Abstract
Distal and proximal colon tumors have distinct incidence trends and embryonic origins; whether these sub-sites have distinct susceptibilities to known risk factors is unclear. We used pooled data from 407,270 participants in three US-based studies, with overall median follow-up of 13.8 years. We used adjusted Cox models to analyze the association between dietary intakes (from diet history questionnaire) of total, processed and unprocessed red meat; total white meat, poultry and fish; and meat-related compounds: heme iron, nitrate, nitrite, the heterocyclic amines (HCAs), and benzo(a)pyrene (B(a)P) and incidence of colorectal cancer subsites. The risk of colorectal cancer (n=6,640) increased by 35% for each 50 g/1000 kcal higher daily intake of total red meat, with a significant right-to-left trend from proximal colon (HR:1.24; 95%CI:1.09-1.39) to distal colon (HR:1.34; 95%CI:1.13-1.55) and rectum (HR:1.53; 95%CI:1.28-1.79). Only unprocessed red meat showed a significant right-to-left trend. Each 50 g/1000 kcal increase in white meat intake was associated with a 26% reduction in total colorectal cancer risk (HR: 0.74; 95%CI: 0.68-0.80), with a significant inverse right-to-left trend. The highest quintile of heme iron was associated with increased cancer risk only in the distal colon (HR:1.20; 95%CI: 1.02-1.42) and rectum (HR:1.27; 95%CI: 1.07-1.52). The highest quintile of HCAs, and nitrate/nitrite were associated with increased risk of total colorectal cancer, but these associations did not vary across anatomical subsites. In summary, right and left subsites of the colon may have distinct susceptibilities to meat and possibly other dietary risk factors, suggesting that the causes of colorectal cancer may vary across anatomical subsites.
Introduction
Colorectal cancer is the second most common cause of cancer death among Americans.1 While tumors in the rectum and distal colon have increased more rapidly among the younger population, a “left-to-right shift” (an increase in more proximal cancers) has been reported among older patients. Proximal and distal parts of the large intestine have different embryonic origins. In addition, they have different exposures to bowel contents,2 and they have different microbiota.3 Distal cancers are more often infiltrating, and show aneuploidy and chromosomal instability. Proximal tumors, on the other hand, are more likely to be mucinous, have microsatellite instability, and CpG island methylation.4 Risk factor susceptibility may also differ across subsites. A meta-analysis of cohort studies showed stronger associations between BMI and distal colon cancers, compared with proximal colon and rectal cancers.5 Alcohol has also shown different associations with proximal and distal cancers in the large bowel, but the results have not been consistent.6 Although meat intake is one of the risk factors repeatedly studied in association with colorectal cancers,7–9a recent meta-analysis for the WCRF continuous update project found insufficient data to conduct a dose-response association between red and processed meat with anatomical subsites of colorectal cancer, including rectal cancer.6
Half to 80% of avoidable colorectal cancer deaths are attributable to diet.10 The International Agency for Research on Cancer (IARC) has classified processed meat as carcinogenic to humans (Group 1), and red meat consumption as probably carcinogenic to humans (Group 2A).11 The distinction was based on the fact that despite strong mechanistic evidence, there is more limited evidence that the consumption of red meat causes cancer in humans. Red meat is a major source of animal protein in many populations, and about 80% of the meat consumed in the US is not processed.12 The mechanistic effects of red and processed meat on cancer are thought to be due to natural meat components (such as heme), substances added during curing and processing (nitrates and nitrites), and those formed during cooking (heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs)). There is limited evidence for the association between most of these compounds and colorectal cancer, and whether different parts of the large intestine are affected differently.
We have recently shown increased risk of cancer death associated with the intake of red and processed meat and meat-related compounds among more than half a million participants in the National Institutes of Health-AARP Diet and Health Study.13 The availability of the same data on the intake of meat and meat-related compounds in two other large studies (the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial and Agricultural Health Study) gave us the opportunity to analyze the association between many of the exposures described above and anatomical subsites of colorectal cancer. By pooling the data from these three studies, we have more than 6,500 incident cases of colorectal cancer, which is large enough to analyze each anatomical subsite separately.
Methods
We used data from three large US Cohorts: the NIH-AARP Diet and Health Study (AARP),14 the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO),15 and the Agricultural Health Study (AHS).16 The details and design of each study have been published before, but here we briefly summarize the methods and the data used in this analysis.
NIH-AARP Diet and Health Study (AARP)
In the AARP study, 3.5 million AARP members, aged 50-71 years, from 6 states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, GA, and Detroit, MI) received questionnaires on demographic characteristics, diet and lifestyle in 1995. A total of 617,119 persons returned this baseline questionnaire, including a 124-item food frequency questionnaire, the NCI-Diet History Questionnaire (DHQ). Six months later, 334,907 respondents completed and returned a follow-up risk factor questionnaire, including information on meat-cooking methods. We excluded proxy respondents, individuals who had a cancer diagnosis (except non-melanoma skin cancer) before returning the risk factor questionnaire, and subjects who reported extreme (more than two times the interquartile range of sex-specific log-transformed values) total energy intake, resulting in an analytic cohort of 327,183 participants (191,925 men and 135,258 women). Probabilistic linkage with state cancer registries was used to identify incident cancer cases, and vital status was confirmed by annual linkage of the cohort to the Social Security Administration Death Master File in the US verification of vital status. Follow-up was censored at the date of death, cancer diagnosis, participant relocation out of the registry area, or December 31, 2011, whichever came first. The AARP Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute.
