Abstract
Objective
Mothers of advanced maternal age at childbirth (AMA, age ≥35) may have different perceptions of autism spectrum disorder (ASD) risk, independent of sociodemographic factors, which may affect ASD identification. We aimed to estimate associations between AMA and both age of a child’s first evaluation noting developmental concerns and time from first evaluation to first ASD diagnosis.
Methods
We used data for eight-year-olds identified with ASD in the 2008–2012 Autism and Developmental Disabilities Monitoring Network. We estimated differences in age at first evaluation noting developmental concerns and time to first ASD diagnosis by AMA using quantile and Cox regression.
Results
Of 10,358 children with ASD, 19.7% had mothers of AMA. AMA was associated with higher educational attainment and prior live births compared to younger mothers. In unadjusted analyses, AMA was associated with earlier first evaluation noting developmental concerns (median 37 vs. 40 months) and patterns in time to first evaluation (Hazard ratio: 1.12, 95% Confidence Interval: 1.06, 1.18). Associations between AMA and evaluation timing diminished and were no longer significant after adjustment for socioeconomic and demographic characteristics. Child intellectual disability did not modify associations between AMA and timing of evaluations.
Conclusion
AMA is one sociodemographic factor associated with younger age of first evaluation noting developmental concerns in children with ASD, but AMA was not independently associated likely since it is a consequence or co-factor of maternal education and other sociodemographic characteristics. AMA may be a demographic factor to consider when aiming to screen and evaluate children at risk for ASD.
Keywords: Autism spectrum disorder, advanced maternal age, developmental evaluation
Advanced maternal age (AMA), or childbirth at or after the age of 35, is a risk factor for an array of poor pregnancy and child health outcomes. AMA increases the risk of stillbirth, fetal loss, preterm birth, preeclampsia, and gestational diabetes.1 Children of AMA mothers are at greater risk of being small for gestational age, having low birth weight,1 Down syndrome,2 and congenital malformations.3 AMA is also associated with child intellectual disability4 and autism spectrum disorder (ASD).5 A meta-analysis by Sandin et al.5 of 25 687 ASD cases and 8 655 576 controls pooled from 16 epidemiological studies found an adjusted and statistically significant relative risk for ASD of 1.31 comparing mothers ≥35 to mothers 25–29. Etiologic hypotheses for this increased risk include genomic alterations, environmental exposures, and epigenetic mechanisms.5
Many mothers of AMA are aware of their higher risk for non-optimal pregnancy outcomes compared to younger mothers.6 This awareness may be related to the increased levels of education and higher socio-economic status (SES) associated with pregnancy at older ages.7 Independent of these SES and demographic factors, the increased risk for poorer pregnancy outcomes at older ages are often explicitly communicated to all mothers of AMA. Best obstetrician practice recommends both additional screenings for fetal aneuploidy and clear communication with mothers of AMA about their increased risk.8 Understanding patterns in the awareness of these enhanced risks is important because increased awareness affects how a woman seeks care and adopts certain health behaviors.8
Studies have found that a portion of parents of children with ASD attribute their child’s ASD to maternal age;9,10 however, research has not assessed whether the increased risk of ASD among children with AMA mothers affects screening and evaluation of ASD. Reducing age at ASD evaluation and identification is important as early identification and intervention provide short and long term benefits to the child and family.11 Past studies have evaluated maternal or parental age as a predictor of early age at ASD diagnosis. Some studies have found an association between older maternal age and younger age at child diagnosis12,13 while others have found no such association.14,15 These studies may not capture the lag from first evaluation to diagnosis, which can be an extended period of time.16 Additionally, these studies address maternal age as an additional covariate in models that aim to evaluate another exposure or group of exposures; this approach does not appropriately account for confounding or capture the independent effects of maternal age.
Identifying children with ASD at an early age is associated with improved developmental outcomes and reduced health expenditures.17 Although early screening for ASD is not mandatory in the U.S.,18 universal early screening and evaluation may help identify those at greatest risk and reduce diagnostic disparities.15,19 Understanding how children with ASD get evaluated and diagnosed may help us understand which factors to target when aiming to lower age at identification. AMA is one such factor to assess, as it is both a risk factor for ASD and a demographic characteristic that may affect service usage.
Our objective was to estimate effects of AMA on age when a child with ASD had their first evaluation that noted developmental concerns and time from that evaluation to first ASD diagnosis among a group of children with ASD identified from the Autism and Developmental Disabilities Monitoring Network (ADDM). We hypothesized that after controlling for confounding effects like maternal education and live birth order, we would still see AMA to be associated with younger child age at first evaluation noting developmental concerns, likely through increased knowledge about risk due to AMA. In addition, we explored whether intellectual disability (ID) in the child modified associations, as co-occurring ID may be associated with maternal age.
METHODS
Autism and Developmental Disability Monitoring Network
ADDM is a multi-site, population-based, surveillance program funded by the [REDACTED FOR BLINDING] with the goal of estimating ASD prevalence in eight- and four-year-old children in the U.S. using a standardized methodology.20 Data have been collected biennially since 2000. All participating study sites received institutional review board approval.
