Abstract
Background:
High-level plasma 25-hydroxyvitamin D [25(OH)D] has been associated with lower colorectal cancer incidence and mortality. Considering evidence indicating immunomodulatory effects of vitamin D, we hypothesised that survival benefits from high systemic vitamin D level might be stronger for colorectal carcinoma with lower immune response to tumour.
Methods:
Using 869 colon and rectal cancer cases within the Nurses’ Health Study and Health Professionals Follow-up Study, we assessed the prognostic association of postdiagnosis 25(OH)D score [derived from diet and lifestyle variables to predict plasma 25(OH)D level] in strata of levels of histopathologic lymphocytic reaction. The Cox proportional hazards regression model was adjusted for potential confounders, including microsatellite instability, CpG island methylator phenotype, LINE-1 methylation, PTGS2 (cyclooxygenase-2) expression, and KRAS, BRAF, and PIK3CA mutations.
Results:
The association of postdiagnosis 25(OH)D score with colorectal cancer-specific mortality differed by levels of peritumoural lymphocytic reaction (pinteraction = 0.001). Multivariable-adjusted mortality hazard ratios for a quintile-unit increase of 25(OH)D score were 0.69 [95% confidence interval (CI), 0.54–0.89] in cases with negative/low peritumoural lymphocytic reaction, 1.08 (95% CI, 0.93–1.26) in cases with intermediate peritumoural reaction, and 1.25 (95% CI, 0.75–2.09) in cases with high peritumoural reaction. The survival association of the 25(OH)D score did not significantly differ by Crohn’s-like lymphoid reaction, intratumoural periglandular reaction, or tumour-infiltrating lymphocytes.
Conclusions:
The association between the 25(OH)D score and colorectal cancer survival is stronger for carcinomas with lower peritumoural lymphocytic reaction. Our results suggesting interactive effects of vitamin D and immune response may contribute to personalised dietary and lifestyle intervention strategies.
Keywords: Clinical outcome, Immunology, Molecular pathological epidemiology, Precision medicine, Tumour microenvironment
1. Introduction
In colorectal cancer, high levels of lymphocytic reaction to tumour have been associated with prolonged patient survival [1–5]. Evidence supports the effectiveness of therapeutic antibodies that target immune checkpoint proteins such as PDCD1 (programmed cell death 1, PD-1) and CD274 (PDCD1 ligand 1, PD-L1) in various cancers, including microsatellite instability (MSI)-high colorectal carcinoma [6–8]. Colorectal cancer consists of heterogeneous groups of neoplasms with varying sets of genetic and epigenetic alterations that are influenced by exogenous and endogenous factors [9–12]. A better understanding of inter-individual differences in anti-tumour effects of immunomodulatory factors would help develop personalised immunotherapeutic strategies [13].
High levels of plasma 25-hydroxyvitamin D [25(OH)D] are associated with lower incidence and mortality of colorectal cancer [14–19]. Vitamin D is hydroxylated in the liver to produce 25(OH)D, and plasma 25(OH)D level serves as a standard indicator of vitamin D activity. It is then hydroxylated further in the kidneys to produce a hormonally active metabolite, 1,25-dihydroxyvitamin D (also known as calcitriol) [20]. Some immune cells can also enzymatically convert 25(OH)D to calcitriol [21]. Experimental evidence suggests that calcitriol may modulate the innate and adaptive immunity [22,23], and can activate T lymphocyte-mediated anti-tumour immune response, thereby suppressing tumour progression [24]. Thus, we hypothesised that the association of vitamin D levels with colorectal cancer survival might be stronger for tumours with lower lymphocytic response than for tumours with higher lymphocytic response.
To test our hypothesis, we conducted this study based on two U.S. large prospective cohort studies. We utilised predicted 25(OH)D score derived from dietary and lifestyle data, which comprehensively takes into account both endogenous and exogenous sources of vitamin D, and estimates long-term plasma 25(OH)D levels [25,26].
2. Methods
2.1. Study population and data collection
We used two prospective cohort studies in the U.S., the Nurses’ Health Study (NHS, 121,701 women aged 30–55 years followed since 1976) and the Health Professionals Follow-up Study (HPFS, 51,529 men aged 40–75 years followed since 1986) [27]. Study participants have been sent questionnaires biennially to update information on lifestyle factors and newly-diagnosed diseases. The follow-up rate has been over 90% for each biennial questionnaire cycle. Additional lethal colorectal cancer cases were identified using the National Death Index.
We analysed 869 cases with available data on postdiagnosis predicted 25(OH)D score, tumour tissue, and survival from participants diagnosed with colorectal cancer up to 2008 (Fig. 1 and Table 1). We included cases with colon and rectal carcinoma based on the colorectal continuum model [28]. We excluded patients who had been preoperatively treated. Patients were followed until death or end of follow-up (1 January 2014 for the HPFS; 30 June 2014 for the NHS), whichever came first. Causes of death were determined by study physicians based on a review of medical records. Formalin-fixed paraffin-embedded (FFPE) tissue blocks of surgically-resected colorectal carcinomas were collected from hospitals throughout the U.S.. A single pathologist (S.O.), who was unaware of other data, reviewed haematoxylin and eosin-stained tissue sections and recorded pathological features including tumour differentiation and four components of lymphocytic reaction, namely, Crohn’s-like lymphoid reaction, peritumoural lymphocytic reaction, intratumoural periglandular reaction, and tumour-infiltrating lymphocytes (TIL) [29]. Each lymphocytic reaction component was graded as negative/low, intermediate, or high. A subset of cases (n = 398) were independently reviewed by a second pathologist (J.N. Glickman) with a good inter-observer correlation as previously described [29]. Tumour differentiation was categorised as well to moderate or poor (> 50% vs. ≤ 50% gland formation, respectively).