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO)
The PLCO is a randomized, multicenter clinical trial investigating the efficacy of screening for four cancers. Participants were 55–74 years old, and recruited from 10 centers in the USA (Birmingham, AL; Denver, CO; Washington, DC; Honolulu, HI; Detroit, MI; Minneapolis, MN; St Louis, MO; Pittsburgh, PA; Salt Lake City, UT; and Marshfield, WI) from 1993 to 2001. More than 150,000 individuals were randomized to either a screening arm or a control arm. Since the screening can potentially influence the outcomes under study, we restricted the present study to the individuals in the control arm of the trial (more than 77,000 of the participants). Since 1998, the NCI-Diet History Questionnaire (DHQ) and the cooking method questions were used for the control group at baseline, and at the same time, these questionnaires were offered to those enrolled in the study prior to 1998. After excluding individuals who did not complete the baseline questionnaire, the DHQ or the meat-cooking questionnaire completely, reported extreme total energy intake, or had a history of cancer other than non-melanoma skin cancer before dietary assessment, a total of 49,850 individuals (23,761 men and 26,089 women) were included in the current study. Incident cancers were ascertained through medical record abstraction of suspected cases in annual study update questionnaires, and vital status was confirmed by linkage to the National Death Index. Follow-up extended until the time of death, cancer diagnosis, participant withdrawal, the end of the follow-up period, or December 31, 2009, whichever came first. The PLCO study was approved by the Institutional Review Board at the National Cancer Institute and the 10 study centers.
Agricultural Health Study (AHS)
The AHS is a prospective cohort study of licensed pesticide applicators (farmer and commercial applicators) and spouses of farmer and commercial applicators in Iowa and North Carolina (commercial applicators were enrolled only from Iowa). The baseline questionnaires were sent to 57,311 people (52,395 farmer and 4916 commercial applicators) and 32,347 spouses from December 1993 through December 1997. In addition to basic demographic information, the self-administered take-home questionnaire also sought information about smoking and alcohol consumption, medical history, meat-cooking practices and diet (using the NCI-DHQ). After excluding those who did not complete the DHQ or did not provide information on meat cooking practices, reported extreme total energy intake, had a history of cancer other than non-melanoma skin cancer before returning the take-home questionnaire, or had no follow-up time, a total of 30,237 individuals (16,295 men and 13,942 women) were eligible for this study. Linkage to the cancer registries in Iowa and North Carolina were used for the identification of incident cancers and linkage to the state death registries and the National Death Index were used to ascertain vital status. Follow-up was censored at the time of death, cancer diagnosis, movement out of the state, or December 31, 2013, whichever came first. The institutional review boards of the National Institutes of Health, Battelle Centers for Public Health Research and Evaluation (North Carolina field station), and the University of Iowa (Iowa field station) approved this study.
Exposure Assessment
We extracted information on meat intake from the DHQ in the three cohort studies. These included unprocessed red meat (beef and pork, hamburger, liver, steak, and meats in foods such as chili, lasagna, and stew), processed red meat (bacon, beef cold cuts, ham, hotdogs, and sausage), and white meat (chicken, turkey, fish, and canned tuna, poultry cold cuts, low-fat sausages and low-fat hotdogs made from poultry). Total processed meat included both red and white processed meat.
Heme iron intake was computed using a previously developed database of measured heme iron content. These data included a variety of fresh and processed meats, in conjunction with detailed meat cooking practices.17 Daily intakes of nitrate and nitrite from processed meat were estimated using a similar database of 10 types of processed meats, which represent 90% of processed meats consumed in the United States.18 We used the meat cooking methods (grilled/barbecued, pan-fried, microwaved, baked, or broiled) and doneness levels (very well-done/well-done and medium/rare), in conjunction with the CHARRED database,18 to estimate the intakes of heterocyclic amines (HCAs), including 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), as well as the polycyclic aromatic hydrocarbon (PAH) benzo(a)pyrene (B(a)P).
Study Outcomes
The cancer end points were defined, based on first primary diagnosis, by anatomic site and histologic codes of the International Classification of Diseases for Oncology, third edition. Colorectal cancer included codes C180-C184 (proximal colon), C185-187 (distal colon), C199 and C209 (rectum), and C188, C189 and C260 (overlapping or non-specified regions). We restricted our analysis to adenocarcinomas, excluding lymphomas, sarcomas, neuroendocrine tumors, squamous cell tumors, other non-adenocarcinoma histology types, and cases with unspecified histologies.
Statistical Analysis
All nutritional variables were divided by the daily calorie intake (the nutritional density method). We used Cox proportional hazards regression, after checking the proportionality assumption, to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs). The underlying time metric used in the models was follow-up time. This was calculated from DHQ completion until time of first primary diagnosis of colorectal cancer or censoring time (described for each cohort above). For dose-response associations, we analyzed the effects of each 50 g/1000 kcal increase in the daily intakes on the colorectal cancer risks in each cohort separately, and then combined them by random-effects meta-analysis. To analyze the effect of quintile increases in intakes, since the quintile values were different for each cohort, we pooled the data from the three cohorts into one database, and categorized the calorie-adjusted values into quintiles in the pooled data. The same models were used with the lowest quintile of the calorie-adjusted intakes in the pooled data as the referent category.
