the curve. Snapshots of (B) PD1, (C) PD3, and (D) PD6 in homogeneous E. coli K12 bilayers: PMBN (magenta), PEG-DNP (black), LPS (gray), phosphate groups in lipid A (orange), and phosphate groups in Hep (yellow). The corresponding simulation movies are in supporting Movies S1–3. The estimated z value of the 90% upper bound of (E) K12 core distribution (i.e., z = 43 Å), and the percent of the area between 43 Å to the upper bound of DNP distribution of (F) PD1, (G) PD3, and (H) PD6.
Figure 5.
Effect of LPS mutations. Five designated E. coli mutants were incubated for 2 h with 40 µM of PD6 (A) followed by incubation with FITC-conjugated anti-DNP antibodies or incubated for 2 h with 40 µM of PF6 (B) and analyzed using flow cytometry. Data are represented as mean + SD (n =3). (C) Cartoon representations of the LPS mutants used in this study with the same symbol notation as in Figure 3A.
To further explore the effect of surface composition on antibody recruitment, six additional mutants were tested for both antibody recruitment and surface labeling with PD6 and PF6, respectively (Stokes, et al., 2017). Treatment of E. coli mutants with PD6 demonstrated how size, charge, and composition of LPS can dictate antibody recruitment (Figure 5). Generally, antibody recruitment was higher with the loss of LPS segments. These results suggest that segments that extend away from lipid A may hinder the availability of the hapten. Supportive of this concept was the finding that surface labeling by the similarly sized fluorescent PF6 resulted in more constant labeling levels across LPS mutants. The importance of these finding is that they have implications for the design of immunotherapeutics with a focus on how the LPS structure can control immune responses, including the design of specific antigens for vaccine development. Moreover, our scan across LPS mutants using PF6 represents a comprehensive analysis of how changes to the LPS structure can control PMBN binding to lipid A.
Next, we set out to establish the role of LPS binding by PDn agents by performing a competition experiment with exogenous LPS (Figure S3). A near 2-fold and 8-fold decrease in anti-DNP recruitment was observed in E. coli treated with PF6 in the presence of 0.5 mg/mL and 2 mg/mL of LPS, respectively. These results are suggestive of LPS being the primary target of PF6, as expected based on the PMBN homing moiety. Antibody recruitment was also evaluated directly from pool human serum (PHS). Immunotherapeutic approaches against Gram-negative pathogens based on PD6 must rely on opsonization of bacterial cells by anti-DNP antibodies from PHS, which is a complex mixture of biomacromolecules and diverse antibodies. For this assay, detection of anti-DNP recruitment was performed using a FITC-labeled anti-human antibody (Figure S4). Satisfyingly, treatment of E. coli with PD6 led to a significant increase in cellular fluorescence, which is indicative of anti-DNP recruitment directly from serum.
Cytotoxicity towards mammalian cells was evaluated to establish the potential therapeutic window of PD6. No loss of cellular viability was observed in the presence of up to 100 µM of PD6, which is likely reflective of the lack of lipid A in mammalian cells (Figure S5). We then evaluated the ability of PD6 to promote the entry of polar antibiotics across the OM, which retains the PMBN fragment that is known to make the OM more permeable (Vaara, 1992). More specifically, we tested whether the co-incubation of PD6 with rifamycin would potentiate this antibiotic against Gram-negative bacteria. The OM provides a barrier for the translocation of most polar antibiotics, which can be a major cause of intrinsic resistance by Gram-negative pathogens to entire classes of potent antibiotics. For these reasons, rifamycin displays weak antibacterial properties against Gram-negative pathogens. While PD6 alone displayed a minimum inhibitory concentration (MIC) value >25 µg/mL and the MIC for rifamycin alone was 12.5 µg/mL, the combination of sub-lethal concentrations of PD6 reduced the MIC for rifamycin to 0.78 µg/mL (Figure 6A). This significant decrease in the MIC value indicates that the PMBN fragment within PD6 retains the ability to promote the entry of polar antibiotics. Therefore, in addition to the immunomodulatory properties of PD6 it is also expected to potentiate co-therapeutics.
Figure 6.
Induction of bactericidal activity by PD6. (A) E. coli were incubated with PD6 alone, rifamycin alone, or in combination of the two agents at varying concentrations. Optical density at 600 nm was analyzed to assess bacteria viability. (B) E. coli were treated with either PD6, PHS, PMBN, their combinations at 10 µM, or media to determine complement dependent cytotoxicity (CDC). Colony counts were measured the next day. (C) E. coli were incubated with PD6tail at varying concentrations. Optical density at 600 nm was analyzed to assess bacteria viability. (D) E. coli cells were incubated for 30 min with the stated concentrations of PD6 or PD6tail followed by incubation with FITC-conjugated anti-DNP antibodies and analyzed using flow cytometry. Data are represented as mean + SD (n =3).
Opsonization of foreign and potentially dangerous entities triggers a series of steps within the innate immune system. In complement dependent cytotoxicity (CDC), antigen display on bacteria drives the recruitment of antibodies (Rettig, et al., 2015). Subsequently, opsonized cells elicit a complement cascade that leads to the formation of membrane attack complexes that ultimately lyse target cells. Having shown that PD6 promoted the recruitment of anti-DNP antibodies directly from PHS, we sought to establish its ability to induce the killing of Gram-negative bacteria. Based on our results, treatment of E. coli with PHS, PMBN, or PD6 alone led to no significant change in colony counts (Figure 6B). Similarly, the co-incubation of PMBN (10 µM) and PHS resulted in minimal decrease in colony counts. Satisfyingly, treatment of cells with PD6 and PHS resulted in a greater than one log reduction in colony counts. This increase in bacterial lysis is suggestive of DNP mediated induction of CDC. To further confirm the role of anti-DNP antibodies, a similar assay was performed with PHS depleted of antibodies. When cells were treated with antibody-depleted PHS, there was no induction in cell lysis upon co-incubation with PD6 (Figure S5). In addition, there was no increase in cell killing upon the heat-mediated depletion of complement proteins from PHS (Figure S6).
