Abstract
The extant literature finds that children with type 1 diabetes (T1D) experience mild cognitive alterations compared to healthy age-matched controls. The neural basis of these cognitive differences is unclear but may relate in part to the effects of dysglycemia on the developing brain. We investigated longitudinal changes in hippocampus volume in young children with early-onset T1D. Structural magnetic resonance imaging data were acquired from 142 children with T1D and 65 age-matched control subjects (4–10 years of age at study entry) at two time points, 18 months apart. The effects of diabetes and glycemic exposure on hippocampal volume and growth were examined. Results indicated that although longitudinal hippocampus growth did not differ between children with T1D and healthy control children, slower growth of the hippocampus was associated with both increased exposure to hyperglycemia (interval HbA1c) and greater glycemic variability (MAGE) in T1D. These observations indicate that the current practice of tolerating some hyperglycemia to minimize the risk of hypoglycemia in young children with T1D may not be optimal for the developing brain. Efforts that continue to assess the factors influencing neural and cognitive development in children with T1D will be critical in minimizing the deleterious effects of diabetes.
Keywords: Diabetes Mellitus, Type 1, Hyperglycemia, Blood Glucose, Hippocampus, Brain
Introduction
The developing brain undergoes significant and long-lasting changes during childhood (1) and as a result is vulnerable to deleterious insults (2, 3). As glucose is the brain’s primary energy source (4, 5), extreme fluctuations in fluctuations in blood glucose as experienced by children with type 1 diabetes mellitus (T1D) represent a potential threat to neural development. Indeed, despite careful monitoring of insulin dosing, diet and activity, most children with T1D have difficulty maintaining optimal levels of blood glucose (6). Given these issues, studies of cognition and behavior in children with T1D have received increased attention over the last decade (7, 8).
The extant literature finds that children with T1D, especially those with early-onset T1D or histories of severe dysglycemia, experience mild intellectual and memory deficits compared to controls without diabetes of a similar demographic (7–15). The neural basis of these alterations in cognition is unclear but may relate in part to the effects of dysglycemia on the hippocampus, an area critical to learning and memory (16). Insulin receptors and insulin-sensitive glucose transporters are densely populated in this region (17, 18), making it a viable target for the study of T1D effects on the developing brain. Rodent models of streptozotocin-induced experimental diabetes show that chronic dysglycemia results in learning and memory deficits and that these changes are coupled with apoptosis of neurons in the CA1 and CA3 hippocampal subfields (19–22). Furthermore, hyperglycemia has been found in animal studies of T1D to be associated both with structural changes of hippocampal neurons as well as impaired long-term spatial memory (23). Neuroimaging studies of children with T1D also point to glycemic effects on hippocampal structure (24, 25), with early onset of diabetes shown to be associated with apoptosis of hippocampal pyramidal cells (26) and severe hypoglycemic episodes with abnormal enlargement of the hippocampus (27).
While these findings suggest that chronic dysregulation of blood glucose levels contribute to alterations in development of the hippocampus, no studies to our knowledge have attempted a focused, longitudinal investigation of this structure in young children with T1D. Using high-resolution structural MRI in conjunction with continuous glucose monitoring (CGM), we evaluated the association between hippocampal volume change and exposure to dysglycemia in a large (N=211) sample of diabetic and non-diabetic children followed over the course of 18 months. We hypothesized that a diagnosis of T1D in young children would be associated both with decreased hippocampal volume at baseline as well as reduced hippocampal growth over time. Additionally, we hypothesized that smaller longitudinal gains in hippocampus volume would be correlated with increased exposure to dysglycemia.
Methods
Participants
The study was conducted after institutional review board approval at all centers, and informed written consent was obtained. Participants were recruited at five clinical centers participating in the Diabetes Research in Children Network (DirecNet) study group (Nemours Children’s Clinic, Stanford University, University of Iowa, Washington University in St. Louis, Yale University). The Jaeb Center for Health Research served as the Coordinating Center for the study. The Stanford Center for Interdisciplinary Brain Sciences Research served as the Image Data Coordinating Center (IDCC) for the study and research personnel from the IDCC directed all image processing and analyses.
