Abstract
Alcohol use disorder (AUD) is a chronic, relapsing psychiatric disease characterized by the emergence of negative emotional states and the development of motivational deficits that manifest during alcohol withdrawal. Accordingly, alcohol may be sought after and taken in excessive amounts to alleviate withdrawal-related symptoms. To develop more effective treatments for AUD, it is necessary to identify potential molecular targets that underlie the transition from initial alcohol use to alcohol dependence, and our previous work has implicated a role for potentiated glucocorticoid receptor (GR) signaling in this regard. As a key negative regulator of GR-mediated signaling, the current study first measured c-Jun N-terminal kinase (JNK) phosphorylation in animals following an acute alcohol challenge. We found that JNK phosphorylation (pJNK) was significantly increased in the hippocampus, frontal cortical regions, and striatum of adult male Wistar rats following alcohol challenge, indicating that initial alcohol exposure increases JNK activity across several brain regions. A separate group of adult male Wistar rats were made dependent via chronic, intermittent ethanol vapor exposure and were trained to self-administer alcohol. We found that alcohol-dependent animals consumed significantly more alcohol and escalated their drinking over time compared to non-dependent animals. We then measured alterations in JNK phosphorylation in this alcohol-dependent group during acute withdrawal and found that pJNK was selectively decreased in the dorsal hippocampus, dorsomedial prefrontal cortex, and cingulate cortex. These findings demonstrate that withdrawal from chronic alcohol exposure leads to region-specific deficits in JNK phosphorylation. JNK signaling dysregulation may foster long-lasting behavioral and motivational impairments in alcohol dependence, either as a result of increased GR-mediated stress signaling or via other downstream mechanisms.
INTRODUCTION
Based on the 2014 National Survey on Drug Use and Health, there is an estimated 139.7 million current users of alcohol in the U.S. (SAMHSA, 2014). Most individuals engage in recreational and limited alcohol use. However, select individuals will transition to uncontrolled, excessive alcohol drinking and develop an alcohol use disorder (AUD) (Edwards & Koob, 2010). It is also estimated that 43.6% of current alcohol users in the U.S. are engaging in binge drinking and that 11.7% of current users are classified as heavy users of alcohol (SAMHSA, 2014). Excessive alcohol consumption is estimated to cost the U.S. $223.5 billion dollars in health care expenses, lost productivity, and legal expenses (Bouchery et al., 2011). In 2015, 13.9% of the U.S population met the criteria for AUD (Grant et al., 2015). Alcohol addiction is a chronic, relapsing disease associated with negative affective states (e.g., pain, anxiety, depression) and motivational symptoms of dependence (e.g., increased alcohol intake and craving) (Koob & Le Moal, 1997). It is thought that the emergence of these symptoms coincides with a switch in motivation from positive to negative reinforcement processes whereby individuals will escalate alcohol intake in an attempt to alleviate negative symptoms associated with alcohol withdrawal (Edwards & Koob, 2010). The behavioral and motivational impairments associated with uncontrolled, excessive alcohol use are attributed to long-lasting neuroadaptations and functional changes to neuronal circuitry.
Alcohol dependence (or severe AUD) can be reliably modeled in rodents using chronic, intermittent ethanol vapor exposure, which produces both somatic and motivational symptoms of dependence (Rogers et al., 1979; Gilpin et al, 2008; Vendruscolo & Roberts, 2014). During acute and protracted alcohol withdrawal, a robust escalation of drinking in dependent animals is associated with increases in central brain glucocorticoid receptor (GR) signaling (Vendruscolo et al., 2012; Repunte-Canonigo et al., 2015). Interestingly, central brain glucocorticoids remain elevated in specific brain regions including the hippocampus, prefrontal cortex, and striatum after two months of withdrawal from chronic alcohol exposure (Little et al., 2008). Additionally, reinstatement of ethanol-seeking behavior during protracted alcohol withdrawal is associated with increased GR expression in the medial prefrontal cortex (Somkuwar et al., 2017). At the molecular level, the transcriptional activity of GR is directly controlled via phosphorylation status and the subcellular location of GR within the cytosol or nucleus. Selective activation of c-Jun N-terminal kinase (JNK) phosphorylates GR at serine 246, which decreases its transcriptional activity, partly due to the export of GR from the nucleus (Rogatsky et al., 1998). Importantly, chronic stress has been reported to decrease JNK phosphorylation in the hippocampus and prefrontal cortex, while also increasing GR activity (Adzic et al., 2009). This indicates that chronic stressors may impair the ability of JNK to limit GR function, thereby increasing stress signaling in regions critically vulnerable to the disruptive effects of excessive alcohol exposure (Richardson et al., 2009; de la Monte & Kril, 2014).
