Abstract
Inhibitory cell surface proteins on T cells are often dynamically regulated, which contributes to their physiologic function. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1, CD31) is an inhibitory receptor that facilitates TGFβ-mediated suppression of T cell activity. It is well established in CD4+ T cells that PECAM-1 is expressed in naïve recent thymic emigrants, but is downregulated after acute T cell activation and absent from memory cells. The extent to which PECAM-1 expression is similarly regulated in CD8+ T cells is much less well characterized. We evaluated T cells recovered from mice after infection with a model intracellular pathogen and determined that, in CD8+ T cells, PECAM-1 expression was strongly downregulated during acute infection but re-expressed to intermediate levels in memory cells. Downregulation of PECAM-1 expression in CD8+ T cells was transcriptionally regulated and affected by the strength and nature of TCR signaling. PECAM-1 was also detected on the surface of human activated/memory CD8+, but not CD4+ T cells. These data demonstrate that PECAM-1 expression is dynamically regulated, albeit differently, in both CD4+ and CD8+ T cells. Furthermore, unlike memory CD4+ T cells, memory CD8+ T cells retain PECAM-1 expression and have the potential to be modulated by this inhibitory receptor.
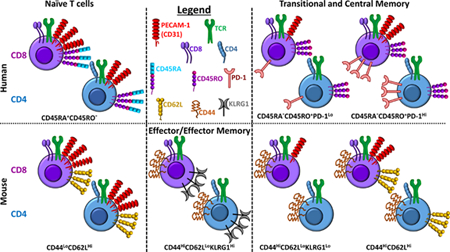
Introduction
T cells express a multitude of cell surface inhibitory receptors, including cytotoxic lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1), some of which have been targeted, either alone or in combination with other therapies, to improve cancer treatment (1,2). We recently demonstrated that Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) engages in a physical interaction with the Transforming Growth Factor β Receptor (TGFβR) and synergizes with TGFβ to potently inhibit CD8+ T cell responses to T cell receptor (TCR) stimulation in vitro and interfere with CD8+ T cell-mediated tumor clearance in vivo (3). These findings suggest that PECAM-1/TGFβR interactions on CD8+ T cells could also be targeted to improve cancer therapy.
Temporal regulation of inhibitory receptor expression on CD8+ T cells is a critical determinant of their function. CTLA-4 is expressed immediately after initial T cell activation, which limits the magnitude of naive T cell responses (4,5). In contrast, PD-1 expression is most highly expressed following chronic exposure of T cells to cognate antigen, when it serves an important role in maintenance of T cell exhaustion (6). Like CTLA-4 and PD-1, PECAM-1 expression is known to be temporally regulated in T cells. Changes in expression of PECAM-1, also known as CD31, has been best characterized in naïve (i.e., CD45RA+) CD4+ T cells, in which CD31 expression distinguishes between CD4+CD45RA+CD31+ thymic naive T cells and CD4+CD45RA+CD31- central naïve T cells (7–11). The majority of CD4+CD45RA+CD31+ thymic naive T cells contain T cell receptor excision circles (TRECs) and express protein tyrosine kinase-7 (PTK7), and therefore have not undergone proliferation since exiting the thymus, whereas CD4+CD45RA+CD31- central naïve T cells do not have TRECs and are thought to comprise cells that have undergone homeostatic proliferation in response to TCR interactions with low affinity self-peptide/MHC complexes. This dichotomy is not perfect, however, since a subset of CD4+CD45RA+CD31+ cells that lack TRECs (12–14) and possess a transcriptional profile that is more characteristic of the activated and/or proliferated state of CD4+CD45RA+CD31- cells has also been described (15). Nevertheless, CD31 has come to be widely used as a marker of recent thymic emigrants within the CD4+CD45RA+ T cell population (15–22).
Importantly, temporal regulation of PECAM-1 expression in T cells other than those that are CD4+CD45RA+ has been much less well studied. Early studies reported that, following its downregulation in response to TCR stimulation, CD31 remains absent from mature CD4+ T cells throughout subsequent maturation events, including establishment of memory (i.e., CD4+CD45RA-CD45RO+) (23,24). In contrast, among CD8+ T cells, a subset of those that were CD45RA- were found to retain CD31 expression, as did CD45RO+ T cells, which expressed CD31 at low to intermediate levels (23,24). These findings, which were subsequently largely ignored, suggested that PECAM-1 expression is regulated differently in CD8+ and CD4+ T cells, and that CD8+ memory T cells may retain the potential to be modulated by PECAM-1.
A thorough understanding of how PECAM-1 expression is temporally regulated in CD8+ T cells is crucial for determining the utility of targeting the PECAM-1/TGFβR inhibitory axis therapeutically, either alone or in combination with TGFβ inhibitors to achieve additive or synergistic effects. We therefore undertook studies to evaluate temporal regulation of PECAM-1 expression in CD8+ T cells. Using mouse models, we found that, similarly to what has been reported for CD4+ T cells, CD8+ T cells express PECAM-1 at high levels in the naïve state and downregulate it to undetectable levels during acute activation, both after engagement of the TCR in vitro and during acute infection in vivo. Unlike CD4+ T cells, however, memory CD8+ T cells express PECAM-1 at levels that are intermediate between naïve and acutely activated cells. Moreover, human CD8+, but not CD4+, T cells that express markers consistent with prior activation also express PECAM-1 at intermediate levels. These data demonstrate that temporal regulation of PECAM-1 expression is different in CD8+ and CD4+ T cells, and that memory CD8+ T cells continue to express PECAM-1, which is unlike memory CD4+ T cells. These findings suggest that, along with CD4+ and CD8+ thymic naive T cells, memory CD8+ T cells may be susceptible to therapeutic strategies designed to target PECAM-1.
Materials and Methods
Mice.
PECAM-1−/− mice (25) were backcrossed for >12 generations onto a C57BL/6J background. Wild type and OT-1 C57BL/6J mice were from Jackson Laboratories. CD45.1 mice were from NCI-Charles River Laboratories. DGKζ−/− mice were described previously (26). DGKζ −/−/OT-I mice were from in-house breeding. Protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Listeria infection.