All models were adjusted for factors previously shown to be associated with colorectal cancer risk, including sex, age at entry into the study, family history of colorectal cancer in first-degree relatives (yesno), regular use of aspirin and/or other NSAIDS in the 12 months before enrollment (yes/no), ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), education (high school graduate or less, post high school training or some college training, college graduate, and postgraduate education), cigarette smoking (never smokers, former smokers who smoked ≤20 cigarettes/day, former smokers who smoked >20 cigarettes/day, current smokers who smoke ≤20 cigarettes/day, and current smokers who smoke >20 cigarettes/day), body mass index (18.5 to <25, 25 to <30, 30 to <35, ≥35), alcohol consumption (none, >0–0.5, >0.5–1, >1–2, >2–4, >4 drinks per day), and daily intakes of fiber, calcium, and total energy. Missing values were entered into the model as a separate category. The models including meat variables were also adjusted for total meat intake, so that increases in the meat variable of interest reflected reductions in other meat types, and the total meat intake remained constant (“substitution model”). We further adjusted the models including meat variables for meat cooking methods (grilled or barbecued, pan-fried, oven broiled, and baked or microwaved), but since the changes in estimates were much less than 10%, we did not include them in the final models. The pooled analyses were also adjusted for the participants’ cohort. In all the models, we used median values of each quintile to test for linear trends in intake. To test the “right-to-left” trend, i.e. the linear trend of the strengths of association as the lesion location became more distal, we defined a “location score” which increased as the lesion was found more distally in the large intestine (1 for proximal colon, 2 for distal colon, and 3 for rectum). We then used variance-weighted least-square regression modelling,19 with beta coefficients from the continuous meat and cancer models (described above) as the outcome variable, the “location score” as the independent variable, and the inverse variances of the beta coefficients as weights. P values for these models were reported as “p value for subsite trend”.
We conducted two types of sensitivity analyses: stratifying by the follow-up duration, and using the residual method for energy adjustment.20
Results
Table 1 summarizes the baseline characteristics of the participants from the three cohorts by quintiles of red meat consumption. The highest amount of red meat (particularly unprocessed red meat) intake was seen among AHS participants: mean intake of total red meat was 33.9±0.04, 33.9±0.09, 49.4±0.1 g/1000 kcal in the AARP, PLCO and AHS cohorts, respectively. AARP cohort participants consumed a higher proportion of their red meat as processed meat (25%) compared with 20% in the PLCO and 19% in the AHS cohorts. In all three cohorts, people eating the least amount of red meat, consumed, on average, slightly more white meat.
Table 1.
Baseline characteristics of the three cohort studies in relation to quintiles of red meat intake
| Red Meat Intake Quintile, g/1000 kcal | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
|
|
||||||
| AARP | Red meat, mean, g/1000 kcal | 9.0 | 20.9 | 30.8 | 42.4 | 66.6 |
| Processed | 2.3 | 5.1 | 7.8 | 11.0 | 17.0 | |
| Unprocessed | 6.7 | 15.8 | 23.0 | 31.4 | 49.5 | |
| White meat, mean, g/1000 kcal | 36.7 | 33.8 | 32.4 | 31.9 | 31.9 | |
| Male, n (%) | 29,686 (45.4) | 33,818 (51.7) | 38,284 (58.5) | 42,506 (65.0) | 47,631 (72.8) | |
| Age, mean, y | 62.6 | 62.6 | 62.5 | 62.2 | 61.7 | |
| Race, n (%) | ||||||
| Non-Hispanic white | 58,375 (90.5) | 60,328 (93.2) | 60,993 (94.2) | 61,668 (95.2) | 61,771 (95.4) | |
| Non-Hispanic black | 3,310 (5.1) | 2,378 (3.7) | 2,001 (3.1) | 1,582 (2.4) | 1,296 (2.0) | |
| Other/missing | 3,751 (5.8) | 2,731 (4.2) | 2,443 (3.8) | 2,187 (3.4) | 2,369 (3.7) | |
| Smoking, n (%) | ||||||
| Never smoker | 26,678 (42.3) | 24,889 (39.4) | 23,241 (36.7) | 21,854 (34.6) | 19,896 (31.5) | |
| Former smoker | 31,277 (49.6) | 31,222 (49.4) | 31,652 (50.0) | 31,847 (50.4) | 31,923 (50.5) | |
| Current smoker or having quit <1 y | 5,131 (8.1) | 7,141 (11.3) | 8,461 (13.4) | 9,555 (15.1) | 11,365 (18.0) | |
| College or postgraduate, n (%) | 30,356 (47.7) | 26,995 (42.3) | 26,192 (41.0) | 25,189 (39.5) | 24,017 (37.6) | |
| BMI, mean, kg/m2 | 25.6 | 26.5 | 27.0 | 27.4 | 28.2 | |
| Energy intake, mean, kcal/d | 1,692.6 | 1,750.6 | 1,822.1 | 1,888.0 | 1,985.8 | |
| Alcohol intake, mean, g/1000 kcal | 5.7 | 6.6 | 6.3 | 6.0 | 5.2 | |
| Fiber intake, mean, g/1000 kcal | 13.7 | 11.5 | 10.7 | 10.0 | 9.1 | |
|
| ||||||
