Abstract
Animal studies have shown that polyunsaturated fatty acids (PUFAs) have antineoplastic and anti-inflammatory properties. Results from epidemiologic studies on specific types of PUFAs for lung cancer risk, however, are inconclusive. We prospectively evaluated the association of specific types of dietary PUFA intakes and lung cancer risk in two population-based cohort studies, the Shanghai Women’s Health Study (SWHS) and Shanghai Men’s Health Study (SMHS) with a total of 121,970 study participants (i.e., 65,076 women and 56,894 men). Dietary fatty acid intakes were derived from data collected at the baseline using validated food frequency questionnaires (FFQs). Cox proportional hazards model was performed to assess the association between PUFAs and lung cancer risk. Total, saturated and monounsaturated fatty acid intakes were not significantly associated with lung cancer risk. Total PUFAs intake was inversely associated with lung cancer risk [HRs and respective 95% CIs for quintiles 2 to 5 versus quintile 1: 0.84 (0.71-0.98), 0.97 (0.83-1.13), 0.86 (0.74-1.01) and 0.85 (0.73-1.00), Ptrend=0.11]. However, DHA intake was positively associated with lung cancer risk [HRs and 95% CIs: 1.01 (0.86-1.19), 1.20 (1.03-1.41), 1.21 (1.03-1.42) and 1.24 (1.05-1.47), Ptrend=0.001]. The ratio of n-6 PUFAs to n-3 PUFAs (i.e., 7:1) was inversely associated with lung cancer risk, particularly among never-smokers and adenocarcinoma patients. Total PUFAs and the ratio between n-6 PUFAs and n-3 PUFAs were inversely associated with lung cancer risk. Our current study highlights an important public health impact of PUFA intakes toward intervention/prevention programs of lung cancer.
Keywords: Polyunsaturated fatty acids, lung cancer risk
INTRODUCTION
Lung cancer is one of the most common cancers worldwide. In the US, it is estimated that in 2016, there were 224,390 new lung cancer cases (accounts for 14% of all cancer diagnosis) and 158,080 deaths due to lung cancer (27% of all cancer deaths)1. Cigarette smoking is by far the most important risk factor for lung cancer, follow by radon exposure. Other risk factors include secondhand smoke, asbestos exposure, certain metal exposures (i.e., chromium, cadmium, arsenic) (review in1) and susceptible genes, including 5p15, 6p21, 15q25 (review by Brennan P et al.2), 12q243 or 2q224. It is estimated that 10-25% of lung cancer cases are never-smokers of whom many are women5. In this particular population (i.e., never-smoking female), hormonal factor might be an important risk factor in addition to other risk factors such as genetic factor, history of infectious diseases, cooking and heating fumes, etc. for lung cancer risk5. Cumulative evidence also suggest that dietary factors may also play important role in lung cancer pathogenesis6.
Polyunsaturated fat acids (PUFAs) have been shown to be important in maintaining cell functions and homeostasis, including signal transduction, cell growth, differentiation and viability7. Depending on the position of the first double-bond from the methyl end of the carbon chain, PUFAs can be divided into two groups: n-6 PUFAs (including linoleic and arachidonic acids) and n-3 PUFAs (including linolenic, EPA and DHA)8. Evidence from studies in animal models or human cell lines shown that n-3 PUFAs have antineoplastic and anti-inflammatory effects9,10. Several studies reported that both n-3 PUFAs (mainly EPA and DHA) and n-6 PUFAs (i.e., arachidonic) inhibit growth of human lung cancer cells8,11,12.
Recent epidemiologic evidence revealed that fish oil supplementation, a rich source of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), may be inversely associated with lung cancer development13. Proposed possible mechanisms include 1) the mitochondrial membrane of cancer cells (i.e., effect of elevated hydrostatic pressure and low temperature on membrane fluidity)14, 2) stimulating polyunsaturated fatty acid (PUFA) metabolism15, and 3) inhibiting angiogenesis16. In addition, n-3 PUFAs have anti-inflammatory effects, a hallmark of cancer17, via different mechanisms such as: disruption of lipid raft, activation of the anti-inflammatory transcription factor NR1C3 (i.e., peroxisome proliferator activated receptor γ) and binding to the G protein coupled receptor GPR120, and alteration of cell membrane phospholipid fatty acid composition18. Another important role of n-3 PUFAs is that they also have anti-estrogenic properties by inhibiting cell growth in several cancers, such as breast cancer19 or ovarian cancer20.
Human data on the role of PUFA intakes and lung cancer risk is inconsistent. A recent study pooling study with data from 10 cohorts by Yang et al.21 found that high intakes of PUFA were associated with decreased risk of lung cancer (HRs and respective 95% CIs for quintiles 2, 3, 4, 5 vs. quintile 1 were 0.96 (0.91-1.00), 0.97 (0.92-1.02), 0.96 (0.91-1.02), and 0.92 (0.81-0.98); Ptrend=0.02). However, a meta-analysis of eight prospective cohort studies (2 US cohorts, 2 Japanese cohorts and 4 European cohorts)22 found that high PUFA intake was not significantly associated with lung cancer risk (RR=0.91, 95%: 0.78-1.06). Most of prior studies have not specifically evaluated the association of n-6 PUFAs and n-3 PUFAs with lung cancer risk. Also, evaluating individual fatty acid at a time is not sufficient given the fact that there is a competition between n-6 and n-3 PUFAs as enzyme substrates and they may have a different role in inflammation23. Research in breast cancer24, colorectal cancer25,26 or prostate cancer27 found that the ratio of n-6 PUFAs to n-3 PUFAs might be more informative as aforementioned, yet no study has evaluated this ratio for its association with lung cancer risk. We, therefore, investigated the association between dietary intakes of PUFAs and lung cancer, focusing on both individual common PUFAs and the ratio of n-6 PUFAs to n-3 PUFAs in the Shanghai Women’s Health Study (SWHS) and the Shanghai Men’s Health Study (SMHS), two on-going prospective cohort studies.
METHODS
Study Population
The SWHS and SMHS are two on-going population-based cohort studies conducted in eight communities of urban Shanghai, China. Detailed information on designs and methods has been described elsewhere28,29. Briefly, between 1996 and 2000, a total of 74,940 women aged 40 to 70 years were recruited for the SWHS and between 2002 and 2006, a total of 61,478 men aged 40 to 74 years were recruited for the SMHS. At baseline, an in-person interview was conducted to collect information on diet habits, disease history, smoking and alcohol history, occupational history, family cancer history, physical activity and anthropometric measurements, including weight, height, and circumferences. For SWHS, additional information on reproductive history and hormone use was collected. The overall participation rate was 92.7% in SWHS and 74.1% in SMHS. Participants in both cohorts provided written informed consent, and the Institutional Review Boards of all participating institutions approved the study protocols.
For the current analysis, we excluded the first two years of follow-up observation and subjects developed cancer or lost to follow-up within 2-years (n=883 in SWHS and 1,094 in SMHS) to minimize the influence of preclinical conditions at baseline on dietary intake. We excluded women who reported ever smoking (n=2,070) in SWHS because the low smoking rate precluded an informative analysis. We further excluded participants who reported any prior history of cancer, missing data of any cancer diagnosis or non-lung incident cancer (n=6,813 in SWHS and 3,420 in SMHS) and those who reported extreme total energy intake [i.e., outside the range of 500-3,500 Kcal/day in SWHS (n=98) in SWHS and ≥4,000Kcal/day (n=70) in SMHS]. The final sample size for current analysis was 121,970 participants, including 65,076 participants for SWHS and 56,894 participants for SMHS.
Outcome Assessment
In both cohorts, study participants were followed up by annual record linkage with the Shanghai Tumor Registry and Shanghai Vital Statistics Registry and in-person surveys every 2 to 4 years. All possible matches from the linkages are checked manually and verified by home visits. Follow-up for survival status in both cohorts was nearly 100% because of the extremely low out-migration rate in Shanghai28,29. For the current analysis, total incident cases of lung cases diagnosed, after exclusion of 2 years of observation to December 2014, was 1,496 (i.e., 714 in SWHS and 782 in SMHS).