Children with T1D (N=143) and healthy, nondiabetic control children (N=69) between the ages of 4 and 10 years were enrolled in the study (see Supporting Information for details on study recruitment and inclusion and exclusion criteria). Usable imaging data were acquired from 142 individuals with T1D and 65 control participants.
MRI acquisition
All participants were prepared for unsedated MRI scans through scan simulations as previously described (28). Glucose levels were monitored for participants with T1D immediately before and after each scan session to ensure the glucose level was between 70 and 300 mg/dL. These two measurements were averaged together to compute the blood glucose level at the time of scan. T1 weighted images of the brain were acquired using a Siemens 3T Tim Trio whole body MR systems with a 12-channel head coil. Details on scanning and the assessment of inter-scanner reliability are presented in the Supporting Information.
Hippocampus delineation
An initial segmentation of the hippocampus was obtained with FreeSurfer software version 5.3 (http://surfer.nmr.mgh.harvard.edu/). Automated hippocampal segmentations were reviewed and corrected by a single rater (L.F.R) who was blinded to subject group. Ten different randomly selected brains were re-traced and intraclass correlation coefficients were computed. Results from intra-rater testing revealed excellent reliability, with intraclass correlations >0.90.
Experimental Design and Statistical Analysis
Hippocampal volumes were analyzed using Repeated Measure Analyses of Covariance (ANCOVA). Hippocampal volumes were entered as the dependent variable; time point and hemisphere were entered as within-subjects factors. Diagnosis (T1D vs. control) and sex were entered as between-subject factors. Total brain volume (TBV) at baseline and age at baseline were entered as covariates. Analyses were conducted using the Statistical Package for Social Sciences (SPSS version 22.0, IBM Corp., Armonk, N.Y., USA). Significance was established based on a two-tailed α level of .05 for all tests.
Glycemic measures
Within the T1D group, glycated hemoglobin (HbA1c) was determined every 3 months between the baseline and 18-month follow-up evaluation. Continuous glucose monitoring (CGM) was performed to collect glycemic data over the 18 months between baseline and follow-up scans. CGM data were acquired every 3 months for a minimum of 72 hours (at least 24 of which were acquired overnight) at each 3-month time point using either the patient’s clinically prescribed devices, or a study-provided iPro2 (Medtronic MiniMed, Northridge, CA) or Dexcom SEVEN Plus (Dexcom, San Diego, CA). An average of 1423.5 hours (59.3 days) of CGM data were collected on each subject.
The primary glycemic covariate of interest was total hyperglycemia exposure, occurring between the baseline and the 18-month follow-up scans. Total cumulative hyperglycemia exposure from diagnosis until assessment (lifetime HbA1cAUC6%) was determined using all available HbA1c values and computing the area under the curve >6.0% according to the trapezoid rule. Lifetime values were computed at baseline and follow-up. Hyperglycemia exposure occurring over the course of study, henceforth referred to as incremental HbA1cAUC6%, was computed by subtracting the lifetime value at baseline from the lifetime value at follow-up.
Other glycemic covariates of interest were computed from the total CGM wears for a given participant (usually seven per participant) during the 18-month interval and included mean blood glucose (avgMean), two measures of glycemic variability: the standard deviation (SD) of the CGM glucoses and mean amplitude of glycemic excursions (MAGE), and two measures reflecting percentage and severity of blood glucose values in hyperglycemic and hypoglycemic ranges: area under the curve above blood glucose 180 mg/dL (AUC180) and area over the curve below blood glucose 70 mg/dL (AOCBelow70), respectively.
Associations between hippocampus volume and glycemic measures
Associations between hippocampal volume, incremental HbA1cAUC6%, age of T1D onset, T1D duration, blood glucose at scan and CGM measures of hyperglycemia (AUC180), hypoglycemia (AOCBelow70) and glycemic variability (SD and MAGE) within the T1D group were explored in secondary analyses by including each variable as a covariate of interest in separate repeated measures ANCOVA models that controlled for age, TBV, sex and scan interval. Analyses with SD or MAGE included avgMean as a covariate of non-interest to discriminate between the effects of mean glucose and glucose variability. Likewise, analyses with incremental HbA1cAUC6% included baseline HbA1c as a covariate of non-interest to discriminate between the effects of 18-month hyperglycemia exposure and initial mean glucose levels. Analyses with DKA were examined in repeated measures ANCOVA that controlled for age, TBV, sex and scan interval and which compared hippocampus volumes between children with no history of a DKA event and children that had experienced one or more DKA events.