The present study was designed to test how repeated alcohol vapor exposure and withdrawal alters alcohol drinking behavior and JNK phosphorylation in alcohol-dependent animals compared to non-dependent (air-exposed) animals. In comparison, to determine how acute alcohol exposure in previously alcohol-naïve rats alters JNK activity in regions prone to the effects of alcohol (including the hippocampus, frontal cortical regions, and striatum), we also measured central brain JNK phosphorylation (pJNK) in animals that were given an intoxicating bolus injection of alcohol. Rats were made dependent via chronic, intermittent ethanol vapor (CIEV) exposure. With this procedure animals underwent daily cycles of alcohol exposure (14 h) and withdrawal (10 h) over several weeks. To test the hypothesis that alcohol-dependent animals would escalate their alcohol drinking compared to non-dependent animals, we employed operant self-administration to assess alcohol-drinking behavior when animals were in acute withdrawal (corresponding to 8 h post-CIEV in dependent rats).
MATERIALS AND METHODS
Animals
Adult male Wistar rats weighing 200–300 grams at the time of arrival were purchased from Charles River (Wilmington, MA). Rats were pair-housed and given ad libitum access to food (Purina Rat Chow, Ralston Purina, St. Louis, MO) and water throughout all experimental procedures. Rats were maintained on a reverse 12 h light/dark cycle (lights off at 8:00 am) and were handled regularly. Rats were given one wk to acclimate to the colony room prior to the start of experimental procedures. All animal care, use, and procedures in this study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center (LSUHSC) and were in accordance with the National Institute of Health guidelines.
Alcohol Self-Administration and Ethanol Vapor Inhalation Protocol
Prior to alcohol self-administration, animals were given 2-bottle choice access to 10% (w/v) ethanol and water in the home cage for 24 h to habituate them to the taste of alcohol. The following day rats were given an overnight session in the operant chambers (Med Associates) with access to one lever that delivers water. Regular food was available ad libitum in the operant chambers during this overnight session. One day later rats were placed in the operant chambers with access to one lever that delivers 10% (w/v) ethanol for 2 h, 1 h, and 30 min over the course of three days. All subsequent sessions were 30-min sessions with access to one lever that delivers 10% (w/v) ethanol and one lever that delivers water. Operant alcohol self-administration was conducted under a fixed-ratio 1 (FR-1) schedule of reinforcement, in which each lever press results in a 0.1 ml fluid delivery of 10% (w/v) ethanol. Rats were trained to self-administer 10% (w/v) alcohol and water solution in operant chambers (Med Associates) until stable responding was maintained, as previously described (Edwards et al., 2012).
Following stable acquisition of operant alcohol self-administration, rats were split into two groups, which were matched for alcohol self-administration over the last three sessions of self-administration. One group was designated as “alcohol-dependent” and the other as “non-dependent”. For CIEV, animals were pair-housed in plexiglass vapor delivery chambers. CIEV procedures in which animals undergo cycles of alcohol exposure and abstinence over several weeks were the same as previously described (Edwards et al., 2012; Gilpin et al., 2008). Here, the intermittent procedure entails daily cycles of alcohol vapor exposure (14 h, producing target blood alcohol levels (BALs) of 150–250 mg/dL) and alcohol withdrawal (10 h, where BALs are reduced to near zero, and physical and negative motivational symptoms emerge). Tail blood samples were collected and analyzed one to two times per week to maintain BALs of 150–250 mg/dL, as previously described (Gilpin et al., 2008). This procedure has been reliably used to produce both somatic and motivational-like symptoms of dependence (Gilpin et al., 2008; Rogers et al., 1979; Vendruscolo & Roberts, 2014).
Measurement of BALs
Tail blood (0.2 ml) was collected and centrifuged to extract the plasma. The plasma was injected into an oxygen-rate alcohol analyzer (Analox Instruments, London, UK) for Blood Alcohol Level (BAL) determination via an alcohol oxidation reaction as previously described (McGinn et al., 2016). Single point calibrations were done for each set of samples with reagents provided by Analox Instruments (25–400 mg%).
Alcohol Drinking Behavior
Following acquisition of alcohol self-administration and induction of alcohol dependence by four weeks of CIEV exposure, alcohol-dependent (n=21) and non-dependent (air-exposed) animals (n=25) were tested until post-vapor intake stabilization was reached (25 sessions). Responses for both alcohol and water (a natural reinforcer) were recorded. For post-vapor alcohol drinking behavior, the number of alcohol reinforcers (0.1 ml fluid delivery of 10% (w/v) ethanol) and the number of lever presses from the last three 30-min sessions were recorded. All post-vapor testing for alcohol self-administration was conducted when alcohol-dependent animals were in acute withdrawal (8 h after removal from vapor when BALs are near zero).
Effects of Chronic Alcohol and Withdrawal on JNK Phosphorylation
A subset of alcohol-dependent animals (n=9) and non-dependent animals (n=14) who were trained to self-administer alcohol were euthanized by decapitation under anesthesia during acute (8 h) alcohol withdrawal to test for changes in JNK phosphorylation via Western blotting.
Effects of Acute Alcohol Challenge on JNK Phosphorylation
A separate group of rats were given a 1 ml/kg intraperitoneal (IP) injection of saline to habituate them to the injection procedure prior to drug injections. Alcohol-naïve animals received an IP injection of either ethanol (1g/kg of 15% w/v EtOH) (n=12) or saline (1ml/kg) (n=12) and were euthanized by decapitation under anesthesia 15 min later to test for changes in JNK phosphorylation via Western blotting.