Dietary Assessment
Dietary assessment in both cohorts used previously validated semi-quantitative food frequency questionnaires (FFQs)30,31. The SWHS/SMHS FFQs contain 84-87 food items and food groups commonly consumed in urban Shanghai. Study participants were asked how frequently (in 5 categories: daily, weekly, monthly, yearly, or never) they consumed the food or food group, followed by a question on the amount of food consumed in liangs (1 liang = 50g) per unit of time during the previous 12 months29,32. Daily nutrient intakes were then calculated from the FFQs using the nutrient content of each food based on the China Food Composition Tables33.
Total n-3 PUFAs were calculated by combining 18:3 (linolenic acid), 20:5 (EPA), 22:5 (docosapentaenoic acid-DPA), and 22:6 (docosahexaenoic acid-DHA) fatty acids. Total n-6 PUFAs were calculated by combining 18:2 (linoleic acid) and 20:4 (arachidonic acid) fatty acids. Total n-3 highly unsaturated fatty acids (HUFAs), fatty acids with 20 or greater carbon molecules, were calculated by combining EPA, DPA, and DHA. The ratio between total n-6 PUFAs to total n-3 PUFAs was determined by dividing the sum of the reported dietary intake of linoleic acid and arachidonic acids by the sum of the reported dietary intake of linolenic acid, EPA, DPA, and DHA.
Statistical Analysis
Means and standard deviations were calculated for continuous variables while counts and proportions were computed for categorical variables. Cox proportional hazards models were used to determine the association between dietary fatty acids and lung cancer risk. Dietary PUFAs were categorized into quintiles based on the overall distribution of nutrient intakes in individual cohort. These dietary fatty acids and red meat intake were adjusted for energy intake using residual method34. Covariates included in the multivariable models were age at entry (continuous scale), smoking status (ever/never-SMHS only), smoking packs-year (continuous scale-SMHS only), alcohol drinking status (ever/never), physical activity status (yes/no), vitamin supplemental use (ever/never), BMI (continuous scale), use of hormone therapy replacement (ever/never-SWHS only), menopausal status (postmenopausal, premenopausal, or perimenopausal-SWHS only). To test for a linear trend across quintiles of dietary fatty acids, a continuous variable was created with the values of 0, 1, 2, 3, and 4 for the 5 quintiles. We further performed stratified analysis by histologic subtypes (i.e., squamous cell vs. adenocarcinoma) and smoking status (ever vs. never) in both SWHS and SMHS.
Because both red meat and vegetables are sources of n-6 PUFAs and n-3 PUFAs, we checked our models by including these two variables in our models. To evaluate the impact of total n-3 PUFA intakes in the main model(s) in presence of n-6 PUFA intake, we added total n-3 PUFA intakes as another covariate and vice versa (or mutual adjustment of these two types of PUFAs). Accordingly, for models in which total n-6 PUFA intake was treated as independent variable, we adjusted for total energy-adjusted n-3 PUFAs (g/day-continuous scale). Similarly, for models in which total n-3 PUFAs or total n-3 HUFAs was treated as an independent variable, we also adjusted for total energy-adjusted n-6 PUFA intake (g/day-continuous scale). The proportional hazards assumption was evaluated using Schoenfeld residual plots, and no evidence of violation of assumption was found. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc.). All tests were 2-sided, and P < 0.05 was considered statistically significant.
RESULTS
Table 1 presents the distribution of socio-demographic characteristics and fatty intakes of study participants in the current analysis. In both cohorts, cases had significant shorter follow-up time than non-cases (mean±SD in SWHS: 8.3±4.0 years for cases vs. 13.9±1.8 years for non-cases; and SMSH: 4.6±2.6 years for cases vs. 8.4±1.5 years for non-cases; P<0.0001 in both cohorts). Lung cancer cases were older than non-cases in both cohorts. Men who developed cancer were more likely to smoke and drink more and to exercise more than non-cases. Lung cancer women were more likely to exercise and had post-menopausal status than non-cases, two factors that are more prevalent in older than young Chinese. In both sexes, there was no difference between cases and non-cases with regards to vitamin supplemental use.
Table 1.
Socio-demographic Characteristics and Fatty Intakes of Study Participants
| Characteristic | SMHS
|
SWHS (never smoking women only) |
||||
|---|---|---|---|---|---|---|
| Cases (n=782) |
Non-cases (n=56,112) |
P-value | Cases (n=714) |
Non-cases (n=64,362) |
P-value | |
| Categorical Variables | n (%) | n (%) | n (%) | n (%) | ||
| Ever smoking | 667 (85.3) | 39,067 (69.6) | <0.0001 | - | - | - |
| Ever drinking | 336 (43.0) | 18,769 (33.4) | <0.0001 | 13 (1.8) | 1,252 (1.9) | 0.81 |
| Smoking packs-year (Mean±SD) | 34.9±17.9 | 22.3±17.3 | <0.0001 | - | - | - |
| 0 | 115 (14.7) | 17,045 (30.4) | <0.0001 | - | - | - |
| 1-31 | 333 (42.6) | 29,866 (53.2) | - | - | - | |
| ≥32 | 334 (42.7) | 9,201 (16.4) | - | - | - | |
| BMI (kg/m2) (Mean±SD) | 23.2±3.3 | 23.7±3.1 | <0.0001 | 24.3±3.5 | 24.0±3.4 | 0.01 |
| BMI<25 | 551 (70.5) | 37,666 (67.1) | 0.05 | 441 (61.8) | 42,250 (65.6) | 0.03 |
| BMI≥25 | 231 (29.5) | 18,446 (32.9) | 273 (38.2) | 22,112 (34.4) | ||
| Vitamin supplemental use | 123 (15.7) | 8,355 (14.9) | 0.52 | 149 (20.9) | 12,554 (19.5) | 0.36 |
| Post-menopausal | - | - | - | 500 (70.0) | 30,273 (47.0) | <0.0001 |
| Regular hormone replacement therapy use | - | - | - | 27 (3.8) | 2,343 (3.6) | 0.11 |
| Physical activity | 305 (39.0) | 19,112 (34.7) | 0.01 | 298 (41.7) | 22,171 (34.4) | <0.0001 |
|
| ||||||
| Continuous Variables | (Mean±SD) | (Mean±SD) | P-value | (Mean±SD) | (Mean±SD) | P-value |
|
| ||||||
| Follow-up time | 4.6±2.6 | 8.4±1.5 | <0.0001 | 8.3±4.0 | 13.9±1.8 | <0.0001 |
| Age at baseline | 61.5±9.4 | 55.8±9.5 | <0.0001 | 57.2±8.9 | 52.0±8.9 | <0.0001 |
| Total fatty acid (g/d) | 34.2±17.0 | 34.7±16.1 | 0.42 | 28.0±13.3 | 29.7±13.5 | 0.0005 |
| Saturated fatty acid (g/d) | 10.1±5.3 | 10.3±5.0 | 0.39 | 8.0±4.1 | 8.6±4.2 | 0.0003 |
| Monounsaturated fatty acid (g/d) | 15.1±8.3 | 15.3±7.9 | 0.58 | 12.1±6.3 | 12.9±6.4 | 0.0003 |
| Polyunsaturated fatty acid (g/d) | 8.5±4.1 | 8.6±3.8 | 0.28 | 7.4±3.7 | 7.7±3.5 | 0.03 |
| Linoleic acid (g/d) | 7.1±3.4 | 7.2±3.2 | 0.35 | 6.2±3.1 | 6.4±3.0 | 0.05 |
| Linolenic acid (g/d) | 1.0±0.5 | 1.1±0.5 | 0.08 | 0.9±0.5 | 0.9±0.4 | 0.03 |
| Arachidonic acid (g/d) | 0.05±0.03 | 0.05±0.03 | 0.17 | 0.05±0.03 | 0.05±0.03 | <0.0001 |
| Timnodonic acid-EPA (g/d) | 0.03±0.03 | 0.03±0.03 | 0.11 | 0.03±0.03 | 0.03±0.03 | 0.12 |
| Docosahexaenoic acid-DHA (g/d) | 0.07±0.08 | 0.07±0.08 | 0.22 | 0.06±0.07 | 0.07±0.07 | 0.26 |
| Total n-3 PUFAs (g/d) | 1.1±0.6 | 1.2±0.5 | 0.06 | 1.0±0.5 | 1.0±0.5 | 0.02 |
| Total n-3 HUFAs (g/d) | 0.1±0.1 | 0.1±0.1 | 0.20 | 0.09±0.1 | 0.1±0.1 | 0.19 |
| Total n-6 PUFAs (g/d) | 7.1±3.4 | 7.2±3.2 | 0.35 | 6.2±3.1 | 6.5±3.0 | 0.04 |
| Total energy intake (Kcal/d)a | 1,836.0±479.4 | 1,911.3±476.5 | 0.02 | 1,647.3±374.7 | 1,676.1±391.0 | 0.43 |
| Red meat intake (g/d)b | 61.5±44.5 | 63.1±43.6 | 0.0002 | 47.0±33.5 | 51.2±35.9 | 0.68 |
| Fish intake (g/d)b | 48.9±46.8 | 52.0±46.3 | 0.13 | 48.0±42.2 | 51.1±44.5 | 0.32 |
| Vegetable intake (g/d)b | 316.6±185.6 | 345.0±192.8 | 0.01 | 290.2±174.5 | 296.9±168.7 | 0.73 |
| Peanuts intake (g/d)b | 2.7±6.5 | 2.4±4.4 | 0.08 | 1.6±3.5 | 1.6±3.4 | 0.52 |
| Soy intake (g/d)b | 148.5±109.8 | 156.1±118.1 | 0.05 | 142.8±126.0 | 139.6±122.4 | 0.60 |
Adjusted for age at baseline;
Adjusted for age at baseline and total energy intake
Abbreviation: PUFA-polyunsaturated fatty acid; HUFA-highly unsaturated fatty acid.
