Abstract
Biologists have long marveled at the ability of planarian flatworms to regenerate any parts of their bodies in just a little over a week. While great progress has been made in deciphering the mechanisms by which new tissue is formed at sites of amputation, we know relatively little about the complementary remodeling response that occurs in uninjured tissues to restore anatomical scale and proportion. This review explores the mysterious biology of this process, first described in hydra by the father of experimental zoology, Abraham Trembley, and later termed ‘morphallaxis’ by the father of experimental genetics, Thomas Hunt Morgan. The perceptive work of these early pioneers, together with recent studies using modern tools, has revealed some of the key features of regenerative tissue remodeling, including repatterning of the body axes, reproportioning of organs like the brain and gut, and a major increase in the rate of cell death. Yet a mechanistic solution to this longstanding problem in the field will require further study by the next generation of planarian researchers.
Keywords: Regeneration, Planarian, Epimorphosis, Morphallaxis
1. INTRODUCTION
If I have seen further it is by standing on the shoulders of giants
- Isaac Newton, 1675
The capacity of stem cells to produce new tissue, and their attendant clinical potential, has emerged as a major focus of modern developmental biology. In the field of regeneration, many basic scientists study adult stem cells and their roles in naturally occurring regenerative phenomena, while translational researchers and clinicians seek to harness this biology to repair tissues and organs damaged by injury or disease. It is rather ironic, then, that the field of regeneration was first established by work on a far less heralded way of replacing lost body parts that has more to do with how differentiated cells respond to amputation.
Abraham Trembley was born in Geneva, Switzerland in 1710. While tutoring students in natural history, he came across the polyp, hydra, in a sample of pond water. Unaware of their discovery by Antoni van Leeuwenhoek some 40 years earlier, Trembley set out to determine whether these “new” organisms were animals or plants. His careful observations of their movements and feeding behavior led him to believe they were animals, yet he also noticed the polyps had differing numbers of arms, which reminded him of the variability in the branches and roots of plants. He eventually decided to cut the polyps in half, reasoning that each piece might give rise to a new polyp if they were plants, but would die if they were animals. To his surprise, not only did each half survive and give rise to a fully formed individual, but smaller fragments did the same. Despite these unexpected results, Trembley remained convinced that hydras were animals and therefore proposed that regeneration might not be restricted to the known examples previously described in the plant kingdom [1,2].
Trembley went on to conduct many variations on his initial experiments, thus launching the era of experimental zoology (and sealing the demise of a wide range of animals subjected to all manner of amputations for centuries to come). The detailed descriptions in his memoirs, published in 1744 [1], evidence his meticulous observational skills, but an in-depth theoretical analysis of his work would not come until much later, when other scientists began asking how small fragments of invertebrates like hydra regain the form and function of the animals from which they are derived. In this regard, the work of the American geneticist Thomas Hunt Morgan at the turn of the twentieth century represented a key chapter in the history of the field.
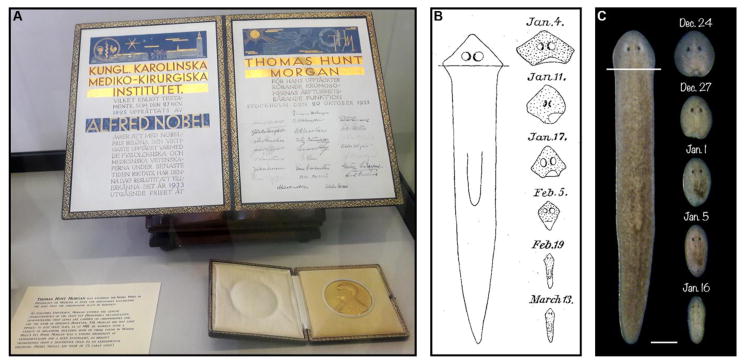
Most well-known for his work on Drosophila that confirmed the chromosomal theory of inheritance (Figure 1A), Morgan also studied various species of planarians earlier in his career [3,4]. These free-living members of the phylum Platyhelminthes (the flatworms) have a substantially more complex anatomy than hydra, with derivatives of all three germ layers, including an epidermis, a brain, a pair of ventral nerve cords, light-sensing photoreceptors, body wall and enteric muscles, a pharynx, a highly branched gut, and a protonephridial system that filters fluid waste. Despite this relative anatomical complexity, planarians share hydra’s remarkable ability to regenerate entire individuals from small body fragments [4–8]. In fact, Morgan showed fragments less than 1/100th an animal’s original size could form complete new, albeit much smaller, planarians [9].
Figure 1. T. H. Morgan’s concept of morphallaxis.
(A) Morgan’s 1933 Nobel Prize in Physiology or Medicine (for his work on the chromosomal theory of heredity), photographed during the 2017 North American Planarian Meeting at the Marine Biological Laboratory in Woods Hole, Massachusetts. Morgan conducted summer research at the MBL for much of his career. (B) An 1898 illustration from Morgan’s analysis of regenerative tissue remodeling in Planaria maculata (adapted from [22]). Note the gradual narrowing and elongation of the head fragment over time, as anatomical scale and proportion are restored. (C) Reproduction of the experiment using a similar amputation scheme in Schmidtea mediterranea. Scale bar = 500 μm.
Like Trembley before him, Morgan conducted nearly every imaginable amputation and carefully documented the results. Unlike Trembley, he also thought and wrote extensively about what those results might indicate regarding the underlying mechanisms of regeneration. He noted that the regenerative response in planarians encompassed both an “addition of new material at the cut-end” and a “change in the form of the old part” [10]. The former process resembled accounts of limb regeneration in organisms like salamanders, in that it involved the formation of what is now called a ‘blastema,’ or mass of undifferentiated tissue – easily identifiable by its initial lack of pigmentation – at the wound site [11]. The latter process, on the other hand, was reminiscent of the regenerative response Trembley had described in hydra. It was this striking transformation of uninjured tissues that occupied much of Morgan’s attention, perhaps because it related to the ongoing debate amongst his contemporaries Wilhelm Roux, Hans Driesch, and others over regulative (conditional) vs. mosaic (autonomous) modes of embryonic development [3,12]. Was developmental cell fate fixed or plastic? Regeneration might provide an alternative context in which to address this important question. In any event, Morgan emphasized the distinction between the kind of regeneration observed in salamander limbs and remodeling of existing structures in organisms like hydra and planarians in his seminal 1901 work, Regeneration, by proposing new terms for each process:
I propose to call those cases of regeneration in which a proliferation of material precedes the development of the new part, “epimorphosis.” The other mode, in which a part is transformed directly into a new organism, or part of an organism without proliferation at the cut-surfaces, “morphallaxis” [10].