Western Blot Analysis
Western blot analyses for brain regional changes in protein phosphorylation were conducted and analyzed as previously described (McGinn et al., 2016; Pahng et al., 2017). After the rats were euthanized by decapitation under light isoflurane anesthesia, the brains were rapidly removed, snap-frozen in isopentane, and stored at −80°C until dissection. During the dissection, the brains were mounted and sliced using a cryostat. Regional brain punches (0.5 mm thick) were taken from frozen tissue using a 13–16 gauge needle (inner diameter: 1.19–1.80mm) according to (Paxinos and Watson, 1998) (Figure 1). Brain punches were homogenized by sonication in a lysis buffer (320 mm sucrose, 5 mm HEPES, 1 mm EGTA, 1 mm EDTA, 1 %SDS, protease inhibitor cocktail (diluted 1:100), and phosphatase inhibitor cocktails II and III (diluted 1:100); Sigma, St. Louis, MO, USA). Tissue homogenates were heated at 100°C for 5 min and stored at −80°C until the total protein concentration was measured using a detergent-compatible Lowry method (Bio-Rad, Hercules, CA, USA). Samples of protein (20 μg) were separated by SDS-polyacrylamide gel electrophoresis on 8% acrylamide gels using a Tris/Glycine/SDS buffer system (Bio-Rad). The gels were electrophoretically transferred to polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA). Membranes were blocked for 1 h in 5% non-fat milk at room temperature and incubated overnight in 2.5% non-fat milk with primary antibody, phospho JNK (1:10000; Cell Signaling; Cat # 9251), at 4°C. Membranes were washed and incubated with species-specific peroxidase-conjugated secondary antibody (1:10000; Bio-Rad) for 1 h at room temperature. Membranes were washed and incubated in a chemiluminescent reagent (SuperSignal West Pico; Thermo Scientific, Rockford, IL, USA), and exposed to film. Following film development, membranes were stripped for 30 min at room temperature (Restore; Thermo Scientific) and reprobed for total JNK (1:20000; Cell Signaling; Cat # 9251) levels. The immunoreactivity of the bands was detected using densitometry (Image J 1.45S; Bethesda, MD). To normalize the data across the blots, the densitized values were expressed as a percentage of the mean of the non-dependent or saline-injected controls for each gel. Individual phosphorylated JNK levels were normalized to individual total JNK protein levels to generate phosphorylation: total ratio values for statistical comparison. There was no change in total JNK between groups (saline-challenged, alcohol-challenged, alcohol-dependent, non-dependent) in the acute alcohol challenge and alcohol-dependence experiments for any region.
Figure 1.
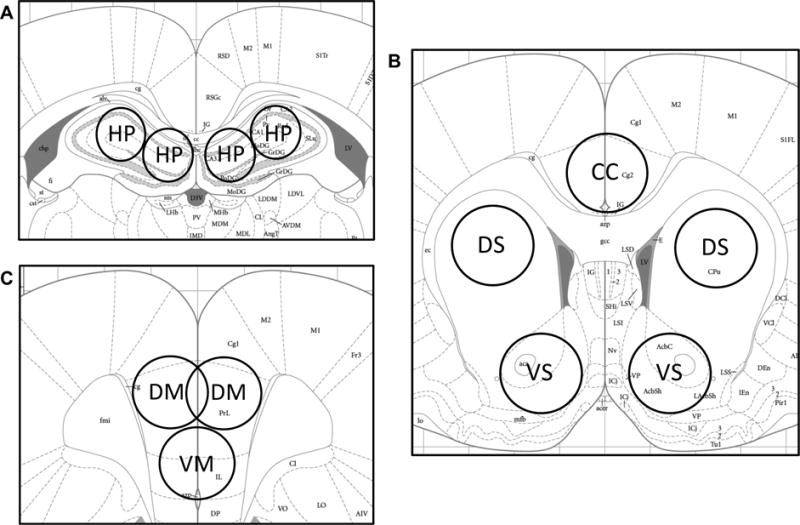
Schematic representation of sub-regional brain sample collected (Paxinos and Watson, 1998). (A) HP = dorsal hippocampus; (B) CC = cingulate cortex; DS = dorsal striatum; VS = ventral striatum; (C) DM = dorsomedial prefrontal cortex; VM = ventromedial prefrontal cortex.
Intracerebral Surgery
A separate group of rats (n=13) were trained to self-administer alcohol. Following acquisition of alcohol self-administration, rats were implanted with indwelling cannulae directed unilaterally at the lateral ventricle. The rats were anesthetized with isoflurane (1.5–2%) and secured in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and a 22-gauge stainless steel cannula (Plastics One, Roanoke, VA) was aimed 1 mm above the lateral ventricle and secured to the skull with four stainless steel screws and Silux dental cement. The stereotaxic coordinates were the following: anterior/posterior, −0.6 mm; medial/lateral, ± 2.0 mm relative to bregma; dorsal/ventral, −3.2 mm from skull surface. A 7 mm dummy stylet (Plastics One, Roanoke, VA) filled the cannula and maintained patency. The animals were allowed 1 week to recover from surgery before alcohol self-administration.