Our initial design of surface homing agents focused primarily on PMBN, which is devoid of the fatty acid tail on the N-terminus that is proposed to be responsible for OM disruption. We reasoned that it would be challenging to measure antibody recruitment and isolate the effect of opsonization with an agent that inherently destabilizes the OM structure. Having successfully demonstrated the ability of PD6 to graft DNP antigens onto the surface of Gram-negative bacteria, we sought to re-introduce the fatty acid tail. For the design of this agent, the DNP epitope was conjugated on the sidechain of a lysine residue that bridged PMBN and the fatty acid tail (PD6tail, Figure 6C inset). The inherent antimicrobial activity of PD6tail (MIC ~ 3.25 µg/mL) was similar to the parent PMB (MIC ~ 1 µg/mL) and considerably lower than PD6 (Figure 6C). From these results, we can conclude that the re-introduction of the fatty acid tail recovers most of the antimicrobial activity of the parent PMB. We next set out to establish the antibody recruitment properties of PD6tail. Our initial attempts to perform the assay at 37°C failed due to extensive cellular disruption as measured in the scatter plot during flow cytometry analysis. Instead, the temperature was lowered to room temperature to minimize lysis prior to antibody recruitment. A near 2-fold increase in cellular fluorescence was observed for cells treated with PD6tail at 10 µM over background fluorescence (Figure 6D). Using similar conditions, there was no observable increase in cellular fluorescence in cells treated with PD6. These results may reflect higher surface retention by PD6tail relative to PD6 due to better membrane anchorage, which should reduce the concentration required to decorate cell surfaces with DNP epitopes. Most significantly, we anticipate that PD6tail can operate in two distinct antimicrobial modes. Therefore, bacteria treated with PD6tail that are not initially destroyed by direct cell lysis would, in turn, be targeted by the immune system.
We then assessed how our antibody recruitment strategy operates in other Gram-negative pathogens. More specifically, Pseudomonas aeruginosa (P. aeruginosa), Acinetobacter baumannii (A. baumannii), and Klebsiella pneumoniae (K. pneumoniae) were chosen as a panel of Gram-negative bacteria that have been identified by the WHO and CDC as high priority due to the low number of available drugs. Interestingly, there was a wide range in opsonization levels across all four species treated with PD6 (Figure 7A). Reduced cellular fluorescence was observed for both K. pneumoniae and A. baumannii despite their well-established sensitivity to PMB (Levin, et al., 1999). We attribute this difference to the diversity in cell surface architecture between Gram-negative bacteria despite the common lipid A anchor. In the case of P. aeruginosa, cells treated with PD6 resulted in fluorescence levels 11-fold higher than untreated cells. Given the possibility that the surface composition of E. coli and P. aeruginosa are unique to each species, this difference may also reflect on the optimum PEG spacer length and epitope availability. For these reasons, the entire PDn panel of agents was tested against P. aeruginosa to empirically identify the most appropriate spacer length (Figure 7B). Interestingly, PD6 was also the most efficient inducer of opsonization against P. aeruginosa. We propose two possible explanations for the similar pattern of recruitment to E. coli. First, it is possible that PD6 represents an idealized combination of a spacer long enough to display DNP epitopes while minimizing steric interference that is common for longer PEG spacers (Fishburn, 2008). Therefore, it provides the best induction in antibody recruitment regardless of surface composition. Alternatively, differences in surface composition between the two types of bacteria may favor the same spacer length for unrelated reasons.
Figure 7.
Recruitment across Gram-negative bacterial species. (A) Specified bacteria were incubated for 2 h with 40 µM of PD6 followed by incubation with FITC-conjugated anti-DNP antibodies and analyzed using flow cytometry. (B) P. aeruginosa were incubated for 2 h with 40 µM of PDn followed by incubation with FITC-conjugated anti-DNP antibodies and analyzed using flow cytometry. Data are represented as mean + SD (n =3). (C) P. aeruginosa were incubated with PD6tail at varying concentrations. Optical density at 600 nm was analyzed to assess bacteria viability. (D) C. elegans infected with P. aeruginosa were treated with PF6 (50 µM), washed, anesthetized, mounted on a bed of agarose, and imaged using confocal microscopy. Scale bar presents 25 µm.
The sensitivity of P. aeruginosa against the most efficient opsonizing agents was evaluated next (Figure 7C). PD6 displayed a lower MIC value than the parent PMBN. Interestingly, P. aeruginosa was considerably more sensitive to PD6 than E. coli displaying a MIC value of 12.5 µg/ml. As expected based on our results with E. coli, the introduction of the fatty acid tail in PD6tail further lowers the MIC value to 6.25 µg/ml. Additionally, PD6tail displayed no toxicity towards mammalian cells at all concentrations tested (Figure S7). Finally, we set out to show that the specificity endowed by PMBN towards lipid A could be exploited within a living host to target Gram-negative bacteria for imaging and, potentially, therapeutic interventions. Towards these goals, we used the nematode Caenorhabditis elegans (C. elegans), which is a widely used model animal to study bacterial pathogenesis. Profound understanding of host-pathogen interactions in P. aeruginosa infections can be directly attributed to the use of C. elegans (Mahajan-Miklos, et al., 1999). In our assay, C. elegans (at primarily L4 larval stage) were pre-incubated with P. aeruginosa to establish bacterial colonization. Residual bacteria were gently washed away and P. aeruginosa infected C. elegans were treated with PF6. At the end of the assay, the worms were anesthetized and mounted on a bed of agarose to be imaged via confocal fluorescence microscopy (Figure 7D). Remarkably, bacterial cells were readily visualized inside the host C. elegans. These results demonstrate that polymyxins can track bacterial infections in a live host. Most importantly, they demonstrate the homing properties of our designed agents towards Gram-negative bacteria. Given the demonstrated ability of our agents to target bacteria in culture and in vivo, we anticipate that PD6 (or similar scaffolds) can guide the design of potential drug leads.
In conclusion, we have designed and synthesized a unique class of immunotherapeutic agents that exploits the lipid A binding scaffold of polymyxins to decorate the surface of Gram-negative bacteria with haptens. We showed that the most potent members of this panel trigger the opsonization of E. coli and P. aeruginosa. Most significantly, the lead agent induced CDC-based killing of E. coli. Re-introduction of the membrane disrupting fatty acid tail restored its inherent antimicrobial activity. Finally, we showed that this agent can target and label the surface of Gram-negative pathogens in a live host. In the future, we plan to expand our in vivo studies to complex animals to establish the suitability of this class of molecules to fight infections. Moreover, we will explore how our strategy can be used to induce the grafting of exogenous haptens onto bacterial cell surfaces with the goal of providing finer control on antibody levels.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author Marcos Pires (map311@lehigh.edu).
Experimental Model and Subject Details
Cell Culture and Generation of Stable Cell Lines
HEK293 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% bovine calf serum, penicillin, and streptomycin.