Fig. 1.
Flow diagram of the study population in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). 25(OH)D, 25-hydroxyvitamin D.
Table 1.
Clinical, pathological, and molecular characteristics of colorectal cancer cases according to postdiagnosis predicted 25(OH)D score.
| Postdiagnosis predicted 25(OH)D score (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| All cases | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Characteristica | (n = 869) | (n = 173) | (n = 171) | (n = 179) | (n = 172) | (n = 174) | pb |
| Postdiagnosis predicted 25(OH)D score (ng/mL), median (range) |
|||||||
| Female (n = 454, NHS) | 27.4 (18.3–35.3) |
23.9 (18.3–25.2) |
26.2 (25.3–27.0) |
27.4 (27.0–28.4) |
29.4 (28.4–30.4) |
31.7 (30.4–35.3) |
- |
| Male (n = 415, HPFS) | 28.4 (20.5–36.0) |
25.3 (20.5–26.4) |
27.3 (26.4–28.0) |
28.4 (28.0–29.2) |
29.9 (29.2–30.9) |
32.4 (30.9–36.0) |
- |
| Mean age ± SD (years) | 68.3 ± 8.6 | 69.2 ± 8.7 | 68.2 ± 8.8 | 68.4 ± 8.7 | 67.7 ± 8.3 | 67.8 ± 8.6 | 0.53 |
| Year of diagnosis | 0.25 | ||||||
| 1995 or before | 347 (40%) | 62 (36%) | 76 (44%) | 59 (33%) | 71 (41%) | 79 (45%) | |
| 1996–2000 | 277 (32%) | 55 (32%) | 54 (32%) | 66 (37%) | 51 (30%) | 51 (29%) | |
| 2001–2008 | 245 (28%) | 56 (32%) | 41 (24%) | 54 (30%) | 50 (29%) | 44 (25%) | |
| Family history of colorectal cancer in first-degree relative(s) |
0.47 | ||||||
| Absent | 690 (79%) | 132 (76%) | 141 (82%) | 140 (78%) | 142 (83%) | 135 (78%) | |
| Present | 179 (21%) | 41 (24%) | 30 (18%) | 39 (22%) | 30 (17%) | 39 (22%) | |
| Tumour location | 0.78 | ||||||
| Caecum | 161 (19%) | 31 (18%) | 29 (17%) | 40 (22%) | 32 (19%) | 29 (17%) | |
| Ascending to transverse colon | 238 (27%) | 45 (26%) | 48 (28%) | 48 (27%) | 44 (26%) | 53 (30%) | |
| Splenic flexure to sigmoid colon | 284 (33%) | 51 (29%) | 61 (36%) | 53 (30%) | 59 (34%) | 60 (34%) | |
| Rectum | 186 (21%) | 46 (27%) | 33 (19%) | 38 (21%) | 37 (22%) | 32 (18%) | |
| Tumour differentiation | 0.92 | ||||||
| Well to moderate | 797 (92%) | 158 (91%) | 158 (93%) | 167 (93%) | 158 (93%) | 156 (91%) | |
| Poor | 66 (7.7%) | 15 (8.7%) | 12 (7.1%) | 12 (6.7%) | 12 (7.1%) | 15 (8.8%) | |
| AJCC disease stage | 0.53 | ||||||
| I | 245 (31%) | 45 (28%) | 51 (33%) | 59 (36%) | 44 (29%) | 46 (28%) | |
| II | 294 (37%) | 60 (37%) | 49 (32%) | 60 (37%) | 58 (38%) | 67 (41%) | |
| III | 220 (28%) | 45 (28%) | 48 (31%) | 39 (24%) | 42 (28%) | 46 (28%) | |
| IV | 36 (4.5%) | 12 (7.4%) | 7 (4.5%) | 4 (2.5%) | 7 (4.6%) | 6 (3.6%) | |
| MSI status | 0.26 | ||||||
| Non-MSI-high | 652 (83%) | 126 (78%) | 135 (87%) | 137 (86%) | 122 (82%) | 132 (84%) | |
| MSI-high | 131 (17%) | 35 (22%) | 21 (13%) | 22 (14%) | 27 (18%) | 26 (16%) | |
| CIMP status | 0.75 | ||||||
| CIMP-low/negative | 621 (83%) | 119 (80%) | 128 (84%) | 123 (82%) | 118 (84%) | 133 (85%) | |
| CIMP-high | 127 (17%) | 30 (20%) | 24 (16%) | 27 (18%) | 23 (16%) | 23 (15%) | |
| Mean LINE-1 methylation level ± SD (%) |
62.8 ± 9.6 | 63.5 ± 10.2 | 61.4 ± 9.2 | 62.9 ± 10.3 | 63.3 ± 9.3 | 63.0 ± 9.1 | 0.35 |
| KRAS mutation | 0.056 | ||||||
| Wild type | 465 (60%) | 106 (68%) | 85 (54%) | 98 (62%) | 90 (62%) | 86 (55%) | |
| Mutant | 310 (40%) | 49 (32%) | 73 (46%) | 61 (38%) | 56 (38%) | 71 (45%) | |
| BRAF mutation | 0.85 | ||||||
| Wild type | 690 (87%) | 139 (87%) | 141 (89%) | 136 (85%) | 134 (89%) | 140 (87%) | |
| Mutant | 100 (13%) | 21 (13%) | 18 (11%) | 24 (15%) | 17 (11%) | 20 (13%) | |
| PIK3CA mutation | 0.74 | ||||||