JNK Inhibition on Alcohol Self-Administration
After four weeks of alcohol self-administration, animals were infused with ascending doses of a JNK Inhibitor (SP600125; 0, 1, 2, 3μL/side, 1 h pretreatment) (n=6) or vehicle (n=7) using a Hamilton microsyringe and a 30-gauge stainless steel injector attached to polyethylene 20 tubing. The injector projected 1 mm beyond the end of the cannula. SP600125 was dissolved in 100% DMSO to make a 20μg/μL stock solution, which was diluted with PBS to reach a final concentration of 1μg/μL in 5% DMSO. The vehicle and doses were chosen based on Benzler et al. (2013), where it was demonstrated that intracerebroventricular injection of our lowest SP600125 dose tested (1ug/uL) increased downstream JNK signaling in the brain.
Statistical Analysis
All data were analyzed using Prism 6 (GraphPad Software, Inc; La Jolla, CA). Alcohol and water drinking data were analyzed using two-way repeated measures ANOVAs with the between-subjects factor of group (dependent vs. non-dependent) and the within-subjects factor of drinking session (baseline drinking vs. post-vapor drinking). Western blot data were analyzed using two-way between-subjects ANOVAs with alcohol exposure (dependent vs. non-dependent or acute alcohol vs. saline) and pJNK bands normalized to total JNK (46kDa, 54kDa) as factors. The JNK inhibitor data were analyzed using two-way repeated measures ANOVA with the between-subjects factor of drug treatment (SP600125 vs. DMSO) and the within-subjects factor of drug dose (0, 1, 2, 3μL/side). Significance levels for statistical tests was set at p<0.05.
RESULTS
Effects of Acute Alcohol Challenge on JNK Phosphorylation in the Hippocampus, Frontal Cortical Regions, and Striatum
We first investigated acute alcohol-induced alterations in pJNK (both 46kDa & 54kDa isoforms) in the hippocampus, frontal cortex, and striatum, as these are regions prone to the damaging effects of alcohol. Specifically, we measured alcohol-induced alterations in pJNK in the dorsal hippocampus (HP), dorsomedial prefrontal cortex (dmPFC), ventromedial prefrontal cortex (vmPFC), cingulate cortex (CC), dorsal striatum (DS), and ventral striatum (VS). pJNK was significantly increased in the HP of acute alcohol challenge animals compared to saline injected controls [F(1,44)=16.91, p=0.0002] (Figure 2A). In addition, acute alcohol challenge significantly increased pJNK in frontal cortical regions including dmPFC [F(1,44)=5.777, p=0.0205] (Figure 2B), vmPFC [F(1,44)=5.526, p=0.0233] (Figure 2C), and CC [F(1,44)=17.02, p=0.0002] (Figure 2D). Finally, there were significant increases in striatal pJNK in the DS [F(1,44)=34.11, p<0.0001] (Figure 2E) and VS [F(1,44)=10.71, p=0.0021] (Figure 2F) of acute alcohol challenge animals compared to saline controls. In summary, an intoxicating bolus of alcohol significantly increased JNK phosphorylation in the HP, dmPFC, vmPFC, CC, DS, and VS.
Figure 2.
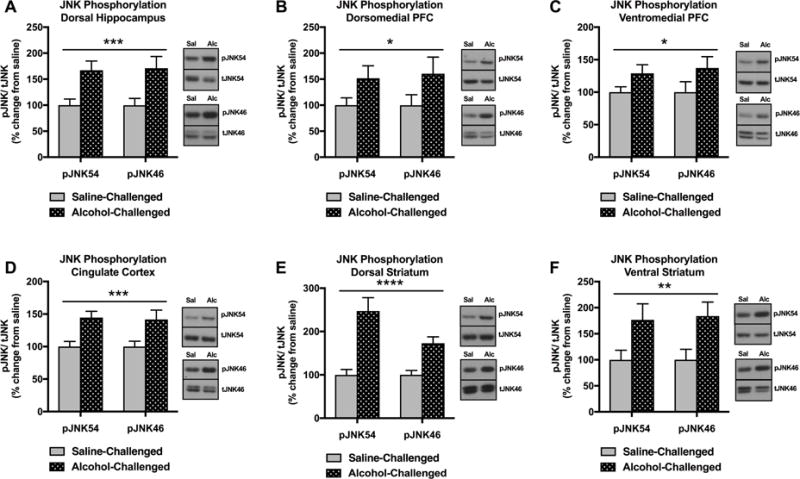
Effect of acute alcohol challenge on pJNK (15 min after injections) in the HP, dmPFC, vmPFC, CC, DS, VS. (A) pJNK was significantly increased in the HP of alcohol challenge animals compared to saline controls (***p<0.001). (B) pJNK was significantly increased in the dmPFC of alcohol challenge animals compared to saline controls (*p<0.05). (C) pJNK was significantly increased in the vmPFC of alcohol challenge animals compared to saline controls (*p<0.05). (D) pJNK was significantly increased in the CC of alcohol challenge animals compared to saline controls (***p<0.001). (E) pJNK was significantly increased in the DS of alcohol challenge animals compared to saline controls (****p<0.0001). (F) pJNK was significantly increased in the VS of alcohol challenge animals compared to saline controls (**p<0.01). Significant effects were observed in both p46 and p54 molecular weight bands representing JNK1-3 phosphoisoforms. Data are expressed as pJNK levels normalized to total JNK levels. Representative bands indicate changes in pJNK54 (single band), tJNK54 (single band), pJNK46 (single band), and tJNK46 (doublet).