Method Details
Reagents
Fluorescein 5-isothiocyanate and 5, 6-carboxyfluorescein were purchased from Chem-Impex. Amino-PEG16-24-acid, DNP-PEG2-12-acid, and Fmoc-N-amido-PEG12-acid and amino-PEG6-acid compounds were purchased from Broadpharm. Antibody reagents were purchased from Vector Laboratories. Purified Human IgG, Normal Serum was purchased from Bethyl Laboratories. LPS-EK (Lot: LEK-38-02(1)) was purchased from InvivoGen. Dimethyl sulfoxide (DMSO) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Thermo Fisher Scientific Inc. Fetal Bovine Serum (FBS) was purchased from Corning. Penicillin-Streptomycin was purchased from Sigma-Aldrich. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from EMD Millipore. Anti-DNP was purchased from Vector Labs (RRID:AB_2336117). All other organic chemical reagents were purchased from Fisher Scientific or Sigma Aldrich and used without further purification.
Antibodies
Rabbit anti-DNP antibodies were purchased from Vector Labs (RRID:AB_2336117).
Bacteria Cell Culture
Bacterial cells were cultured in specific media in an aerobic environment shaking at 250 rpm at 37 °C. E. coli (MG1655) E. coli (ATCC 25922), Acinetobacter baumannii (ATCC 19606), Klebsiella pneumoniae (ATCC 13883) were all grown in Luria Bertani (LB) medium. E. coli mutants of the Keio Collection (ΔC, ΔP, ΔY, ΔG, ΔO) were also grown in LB medium. P. aeruginosa (ATCC 27853) was grown in Trypsin Soy Broth (TSB) medium.
Mammalian Cell Culture
Human embryonic kidney 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37 °C.
Overnight PF Fluorescent Labeling
E. coli (OD600 = 0. 014) were grown overnight in LB media supplemented with varying concentrations of PF (0 – 100 µM). After treatment, bacteria were harvested, washed with 1× phosphate buffered saline (PBS) 3×, and fixated with 2% formaldehyde solution and analyzed via flow cytometry on a BDFacs Canto II flow cytometer (BD Biosciences, San Jose, CA) equipped with a 488 nm argon laser and a 530 bandpass filter (FL1). A minimum of 10,000 events were counted for each data point. The data were analyzed using the FACSDiva version 6.1.1 software. Treatment with higher concentrations of PF (80 µM, 100 µM) resulted in nonviable bacteria.
PF Fluorescent Labeling
E. coli (OD600 = 0. 014) were grown overnight in LB media at 37 °C with shaking. Stationary E. coli (OD600 = 1.4) was incubated with LB media supplemented with 40 µM PF3 or PF6 for 2 h at 37 °C. After treatment, bacteria were harvested, washed with 1× PBS (3×), and fixated with 2% formaldehyde solution and analyzed via flow cytometry on a BDFacs Canto II flow cytometer as previously stated.
PF Dissociation
Stationary phase E. coli (OD600 = 1.4) (2 mL) were treated with 40 µM PF for 2 h in LB media at 37 °C. After treatment, 2 mL of cells were harvested, washed with 1× PBS, and resuspended in 2 mL of PBS. Cells were shaken at 250 rpm at 37 °C. At designated time points, 0 – 24 h, 100 µL of cells were harvested, washed and fixated with 2% formaldehyde solution. All fixed samples were analyzed via flow cytometry as previously stated.
Antibody Binding Assay
Stationary phase bacteria (E. coli OD600 = 1.4, P. aeruginosa OD600 = 2.4, K. pneumoniae OD600 = 1.2, A. baumannii OD600 = 1.4) from starter cultures were treated with 40 µM PDn supplemented media for 2 h at 37 °C. Depending on the construct tested, concentrations and times are as indicated in the respective figure legend. After treatment, cells were harvested and washed with 1× PBS (3×). 2 × 106 colony forming units (CFU) were then incubated in 100 µL of PBS containing 10% (v/v) FBS and 0.02 µg / mL of FITC-conjugated rabbit anti-dinitrophenyl IgG. All experiments were protected from light and incubated at 37 °C for 1 h. Cells were washed 1× PBS and fixated in 2% formaldehyde. Samples were then analyzed by flow cytometry. Fluorescence data are expressed as mean arbitrary fluorescence units and were gated to include all healthy bacteria.
Fluorescent Imaging
Medium containing fluorescent conjugate was prepared to desired concentration. Bacteria were inoculated (1:100) in the corresponding medium and allowed to grow at designated time points or overnight at 37 °C. The bacteria were harvested at 1,000g and washed with 1× PBS (3×). The bacteria were analyzed on a glass slide by fluorescent confocal microscopy using a B-2E/C filter (ex 465–495/em 515–555) for bacteria labeled with FITC fluorophore.
LPS Competition Assay
LPS-EK (5 mg) was dissolved in LAL (endotoxin-free) water (1 mL) according to InvivoGen protocol. Stationary phase bacteria were treated with 40 µM PF6 in LB supplemented with increasing concentrations of LPS. Aliquots of each sample were removed at indicated time points. Bacteria was harvested, washed, and fixed in 2% formaldehyde and analyzed via flow cytometry.
Bacterial Opsonization with Pooled Human Serum
Bacteria was grown at 37 °C in LB broth with shaking. Stationary phase bacteria (OD = 1.4) were incubated with construct for time indicated. The bacteria were harvested and washed 3× with PBS solution, and in the treatment with PD6, fixated in 2% formaldehyde. Bacteria were then washed 2× with LB media. The bacteria were then washed with PBS and 2 × 106 colony forming units (CFU) were incubated with 25% PHS in PBS solution with 10 % FBS and incubated with bacteria at 4 °C for 20 min. The opsonized bacteria were then washed with PBS and 2 × 106 colony forming units (CFU) were incubated with Anti-Human IgG-FITC diluted 1:1000 in PBS containing 10% FBS at 4 °C for 30 min protected from light. Cells were washed 1× PBS and fixated in 2% formaldehyde. Samples were then analyzed by flow cytometry.
Minimal Inhibitory Concentration Assay
The MICs of conjugates were determined by broth microdilution assays. Experiments were performed with Cation-adjusted Mueller-Hinton Broth (CaMHB) in 96-well polypropylene microtiter plates. Wells were inoculated with 200 µL of bacterial suspension prepared in CaMHB (containing ~106 colony forming units (CFU) / mL) and 200 µL of CaMHB containing increasing concentrations of the conjugates (0 to 100 µg/mL). The MIC was defined as the lowest concentration at which visible growth was inhibited following 18 h incubation at 37 °C. Test wells were diluted 2-fold and absorbance was measured at 580 nm using an Infinite 200 PRO microplate reader (Tecan).