Total, saturated and monounsaturated fatty acid intake were not significantly associated with lung cancer risk. The pattern of association between fatty acids and lung cancer risk in male cohort analysis was comparable to that in the pooled analysis. In addition, there was an inverse association between PUFAs and the risk of lung cancer in both cohorts and in female particularly. The hazard ratios (HRs) and respective 95% CIs for quintiles 2, 3, 4, and 5 compared to quintile 1 were 0.84 (0.71-0.98), 0.97 (0.83-1.13), 0.86 (0.74-1.01), and 0.85 (0.73-1.00), Ptrend=0.11-both cohorts and 0.82 (0.65-1.02), 0.94 (0.76-1.18), 0.78 (0.62-0.99) and 0.80 (0.64-1.01), Ptrend=0.06-female (Table 2–Figure 1).
Table 2.
Association Between Fatty Acid Intake and Lung Cancer Risk in the SMHS and SWHS Overalla
| Pooled Analysis (SWHS+SMHS) |
Analysis by Cohort
|
|||||
|---|---|---|---|---|---|---|
| SMHS | SWHS | |||||
| # lung cancer cases | HR (95%CI) | # lung cancer cases | HR (95%CI) | # lung cancer cases | HR (95%CI) | |
| Total Fatty Acid | ||||||
| Q1 | 314 | Ref. | 134 | Ref. | 180 | Ref. |
| Q2 | 302 | 0.97 (0.83-1.14) | 154 | 1.09 (0.86-1.37) | 148 | 0.90 (0.72-1.11) |
| Q3 | 277 | 0.91 (0.78-1.07) | 150 | 1.05 (0.83-1.33) | 127 | 0.81 (0.64-1.02) |
| Q4 | 308 | 1.01 (0.86-1.19) | 168 | 1.14 (0.90-1.43) | 140 | 0.92 (0.73-1.15) |
| Q5 | 295 | 0.97 (0.82-1.14) | 176 | 1.14 (0.91-1.43) | 119 | 0.81 (0.64-1.03) |
| Ptrend | 0.87 | 0.24 | 0.13 | |||
| Saturated Fatty Acid | ||||||
| Q1 | 316 | Ref. | 132 | Ref. | 184 | Ref. |
| Q2 | 299 | 0.98 (0.83-1.14) | 162 | 1.18 (0.94-1.49) | 137 | 0.82 (0.66-1.02) |
| Q3 | 296 | 0.99 (0.85-1.16) | 166 | 1.21 (0.96-1.52) | 130 | 0.83 (0.66-1.04) |
| Q4 | 289 | 0.96 (0.81-1.12) | 151 | 1.04 (0.83-1.33) | 138 | 0.90 (0.72-1.13) |
| Q5 | 296 | 1.00 (0.85-1.17) | 171 | 1.18 (0.93-1.48) | 125 | 0.86 (0.68-1.09) |
| Ptrend | 0.90 | 0.48 | 0.37 | |||
| Monounsaturated Fatty Acid | ||||||
| Q1 | 307 | Ref. | 139 | Ref. | 168 | Ref. |
| Q2 | 300 | 0.98 (0.84-1.15) | 143 | 0.95 (0.75-1.20) | 157 | 1.02 (0.82-1.27) |
| Q3 | 289 | 0.98 (0.83-1.15) | 155 | 1.05 (0.83-1.32) | 134 | 0.91 (0.73-1.15) |
| Q4 | 310 | 1.06 (0.90-1.24) | 173 | 1.13 (0.91-1.42) | 137 | 0.98 (0.78-1.24) |
| Q5 | 290 | 1.01 (0.86-1.19) | 172 | 1.11 (0.88-1.39) | 118 | 0.90 (0.71-1.15) |
| Ptrend | 0.60 | 0.14 | 0.38 | |||
| Polyunsaturated Fatty Acid | ||||||
| Q1 | 323 | Ref. | 151 | Ref. | 172 | Ref. |
| Q2 | 270 | 0.84 (0.71-0.98) | 138 | 0.86 (0.68-1.08) | 132 | 0.82 (0.65-1.02) |
| Q3 | 313 | 0.97 (0.83-1.13) | 162 | 0.99 (0.79-1.24) | 151 | 0.94 (0.76-1.18) |
| Q4 | 288 | 0.86 (0.74-1.01) | 162 | 0.94 (0.76-1.18) | 126 | 0.78 (0.62-0.99) |
| Q5 | 302 | 0.85 (0.73-1.00) | 169 | 0.90 (0.72-1.12) | 133 | 0.80 (0.64-1.01) |
| Ptrend | 0.11 | 0.62 | 0.06 | |||
| Linoleic Acid | ||||||
| Q1 | 312 | Ref. | 152 | Ref. | 160 | Ref. |
| Q2 | 279 | 0.89 (0.75-1.04) | 132 | 0.81 (0.64-1.03) | 147 | 0.96 (0.77-1.20) |
| Q3 | 306 | 0.96 (0.82-1.13) | 167 | 1.01 (0.81-1.26) | 139 | 0.91 (0.72-1.14) |
| Q4 | 299 | 0.91 (0.77-1.06) | 162 | 0.91 (0.73-1.14) | 137 | 0.89 (0.71-1.12) |
| Q5 | 300 | 0.85 (0.72-1.00) | 169 | 0.86 (0.69-1.08) | 131 | 0.81 (0.64-1.03) |
| Ptrend | 0.09 | 0.46 | 0.07 | |||
| Linolenic Acid | ||||||
| Q1 | 318 | Ref. | 154 | Ref. | 164 | Ref. |
| Q2 | 286 | 0.92 (0.78-1.08) | 152 | 0.96 (0.76-1.20) | 134 | 0.88 (0.70-1.11) |
| Q3 | 300 | 0.96 (0.82-1.13) | 149 | 0.91 (0.73-1.14) | 151 | 1.02 (0.81-1.27) |
| Q4 | 291 | 0.94 (0.80-1.10) | 153 | 0.95 (0.76-1.19) | 138 | 0.93 (0.74-1.17) |
| Q5 | 301 | 1.07 (0.82-1.13) | 174 | 1.07 (0.86-1.33) | 127 | 0.84 (0.67-1.07) |
| Ptrend | 0.76 | 0.58 | 0.28 | |||
| Arachidonic Acid | ||||||
| Q1 | 335 | Ref. | 154 | Ref. | 181 | Ref. |
| Q2 | 316 | 1.01 (0.87-1.18) | 158 | 1.04 (0.83-1.30) | 158 | 1.00 (0.81-1.24) |
| Q3 | 311 | 1.03 (0.88-1.20) | 156 | 1.02 (0.82-1.28) | 155 | 1.05 (0.85-1.31) |
| Q4 | 260 | 0.89 (0.76-1.05) | 149 | 0.97 (0.78-1.22) | 111 | 0.81 (0.63-1.03) |
| Q5 | 274 | 0.98 (0.83-1.15) | 165 | 1.09 (0.87-1.36) | 109 | 0.86 (0.68-1.10) |
| Ptrend | 0.35 | 0.69 | 0.09 | |||
| Timnodonic Acid-EPA | ||||||
| Q1 | 303 | Ref. | 151 | Ref. | 152 | Ref. |
| Q2 | 299 | 1.01 (0.86-1.18) | 168 | 1.08 (0.86-1.34) | 131 | 0.92 (0.73-1.11) |
| Q3 | 325 | 1.19 (1.02-1.39) | 162 | 1.13 (0.90-1.41) | 163 | 1.26 (1.01-1.57) |
| Q4 | 290 | 1.17 (1.00-1.38) | 144 | 1.11 (0.88-1.39) | 146 | 1.25 (0.99-1.57) |
| Q5 | 279 | 1.20 (1.02-1.41) | 157 | 1.29 (1.03-1.62) | 122 | 1.10 (0.87-1.40) |
| Ptrend | 0.005 | 0.04 | 0.06 | |||