Effects of Alcohol Dependence and Withdrawal on Alcohol Drinking Behavior
Following acquisition of alcohol self-administration and induction of alcohol dependence, alcohol-dependent and non-dependent animals were tested until post-vapor intake stabilization was reached. We compared the number of alcohol reinforcers (0.1 ml fluid delivery of 10% (w/v) ethanol) during baseline drinking and post-vapor drinking sessions in both alcohol-dependent and non-dependent animals. For alcohol drinking behavior, there was a significant main effect of group [F(1,44)=16.57, p=0.0002] and a significant group × drinking session interaction [F(1,44)=21.91, p<0.0001] (Figure 3). During the last three 30-min sessions of post-vapor alcohol drinking, alcohol-dependent rats consumed significantly more alcohol than non-dependent rats [Sidak’s multiple comparisons test, p<0.0001] (Figure 3), reflective of excessive alcohol drinking in dependent animals during acute withdrawal (corresponding to 8 h post-CIEV in dependent rats). This increase in drinking was specific to alcohol, because there was no difference in water consumption (number of 0.1 ml water deliveries) between dependent animals and non-dependent animals (group [F(1,44)=0.6875, p=0.4115]; group x drinking session interaction [F(1,44)=0.2591, p=0.6133]) (data not shown). There was also a significant increase in post-vapor alcohol drinking in alcohol-dependent animals compare to baseline levels [Sidak’s multiple comparisons test, p=0.0001] (Figure 3). In contrast, there was no difference in drinking between post-vapor (air) alcohol drinking and baseline drinking in non-dependent animals [Sidak’s multiple comparisons test, p=0.0778] (Figure 3). This demonstrates that alcohol-dependent animals significantly escalated their alcohol intake from baseline drinking levels, while non-dependents animals did not.
Figure 3.
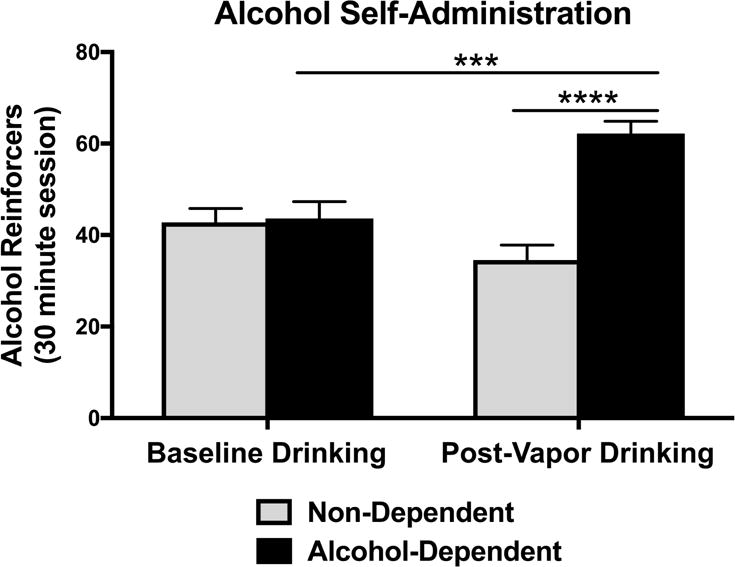
Effect of alcohol dependence and withdrawal on alcohol drinking behavior. The number of alcohol reinforcers (0.1 ml fluid delivery of 10% (w/v) ethanol) were recorded during baseline drinking and post-vapor drinking sessions in both alcohol-dependent and non-dependent animals. During the last three 30-min sessions of post-vapor alcohol drinking, alcohol-dependent rats consumed significantly more alcohol than non-dependent rats (****p<0.0001) during acute withdrawal (corresponding to 8 h post-CIEV in dependent rats). During alcohol self-administration, alcohol-dependent animals significantly escalated their alcohol intake from baseline drinking levels (***p=0.0001), while non-dependents animals did not (p>0.05).