Mice were injected periorbitally with 5,000 cfu of Listeria monocytogenes engineered to express ovalbumin (LM-OVA), which contains the H-2Kb-restricted T cell epitope SIINFEKL (gift of Michael Bevan, University of Washington). Naïve (CD44LoCD62LHi), memory (CD44HiCD62LHi), and activated effector (CD44HiCD62LLo) CD8+ T cells were purified by FACS from pooled spleen cells 1–4 weeks later. Cells were analyzed for expression of PECAM-1 using anti-mouse CD31 (11–0311; eBioscience) and for OVA-specific TCRs using H-2Kb/SIINFEKL tetramers (gift of John Wherry, University of Pennsylvania). The cell pellets were directly lysed in 2 x SDS gel loading buffer which contained 4% SDS and cell lysates were western blotted using an antibody specific for the PECAM-1 C-terminus (sc-1506-R; Santa Cruz Biotechnology). For adoptive transfer experiments, naïve splenic CD8+CD44Lo cells from wild-type OT-I mice were injected via tail vein into CD45.1 recipients that were injected 24 hours later with 20,000 cfu of LM-OVA expressing the high affinity SIINFEKL (N4) epitope, or a progressively less potent variant (Q4, T4, or V4). Spleen cells isolated 4 days later were analyzed for PECAM-1 expression.
Quantification of PECAM-1 expression.
cDNA was synthesized using SuperScript® III First-Strand Synthesis System (18080–051; Thermo Fisher Scientific) from RNA extracted from splenic naïve, memory, and activated effector CD8+ T cells using Qiagen RNeasy Plus Minikit (74134; Qiagen). Pecam-1 cDNA was quantified using validated TaqMan primer sets (137710319; Integrated DNA Technologies) and Brilliant II QPCR Master Mix (600804; Agilent Technologies) on a 7500 Real-Time PCR System (Applied Biosystems) and normalized to β-actin.
In-vitro stimulation of CD8+ cells.
Naïve CD8+ cells were isolated from spleens of OT-I or DGKζ−/−/OT-I mice using Mouse Naïve CD8+ T Cell Isolation Kit (19858; StemCell Technologies), and stimulated in the presence of irradiated (2500 Rads) spleen cells with a SIINFEKL peptide variant (5 μM), soluble anti-mouse CD3ε (1 or 5 or 15 μg/ml; c553058; BD Biosciences), anti-CD3ε (1 μg/ml) plus anti-CD28 (1 μg/ml; 553294; BD Biosciences), or medium alone. Results shown are from cells analyzed 24 hours later for PECAM-1 expression after gating out dead cells using LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (L34957; ThermoFisher Scientific).
Evaluation of PECAM-1 expression from RNA-seq data sets of murine LM-OVA infection.
Microarray data were retrieved from the Gene Expression Omnibus (GEO) series with GSE15907 (27). The R/Bioconductor package oligo (28) was used for microarray analysis, including background subtraction, quantile normalization, and generation of expression values using the Robust Multi-array Average (RMA) methodology.
PECAM-1 expression on human T cells.
PBMCs obtained from de-identified volunteer blood donors were isolated using Ficoll-paque density gradient centrifugation. Cells were stained with 7-aminoactinomycin D (7-AAD, eBiosciences) to assess cell viability and with antibodies specific for human CD4 (OKT4), CD8 (RPA-T8), CD45RO (UCHL1), and PD-1 (eBioJ105) (all from Thermo Fisher), or CD31 (MBC 78.2 or WM59, BD Biosciences), and analyzed using an LSR II (BD Biosciences) and FlowJo version 8.8.7 software (FlowJo, LLC).
Evaluation of PECAM-1 expression from Microarray data sets.
Microarray data were retrieved from GSE23321 (29). GEO2R was used to calculate log2 transformed RMA levels of PECAM-1 expression from microarray data of sorted populations of human naïve (CD3+CD8+CD45RO-CCR7+CD45RA+CD62L+CD27+CD28+IL7Rα+CD95-), central memory (CD3+CD8+CD45RO+CCR7+CD45RA-CD62L+), and effector memory (CD3+CD8+CD45RO+CCR7-CD45RA-CD62L-) T cell subsets from peripheral blood of three healthy donors (29). Independent replicates were averaged, and statistical analysis performed as described below.
Statistical analysis.
Data were analyzed by two-way ANOVA followed by Bonferroni’s multiple comparisons test, one-way ANOVA followed by Tukey’s multiple comparison test, or 2-tailed Student’s t test using GraphPad Prism 6 software.
Results and Discussion
PECAM-1 is expressed in naïve CD8+ T cells but is downregulated acutely after activation
The potential for PECAM-1 to function as a regulator of T cell function relies on its expression in T cells. PECAM-1 expression in CD4+ T cell populations is known to be affected by the activation state of the T cell; thus PECAM-1 is expressed at high levels in naïve CD4+ T cells soon after their emigration from the thymus but is subsequently downregulated after antigen exposure (7–11). To determine whether PECAM-1 expression in CD8+ T cells also changes as a function of activation, we infected mice with Listeria monocytogenes-ovalbumin (LM-OVA), which is a bacterial pathogen that generates CD8+ T cell responses to numerous uncharacterized bacterial antigens along with strong responses to ovalbumin (30). As expected (31), infection of C57BL6/J mice with LM-OVA resulted in significant activation of CD8+ T cells, as demonstrated by an increased number of cells expressing high levels of CD44 (CD44Hi) and low levels of CD62L (CD62LLo) (Supplementary Figure 1, middle panels), especially among CD8+ T cells bearing a TCR specific for the H-2Kb-restricted, ovalbumin-derived peptide, SIINFEKL (CD8+OVApos; Supplementary Figure 1, right panels). In uninfected mice, PECAM-1 was expressed at high levels in naive (CD44LoCD62LHi) CD8+ T cells, at lower but still substantial levels in central memory (CD44HiCD62LHi) CD8+ T cells, and at still lower but detectable levels in effector/memory precursor (CD44HiCD62LLo) CD8+ T cells (Figure 1A,B). Seven days after infection with LM-OVA, PECAM-1 expression remained unchanged in naïve CD8+ T cells, but decreased slightly but significantly in CD44HiCD62LHi CD8+ T cells and dramatically in CD44HiCD62LLo CD8+ T cells (Figure 1A,B), in the latter especially among T cells specific for H2Kb/SIINFEKL (OVApos; Figure 1C,D). PECAM-1 expression on CD4+ T cells did not differ between uninfected and infected mice (Supplementary Figure 2). The broad distribution of PECAM-1 expression on CD44HiCD62LLo CD8+ T cells seven days after infection with LM-OVA was attributable to different levels of expression of PECAM-1 on different subsets of cells within this population. Thus, PECAM-1 expression was lost on both long-lived (CD127Hi) and short-lived (CD127Lo) effector (KLRG1Hi) CD8+ T cells, regardless of OVA-specificity, but was retained on memory precursor (CD127HiKLRG1Lo) CD8+ T cells, albeit to a greater extent in OVAneg than in OVApos cells (Figure 1E,F). These results indicate that PECAM-1 expression changes similarly as a function of activation in effector CD8+ and CD4+ T cells; however, central memory and memory precursor CD8+ T cells are different from their CD4+ counterparts in that they continue to express significant amounts of PECAM-1 after exposure to antigen.