Results
Participants
There were no significant differences between the T1D and control group in age, t(205)=0.285, p=0.776, or sex, χ2 =1.27, p=0.196 (Table 1). The duration of time between baseline and follow-up scans was slightly higher in the control (1.53 ± 0.07 years) relative to the T1D group (1.47 ± 0.09 years), t(205)=5.112, p<0.001. Although the interval between scans was significantly longer in the control group, the absolute difference is equivalent to only 23 days and is not felt to be clinically significant. BMI percentile was significantly higher in the T1D group, t(93.9)=-3.225, p=0.002. Fourteen sibling pairs were included in the study sample; 13 pairs were discordant for T1D and two participants without T1D comprised another pair. As we describe below, analyses were conducted to assess the influence of these variables on our primary findings.
Table 1.
Clinical characteristics of study participants
| Type 1 Diabetes Participants | Control Participants | |
|---|---|---|
| General Information | ||
| N (Female/Male) | 142 (66/76) | 65 (32/33) |
| Race/ethnicitya | 85% W, 7% H, 4% AA, 1% A, 10% O | 88% W, 6% H, 6% AA, 0% A, 6% O |
| BMI percentile, mean ± SD * | 69.4 ± 21.1 | 56.3 ± 28.9 |
| Age at diabetes onset (years) | 4.06 ± 1.86 | - |
| Diabetes duration (years), median (25th, 75th percentile) | 2.6 (1.1, 4.5) | - |
| Scan interval (years) ** | 1.47 ± 0.09 | 1.53 ± 0.07 |
|
| ||
| Baseline characteristics | ||
| Age (years), mean ± SD | 7.0 ± 1.7 | 7.1 ± 1.8 |
| DKA history, n (%)b | 42 (30) | - |
| Severe hypoglycemia history, n (%)c | 24 (17) | - |
| Glycated hemoglobin, % (mmol/mol), mean ± SD | 7.9 ± 0.9 (63 ± 10) | 5.2 ± 0.2 (33 ± 2) |
| Lifetime averaged HbA1cAUC6%, mean ± SD | 2.0 ± 0.8 | - |
| Blood glucose level at scan (mg/dL) | 175.9 ± 54.1 | - |
|
| ||
| 18-month characteristics | ||
| Age (years), mean ± SD | 8.4 ± 1.7 | 8.6 ±1.8 |
| Interval DKA history, n (%)d | 4 (2.8) | - |
| Interval severe hypoglycemia history, n (%)e | 5 (3.5) | - |
| Glycated hemoglobin, % (mmol/mol), mean ± SD | 7.9 ± 0.9 (63 ± 10) | 5.2 ± 0.3 (33 ± 3) |
| Lifetime averaged HbA1cAUC6%, mean ± SD | 2.1 ± 0.8 | - |
| Blood glucose level at scan (mg/dL) | 176.4 ± 56.2 | - |
|
| ||
| Average CGM indices over 18 months, median (IQR) | ||
| % Glucose within target range (70–180 mg/dL) | 45 (37, 51) | - |
| % Glucose > 180 mg/dL | 50 (41, 58) | - |
| % Glucose < 70 mg/dL | 5 (3, 7) | - |
| Mean glucose (mg/dL) | 192 (176, 210) | - |
| Glucose SD (mg/dL) | 83 (74, 90) | - |
| MAGE (mg/dL) | 160 (141, 175) | - |
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; IQR, interquartile range; MAGE, mean amplitude of glycemic excursions; AA, African American; A, Asian; H, Hispanic; O, other/more than one race; W, white.
Includes 50 participants with one episode each.
Includes 19 participants with one episode, 3 participants with two episodes and 1 participant with three episodes.