| DHA | ||||||
| Q1 | 295 | Ref. | 152 | Ref. | 143 | Ref. |
| Q2 | 298 | 1.01 (0.86-1.19) | 161 | 1.00 (0.80-1.25) | 137 | 1.02 (0.80-1.28) |
| Q3 | 322 | 1.20 (1.03-1.41) | 163 | 1.12 (0.90-1.40) | 159 | 1.29 (1.03-1.62) |
| Q4 | 295 | 1.21 (1.03-1.42) | 148 | 1.12 (0.89-1.41) | 147 | 1.30 (1.03-1.64) |
| Q5 | 286 | 1.24 (1.05-1.47) | 158 | 1.27 (1.01-1.59) | 128 | 1.21 (0.95-1.54) |
| Ptrend | 0.001 | 0.02 | 0.02 | |||
| Total n-3 PUFA | ||||||
| Q1 | 323 | Ref. | 164 | Ref. | 164 | Ref. |
| Q2 | 282 | 0.88 (0.75-1.04) | 137 | 0.81 (0.64-1.01) | 145 | 0.97 (0.77-1.21) |
| Q3 | 285 | 0.91 (0.77-1.07) | 148 | 0.88 (0.70-1.10) | 137 | 0.94 (0.75-1.18) |
| Q4 | 303 | 0.98 (0.84-1.15) | 164 | 0.98 (0.79-1.22) | 139 | 0.97 (0.77-1.22) |
| Q5 | 298 | 0.97 (0.83-1.14) | 169 | 1.03 (0.82-1.28) | 129 | 0.91 (0.72-1.15) |
| Ptrend | 0.82 | 0.33 | 0.47 | |||
| Total n-3 PUFA | ||||||
| Q1 | 313 | Ref. | 160 | Ref. | 153 | Ref. |
| Q2 | 282 | 0.94 (0.80-1.10) | 154 | 0.95 (0.76-1.18) | 128 | 0.92 (0.73-1.16) |
| Q3 | 324 | 1.18 (1.01-1.38) | 166 | 1.12 (0.90-1.40) | 158 | 1.24 (0.99-1.55) |
| Q4 | 289 | 1.15 (0.97-1.35) | 142 | 1.05 (0.83-1.31) | 147 | 1.26 (1.00-1.58) |
| Q5 | 288 | 1.22 (1.04-1.43) | 160 | 1.26 (1.01-1.58) | 128 | 1.17 (0.92-1.48) |
| Ptrend | 0.001 | 0.03 | 0.02 | |||
| Total n-6 PUFA | ||||||
| Q1 | 314 | Ref. | 151 | Ref. | 163 | Ref. |
| Q2 | 275 | 0.87 (0.74-1.02) | 132 | 0.82 (0.65-1.04) | 143 | 0.92 (0.73-1.15) |
| Q3 | 305 | 0.95 (0.81-1.12) | 167 | 1.02 (0.81-1.27) | 138 | 0.89 (0.71-1.11) |
| Q4 | 305 | 0.92 (0.79-1.08) | 163 | 0.92 (0.74-1.16) | 142 | 0.91 (0.73-1.14) |
| Q5 | 297 | 0.84 (0.71-0.98) | 169 | 0.87 (0.70-1.09) | 128 | 0.78 (0.62-0.99) |
| Ptrend | 0.10 | 0.52 | 0.07 | |||
| Ratio n-6 PUFA/n-3 PUFA | ||||||
| Q1 | 314 | Ref. | 161 | Ref. | 153 | Ref. |
| Q2 | 315 | 1.02 (0.87-1.20) | 164 | 1.07 (0.86-1.33) | 151 | 0.98 (0.79-1.23) |
| Q3 | 278 | 0.90 (0.77-1.06) | 152 | 1.03 (0.82-1.29) | 126 | 0.79 (0.62-1.00) |
| Q4 | 279 | 0.86 (0.73-1.01) | 141 | 0.91 (0.72-1.14) | 138 | 0.82 (0.65-1.04) |
| Q5 | 310 | 0.79 (0.68-0.93) | 164 | 0.84 (0.68-1.05) | 146 | 0.74 (0.59-0.93) |
| Ptrend | 0.0005 | 0.04 | 0.003 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; HUFA, highly unsaturated fatty acid; HR, hazard ratio; PUFA, polyunsaturated fatty acid; SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study;
Model adjusted for: age, ever smoking status, smoking packs-year (SMHS only), ever drinking status, BMI, physical activity status, vitamin supplemental use, menopausal status, hormone replacement therapy (SWHS only).
Figure 1.
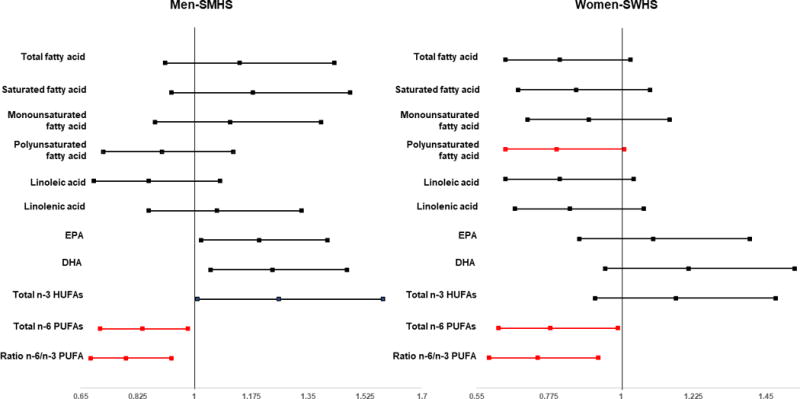
Association Between Highest Quintiles of Selected Fatty Acids and Lung Cancer Risk by Sex
On the other hand, we found a positive association between EPA intake and lung cancer risk in pooled analysis of both cohort [HRs and 95% CIs: 1.01 (0.86-1.18), 1.19 (1.02-1.39), 1.17 (1.00-1.38) and 1.20 (1.02-1.41); Ptrend=0.005 for quintiles 2 to 5 versus quintile 1]. In a separate analysis by sex (or cohort), no association was observed in neither SWHS nor SMHS (Tables 2). We also found that DHA intake was positively associated with lung cancer risk, particularly in female [HRs and 95% CIs: 1.01 (0.86-1.19), 1.20 (1.03-1.41), 1.21 (1.03-1.42), and 1.24 (1.05-1.47), Ptrend=0.001-both cohorts; 1.02 (0.80-1.28), 1.29 (1.03-1.62), 1.30 (1.03-1.64), 1.21 (0.95-1.54), Ptrend=0.02-female; for quintiles 2 to 5 versus quintile 1; respectively] (Tables 2).
No significant associations between total, saturated and monounsaturated fatty acids and lung cancer risk were found in stratified analyses by lung cancer types or smoking status (Table 3). A positive association between EPA intake and lung cancer risk was found among adenocarcinoma patients and never-smokers [HRs and 95% CIs: 0.96 (0.73-1.20), 1.37 (1.06-1.76), 1.59 (1.24-2.04) and 1.26 (0.96-1.64); Ptrend=0.001; and 0.97 (0.78-1.21), 1.30 (1.05-1.60), 1.29 (1.04-1.60) and 1.19 (0.95-1.49); Ptrend=0.01, for quintiles 2 to 5 versus quintile 1; respectively] (Tables 3 and Figure 2). Similar pattern was found in the positive association between DHA intake and with lung cancer risk adenocarcinoma patients and never-smokers. The HRs and respective 95% CIs were 0.99 (0.75-1.30), 1.44 (1.12-1.86), 1.58 (1.23-2.04), and 1.36 (1.04-1.77), Ptrend=0.003; and 1.08 (0.87-1.34), 1.37 (1.11-1.70), 1.37 (1.10-1.71), and 1.34 (1.07-1.68), Ptrend=0.0009, for quintiles 2 to 5 versus quintile 1] (Tables 3 and Figure 2).