Figure 1. Central memory and memory precursor CD8+ T cells express PECAM-1 at levels that are intermediate between naïve and activated effector CD8+ T cells one week after bacterial infection in mice.
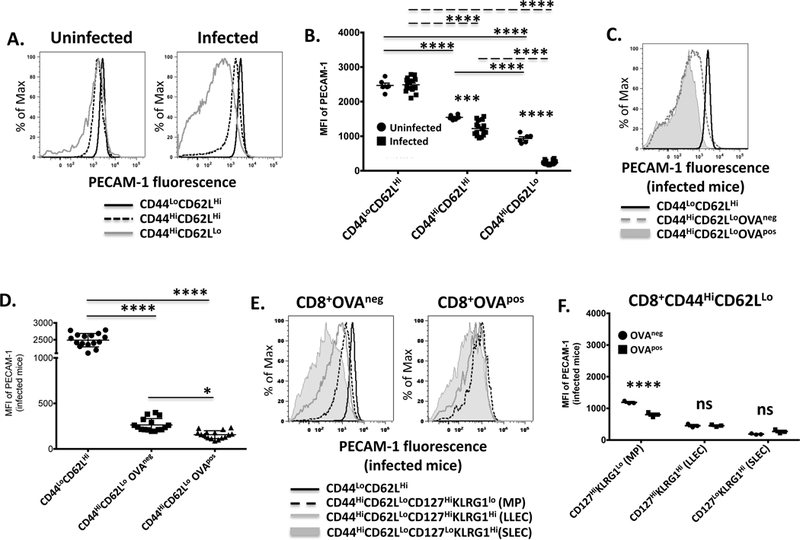
PECAM-1 expression on splenic CD8+ T cells was assessed one week after infection of C57BL/6 mice with Listeria monocytogenes expressing ovalbumin. (A) Fluorescence intensity of PECAM-1 expression on naïve (CD44LoCD62LHi), central memory (CD44HiCD62LHi), and activated effector (CD44HiCD62LLo) CD8+ T cells from an uninfected and infected mouse in one representative experiment. (B) Statistical analysis of mean fluorescence intensity (MFI) of PECAM-1 expression on naïve, memory and activated effector CD8+ T cells from uninfected (n=6) and infected (n=16) mice in 4 independent experiments. Symbols represent individual data points and lines represent means ± SEM. Differences between means were determined by 2-way ANOVA and Bonferroni’s multiple comparison test; ****p < 0.0001, ***p < 0.001. (C) Fluorescence intensity of PECAM-1 expression on naïve and activated effector CD8+ T cells with TCRs specific for the H-2Kb-specific, ovalbumin-derived SIINFEKL peptide (OVApos) or for non-ovalbumin bacterial antigens (OVAneg) from an infected mouse in one representative experiment. (D) Statistical analysis of MFI of PECAM-1 expression on naïve and OVAneg or OVApos activated effector CD8+ T cells from infected mice in 4 independent experiments. Symbols represent individual data points and lines represent means ± SEM. Differences between means were determined by 1-way ANOVA and Tukey’s multiple comparison test; *p < 0.05, ****p < 0.0001. (E) Fluorescence intensity of PECAM-1 expression on OVAneg or OVApos memory precursors (MP; CD127HiKLRG1Lo), long-lived effector cells (LLEC; CD127HiKLRG1Hi), or short-lived effector cells (SLEC; CD127LoKLRG1Hi) among activated effector (CD44HiCD62LLo) CD8+ T cells from an infected mouse in one representative experiment. PECAM-1 expression on OVAneg naïve (CD44LoCD62LHi) CD8+ T cells is shown as a reference. (F) Statistical analysis of MFI of PECAM-1 expression on OVAneg or OVApos MP, LLEC, or SLEC among activated effector CD8+ T cells from infected mice in 3–4 independent experiments. Symbols represent individual data points and lines represent means ± SEM. Differences between means were determined by 2-way ANOVA and Sidak’s multiple comparison test; ****p < 0.0001, ns = not significant.
PECAM-1 is expressed at intermediate levels in memory CD8+ T cells in mice
In CD4+ T cells, PECAM-1 expression has been shown to be dramatically decreased following activation to levels that are comparably low in acutely activated effector cells and memory cells, which suggests that the downregulation of PECAM-1 that occurs upon activation of CD4+ T cells is durable (23,32,33). To determine whether the same is true for CD8+ T cells, we evaluated PECAM-1 expression levels on CD8+ T cells recovered from mice four weeks after infection with LM-OVA, a time point when formation of memory CD8+ T cell populations is firmly established (34). PECAM-1 expression remained significantly lower in central memory (CD44HiCD62LHi) relative to naïve (CD44LoCD62LHi) CD8+ T cells four weeks after infection with LM-OVA (Figure 2A,B). The slightly but significantly lower level of PECAM-1 expression that was observed in central memory CD8+ T cells from infected relative to uninfected mice at one week post-infection (Figure 1A,B) was no longer present by four weeks post-infection (Figure 2A,B), although the slightly lower level of PECAM-1 expression on OVApos relative to OVAneg central memory CD8+ T cells remained at four weeks post-infection (Figure 2C,D). The broad distribution of PECAM-1 expression that had characterized CD44HiCD62LLo CD8+ T cells one week after infection (Figure 1A) changed to a bimodal distribution by four weeks post-infection (Figure 2A). The PECAM-1 expressing cells within this population were identified as transitional memory (KLRG1Lo) cells (Supplementary Figure 3), which expressed PECAM-1 at levels that were slightly but significantly lower in OVApos than in OVAneg populations (Figure 2C,D). The PECAM-1-negative CD44HiCD62LLo CD8+ T cells four weeks after infection were effector memory (KLRG1Hi) cells (Supplementary Figure 3), which failed to express PECAM-1 regardless of OVA specificity (Figure 2C,D). These results indicate that failure of effector/effector memory cells to express PECAM-1 and expression of PECAM-1 at low levels and intermediate levels, respectively, on memory precursor/transitional memory and central memory cells are durable properties of these subsets of CD8+ T cells.