ADDM case ascertainment
A child was eligible to have their record reviewed in ADDM if he or she was eight-years-old and had at least one parent who resided in the site’s defined geographic area during the surveillance year. Trained abstractors screened a child’s special education records and/or health records from community providers that serve children with developmental disabilities for ADDM specified behavioral and diagnostic triggers. Behavioral triggers include impairment in social gestures and expressions, joint attention problems, lack of a social use of language, and social delay before age 3. Diagnostic triggers include a past diagnosis of ASD, a special education eligibility of autism, or an autism test. If at least one trigger was present, then a study clinician reviewed all of a child’s developmental evaluations in special education and health records and used a highly structured standardized scoring protocol based on the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition-Text Revision (DSM-IV-TR) to deduce ASD case status. This protocol examined whether there is sufficient information on the record to conclude that the child meets DSM-IV-TR criteria for autistic disorder, Asperger’s syndrome, or pervasive developmental disability not otherwise specified; a prior diagnosis for one of these conditions was not necessary to receive case confirmation in the ADDM network. DSM-IV-TR criteria were used because data collection preceded implementation of DSM5. Initial inter-rater reliability was set at a minimum of 90% agreement on final case status and 80–90% agreement for individual variables scored.20 Further information about ADDM Network methods and scoring protocols can be found in Rice et al.20 and Christensen et al.21
Study population
For this secondary analysis of ADDM data, we included all eight-year-old children with ASD identified in surveillance years 2008, 2010, and 2012 from 13 ADDM sites that participated in any of the three surveillance years (Alabama, Arkansas, Arizona, Colorado, Georgia, Maryland, Missouri, North Carolina, New Jersey, Pennsylvania, South Carolina, Utah, and Wisconsin) (n=14 416). We restricted to these surveillance years to minimize differences in ASD prevalence and geographic surveillance area over time. These surveillance years correspond to birth years of 2000, 2002, and 2004. Additionally, we excluded children without linked birth certificates, who we did not have maternal age information on (N=4 058)
Outcome variable
We defined the child’s earliest evaluation noting developmental concern as the first evaluation conducted by a trained professional that noted an ADDM behavioral or developmental trigger or a DSM-IV-TR defined associated feature of ASD.22 Associated features included aggression, self-injurious behaviors, hyperactivity, and odd response to sensory stimuli and were abstracted by the ADDM network and indicate developmental issues. Evaluations were conducted most frequently by occupational therapists, speech language pathologists, psychologists, and developmental pediatricians. In 165 cases, children had a developmental evaluation before the age of 8 months. We excluded these observations since measuring ASD and other developmental traits at this age is often unreliable17, as many of these traits may not emerge until the latter parts of the first and second year.23 Additionally, no evaluation prior to 8 months in our data indicated specific measurement tool used. To evaluate this assumption, we conducted sensitivity analyses that included evaluations before eight months.
ADDM clinicians determined first ASD diagnosis as the earliest date on the child’s record of an ASD diagnosis given by a qualified professional. In certain instances, the date at first evaluation noting developmental concerns was also the date of ASD diagnosis. To determine time from first evaluation that noted ASD traits to first diagnosis, we subtracted age at first diagnosis in months from age at first evaluation in months.
Advanced maternal age
Maternal age at childbirth was derived from the child’s birth certificate. AMA was defined as age at childbirth ≥ 35 based on standard convention. There were no implausible values and minimal missing data for this variable (N=7).
Intellectual disability
As a secondary objective, we examined whether effects of AMA differed by whether the child had ID. Child ID status was abstracted during the record review process based on whether there was indication of an IQ test in the record. Not all children in our sample had results for ID tests, largely due to differences in whether health records, education records, or both were collected at an individual site. We elected to restrict this sub-analysis to children from sites that had collected data on ID in >60% of children (Alabama, Arkansas, Arizona, Colorado, Georgia, Maryland, North Carolina, New Jersey, South Carolina, and Utah). The ADDM network defined ID as an IQ test with a score ≤70 or a statement from an examiner indicating ID.24 We present results from this analysis in three strata: ID, no ID, and missing ID status.
Covariates
We utilized demographic variables from the child’s birth certificate to describe sample characteristics and control for confounding. To account for missing paternal age data, we used the multiple imputation procedure in SAS 9.4 using a fully conditional specification method. We assumed that these data were missing at random conditional on known covariates; therefore we included all other covariates and child age at first evaluation that noted ASD traits in our imputation model. Twenty-five data sets were imputed and pooled for our adjusted analyses. Imputation was redone for the sub-analysis that restricted analyses to sites that collected adequate ID data. As a sensitivity analysis, we restricted to just those with parental age data and found that point estimates changed by less than 5% (data not shown); therefore, we elected to present results using the imputed data to maximize precision.