Mammalian Cell Viability Assay
HEK293 cells were seeded in 96-well plates at a density of 10, 000 cells/well and incubated overnight. Prior to treatment, constructs were dissolved in DMSO to obtain desired aliquoted stock solutions. Appropriate volumes of these stock solutions were added to DMEM media so the final concentration of DMSO was equal to 1%. After removal of cell media, 200 µL of treatment solutions were added to each well and incubated at 37 °C for 72 h. After treatment, the media was removed and the cells were washed with 100 µL of complete DMEM. Next, 100 µL of complete DMEM was added to each well. Cell viability was determined using the colorimetric 3-(4,5-dimethylthiazol-2yl)-2,5 diphenyltetrazolium bromide (MTT) assay, in which 10 µL of a 5 mg/mL MTT stock solution was added to the treated cells and incubated for 2 h at 37 °C. The resulting formazan crystals were solubilized in 200 µL of DMSO. Absorbance was measured at 580 nm using an Infinite 200 PRO microplate reader (Tecan). Cell viability was calculated against control cells treated with 1% DMSO complete medium.
Antibiotic Potentiation Assay
Antibiotic synergy was determined by broth microdilution assays. Experiments were performed with cation-adjusted Mueller-Hinton Broth (CaMHB) in 96-well polypropylene microtiter plates. Wells were inoculated with 200 µL of bacterial suspension prepared in CaMHB (containing ~106 colony forming units (CFU) / mL) and 200 µL of CaMHB containing increasing concentrations of the antibiotics (0 to 100 µg/mL) and 25 µg / mL of PMBN-PEG12-DNP. The MIC was defined as the lowest concentration at which visible growth was inhibited following 18 h incubation at 37 °C. Data are representative of at least three biological replicates. Fractional inhibitory concentration (FIC) indices were calculated according to:
where MICa is the minimum inhibitory concentration (MIC) of compound A alone; MICac is the MIC of compound A in combination with compound B; MICb is the MIC of compound B alone; MICbc is the MIC of compound B in combination with compound A; FICa is the FIC of compound A; FICb is the FIC of compound B. Synergy is defined as an FIC index of ≤0.5. Antagonism is defined as an FIC index of ≥4.
CDC Killing using Depleted PHS
E. coli WT (ATCC 25922) was grown at 37 °C in LB broth with shaking. Stationary phase E. coli (OD600 = 1.4) were incubated with 10 µM of PD6 for 30 min. The bacteria were then resuspended in HBSS with and without 5% depleted PHS and incubated for 1 h at 37 °C. Depleted PHS was obtained by treating PHS with bentonite (pre-washed with 10mM NaCl) for 30 min while shaking at 37 °C. After centrifugation, the supernatant was diluted 2-fold with HBSS and incubated with protein A source, Staphylococcus aureus (200 µL) for 30 min. After three centrifugations, the supernatant was diluted to 5% PHS with HBSS. Bacteria were serially diluted in 1× PBS (pH 7.4 to 7.6), plated (50 µL) on LB agar plates and incubated overnight in a 37 °C incubator. Cell viability was assessed by enumerating the CFU per milliliter. Bactericidal activity was defined as a log reduction with combination (compound and 5% PHS) treatment compared with the untreated control at the start of each assay. All data are representative of at least two biological replicates.
CDC Killing
E. coli WT (ATCC 25922) was grown at 37 °C in LB broth with shaking. Stationary phase E. coli (OD600 = 1.4) were incubated with 10 µM of PD6 for 30 min. The bacteria were then resuspended in HBSS with and without 5% depleted PHS (pre-treated with bentonite and protein A) and incubated for 1 h at 37 °C. Bacteria were serially diluted in 1× PBS (pH 7.4 to 7.6), plated (50 µL) on LB agar plates and incubated overnight in a 37 °C incubator. Cell viability was assessed by enumerating the CFU per milliliter. Bactericidal activity was defined as a log reduction with combination (compound and 5% PHS) treatment compared with the untreated control at the start of each assay. All data are representative of at least two biological replicates.
CDC Killing using Heat Killed Serum
For pooled human serum (PHS) complement inactivation, frozen serum was thawed under refrigerated conditions and then placed in a 56 °C water bath for 30 min with mixing at 5 min intervals. After 30 min, the heat inactivated serum was transferred to ice and the remaining experiment was proceeded as described in the previous CDC killing experiments. E. coli WT (ATCC 25922) was grown at 37 °C in LB broth with shaking at 250 rpm. The stationary phase E. coli (OD600 = 1.4) were incubated with or without 10 µM of PD6 for 30 min at 37 °C shaking at 250 rpm. The bacteria were harvested by centrifugation, resuspended in Hank’s balanced salt solution (HBSS) with 5% PHS (pretreated with bentonite), without PHS (HBSS alone), or with heat inactivated 5% PHS (pretreated with bentonite). The bacteria were incubated for 1 h at 37 °C with shaking at 250 rpm. Bacteria were serially diluted in HBSS, plated (50 µL) on LB agar plates and incubated overnight in a 37 °C incubator. Cell viability was assessed by enumerating the CFU per milliliter. Bactericidal activity was defined as a log reduction with combination (compound and 5% PHS) treatment compared with the untreated control at the start of each assay. All data are representative of at least two biological replicates.
Bacterial Labeling in live C. elegans
N2 C. elegans were maintained by standard protocol using nematode growth agar with bacterial lawns of E. coli OP50 (source) on a 60 mm × 15mm cell culture dish. For bacterial labeling assays, C. elegans were grown to contain primarily L4 larval stage nematodes by incubation at 25 °C for ~48–52 h. On the day of experiments, C. elegans were washed off the plates with M9 buffer, and washed three times with M9 buffer. For washing steps, the C. elegans were pelleted at 1000g. C. elegans were resuspended in 450 µL of M9 buffer containing 10% LB broth and transferred to a sterile 24 multiwell plate. For infection, P. aeruginosa of an overnight growth (OD600 = 2.4) was harvested at 6000g and washed three times with original culture volume of M9 buffer. The bacteria were resuspended in original culture volume in M9 buffer containing 10% LB broth and 50 µL of the bacterial cells were added to the 450 µL suspension of C. elegans. C. elegans were incubated at 25 °C for 4 h, harvested at 1000g and washed three times with M9 buffer to remove excess bacteria in the extracellular space. C. elegans were then resuspended in 500 µL of M9 buffer containing 10% LB broth and 50 µM PD6. C. elegans were incubated for an additional 30 min at 25 °C. The C. elegans were harvested at 1000g, and washed three times with M9 buffer, and put into a final suspension of 10 mM sodium azide in M9 buffer and analyzed by confocal microscopy. (Source) Lewis, J. A. & Fleming, J. T. (1995) Caenorhabditis elegans: Modern Biological Analysis of an Organism, eds. Epstein, H. F. & Shakes, D. C. (Academic, San Diego), Vol. 48, pp. 3–29.