Figure 2. Central memory and transitional memory CD8+ T cells express PECAM-1 at levels that are intermediate between naïve and effector/effector memory CD8+ T cells four weeks after bacterial infection in mice.
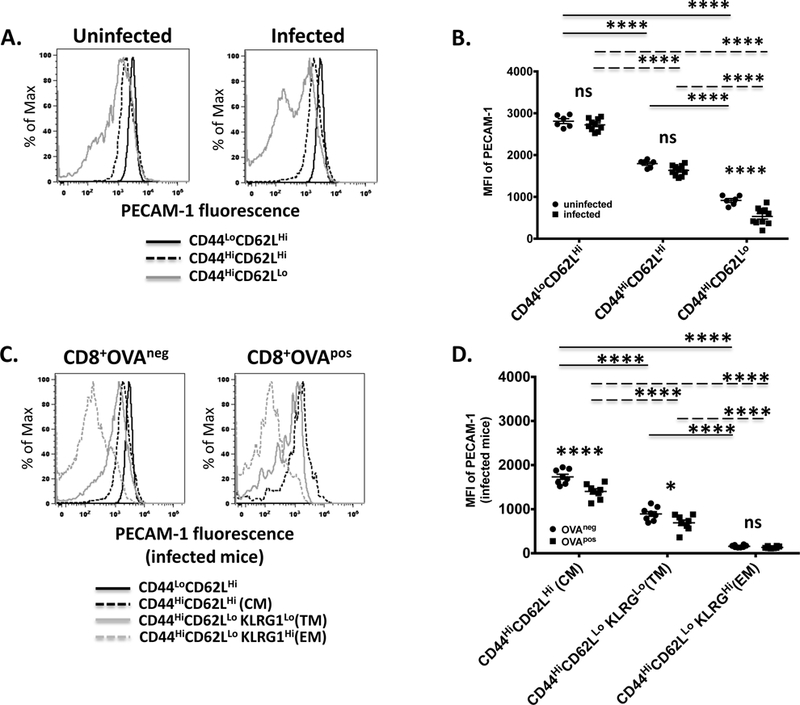
PECAM-1 expression on splenic CD8+ T cells was assessed four weeks after infection of C57BL/6 mice with Listeria monocytogenes expressing ovalbumin. (A) Fluorescence intensity of PECAM-1 expression on naïve (CD44LoCD62LHi), central memory (CD44HiCD62LHi) and effector memory (CD44HiCD62LLo) CD8+ T cells from an uninfected and infected mouse in one representative experiment. (B) Statistical analysis of mean fluorescence intensity (MFI) of PECAM-1 expression on naïve, memory, and effector memory CD8+ T cells from uninfected (n=6) and infected (n=10) mice in 2 independent experiments. Symbols represent individual data points and lines represent means ± SEM. Differences between means were determined by 2-way ANOVA and Bonferroni’s multiple comparison test; **** p<0.0001; ns, not significant. (C) Fluorescence intensity of PECAM-1 expression on central memory (CM; CD44HiCD62LHi), transitional memory (TM; CD62LLoKLRG1Hi), and effector memory (EM; CD62LLoKLRG1Lo) CD8+CD44Hi T cells with TCRs specific for the H-2Kb-specific, ovalbumin-derived SIINFEKL peptide (OVApos) or those specific for non-ovalbumin bacterial antigens (OVAneg) from an infected mouse in one representative experiment. PECAM-1 expression on OVAneg naïve (CD44LoCD62LHi) CD8+ T cells is shown as a reference. (D) Statistical analysis of MFI of PECAM-1 expression on CM, TM and EM CD8+ T cells from infected mice in two pooled independent experiments. Symbols represent individual data points and lines represent means ± SEM. Differences between means were determined by 1-way ANOVA and Sidak’s multiple comparison test; **** p<0.0001; * p < 0.05; ns, not significant.
Decreased PECAM-1 expression in activated CD8+ T cells is regulated transcriptionally
The mechanism underlying loss of PECAM-1 from the T cell surface after activation is incompletely understood. There is evidence that, in activated human T cells, the PECAM-1 extracellular domain is cleaved, resulting in retention on the surface of a truncated form of PECAM-1 that contains a small portion of the extracellular domain, the transmembrane domain, and the cytoplasmic domain (35). Since the PECAM-1 cytoplasmic domain is responsible for its inhibitory function (3,36), such a truncated form of PECAM-1 may still be capable of inhibiting T cell responses. We therefore sought to determine whether downregulation of PECAM-1 expression in activated effector and memory CD8+ T cells is due to cleavage of the extracellular domain or is instead regulated at the level of transcription. To this end, CD8+ T cells were recovered from mice seven days after infection with LM-OVA and sorted into activated (CD44HiCD62LLo), memory (CD44HiCD62LHi), and naïve (CD44LoCD62LHi) populations. As shown in Figure 3A, PECAM-1 was expressed at low, intermediate and high levels on the surfaces of activated, memory, and naive CD8+ T cells, respectively (Figure 3A). Interestingly, Western blot analysis using an antibody specific for the PECAM-1 C-terminus detected only the full-length form of PECAM-1 (apparent MW = 130 kDa), which was present at low, intermediate, and high levels, respectively, in lysates of activated, memory and naïve CD8+ T cells (Figure 3B). In contrast, real-time PCR analysis of mRNA purified from the same populations revealed good correlation between PECAM-1 protein and mRNA levels, in that PECAM-1 mRNA levels were significantly higher in naïve cells relative to both memory and activated cells, and in memory cells relative to activated cells (Figure 3C). As an additional means to evaluate PECAM-1 mRNA levels, we probed a published microarray data set that evaluated gene expression in OVA-specific CD8+ T cells at various time points after infection with LM-OVA (27), and found good agreement between our data and the previously published analysis in that PECAM-1 mRNA levels declined during acute infection (Figure 3D, day 8), before becoming re-expressed at later time points to levels intermediate between naïve and acutely activated T cells (Figure 3D, day 45 and day 100). On the basis of these results, we conclude that transcriptional downregulation, and not extracellular domain cleavage, is responsible for decreased levels of expression of PECAM-1 in activated effector and memory CD8+ T cells.
Figure 3. Transcriptional down-regulation is responsible for decreased levels of PECAM-1 expression in activated effector and memory relative to naïve CD8+ T cells.