| PLCO | Red meat, mean, g/1000 kcal | 10.5 | 21.8 | 30.9 | 41.8 | 65.5 |
| Processed | 2.0 | 4.2 | 6.2 | 8.6 | 12.8 | |
| Unprocessed | 8.5 | 17.6 | 24.7 | 33.2 | 52.6 | |
| White meat, mean, g/1000 kcal | 32.7 | 29.5 | 29.0 | 29.0 | 28.8 | |
| Male, n (%) | 3,390 (33.2) | 3,927 (39.5) | 4,657 (47.2) | 5,304 (53.5) | 6,483 (65.4) | |
| Age, mean, y | 66.1 | 65.7 | 65.3 | 64.9 | 64.2 | |
| Race, n (%) | ||||||
| Non-Hispanic white | 8,705 (85.3) | 8,990 (90.4) | 9,104 (92.2) | 9,209 (93.0) | 9,334 (94.2) | |
| Non-Hispanic black | 546 (5.4) | 351 (3.5) | 272 (2.8) | 249 (2.5) | 173 (1.8) | |
| Other/missing | 957 (9.4) | 607 (6.1) | 496 (5.0) | 449 (4.5) | 408 (4.1) | |
| Smoking, n (%) | ||||||
| Never smoker | 5,476 (54.2) | 5,106 (51.8) | 4,738 (48.5) | 4,446 (45.4) | 3,949 (40.3) | |
| Former smoker | 3,990 (39.5) | 3,949 (40.0) | 4,058 (41.5) | 4,224 (43.1) | 4,431 (45.2) | |
| Current smoker or having quit <1 y | 635 (6.3) | 811 (8.2) | 974 (10.0) | 1,131 (11.5) | 1,421 (14.5) | |
| College or postgraduate, n (%) | 4,332 (42.6) | 3,764 (38.0) | 3,561 (36.2) | 3,294 (33.4) | 3,043 (30.8) | |
| BMI, mean, kg/m2 | 25.9 | 26.8 | 27.3 | 27.8 | 28.6 | |
| Energy intake, mean, kcal/d | 1,607.7 | 1,644.5 | 1,702.8 | 1,780.4 | 1,924.4 | |
| Alcohol intake, mean, g/1000 kcal | 4.9 | 5.2 | 5.1 | 4.9 | 4.5 | |
| Fiber intake, mean, g/1000 kcal | 12.8 | 11.2 | 10.5 | 9.9 | 9.0 | |
|
| ||||||
| AHS | Red meat, mean, g/1000 kcal | 20.0 | 34.9 | 46.1 | 59.0 | 87.0 |
| Processed | 4.8 | 7.6 | 9.4 | 11.1 | 13.8 | |
| Unprocessed | 15.2 | 27.2 | 36.7 | 47.9 | 73.2 | |
| White meat, mean, g/1000 kcal | 23.9 | 22.3 | 22.2 | 22.0 | 21.1 | |
| Male, n (%) | 2,603 (43.1) | 2,947 (48.7) | 3,209 (53.1) | 3,544 (58.6) | 3,992 (66.0) | |
| Age, mean, y | 56.9 | 53.5 | 52.2 | 51.6 | 50.7 | |
| Race, n (%) | ||||||
| Non-Hispanic white | 5,795 (97.9) | 5,892 (98.6) | 5,955 (99.4) | 5,961 (99.5) | 5,970 (99.6) | |
| Non-Hispanic black | 100 (1.7) | 60 (1.0) | 21 (.4) | 16 (.3) | 15 (.3) | |
| Other/missing | 151 (2.5) | 95 (1.6) | 71 (1.2) | 70 (1.2) | 62 (1.0) | |
| Smoking, n (%) | ||||||
| Never smoker | 3,860 (64.3) | 3,879 (64.5) | 3,827 (63.6) | 3,757 (62.5) | 3,735 (62.0) | |
| Former smoker | 1,575 (26.2) | 1,526 (25.4) | 1,536 (25.5) | 1,552 (25.8) | 1,531 (25.4) | |
| Current smoker or having quit <1 y | 573 (9.5) | 612 (10.2) | 653 (10.9) | 702 (11.7) | 760 (12.6) | |
| College or postgraduate, n (%) | 1,329 (24.0) | 1,357 (24.3) | 1,366 (24.1) | 1,279 (22.5) | 1,164 (20.3) | |
| BMI, mean, kg/m2 | 24.8 | 25.2 | 25.4 | 25.6 | 25.6 | |
| Energy intake, mean, kcal/d | 1,811.0 | 1,922.1 | 1,997.7 | 2,072.7 | 2,182.0 | |
| Alcohol intake, mean, g/1000 kcal | 1.9 | 2.0 | 2.2 | 2.2 | 2.3 | |
| Fiber intake, mean, g/1000 kcal | 10.5 | 9.4 | 9.0 | 8.7 | 8.1 | |
AARP: the NIH-AARP Diet and Health Study; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; AHS: Agricultural Health Study. BMI: body mass index.
In all three cohorts, those with higher red meat consumption were slightly younger, and were more likely to be male, non-Hispanic white, a current smoker, and not have a college degree. Meat consumption was also associated with higher BMI and energy intake, and lower dietary fiber intake. In the AHS, differences in smoking prevalence, education and BMI across meat intake categories were less pronounced. AHS participants were also younger, more likely to be non-Hispanic white and non-smokers, less likely to have college or postgraduate education, and consumed less alcohol. PLCO participants were the oldest at study entry (3 years older than AARP participants, and 12 years older than the AHS participants, on average).
Meat consumption and colorectal cancer
The average follow-up duration was 11.3 years (median: 13.8 years): 11.7 for AARP, 8.2 years for PLCO, and 11.7 years for AHS. During this period, a total of 6,640 cases of colorectal cancer occurred in the three studies. Table 2 shows the number of cases by anatomical subsite in the three cohorts.
Table 2.
The number of participants, follow-up time and colorectal cancer cases in the three cohort studies
| AARP | PLCO | AHS | Total | |
|---|---|---|---|---|
| Participants included in the study, n | 327,183 | 49,850 | 30,237 | 407,270 |
| Person-years of follow-up | 3,842,602 | 408,080 | 354,629 | 4,605,311 |
| All colorectal cancers, n* | 5,737 | 604 | 299 | 6,640 |
| All Colon cancers, n* | 4,306 | 472 | 219 | 4,997 |
| Proximal colon cancers, n | 2,624 | 301 | 135 | 3,060 |
| Distal colon cancers, n | 1,558 | 169 | 80 | 1,807 |
| Rectal cancers, n | 1,431 | 132 | 80 | 1,643 |
AARP: the NIH-AARP Diet and Health Study; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; AHS: Agricultural Health Study.
the numbers do not add up because of a few overlapping or unknown tumor locations.