Effects of Alcohol Dependence and Withdrawal on JNK Phosphorylation in the Hippocampus, Frontal Cortical Regions, and Striatum
We next investigated chronic alcohol and withdrawal-induced neuroadaptations in the hippocampus, frontal cortical regions, and striatum, which are regions critically vulnerable to the disruptive effects of excessive alcohol exposure. Specifically, we measured pJNK (both 46kDa & 54kDa isoforms) in the HP, dmPFC, vmPFC, CC, DS, and VS of alcohol-dependent animals during acute alcohol withdrawal (corresponding to 8 h post-CIEV) and non-dependent animals. pJNK was significantly decreased in the HP of alcohol-dependent animals in withdrawal compared to non-dependent animals [F(1,42)=12.11, p=0.0012] (Figure 4A). pJNK was also decreased in the dmPFC of alcohol-dependent animals in withdrawal compared to non-dependent animals [F(1,42)=9.815, p=0.0032] (Figure 4B). However, there was no difference in pJNK in the vmPFC of alcohol-dependent animals in withdrawal compared to non-dependent animals [F(1,42)=1.437, p=0.2373] (Figure 4C). There was a significant decrease in pJNK in the CC of alcohol-dependent animals in withdrawal compared to non-dependent animals [F(1,42)=5.557, p=0.0231] (Figure 4D). There was no difference in pJNK in the DS [F(1,42)=2.923, p=0.0947] (Figure 4E) or VS [F(1,42)=1.394, p=0.244] (Figure 4F) between alcohol-dependent and non-dependent animals. In contrast to the acute alcohol challenge findings, there were significant decreases in JNK phosphorylation in the HP, dmPFC, and CC of alcohol-dependent rats in withdrawal and no change in JNK phosphorylation in the vmPFC, DS, and VS.
Figure 4.
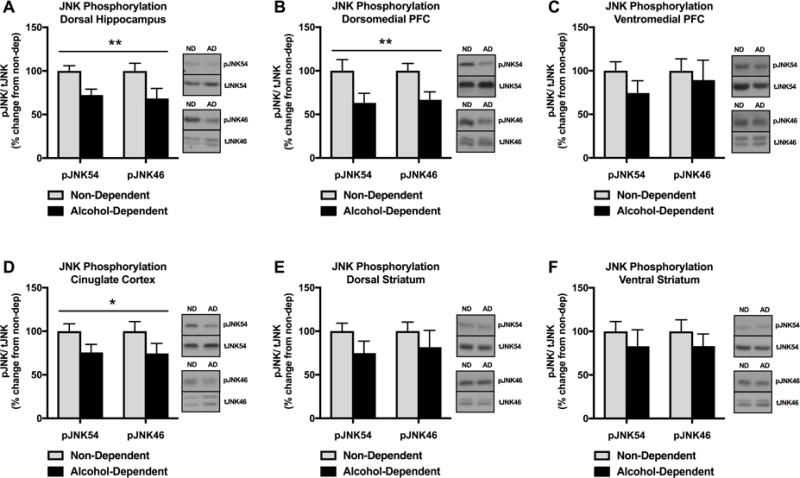
Effect of alcohol dependence and acute (8 h) withdrawal on JNK phosphorylation in in the HP, dmPFC, vmPFC, CC, DS, VS. (A) pJNK was significantly decreased in the HP of alcohol-dependent animals in withdrawal compared to non-dependent animals (**p<0.01). (B) pJNK was significantly decreased in the dmPFC of alcohol-dependent animals in withdrawal compared to non-dependent animals (**p<0.01). (C) There was no difference in pJNK in the vmPFC between alcohol-dependent animals in withdrawal and non-dependent animals (p>0.05). (D) pJNK was significantly decreased in the CC of alcohol-dependent animals in withdrawal compared to non-dependent animals (*p<0.05). (E) There was no difference in pJNK in the DS between alcohol-dependent animals in withdrawal and non-dependent animals (p>0.05). (F) There was no difference in pJNK in the VS between alcohol-dependent animals in withdrawal and non-dependent animals (p>0.05). Significant effects were observed in both p46 and p54 molecular weight bands representing JNK1-3 phosphoisoforms. Data are expressed as pJNK levels normalized to total JNK levels. Representative bands indicate changes in pJNK54 (single band), tJNK54 (single band), pJNK46 (single band), and tJNK46 (doublet).
Effects of JNK Inhibition on Alcohol Drinking Behavior in Non-Dependent Animals
We found that there were significant decreases in pJNK in the HP, dmPFC, and CC of alcohol-dependent rats during withdrawal. In the current experiment, we tested whether inhibition of JNK signaling in non-dependent animals would produce increases in alcohol drinking. Non-dependent animals were trained to self-administer alcohol and, following stable acquisition of operant alcohol self-administration, were surgically implanted with intracerebroventricular (ICV) cannulas. After post-surgery stabilization of alcohol drinking, animals were infused with ascending doses of a JNK Inhibitor (0, 1, 2, 3 μL/side of SP600125 (1μg/μl), 1h pretreatment). Each dose was repeated 3 times over consecutive sessions of alcohol self-administration. For alcohol drinking behavior, there was no main effect of drug treatment [F(1,11)=0.0291, p=0.8678], drug dose [F(3,33)=1.018, p=0.3973], or drug treatment × dose interaction [F(3,33)=0.5858, p=0.6286] (Figure 5). In summary, intracerebral infusion of a JNK inhibitor did not alter alcohol-drinking behavior in non-dependent animals.