Data analysis
Chi square tests were conducted comparing AMA and categorical demographic covariates to assess correlation between AMA and confounders. T-tests were used for normally distributed continuous variables and Wilcoxon signed-rank test for non-normally distributed continuous variables. We then ran unadjusted and adjusted models to assess differences in age at first evaluation in two ways. Since outcome measures were not normally distributed, we used quantile regression to estimate median age differences at the deciles for first evaluation and time to diagnosis (due to 39% of those with a formal diagnosis having first diagnosis at the time of first evaluation, we only present the 4th through 9th deciles). Secondly, we examined differences in a time-to-event framework using Cox proportional hazard models. This allowed us to assess whether the patterns in timing to evaluation differed by AMA. Children without ASD diagnoses on their record but with ASD as determined by ADDM methodology were considered administratively censored. These children had not had a formal diagnosis by the end of data collection (age 8) so they were right censored. An advantage to this approach is that we are able to incorporate these observations into our semi-parametric methods even though they do not have a time of diagnosis; however, if there was missing data on time of diagnosis, it may be incorrectly censored. We used the imputed data sets and included covariates identified as confounders a priori: maternal education, maternal race/ethnicity, paternal age, parity, birth order, gestational age, child year of birth, and study site. Additionally, we reran these analyses with our outcome being difference from the first evaluation to first ASD diagnosis. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary NC).
RESULTS
Of 10 358 children that met our entry criteria, 2432 were identified in surveillance year 2008, 4066 in 2010, and 3860 in 2012 (Table 1). Overall, 2036 (19.7%) of mothers were of AMA at time of childbirth. A larger percentage of AMA mothers were white and had a college degree compared to non-AMA mothers. AMA mothers were also more likely to have had more previous live births, have their child be a multiple birth, and have an older father of the child than non-AMA mothers. Children of AMA mothers had a mean age of first evaluation noting developmental concern of 42.2 months (median=37 months) and a mean time from first evaluation to first diagnosis of 11.52 months (median=3 months). Of these children, 20.9% of children met ADDM ASD criteria but did not have an ASD diagnosis reported in their records. Children of non-AMA mothers had a mean age of 44.65 months (median=40 months) at their first evaluation noting developmental concern and it took an average of 11.70 months (median=3 months) from first evaluation to first diagnosis. Of these children, 26.3% met ADDM ASD criteria but did not have an ASD diagnosis on their record. Our imputed paternal age variable was associated with (P<0.0001) and strongly correlated with (Pearson correlation=0.73) AMA.
Table 1.
Characteristics of children with autism spectrum disorder identified from the Autism and Developmental Disabilities Monitoring Network surveillance years 2008, 2010, and 2012, by advanced maternal age at childbirth.
| Maternal age
<35 N=8322 |
Maternal age
≥35 N=2036 |
||||
|---|---|---|---|---|---|
|
|
|||||
| N | % | N | % | P value* | |
|
|
|||||
| Maternal race / ethnicity | <0.001 | ||||
| White | 5071 | 61.1 | 1399 | 69.4 | |
| Black | 1754 | 21.1 | 361 | 17.9 | |
| Other | 366 | 4.4 | 88 | 4.4 | |
| Hispanic | 1108 | 13.4 | 169 | 8.4 | |
| Missing | 23 | 19 | |||
| Maternal education (years) | <0.0001 | ||||
| Teen pregnancy | 155 | 1.9 | - | ||
| <12 | 1007 | 12.5 | 96 | 4.9 | |
| 12–<16 | 4337 | 53.8 | 817 | 41.7 | |
| >=16 | 2557 | 31.7 | 1048 | 53.4 | |
| Missing | 266 | 75 | |||