We now know that purely epimorphic or morphallactic regeneration are unlikely to occur in nature. For instance, limb regeneration is often described as epimorphic, yet amputation triggers re-specification of positional identities along the proximal-distal axis in the preexisting tissue, in conjunction with blastema formation [13]. Similarly, it has recently been shown that two waves of cell division contribute to regeneration in the classic morphallactic model, hydra [14,15]. There are also clear mechanistic and morphological differences between regenerative phenomena typically grouped together under one heading or the other; not all proliferation produces a blastema, for example [16,17]. In light of these considerations, it can be misleading to use Morgan’s terminology when referring to a regenerative process in its entirety [17–20]. Nevertheless, I believe there is justification for continued use of the classic terms, particularly if we allow for a revision to the definition of ‘morphallaxis.’
At face value, Morgan’s distinction is based solely on the presence or absence of cell division, but a closer examination of his work reveals a broader perspective. The cell biology of regeneration was not well understood in Morgan’s time, and his primary concern was whether regeneration of lost body parts resulted from the formation of new tissue or changes to existing (old) tissue. This issue is of course linked to cell division, but is not equivalent. Morgan was particularly interested in the restoration of anatomical scale and proportion achieved through remodeling of existing body parts (Figure 1B), and returned to this topic repeatedly in his writing. For example:
If a planarian is cut into a number of cross-pieces each piece develops new tissue at its anterior and posterior ends, and at the same time there is a corresponding loss in the material of the old part. The new material at the anterior end very early differentiates into a new head, and the new material at the posterior end makes a new tail. The new worm is much too broad for its length, i.e., it lacks the proportions of length to breadth characteristic of the normal worms, either young or old. In the course of a few weeks changes take place in the worm that remodel it into the characteristic form [21].
If the changes are carefully followed it will be noticed that the new material that is first laid down to form the new head and tail does not increase very much in length, at least not sufficiently to account for the greater part of the entire change in length. The old part on the other hand soon becomes longer than it was at first, and at the same time narrower [21].
In other words, while Morgan clearly saw cell division as a central feature of epimorphosis, passages like these suggest he viewed morphallaxis more in terms of its functional outcome (reproportioning) than what cellular mechanisms it might have involved, much less lacked. Indeed, his first published use of the term in 1898 made no mention of cell division, referring only to a “process of transformation” through which “the relative proportions of the planarian are attained by a remodelling of the old tissue” [22].
Given this context, I propose modifying Morgan’s 1901 definition of morphallaxis by deleting the phrase “without proliferation at the cut-surfaces” and adding a reference to the restoration of scale and proportion in existing tissues (Table 1). This would accommodate recent data indicating that some aspects of tissue remodeling include, or even require, cell division (see below). It would also more clearly reflect Morgan’s focus on the anatomical features of the process, preserving a one-word term for regenerative tissue remodeling that recognizes his pivotal contributions to the field. It is this revised meaning that is intended when the term is used in the remainder of this review.
Table 1. The terminology of regeneration.
Proposed revisions to Morgan’s 1901 definitions for epimorphosis and morphallaxis are intended to: 1) convey that stem or progenitor cells (including those formed via dedifferentiation in some organisms) are the typical source of the “proliferation of material” that forms new parts [82]; 2) accommodate recent observations that at least some tissue remodeling processes include cell division; and 3) emphasize the functional outcomes of morphallaxis, as Morgan did in most of his writing on the subject. Examples of regenerative responses in planarians are intended to illustrate primarily epimorphic or morphallactic processes. PCGs = positional control genes. See text for details.
| Epimorphosis | Morphallaxis | |
|---|---|---|
| Original definition T. H. Morgan, 1901 [10] | Regenerative process “in which a proliferation of material precedes the development of the new part” | Regenerative process “in which a part is transformed directly into a new organism, or part of an organism without proliferation at the cut-surfaces” |
| Proposed revision | Regenerative process in which proliferation of stem/progenitor cells leads to the development of a new part | Regenerative process in which anatomical patterning, scale, and proportion are restored through remodeling of an existing part |
| Examples in planarians |
|
|
That epimorphosis and morphallaxis often coincide – temporally, and to a degree also spatially – by no means diminishes their conceptual significance. Just as conditional and autonomous cell fate specification function together during embryonic development, so to do addition of new tissues and remodeling of existing tissues during regeneration. Morgan himself acknowledged that “the two processes are not sharply separated, and may even appear combined in the same form” [10].
In summary, I would argue Morgan’s distinction between epimorphosis and morphallaxis remains a useful theoretical construct, particularly if we update the definitions of these terms to account for recent observations. The growth of the stem cell field has led to an increased focus on, and consequently better understanding of, epimorphosis. A key challenge for the future will be to develop a corresponding level of insight into the cellular and molecular mechanisms of morphallaxis. Toward that end, I review what we already know about the process in planarians and discuss some key unanswered questions below.
2. FEATURES OF PLANARIAN MORPHALLAXIS
In these planarians the results are somewhat complicated
- T.H. Morgan, Regeneration, 1901
Morgan’s work raised two logical questions, only one of which has been adequately addressed in the more than 100 years that have passed since Regeneration was published: 1) What is the source of the “proliferation of material” that drives epimorphosis? 2) How are existing tissues transformed by morphallaxis? We now know planarians possess a large population of somatic stem cells, or ‘neoblasts,’ that respond to amputation by increasing their rate of division and migrating to the wound site, where they give rise to the blastema (reviewed in [23], this issue). As discussed below, we have also identified and characterized some of the major changes that occur during morphallaxis, but how those changes effect the kinds of physical transformations Morgan so painstakingly documented remains an unsolved mystery.
2.1 Repatterning the Body Axes
The planarian body plan exhibits polarity along the anterior-posterior (AP), dorsoventral (DV), and mediolateral (ML) axes. During regeneration, new cells produced via epimorphosis must adopt the correct axial fates (e.g., cells in an anterior-facing blastema should form head rather than tail structures), while existing tissues must alter – sometimes drastically – their positional identities to match their new anatomical locations (e.g., many of the tissues in a small head fragment like the ones in Figure 1B,C must acquire posterior fates). How, then, are the body axes reset in response to amputation?