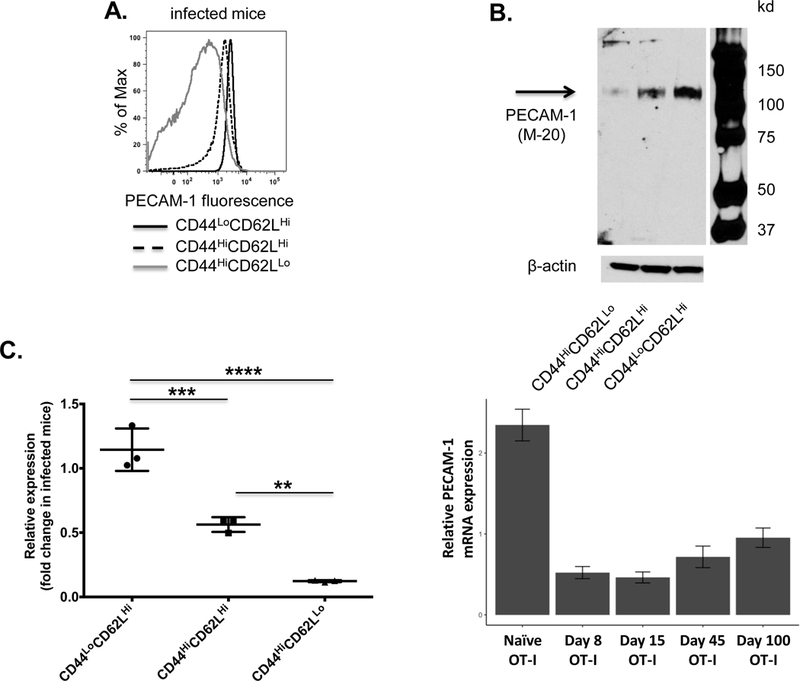
PECAM-1 surface expression, protein levels and transcript levels were measured in activated effector (CD44HiCD62LLo), memory (CD44HiCD62LHi), and naive (CD44LoCD62LHi) CD8+ cells purified by FACS from pooled spleen cells one week after infection of C57BL/6 mice with Listeria monocytogenes expressing ovalbumin. (A) Fluorescence intensity of PECAM-1 expression on the surface of activated effector, memory and naïve CD8+ T cells from one infected mouse is representative of six mice studied in four independent experiments. (B) Western blot analysis of total PECAM-1 in lysates of activated effector, memory and naïve CD8+ T cells purified from pooled splenocytes of fix infected mice is representative of two independent experiments. (C) Statistical analysis of changes in pecam-1 transcript levels in activated effector, memory, and naïve CD8+ T cells. Levels of pecam-1 transcripts in activated effector, memory, and naïve T cells purified from pooled splenocytes of infected mice (n=5), and in naïve CD8+ T cells purified from pooled splenocytes of uninfected mice (n=3), were determined by RT-PCR and normalized to β actin. Fold changes in pecam-1 transcript levels in activated effector, memory and naïve CD8+ T cells from infected mice relative to levels observed in naïve CD8+ T cells from uninfected mice were calculated. Symbols represent individual data points and lines represent mean fold change in relative pecam-1 expression ± SD in three independent experiments. Differences between means were determined by 1-way ANOVA and Tukey’s multiple comparison test; **p < 0.01, ***p < 0.001, ****p < 0.0001. (D) Relative expression of pecam-1 from published microarray data (27) in naive OT-I cells, as well as effector and memory OT-I cells at day 8, 15, 45, and 100 post infection with LM-OVA (mean +/− SEM).
PECAM-1 downregulation in activated CD8+ T cells is mildly affected by TCR ligand affinity
The extent of PECAM-1 downregulation during acute infection with LM-OVA was slightly more extensive in activated CD8+ T cells bearing TCRs specific for the high affinity H-2Kb/SIINFEKL complex (OVApos) than in activated CD8+OVAneg T cells, which presumably bear TCRs that recognize bacterial antigens with a broad range of affinities (Figures 1 & 2). This finding suggests that the strength of the signal emanating from the TCR may affect the magnitude of PECAM-1 downregulation in activated CD8+ T cells. To evaluate this possibility, we made use of OT-I mice, engineered to express TCRs in CD8+ T cells that are specific for the H2Kb/SIINFEKL complex (37). To vary peptide/MHC ligand affinity for the TCR, OT-I mice were infected with variant forms of LM-OVA that express SIINFEKL peptides with progressively lower affinities for the OT-I TCR due to amino acid substitutions at the fourth position of the peptide (N4>Q4>T4>V4) (37). Naïve T cells from CD45.2+OT-I mice were adoptively transferred into recipient CD45.1+ mice, after which mice were infected with the variant forms of LM-OVA. The level of PECAM-1 expression in activated CD45.2+ T cells was assessed 4 days after infection. As shown in Figure 4A, B, PECAM-1 downregulation in activated CD8+ T cells was equally efficient regardless of the form of LM-OVA with which mice were infected.
Figure 4. The extent of PECAM-1 down-regulation on activated effector CD8+ T cells in vivo is not affected by the affinity of the TCR for its ligand.
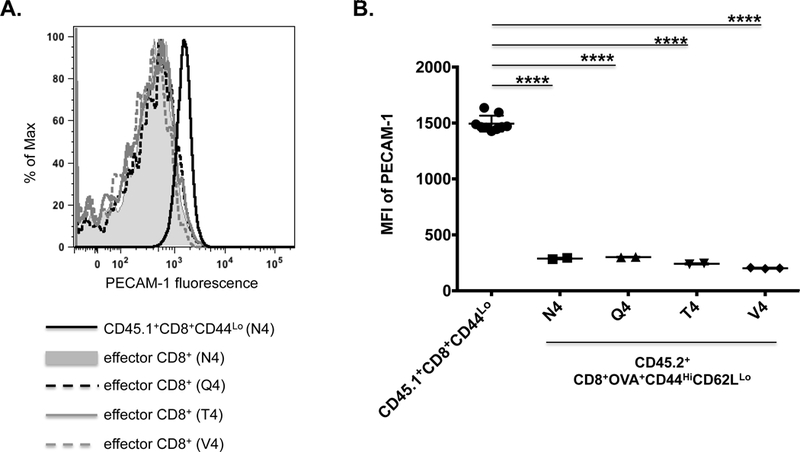
PECAM-1 expression in adoptively transferred CD45.2+ OT-I CD8+ T cells was assessed four days after infection of recipient CD45.1+ mice with Listeria monocytogenes expressing ovalbumin (LM-OVA) containing the native H-2Kb-restricted SIINFEKL peptide (N4), which has high affinity for the OT-I TCR, or its progressively less potent variants SIIQFEKL (Q4), SIITFEKL (T4), and SIIVFEKL (V4). (A) Fluorescence intensity of PECAM-1 expression on splenic CD45.2+ effector (CD44HiCD62LLo) CD8+ T cells from infected mice in one representative experiment; splenic CD45.1+ naïve (CD44Lo) CD8+ T cells from a mouse infected with the N4 variant of LM-OVA served as a control. (B) Statistical analysis of mean fluorescence intensity (MFI) of PECAM-1 expression on splenic CD45.2+ effector (CD44HiCD62LLo) CD8+ T cells from infected mice (n=2 for each variant) relative to CD45.1+ naïve (CD44Lo) CD8+ T cells from the same mice (n=8). Symbols represent individual data points and lines represent means ± SD. Differences between means were determined by 1-way ANOVA and Tukey’s multiple comparison test; ****p < 0.0001.