Figure 5.
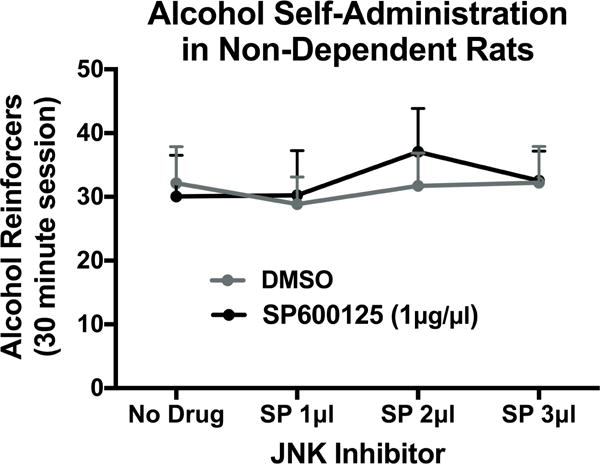
Effect of JNK inhibition on alcohol drinking in non-dependent animals. Intracerebral inhibition of JNK (0, 1, 2, 3μL/side of SP600125 (1μg/1μl), 1 h pretreatment) had no effect on alcohol-drinking behavior in non-dependent animals at any dose (p>0.05).
DISCUSSION
The present study was designed to test how repeated alcohol vapor exposure and withdrawal alters alcohol drinking behavior and JNK phosphorylation in alcohol-dependent animals compared to non-dependent (air-exposed) animals. We found that alcohol-dependent animals consumed significantly more alcohol versus non-dependent animals. As a key mediator of glucocorticoid-regulated stress signaling, we also tested if acute and chronic alcohol exposure significantly altered JNK activity in brain regions critically vulnerable to the disruptive effects of excessive alcohol exposure and chronic stress. We found that pJNK was significantly increased in the HP, dmPFC, vmPFC, CC, DS, and VS of previously alcohol-naïve animals following acute alcohol administration. In contrast, pJNK was significantly decreased in the HP, dmPFC, and CC of alcohol-dependent animals during acute withdrawal, while we observed no significant changes in pJNK in the vmPFC, DS, and VS. These findings suggest that while acute alcohol challenge increases neuronal JNK activity, withdrawal from chronic alcohol exposure leads to region-specific deficits in JNK activity, which may contribute to the transition to alcohol dependence.
In the present study, we first measured region-specific changes in pJNK following acute alcohol exposure in alcohol-naïve animals. We found that there were widespread and significant increases in pJNK across the HP, dmPFC, vmPFC, CC, DS, and VS of animals that were given an intoxicating bolus of alcohol compared to saline-injected controls. This demonstrates that an intoxicating dose of alcohol can increase JNK activity in multiple brain regions. JNK is a kinase that mediates a negative regulatory effect on GR, by promoting export of the receptor from the nucleus and decreasing its activity (Rogatsky et al., 1998; Adzic et al., 2009). Based on evidence that JNK can regulate the activity of GR-mediated stress signaling, our findings suggest that the initial alcohol exposure increases central JNK activity, which may initially dampen GR activity in several regions prone to the damaging effects of alcohol.
Alcohol dependence is associated with an escalation of alcohol intake and the development of negative affective states (Koob & Le Moal, 1997; Edwards & Koob, 2010). We used chronic, intermittent ethanol vapor exposure (CIEV) to model alcohol dependence in rodents (Rogers et al., 1979; Gilpin et al., 2008; Vendruscolo & Roberts, 2014), and found that alcohol-dependent animals consumed significantly more alcohol than non-dependent animals during acute alcohol withdrawal. This effect was specific to alcohol drinking as there was no difference in water consumption between groups. We also found that alcohol-dependent animals significantly escalated their alcohol drinking from baseline drinking levels, which reflects a key DSM-V criterion for AUD.