Table 3.
Association Between Fatty Acid Intake and Lung Cancer Risk in the SWHS and SMHS, Stratified Analysis by Lung Cancer Type and Smoking Statusa
| By Lung Cancer Type (SWHS + SMHS)
|
By Smoking Status (SWHS + SMHS)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Squamous Cell | Adenocarcinoma | Ever Smoking | Never Smoking | |||||
|
|
|
|||||||
| # | HR (95%CI) | # | HR (95%CI) | # | HR (95%CI) | # | HR (95%CI) | |
| Total Fatty Acid | ||||||||
| Q1 | 16 | Ref. | 119 | Ref. | 120 | Ref. | 194 | Ref. |
| Q2 | 28 | 1.75 (0.95-3.25) | 136 | 1.15 (0.90-1.47) | 128 | 1.02 (0.79-1.31) | 174 | 0.95 (0.77-1.16) |
| Q3 | 21 | 1.30 (0.67-2.50) | 107 | 0.92 (0.70-1.19) | 125 | 1.00 (0.77-1.28) | 152 | 0.85 (0.69-1.06) |
| Q4 | 32 | 1.83 (1.00-3.36) | 134 | 1.14 (0.89-1.47) | 142 | 1.08 (0.84-1.38) | 166 | 0.96 (0.78-1.18) |
| Q5 | 30 | 1.55 (0.84-2.88) | 120 | 1.02 (0.79-1.32) | 152 | 1.07 (0.84-1.37) | 143 | 0.88 (0.70-1.09) |
| Ptrend | 0.23 | 0.90 | 0.48 | 0.31 | ||||
| Pinteraction | 0.99* | |||||||
| Saturated Fatty Acid | ||||||||
| Q1 | 17 | Ref. | 120 | Ref. | 118 | Ref. | 198 | Ref. |
| Q2 | 22 | 1.33 (0.71-2.51) | 123 | 1.04 (0.81-1.34) | 135 | 1.11 (0.87-1.43) | 164 | 0.88 (0.72-1.09) |
| Q3 | 33 | 1.92 (1.07-3.47) | 119 | 1.02 (0.79-1.32) | 140 | 1.16 (0.91-1.49) | 156 | 0.87 (0.71-1.08) |
| Q4 | 23 | 1.27 (0.67-2.39) | 123 | 1.05 (0.81-1.36) | 128 | 1.01 (0.78-1.30) | 161 | 0.92 (0.74-1.14) |
| Q5 | 32 | 1.69 (0.93-3.08) | 131 | 1.13 (0.87-1.45) | 146 | 1.13 (0.88-1.44) | 150 | 0.91 (0.73-1.13) |
| Ptrend | 0.16 | 0.40 | 0.64 | 0.49 | ||||
| Pinteraction | 0.89* | |||||||
| Monounsaturated Fatty Acida | ||||||||
| Q1 | 18 | Ref. | 114 | Ref. | 123 | Ref. | 184 | Ref. |
| Q2 | 22 | 1.19 (0.64-2.23) | 126 | 1.12 (0.86-1.44) | 112 | 0.85 (0.65-1.09) | 188 | 1.08 (0.88-1.32) |
| Q3 | 26 | 1.45 (0.79-2.65) | 120 | 1.08 (0.83-1.39) | 137 | 1.06 (0.83-1.35) | 152 | 0.90 (0.73-1.12) |
| Q4 | 32 | 1.58 (0.88-2.83) | 131 | 1.19 (0.92-1.53) | 142 | 1.04 (0.82-1.32) | 168 | 1.06 (0.86-1.30) |
| Q5 | 29 | 1.36 (0.75-2.47) | 125 | 1.15 (0.89-1.49) | 153 | 1.07 (0.84-1.35) | 137 | 0.94 (0.75-1.17) |
| Ptrend | 0.20 | 0.24 | 0.23 | 0.58 | ||||
| Pinteraction | 0.85* | |||||||
| Polyunsaturated Fatty Acid | ||||||||
| Q1 | 22 | Ref. | 131 | Ref. | 135 | Ref. | 188 | Ref. |
| Q2 | 17 | 0.72 (0.38-1.35) | 117 | 0.89 (0.70-1.15) | 114 | 0.80 (0.63-1.03) | 156 | 0.86 (0.69-1.06) |
| Q3 | 28 | 1.20 (0.69-2.11) | 136 | 1.03 (0.81-1.31) | 133 | 0.92 (0.72-1.17) | 180 | 0.99 (0.81-1.22) |
| Q4 | 29 | 1.15 (0.66-2.00) | 114 | 0.85 (0.66-1.10) | 136 | 0.90 (0.70-1.14) | 152 | 0.83 (0.67-1.03) |
| Q5 | 31 | 1.04 (0.60-1.82) | 118 | 0.85 (0.66-1.09) | 149 | 0.88 (0.69-1.11) | 153 | 0.82 (0.66-1.02) |
| Ptrend | 0.43 | 0.19 | 0.56 | 0.08 | ||||
| Pinteraction | 0.90* | |||||||
| Linoleic Acid | ||||||||
| Q1 | 23 | Ref. | 127 | Ref. | 136 | Ref. | 176 | Ref. |
| Q2 | 18 | 0.73 (0.39-1.35) | 110 | 0.94 (0.73-1.20) | 111 | 0.77 (0.60-0.99) | 168 | 0.97 (0.78-1.20) |
| Q3 | 24 | 0.98 (0.55-1.74) | 133 | 1.03 (0.81-1.31) | 136 | 0.93 (0.73-1.18) | 170 | 0.97 (0.79-1.20) |
| Q4 | 30 | 1.10 (0.64-1.91) | 116 | 0.88 (0.68-1.14) | 135 | 0.86 (0.67-1.09) | 164 | 0.93 (0.75-1.16) |
| Q5 | 32 | 1.02 (0.59-1.75) | 120 | 0.87 (0.68-1.12) | 149 | 0.84 (0.66-1.07) | 151 | 0.83 (0.67-1.04) |
| Ptrend | 0.49 | 0.25 | 0.37 | 0.11 | ||||
| Pinteraction | 0.91* | |||||||
| Linolenic Acid | ||||||||
| Q1 | 21 | Ref. | 116 | Ref. | 138 | Ref. | 180 | Ref. |
| Q2 | 25 | 1.17 (0.66-2.10) | 132 | 1.15 (0.90-1.48) | 125 | 0.89 (0.70-1.13) | 161 | 0.94 (0.76-1.16) |
| Q3 | 26 | 1.17 (0.66-2.09) | 124 | 1.08 (0.63-1.39) | 127 | 0.89 (0.70-1.13) | 173 | 1.02 (0.82-1.25) |
| Q4 | 24 | 1.10 (0.61-1.98) | 129 | 1.12 (0.87-1.44) | 126 | 0.89 (0.70-1.13) | 165 | 0.97 (0.71-1.20) |
| Q5 | 31 | 1.32 (0.75-2.32) | 115 | 0.99 (0.76-1.28) | 151 | 1.05 (0.83-1.33) | 150 | 0.88 (0.71-1.10) |
| Ptrend | 0.44 | 0.85 | 0.69 | 0.39 | ||||
| Pinteraction | 0.75* | |||||||
| Arachidonic Acid | ||||||||
| Q1 | 23 | Ref. | 116 | Ref. | 138 | Ref. | 197 | Ref. |
| Q2 | 22 | 1.02 (0.57-1.84) | 127 | 1.14 (0.89-1.47) | 131 | 0.97 (0.76-1.23) | 185 | 1.04 (0.85-1.28) |
| Q3 | 28 | 1.21 (0.70-2.11) | 140 | 1.28 (1.00-1.64) | 133 | 0.99 (0.78-1.26) | 178 | 1.06 (0.86-1.30) |
| Q4 | 26 | 1.20 (0.68-2.12) | 112 | 1.04 (0.80-1.36) | 125 | 0.93 (0.73-1.19) | 135 | 0.84 (0.67-1.05) |
| Q5 | 28 | 1.17 (0.67-2.06) | 121 | 1.15 (0.89-1.50) | 140 | 1.03 (0.81-1.30) | 134 | 0.92 (0.74-1.15) |
| Ptrend | 0.47 | 0.51 | 0.94 | 0.16 | ||||
| Pinterac | 0.16* | |||||||