To ascertain the nature of the conditions responsible for PECAM-1 downregulation in CD8+ T cells, we exposed naïve OT-I-transgenic CD8+ T cells to different types of stimuli in vitro. To determine the requirements for co-stimulation, we cultured naïve CD8+ T cells from wild-type OT-I mice with αCD3 + αCD28 to mimic ligation of the TCR in the presence of co-stimulation, or with αCD3 alone to mimic TCR stimulation in the absence of co-stimulation. Stimulation of naïve CD8+ T cells for 24 hours with αCD3 + αCD28, but not αCD3 alone, resulted in significantly lower levels of expression of PECAM-1 on the surfaces of stimulated (CD69+) relative to unstimulated (CD69-) cells (Figure 5A, B). Stimulation with higher concentrations of αCD3 did not significantly reduce the level of PECAM-1 expression by CD8+ T cells, indicating that costimulation is necessary for PECAM-1 downregulation (Supplementary Figure 4). PECAM-1 downregulation induced by αCD3 + αCD28 at 24 hours was accompanied by detection of trace amounts of a truncated form of PECAM-1 in cell lysates (Supplementary Figure 5A) and significantly lower levels of expression of PECAM-1 mRNA (Supplementary Figure 5B), indicating the PECAM-1 cleavage and transcriptional downregulation are both early events after TCR ligation in the presence of costimulation. To determine the effect of TCR avidity for peptide/MHC ligands on PECAM-1 downregulation, we cultured naïve CD8+ T cells from wild-type OT-I mice with peptide/MHC complexes that encompassed native (N4) and variant forms (Q4, T4, and V4) of the SIINFEKL peptide, the latter of which have increasingly lower avidities for the OT-I TCR. We found that the native SIINFEKL peptide induced PECAM-1 down regulation to a similar extent relative to those observed with αCD3 + αCD28 and with peptide variants with lower avidities for the OT-I TCR (Fig. 5A,B). Finally, to further explore the effect of TCR signal strength on PECAM-1 downregulation, we obtained naïve CD8+ T cells from OT-I mice that were crossed with mice deficient in Diacylglycerol kinase ζ (DGKζ). DGKζ phosphorylates and enhances degradation of DAG, which is a second messenger generated in response to TCR ligation (38). In the absence of DGKζ, TCR signal strength is increased and the threshold for T cell activation is lowered (39). Interestingly, DGKζ deficiency abolished both the effect of CD28 co-stimulation, and the subtle effects of SIINFEKL peptide variant affinity, on the extent to which naïve CD8+ OT-I T cells downregulated PECAM-1 in response to stimulation via the TCR (Fig. 5C, D). Collectively, our studies of CD8+ T cells obtained from mice infected with LM-OVA in vivo (Figs. 3 and 4) and of those stimulated with antibodies or peptide/MHC complexes in vitro (Fig. 5) reveal that effector and effector memory cells generated from naïve CD8+ T cells that receive a costimulatory signal and even a low avidity signal from the TCR rapidly downregulate PECAM-1 both through a cleavage event, which results in transient expression of a truncated form of PECAM-1 on the cell surface, and suppression of gene transcription, which results in permanent downregulation of PECAM-1 expression. Memory precursor, transitional memory and central memory CD8+ T cells, in contrast, continue to express PECAM-1 at levels that are intermediate between naïve and effector CD8+ T cells.
Figure 5. PECAM-1 down-regulation on activated effector CD8+ T cells in vitro requires co-stimulation and is weakly affected by TCR ligand affinity.
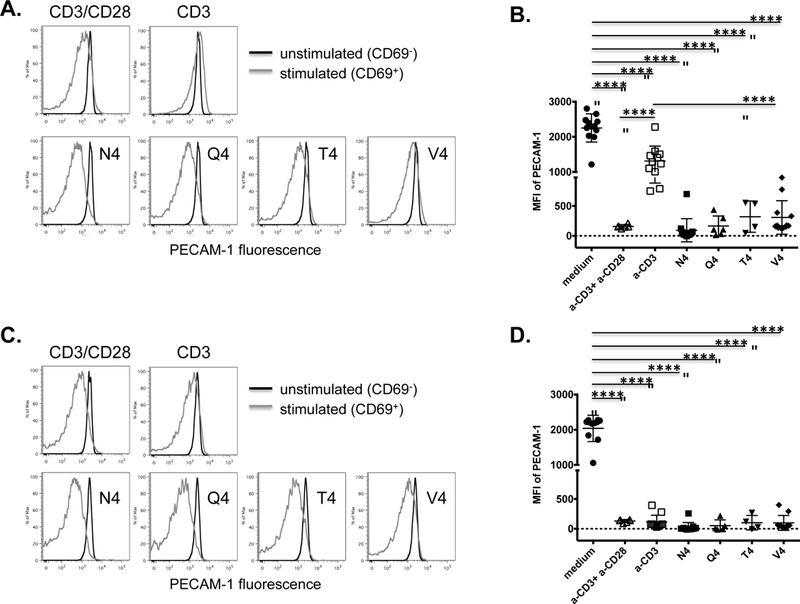
PECAM-1 expression on naïve OT-I CD8+ T cells from wild-type (A and B) or DGKζ−/− (C and D) mice was assessed 24 hours after in vitro stimulation, in the presence of antigen presenting cells, with anti-CD3 plus anti-CD28 (αCD3+αCD28), anti-CD3 alone (αCD3), or SIINFEKL peptide variants with progressively lower affinities for the OT-I TCR including native peptide (N4), SIIQFEKL (Q4), SIITFEKL (T4), and SIIVFEKL (V4). Cells cultured in medium alone served as a negative control. (A and C) Fluorescence intensity of PECAM-1 expression on unactivated (CD69-) and activated (CD69+) cells from a wild-type (A) or DGKζ−/− (C) mouse in one representative experiment. (B and D) Statistical analysis of mean fluorescence intensity (MFI) of PECAM-1 expression on stimulated cells from wild-type (B) or DGKζ−/− (D) mice relative to unstimulated cells cultured in medium alone (n=4–11 per condition). Symbols represent individual data points and lines represent means ± SD from at least four independent experiments. Differences between means were determined by 1-way ANOVA and Tukey’s multiple comparison test; ****p < 0.0001.