Includes 4 participants with one episode each.
Includes 3 participants with one episode each and 2 participants with two episodes. Lifetime averaged HbA1cAUC is the cumulative lifetime value divided by duration.
p=0.002.
p<0.001
Blood glucose at the time of scan was similar at both study time points, F(1,139)=0.007, p=0.934. Within-subject differences in blood glucose levels between scan time points ranged from −240.5 to190.0 mg/dL. Sex was a significant predictor of blood glucose at scan, F(1,138)=5.474, p=0.021; higher mean blood glucose at scan was present in males (184.3 ± 41.5 mg/dL) relative to females (167.6 ± 39.2 mg/dL), even though the HbA1c levels were well matched across the sexes. The interaction of sex and time was not significant, F(1,138)=0.011, p=0.915, indicating that sex differences in glucose at scan were stable across time points. Sex was not a significant predictor of DKA, age of onset, T1D duration, avgMean, lifetime HbA1cAUC6%, AUC180, AOCBelow70, SD or MAGE.
Volumetric Analyses
Repeated measures ANCOVA indicated that change in hippocampal volume did not differ between T1D and control groups after adjusting for total brain volume, age, and sex, F(1,200)=1.111, p=0.293. Moreover, hippocampal volume did not differ between groups overall, F(1,201)=0.443, p=0.507. Across T1D and controls, hippocampal volume significantly increased over time, F(1,201)=4.351, p=0.038. A significant interaction between time and age at baseline was also observed, F(1,201)=19.106, p<0.001; across both groups, younger participants exhibited greater relative volume increases over time compared to older participants. The interaction between diagnosis and sex was significant, F(1,201)=5.203, p=0.024. Post-hoc tests indicated significantly lower hippocampal volume in males with T1D relative to control males, F(1,105)=4.480, p=0.030; this difference was not seen in females, F(1,94)=1.068, p=0.304 (Figure 1).
Figure 1.
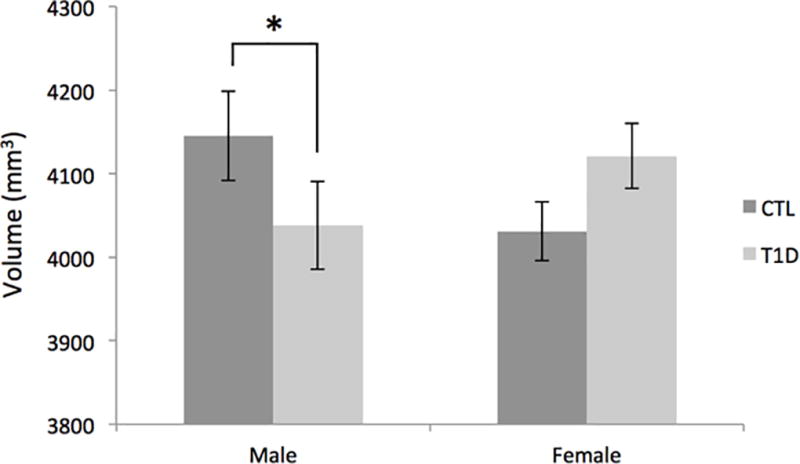
Mean volume of the hippocampus (averaged across hemispheres and time points), adjusted for age at baseline and total brain volume. Error bars represent standard deviation. *p < 0.05.
All findings remained essentially unchanged when scan interval and BMI were entered as covariates in the model and when unaffected siblings from each of the 14 sibling pairs were removed from the analyses. Moreover, intraclass correlations of hippocampal volumes between siblings (adjusted for TBV and age) were low (<0.350), indicating that hippocampal volume was not tightly linked between siblings. As we note below, supplementary analyses were also conducted to assess the influence of blood glucose at the time of scan on hippocampal volumes.