| Wild type | 606 (83%) | 124 (84%) | 120 (82%) | 126 (86%) | 114 (81%) | 122 (81%) | |
| Mutant | 125 (17%) | 24 (16%) | 26 (18%) | 20 (14%) | 27 (19%) | 28 (19%) | |
| PTGS2 (cyclooxygenase-2) expression | 0.82 | ||||||
| Negative | 297 (38%) | 62 (39%) | 66 (42%) | 57 (37%) | 53 (36%) | 59 (38%) | |
| Positive | 476 (62%) | 96 (61%) | 91 (58%) | 96 (63%) | 96 (64%) | 97 (62%) | |
| Crohn’s-like lymphoid reaction | 0.49 | ||||||
| Negative/low | 508 (72%) | 99 (71%) | 92 (69%) | 115 (77%) | 90 (69%) | 112 (77%) | |
| Intermediate | 130 (19%) | 26 (19%) | 27 (20%) | 22 (15%) | 28 (21%) | 27 (18%) | |
| High | 63 (9.0%) | 15 (11%) | 15 (11%) | 13 (8.7%) | 13 (9.9%) | 7 (4.8%) | |
| Peritumoural lymphocytic reaction | 0.15 | ||||||
| Negative/low | 92 (11%) | 28 (16%) | 19 (11%) | 18 (10%) | 15 (8.8%) | 12 (6.9%) | |
| Intermediate | 639 (74%) | 121 (70%) | 119 (70%) | 131 (74%) | 130 (76%) | 138 (80%) | |
| High | 133 (15%) | 24 (14%) | 33 (19%) | 28 (16%) | 25 (15%) | 23 (13%) | |
| Intratumoural periglandular reaction | 0.40 | ||||||
| Negative/low | 88 (10%) | 24 (14%) | 18 (11%) | 18 (10%) | 15 (8.7%) | 13 (7.5%) | |
| Intermediate | 662 (76%) | 125 (72%) | 123 (72%) | 137 (77%) | 136 (79%) | 141 (82%) | |
| High | 118 (14%) | 24 (14%) | 30 (18%) | 23 (13%) | 21 (12%) | 20 (11%) | |
| Tumour-infiltrating lymphocytes | 0.25 | ||||||
| Negative/low | 638 (73%) | 122 (71%) | 126 (74%) | 120 (67%) | 134 (78%) | 136 (78%) | |
| Intermediate | 128 (15%) | 30 (17%) | 22 (13%) | 34 (19%) | 19 (11%) | 23 (13%) | |
| High | 103 (12%) | 21 (12%) | 23 (13%) | 25 (14%) | 19 (11%) | 15 (8.6%) | |
25(OH)D, 25-hydroxyvitamin D; AJCC, American Joint Committee on Cancer; CIMP, CpG island methylator phenotype-specific promoters; HPFS, Health Professionals Follow-up Study; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; NHS, Nurses’ Health Study; SD, standard deviation.
Percentage indicates the proportion of cases with a specific clinical, pathological, or molecular characteristic in all cases or in strata of quintiles of postdiagnosis predicted 25(OH)D score.
To compare characteristics between subgroups, we used the chi-square test for categorical variables, and the analysis of variance for continuous variables.
Informed consent was obtained from all participants. This study was approved by the institutional review boards at Harvard T.H. Chan School of Public Health, and Partner’s Healthcare (Boston, MA, USA).
2.2. Predicted 25(OH)D score
The prediction model for plasma 25(OH)D level was described elsewhere [25]. Briefly, linear regression analysis was performed on 1,095 cancer-free male participants with available plasma 25(OH)D levels from the HPFS. The model identified race, region of residence, physical activity, body mass index (BMI), and dietary and supplementary vitamin D intake as independent predictors of plasma 25(OH)D level. The derived regression coefficients were used to estimate plasma 25(OH)D level. In an independent sample of 542 men with available plasma 25(OH)D levels from the HPFS [25], plasma 25(OH)D level increased according to the increase in deciles of predicted 25(OH)D score (ptrend < 0.001). The difference in the mean plasma 25(OH)D level between extreme deciles was 10.0 ng/mL, similar to the difference of 11.1 ng/mL in the derivation cohort. A similar approach was used to derive predicted 25(OH)D scores in the NHS [26]. We calculated postdiagnosis predicted 25(OH)D score using the earliest questionnaire returned between 6 and 48 months after colorectal cancer diagnosis.