| Site | <0.0001 | ||||
| AL | 220 | 2.6 | 31 | 1.5 | |
| AR | 571 | 6.9 | 66 | 3.2 | |
| AZ | 916 | 11.0 | 198 | 9.7 | |
| CO | 476 | 5.7 | 137 | 6.7 | |
| GA | 1158 | 13.9 | 342 | 16.8 | |
| MD | 658 | 7.9 | 212 | 10.4 | |
| MO | 692 | 8.3 | 163 | 8.0 | |
| NC | 1035 | 12.4 | 254 | 12.5 | |
| NJ | 874 | 10.5 | 337 | 16.6 | |
| PA | 132 | 1.6 | 32 | 1.6 | |
| SC | 311 | 3.7 | 66 | 3.2 | |
| UT | 620 | 7.5 | 63 | 3.1 | |
| WI | 659 | 7.9 | 135 | 6.6 | |
| Study year | 0.1 | ||||
| 2008 | 1956 | 23.5 | 476 | 23.4 | |
| 2010 | 3304 | 39.7 | 762 | 37.4 | |
| 2012 | 3062 | 36.8 | 798 | 39.2 | |
| Record type | 0.3 | ||||
| School | 2869 | 34.5 | 732 | 36.0 | |
| Health | 3306 | 39.7 | 775 | 38.1 | |
| Both | 2147 | 25.8 | 529 | 26.0 | |
| Child Sex | 0.8 | ||||
| Male | 6870 | 82.6 | 1676 | 82.3 | |
| Female | 1452 | 17.4 | 360 | 17.7 | |
| Birth-order | <0.0001 | ||||
| First born | 4200 | 50.6 | 669 | 32.9 | |
| Second born | 2551 | 30.7 | 703 | 34.5 | |
| Third or greater | 1557 | 18.7 | 664 | 32.6 | |
| Missing | 14 | - | |||
| Plurality | <0.0001 | ||||
| Singleton | 7939 | 95.4 | 1853 | 91.0 | |
| Multiple | 383 | 4.6 | 183 | 9.0 | |
| Child gestational age (weeks) | <0.0001 | ||||
| ≥37 | 7099 | 85.3 | 1664 | 81.7 | |
| ≥35–37 | 650 | 7.8 | 180 | 8.8 | |
| ≥32–35 | 285 | 3.4 | 108 | 5.3 | |
| <32 | 288 | 3.4 | 84 | 4.1 | |
| Paternal age | <0.0001† | ||||
| Mean, SD | 30.1 | 6.0 | 38.7 | 5.5 | |
| Missing | 1003 | 133 | |||
| Age at first evaluation noting developmental concern (mean months, SD) | |||||
| Overall | 44.7 | 0.2 | 41.3 | 0.5 | <0.0001‡ |
| 10th percentile | 21.0 | 0.2 | 21.0 | 0.2 | |
| 20th percentile | 27.0 | 0.3 | 26.0 | 0.5 | |
| 30th percentile | 31.0 | 0.2 | 30.0 | 0.4 | |
| 40th percentile | 35.0 | 0.2 | 34.0 | 0.4 | |
| 50th percentile | 40.0 | 0.3 | 37.0 | 0.6 | |
| 60th percentile | 47.0 | 0.4 | 42.0 | 0.8 | |
| 70th percentile | 54.0 | 0.4 | 49.0 | 0.9 | |
| 80th percentile | 63.0 | 0.4 | 59.0 | 0.8 | |
| 90th percentile | 75.0 | 0.4 | 72.0 | 0.8 | |
| Missing | 141 | 44 | |||
| Time from first evaluation to first diagnosis (mean months, SD)^ | |||||
| Overall | 11.7 | 0.4 | 11.5 | 0.2 | 0.1‡ |
| 10th percentile | 0.0 | 0.0 | 0.0 | 0.0 | |
| 20th percentile | 0.0 | 0.0 | 0.0 | 0.0 | |
| 30th percentile | 0.0 | 0.0 | 0.0 | 0.0 | |
| 40th percentile | 1.0 | 0.1 | 0.0 | 0.1 | |
| 50th percentile | 3.0 | 0.2 | 3.0 | 0.4 | |
| 60th percentile | 8.0 | 0.3 | 7.0 | 0.5 | |
| 70th percentile | 14.0 | 0.5 | 13.0 | 0.1 | |
| 80th percentile | 23.0 | 0.5 | 23.0 | 1.1 | |
| 90th percentile | 37.0 | 0.7 | 38.0 | 1.3 | |
| Censored (N, %) | 1693 | 26.3 | 360 | 20.9 | |
| Missing | 141 | 44 | |||
P value calculated by chi-square tests
P value calculated using student t-test
P value calculated using Wilcoxon test for non-normally distributed continuous data
For deciles 10, 20, and 30, all observations were 0
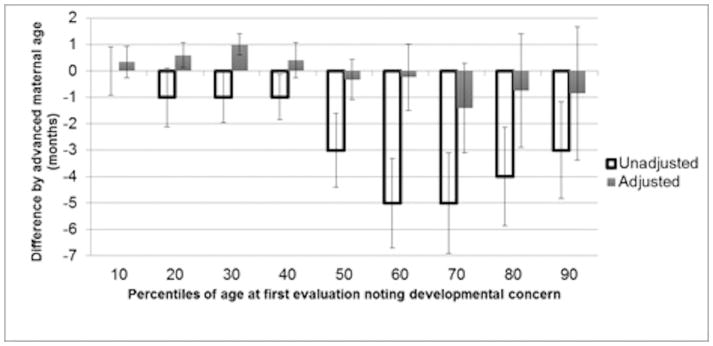
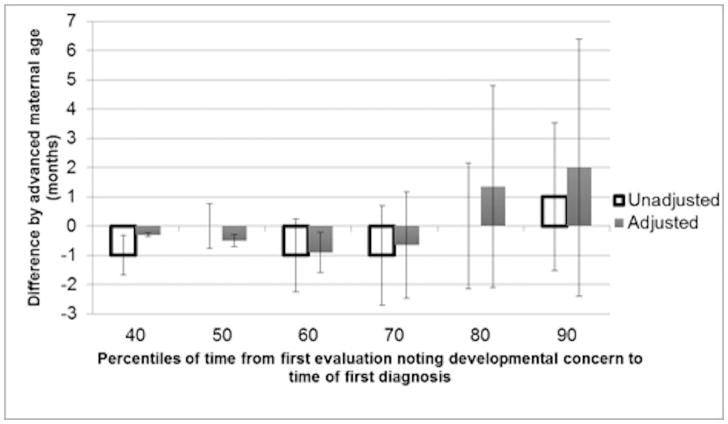
In the unadjusted model (Figure 1), there was a difference in age at first evaluation noting developmental concern by AMA, with the median time for children of AMA mothers being identified between 1 and 5 months earlier than in non-AMA mothers (depending on decile). These differences were statistically significant in all but the first decile. After adjustment for socioeconomic and demographic confounders, differences in medians ranged from 0.6 months later to 1.4 months earlier, with only the 20th and 30th percentile groups meeting statistical significance. When the child had a formal diagnosis of ASD on their record, median time from first evaluation noting developmental concern to ASD diagnosis did not differ in deciles by AMA in most unadjusted and all adjusted analyses (Figure 2). In our sensitivity analysis that included children with first evaluation age that noted ASD traits <8 months, results were similar (data not shown).