The field has made significant progress in this area over the last decade. Several key signaling pathways – Wnt, FGF, BMP, and Hedgehog (Hh) – regulate the patterning of adult tissues during both homeostasis and regeneration [24–27]. Importantly, some of the genes encoding components of these pathways display regionalized expression along one or more body axes. Together, these so-called ‘positional control genes’ (PCGs) are thought to comprise a kind of coordinate system that provides a positional frame of reference for both stem cells and differentiated cells throughout the body [28]. The importance of this system is illustrated by the dramatic homeotic-like transformations that arise when some PCGs are knocked down by RNA interference (RNAi), such as heads developing at posterior-facing wounds instead of tails [25,29,30].
Intriguingly, PCGs are expressed in the body wall muscles, revealing simultaneous contractile and signaling roles for this tissue [28,31]. Following amputation, muscle fibers rapidly alter their PCG expression profiles to reestablish a complete coordinate system. This encompasses two distinct responses. Some genes are induced at wound sites, while others adopt a more restricted expression pattern, recreating a gradient along a given body axis with peak expression distal to the plane of amputation. For example, the Wnt family gene wntP-2/wnt11-5 exhibits enriched expression in the posterior of intact animals, whereas the putative Wnt antagonist sFRP-1 is expressed at the anterior pole [32,33]. In a tail fragment generated by a transverse amputation posterior to the pharynx, sFRP-1 is induced at the wound site (the future anterior), whereas the wntP-2/wnt11-5 expression domain contracts, restoring the posterior-to-anterior gradient that existed prior to amputation (Figure 2A). Similar scenarios have been described for PCGs that exhibit polarized expression along the DV and/or ML axes and reset in response to lateral amputations [25,34–38]. Because these changes occur within the first several days of regeneration, normal PCG expression patterns are largely reestablished prior to the extensive physical remodeling of the fragment that must subsequently occur to restore anatomical scale and proportion (see below).
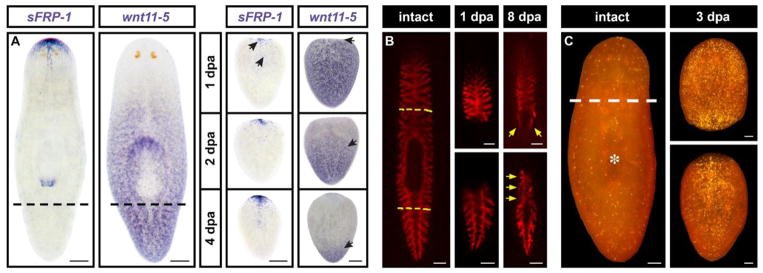
Figure 2. Features of morphallaxis in planarians.
(A) Changes in PCG expression. sFRP-1 is an anterior marker; wnt11-5 (also called wntP-2) exhibits graded expression along the AP axis, with higher levels toward the posterior. Tail fragments activate
sFRP-1 expression at the anterior pole within 1 day post-amputation (dpa; arrows). wnt11-5 expression retracts toward the posterior (and later expands to reach the anterior end of the pharynx). Adapted from [33]. (B) Gut remodeling. The gut was labeled with a fluorescent dextran prior to amputation, and head and tail fragments were photographed 1 and 8 days later. Note the extensive contribution of existing anterior gut tissue to the newly formed posterior branches and vice versa (arrows). Adapted from [61]. (C) Systemic cell death response, visualized by whole-mount TUNEL. Asterisk denotes position of the pharynx. The magnitude of the increase in cell death following amputation is proportional to the amount of tissue removed. Adapted from [76]. Scale bars: A = 200 μm; B = 250 μm; C = 100 μm.
It is notable that a subset of amputation-induced changes in PCG expression still take place after the stem cell population has been ablated by irradiation [28,32–34]. This shows initial repatterning of the body axes occurs independently of epimorphosis (some later changes in gene expression are radiation-sensitive, presumably reflecting the acquisition of positional identity by differentiating neoblast division progeny). If one views “without proliferation” as synonymous to “independent of proliferation,” axial repatterning formally meets Morgan’s 1901 criteria for morphallaxis. In any case, this component of the remodeling response is clearly separable from the formation of new body parts, underscoring the conceptual importance of the epimorphosis-morphallaxis distinction.
2.2 Restoring Scale and Proportion
In planarian species such as Schmidtea mediterranea and Dugesia japonica, the size of adult animals can vary by more than an order of magnitude [22,39–43]. This reflects not only differences in regenerative status – small body fragments are regularly formed by fissioning for asexual reproduction – but also variability in nutrient intake. During extended periods of starvation, animals can shrink or ‘degrow’ (reviewed in [44], this issue). Growth, and eventual fissioning, resumes with feeding. Remarkably, anatomical proportions are maintained even as animal size changes, indicating that tissues and organs can be resized and, if necessary, reshaped in response to multiple external cues (i.e. tissue loss or altered nutrient levels).
Body mass is predominantly a function of cell number rather than cell size in the freshwater planarian species analyzed to date [39–43]. In situ hybridization and immunostaining approaches have allowed for the labeling and quantification of specific cell types in whole-mounted animals. These analyses have generally revealed a linear relationship between the number of cells expressing a given marker and body length, suggesting planarians are not only able to “count” a wide variety of differentiated cell types, but can also increase or decrease their numbers on demand in order to maintain or restore proportionality [41,42]. The mechanisms underlying this plasticity are not well understood. However, elegant studies of morphallaxis in the brain and gut have yielded some valuable insights, as described below.
2.2.1 Remodeling the Brain
The planarian brain is a bi-lobed structure composed of glia and multiple neuronal subtypes that exhibits a high rate of physiological cell turnover [45–49]. When an animal is subjected to a transverse, cephalic amputation (see Figure 1B,C), the resulting trunk/tail fragment generates a new brain via epimorphosis, while the head fragment must reduce the size of the existing brain through morphallaxis to restore scale and proportion.