2.3. Immunohistochemistry
We constructed tissue microarrays to include up to four cores from colorectal cancer and up to two cores from normal tissue blocks. We performed immunohistochemistry for CD3, CD8, CD45RO (one of PTPRC protein isoforms), and FOXP3 as previously described [30]. We used an automated scanning microscope and the Ariol image analysis system (Genetix, San Jose, CA, USA) to measure densities (cells/mm2) of CD3+ cells, CD8+ cells, CD45RO+ cells, and FOXP3+ cells in colorectal cancer tissue [30]. We conducted immunohistochemical analysis for PTGS2 (cyclooxygenase-2) using an anti-PTGS2 antibody (Cayman Chemical, Ann Arbor, MI, USA) [31].
2.4. Analyses of tumour molecular markers
DNA was extracted from FFPE tissue blocks. MSI status was determined using 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487), and MSI-high was defined as presence of instability in ≥ 30% of the markers [28]. Using bisulphite-treated DNA, methylation status of eight CpG island methylator phenotype (CIMP)-specific promoters (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) and long interspersed nucleotide element-1 (LINE-1) was analysed [28]. CIMP-high was defined as methylation in ≥ 6 of eight promoters [28]. Polymerase chain reaction and pyrosequencing were performed for KRAS (codons 12, 13, 61, and 146), BRAF (codon 600), and PIK3CA (exons 9 and 20) [28].
2.5. Statistical analysis
All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA), and all p values were two-sided. In our primary hypothesis testing, we examined the statistical interaction between postdiagnosis predicted 25(OH)D score (cohort-specific quintiles, ordinal) and each lymphocytic reaction component (three-tiered, ordinal) using the Wald test in the multivariable-adjusted Cox proportional hazards regression model for colorectal cancer mortality. In addition, we assessed the interaction between postdiagnosis predicted 25(OH)D score and the density (ordinal quartile variable) of CD3+ cells, CD8+ cells, CD45RO+ cells, or FOXP3+ cells. In our primary hypothesis testing on new discoveries, we used the α level of 0.005 [32]. All other analyses represented secondary analyses, and we used the α level of 0.005. We estimated hazard ratio for a quintile-unit increase of postdiagnosis predicted 25(OH)D score in strata of levels of lymphocytic reaction components using a re-parameterisation of the interaction term in a single regression model [33]. In the Cox regression model, survival time was left-truncated at the date of return of the first postdiagnosis questionnaire. In colorectal cancer-specific mortality analyses, participants were censored at the time of deaths from other causes.
In all survival analyses, the inverse probability weighting (IPW) method was applied to reduce the potential bias due to the availability of postdiagnosis questionnaire data [34,35]. Cumulative survival probabilities were estimated using the IPW-adjusted Kaplan-Meier method, and a linear trend in survival probabilities across ordinal categories of postdiagnosis predicted 25(OH)D score was assessed using the weighted log-rank test for trend. The multivariable IPW-adjusted Cox regression model initially included the variables described in Table 2, and a backward elimination with a threshold p of 0.05 was used to select variables for the final models. The Cox regression model was stratified by the time between colorectal cancer diagnosis and the first questionnaire return (≤ 1 year vs. 1.1–2.0 years vs. 2.1–3.0 years vs. 3.1–4.0 years). Cases with missing data were assigned to the majority category of a given categorical covariate: tumour differentiation (0.7%), MSI status (9.9%), CIMP status (14%), PTGS2 expression (11%), KRAS mutation (11%), BRAF mutation (9.1%), and PIK3CA mutation (16%). Cases with missing data on prediagnosis predicted 25(OH)D score (6.1%) were included in the middle quintile. For cases with missing data on LINE-1 methylation level (12%), we assigned a separate indicator variable. We confirmed that excluding cases with missing data on any of the covariates did not substantially alter our results (data not shown). The Cox regression model without IPW yielded similar results to the IPW-adjusted model (Supplementary Table 1). The assumption of proportional hazards was generally satisfied using the assessment of a time-varying covariate; i.e., the cross-product of postdiagnosis predicted 25(OH)D score and log-transformed survival time in strata of each lymphocytic reaction component (p > 0.05).
Table 2.
Colorectal cancer mortality according to postdiagnosis predicted 25(OH)D score in all cases or in strata of levels of lymphocytic reaction components.