Table 3 shows the pooled estimate (using random-effects meta-analysis of the three cohort studies) for the dose-response association of red and white meat with colorectal cancer and its anatomical subtypes. As the table shows, for each 50 g/1000 Kcal daily intake of total red meat, total colorectal cancer risk increased by 35% (HR: 1.35; 95%CI: 1.24-1.46). The risk was the lowest for cancers of proximal colon (HR:1.24; 95%CI: 1.09-1.39), and progressively increased when the lesion was in the distal colon (HR: 1.34; 95%CI: 1.13-1.55) or the rectum (HR: 1.53; 95%CI: 1.28-1.79). This right-to-left trend was statistically significant (p<0.05). The positive association with cancer was observed with both processed and unprocessed red meat, but the right-to-left trend was only statistically significant for unprocessed red meat (Table 3). The effect sizes were lower when we combined red and white processed meat into total processed meat. Each 50 g/1000 kcal increase in the intake of white meat, on the other hand, was associated with a 26% reduction in the risk of total colorectal cancer (HR: 0.74; 95%CI: 0.68-0.80). Again, the association became significantly stronger when the tumor was more distal. Both poultry (HR:0.73; 95%CI: 0.56,0.89) and fish (HR:0.79; 95%CI: 0.68,0.89) were associated with decreased risk, but the association with poultry intake was generally stronger, and showed an inverse right-to-left trend. Supplementary table 1 shows the results for each cohort separately. There was little evidence for heterogeneity across the three cohorts, except for poultry intake. Two of the cohorts showed very similar results, while in the AHS the effect was somewhat stronger in the proximal colon. However, the number of cases in the AHS was too small for an independent assessment.
Table 3.
Dose response associations (for each 50 g/1000 kcal per day increase) between different types of meat intake and colorectal cancer (total and subsites) in substitution models
| All colorectal cancers | All colon | Proximal | Distal | Rectum | p value for subsite trend† | |
|---|---|---|---|---|---|---|
| Total red meat | 1.35 (1.24,1.46)* | 1.29 (1.17,1.42)* | 1.24 (1.09,1.39)* | 1.34 (1.13,1.55)* | 1.53 (1.28,1.79)* | 0.04 |
| Processed red meat | 1.30 (1.10,1.49)* | 1.19 (0.85,1.53) | 1.27 (0.98,1.56) | 1.08 (0.75,1.41) | 1.41 (0.99,1.83)* | 0.6 |
| Unprocessed red meat | 1.34 (1.22,1.46)* | 1.29 (1.15,1.43)* | 1.21 (1.04,1.37)* | 1.40 (1.15,1.64)* | 1.47 (1.20,1.74)* | 0.05 |
| White meat | 0.74 (0.68,0.80)* | 0.77 (0.70,0.84)* | 0.80 (0.71,0.90)* | 0.74 (0.63,0.86)* | 0.65 (0.54,0.76)* | 0.04 |
| Poultry | 0.73 (0.56,0.89)* | 0.73 (0.53,0.94)* | 0.73 (0.37,1.08) | 0.72 (0.53,0.91)* | 0.71 (0.57,0.85)* | 0.05 |
| Fish | 0.79 (0.68,0.89)* | 0.80 (0.67,0.92)* | 0.78 (0.62,0.93)* | 0.86 (0.64,1.08) | 0.74 (0.54,0.95)* | 0.7 |
| Total processed meat | 1.14 (1.00,1.30)* | 1.16 (1.00,1.36)* | 1.16 (0.95,1.41) | 1.13 (0.88,1.46) | 1.06 (0.81,1.38) | 0.8 |
Using variance-weighted least-square regression modelling, with the beta coefficients from meat and cancer models (described below) as the outcome variable, the subsite location as the independent variable, and the inverse variances of the beta coefficients as weights.
p<0.001
Numbers represent hazard ratios (95% confidence intervals) for each 50 g/1000 kcal per day increased intake in adjusted models. Models were built for each of the 3 cohorts separately and combined by random-effects meta-analysis. Details for each cohort are presented in supplementary table 1. Models were adjusted for sex, age at entry to study, family history of colorectal cancer, ethnicity, regular use of aspirin and other NSAIDS, education, smoking history, body mass index, alcohol consumption, and daily intakes of fiber, calcium, total energy and total meat.
Figure 1 shows the results for the highest versus lowest quintile of intake in the pooled data. Details of the quintile model results are presented in the supplementary table 2. The pattern of the associations was similar to what we observed with the meta-analysis of the dose-response models. The associations between the highest quintiles of total and unprocessed red meat intake were strongest for rectal cancer, followed by distal colon cancer. The associations with processed red meat were similar across the subsites. The highest quintile of white meat intake was associated with decreased risk, which was strongest for rectal cancer.
Figure 1. The association between the intake of different types of A. red meat and B. white meat and colorectal cancer risk by anatomical subsites in the pooled data from the AARP, PLCO and AHS cohorts using substitution models.