| Timnodonic Acid-EPA | ||||||||
| Q1 | 20 | Ref. | 108 | Ref. | 134 | Ref. | 169 | Ref. |
| Q2 | 27 | 1.34 (0.75-2.40) | 102 | 0.96 (0.73-1.26) | 143 | 1.03 (0.81-1.30) | 156 | 0.97 (0.78-1.21) |
| Q3 | 27 | 1.33 (0.74-2.38) | 138 | 1.37 (1.06-1.76) | 136 | 1.06 (0.83-1.35) | 189 | 1.30 (1.05-1.60) |
| Q4 | 28 | 1.54 (0.86-2.75) | 152 | 1.59 (1.24-2.04) | 120 | 1.02 (0.80-1.31) | 170 | 1.29 (1.04-1.60) |
| Q5 | 25 | 1.37 (0.75-2.50) | 116 | 1.26 (0.96-1.64) | 134 | 1.21 (0.95-1.54) | 145 | 1.19 (0.95-1.49) |
| Ptrend | 0.26 | 0.001 | 0.18 | 0.01 | ||||
| Pinteraction | 0.29* | |||||||
| DHA | ||||||||
| Q1 | 25 | Ref. | 104 | Ref. | 139 | Ref. | 156 | Ref. |
| Q2 | 25 | 0.96 (0.55-1.66) | 102 | 0.99 (0.75-1.30) | 136 | 0.92 (0.73-1.17) | 162 | 1.08 (0.87-1.34) |
| Q3 | 25 | 1.00 (0.57-1.74) | 141 | 1.44 (1.12-1.86) | 135 | 1.01 (0.79-1.28) | 187 | 1.37 (1.11-1.70) |
| Q4 | 28 | 1.22 (0.71-2.10) | 147 | 1.58 (1.23-2.04) | 125 | 1.02 (0.80-1.30) | 170 | 1.37 (1.10-1.71) |
| Q5 | 24 | 1.06 (0.60-1.87) | 122 | 1.36 (1.04-1.77) | 132 | 1.13 (0.88-1.44) | 154 | 1.34 (1.07-1.68) |
| Ptrend | 0.57 | 0.003 | 0.23 | 0.0009 | ||||
| Pinteraction | 0.44* | |||||||
| Total n-3 PUFAc | ||||||||
| Q1 | 22 | Ref. | 119 | Ref. | 145 | Ref. | 183 | Ref. |
| Q2 | 25 | 1.12 (0.63-2.00) | 124 | 1.06 (0.82-1.36) | 113 | 0.76 (0.59-0.97) | 169 | 0.98 (0.79-1.21) |
| Q3 | 22 | 0.97 (0.54-1.76) | 128 | 1.10 (0.85-1.41) | 127 | 0.87 (0.68-1.10) | 158 | 0.93 (0.75-1.15) |
| Q4 | 29 | 1.28 (0.73-2.25) | 126 | 1.09 (0.84-1.40) | 135 | 0.93 (0.73-1.18) | 168 | 1.01 (0.81-1.24) |
| Q5 | 29 | 1.22 (0.69-2.14) | 119 | 1.03 (0.79-1.33) | 147 | 1.02 (0.81-1.29) | 151 | 0.93 (0.75-1.16) |
| Ptrend | 0.41 | 0.78 | 0.42 | 0.63 | ||||
| Pinteraction | 0.92* | |||||||
| Total n-3 HUFAc | ||||||||
| Q1 | 24 | Ref. | 112 | Ref. | 145 | Ref. | 168 | Ref. |
| Q2 | 26 | 1.09 (0.62-1.90) | 91 | 0.84 (0.64-1.11) | 130 | 0.88 (0.70-1.12) | 152 | 0.97 (0.78-1.21) |
| Q3 | 26 | 1.13 (0.65-1.97) | 144 | 1.41 (1.10-1.80) | 138 | 1.02 (0.81-1.29) | 186 | 1.31 (1.06-1.62) |
| Q4 | 26 | 1.18 (0.67-2.07) | 148 | 1.52 (1.19-1.95) | 118 | 0.94 (0.74-1.21) | 171 | 1.32 (1.06-1.64) |
| Q5 | 25 | 1.19 (0.67-2.10) | 121 | 1.29 (0.99-1.67) | 136 | 1.15 (0.91-1.46) | 152 | 1.27 (1.02-1.59) |
| Ptrend | 0.51 | 0.0003 | 0.22 | 0.002 | ||||
| Pinteraction | 0.36* | |||||||
| Total n-6 PUFA | ||||||||
| Q1 | 23 | Ref. | 127 | Ref. | 135 | Ref. | 179 | Ref. |
| Q2 | 18 | 0.73 (0.39-1.35) | 119 | 0.93 (0.72-1.20) | 111 | 0.78 (0.60-1.00) | 164 | 0.93 (0.75-1.15) |
| Q3 | 24 | 0.98 (0.55-1.74) | 131 | 1.01 (0.79-1.30) | 136 | 0.94 (0.74-1.19) | 169 | 0.95 (0.77-1.18) |
| Q4 | 30 | 1.11 (0.64-1.91) | 121 | 0.92 (0.72-1.18) | 137 | 0.87 (0.69-1.11) | 168 | 0.94 (0.76-1.17) |
| Q5 | 32 | 1.02 (0.59-1.76) | 118 | 0.86 (0.67-1.11) | 148 | 0.85 (0.67-1.07) | 149 | 0.81 (0.65-1.01) |
| Ptrend | 0.48 | 0.28 | 0.41 | 0.11 | ||||
| Pinteraction | 0.90* | |||||||
| Ratio n-6 PUFA/n-3 PUFA | ||||||||
| Q1 | 24 | Ref. | 147 | Ref. | 135 | Ref. | 179 | Ref. |
| Q2 | 32 | 1.47 (0.86-2.50) | 140 | 0.96 (0.76-1.21) | 140 | 1.12 (0.88-1.42) | 175 | 0.96 (0.78-1.18) |
| Q3 | 24 | 1.18 (0.67-2.08) | 122 | 0.83 (0.65-1.06) | 128 | 1.08 (0.85-1.38) | 150 | 0.79 (0.64-0.98) |
| Q4 | 23 | 1.06 (0.60-1.88) | 103 | 0.68 (0.53-0.88) | 117 | 0.94 (0.73-1.20) | 162 | 0.81 (0.65-1.00) |
| Q5 | 24 | 0.92 (0.52-1.64) | 104 | 0.62 (0.48-0.80) | 147 | 0.92 (0.73-1.17) | 163 | 0.70 (0.57-0.87) |
| Ptrend | 0.42 | <0.0001 | 0.22 | 0.003 | ||||
| Pinteraction | 0.17* | |||||||
Abbreviations: BMI, body mass index; CI, confidence interval; HUFA, highly unsaturated fatty acid; HR, hazard ratio; PUFA, polyunsaturated fatty acid; SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study;
Model adjusted for: age, smoking packs-year (SMHS only), ever drinking status, physical activity status, total energy (kcal/day), vitamin supplemental use, menopausal status, hormone replacement therapy (SWHS only).
Pinteraction were calculated in Shanghai Men’s Health Study (SMHS) only
Figure 2.
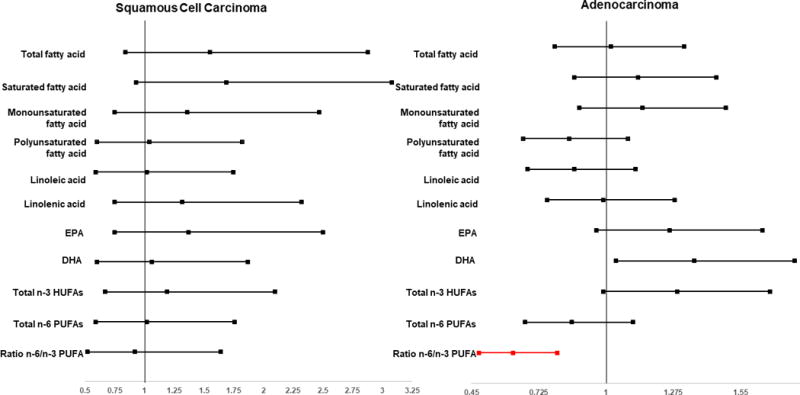
Association Between Highest Quintiles of Selected Fatty Acids and Lung Cancer Risk by Histologic Subtypes
We also found an association between total n-3 HUFAs with increased risk of lung cancer among adenocarcinoma patients and never-smokers [HRs and 95% CIs: 0.84 (0.64-1.11), 1.41 (1.10-1.80), 1.52 (1.19-1.95), and 1.29 (0.99-1.67), Ptrend=0.00031; and 0.97 (0.78-1.21), 1.31 (1.06-1.62), 1.32 (1.06-1.64), 1.27 (1.02-1.59), Ptrend=0.002; for quintiles 2 to 5 versus quintile 1; respectively] (Table 3).