Hill and Petersen recently identified a Wnt signaling pathway that exerts control over the number of neurons in the regenerating brain [50]. Specifically, they determined the Wnt family member wnt11-6 and secreted Wnt inhibitor notum form a negative feedback loop that controls brain size during both epimorphosis and morphallaxis. RNAi knockdown of wnt11-6 increased the numbers of cholinergic, GABAergic, and putative chemosensory neurons in both head and trunk fragments, whereas notum knockdown had the opposite effect. Intriguingly, stem cell ablation abrogated these phenotypes in remodeling head fragments (the effects on epimorphosis could not be assessed because irradiated animals are unable to form a blastema). Thus, despite the fact that remodeling of the head entails a reduction in the brain:body ratio, these results support the somewhat surprising conclusion that wnt11-6 and notum regulate brain size through an effect on the generation of new brain cells (a finding further supported by BrdU labeling data) [50]. In other words, new neurons are formed during remodeling of the existing brain as well as de novo brain formation in the blastema, and perturbation of neurogenesis disrupts brain:body proportion in both contexts. This illustrates the need for a revised definition of morphallaxis (see Introduction), as transformation of the existing part clearly entails some degree of cell division.
Other studies have revealed additional regulators of brain size. RNAi knockdown of the Hippo signaling pathway effector yorkie leads to the same kind of increase in the brain:body ratio as wnt11-6 knockdown in remodeling head fragments [50,51]. However, this is only one of a broad spectrum of phenotypes observed in yorkie(RNAi) animals (including other morphallaxis defects – see below); these may be a secondary consequence of a hyperactivated transcriptional response to wounding, whereas the wnt11-6/notum feedback loop appears to play a more specific role in controlling brain proportion [50–52]. Canonical Wnt, FGF, BMP, and Hh signaling pathways have also been shown to regulate various aspects of anterior patterning, neurogenesis, and brain development during homeostasis and epimorphic regeneration, though their roles in morphallaxis are not as well characterized [25,27,29,30,53,54].
2.2.2 Remodeling the Gut
The planarian gut has a highly branched morphology enabling distribution of metabolites throughout the body [55]. Thus, it partly assumes the function of the vasculature in more complex organisms, and is accordingly referred to as the gastrovascular system. A single, primary anterior branch joins two primary posterior branches just in front of the pharynx. Secondary, tertiary, and quaternary branches extend outward from the primary branches. The gut is composed of absorptive phagocytes and secretory goblet cells; these form a single epithelial layer surrounded by a basal lamina and enteric muscle [56–61].
Following amputation, the remaining branch(es) of the gut are extensively remodeled to restore gastrovascular form and function. In head fragments, the single anterior branch extends and diverges to form two new posterior branches. In tail fragments, the posterior branches converge at the midline in front of the pharynx to establish a new anterior branch. Elongation of both new and existing branches is accompanied by branching morphogenesis to elaborate the normal intestinal morphology [33,61]. Until recently, it was unclear to what extent these aspects of the regenerative response are driven by reorganization of existing, differentiated cells vs. formation of new tissue in the preexisting gut through stem cell division. Forsthoefel, Park, and Newmark took a clever approach to address this problem [61]. They found intestinal phagoyctes ingest and retain fluorescent dextrans. By feeding a red dextran prior to amputation, and then feeding a green dextran 10 days after amputation (when regeneration was complete), they were able to distinguish cells that existed prior to injury (those with red and green fluorescence) from newly differentiated cells generated via stem cell division (those with green fluorescence only). As expected, gut branches in the blastema were only labeled green, indicating they were comprised entirely of newly differentiated cells. Outside the blastema (i.e. in tissue that underwent morphallactic regeneration), it was clear that the preexisting (red) posterior branches had contributed extensively to formation of the anterior gut, and vice versa (Figure 2B) [61]. However, irradiation severely delayed or blocked gut remodeling in both head and tail fragments, consistent with results from a prior study [33,61]. Taken together, these observations indicate that regeneration of the gastrovascular system entails a combination of epimorphosis and morphallaxis. The addition of new gut branches in the blastema clearly qualifies as epimorphosis. Remodeling of existing gut branches, while morphallactic with respect to the reorganization of uninjured tissue, could also be described as epimorphic in that it involves substantial incorporation of stem cell-derived, new tissue. Morgan would likely have viewed this as an example of both phenomena being “combined in the same form.”
Several studies have identified genes controlling growth and remodeling of the gastrovascular system during tissue homeostasis and regeneration. An RNAi screen revealed roles for the tropomyosin family member tpm-1 and the Rho-family GTPase rho-A in branching morphogenesis [62]. While both genes are putative regulators of the actin cytoskeleton, the tpm-1(RNAi) and rho-A(RNAi) phenotypes are specific to uninjured animals and regenerating tail fragments, respectively, hinting at unexpected complexity (Morgan’s above observation not withstanding) in the associated regulatory networks. RNAi knockdown of the EGF receptor egfr-1 or its putative ligand nrg-1 also disrupts elongation and branching of the gut during regeneration. However, these phenotypes are apparently secondary to defects in the differentiation of gut progenitors, providing further evidence for the importance of new tissue production during gut remodeling [63]. Additional regulators of the gut lineage include the nuclear receptor hnf4 and transcription factors gata4/5/6 and nkx2.2 [64–66]. Planarian raf and mek homologs have also been implicated in control of gut branching [67]; these genes play key roles in EGF signal transduction, raising the possibility they function downstream of egfr-1 and nrg-1. Finally, RNAi knockdown of the novel gene phred-1 interferes with the regeneration of both muscle and gut. Given that similar defects were observed following knockdown of the transcription factor myoD (a central regulator of muscle differentiation), these data raise the interesting hypothesis that muscle forms a kind of scaffold necessary for gastrovascular remodeling [68].
2.3 Pharynx Regeneration
The pharynx is a complex, cylindrical organ that connects to the gut, providing a route for both ingestion of food and excretion of solid waste [69]. It has long been known that head and tail fragments devoid of a pharynx regenerate a new one de novo, yet it is also clear this happens outside the blastema [21,70]. Is this epimorphosis or morphallaxis? A closer look at the anatomy of the organ argues for the involvement of both processes, though not in the same way as occurs in the gut. The pharynx is an appendage that can be extended into the external environment, but is normally contained within a body cavity referred to as the ‘pharyngeal pouch.’ Regeneration necessitates remodeling of the gut branches and overlying epidermis to reestablish the pouch [33,61], and in this sense, can be considered morphallactic. However, multiple lines of evidence indicate the organ itself is generated from new, stem cell division progeny that differentiate within the parenchyma (loosely organized mesenchymal tissue surrounding the gut) to form new neurons, muscle, epithelial, and secretory cells [70–75]. This entails a localized increase in stem cell division similar to the one that accompanies blastema formation [75]. With respect to the pharynx proper, then, the regenerative response is reminiscent of vertebrate limb regeneration (i.e. it involves epimorphic outgrowth of a new appendage). The forkhead transcription factor FoxA is a master regulator of this process that controls the differentiation of neoblasts into pharyngeal tissues [75].