Simulation systems
We first modeled a homogeneous E. coli K12 bilayer that consists of lipid A and K12 core. The E. coli K12 LPS modeling, its assembly to a bilayer, and simulation protocols followed the CHARMM-GUI LPS Modeler (Jo, et al., 2017; Jo, et al., 2015) and Membrane Builder step-by-step protocol (Jo, et al., 2008; Jo, et al., 2009; Wu, et al., 2014), which were successfully generalized and applied in the previous LPS simulation (Lee, et al., 2017; Patel, et al., 2016; Wu, et al., 2013). The CHARMM36 force field (Guvench, et al., 2008; Guvench, et al., 2011; Pastor and Mackerell, 2011) was assigned for all simulation. 72 LPS were assembled in the system (36 LPS per each leaflet) and neutralized by Ca2+ ions with 150 mM KCl bulk solution. Note that, all simulations were performed using NAMD simulation package (Phillips, et al., 2005), and additional dihedral angle restraints for all sugar rings were applied during all simulation to keep the chair conformation. After equilibrated, a 500-ns NPT (constant particle number, pressure, and temperature) production simulation was performed at 310.15 K and 1 bar. Using the last snapshot of the K12 bilayer system, the three different PDn (PD1, PD3, and PD6) systems were generated by placing the same kind of two PDn (one above and the other below the K12 bilayer). The force field parameters for PMBN, PEG, and DNP were generated and assembled by analogy from the CHARMM36 force field. After short minimization, 50-ps equilibration simulations were performed for stabilization of newly added PDn. By randomly assigning initial velocities, three replicas for each PDn system were built to improve conformational sampling and check the convergence.
Next, a collective variable-based steered molecular dynamics (CVSMD) simulation was performed for each system in NPT ensemble to bring the PMBN moiety near the lipid A head region; the bilayer center was located at z = 0, and z = ±18 Å were used as the target z values for the upper and lower PDn, respectively. In the CVSMD simulation, the PMBN Cα atoms were smoothly steered with a force constant of 50 kcal/mol toward the lipid A head region (Δz; 50~55 A) for 10 ns with a 2-fs time step. The positional restraints with a force constant of 10 kcal/mol were applied to the LPS bilayer to keep the bilayer center near z = 0. After the CVSMD simulation, additional 200-ns NPT production simulations were performed without any restraint except the dihedral angle restraints for sugar rings, and the last 150-ns trajectories were used for the analysis.
Chemical Synthesis
General Methods for Chemical Synthesis
All solvents and reagents were obtained from commercial sources and were used as such.
Synthesis of FITC-conjugated anti-DNP antibody
Anti-DNP rabbit (1 mg/mL) was suspended in 1 mL cold solution of 0.05 M boric acid, 0.2 M NaCl at a pH = 9.2 in a 30 KDa molecular weight cut-off centrifuge tube. Antibody was spun at 5,000g for 10 min at 4 °C (4×) to complete the wash process. A solution of 5 mg/mL of fluorescein 5-isothiocyanate (40 µL) was added to the washed antibody solution (1mL). Solution rotated for 2 h protected from light. After reaction time, solution was washed in with 10 mM phosphate, 0.15 M NaCl, 0.08% NaN3 at pH 7.8, as before. Concentration was determined by absorbance at 280 nm and 480 nm via Shimadzu Biotech BioSpec-nano spectrophotometer.
Synthesis of Boc-protected PMBN
Boc-protected PMBN was synthesized according to the published protocol: O’Dowd H.; Kim B.; Margolis P.; Wang W.; Wu C.; Lopez S. L.; Blais J. Preparation of tetra-Boc-protected polymyxin B nonapeptide. Tetrahedron Lett. 2007, 48, 2003–2005.
Briefly, a solution was prepared from papain (30 mg, 13 units/mg, Sigma-Aldrich), NaCl (33 mg, 0.6 mmol) and cysteine (14 mg, 0.115mmol) and 44 mg EDTA (44 mg, 0,15mmol) in 15 mL of sodium phosphate buffer (PB buffer, pH 7.0). After incubating this solution for 25 min at 37 °C, it was then added, directly, to a solution that was made from 75 mL of sodium phosphate buffer (PB buffer pH 7.0) that contain Polymyxin B sulfate (1g, 0.84 mmol), which was maintain at 37°C. After gentle stirring at 37°C overnight, the mixture was heated to reflux briefly (about 15 min), cooled, and filtered to remove denatured enzyme. The filtrate was then concentrated under reduced pressure. The resulting solid was then dissolved in 100 mL of H2O/MeOH/NEt3 = 1/1/1. To this solution was added Boc-ON (1.036 g, 4.21 mmol) and the solution stirred for 2h at room temperature. After reaction completed, all solvent were removed under reduced pressure. The resulting solid was then purified by column chromatography using CHCl3/MeOH = 100/1 to CHCl3/MeOH = 20/1 as eluent and to give 1.02 g (0.69 mmol) penta-Boc-PMBN. The penta-Boc-PMBN was then dissolved in 30 mL of 20% TFA in DCM and stirred for 1.5 h at room temperature. After reaction completed, solvents was removed under reduced pressure and give TFA salt of PMBN (confirmed by MS spectra).
In a 100 mL flask was added the TFA salt of PMBN (520 mg, 0.366 mmol), 10 mL of MeOH, 10 mL of H2O, and NEt3 (550 mg, 5.1 mmol, 0.7 mL). Then Boc-ON (360 mg, 1.46 mmol) was added into the solution and the reaction stirred for 24 hours. The solvent was removed under reduced pressure and CH2Cl2 was added and washed with saturated NaHCO3. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The product was purified by chromatography (0 – 20% MeOH in CH2Cl2) to afford tetra-Boc-PMBN (251 mg, yield: 50%) as a white solid having 1H NMR (400 MHz, CD3OD) δ 7.33 – 7.15 (m, 5H), 4.58 – 3.93 (m, 10H), 3.64 – 3.41 (m, 1H), 3.26 – 2.81 (m, 11H), 2.37 – 0.99 (m, 56H), 0.65 (d, J = 25.2 Hz, 6H). LC-MS-MS, ESI MS: for C63H107N14O19, (M+H) calculated: 1364.89; found: 1364.52.