Secondary analyses with glycemic and illness variables
Within the T1D group, a significant interaction of time and incremental HbA1cAUC6% was observed, F(1,133)=7.412, p =0.007 (Figure 2). Tests of simple slopes indicated greater incremental HbA1cAUC6% was associated with slower growth in hippocampus volume over time, β=-0.347, t(133)=-2.723. The interaction of time and MAGE was also significant, F(1,133)=7.023, p=0.009; increased glucose variability (as determined by MAGE) was associated with reduced hippocampal volume growth over time, β=-0.283, t(133)=-2.650. No associations were observed between hippocampus volume and avgMean, SD, AUC180, AOCBelow70, age of T1D onset, T1D duration or history of DKA. Supplementary analyses were conducted to better understand state-related osmolality effects of blood glucose levels on our findings (see Supporting Information). Results showed that findings involving lifetime hyperglycemia exposure, MAGE, diagnosis and the interaction of diagnosis by time were unchanged when controlling for blood glucose levels at scan.
Figure 2.
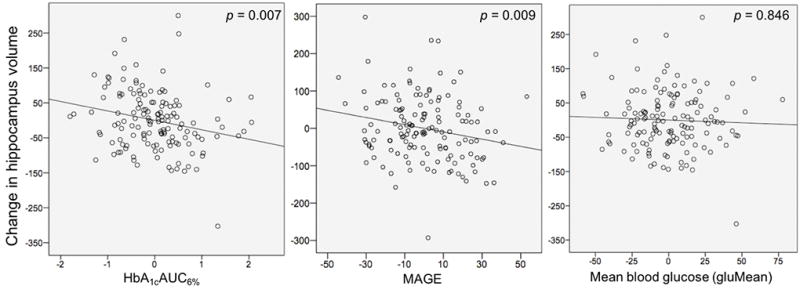
Associations between change in hippocampus volume from time 1 to time 2 and total exposure to hyperglycemia (incremental change in HbA1cAUC6%, left), mean amplitude of glycemic excursions (MAGE, middle) and mean blood glucose (avgMean, right) experienced between these two time points. Values are adjusted for covariates in the model (see text).
Discussion
Using one of the largest neuroimaging samples of children with T1D available to date, results of our analyses indicate that longitudinal hippocampus growth over 18 months did not differ between children with T1D and healthy control children. However, slower hippocampal growth among children with T1D was associated with both increased exposure to hyperglycemia (as measured by incremental HbA1cAUC6%) and with greater glycemic variability (as measured by MAGE). Our findings confirm prior reports of a possible metabolic effect of dysglycemia on the developing hippocampus (19–22, 24), and suggest that both chronic hyperglycemia and large glucose fluctuations may be detrimental to the developing brain.
Although Pell et al (29) reported decreased parahippocampal gray matter volume in a cross-sectional study of young adults with diabetes of longer duration, other cross-sectional studies targeting the hippocampal region in children with T1D have also reported an absence of a main effect of diagnosis. Hershey et al. found no evidence for a group difference in hippocampus volume between 95 youth with T1D and 49 sibling control subjects, ages 7–17 (27). Similarly, Aye et al. (30) observed no differences in hippocampus structure between 25 children with T1D and 16 healthy control children, ages 3–10. In a study of adults, Lobnig et al. (13) found no volumetric differences between 13 participants with T1D and 13 healthy age-matched controls. Our longitudinal results extend these findings through showing that T1D is not associated with alterations in hippocampal growth rate over time.
Results of our analyses relating hippocampus change with measures of blood glucose are of potential importance and emphasize the deleterious effects of dysglycemia on brain development. Both increased exposure to hyperglycemia and greater variability in glucose levels, measured during the months between baseline and follow-up scans, were associated with decreased growth in the hippocampus across these two time points. Such a pattern is consistent with results in the hippocampal region from whole brain voxel based morphometry analyses conducted by our group in a partially overlapping sample of children (24), and highlights the importance of conducting more detailed assessments of brain structure in T1D. Indeed, future studies aimed at clarifying T1D’s effect on the brain should take glycemic measures into consideration, particularly since current clinical practice involves the toleration of some hyperglycemia to minimize the risk of hypoglycemia in young children with T1D.