In trunk fragments, the existing pharynx is remodeled to restore scale and proportion. At an organismal level, this is analogous to remodeling of the brain and photoreceptors in head fragments, in that the organ exhibits a decrease in size proportional to the amount of tissue removed [76]. It is unclear whether new cells are added to the pharynx during remodeling, as has been described for the brain and gut (see above). Indeed, whether there are universal scaling mechanisms that apply across organs remains a key, unanswered question (see Conclusions).
2.4 Cell Death
One of the most conspicuous features of morphallaxis is the reduction in size of organs that are too large for the eventual size of the newly regenerated animal (e.g., the photoreceptors in a head fragment). The greater the amount of tissue removed, the greater the size reduction required to restore proportion. It is not entirely clear how this aspect of tissue remodeling occurs, but cell death appears to play an important role.
A whole-mount TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay revealed two waves of cell death in response to amputation – an initial, localized increase near the wound site and a later, systemic increase that peaked at around 3 days post-amputation (Figure 2C) [76]. Importantly, the former response was triggered by incision wounds that did not remove any tissue, whereas the latter was only induced by amputation and was clearly apparent in uninjured tissues. Furthermore, the systemic increase in cell death was proportional to the amount of tissue removed, and also occurred during degrowth. Taken together, these observations suggest programmed cell death contributes to the reduction in size of organs that need to be reproportioned during morphallaxis. As with repatterning of the body axes, amputation-induced cell death is unaffected by irradiation (i.e. the response occurs in differentiated cell types, independent of blastema formation) [76].
Biochemical and genetic evidence indicates planarians utilize a mitochondrial pathway of apoptosis, in which cytochrome c release from mitochondria triggers caspase activation [76,77]. Induction of this pathway during regeneration may be regulated by the Hippo signaling effector yorkie and the stress-activated kinase JNK. RNAi knockdown of either of these genes blocks the systemic, amputation-induced increase in cell death, as visualized by whole-mount TUNEL [51,78]. However, the RNAi phenotypes for these genes also include defects in other aspects of the regenerative response, including stem cell division (which, as noted above, is required for some aspects of regenerative tissue remodeling). Thus, conclusive evidence for the function(s) of apoptosis in morphallaxis awaits specific inhibition of cell death in this context.
Autophagy has also been implicated in tissue remodeling during both regeneration and degrowth [79,80]. This could not only contribute to a reduction in the size of organs that need to be reproportioned, but could provide a source of energy for other aspects of the regenerative response. Acting in this capacity, autophagy might explain the surprising fact that both blastema formation and remodeling occur even in animals amputated following prolonged periods of starvation [9].
3. CONCLUSIONS
We cannot work better to explain the facts we know than by trying to discover new ones
- Abraham Trembley, Memoirs, 1744
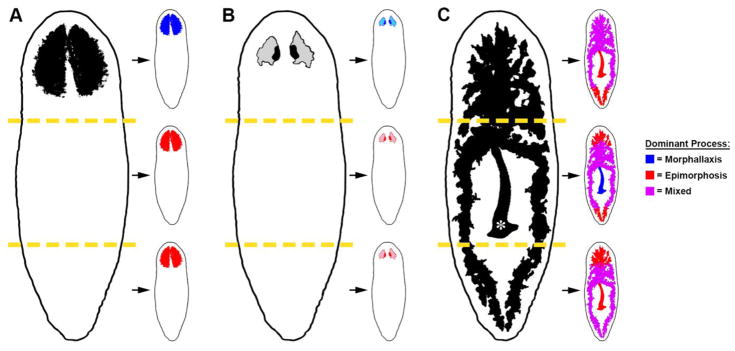
The facts we know about planarian morphallaxis are growing. Altered expression of positional control genes in muscle repatterns the body axes. Organs like the brain, gut, and pharynx are extensively remodeled to restore anatomical scale and proportion (Figure 3). And cell death is systemically elevated in preexisting, differentiated tissues. Some of these processes occur independently of epimorphosis (e.g., altered expression of PCGs and cell death), and are thus consistent with Morgan’s classic definition of morphallaxis. Other aspects of tissue remodeling entail addition of newly differentiated cells (e.g., remodeling of the brain), or require the wholesale integration of new tissue (e.g., remodeling of the gut). These cases necessitate revision of Morgan’s terminology (Table 1), so that epimorphosis and morphallaxis are not construed as mutually exclusive processes. Indeed, it is now apparent that these complementary amputation responses function together not just at the level of entire organisms, but also in the regeneration of individual tissues and organs (Figure 3).
Figure 3. Schematic illustration of morphallactic and epimorphic regeneration in planarians.
Head fragments reduce the size of the existing brain (A) and photoreceptors (B) through morphallaxis (blue), whereas trunk and tail fragments form these organs de novo in the blastema (a predominantly epimorphic response; red). Regeneration of the gut and pharynx (C) requires the combined action of both processes. The pharynx (asterisk) is regenerated from stem cell division progeny in head and tail fragments, whereas the existing pharynx in the trunk is remodeled to restore scale and proportion. These events are accompanied by remodeling of existing tissues to form or resize the pharyngeal pouch (not illustrated for clarity). All fragments form some new gut tissue in the blastema(s); additionally, simultaneous remodeling of existing gut branches and incorporation of new tissue (magenta) is required to restore gastrovascular form and function. Regeneration of other organs that extend throughout the body (e.g., the epidermis and protonephridial system) is likely to involve a similar mix of epimorphic and morphallactic processes.
While it is tempting to speculate about the mechanisms of morphallaxis, we would do well to heed Trembley’s advice and continue to more thoroughly investigate the changes amputation triggers in uninjured tissues before attempting to develop general models. A few of the many questions yet to be answered are briefly discussed below to frame key unresolved issues.
Why are stem cells required for remodeling of the brain and gut? What roles, if any, do stem cells play in remodeling of other organs? Some aspects of morphallaxis may be induced by newly formed body parts, and the degree of morphological change observed in processes like branching morphogenesis may simply be impossible without the bulk addition of new tissue [61]. Alternatively, some events may require communication between newly formed and preexisting organs (e.g., the pharynx and gut, or pharynx and muscle [61,68]). It is becoming increasingly clear that neoblasts play important roles in epimorphosis and morphallaxis alike, but their functions in the latter context are not nearly as well understood.