Synthesis of PF (PMBN-FITC)
Fluorescein 5-isothiocyanate (FITC) (1.5 eq., 4.08 mg, 0.0105 mmol) was dissolved in DMF (100 µL). (BOC)PMBN (1eq., 10 mg, 0.007 mmol) was added to the fluorophore solution. DIEA (3eq., 3.65 µL, 0.021 mmol) was then added to the solution. Reaction progressed for 2 h while rotating protected from light. Reaction solution was purified on normal phase silica column with an eluent consisting of solvent A (CH2Cl2 / 0.01% DIEA) and solvent B (MeOH) with a 20 minute gradient transitioning from 0% B to 20% B at a flow rate of 18 ml min-1 and monitored at 280 nm and 254 nm. Product eluted at 10% MeOH and molecular weight was confirmed using ESI-MS. Solvent was removed under reduced pressure and yellow oil was treated with acidic cocktail (50%, TFA, 2.5% TIPS, and 47.5 % CH2Cl2) for 2 h at room temperature to remove protecting groups. After reaction time, solvent was removed to yield 11 mg of PF.
Synthesis of PEGn-FITC
Amino-PEG6-acid (Broadpharm 20424) or Amino-PEG12-acid (Broadpharm 21114) (20.0 mg, 0.057 mmol), fluorescein 5-isothiocyanate (14.69 mg, 0.0377 mmol) and DIEA (26 µL, 0.152 mmol) was dissolved in 200 µL of DMF and progressed for 2 h at room temperature protected from light. After reaction time, product was extracted in CH2Cl2: 2 M HCl and organic layer was isolated. Solvent was removed under reduced pressure and product was used without further purification.
Synthesis of PDn
Example: Amino-PEG16-acid (Broadpharm 21880) (12.8 mg, 0.016 mmol) was dissolved in 200 µL of MeCN:0.1 M NaHCO3. 2, 4-dinitrofluorobenzene (1.5 eq, 3 µL) was added to the reaction and progressed for 2 h at room temperature protected from light. After reaction time, product was extracted in CH2Cl2: 2 M HCl and organic layer was isolated. Solvent was removed under reduced pressure and product was used without further purification. Synthesis repeated for DNP- PEG20-acid and PEG24-acid. DNP-PEG(2–12)-acid compounds were purchased from Broadpharm.
DNP-PEG12-acid (1.5 eq., 12 mg, 0.012 mmol) was dissolved in DMF (50 µL). HATU (1.4 eq., 4.7 mg, 0.0112 mmol) and DIEA (3 eq., 4.6 µL) were added to DNP-PEG12-acid solution. (BOC)PMBN (1 eq., 10.9 mg, 0.0079 mmol) was dissolved in DMF (100 µL). Solutions were mixed together and rotated for 2 h protected from light. After reaction time, product was resuspended in CH2Cl2:NaHCO3 and organic layer was isolated. Solvent was removed under reduced pressure. The resulting yellow oil was purified on normal phase silica column with an eluent consisting of solvent A (CH2Cl2 / 0.01% DIEA) and solvent B (MeOH) with a 20 minute gradient transitioning from 0% B to 20% B at a flow rate of 18 ml min-1 and monitored at 280 nm and 254 nm. Product eluted at 19% MeOH and molecular weight was confirmed using MALDI-TOF MS. Solvent was removed under reduced pressure and yellow oil was treated with acidic cocktail (50%, TFA, 2.5% TIPS, and 47.5 % CH2Cl2) for 2 h at room temperature to remove protecting groups. After reaction time, solvent was removed to yield 13 mg of PD6.
Synthesis of PD6tail
A 25 mL vessel was charged with 1 g (0.9 mmol) of 2-Chlorotrityl chloride resin. Initial loading with N-α-Fmoc-N-ε-4-methyltrityl-L-lysine (Fmoc-L-Lys(Mtt)-OH) (2 eq., 1.8 mmol) and DIEA (4eq., 3.6 mmol) in anhydrous CH2Cl2 (10 mL) was performed. The vessel was agitated for 2 h at room temperature. The resin was washed with DMF, CH2Cl2, MeOH, CH2Cl2, and DMF (3 × 5 ml each). The N-terminus of lysine was deprotected in a solution of 6 M piperazine/100 mM HOBt in DMF (7 ml) for 30 min. Caprylic acid (5 eq.) was added to the vessel in DMF (10 mL) with HCTU (4.9 eq.) and DIEA (10 eq.). The vessel was agitated for 2 h at room temperature. The resin was washed as previously stated. Selective unmasking of the Nε-methyltrityl protecting group was completed in mild acidic conditions of 2 % TFA in CH2Cl2. Fmoc-N-amido-PEG12-acid (2 eq.) was added to the resin in DMF (10 mL) with HCTU (1.9 eq.) and DIEA (4 eq.). After shaking for 4 h, the resin was washed and deprotected, as previously stated. 2,4-dinitrofluorobenzene (8 eq.) was added to the reaction and progressed for 3 h at room temperature protected from light. After reaction time, resin was again washed and cleaved from resin in acidic conditions. After removing solvent, product was isolated and used without further purification. Lys(PEG12)-DNPtail was conjugated to (BOC)PMBN following reaction steps as mentioned above.
Quantification and Statistical Analysis
Statistical parameters are reported in Figure Legends and in Method Details.
Data and Software Availability
Raw data of this study will be available upon request.
Supplementary Material
Movies 1–3 related to Figure 4. The corresponding simulations movies (S1–3) are the last 10 ns of the MD simulation for the three different PDn (PD1, PD3, and PD6) systems without any restraint except the dihedral angle restraints for sugar rings: PMBN (magenta), PEG-DNP (black), LPS (gray), phosphate groups in lipid A (orange), and phosphate groups in Hep (yellow). Movie S1. Dynamics of PD1 in homogeneous E. coli K12 bilayers. Movie S2. Dynamics of PD3 in homogeneous E. coli K12 bilayers. Movie S3. Dynamics of PD6 in homogeneous E. coli K12 bilayers.
Significance.
Traditional antibiotic agents paved the way for massive advances in human health but we need additional strategies to maintain the upper hand in the battle against pathogenic bacteria. In this work, we have developed a powerful method to re-engage components of the immune system to induce a targeted immunological response to Gram-negative pathogens. Specifically, we have established the framework for the use of small molecules to trigger the recruitment of endogenous antibodies to the surface of bacterial cells.
Highlights.