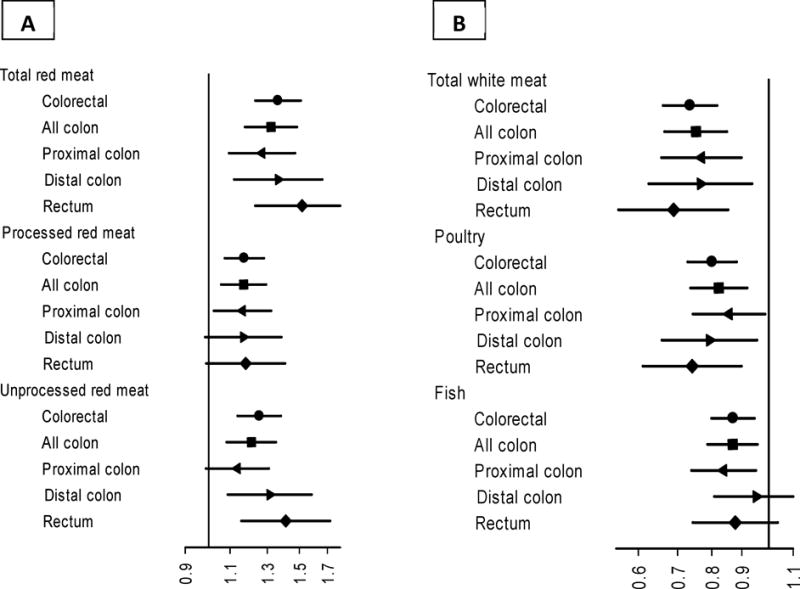
The point estimates are hazard ratios for highest vs. lowest quintiles of calorie-adjusted intakes, and the lines represent 95% confidence intervals in adjusted Cox models. Detailed results are shown in supplementary table 2. Models are adjusted for sex, age at entry to study, family history of colorectal cancer, ethnicity, regular use of aspirin and other NSAIDS, education, smoking history, body mass index, alcohol consumption, cohort, and daily intakes of fiber, calcium, total energy and total meat.
Meat-related compounds and colorectal cancer
Figure 2 and supplementary table 3 show the results for the highest versus lowest quintile of intake for each meat compound in the pooled data. These meat compounds included heme iron, processed meat nitrate, processed meat nitrite, the HCAs MeIQx, DiMeIQx, and PhIP, and the PAH B(a)P. The highest quintile of heme iron intake was associated with increased risk of colorectal cancer (HR: 1.14; 95%CI: 1.05-1.24), particularly in distal colon (HR: 1.20; 95%CI: 1.02-1.42) and rectum (HR: 1.27; 95%CI: 1.07-1.52), with a significant right-to-left trend (p<0.05). Nitrate from processed meat showed associations with colorectal cancer (HR: 1.18; 95%CI: 1.08-1.28) and its subsites which did not vary greatly across subsites. The same was true for processed meat nitrite, though the associations were generally weaker than those for nitrate. Overall, the associations of HCAs with colorectal cancer risk seemed uniform across anatomical subsites (Figure 2). Two HCAs (MeIQx and DiMeiQx) showed associations with all anatomical subsites and significant trends across quintiles of intake (Supplementary table 3). The highest quintiles of MeIQx and DiMeIQx increased the risk of colorectal cancer by about 21% (HR: 1.21; 95%CI: 1.12-1.31) and 16% (HR: 1.16; 95%CI: 1.08-1.24), respectively. The highest quintile of PhIP intake showed a weaker association with colorectal cancer (HR: 1.09; 95%CI: 1.00-1.18), and all colon cancers (HR: 1.09; 95%CI: 1.00-1.20). The two highest quintiles (particularly the 4th quintile) of PhIP also showed associations with distal colon cancer, but the trend did not reach statistical significance (supplementary table 3). We found no evidence for an association between the B(a)P and colorectal cancer risk (HR: 1.00; 95%CI: 0.93-1.08) or any of the subsites.
Figure 2. The association between the intake of meat-associated compounds and colorectal cancer risk by anatomical subsites in the pooled data from the AARP, PLCO and AHS cohorts.
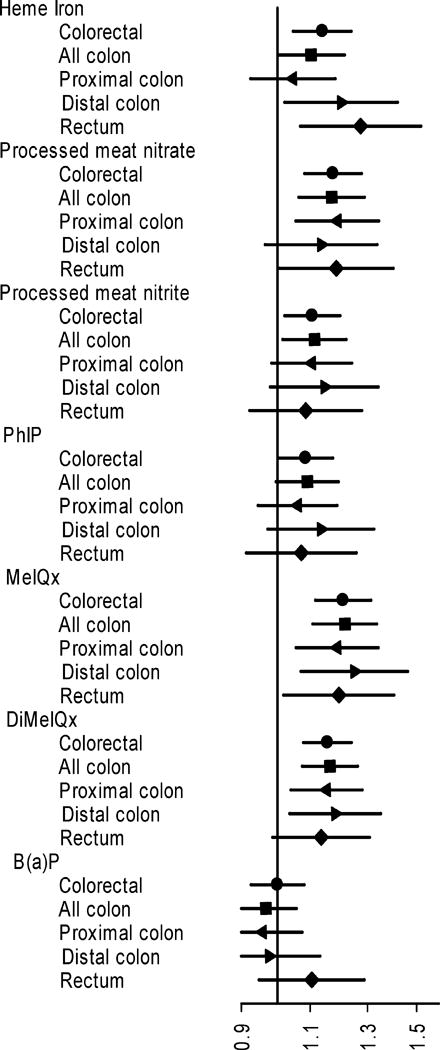
The point estimates are Hazard Ratios for highest vs. lowest quintile of calorie-adjusted intakes, and the lines represent 95% CI in adjusted Cox models. Detailed results are shown in supplementary table 3. Models are adjusted for sex, age at entry to study, family history of colorectal cancer, ethnicity, regular use of aspirin and other NSAIDS, education, smoking history, body mass index, alcohol consumption, cohort, and daily intakes of fiber, calcium, and total energy.
Dropping the first 5 years of follow-up attenuated the associations between meat types and colorectal cancer, however the gradient across the subsites was still present (data not shown). Using the residual method instead of the nutritional density method of energy adjustment made little change to the results (data not shown).
Discussion
We found a 35% higher risk of colorectal cancer per 50 grams/1000 kcal intake of red meat, which increased significantly when the tumor was more distal (a “right-to-left” trend), reaching 53% for rectal cancer. The association was present with the consumption of both processed and unprocessed red meat, but the right-to-left trend was only significant for unprocessed red meat. Heme iron showed a similar gradient across anatomical subsites. Substituting white meat, particularly poultry, was associated with reduced risk, which became significantly stronger for more distal tumors. Nitrate/nitrite from processed meat and HCAs produced during cooking were all associated with increased risk of colorectal cancer, but their associations were similar across subsites.