How are cell division and cell death coordinated during tissue homeostasis and repair? Cell death occurs in the absence of stem cell division (before and after amputation) [76], but it is not yet known whether the converse is true. Cell division is triggered by apoptosis in developmental phenomena like compensatory proliferation [81]. Whether planarians have similar mechanisms or not, they must have some means of regulating the balance between proliferation and apoptosis to control cell number during growth, degrowth, and regeneration.
What is the role of cell migration in morphallaxis? For instance, the dramatic increase in cell death observed when a pharynx decreases its size in a regenerating trunk fragment [76] would create a “Swiss cheese” morphology without extensive rearrangement of the remaining cells. How is this reorganization achieved at a cellular level? And on a (potentially) related note, how are cells and tissues reorganized throughout the body to narrow and elongate small fragments resulting from transverse amputations?
To what extent are remodeling mechanisms universal, vs. specific to individual tissues or organs? And what are the links, if any, between morphallaxis and degrowth? Tissue- and context-specific processes are inevitable, but at least some commonalities seem likely too – for example, cell “counting” mechanisms might be the same for different body parts and/or under different conditions (growth, degrowth, epimorphosis, morphallaxis).
How are the new body parts formed through epimorphosis integrated with the dynamically remodeling, preexisting anatomy? For example, is the nervous system of a decapitated animal in fact “rewired” during regeneration, to connect the newly formed brain with the existing ventral nerve cords? And if so, how? Integration of old and new tissues is an oft-cited function of morphallaxis, yet data addressing this issue are, at best, limited.
In conclusion, it is clear that transformation of existing anatomical structures is every bit as important to planarian regeneration as the formation of new ones, yet we know far less about the former process than the latter. Further research will be critical to answering questions like the ones posed above, and ultimately, to providing a satisfactory, mechanistic solution to the longstanding problem of morphallaxis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R15GM126456-01) and National Science Foundation (1656793). I thank Haley Zanga and Kaleigh Powers for the photographs in Figure 1C, and students in my lab for their independence during the extensive remodeling of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lenhoff SG, Lenhoff HM. Hydra and the Birth of Experimental Biology -1744: Abraham Trembley’s Memoirs Concerning the Natural History of a Type of Freshwater Polyp With Arms Shaped Like Horns. The Boxwood Press; Pacific Grove, CA: 1986. [Google Scholar]
- 2.Sunderland ME. Abraham Trembley (1710–1784) [Accessed January 1, 2018];Embryo Proj Encycl. 2007 http://embryo.asu.edu/handle/10776/1695.
- 3.Sunderland ME. Regeneration: Thomas Hunt Morgan’s window into development. J Hist Biol. 2010;43:325–361. doi: 10.1007/s10739-009-9203-2. [DOI] [PubMed] [Google Scholar]
- 4.Newmark PA, Sánchez Alvarado A. Not your father’s planarian: a classic model enters the era of functional genomics. Nat Rev Genet. 2002;3:210–219. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- 5.Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- 6.Saló E, Abril JF, Adell T, Cebrià F, Eckelt K, Fernandez-Taboada E, Handberg-Thorsager M, Iglesias M, Molina MD, Rodriguez-Esteban G. Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol. 2009;53:1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- 7.Roberts-Galbraith RH, Newmark PA. On the organ trail: insights into organ regeneration in the planarian. Curr Opin Genet Dev. 2015;32:37–46. doi: 10.1016/j.gde.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Aboobaker AA. Planarian stem cells: a simple paradigm for regeneration. Trends Cell Biol. 2011;21:304–311. doi: 10.1016/j.tcb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Morgan TH. Growth and regeneration in Planaria lugubris. Arch Für Entwicklungsmechanik Der Org. 1901;13:179–212. doi: 10.1007/BF02161982. [DOI] [Google Scholar]
- 10.Morgan TH. Regeneration. The Macmillan Company; London: 1901. [Google Scholar]
- 11.Tsonis PA, Fox TP. Regeneration according to Spallanzani. Dev Dyn. 2009;238:2357–2363. doi: 10.1002/dvdy.22057. [DOI] [PubMed] [Google Scholar]
- 12.Morgan TH. The Development of the Frog’s Egg: an Introduction to Experimental Embryology. The Macmillan Company; London: 1897. [Google Scholar]
- 13.Alwes F, Enjolras C, Averof M. Live imaging reveals the progenitors and cell dynamics of limb regeneration. Elife. 2016;5:e19766. doi: 10.7554/eLife.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindasamy N, Murthy S, Ghanekar Y. Slow-cycling stem cells in hydra contribute to head regeneration. Biol Open. 2014;3:1236–1244. doi: 10.1242/bio.201410512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzgariu W, Wenger Y, Tcaciuc N, Catunda-Lemos AP, Galliot B. Impact of cycling cells and cell cycle regulation on Hydra regeneration. Dev Biol. 2018;433:240–253. doi: 10.1016/J.YDBIO.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez Alvarado A. Regeneration in the metazoans: why does it happen? BioEssays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Tiozzo S, Copley RR. Reconsidering regeneration in metazoans: an evo-devoapproach. Front Ecol Evol. 2015;3:1–12. doi: 10.3389/fevo.2015.00067. [DOI] [Google Scholar]
- 18.Saló E, Bagu˜nà J. Regeneration in planarians and other worms: new findings, new tools, and new perspectives. J Exp Zool. 2002;292:528–539. doi: 10.1002/jez.90001. [DOI] [PubMed] [Google Scholar]
- 19.Agata K, Tanaka T, Kobayashi C, Kato K, Saitoh Y. Intercalary regeneration in planarians. Dev Dyn. 2003;226:308–316. doi: 10.1002/dvdy.10249. [DOI] [PubMed] [Google Scholar]
- 20.Agata K, Saito Y, Nakajima E. Unifying principles of regeneration I: epimorphosis versus morphallaxis. Dev Growth Differ. 2007;49:73–78. doi: 10.1111/j.1440-169X.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TH. Regeneration in planarians. Arch Für Entwicklungsmechanik Der Org. 1900;10:58–119. doi: 10.1007/BF02156347. [DOI] [Google Scholar]