PECAM-1 is expressed on human CD8+, but not CD4+, T cells that co-express markers of prior activation
In humans, expression of different CD45 isoforms differentiates naïve T cells, which express CD45RA but not CD45RO (CD45RO-), from activated/memory T cells, which express CD45RO (CD45RO+) but not CD45RA (40). Additionally, in healthy human volunteers, the inhibitory cell surface receptor PD-1 represents an additional robust marker of prior activation (41). Consistent with these past observations, we found that all CD45RO+ cells expressed either intermediate or high levels of PD-1 (Fig. 6A). As reported in past studies evaluating CD4+ T cells, we detected PECAM-1 expression on CD45RO- but not CD45RO+CD4+ T cells (Fig. 6B). In contrast, PECAM-1 was expressed on both CD45RO- and CD45RO+CD8+ T cells, as assessed by flow cytometry using antibodies specific for either the membrane proximal Ig domain 6 (IgD6) (Fig. 6B) or the membrane distal IgD1 (Supplementary Fig. 6). Of note, the IgD6-specific (Fig. 6B), but not the IgD1-specific (Supplementary Fig. 6), antibody detected higher levels of PECAM-1 on CD45RO- compared to CD45RO+ CD8+ T cells, suggesting that naïve, but not activated/memory, human CD8+ T cells express a form of PECAM-1 that possesses membrane-proximal IgD6 and either lacks, or is not recognizable by an antibody specific for, membrane-distal IgD1. Interestingly, in CD45RO+CD8+ cells, the level of PECAM-1 expression was affected by the extent of prior activation in that cells with the highest level of PD-1 expressed PECAM-1 at higher levels than did those with the lowest levels of PD-1 expression (Fig. 6C). These data indicate that, similar to mice, expression of PECAM-1 differs between CD4+ and CD8+ T cell subsets in humans. To determine whether PECAM-1 mRNA expression varies among human CD8+ T cell subsets, we probed a microarray data set generated by others from sorted populations of naïve, central memory, and effector memory CD8+ T cells (29). Consistent with our results from flow cytometry, we observed expression of PECAM-1 mRNA in memory CD8+ T cell data sets at levels that were slightly but significantly less than those observed in naïve CD8+ T cell populations (Fig. 6D). This expression pattern was in contrast to Granzyme B, which was only expressed in effector memory subsets, or L-selectin (CD62L), which was only expressed in naïve and central memory subsets (data not shown). Furthermore, there was a statistically insignificant trend towards increased expression of PECAM-1 mRNA in human central memory T cells relative to effector memory T cells (Fig. 6D), which is consistent with our observation that PECAM-1 is expressed at higher levels in central memory relative to effector memory CD8+ T cell subsets in mice (Figure 2). Differences in magnitude of PECAM-1 expression levels between central memory and effector memory CD8+ T cells in humans relative to mice may be attributable to assessment of mRNA versus protein levels, respectively, or because the effector memory population generated in murine experiments resulted from a recent infection.
Figure 6. PECAM-1 is expressed at high levels in activated CD8+ but not CD4+ human T cells.
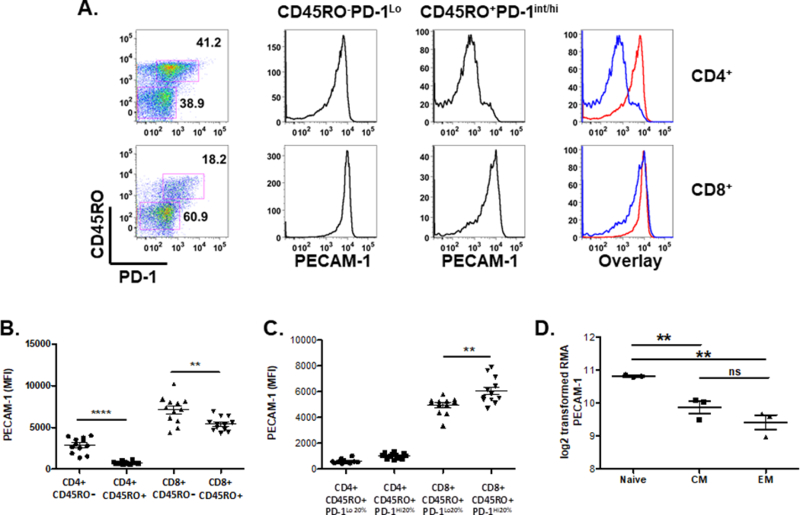
Buffy coat cells from healthy blood donors were evaluated for expression of PECAM-1 in naïve (CD45RO-PD-1Lo/Int) and activated (CD45RO+PD-1Int/Hi) CD4+ and CD8+ T cells. (A) Left panel identifies gating strategy for CD45RO-PD-1Lo/Int and CD45RO+PD-1Int/Hi subgroups in CD4+ (top panel) and CD8+ (lower panel) T cells. Middle panels demonstrate representative histograms of PECAM-1 expression from indicated gates, with an overlay of CD45RO-PD-1Lo/Int (red) and CD45RO+PD-1Int/Hi (blue) depicted in far right panel. (B) Average mean fluorescence intensity (MFI) of PECAM-1 expression was pooled from 12 donor samples from gates depicted in (A). (C) CD45RO+ CD4+ or CD45RO+CD8+ T cells from (A) were distributed into quintiles based on PD-1 expression, and MFI of PECAM-1 was calculated from cells within the lowest quintile (PD-1Lo20%) or highest quintile (PD-1Hi20%) of PD-1 expression. (B and C) **p < 0.01, ****p < 0.0001. (D) PECAM-1 mRNA expression was probed from a microarray dataset (GEO series GSE23321) of sorted human naïve, central memory (CM), and effector memory (EM) T cells from three healthy donors (29). **p<0.01, ns – not significant. ***P < 0.004, ****P < 0.0001.