The mechanisms mediating our observed associations between blood glucose measures and hippocampus growth are unclear. Posited mechanisms of hyperglycemia-based tissue damage include increased flux of glucose through the polyol pathway, increased formation of advanced glycation end-products (AGEs), activation of protein kinase C (PKC) isoforms and overactivity of the hexosamine pathway (see 31 for a review). It has been suggested that such events may be triggered by a single upstream occurrence of mitochondrial overproduction of reactive oxygen species, or oxidative stress (32). In line with this formulation, markers of oxidative stress, such as 8-iso prostaglandin F2α (8-iso PGF2α), are abnormally increased in patients with T1D (33, 34), and improvements in the control of blood glucose levels result in a reduction of these markers (34). Moreover, spatial learning, a hippocampal-dependent cognitive function, is impaired in streptozotocin (STZ)-diabetic rats (35) and improves following treatments that reduce oxidative stress in the hippocampus (36).
Some have posited that, in addition to hyperglycemia, glucose variability increases risk of microvascular complications. In type 2 diabetes, Monnier and colleagues (37) found that excretion of 8-iso PGF2α was not correlated with sustained hyperglycemia, but rather with glucose fluctuations. A subsequent report by Kilpatrick et al (38) and a recent meta-analysis by Gorst et al, (39), found that microvascular complications in T1D were associated with variability in HbA1c, independent of mean HbA1c levels. Importantly, however, variability of HbA1c is not a reflection of actual glucose variability as measured by CGM, as in our study. Whether glycemic variability, as reflected in fluctuations of actual glucose levels contributes to microvascular complications remains controversial (40, 41). Nevertheless, our data herein, and our previously published data (24), suggest an association between glycemic variability and neuroimaging results, including hippocampal size. Further studies examining the relative associations between glucose variability, mean glucose, oxidative stress and hippocampal structure are warranted.
The current study is the first, to our knowledge, to report on a sex-specific effect of T1D on brain structure. However, because blood glucose levels at the time of scan were higher in boys versus girls in our T1D sample, we cannot exclude the possibility that this finding was at least partially influenced by glucose-related hydration effects on the brain. Indeed, excess blood glucose is a known cause of dehydration in children with diabetes and studies of healthy adults show that dehydration is associated with significant, reversible reductions in brain tissue volume (42, 43). Interactions between sex and diagnosis have nevertheless been noted in some prior cognitive studies of T1D. Schonle et al (44) found that boys with early-onset T1D showed declines in IQ relative to boys with later onset T1D and to females with an early- or later-onset. Similarly, Ghetti et al. (45) found that males with T1D performed significantly worse on two measures of memory relative to females with T1D. And, Fox et al. (46) found that girls, but not boys, with T1D made expected gains in verbal learning over time. Whether these differences in cognition may be caused by sex-specific alterations in hippocampal volume or blood glucose is unknown. Additional research designed to clarify the influence of sex and blood glucose levels on cognition, and glucose-related dehydration effects on brain volume in T1D would be helpful in teasing apart the effects of these factors.
The current study had minor limitations. First, this study did not consider the association between hippocampal volume and performance on measures assessing cognitive and behavioral domains putatively subserved by this brain region. Second, MAGE and total hyperglycemia exposure in our participants were significantly correlated. Thus, we cannot determine with certainty from the current study whether one or both measures were driving slower hippocampus growth in children with T1D. Additional studies will be required to more robustly address glycemic contributions to changes in brain structure. Third, because power calculations were not conducted a priori, we cannot exclude the possibility of Type II error. Fourth, although supplementary analyses suggest that our primary study findings were not influenced by state-related effects of blood glucose at scan, we cannot exclude the possibility that hippocampal volumes were influenced by glucose-related hydration effects. Future studies that directly address the influence of glucose-based changes in hydration on brain volume in the context of T1D are needed.
In summary, in this first-ever focused longitudinal study of the hippocampus in T1D, we found that, although overall hippocampal volume did not differ between children with T1D and healthy control children, greater severity of glycemic variability as well as increased exposure to hyperglycemia among affected children were associated with slowed growth of the hippocampus over time. These observations indicate that the current practice of tolerating some hyperglycemia to minimize the risk of hypoglycemia in young children with T1D may not be an optimal strategy for the developing brain. Efforts that continue to assess the factors influencing neural and cognitive development in children with T1D will be critical in minimizing the deleterious effects of this disorder.