- 22.Morgan TH. Experimental studies of the regeneration of Planaria maculata. Arch Für Entwickelungsmechanik Der Org. 1898;7:364–397. doi: 10.1007/BF02161491. [DOI] [Google Scholar]
- 23.Tran Thao Anh, Gentile Luca. A lineage CLOUD for neoblasts. Semin Cell Dev Biol. 2018 doi: 10.1016/j.semcdb.2018.04.012. (submitted to journal) [DOI] [PubMed] [Google Scholar]
- 24.Adell T, Cebrià F, Saló E. Gradients in planarian regeneration and homeostasis. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000505. , a000505–a000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddien PW. Constitutive gene expression and specification of tissue identity in adult planarian biology. Trends Genet. 2012;27:277–285. doi: 10.1016/j.tig.2011.04.004.Constitutive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umesono Y, Tasaki J, Nishimura Y, Hrouda M, Kawaguchi E, Yazawa S, Nishimura O, Hosoda K, Inoue T, Agata K. The molecular logic for planarian regeneration along the anterior–posterior axis. Nature. 2013;500:73–76. doi: 10.1038/nature12359. [DOI] [PubMed] [Google Scholar]
- 27.Owlarn S, Bartscherer K. Go ahead, grow a head! A planarian’s guide to anterior regeneration. Regeneration. 2016;3:139–155. doi: 10.1002/reg2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witchley JN, Mayer M, Wagner DE, Owen JH, Reddien PW. Muscle cells provide instructions for planarian regeneration. Cell Rep. 2014;4:633–641. doi: 10.1016/j.celrep.2013.07.022.Muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurley KA, Rink JC, Sánchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;80(319):323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;80(319):327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 31.Scimone ML, Cote LE, Reddien PW. Orthogonal muscle fibres have different instructive roles in planarian regeneration. Nature. 2017 doi: 10.1038/nature24660. [DOI] [PMC free article] [PubMed]
- 32.Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci. 2009;106:17061–17066. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurley KA, Elliott SA, Simakov O, Schmidt HA, Holstein TW, SánchezAlvarado A. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol. 2010;347:24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddien PW, Bermange AL, Kicza AM, Sánchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134:4043–4051. doi: 10.1242/dev.007138. [DOI] [PubMed] [Google Scholar]
- 35.Molina MD, Saló E, Cebrià F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol. 2007;311:79–94. doi: 10.1016/j.ydbio.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Cebrià F, Guo T, Jopek J, Newmark PA. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307:394–406. doi: 10.1016/j.ydbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molina MD, Neto A, Maeso I, Gómez-Skarmeta JL, Saló E, Cebrià F. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol. 2011;21:300–305. doi: 10.1016/j.cub.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Gavi˜no MA, Reddien PW. A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. CurrBiol. 2011;21:294–299. doi: 10.1016/j.cub.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagu˜nà J, Romero R. Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia. 1981;84:181–194. doi: 10.1007/BF00026179. [DOI] [Google Scholar]
- 40.Romero R, Bagu˜nà J. Quantitative cellular analysis of growth and reproduction in freshwater planarians (Turbellaria; Tricladida). I. A cellular description of the intact organism. Invertebr Reprod Dev. 1991;19:157–165. doi: 10.1080/07924259.1991.9672170. [DOI] [Google Scholar]
- 41.Oviedo NJ, Newmark PA, Sánchez Alvarado A. Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev Dyn. 2003;226:326–333. doi: 10.1002/dvdy.10228. [DOI] [PubMed] [Google Scholar]
- 42.Takeda H, Nishimura K, Agata K. Planarians maintain a constant ratio of different cell types during changes in body size by using the stem cell system. Zoolog Sci. 2009;26:805–813. doi: 10.2108/zsj.26.805. [DOI] [PubMed] [Google Scholar]
- 43.González-Estévez C, Felix DA, Rodríguez-Esteban G, Aboobaker AA. Decreased neoblast progeny and increased cell death during starvation-induced planarian degrowth. Int J Dev Biol. 2012;56:83–91. doi: 10.1387/ijdb.113452cg. [DOI] [PubMed] [Google Scholar]
- 44.Felix Daniel A, Gutiérrez-Gutiérrez Óscar, Espada Lilia, Thems Anne, González-Estévez Cristina. It is not all about regeneration: planarians striking power to stand starvation. Semin Cell Dev Biol. 2018 doi: 10.1016/j.semcdb.2018.04.010. (submitted to journal) [DOI] [PubMed] [Google Scholar]
- 45.Agata K, Umesono Y. Brain regeneration from pluripotent stem cells in planarian. Philos Trans R Soc B Biol Sci. 2008;363:2071–2078. doi: 10.1098/rstb.2008.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts-Galbraith RH, Brubacher JL, Newmark PA. A functional genomics screen in planarians reveals regulators of whole-brain regeneration. Elife. 2016;5:e17002. doi: 10.7554/eLife.17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang IE, Lapan SW, Scimone ML, Clandinin TR, Reddien PW. Hedgehog signaling regulates gene expression in planarian glia. Elife. 2016;5 doi: 10.7554/eLife.16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross KG, Currie KW, Pearson BJ, Zayas RM. Nervous system development and regeneration in freshwater planarians. Wiley Interdiscip Rev Dev Biol. 2017;6:e266. doi: 10.1002/wdev.266. [DOI] [PubMed] [Google Scholar]
- 49.Brown DDR, Pearson BJ. A brain unfixed: unlimited neurogenesis and regeneration of the adult planarian nervous system. Front Neurosci. 2017;11:289. doi: 10.3389/fnins.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill EM, Petersen CP. Wnt/Notum spatial feedback inhibition controls neoblast differentiation to regulate reversible growth of the planarian brain. Development. 2015;142:4217–4229. doi: 10.1242/dev.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin AYT, Pearson BJ. Yorkie is required to restrict the injury responses in planarians. PLOS Genet. 2017;13:e1006874. doi: 10.1371/journal.pgen.1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin AYT, Pearson BJ. Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development. 2014;141:1197–1208. doi: 10.1242/dev.101915. [DOI] [PubMed] [Google Scholar]