Polymyxin was modified to include endogenous haptens
Agents triggered the recruitment of antibodies to cell surfaces
Targeting of Gram-negative pathogens was observed in culture and in live C. elegans
Acknowledgments
This study was supported by GM124893-01 (MMP), NSF MCB-1727508, and XSEDE MCB070009 (W.I). We would like to thank Dr. Eric Brown (McMaster University) for the kind gifts of E. coli strains with deletions in the LPS biosynthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.S.F. and S.E.P. performed cellular and biochemical experiments. M.S.F. and G.M.O. synthesized, purified, and characterized all PMB analogs. Y.Y. synthesized and purified the Boc-protected PMB precursor. S.E.P. performed the microscopy imaging data. S.K. and D.S.P. performed the simulation experiments. M.S.F. and M.M.P wrote the manuscript with input from other authors.
Declaration of Interests
M. M. P and M. S. F. have filed a patent application based on this work.
References
- Amato RJ, Shetty A, Lu Y, Ellis PR, Mohlere V, Carnahan N, Low PS. A Phase I/Ib study of folate immune (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) with interferon-alpha and interleukin-2 in patients with renal cell carcinoma. J Immunother. 2014;37:237–244. doi: 10.1097/CJI.0000000000000029. [DOI] [PubMed] [Google Scholar]
- Amato RJ, Shetty A, Lu Y, Ellis R, Low PS. A phase I study of folate immune therapy (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J Immunother. 2013;36:268–275. doi: 10.1097/CJI.0b013e3182917f59. [DOI] [PubMed] [Google Scholar]
- Bentley AT, Klebba PE. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J Bacteriol. 1988;170:1063–1068. doi: 10.1128/jb.170.3.1063-1068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi C, Bednarski M. C-glycosyl compounds bind to receptors on the surface of Escherichia coli and can target proteins to the organism. Carbohydr Res. 1992;223:243–253. doi: 10.1016/0008-6215(92)80021-r. [DOI] [PubMed] [Google Scholar]
- Brade H, Steven OM, Stefanie VN, David MC. Endotoxin in health and disease. New York: Marcel Dekker; 1999. [Google Scholar]
- Brown P, Dawson MJ. Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections. J Antibiot (Tokyo) 2017;70:386–394. doi: 10.1038/ja.2016.146. [DOI] [PubMed] [Google Scholar]
- Chihara ST, Takashi, Yahata Masahiro, Ito Akira, Koyama Yasuo. Enzymatic degradation of colistin. Isolation and identification of α-N-acyl α,γ-diaminobutyric acid and colistin nonapeptide. Agricultural and Biological Chemistry. 1973;37:2455–2463. [Google Scholar]
- Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Hancock RE. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- Fura JM, Pidgeon SE, Birabaharan M, Pires MM. Dipeptide-Based Metabolic Labeling of Bacterial Cells for Endogenous Antibody Recruitment. ACS Infect Dis. 2016;2:302–309. doi: 10.1021/acsinfecdis.6b00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fura JM, Pires MM. D-amino carboxamide-based recruitment of dinitrophenol antibodies to bacterial surfaces via peptidoglycan remodeling. Biopolymers. 2015;104:351–359. doi: 10.1002/bip.22618. [DOI] [PubMed] [Google Scholar]
- Fura JM, Sabulski MJ, Pires MM. D-amino acid mediated recruitment of endogenous antibodies to bacterial surfaces. ACS Chem Biol. 2014;9:1480–1489. doi: 10.1021/cb5002685. [DOI] [PubMed] [Google Scholar]
- Fura JM, Sarkar S, Pidgeon SE, Pires MM. Combatting Bacterial Pathogens with Immunomodulation and Infection Tolerance Strategies. Curr Top Med Chem. 2017;17:290–304. doi: 10.2174/1568026616666160829160707. [DOI] [PubMed] [Google Scholar]
- Gallardo-Godoy A, Muldoon C, Becker B, Elliott AG, Lash LH, Huang JX, Butler MS, Pelingon R, Kavanagh AM, Ramu S, et al. Activity and Predicted Nephrotoxicity of Synthetic Antibiotics Based on Polymyxin B. J Med Chem. 2016;59:1068–1077. doi: 10.1021/acs.jmedchem.5b01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvench O, Greene SN, Kamath G, Brady JW, Venable RM, Pastor RW, Mackerell AD., Jr Additive empirical force field for hexopyranose monosaccharides. J Comput Chem. 2008;29:2543–2564. doi: 10.1002/jcc.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvench O, Mallajosyula SS, Raman EP, Hatcher E, Vanommeslaeghe K, Foster TJ, Jamison FW, 2nd, Mackerell AD., Jr CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J Chem Theory Comput. 2011;7:3162–3180. doi: 10.1021/ct200328p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- Jakobsche CE, Parker CG, Tao RN, Kolesnikova MD, Douglass EF, Jr, Spiegel DA. Exploring binding and effector functions of natural human antibodies using synthetic immunomodulators. ACS Chem Biol. 2013;8:2404–2411. doi: 10.1021/cb4004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Cheng X, Lee J, Kim S, Park SJ, Patel DS, Beaven AH, Lee KI, Rui H, Park S, et al. CHARMM-GUI 10 years for biomolecular modeling and simulation. J Comput Chem. 2017;38:1114–1124. doi: 10.1002/jcc.24660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- Jo S, Lim JB, Klauda JB, Im W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys J. 2009;97:50–58. doi: 10.1016/j.bpj.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Wu EL, Stuhlsatz D, Klauda JB, MacKerell AD, Jr, Widmalm G, Im W. Lipopolysaccharide membrane building and simulation. Methods Mol Biol. 2015;1273:391–406. doi: 10.1007/978-1-4939-2343-4_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Kieser KJ, Kagan JC. Multi-receptor detection of individual bacterial products by the innate immune system. Nat Rev Immunol. 2017;17:376–390. doi: 10.1038/nri.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killock D. Immunotherapy: the treatment bug--fighting cancer with bacterial infection. Nat Rev Clin Oncol. 2014;11:562. doi: 10.1038/nrclinonc.2014.151. [DOI] [PubMed] [Google Scholar]
- Kobertz WR, Bertozzi CR, Bednarski MD. C-Glycosyl Aldehydes: Synthons for C-Linked Disaccharides. J Org Chem. 1996;61:1894–1897. doi: 10.1021/jo9517095. [DOI] [PubMed] [Google Scholar]
- Koch AL. Bacterial wall as target for attack: past, present, and future research. Clin Microbiol Rev. 2003;16:673–687. doi: 10.1128/CMR.16.4.673-687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Patel DS, Kucharska I, Tamm LK, Im W. Refinement of OprH-LPS Interactions by Molecular Simulations. Biophys J. 2017;112:346–355. doi: 10.1016/j.bpj.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sega E, Low PS. Folate receptor-targeted immunotherapy: induction of humoral and cellular immunity against hapten-decorated cancer cells. Int J Cancer. 2005;116:710–719. doi: 10.1002/ijc.21126. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 1999;96:47–56. doi: 10.1016/s0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Masihi KN. Fighting infection using immunomodulatory agents. Expert Opin Biol Ther. 2001;1:641–653. doi: 10.1517/14712598.1.4.641. [DOI] [PubMed] [Google Scholar]