Most studies and meta-analyses support the presence of an association between red meat intake and colorectal cancer,7–9 even in populations with low intake,21,22 although there are also a few studies which have not shown such an association.23,24 However, to our knowledge, this is the first study to formally test the trend of the association between red meat and colorectal cancer across anatomical subsites. The observed “right-to-left” trend may be due to several factors, including different exposures to bowel contents, variations in the gut microbiome, and/or differences in susceptibility to carcinogens by anatomical subsite. Mami et al. have shown that F. nucleatum-high colorectal cancers gradually increase from rectal cancers to proximal cancers.25 in another study, Flemer and colleagues observed notable differences both at the community level and single operational taxonomic units between proximal cancers, and distal and rectal tumors.3 In the large intestine, bacterial decarboxylation is the first stage of events converting endogenous NO and heme to form carcinogenic N-nitroso compounds (NOCs).26 Susceptibility to NOCs also seems to vary throughout the large intestine.27 NOCs produce O6-methyldeoxyguanine (O6 MEG), which has cytotoxic, mutagenic, clastogenic and carcinogenic activities,28 and O6 MEG adduct levels correlate with red meat intake and NOC formation. O6-MEG has been found to be higher in the normal mucosa of the distal colon in colorectal cancer patients.29 In addition, the levels of the repair enzyme O6-methylguanine-DNA methyltransferase (MGMT), which is responsible for the removal of O6 MEG adducts, is higher in normal tissue of the rectum compared to the sigmoid colon and proximal colon.30 MGMT levels in tumors from rectum and sigmoid colon have also been shown to be correlated with their levels in the normal tissue from the same individuals, while such correlation was absent in the proximal colon.30 These changes suggest increased exposure to endogenously formed alkylating NOCs in the distal colon and rectum, and possibly a more prominent role for the resulting O6 MEG adducts in tumors arising in these subsites.
This study shows associations between both processed and unprocessed red meat and colorectal cancer. Our findings are consistent with a previous report from AARP showing significant associations between unprocessed red meat and colorectal cancer,9 which is now replicated in two other large cohorts. This observation may be due to the high content of heme iron in both processed and unprocessed red meat. Bastide et al.31 have reviewed the evidence for the association between heme iron and colorectal cancer, and have suggested the mechanistic explanation involving the catalytic role of heme iron on two types of reactions: the formation of N-nitroso compounds (described above) and lipid peroxidation. We also found a reduced risk of colorectal cancer associated with replacing total red meat with white meat. Previous studies have also shown beneficial associations for poultry,32 and fish intake.33,34 Unlike red meat, which is abundant in heme iron, white meat has low heme levels.35 White meat does not seem to stimulate endogenous intestinal N-nitrosation,36 and thus has little effect on the NOCs,28 presumably because of the low level of heme.35 We also found a right-to-left subsite-specific gradient for the inverse association of white meat with colorectal cancer, which can also be explained by a substitution effect: parts of the large intestine which are more susceptible to red meat and heme iron effects are the same ones benefiting most from replacing red meat with low-heme white meat.
HCAs are mutagenic compounds produced during high-temperature cooking. Although case-control studies have shown significant associations between HCAs and distal and rectal cancers,37 most prospective studies have been underpowered to detect an association by anatomical subsites.38,39 We were powered enough to detect significant associations between MeIQx and DiMeIQx and all anatomical subsites of colorectal cancer (including rectal cancer). PhIP intake also showed significant associations with total colorectal cancers and all colon cancers. The magnitudes of the associations we observed in our study closely correlate with the in vitro mutagenic potency of these compounds, with MeIQx having more potency in the Ames bacterial reversion assay,40 followed by DiMeIQx and PhIP, respectively.41 Based on our results, it seems that the risk imposed by HCA exposure is not very different by anatomical subsite, but increases with higher mutagenic potency of the compound. Mechanistic data suggest a rationale for an association between PAH exposure and colorectal cancer,42 and a case-control study has shown an association between B(a)P intake and distal colon and rectal cancers.37 However, there is little evidence from prospective studies to support such a link. We also did not observe any association between ingested B(a)P and any of the colorectal cancer subsites. However, this does not completely preclude the possibility of such an association, as the quantification of PAH in the diet is far from perfect. Besides, PAH carcinogenicity depends on many genetic factors affecting PAH metabolism,43 which further complicate studying such an association.
The main strength of our study was the large number of cases, from three prospective studies, which made it possible to analyze each anatomical subsite of colorectal cancer with excellent statistical power. We also adjusted for a wide range of plausible confounders, due to the availability of detailed individual exposure and lifestyle data. There was little evidence for heterogeneity of the associations across the cohorts, except for poultry intake, and by combining these large cohorts, we were also able to increase the diversity of our study population to some extent. However, all three cohorts included mainly non-Hispanic white populations, and in this way our sample may not be representative of populations with a more varied ethnic composition. Finally, we had only a single assessment of dietary habits at the baseline, and could not account for any changes during the follow-up period. Changes in dietary habits during this period could have potentially resulted in exposure misclassification, and led to the attenuation of our effect sizes. However, we don’t think such changes have had a strong influence on our findings; the HRs for AARP study are comparable with an earlier publication, analyzed 7 years into the study, when the likelihood of such dietary change was lower.39
In summary, we observed that total and unprocessed red meat and heme iron had a “right-to-left” trend in their association with colorectal cancer, with the distal colon and rectum observed to be more susceptible. Substitution of red meat with white meat was associated with a lower risk of colorectal cancer, with a similar right-to left trend. These results, in combination with previous observations of sub-site heterogeneity in associations with other risk factors, pathophysiology, and incidence trends, suggest that the causes of colorectal cancer may vary across subsite.