Supplementary Material
Acknowledgments
The authors thank the participants and their families and the clinical and imaging staff at all of the investigator sites, as well as external collaborators for the use of their imaging facilities, including University of California at San Francisco (San Francisco, CA), El Camino Hospital (Mountain View, CA), University of Florida and Shands Jacksonville Medical Center (Jacksonville, FL). The authors also thank Karen Winer, MD, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development for advice and support. This research was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (DIRECNET: U01-HD-41890, HD-41906, HD-41908, HD-41915, HD-41918, HD-56526, R01-HD-078463 and U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University) and by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Appendix
The Diabetes Research in Children Network (DirecNet): The Clinical Centers, Principal Investigators (PI), Co-Investigators (I), and Coordinators (C) are as follows: Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI), Michael J. Tansey, MD (I), Julie Coffey, MSN (C), Joanne Cabbage (C), and Sara Salamati (C); Nemours Children’s Health System, Jacksonville, FL: Nelly Mauras, MD (PI), Larry A. Fox, MD (I), Allison Cato, PhD (I), Kim Englert, RN, BSN, CDE (C), Kaitlin Sikes, ARNP, MSN (C), and Tina Ewen (C); Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI), Darrell M. Wilson, MD (I), Tandy Aye, MD (I), and Kimberly Caswell, ARNP (C); Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI), William V. Tamborlane, MD (I), Amy Steffen, BS (C), Kate Weyman, MSN (C), Melinda Zgorski, BSN (C), and Jodie Ambrosino, PhD (I); Washington University in St. Louis, St. Louis, MO: Neil H. White, MD, CDE (PI), Ana Maria Arbelaez, MD (I), Lucy Levandoski, PA-C (C), Angie Starnes, RN, BSN, CDE (C), and Tamara Hershey, PhD (I); Imaging and Data Coordinating Center, Stanford University, Stanford, CA: Allan L. Reiss, MD, Lara Foland-Ross, PhD, Matthew J. Marzelli, BS, Paul K. Mazaika, PhD, Daniel X. Peng, BS and Gabby Tong, BA; Clinical Coordinating Center, Jaeb Center for Health Research, Tampa Florida: Roy Beck, MD, PhD (PI), Craig Kollman, PhD (Co-I), Katrina Ruedy, MPH (Co-I); Data and Safety Monitoring Board: Mark Sperling, MD, Dorothy M. Becker, MBBCh, Patricia Cleary, MS, Carla Greenbaum, MD, and Antoinette Moran, MD.
Footnotes
Conflict of Interest
S.A.W. reports receiving consulting fees from Insulet and payment to his institution from a Medtronic grant. N.H.W. has received payment for consultancy from Novo Nordisk and Daiichi Sankyo. N.M. reports a research device supply agreement with her institution from Medtronic and Lifescan and research grant support from Medtronic, and consultancy from PicoLife Technologies. TA reports payment to her institution from a Medtronic Grant. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–34. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–82. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- 3.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011 doi: 10.1093/brain/awr103. awr103. [DOI] [PubMed] [Google Scholar]
- 4.Amiel S. Hypoglycaemia in diabetes mellitus–protecting the brain. Diabetologia. 1997;40:S62–S8. doi: 10.1007/s001250051404. [DOI] [PubMed] [Google Scholar]
- 5.Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochemistry international. 2013;63:331–43. doi: 10.1016/j.neuint.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to< 10 years. Diabetes Care. 2012;35:204–10. doi: 10.2337/dc11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershey TCA, Bondurant A, Aye T, Conrad A, Ambrosino J, White NH, Mauras N, Kollman C, Ruedy K, Xing D. for the Diabetes Research in Children Network (DirecNet). Cognitive and behavioral differences in young children with type 1 diabetes mellitus: Preliminary Results. Diabetes. 2012;61 [Google Scholar]
- 8.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes a meta-analysis. Diabetes care. 2008;31:1892–7. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. Journal of child neurology. 2011 doi: 10.1177/0883073811406730. 0883073811406730. [DOI] [PubMed] [Google Scholar]
- 10.Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychology. 2004;10:36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- 11.Hershey T, Craft S, Bhargava N, White NH. Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. Journal of the International Neuropsychological Society. 1997;3:509–20. [PubMed] [Google Scholar]