- Metallo SJ, Kane RS, Holmlin RE, Whitesides GM. Using bifunctional polymers presenting vancomycin and fluorescein groups to direct antifluorescein antibodies to self-assembled monolayers presenting d-alanine-d-alanine groups. J Am Chem Soc. 2003;125:4534–4540. doi: 10.1021/ja030045a. [DOI] [PubMed] [Google Scholar]
- Moore RA, Bates NC, Hancock RE. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986;29:496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Murelli RP, Zhang AX, Michel J, Jorgensen WL, Spiegel DA. Chemical control over immune recognition: a class of antibody-recruiting small molecules that target prostate cancer. J Am Chem Soc. 2009;131:17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- O’Dowd H, Kim B, Margolis P, Wang W, Wu C, Lopez SL, Blais J. Preparation of tetra-Boc-protected polymyxin B nonapeptide. Tetrahedron Letters. 2007;48:2003–2005. [Google Scholar]
- Owen RM, Carlson CB, Xu J, Mowery P, Fasella E, Kiessling LL. Bifunctional ligands that target cells displaying the alpha v beta3 integrin. Chembiochem. 2007;8:68–82. doi: 10.1002/cbic.200600339. [DOI] [PubMed] [Google Scholar]
- Parker CG, Domaoal RA, Anderson KS, Spiegel DA. An antibody-recruiting small molecule that targets HIV gp120. J Am Chem Soc. 2009;131:16392–16394. doi: 10.1021/ja9057647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor RW, Mackerell AD., Jr Development of the CHARMM Force Field for Lipids. J Phys Chem Lett. 2011;2:1526–1532. doi: 10.1021/jz200167q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DS, Re S, Wu EL, Qi Y, Klebba PE, Widmalm G, Yeom MS, Sugita Y, Im W. Dynamics and Interactions of OmpF and LPS: Influence on Pore Accessibility and Ion Permeability. Biophys J. 2016;110:930–938. doi: 10.1016/j.bpj.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig TA, Harbin JN, Harrington A, Dohmen L, Fleming SD. Evasion and interactions of the humoral innate immune response in pathogen invasion, autoimmune disease, and cancer. Clin Immunol. 2015;160:244–254. doi: 10.1016/j.clim.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J, Gupta D, Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat Rev Immunol. 2011;11:837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- Sahalan AZ, Dixon RA. Role of the cell envelope in the antibacterial activities of polymyxin B and polymyxin B nonapeptide against Escherichia coli. Int J Antimicrob Agents. 2008;31:224–227. doi: 10.1016/j.ijantimicag.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Schindler PR, Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975;8:95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan RT, Hudon J, Hank JA, Sondel PM, Kiessling LL. Rhamnose glycoconjugates for the recruitment of endogenous anti-carbohydrate antibodies to tumor cells. Chembiochem. 2014;15:1393–1398. doi: 10.1002/cbic.201402019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silipo A, Castro DC, Lanzetta R, Parrilli M, Molinaro A. Lipopolysaccharides. In: König H, Claus H, Varma A, editors. Prokaryotic cell wall compounds. Berlin; Springer-Verlag: 2010. pp. 133–153. [Google Scholar]
- Smit J, Kamio Y, Nikaido H. Outer Membrane of Salmonella-Typhimurium - Chemical-Analysis and Freeze-Fracture Studies with Lipopolysaccharide Mutants. J Bacteriol. 1975;124:942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JM, MacNair CR, Ilyas B, French S, Cote JP, Bouwman C, Farha MA, Sieron AO, Whitfield C, Coombes BK, et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol. 2017;2:17028. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Wolchok JD, Chan TA, Mellman I, Palucka K, Banchereau J, Rosenberg SA, Dane Wittrup K. Immunotherapy: The path to win the war on cancer? Cell. 2015;161:185–186. doi: 10.1016/j.cell.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubery H, Ofek I, Cohen S, Fridkin M. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of gram-negative bacteria. J Med Chem. 2000;43:3085–3092. doi: 10.1021/jm0000057. [DOI] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M, Viljanen P. Binding of polymyxin B nonapeptide to gram-negative bacteria. Antimicrob Agents Chemother. 1985;27:548–554. doi: 10.1128/aac.27.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen P, Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother. 1984;25:701–705. doi: 10.1128/aac.25.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Endotoxins: lipopolysaccharides of gram-negative bacteria. In: Wang X, Quinn PJ, editors. Endotoxins: Structure, Function and Recognition, Subcellular Biochemistry. Dordrecht: Springer Science+Business Media B.V.; 2010. pp. 3–25. [DOI] [PubMed] [Google Scholar]
- Wu EL, Cheng X, Jo S, Rui H, Song KC, Davila-Contreras EM, Qi Y, Lee J, Monje-Galvan V, Venable RM, et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J Comput Chem. 2014;35:1997–2004. doi: 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu EL, Engstrom O, Jo S, Stuhlsatz D, Yeom MS, Klauda JB, Widmalm G, Im W. Molecular dynamics and NMR spectroscopy studies of E. coli lipopolysaccharide structure and dynamics. Biophys J. 2013;105:1444–1455. doi: 10.1016/j.bpj.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Hancock RE. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- Yuriy KA, Miguel VA. Structure, chemical synthesis, biogenesis and interaction with host cells. Wien: Springer-Verlag; 2011. Bacterial polysaccharides. [Google Scholar]
- Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movies 1–3 related to Figure 4. The corresponding simulations movies (S1–3) are the last 10 ns of the MD simulation for the three different PDn (PD1, PD3, and PD6) systems without any restraint except the dihedral angle restraints for sugar rings: PMBN (magenta), PEG-DNP (black), LPS (gray), phosphate groups in lipid A (orange), and phosphate groups in Hep (yellow). Movie S1. Dynamics of PD1 in homogeneous E. coli K12 bilayers. Movie S2. Dynamics of PD3 in homogeneous E. coli K12 bilayers. Movie S3. Dynamics of PD6 in homogeneous E. coli K12 bilayers.