The behavioral and motivational impairments associated with uncontrolled, excessive alcohol drinking are thought attributed to long-lasting neuroadaptations and functional changes to neuronal circuitry. Repeated and excessive use of alcohol and other drugs of abuse act upon a variety of brain regions to alter intracellular signaling and neurotransmission (Edwards & Koob, 2013), which can result in functional changes within different brain regions. In the present study, we investigated chronic alcohol and withdrawal-induced neuroadaptations in JNK activity in the regions critically vulnerable to the disruptive effects of excessive alcohol exposure. In contrast to the acute alcohol findings, pJNK was significantly decreased in the HP, dmPFC, and CC of alcohol-dependent animals in withdrawal compared to non-dependent animals. This decrease in JNK phosphorylation was specific to the HP, dmPFC, and CC, because there was no difference in pJNK between groups in the vmPFC, DS, and VS. These findings indicate that withdrawal from chronic alcohol exposure after escalation of alcohol intake leads to region-specific deficits in JNK activity. Decreased pJNK activity in alcohol dependence is hypothesized to result in greater GR activity. This is supported by evidence that chronic stress decreases pJNK in the hippocampus and prefrontal cortex, while increasing the GR activity in these regions (Adzic et al., 2009), indicating the ability of JNK activation to limit GR activity. Dysregulation of GR signaling has been implicated in the transition from initial alcohol use to alcohol dependence (Richardson et al., 2008; Vendruscolo et al., 2012; Lu & Richardson, 2014; Repunte-Canonigo et al., 2015; Vendruscolo et al., 2015; Somkuwar et al., 2017). Accordingly, decreased JNK activity during withdrawal from alcohol dependence and may promote escalation of alcohol drinking through dysregulation of GR-mediated stress signaling. Based on our findings, it is possible that escalated drinking may partly be driven by attempts to restore JNK-mediated regulation of GR and decrease GR activity in the HP, dmPFC, and CC. This is supported by reports that GR antagonism via mifepristone reduces escalated drinking in both preclinical animal models and early clinical trials (Vendruscolo et al., 2015). These relationships between JNK and GR activity in the context of alcohol dependence will be explored in our future studies, although alternative signaling mechanisms downstream of JNK should not be ruled out.
We then tested the hypothesis that intracerebral administration of SP600125 (JNK inhibitor) would promote escalated drinking in non-dependent animals. Animals were given an ascending dose regimen of SP600125 and tested for alcohol drinking behavior. Contrary to our hypothesis, we found that the intracerebral JNK inhibition had no effect on alcohol-drinking behavior in non-dependent animals. It is possible that changes in alcohol drinking behavior may only be revealed by region-specific manipulations to brain areas where we found significant changes in JNK activity. Alternatively, changes to central brain JNK activity may only have behavioral effects in the context of dependence. Future studies will investigate pharmacological rescue of JNK deficits on excessive alcohol drinking behavior in alcohol-dependent animals, whereby JNK activation would be expected to reduce escalated drinking.
In conclusion, these findings demonstrate that acute alcohol challenge increases JNK activity in the hippocampus, frontal cortical regions, and striatum, while withdrawal from chronic alcohol exposure leads to region-specific (HP, dmPFC, CC) deficits in JNK activity. JNK signaling dysregulation may promote escalation of alcohol drinking over time in alcohol dependence, either as a result of increased GR-mediated stress signaling or via other downstream mechanisms. Restoration of JNK activity in the HP, dmPFC, and CC following alcohol drinking could potentially alleviate escalated drinking produced by dependence and withdrawal. Future studies should examine these hypotheses while attempting to more definitively link JNK-GR activity in the manifestation of alcohol dependence.
Highlights.
Neuronal JNK phosphorylation is increased following acute alcohol administration
Alcohol-dependent animals escalate alcohol drinking behavior over time
Neuronal JNK phosphorylation is decreased during withdrawal in dependent animals
Acknowledgments
This work was generously supported by research and training grants from the National Institute on Alcohol Abuse and Alcoholism (T32AA007577, ARP; R00AA020839, SE) and by LSUHSC School of Medicine start-up funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
The authors declare no competing financial interests or potential conflicts of interest.
References
- Adzic M, Djordjevic J, Djordjevic A, Niciforovic A, Demonacos C, Radojcic M, Krstic-Demonacos M. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol. 2009;202(1):87–97. doi: 10.1677/JOE-08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzler J, Ganjam GK, Legler K, Stöhr S, Krüger M, Steger J, Tups A. Acute inhibition of central c-Jun N-terminal kinase restores hypothalamic insulin signalling and alleviates glucose intolerance in diabetic mice. J Neuroendocrinol. 2013;25(5):446–454. doi: 10.1111/jne.12018. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5(3):393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of addiction liability. Behavioural Pharmacology. 2013;24(5–6):356–365. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim O, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction Biology. 2012;17(1):76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0929s44. Chapter 9, Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156(4):1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MA, Paulsen RI, Itoga CA, Farooq MA, Reppel JE, Edwards KN, Whitaker AM, Gilpin NW, Edwards S. Withdrawal from Chronic Nicotine Exposure Produces Region-Specific Tolerance to Alcohol-Stimulated GluA1 Phosphorylation. Alcohol Clin Exp Res. 2016;40(12):2537–2547. doi: 10.1111/acer.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Edwards KN, Edwards S. Neurobiological correlates of pain avoidance-like behavior in morphine-dependent and non-dependent rats. Neuroscience. 2017;366:1–14. doi: 10.1016/j.neuroscience.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Shin W, Vendruscolo LF, Lefebvre C, van der Stap L, Kawamura T, Scholsburg JE, Alvarez M, Koob GF, Califano A, Sanna PP. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol. 2015;16:68. doi: 10.1186/s13059-015-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28(8):1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36(1):1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Logan SK, Garabedian MJ. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci U S A. 1998;95(5):2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27(4):466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- SAMHSA releases Behavioral Health, United States, 2012. Psychiatr Serv. Vol. 64. United States: 2013. p. 1281. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32(22):7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125(8):3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