Supplementary Material
Novelty and impact.
We found a significant “right-to-left” trend of increasing associations with colorectal cancer risk for unprocessed red meat and heme iron. Substituting white meat for red meat was associated with a “right-to-left” trend of reduced colorectal cancer risk. Non-subsite-specific associations were seen for processed red meat, nitrate/nitrite additives and heterocyclic amines produced during cooking. Right and left subsites of the colon may have distinct susceptibilities to meat and possibly other dietary risk factors, suggesting that the causes of colorectal cancer vary across subsites.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. The full acknowledgement of NIH-AARP Diet and Health Study cancer registries and data management team can be found here: https://dietandhealth.cancer.gov/acknowledgement.html. The Agricultural Health Study was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES049030) and National Cancer Institute (Z01-CP010119). We used AHS data releases P1REL201701.00, P3REL210701 and AHSREL201701.00. Last, but not least, we are grateful to the study participants in the NIH-AARP Diet and Health Study, The Prostate, Lung, Colorectal and Ovary Screening Trial, and the Agricultural Health Study for their outstanding cooperation.
Footnotes
Conflicts of interest: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 3.Flemer B, Lynch DB, Brown JM, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–Medicare data. J Clin Oncol. 2011;29(33):4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robsahm TE, Aagnes B, Hjartaker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22(6):492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- 6.Vieira AR, Abar L, Chan D, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017 doi: 10.1093/annonc/mdx171. [DOI] [PubMed] [Google Scholar]
- 7.Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293(2):172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol. 2016;97:1–14. doi: 10.1016/j.critrevonc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4(12):e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 1995;103(Suppl 8):165–170. doi: 10.1289/ehp.95103s8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 12.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14(4):575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemadi A, Sinha R, Ward MH, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 15.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 16.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross AJ, Harnly JM, Ferrucci LM, Risch A, Mayne ST, Sinha R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr Sci. 2012;3(7):905–913. doi: 10.4236/fns.2012.37120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha R, Cross A, Curtin J, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res. 2005;49(7):648–655. doi: 10.1002/mnfr.200500018. [DOI] [PubMed] [Google Scholar]
- 19.Grizzle JE, Starmer CF, Koch GG. Analysis of categorical data by linear models. Biometrics. 1969;25(3):489–504. [PubMed] [Google Scholar]
- 20.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 21.Takachi R, Tsubono Y, Baba K, et al. Red meat intake may increase the risk of colon cancer in Japanese, a population with relatively low red meat consumption. Asia Pac J Clin Nutr. 2011;20(4):603–612. [PubMed] [Google Scholar]
- 22.Hirayama T. Association between alcohol consumption and cancer of the sigmoid colon: observations from a Japanese cohort study. Lancet. 1989;2(8665):725–727. doi: 10.1016/s0140-6736(89)90782-4. [DOI] [PubMed] [Google Scholar]
- 23.Spencer EA, Key TJ, Appleby PN, et al. Meat, poultry and fish and risk of colorectal cancer: pooled analysis of data from the UK dietary cohort consortium. Cancer Causes Control. 2010;21(9):1417–1425. doi: 10.1007/s10552-010-9569-7. [DOI] [PubMed] [Google Scholar]
- 24.Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the Multiethnic Cohort Study. Int J Cancer. 2012;131(7):E1125–1133. doi: 10.1002/ijc.27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol. 2016;7(11):e200. doi: 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg JO, Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62(4):616–629. doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 27.Bingham SA, Pignatelli B, Pollock JR, et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17(3):515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 28.Fahrer J, Kaina B. O6-methylguanine-DNA methyltransferase in the defense against N-nitroso compounds and colorectal cancer. Carcinogenesis. 2013;34(11):2435–2442. doi: 10.1093/carcin/bgt275. [DOI] [PubMed] [Google Scholar]
- 29.Povey AC, Hall CN, Badawi AF, Cooper DP, O’Connor PJ. Elevated levels of the pro-carcinogenic adduct, O(6)-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut. 2000;47(3):362–365. doi: 10.1136/gut.47.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Povey AC, Hall CN, Cooper DP, O’Connor PJ, Margison GP. Determinants of O(6)-alkylguanine-DNA alkyltransferase activity in normal and tumour tissue from human colon and rectum. Int J Cancer. 2000;85(1):68–72. doi: 10.1002/(sici)1097-0215(20000101)85:1<68::aid-ijc12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) 2011;4(2):177–184. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 32.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. Int J Cancer. 2005;113(5):829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 33.Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97(12):906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamia C, Lagiou P, Buckland G, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–328. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]
- 35.Carr PR, Walter V, Brenner H, Hoffmeister M. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int J Cancer. 2016;138(2):293–302. doi: 10.1002/ijc.29423. [DOI] [PubMed] [Google Scholar]
- 36.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132(11 Suppl):3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 37.Miller PE, Lazarus P, Lesko SM, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65(2):202–226. doi: 10.1080/01635581.2013.756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le NT, Michels FA, Song M, et al. A Prospective Analysis of Meat Mutagens and Colorectal Cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Environ Health Perspect. 2016;124(10):1529–1536. doi: 10.1289/EHP238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70(6):2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turesky RJ. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol Lett. 2007;168(3):219–227. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95(4):290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44(1):44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 43.Etemadi A, Islami F, Phillips DH, et al. Variation in PAH-related DNA adduct levels among non-smokers: the role of multiple genetic polymorphisms and nucleotide excision repair phenotype. Int J Cancer. 2013;132(12):2738–2747. doi: 10.1002/ijc.27953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