Figure 1.
Differences in median age at first evaluation noting developmental concern in children with autism spectrum disorder at the deciles and corresponding 95% confidence intervals comparing children of mothers of advanced maternal age to those with mothers not of advanced maternal age, identified by the Autism and Developmental Disabilities Monitoring Network in 2008, 2010, and 2012
Advanced maternal age (AMA) defined as childbirth at ≥35 years of age
Difference is age at first evaluation noting developmental concern for children of AMA mothers – non-AMA mothers
Adjusted model controlled for maternal race/ethnicity, maternal education, study site, child year of birth, child gestational age, paternal age, parity, birth order, and record type with multiple imputation to account for missing paternal age data.
Figure 2.
Differences in median time from first evaluation noting developmental concern to first autism diagnosis by decile and 95% confidence intervals by advanced maternal age in children with a past autism diagnosis identified by the Autism and Developmental Disabilities Monitoring Network in 2008, 2010, and 2012
Advanced maternal age defined as childbirth at ≥35 years of age
Difference is the number of months for time from first evaluation noting developmental concern to first autism diagnosis in children of AMA mothers compared to children with non-AMA mothers
Adjusted model controlled for maternal race/ethnicity, maternal education, study site, child year of birth, child gestational age, paternal age, parity, birth order, and record type with multiple imputation to account for missing paternal age data
Deciles 10–30 not shown due to time difference being 0 for all observations
When assessing these differences using Cox proportional hazard models (Table 2) the patterns in age at first evaluation noting developmental concern significantly differed in the unadjusted model (Hazard ratio (HR): 1.12, 95% CI: 1.06, 1.18) but not in the adjusted model (HR: 1.02, 95% CI: 0.96, 1.08). When assessing time from first evaluation to first ASD diagnosis, we found an unadjusted HR of 1.08 (95% CI: 1.02, 1.14) that was no longer significant after adjustment (HR: 1.03, 95% CI: 0.96, 1.10), indicating that the distributions over time did not differ by AMA status.
Table 2.
Hazard ratios for time from first evaluation noting developmental concern to time to first autism spectrum diagnosis comparing children of mothers of advanced maternal age to child whose mothers were not of advanced maternal among children with autism spectrum disorder identified by the Autism and Developmental Disabilities Monitoring Network in 2008, 2010, and 2012
| Age at first evaluation that noted developmental concern (Months) | Time from evaluation to ASD diagnosis (Months) | |||
|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
|
|
||||
| Unadjusted | 1.12* | 1.06, 1.18 | 1.08* | 1.02, 1.14 |
| Adjusted | 1.02 | 0.96, 1.08 | 1.03 | 0.96, 1.10 |
Statistically significant at an alpha=0.05 level
Advanced maternal age defined as childbirth at ≥35 years of age
ASD autism spectrum disorder
CI confidence interval
Adjusted model controlled for maternal race/ethnicity, maternal education, study site, child year of birth, child gestational age, paternal age, parity, birth order, and record type with multiple imputation to account for missing paternal age data
Lastly, we stratified our analysis by ID status. After sub-setting our sample to sites that collected ID status for >60% of children, 8412 children were included in this sub-analysis: 2332 (27.7%) had ID, 4608 (54.8%) did not, and 1472 (17.5%) were missing ID data. Children with ID had a first evaluation noting developmental concern earlier compared to children without ID (mean/median age with ID: 37.9/35 months; mean/median age without ID: 47.5/44 months) and in unadjusted analyses there were significant earlier evaluations noting first concerns by AMA in both groups with and without ID. However, there were no significant differences by AMA in any ID group in any decile after adjustment (Table 3). Further, there were no associations between AMA and time between first evaluation noting developmental concern and first diagnosis of ASD in any ID group. As an additional post-hoc analysis, we examined effect modification by stratifying on maternal education or child birth-order; no strata found significant associations between AMA and age at first evaluation that noted ASD traits.
Table 3.