| Colorectal cancer-specific mortality HR for a quintile-unit increase of postdiagnosis predicted 25(OH)D score |
Overall mortality HR for a quintile-unit increase of postdiagnosis predicted 25(OH)D score |
||||||
|---|---|---|---|---|---|---|---|
| No. of cases |
No. of events |
Univariable HRa (95% CI) |
Multivariable HRa,b (95% CI) |
No. of events |
Univariable HRa (95% CI) |
Multivariable HRa,b (95% CI) |
|
| All colorectal cancer cases | 869 | 122 | 0.95 (0.81–1.10) | 1.06 (0.88–1.26) | 480 | 0.92 (0.86–0.99) | 0.94 (0.88–0.99) |
| Crohn’s-like lymphoid reaction | |||||||
| Negative/low | 508 | 84 | 0.88 (0.74–1.04) | 1.01 (0.83–1.25) | 276 | 0.93 (0.85–1.02) | 0.95 (0.87–1.02) |
| Intermediate | 130 | 13 | 1.01 (0.67–1.53) | 1.21 (0.83–1.76) | 75 | 0.93 (0.78–1.10) | 0.98 (0.86–1.12) |
| High | 63 | 5 | 1.46 (0.90–2.34) | 1.95 (1.01–3.77) | 34 | 0.86 (0.69–1.07) | 0.80 (0.64–1.01) |
| pinteractionc | 0.13 | 0.092 | 0.59 | 0.39 | |||
| Peritumoural lymphocytic reaction | |||||||
| Negative/low | 92 | 29 | 0.73 (0.53–1.02) | 0.69 (0.54–0.89) | 51 | 0.79 (0.61–1.03) | 0.84 (0.68–1.03) |
| Intermediate | 639 | 87 | 1.06 (0.90–1.25) | 1.08 (0.93–1.26) | 358 | 0.96 (0.89–1.04) | 0.98 (0.91–1.05) |
| High | 133 | 5 | 1.18 (0.73–1.91) | 1.25 (0.75–2.09) | 70 | 0.85 (0.72–1.01) | 0.85 (0.74–0.99) |
| pinteractionc | 0.022 | 0.001 | 0.54 | 0.98 | |||
| Intratumoural periglandular reaction | |||||||
| Negative/low | 88 | 24 | 0.77 (0.53–1.12) | 0.74 (0.57–0.96) | 43 | 0.83 (0.62–1.11) | 0.80 (0.64–0.99) |
| Intermediate | 662 | 93 | 1.02 (0.88–1.19) | 1.05 (0.91–1.21) | 375 | 0.97 (0.90–1.04) | 0.98 (0.92–1.06) |
| High | 118 | 5 | 1.16 (0.73–1.83) | 1.27 (0.77–2.08) | 62 | 0.77 (0.65–0.91) | 0.83 (0.72–0.94) |
| pinteractionc | 0.10 | 0.007 | 0.64 | 0.98 | |||
| Tumour-infiltrating lymphocytes | |||||||
| Negative/low | 638 | 102 | 0.88 (0.75–1.04) | 0.98 (0.82–1.18) | 347 | 0.91 (0.84–0.99) | 0.93 (0.86–0.99) |
| Intermediate | 128 | 15 | 1.34 (0.98–1.82) | 1.64 (1.17–2.30) | 74 | 1.02 (0.87–1.18) | 1.00 (0.87–1.15) |
| High | 103 | 5 | 1.23 (0.62–2.43) | 1.66 (0.81–3.44) | 59 | 0.84 (0.70–0.99) | 0.91 (0.78–1.07) |
| pinteractionc | 0.036 | 0.008 | 0.83 | 0.87 | |||
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; HR, hazard ratio; IPW, inverse probability weighting.
IPW was applied to reduce a bias due to the availability of questionnaire data after cancer diagnosis (see “Statistical analysis” subsection for details).
The multivariable Cox regression model initially included sex (female vs. male), age at diagnosis (continuous), year of diagnosis (continuous), family history of colorectal cancer (absent vs. present), prediagnosis predicted 25(OH)D score (cohort-specific quintiles of cumulative average, ordinal), tumour location (proximal colon vs. distal colon vs. rectum), tumour differentiation (well to moderate vs. poor), disease stage (I-II vs. III-IV vs. missing), microsatellite instability status (high vs. non-high), CpG island methylator phenotype-specific promoter status (high vs. low/negative), long interspersed nucleotide element-1 methylation level (continuous), KRAS mutation (wild-type vs. mutant), BRAF mutation (wild-type vs. mutant), PIK3CA mutation (wild-type vs. mutant), and PTGS2 (cyclooxygenase-2) expression (negative vs. positive). A backward elimination with a threshold p of 0.05 was used to select variables for the final models. The variables which remained in the final models for peritumoural lymphocytic reaction are described in Supplementary Table 2.
pinteraction (two-sided) was calculated using the Wald test for the cross-product of postdiagnosis predicted 25(OH)D score (ordinal quintile variable) and each of the lymphocytic reaction variables (ordinal) in the IPW-adjusted Cox regression model.
3. Results
We included 869 colorectal cancer cases (Fig. 1 and Table 1). Postdiagnosis predicted 25(OH)D score modestly correlated with prediagnosis predicted 25(OH)D score (Spearman r = 0.68). During the median follow-up time of 13.3 years (interquartile range, 9.8–17.8 years) for censored cases, there were 480 all-cause deaths, including 122 colorectal cancer-specific deaths.
The association of postdiagnosis predicted 25(OH)D score with colorectal cancer-specific mortality statistically significantly differed by levels of peritumoural lymphocytic reaction (pinteraction = 0.001; with the α level of 0.005; Table 2 and Supplementary Table 2). The multivariable-adjusted HRs for colorectal cancer-specific mortality for a quintile-unit increase in postdiagnosis predicted 25(OH)D score were 0.69 [95% CI (confidence interval), 0.54–0.89] in patients with negative to low peritumoural lymphocytic reaction, 1.08 (95% CI, 0.93–1.26) in patients with intermediate peritumoural reaction, and 1.25 (95% CI, 0.75–2.09) in patients with high peritumoural reaction. In Kaplan-Meier survival analyses, a trend towards lower colorectal cancer-specific mortality associated with higher postdiagnosis predicted 25(OH)D score was observed in tumours with negative to low peritumoural lymphocytic reaction, but did not reach statistical significance (p = 0.032; with the α level of 0.005; Fig. 2). In contrast, no such trend was observed in tumours with intermediate to high peritumoural lymphocytic reaction (p = 0.33, Fig. 2). We did not observe a statistically significant interaction of postdiagnosis predicted 25(OH)D score with other lymphocytic reaction components (pinteraction > 0.006). We did not observe a statistically significant interaction between postdiagnosis predicted 25(OH)D score and lymphocytic reaction in relation to overall mortality (pinteraction > 0.3).