- 53.Currie KW, Molinaro AM, Pearson BJ. Neuronal sources of hedgehog modulate neurogenesis in the adult planarian brain. Elife. 2016;5:1–18. doi: 10.7554/eLife.19735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cebrià F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sánchez Alvarado A, Agata K. FGFR-related genenou-darake restricts brain tissues to the head region of planarians. Nature. 2002;419:620–624. doi: 10.1038/nature01042. [DOI] [PubMed] [Google Scholar]
- 55.Hyman LH. The Invertebrates: Platyhelminthes and Rhynchocoela, the Acoelomate Bilateria., Invertebr. Platyhelminthes Rhynchocoela, Acoelomate Bilateria. McGraw-Hill Book Co.; New York: 1940. [Google Scholar]
- 56.Ishii S. Electron microscopic observations on the planarian tissues II. The intestine., Fukushima. J Med Sci. 1965;12:67–87. [PubMed] [Google Scholar]
- 57.Bowen ID, Ryder TA, Thompson JA. The fine structure of the planarian Polycelis tenuis Iijima. Protoplasma. 1974;79:1–17. doi: 10.1007/BF02055779. [DOI] [PubMed] [Google Scholar]
- 58.Bowen ID. Metazoa. Elsevier; 1980. Phagocytosis in polycelis tenuis, in: nutr, in: Low; pp. 1–14. [DOI] [Google Scholar]
- 59.Garcia-Corrales P, Gamo J. The ultrastructure of the gastro dermal gland cells in the freshwater planarian Dugesia gonocephala s.l. Acta Zool. 1986;67:43–51. doi: 10.1111/j.1463-6395.1986.tb00848.x. [DOI] [Google Scholar]
- 60.Garcia-Corrales P, Gamo J. Ultrastructural changes in the gastrodermal phagocytic cells of the planarian Dugesia gonocephala s.l. during food digestion (Plathelminthes) Zoomorphology. 1988;108:109–117. doi: 10.1007/BF00539786. [DOI] [Google Scholar]
- 61.Forsthoefel DJ, Park AE, Newmark PA. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev Biol. 2011;356:445–459. doi: 10.1016/j.ydbio.2011.05.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forsthoefel DJ, James NP, Escobar DJ, Stary JM, Vieira AP, Waters FA, Newmark PA. An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Dev Cell. 2012;23:691–704. doi: 10.1016/j.devcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barberán S, Fraguas S, Cebrià F. The EGFR signaling pathway controls gut progenitor differentiation during planarian regeneration and homeostasis. Development. 2016;143:2089–2102. doi: 10.1242/dev.131995. [DOI] [PubMed] [Google Scholar]
- 64.Flores NM, Oviedo NJ, Sage J. Essential role for the planarian intestinal GATA transcription factor in stem cells and regeneration. Dev Biol. 2016;418:179–188. doi: 10.1016/j.ydbio.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Wolfswinkel JC, Wagner DE, Reddien PW. Single-cell analysis reveals functionally distinct classes within the planarian stem cell compartment. Cell Stem Cell. 2014;15:326–339. doi: 10.1016/j.stem.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332:811–816. doi: 10.1126/science.1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosoda K, Morimoto M, Motoishi M, Nishimura O, Agata K, Umesono Y. Simple blood-feeding method for live imaging of gut tube remodeling in regenerating planarians. Dev Growth Differ. 2016;58:260–269. doi: 10.1111/dgd.12270. [DOI] [PubMed] [Google Scholar]
- 68.Adler CE, Sánchez Alvarado A. PHRED-1 is a divergent neurexin-1 homolog that organizes muscle fibers and patterns organs during regeneration. Dev Biol. 2017;427:165–175. doi: 10.1016/J.YDBIO.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacRae EK. Observations on the fine structure of pharyngeal muscle in the planarian Dugesia tigrina. J Cell Biol. 1963;18:651–662. doi: 10.1083/JCB.18.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobayashi C, Watanabe K, Agata K. The process of pharynx regeneration in planarians. Dev Biol. 1999;211:27–38. doi: 10.1006/dbio.1999.9291. [DOI] [PubMed] [Google Scholar]
- 71.Bueno D, Espinosa L, Bagu˜nà J, Romero R. Planarian pharynx regeneration in regenerating tail fragments monitored with cell-specific monoclonal antibodies. Dev Genes Evol. 1997;206:425–434. doi: 10.1007/s004270050072. [DOI] [PubMed] [Google Scholar]
- 72.Cebrià F, Bueno D, Reigada S, Romero R. Intercalary muscle cell renewal in planarian pharynx. Dev Genes Evol. 1999;209:249–253. doi: 10.1007/s004270050249. [DOI] [PubMed] [Google Scholar]
- 73.Ito H, Saito Y, Watanabe K, Orii H. Epimorphic regeneration of the distal part of the planarian pharynx. Dev Genes Evol. 2001;211:2–9. doi: 10.1007/s004270000115. [DOI] [PubMed] [Google Scholar]
- 74.Sakai T, Kato K, Watanabe K, Orii H. Planarian pharynx regeneration revealed by the expression of myosin heavy chain-A. Int J Dev Biol. 2002;46:329–332. doi: 10.1006/dbio.1999.9291. [DOI] [PubMed] [Google Scholar]
- 75.Adler CE, Seidel CW, McKinney SA, Sánchez Alvarado A. Selective amputation of the pharynx identifies a FoxA-dependent regeneration program in planaria. Elife. 2014;3:e02238. doi: 10.7554/eLife.02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pellettieri J, Fitzgerald P, Watanabe S, Mancuso J, Green DR, SánchezAlvarado A. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2010;338:76–85. doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bender CE, Fitzgerald P, Tait SWG, Llambi F, McStay GP, Tupper DO, Pellettieri J, Sánchez Alvarado A, Salvesen GS, Green DR. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc Natl Acad Sci U S A. 2012;109:4904–4909. doi: 10.1073/pnas.1120680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Almuedo-Castillo M, Crespo X, Seebeck F, Bartscherer K, Saló E, Adell T. JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling. PLoS Genet. 2014;10:e1004400. doi: 10.1371/journal.pgen.1004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González-Estévez C, Felix DA, Aboobaker AA, Saló E. Gtdap-1 promotes autophagy and is required for planarian remodeling during regeneration and starvation. Proc Natl Acad Sci U S A. 2007;104:13373–13378. doi: 10.1073/pnas.0703588104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Estévez C. Autophagy in freshwater planarians. Methods Enzymol. 2008;451:439–465. doi: 10.1016/S0076-6879(08)03227-8. [DOI] [PubMed] [Google Scholar]
- 81.Pellettieri J, Sánchez Alvarado A. Cell turnover and adult tissue homeostasis: from humans to planarians. Annu Rev Genet. 2007;41:83–105. doi: 10.1146/annurev.genet.41.110306.130244. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21:172–185. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]