In sum, these studies demonstrate that PECAM-1 expression is regulated differently in CD8+ T cells than in CD4+ T cells in that, unlike memory CD4+ T cells, memory precursor and central memory CD8+ T cells continue to express PECAM-1. This suggests that therapeutic targeting of PECAM-1 to relieve T cell inhibition will impact both naïve and memory CD8+ T cell subsets. Future experiments will discern the relative importance of PECAM-1 inhibition of specific CD8+ T cell subsets toward regulating overall immune responses.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (D.K.N., P.J.N., M.J.R.) and an American Society for Hematology Bridge Award (M.J.R.). D.S, is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM080202. This work benefitted from data assembled by the ImmGen consortium (42).
Abbreviations
- PECAM-1
Platelet Endothelial Cell Adhesion Molecule-1
- TCR
T cell receptor
- TGFβ
Transforming growth factor beta
- DGKζ
diacylglycerol kinase zeta
Footnotes
Conflict of Interest Disclosure: The authors declare no competing financial interests.
References
- 1.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol 2015. April;33:23–35. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Sznol M. Therapeutic combinations of immune-modulating antibodies in melanoma and beyond. Semin Oncol 2015. June;42(3):488–94. [DOI] [PubMed] [Google Scholar]
- 3.Newman DK, Fu G, Adams T, Arumugam V, Bluemn T, Riese MJ. An unexpected role for PECAM-1 in TGF beta-mediated inhibition of T cell function. Science Signaling [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol 1999. May 15;162(10):5813–20. [PubMed] [Google Scholar]
- 5.Gajewski TF, Fallarino F, Fields PE, Rivas F, Alegre ML. Absence of CTLA-4 lowers the activation threshold of primed CD8+ TCR-transgenic T cells: lack of correlation with Src homology domain 2-containing protein tyrosine phosphatase. J Immunol 2001. March 15;166(6):3900–7. [DOI] [PubMed] [Google Scholar]
- 6.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell 2009. July 10;138(1):30–50. [DOI] [PubMed] [Google Scholar]
- 7.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Möwes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med 2002. March 18;195(6):789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere J-C, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol 2007. November;37(11):3270–80. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol 2008. February 1;180(3):1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines CJ, Giffon TD, Lu L-S, Lu X, Tessier-Lavigne M, Ross DT, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med 2009. February 16;206(2):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood 2009. January 22;113(4):769–74. [DOI] [PubMed] [Google Scholar]
- 12.Hassan J, Reen DJ. Human recent thymic emigrants--identification, expansion, and survival characteristics. J Immunol 2001. August 15;167(4):1970–6. [DOI] [PubMed] [Google Scholar]
- 13.Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008. July 7;205(7):1701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka K-I, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest 2010. May;120(5):1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Broek T, Delemarre EM, Janssen WJM, Nievelstein RAJ, Broen JC, Tesselaar K, et al. Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J Clin Invest 2016. March 1;126(3):1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringhoffer S, Rojewski M, Döhner H, Bunjes D, Ringhoffer M. T-cell reconstitution after allogeneic stem cell transplantation: assessment by measurement of the sjTREC/βTREC ratio and thymic naive T cells. Haematologica 2013. October;98(10):1600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greinix HT, Kuzmina Z, Weigl R, Körmoczi U, Rottal A, Wolff D, et al. CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2015. February;21(2):250–8. [DOI] [PubMed] [Google Scholar]
- 18.Choi SW, Gatza E, Hou G, Sun Y, Whitfield J, Song Y, et al. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood 2015. January 29;125(5):815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheible KM, Emo J, Yang H, Holden-Wiltse J, Straw A, Huyck H, et al. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin Immunol 2015. December;161(2):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier FM, Tang MLK, Martino D, Saffery R, Carlin J, Jachno K, et al. The ontogeny of naïve and regulatory CD4(+) T-cell subsets during the first postnatal year: a cohort study. Clin Transl Immunology 2015. March;4(3):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmiedeberg K, Krause H, Röhl F-W, Hartig R, Jorch G, Brunner-Weinzierl MC. T Cells of Infants Are Mature, but Hyporeactive Due to Limited Ca2+ Influx. PLoS ONE 2016;11(11):e0166633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fike AJ, Nguyen LT, Kumova OK, Carey AJ. Characterization of CD31 expression on murine and human neonatal T lymphocytes during development and activation. Pediatr Res 2017. April 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, et al. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med 1992. July 1;176(1):245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockinger H, Schreiber W, Majdic O, Holter W, Maurer D, Knapp W. Phenotype of human T cells expressing CD31, a molecule of the immunoglobulin supergene family. Immunology 1992. January;75(1):53–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, et al. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 1999. March 1;162(5):3022–30. [PubMed] [Google Scholar]
- 26.Zhong X-P, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol 2003. September;4(9):882–90. [DOI] [PubMed] [Google Scholar]
- 27.Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol 2013. April;14(4):404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010. October 1;26(19):2363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011. September 18;17(10):1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condotta SA, Richer MJ, Badovinac VP, Harty JT. Probing CD8 T cell responses with Listeria monocytogenes infection. Adv Immunol 2012;113:51–80. [DOI] [PubMed] [Google Scholar]
- 31.Khan SH, Badovinac VP. Listeria monocytogenes: a model pathogen to study antigen-specific memory CD8 T cell responses. Semin Immunopathol 2015. May;37(3):301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, et al. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol 1990. December 1;145(11):3889–97. [PubMed] [Google Scholar]
- 33.Torimoto Y, Rothstein DM, Dang NH, Schlossman SF, Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol 1992. January 15;148(2):388–96. [PubMed] [Google Scholar]
- 34.Serbina N, Pamer EG. Quantitative studies of CD8+ T-cell responses during microbial infection. Curr Opin Immunol 2003. August;15(4):436–42. [DOI] [PubMed] [Google Scholar]
- 35.Fornasa G, Groyer E, Clement M, Dimitrov J, Compain C, Gaston A-T, et al. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol 2010. May 15;184(10):5485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci 2013. June 1;126(Pt 11):2343–52. [DOI] [PubMed] [Google Scholar]
- 37.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature 2009. March 12;458(7235):211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishna S, Zhong X. Role of diacylglycerol kinases in T cell development and function. Crit Rev Immunol 2013;33(2):97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riese MJ, Grewal J, Das J, Zou T, Patil V, Chakraborty AK, et al. Decreased diacylglycerol metabolism enhances ERK activation and augments CD8+ T cell functional responses. J Biol Chem 2011. February 18;286(7):5254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol 1998;16:201–23. [DOI] [PubMed] [Google Scholar]
- 41.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol 2011. April 1;186(7):4200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heng TSP, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 2008. October;9(10):1091–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