We did not find a significant association between the ratio of n-6 to n-3 PUFAs in SMHS, however, there appeared an inverse association between this ratio and lung cancer risk in SWHS in which two quintiles reached significant levels [i.e., HRs and respective 95% CIs for quintile 3 was 0.79 (0.62-1.00) and for quintile 5 was 0.74 (0.59-0.93)]. This association was similar in adenocarcinoma patients and was stronger in never smokers (both men and women). (Table 3–Figure 2). In stratified analysis by smoking status in SMHS, similar patterns of this association were found (data not shown).
Models that included both red meat and vegetable intakes provided similar results as models without these two variables (Supplemental Table 1). Also, in the models to evaluate the impact of total n-3 PUFA intakes in the presence of total n 6 PUFA intakes and vice versa (or mutual adjustment models), we found similar patterns as models without mutual adjustments. Additionally, we observed inverse association between lung cancer risk with PUFAs in males and with linoleic acid in both sexes (Supplemental Table 2).
DISCUSSION
In this analysis of 121,970 study participants (i.e., 65,076 participants of SWHS-714 lung cancer cases and 56,894 participants of SMHS-782 lung cancer cases), we evaluated the association between fatty acids intake and lung cancer risk. We found that while total, saturated and monounsaturated fatty acid intakes were not significantly associated with lung cancer risk, total PUFAs intake was inversely associated with the lung cancer risk, particularly in female. No significant associations were found in stratified analyses by lung cancer types or smoking status. Unexpectedly, we found that high intake of DHA in both cohorts in general and never-smokers in particularly were associated with increased risk of lung cancer.
To our knowledge, our study is the first to show an association between both individual polyunsaturated fatty acids and the n-6 to n-3 ratio PUFAs and lung cancer risk. Our finding of an inverse association between PUFAs with lung cancer risk is consistent with the finding from the recent pooling study21 with data from 10 cohorts, two of which from our two current cohorts (i.e., SWHS and SMHS), which found that high intakes of PUFA were associated with decreased risk of lung cancer.
An unexpected finding of our analysis is that DHA intake was associated with increased risk of lung cancer in Chinese women who are all never-smokers. We also found that EPA was associated with increased risk of lung cancer in female never-smokers. There were recent reports that both EPA and DHA, instead of providing protective effect, were associated with increased risk of cancer, such as prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT)35 or in the Prostate Cancer Prevention Trial (PCPT)36 and endometrial cancer in the VITamins And Lifestyle Cohort (VITAL)37. For example, in the VITAL cohort, Brasky et al.37 reported that women in the highest compared with the lowest quintile of dietary EPA and DHA intake had an approximately 80% increased risk of endometrial cancer. Long-chain ω-3 PUFA, including EPA and DHA, has been previously shown to have anti-inflammatory effect such as inhibition of TNF-α and modification of eicosanoid activity as well as its influence to cell permeability, gene expression or signal transduction38.
Trombetta et al.8 shown that arachidonic, a major source for n-6 PUFAs, inhibits the growth of adenocarcinoma cells by increasing percentage of cells in the G0/G1 phase and by decreasing percentage of cells in S phase. The underlying mechanism might be related to the mediation of lipid peroxided productions, including PPARα-regulating fatty acid catabolism and inflammatory process; PPARβ-playing an important role in epidermal wound healing and PPARγ-involving in lipid storage, adipocyte differentiation and inflammatory processes39. While some studies of animal models have shown that both n-3 PUFAs and n-6 PUFAs inhibit tumor growth of human lung cancer cells8,11,12, several other studies shown that α-linoleic acid (ALA), a precursor molecule for EPA and DHA metabolism, has adverse biological functions in other cancers. For example, ALA was found to induce MEK1 and MEKK1 gene expression in hepatocellular carcinoma cells, both of which are drivers of cell proliferation40,41. Further studies are, therefore, warranted to better understand the mechanism underlying our findings.
Another interesting finding is that the ratio of n-6 PUFAs to n-3 PUFAs was inversely associated with decreased risk of lung cancer in women (i.e., higher ratio, low risk). The mean ratio of n-6 PUFAs to n-3 PUFAs in our study is 7:1. This ratio was reported to be 16.74:1 in US population42, 15:1 in populations of UK and Northern Europe43, and 4:1 in Japanese population44. It is important to note that in our analysis, linoleic acid was the major source of total n-6 PUFAs. It is well known that n-6 PUFAs competes to n-3 PUFAs for the elongation and desaturation enzymes; however, n-3 PUFAs have greater enzyme-substrate affinities than n-6 PUFAs45. Prior study has shown that increasing dietary n-3 PUFAs, EPA, DHA and linolenic acids leads to decreasing the desaturation of linoleic acid, consequently, the production of arachidonic acid or n-6 PUFAs46. Changes in n-6 to n-3 PUFAs ratio were reported to affect the stability and functionality of cell membrane, particularly the ratio of cholesterol:phospholipid47. Once the balance between cholesterol and phospholipid is altered, cell signaling events such as apoptosis, may be affected48. Since our study is the first one examining this ratio with the risk of lung cancer, finding a comparable study is not possible. For this reason, replicative studies in other populations are warranted.
Our study has several strengths. Both SWHS and SMHS are prospective cohort studies, thus the potential recall biases are avoided. Another strength is that both studies have large sample size that allows us to detect even a weaker association. The other strengths are the usage of FFQ and high follow-up rate as well as large sample size for female non-smoking related lung cancer.
One limitation in our analysis is that there was no direct measurement of fatty acids in blood. However, several studies found that fatty acid levels in adipose tissue were correlated with EPA levels (r=0.47;49), PUFA (r=0.50,49) and trans fatty acids (r=0.51) estimated from FFQ50. Another concern is that preclinical conditions at baseline might have influenced baseline dietary intake; however, as we excluded the first 2 years of follow-up observation, this limitation was minimized. The other limitation is fish oil, a main source for n-3 FUFAs51, was not available for analysis. In our cohorts, we did not collect information on supplemental fish oil intake; however, we did collect fish intake from our food frequency questionnaire. In the multivariable models that also accounted for fish intake, results were not materially changed compared with current findings (data not shown).
In summary, we found that there was an inverse association between polyunsaturated fatty acid with lung cancer risk in Chinese women. The risk of lung cancer is, however, particularly increased with dietary intake of DHA in Chinese women and those who never smoked. Dietary EPA intake was also observed to be associated with increased risk of lung cancer, particularly in never-smokers and adenocarcinoma patients. Total n-3 HUFAs were associated with increased risk of lung cancer in never-smokers and adenocarcinoma patients We also found an inverse association between the ratio n-6 to n-3 PUFAs and lung cancer risk in Chinese women (i.e., higher quintiles), particularly in never-smokers and in adenocarcinoma patients. Our current study highlights an important public health impact of polyunsaturated fatty acid intakes toward intervention/prevention programs of lung cancer.
Supplementary Material
Novelty and Impact.
We found total PUFAs and the ratio between n-6 PUFAs and n-3 PUFAs were inversely associated with lung cancer risk while DHA or EPA intakes were associated with an increased risk of lung cancer. Results from this large prospective study highlights an important public health impact of polyunsaturated fatty acid intakes toward intervention/prevention programs of lung cancer.
Acknowledgments
We thank all research team members and participants of the Shanghai Men’s Health Study (SMHS), and Shanghai Women’s Health Study (SWHS). We also thank Nan Kennedy for editing the manuscript.