- 12.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–7. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 13.Lobnig B, Krömeke O, Optenhostert-Porst C, Wolf O. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabetic Medicine. 2006;23:32–9. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- 14.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes care. 2001;24:1541–6. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 15.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatric diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 16.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99:195. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. The Lancet Neurology. 2004;3:169–78. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. European journal of pharmacology. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, et al. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behavioural brain research. 2009;198:224–30. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Huang H-J, Chen Y-H, Liang K-C, Jheng Y-S, Jhao J-J, Su M-T, et al. Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One. 2012;7:e39656. doi: 10.1371/journal.pone.0039656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z-G, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain research. 2002;946:221–31. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- 22.Ye L, Wang F, Yang R-H. Diabetes impairs learning performance and affects the mitochondrial function of hippocampal pyramidal neurons. Brain research. 2011;1411:57–64. doi: 10.1016/j.brainres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, et al. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatric diabetes. 2008;9:531–9. doi: 10.1111/j.1399-5448.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 24.Mauras N, Mazaika P, Buckingham B, Weinzimer S, White NH, Tsalikian E, et al. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2014 doi: 10.2337/db14-1445. DB_141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazaika PK, Weinzimer SA, Mauras N, Buckingham B, White NH, Tsalikian E, et al. Variations in brain volume and growth in young children with type 1 diabetes. Diabetes. 2016;65:476–85. doi: 10.2337/db15-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho MS, Weller NJ, Ives FJ, Carne CL, Murray K, vanden Driesen RI, et al. Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. The Journal of pediatrics. 2008;153:385–90. doi: 10.1016/j.jpeds.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59:236–41. doi: 10.2337/db09-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–40. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pell GS, Lin A, Wellard RM, Werther GA, Cameron FJ, Finch SJ, et al. Age-related loss of brain volume and T2 relaxation time in youth with type 1 diabetes. Diabetes care. 2012;35:513–9. doi: 10.2337/dc11-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aye T, Reiss AL, Kesler S, Hoang S, Drobny J, Park Y, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes care. 2011;34:1458–62. doi: 10.2337/dc10-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences. 2000;97:12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davì G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, et al. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus. Circulation. 2003;107:3199–203. doi: 10.1161/01.CIR.0000074205.17807.D0. [DOI] [PubMed] [Google Scholar]
- 34.Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. In vivo formation of 8-Iso-prostaglandin F2α and platelet activation in diabetes mellitus. Circulation. 1999;99:224–9. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 35.Biessels G-J, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, et al. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–66. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 36.Baluchnejadmojarad T, Roghani M. Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behavioural brain research. 2011;224:305–10. doi: 10.1016/j.bbr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol J-P, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Jama. 2006;295:1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2008;31:2198–202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38:2354–69. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 40.Ceriello A, Kilpatrick ES. Glycemic variability: both sides of the story. Diabetes Care. 2013;36:S272–S5. doi: 10.2337/dcS13-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilpatrick ES. Arguments for and against the role of glucose variability in the development of diabetes complications. Journal of diabetes science and technology. 2009;3:649–55. doi: 10.1177/193229680900300405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duning T, Kloska S, Steinsträter O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–50. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- 43.Streitbürger D-P, Möller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PloS one. 2012;7:e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenle E, Schoenle D, Molinari L, Largo R. Impaired intellectual development in children with Type I diabetes: association with HbA1 c, age at diagnosis and sex. Diabetologia. 2002;45:108–14. doi: 10.1007/s125-002-8250-6. [DOI] [PubMed] [Google Scholar]
- 45.Ghetti S, Lee JK, Sims CE, DeMaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. The Journal of pediatrics. 2010;156:109–14. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 46.Fox MA, San Chen R, Holmes CS. Gender differences in memory and learning in children with insulin-dependent diabetes mellitus (IDDM) over a 4-year follow-up interval. Journal of pediatric psychology. 2003;28:569–78. doi: 10.1093/jpepsy/jsg047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