Stratified results by intellectual disability status for differences in decile means and hazard ratios comparing age at first evaluation noting developmental concern and time from first evaluation to first autism spectrum disorder diagnosis by advanced maternal age at child birth among children identified with autism spectrum disorder by the Autism and Developmental Disabilities Monitoring Network, 2008, 2010, and 2012
| With ID (N=2332) | Without ID (N=4608) | Missing ID (N=1472) | ||||
|---|---|---|---|---|---|---|
| Age at first evaluation (months) | ||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
|
|
||||||
| Unadjusted | ||||||
| Median change | ||||||
| 20th percentile | −2.00* | −0.34, −0.57 | −1.00 | −2.66, 0.66 | −1.00 | −3.60, 1.60 |
| 40th percentile | −2.00* | −3.46, −0.54 | −3.00* | −4.91, −1.09 | −2.00 | −4.80, 0.80 |
| 60th percentile | −3.00* | −5.56, −0.43 | −5.00* | −7.55, −2.45 | −5.00 | −8.59, −1.41 |
| 80th percentile | −5.00 | −8.34, 1.66 | −3.00* | −5.49, −0.51 | 0.00 | −5.73, 5.73 |
| Hazard ratio | 1.16* | 1.05, 1.29 | 1.14* | 1.06, 1.23 | 1.07 | 0.94, 1.22 |
| Adjusted | ||||||
| Median change | ||||||
| 20th percentile | −0.79 | −2.38, 0.80 | −0.77 | −1.72, 0.17 | −0.76 | −3.36, 1.83 |
| 40th percentile | −0.71 | −2.27, 0.85 | −0.65 | −2.36, 1.06 | 0.56 | −3.45, 4.57 |
| 60th percentile | −0.36 | −2.45, 1.72 | 0.29 | −2.36, 2.94 | 0.19 | −8.09, 8.47 |
| 80th percentile | 1.81 | −2.31, 5.93 | 2.25 | −0.75, 5.72 | −1.45 | −15.71, 12.82 |
| Hazard ratio | 0.96 | 0.85, 1.08 | 1.03 | 0.95, 1.12 | 0.99 | 0.85, 1.08 |
| Time from evaluation to diagnosis (months) | ||||||
| % Censored (AMA, non-AMA) | 20.91 | 26.30 | 32.51 | 34.69 | 24.46 | 26.17 |
| Unadjusted | ||||||
| Median change | ||||||
| 40th percentile | −1.00 | −2.13, 0.13 | −1.00 | −2.02, 0.02 | 0.00 | - |
| 60th percentile | −1.00 | −4.02, 2.02 | −3.00* | −5.37, −0.63 | 1.00 | −0.67, 2.67 |
| 80th percentile | 1.00 | −4.23, 6.23 | −2.00 | −6.00, 1.98 | 2.00 | −4.00, 8.00 |
| Hazard ratio | 1.13* | 1.00, 1.27 | 1.07 | 0.98, 1.17 | 1.02 | 0.88, 1.19 |
| Adjusted | ||||||
| Median change† | ||||||
| 40th percentile | 0.73 | 0.22, 1.22 | 0.63 | 0.31, 0.95 | 0.00 | - |
| 60th percentile | 2.23 | −2.00, 6.46 | 1.6.0 | −0.54, 3.73 | −1.19 | −8.52, 6.13 |
| 80th percentile | 0.77 | −13.88, 15.43 | 1.41 | −5.59, 8.41 | 0.42 | −1.95, 2.79 |
| Hazard ratio | 1.03 | 0.90, 1.18 | 1.03 | 0.93, 1.14 | 0.97 | 0.81, 1.16 |
Advanced maternal age defined as childbirth at ≥35 years of age
ID Intellectual disability, defined as IQ test score ≤70 or indication of ID on the child’s record
Sample subset to sites that collected ID data for >60% of children (Alabama, Arkansas, Arizona, Colorado, Georgia, Maryland, North Carolina, New Jersey, South Carolina, and Utah)
Adjusted model controlled for maternal race/ethnicity, maternal education, study site, child year of birth, child gestational age, paternal age, parity, birth order, and record type with multiple imputation to account for missing paternal age data
Statistically significant at an alpha=0.05 level
Deciles 10–30 not shown due to time difference being 0 for all observations in those deciles
DISCUSSION
In an analysis of three years of prevalence data for eight-year-old children with ASD across the U.S., we found that all observed associations between AMA and the age at which a child with ASD first had an evaluation noting developmental concern were largely explained by other sociodemographic factors, namely maternal education. Additionally, we did not see significant associations after adjusting for other maternal characteristics when evaluating time from first evaluation noting developmental concern to first diagnosis of ASD, including after stratification by child ID.
We hypothesized that age at first evaluation noting developmental concern would be independently associated with AMA because of a potential increased perception of risk due to being of AMA. Ultimately, this was not the case as significant differences in medians at the deciles and hazard ratios were attenuated to the null after adjustment for SES and demographic factors. A key factor in the association between timing of developmental evaluations or ASD diagnoses is race and ethnicity. Age at first ASD diagnosis is associated with race in population-based studies, with black children sometimes found to be diagnosed later than white children.13,25 The race/ethnicity disparity in age at diagnosis may be attributable to diagnostic bias26 or access to care.27 In our data, we saw that AMA mothers were significantly more likely to be white, which supports race/ethnicity as a factor that could have led to increased crude estimates. Whether attributable to AMA or race, we see clear evidence of disparity with children of younger, non-white mothers getting evaluated later.
Similarly, education is highly associated with disparity and access to care. Pettygrove et al.28 found that children in the 2002 ADDM surveillance year who were missing health records (likely due to lack of access to healthcare) were from census blocks with lower levels of parental education, fewer parents with a college degree, and had children with later first diagnoses of ASD. Again, our data illustrate disparity by a factor associated with AMA as younger mothers who are more likely to have lower education levels had their children with ASD identified later.
Birth order may also have confounded the unadjusted associations we saw. A mother with previous children may be more acutely aware of atypical development compared to a first time mother as concerns about a child’s development arise sooner when there is an older child for comparison.29 Further, ASD is highly heritable30 and it is likely that some children with ASD identified in ADDM have an older sibling with ASD. When this is the case, mothers tend to have earlier developmental concerns for the later child.29 These familial dynamics need to be further explored and accounted for in order to better understand ways to lower age at ASD diagnosis.