Financial Support: This work was supported by grants from the US National Institutes of Health/National Cancer Institute (R37 CA070867 and UM1 CA182910 - to Wei Zheng; R01 CA082729, UM1 CA173640, and R25 CA160056 - to Xiao-Ou Shu)
Footnotes
Conflict of Interest: None declared.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016 [Internet] Atlanta: American Cancer Society; 2016. p. 72. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- 2.Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408. doi: 10.1016/S1470-2045(10)70126-1. [DOI] [PubMed] [Google Scholar]
- 3.Weissfeld JL, Lin Y, Lin H-M, Kurland BF, Wilson DO, Fuhrman CR, Pennathur A, Romkes M, Nukui T, Yuan J-M, Siegfried JM, Diergaarde B. Lung cancer risk prediction using common SNPs located in GWAS-identified susceptibility regions. J Thorac Oncol. 2015;10:1538–45. doi: 10.1097/JTO.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung RJ, Ulrich CM, Goode EL, Brhane Y, Muir K, Chan AT, Marchand LL, Schildkraut J, Witte JS, Eeles R, Boffetta P, Spitz MR, et al. Cross Cancer Genomic Investigation of Inflammation Pathway for Five Common Cancers: Lung, Ovary, Prostate, Breast, and Colorectal Cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv246. pii: djv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couraud S, Zalcman G, Milleron B, Morin F, Souquet P-J. Lung cancer in never smokers—a review. Eur J Cancer 1990. 2012;48:1299–311. doi: 10.1016/j.ejca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 7.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact. 2007;165:239–50. doi: 10.1016/j.cbi.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 9.McEntee MF, Whelan J. Dietary polyunsaturated fatty acids and colorectal neoplasia. Biomed Pharmacother. 2002;56:380–7. doi: 10.1016/s0753-3322(02)00254-8. [DOI] [PubMed] [Google Scholar]
- 10.Klurfeld DM, Bull AW. Fatty acids and colon cancer in experimental models. Am J Clin Nutr. 1997;66:1530S–1538S. doi: 10.1093/ajcn/66.6.1530S. [DOI] [PubMed] [Google Scholar]
- 11.Kudryavtsev IA, Golenko OD, Gudkova MV, Myasishcheva NV. Arachidonic acid metabolism in growth control of A549 human lung adenocarcinoma cells. Biochem Biokhimii͡a. 2002;67:1021–6. doi: 10.1023/a:1020526119866. [DOI] [PubMed] [Google Scholar]
- 12.Maehle L, Lystad E, Eilertsen E, Einarsdottír E, Høstmark AT, Haugen A. Growth of human lung adenocarcinoma in nude mice is influenced by various types of dietary fat and vitamin E. Anticancer Res. 1999;19:1649–55. [PubMed] [Google Scholar]
- 13.Song J, Su H, Wang B-L, Zhou Y-Y, Guo L-L. Fish consumption and lung cancer risk: systematic review and meta-analysis. Nutr Cancer. 2014;66:539–49. doi: 10.1080/01635581.2014.894102. [DOI] [PubMed] [Google Scholar]
- 14.Chapkin RS, Hong MY, Fan Y-Y, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–9. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 15.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D, Steward W, Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer 1990. 2009;45:2077–86. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W, Ma Z, Rasenick MM, Yeh S, Yu J. N-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PloS One. 2012;7:e52838. doi: 10.1371/journal.pone.0052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason JK, Kharotia S, Wiggins AKA, Kitson AP, Chen J, Bazinet RP, Thompson LU. 17β-estradiol increases liver and serum docosahexaenoic acid in mice fed varying levels of α-linolenic acid. Lipids. 2014;49:745–56. doi: 10.1007/s11745-014-3913-8. [DOI] [PubMed] [Google Scholar]
- 21.Yang JJ, Yu D, Takata Y, Smith-Warner SA, Blot W, White E, Robien K, Park Y, Xiang Y-B, Sinha R, Lazovich D, Stampfer M, et al. Dietary Fat Intake and Lung Cancer Risk: A Pooled Analysis. J Clin Oncol. 2017;35:3055–64. doi: 10.1200/JCO.2017.73.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y-F, Lu J, Yu F-F, Gao H-F, Zhou Y-H. Polyunsaturated fatty acid intake and risk of lung cancer: a meta-analysis of prospective studies. PloS One. 2014;9:e99637. doi: 10.1371/journal.pone.0099637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bougnoux P, Giraudeau B, Couet C. Diet, cancer, and the lipidome. Cancer Epidemiol Biomark Prev. 2006;15:416–21. doi: 10.1158/1055-9965.EPI-05-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang B, Ren X-L, Fu Y-Q, Gao J-L, Li D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer. 2014;14:105. doi: 10.1186/1471-2407-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murff HJ, Shu X-O, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao Y-T, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomark Prev. 2009;18:2283–91. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel CR, McCullough ML, Patel RC, Jacobs EJ, Flanders WD, Thun MJ, Calle EE. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomark Prev. 2009;18:516–25. doi: 10.1158/1055-9965.EPI-08-0750. [DOI] [PubMed] [Google Scholar]
- 27.Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res N Y N. 2011;31:1–8. doi: 10.1016/j.nutres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang Y-B. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol. 2015;44:810–8. doi: 10.1093/ije/dyv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Q, Shu X-O, Gao Y-T. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 30.Villegas R, Yang G, Liu D, Xiang Y-B, Cai H, Zheng W, Shu XO. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men’s health study. Br J Nutr. 2007;97:993–1000. doi: 10.1017/S0007114507669189. [DOI] [PubMed] [Google Scholar]
- 31.Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao Y-T, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58:17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 32.Cai H, Zheng W, Xiang Y-B, Xu WH, Yang G, Li H, Shu XO. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men’s Health Study. Br J Nutr. 2007;98:1006–13. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Wang G, Pan X. Chinese Food Composition Tables. Beijing, China: Peking University Medical Press; 2002. [Google Scholar]
- 34.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 35.Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Goodman GE, Minasian LM, Parnes HL, Klein EA, Kristal AR. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132–41. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, Thompson IM, King IB, Albanes D, Kristal AR. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173:1429–39. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brasky TM, Neuhouser ML, Cohn DE, White E. Associations of long-chain ω-3 fatty acids and fish intake with endometrial cancer risk in the VITamins And Lifestyle cohort. Am J Clin Nutr. 2014;99:599–608. doi: 10.3945/ajcn.113.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors. 2009;35:266–72. doi: 10.1002/biof.42. [DOI] [PubMed] [Google Scholar]
- 39.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 40.Harnack K, Andersen G, Somoza V. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr Metab. 2009;6:8. doi: 10.1186/1743-7075-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carey A-M, Pramanik R, Nicholson LJ, Dew TK, Martin FL, Muir GH, Morris JDH. Ras-MEK-ERK signaling cascade regulates androgen receptor element-inducible gene transcription and DNA synthesis in prostate cancer cells. Int J Cancer. 2007;121:520–7. doi: 10.1002/ijc.22715. [DOI] [PubMed] [Google Scholar]
- 42.Eaton SB, Eaton SB, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev Nutr Diet. 1998;83:12–23. doi: 10.1159/000059672. [DOI] [PubMed] [Google Scholar]
- 43.Sanders TA. Polyunsaturated fatty acids in the food chain in Europe. Am J Clin Nutr. 2000;71:176S–8S. doi: 10.1093/ajcn/71.1.176s. [DOI] [PubMed] [Google Scholar]
- 44.Sugano M, Hirahara F. Polyunsaturated fatty acids in the food chain in Japan. Am J Clin Nutr. 2000;71:189S–96S. doi: 10.1093/ajcn/71.1.189S. [DOI] [PubMed] [Google Scholar]
- 45.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated Fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–44. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 47.Abel S, Riedel S, Gelderblom WCA. Dietary PUFA and cancer. Proc Nutr Soc. 2014;73:361–7. doi: 10.1017/S0029665114000585. [DOI] [PubMed] [Google Scholar]
- 48.Rudolph IL, Kelley DS, Klasing KC, Erickson KL. Regulation of cellular differentiation and apoptosis by fatty acids and their metabolites. Nutr Res. 2001;21:381–93. doi: 10.1016/s0271-5317(00)00285-2. [DOI] [PubMed] [Google Scholar]
- 49.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–27. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 50.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–5. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 51.Martins DA, Custódio L, Barreira L, Pereira H, Ben-Hamadou R, Varela J, Abu-Salah KM. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs. 2013;11:2259–81. doi: 10.3390/md11072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