Fig. 2.
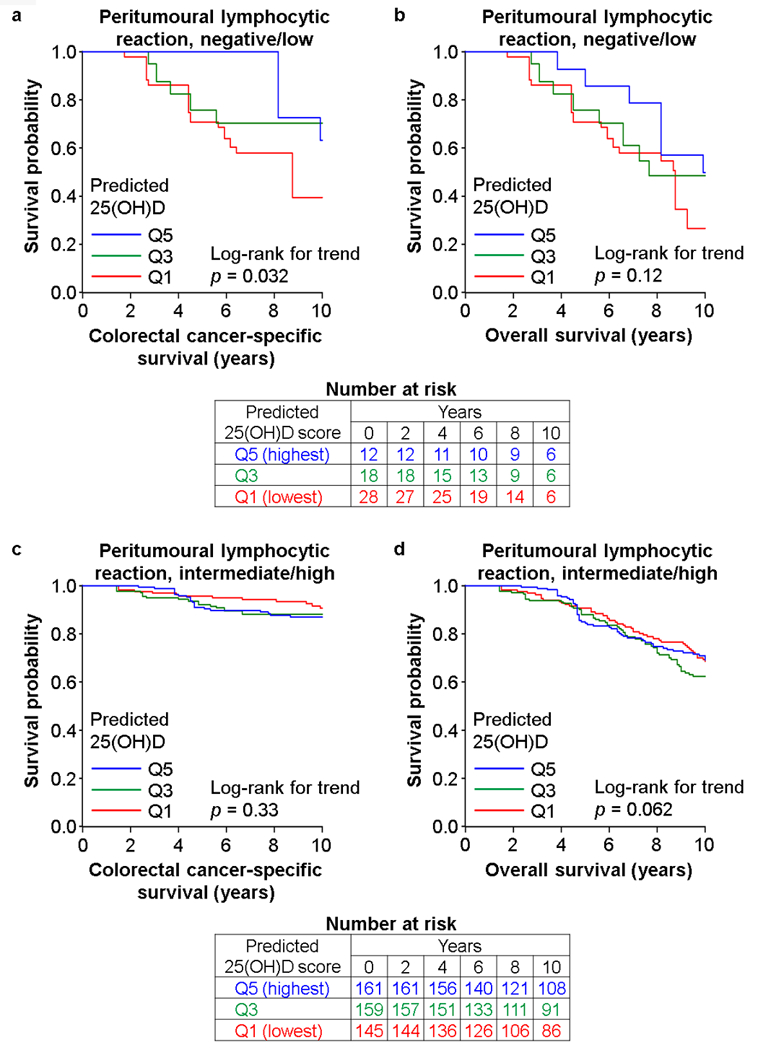
Inverse probability weighting (IPW)-adjusted Kaplan-Meier survival curves of colorectal cancer patients according to postdiagnosis predicted 25(OH)D score in strata of peritumoural lymphocytic reaction. The p values were calculated using the weighted log-rank test for trend (two-sided). a and b, colorectal cancer-specific survival and overall survival, respectively, among patients with tumours accompanying negative to low peritumoural lymphocytic reaction. c and d, colorectal cancer-specific survival and overall survival, respectively, among patients with tumours accompanying intermediate to high peritumoural lymphocytic reaction. 25(OH)D, 25-hydroxyvitamin D; Q1, quintile 1; Q3, quintile 3; Q5, quintile 5.
Considering that predicted 25(OH)D level might reflect any of other factors used in the prediction model, we included postdiagnosis BMI or postdiagnosis physical activity level as an additional covariate in the multivariable models. We observed a similar differential prognostic association of postdiagnosis predicted 25(OH)D score according to peritumoural lymphocytic reaction (pinteraction = 0.001).
In secondary analyses, we did not observe a significant differential association of postdiagnosis predicted 25(OH)D score with colorectal cancer mortality according to the density of any of T cell populations (pinteraction > 0.05, Supplementary Table 3).
4. Discussion
We found that the beneficial survival association of postdiagnosis predicted 25(OH)D score appeared stronger for colorectal cancer with lower peritumoural lymphocytic reaction. In contrast, we did not observe such a differential association for overall mortality, and therefore, a further investigation is warranted considering causes of deaths other than colorectal cancer. Our findings provide evidence for inter-personal heterogeneity of anti-tumour effects of vitamin D according to anti-tumour immune response, potentially contributing to development of tailored dietary and lifestyle intervention strategies for cancer patients.