Additionally, we examined time from first evaluation noting developmental concern to first diagnosis of ASD by AMA. Approximately one-fifth of children who met the standardized criteria for ASD in our data did not have a record indicating they had formally received an ASD diagnosis, but the existence of an ASD diagnosis was not differential by AMA. In a previous ADDM study, Wiggins et al.,16 found a 13-month delay between first evaluation and first diagnosis, with the delay being associated with cognitive impairment. We had hypothesized that mothers of AMA would have more experience and knowledge of the health system that could enable earlier diagnosis. Similarly to age at first evaluation, we saw significant unadjusted associations when evaluating HRs but results were attenuated to the null after adjustment. Again, the crude association was likely attributable to the confounding socioeconomic and demographic factors that affect access to care.
In agreement with past literature,14,16 we found that children with ASD and ID had first evaluations noting developmental concern earlier than children with ASD without ID. AMA may be associated with ASD phenotype (including co-occurring ID)31 and by stratifying by child ID status, we were able to explore effects of co-occurring ID on evaluation and diagnostic timing. For both age at first evaluation and time from first evaluation to first diagnosis, there were significant crude differences but no significant differences by AMA status for any ID strata after adjustment. In our data, the percent of AMA mothers among children with and without ID were similar (19.3% of mothers of children with ID, 20.4% of mothers of children without ID), which may not support ID as a mediating factor. More refined phenotypic measurement, like ASD severity, in children with ASD may be needed to identify the connection between AMA, child presentation, and evaluation and diagnosis.
Our study was limited by having only data on children with ASD, which prevented us from assessing the effect of AMA on ASD screening and evaluation in the general population. We lacked data to fully evaluate what led to the initial ASD evaluation and any reason for a specific delay like lack of access. We were not able to characterize the level of the mother’s concern, identify and account for children who were not raised by the mother indicated on the birth certificate, and account for the influence of the father. Though we used multiple imputation methods to reduce the impact of missing paternal age, the effectiveness of this technique relies on the assumption that data were missing at random conditional on covariates and that the imputation model is correctly specified. We adjusted for appropriate confounders, but some residual confounding may have remained, especially at the level of community-specific factors. In using semi-parametric Cox models, we assumed that those observations that were censored truly did not have a diagnosis of ASD by eight years and were not missing data on a diagnosis. Based on the thorough ADDM methodology, we are confident in this assumption. We were able to use ID status as a representation of child phenotype since it was a co-occurring condition that was consistent over time. It is possible that missing ID status impacted our findings; however, we restricted our analyses to sites that had the least amount of missing ID data and presented our findings for those with missing ID data from those sites.
The strengths of this study include the use of data from ADDM, which enabled us to use a large sample of children with ASD identified from across the U.S. using a standardized surveillance methodology. We assessed two different outcomes (age at first evaluation and time from first evaluation to first diagnosis) to better understand how AMA affects the diagnostic process. Additionally, we modeled these associations in two semi-parametric ways, calculating differences in median at the deciles and calculating HRs, using models appropriately adjusted for confounding. Incorporating the Cox models and a time-to-event framework allowed us to use data on 1/5th of our sample that did not have a formal diagnosis of ASD by eight years old. By stratifying our results among a subset with ID data, we explored the effect of a common co-occurring condition of ASD that may be associated with AMA and age at first evaluation.
These findings highlight the high correlation between AMA and other sociodemographic factors in timing of a child’s ASD evaluations. Children with ASD and older mother were more likely to have earlier evaluations noting developmental concerns and this can be attributed to higher socioeconomic status and racial and ethnic disparity in ASD identification. AMA being a risk factor for ASD does not independently contribute to earlier age at child evaluation and diagnosis. Understanding how risk factors for ASD impact evaluation patterns will help to reduce age at diagnosis in those at highest risk.
Acknowledgments
Funding support: T32HD007490, U54HD090256, Data collection of this work was supported by the Autism and Developmental Disabilities Monitoring Network.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors have no conflicts of interest to report.
Contributor Information
Eric Rubenstein, Waisman Center, University of Wisconsin-Madison, Madison WI.
Maureen S. Durkin, Waisman Center, Department of Population Health Science, University of Wisconsin-Madison, Madison WI.
Rebecca A. Harrington, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore MD.
Russell S. Kirby, Department of Community and Family Health, University of South Florida, Tampa FL.
Laura A. Schieve, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta GA.
Julie Daniels, Department of Epidemiology, University of North Carolina-Chapel Hill, Chapel Hill NC.