Calcitriol exerts anti-neoplastic effects by binding to VDR (vitamin D receptor) [20], which is prevalently expressed in intestinal epithelial cells and immune cells [18,21,36]. Experimental evidence suggests that the anti-inflammatory effects of vitamin D may occur via suppression of the PTGS2 (cyclooxygenase-2), MAPK, and NFKB pathways as well as suppression of several cytokines in cancers [18,37,38]. In addition, the immunomodulatory effects of vitamin D have been proposed as an alternative mechanism through which tumour progression are suppressed [18,36,37]. Vitamin D modulates adaptive immunity by altering responses of B cells, helper T cells, and regulatory T cells [21,22,36], as well as cytotoxic T cells for immune surveillance of cancers [24]. Our study supports the role of the vitamin D-mediated pathway in suppression of human colorectal cancer progression through activation of anti-tumour immune response.
This study supports the potential of lymphocytic reaction status in colorectal cancer as a biomarker for the survival benefits associated with high-level vitamin D. Interestingly, our previous study has shown that the association of plasma 25(OH)D level with low colorectal cancer incidence is stronger for tumours with high intratumoural periglandular reaction [17]. We speculate that carcinomas which have evolved in the presence of a high abundance of lymphocytes may have acquired resistance to calcitriol activated by the lymphocyte-rich microenvironment. In contrast, carcinomas with little lymphocytic response may be more susceptible to immunomodulatory effects of calcitriol. In addition, the multifaceted effects of vitamin D on different tumour subtypes may change during tumour evolution in a continuously changing microenvironment consisting of extra-cellular matrix and non-neoplastic host cells [39].
We observed a trend towards higher colorectal cancer-specific mortality associated with higher postdiagnosis predicted 25(OH)D score in patients with tumours accompanying intermediate/high lymphocytic reaction. However, considering not only little or no evidence for adverse effect of vitamin D on colorectal cancer survival but also multiple comparisons behind the individual hazard ratio estimates, the observed trend might have occurred by chance.
The present study has limitations. First, the retrospective and hypothesis-generating nature of our analyses was a limitation of the current study, and our findings need to be validated in prospective trial studies. Second, data on cancer treatment were limited. However, the selection of cancer treatment was unlikely to be made based on anti-tumour immune response, because such data were not available for treating physicians. Third, the predicted 25(OH)D score inevitably has a measurement error. In addition, we cannot completely exclude the possibility that lower levels of postdiagnosis predicted 25(OH)D score might reflect patient characteristics associated with poor prognosis. Forth, data from postdiagnosis questionnaires used to calculate 25(OH)D score were not available for every colorectal cancer patient in the cohorts. Hence, we applied the IPW method to reduce this potential selection bias.
There are strengths of our current study. A major strength is the use of the molecular pathological epidemiology approach [39,40]. An integrated analysis incorporating prospectively-collected data on epidemiological exposures, clinicopathological features, and tumour molecular markers allowed us to comprehensively examine the interaction between the predicted 25(OH)D score and immune response to tumour. There might be a variety of confounding factors for the association between vitamin D status and colorectal cancer survival. Our results generally became stronger after adjustment for potential confounders. Notably, our study population was drawn from a large number of cases from hospitals throughout the U.S., which increases the generalisability of our findings.
In conclusion, the beneficial survival association of high postdiagnosis vitamin D level is stronger for colorectal carcinoma with lower-level peritumoural lymphocytic reaction than for carcinoma with higher-level reaction. Our study supports differential anti-tumour immunomodulatory effects of vitamin D according to host immune response to tumour. Immune checkpoint inhibition can be effective for treating MSI-high carcinomas but not non-MSI-high colorectal carcinomas. Based on our data supporting the anti-tumour immune-enhancing effects of vitamin D, it is worth examining whether vitamin D can enhance effects of immune checkpoint inhibitors.
Supplementary Material
Acknowledgements:
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; U01 CA167552 to W.C. Willett and L.A. Mucci; P50 CA127003 to C.S.F.; R01 CA118553 to C.S.F.; R01 CA169141 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; R35 CA197735 to S.O.; R01 CA151993 to S.O.; R01 CA205406 to K.Ng; K07 CA190673 to R.N.; and K07 CA188126 to X.Z.); by Nodal Award (2016–02) from the Dana-Farber Harvard Cancer Center (to S.O.); by the Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (to M.Gi. and C.S.F.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. T.H. was supported by a fellowship grant from the Uehara Memorial Foundation and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. L.L. was supported by a scholarship grant from Chinese Scholarship Council and a fellowship grant from Huazhong University of Science and Technology. K.M. was supported by a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japan Society for the Promotion of Science. K.K. was supported by grants from Overseas Research Fellowship (JP2017–775) and Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers, from Japan Society for the Promotion of Science. R.D. was supported by a grant from National Natural Science Foundation of China (31601077). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- FFPE
formalin-fixed paraffin-embedded
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- IPW
inverse probability weighting
- LINE-1
long interspersed nucleotide element-1
- MSI
microsatellite instability
- NHS
Nurses’ Health Study
- SD
standard deviation
- TIL
tumour-infiltrating lymphocytes
Footnotes
Conflict of interest statement: A.T.C. previously served as a consultant for Bayer Healthcare, Pfizer Inc., and Aralez Pharmaceuticals. This study was not funded by Bayer Healthcare, Pfizer Inc., or Aralez Pharmaceuticals. No other conflicts of interest exist. The other authors declare that they have no conflicts of interest.
Use of standardised official symbols: We use HUGO (Human Genome Organisation)-approved official symbols (or root symbols) for genes and gene products, including BRAF, CACNA1G, CD3, CD8, CD274, CDKN2A, CRABP1, FOXP3, IGF2, KRAS, MAPK, MLH1, NEUROG1, NFKB, PDCD1, PIK3CA, PTGS2, PTPRC, RUNX3, SOCS1, and VDR; all of which are described at www.genenames.org. The official symbols are italicised to differentiate from non-italicised colloquial names that are used along with the official symbols. This format enables readers to familiarise themselves with the official symbols for genes and gene products together with common colloquial names.
References
- [1].Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128–39. [DOI] [PubMed] [Google Scholar]
- [2].Grizzi F, Basso G, Borroni EM, et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflamm Res 2018;67:375–89. [DOI] [PubMed] [Google Scholar]
- [3].Berntsson J, Svensson MC, Leandersson K, et al. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A cohort study. Int J Cancer 2017;141:1654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 2017;14:717–34. [DOI] [PubMed] [Google Scholar]
- [5].Rozek LS, Schmit SL, Greenson JK, et al. Tumor-Infiltrating Lymphocytes, Crohn’s-Like Lymphoid Reaction, and Survival From Colorectal Cancer. J Natl Cancer Inst 2016;108:djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bever KM, Le DT. An Expanding Role for Immunotherapy in Colorectal Cancer. J Natl Compr Canc Netw 2017;15:401–10. [DOI] [PubMed] [Google Scholar]
- [7].Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Basile D, Garattini SK, Bonotto M, et al. Immunotherapy for colorectal cancer: where are we heading? Expert Opin Biol Ther 2017;17:709–21. [DOI] [PubMed] [Google Scholar]
- [9].Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011;60:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol 2017;18:843–50. [DOI] [PubMed] [Google Scholar]
- [11].Rajpoot M, Sharma AK, Sharma A, Gupta GK. Understanding the Microbiome: Emerging Biomarkers for Exploiting the Microbiota for Personalized Medicine against Cancer. Semin Cancer Biol 2018. in press. [DOI] [PubMed]
- [12].Morgillo F, Dallio M, Della Corte CM, et al. Carcinogenesis as a Result of Multiple Inflammatory and Oxidative Hits: a Comprehensive Review from Tumor Microenvironment to Gut Microbiota. Neoplasia 2018;20:721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ogino S, Giannakis M. Immunoscore for (colorectal) cancer precision medicine. Lancet 2018;391:2084–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol 2008;26:2984–91. [DOI] [PubMed] [Google Scholar]
- [15].Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol 2014;32:2430–9. [DOI] [PubMed] [Google Scholar]
- [16].Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer 2014;50:1510–21. [DOI] [PubMed] [Google Scholar]
- [17].Song M, Nishihara R, Wang M, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 2016;65:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dou R, Ng K, Giovannucci EL, Manson JE, Qian ZR, Ogino S. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br J Nutr 2016;115:1643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sehdev A, O’Neil BH. The Role of Aspirin, Vitamin D, Exercise, Diet, Statins, and Metformin in the Prevention and Treatment of Colorectal Cancer. Curr Treat Options Oncol 2015;16:43. [DOI] [PubMed] [Google Scholar]
- [20].Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- [21].Veldhoen M, Brucklacher-Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol 2012;12:696–708. [DOI] [PubMed] [Google Scholar]
- [22].von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010;11:344–9. [DOI] [PubMed] [Google Scholar]
- [23].Konijeti GG, Arora P, Boylan MR, et al. Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: Results from a Randomized Control Trial. J Clin Endocrinol Metab 2016;101:533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sarkar S, Hewison M, Studzinski GP, Li YC, Kalia V. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit Rev Clin Lab Sci 2016;53:132–45. [DOI] [PubMed] [Google Scholar]
- [25].Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9. [DOI] [PubMed] [Google Scholar]
- [26].Ng K, Wolpin BM, Meyerhardt JA, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer 2009;101:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009;15:6412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222:350–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007;356:2131–42. [DOI] [PubMed] [Google Scholar]
- [32].Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nature Human Behaviour 2018;2:6–10. [DOI] [PubMed] [Google Scholar]
- [33].Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One 2008;3:e3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu L, Nevo D, Nishihara R, et al. Utility of inverse probability weighting in molecular pathological epidemiology. Eur J Epidemiol 2018;33:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamada T, Cao Y, Qian ZR, et al. Aspirin Use and Colorectal Cancer Survival According to Tumor CD274 (Programmed Cell Death 1 Ligand 1) Expression Status. J Clin Oncol 2017;35:1836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Janakiram NB, Mohammed A, Madka V, Kumar G, Rao CV. Prevention and treatment of cancers by immune modulating nutrients. Mol Nutr Food Res 2016;60:1275–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, Inflammation, and Colorectal Cancer Progression: A Review of Mechanistic Studies and Future Directions for Epidemiological Studies. Cancer Epidemiol Biomarkers Prev 2015;24:1820–8. [DOI] [PubMed] [Google Scholar]
- [38].Meeker S, Seamons A, Paik J, et al. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res 2014;74:4398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut 2018;67:1168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ogino S, Jhun I, Mata DA, et al. Integration of pharmacology, molecular pathology, and population data science to support precision gastrointestinal oncology. NPJ Precis Oncol 2017;1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