References
- 1.Khalil A, Syngelaki A, Maiz N, Zinevich Y, et al. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013 Dec;42(6):634–643. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 2.Coppede F. Risk factors for Down syndrome. Arch Toxicol. 2016 Dec;90(12):2917–2929. doi: 10.1007/s00204-016-1843-3. [DOI] [PubMed] [Google Scholar]
- 3.Ciancimino L, Lagana AS, Chiofalo B, et al. Would it be too late? A retrospective case-control analysis to evaluate maternal-fetal outcomes in advanced maternal age. Arch Gynecol Obstet. 2014 Dec;290(6):1109–1114. doi: 10.1007/s00404-014-3367-5. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Zhu T, Qu Y, Mu D. Prenatal, Perinatal and Neonatal Risk Factors for Intellectual Disability: A Systemic Review and Meta-Analysis. PLoS One. 2016;11(4):e0153655. doi: 10.1371/journal.pone.0153655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandin S, Hultman CM, Kolevzon A, et al. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2012 May;51(5):477–486 e471. doi: 10.1016/j.jaac.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Ayers S, Holden D. A metasynthesis of risk perception in women with high risk pregnancies. Midwifery. 2014 Apr;30(4):403–411. doi: 10.1016/j.midw.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Benzies K, Tough S, Tofflemire K, et al. Factors influencing women’s decisions about timing of motherhood. J Obstet Gynecol Neonatal Nurs. 2006 Sep-Oct;35(5):625–633. doi: 10.1111/j.1552-6909.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 8.Jordan RG, Murphy PA. Risk assessment and risk distortion: finding the balance. J Midwifery Womens Health. 2009 May-Jun;54(3):191–200. doi: 10.1016/j.jmwh.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Selkirk CG, McCarthy Veach P, Lian F, et al. Parents’ perceptions of autism spectrum disorder etiology and recurrence risk and effects of their perceptions on family planning: Recommendations for genetic counselors. J Genet Couns. 2009 Oct;18(5):507–519. doi: 10.1007/s10897-009-9233-0. [DOI] [PubMed] [Google Scholar]
- 10.Mercer L, Creighton S, Holden JJ, et al. Parental perspectives on the causes of an autism spectrum disorder in their children. J Genet Couns. 2006 Feb;15(1):41–50. doi: 10.1007/s10897-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 11.Koegel LK, Koegel RL, Ashbaugh K, et al. The importance of early identification and intervention for children with or at risk for autism spectrum disorders. Int J Speech Lang Pathol. 2014 Feb;16(1):50–56. doi: 10.3109/17549507.2013.861511. [DOI] [PubMed] [Google Scholar]
- 12.Quinlan CA, McVeigh KH, Driver CR, et al. Parental Age and Autism Spectrum Disorders Among New York City Children 0–36 Months of Age. Matern Child Health J. 2015 Aug;19(8):1783–1790. doi: 10.1007/s10995-015-1692-3. [DOI] [PubMed] [Google Scholar]
- 13.Valicenti-McDermott M, Hottinger K, Seijo R, et al. Age at diagnosis of autism spectrum disorders. J Pediatr. 2012 Sep;161(3):554–556. doi: 10.1016/j.jpeds.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Shattuck PT, Durkin M, Maenner M, et al. Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry. 2009 May;48(5):474–483. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fountain C, King MD, Bearman PS. Age of diagnosis for autism: individual and community factors across 10 birth cohorts. J Epidemiol Community Health. 2011 Jun 1;65(6):503–510. doi: 10.1136/jech.2009.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006 Apr;27(2 Suppl):S79–87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 17.Zwaigenbaum L, Bauman ML, Fein D, et al. Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics. 2015 Oct;136(Suppl 1):S41–59. doi: 10.1542/peds.2014-3667D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Preventative Services Task Force. [Accessed December, 21, 2015];Draft Recommendations Statement: Autism Spectrum Disorder in Young Children: Screening. 2015 http://www.uspreventiveservicestaskforce.org/
- 19.Arunyanart W, Fenick A, Ukritchon S, et al. Developmental and autism screening: A survey across six states. Infants & Young Children. 2012;25(3):175–187. [Google Scholar]
- 20.Rice CE, Baio J, Van Naarden Braun K, et al. A public health collaboration for the surveillance of autism spectrum disorders. Paediatr Perinat Epidemiol. 2007 Mar;21(2):179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 21.Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016 Apr 01;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistcal Manual of Mental Disoders, 4th edition, Text Revision. Arlington, VA: American Psychiatric Publishing; 2000. [Google Scholar]
- 23.Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry. 2017 Oct;22(10):1385–1394. doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Autism and Developmental Disabilities Monitoring Network Year 2012 Principal Investigators. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 25.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005 Dec;116(6):1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travers JC, Tincani M, Krezmien MP. A Multiyear National Profile of Racial Disparity in Autism Identification. J Spec Educ. 2013 May;47(1):41–49. [Google Scholar]
- 27.Emerson ND, Morrell HE, Neece C. Predictors of Age of Diagnosis for Children with Autism Spectrum Disorder: The Role of a Consistent Source of Medical Care, Race, and Condition Severity. J Autism Dev Disord. 2016 Jan;46(1):127–138. doi: 10.1007/s10803-015-2555-x. [DOI] [PubMed] [Google Scholar]
- 28.Pettygrove S, Pinborough-Zimmerman J, Meaney FJ, et al. Predictors of ascertainment of autism spectrum disorders across nine US communities. J Autism Dev Disord. 2013 Aug;43(8):1867–1879. doi: 10.1007/s10803-012-1732-4. [DOI] [PubMed] [Google Scholar]
- 29.Herlihy L, Knoch K, Vibert B, Fein D. Parents’ first concerns about toddlers with autism spectrum disorder: effect of sibling status. Autism. 2015 Jan;19(1):20–28. doi: 10.1177/1362361313509731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014 Aug;46(8):881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BK, McGrath JJ. Advancing parental age and autism: multifactorial pathways. Trends Mol Med. 2014 Dec 22;21(2):118–125. doi: 10.1016/j.molmed.2014.11.005. [DOI] [PubMed] [Google Scholar]