Abstract
Sterile alpha motif and histidine/aspartic acid domain containing protein 1 (SAMHD1) limits the efficacy of cytarabine (ara-C) used in AML by hydrolyzing its active metabolite ara-CTP and thus represents a promising therapeutic target. SAMHD1 has also been implicated in DNA damage repair that may impact DNA damage-inducing therapies such as anthracyclines, during induction therapy. To determine whether SAMHD1 limits ara-C efficacy during induction or consolidation therapy, SAMHD1 protein levels were assessed in two patient cohorts of de novo AML from The University of Texas MD Anderson Cancer Center (USA) and the National University Hospital (Singapore), respectively, using immunohistochemistry and tissue microarrays. SAMHD1 was expressed at a variable level by AML blasts but not in a broad range of normal hematopoietic cells in reactive bone marrows. A sizeable patient subset with low SAMHD1 expression (<25% of positive blasts) was identified, which was significantly associated with longer event-free (EFS) and overall (OS) survival in patients receiving high-dose cytarabine (HDAC) during consolidation. Therefore, evaluation of SAMHD1 expression level in AML blasts at diagnosis, may stratify patient groups for future clinical trials combining HDAC with novel SAMHD1 inhibitors as consolidation therapy.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of neoplasms derived from myeloid progenitor cells. Overall survival (OS) after five years in AML patients is age-dependent and ranges from ~70% in children1 to less than 20% in elderly adults2. The most important drugs in the treatment of AML patients are anthracyclines that contribute heavily to the success of remission induction therapy3 and cytarabine (ara-C). Ara-C is particularly effective in high-dose remission consolidation courses4. The inter-patient variability of response to high-dose ara-C (HDAC) regimens correlates with the propensity of AML blasts to accumulate ara-CTP intracellularly5, the main determinant of ara-C efficacy6.
We and others recently identified SAMHD1 as a major negative factor limiting ara-CTP accumulation and retention via a hitherto unknown ara-CTPase activity7–12. SAMHD1 decreases intracellular ara-CTP concentrations, limiting its lethal mis-incorporation into DNA and thus promoting cell survival7,13. Ara-C treatment was more effective in AML xenotransplant mouse models lacking functional SAMHD1 as compared to SAMHD1-proficient counterparts7,8,11. Furthermore, depletion of SAMHD1 in primary AML blasts using the lentiviral protein X (Vpx), which targets SAMHD1 for degradation, increased ara-C sensitivity7.
In our previous report we were able to demonstrate that ara-C-treated patients with higher SAMHD1 mRNA expression at diagnosis had reduced OS and event-free survival (EFS) as compared to patients with lower SAMHD1 expression, in both the adult de novo The Cancer Genome Atlas (TCGA) and the pediatric Therapeutically Applicable Research to Generate Effective Treatments (TARGET) AML cohorts7. However, complete responders versus non-responders showed no significant difference in SAMHD1 mRNA expression in either cohort7. By contrast, high SAMHD1 expression tended to result in better complete response rates7, which might be explained by the role of SAMHD1 as a tumor suppressor9. SAMHD1 also has been implicated in DNA repair and thus modulates the efficacy of DNA damage-inducing agents14,15. Hence, possible advantages of low SAMHD1 levels in patients treated with low-dose ara-C might be mitigated by its combination with anthracyclines, which would rather benefit from higher SAMHD1 expression8,9,11. In addition, we could demonstrate that the effect of SAMHD1 on AML survival varies with time after diagnosis9. Accordingly, for clinical trials aimed at adjusting ara-C doses according to SAMHD1 expression or to combine ara-C with methods to target SAMHD1, it is of utmost importance to understand when to expect the most benefit from these interventions. Dose adjustments and addition of potential strategies to inhibit SAMHD1 at the wrong time might jeopardize possible therapeutic improvements or lead to worse outcomes due to excess toxicity or inhibition of SAMHD1 tumor suppressor functions.
Toward this end, we analyzed two independent and clinically different cohorts of de novo AML to assess whether SAMHD1 protein expression in blasts at the time of diagnosis correlates with clinical endpoints, including complete remission (CR), EFS and OS, as well as the type of ara-C treatment (low-dose vs. high-dose). The data presented here suggest that SAMHD1 expression in AML blasts correlates with clinical outcome after HDAC consolidation therapy.
Patients and methods
Patient groups
Eligible patients had tissue specimens available for immunohistochemical determination of SAMHD1 expression. The diagnosis and subclassification of AML were established according to criteria defined in the World Health Organization classification (2008). A total of 222 de novo AML patients with available diagnostic bone marrow specimens were included, and immunohistochemistry was evaluable in 189 patients. Of these, 98 patients were diagnosed and treated at The University of Texas MD Anderson Cancer Center (MDACC) between 1 June 2007 and 31 December 2015 (87 of which had evaluable IHC results), and 124 patients were diagnosed and treated at the National University Hospital of Singapore (NUH) between 1 February 2001 and 31 December 2011 (102 of which had evaluable IHC results). Research use of these samples was in accord with the Declaration of Helsinki and was approved by the Institutional Review Boards of MDACC (USA) and Domain Specific Review Board, National Healthcare Group (Singapore). The basic demographic and clinical data, including therapy, for both cohorts are shown in Table 1. The cohorts from MDACC and NUH differed substantially with regards to the extent of SAMHD1 expression (percentage of positive blasts), cytogenetic risk group, treatment, response to therapy as well as NPM1 mutation status (Table 1). These two completely different AML cohorts were included in the study on purpose in order to further validate the clinical significance of SAMHD1 expression in AML in various biological backgrounds and different clinical settings. Patients were allocated to favorable-, intermediate- and high-risk cytogenetic risk groups per currently used National Cancer Center Network (NCCN) guidelines (version 3, 2017).
Table 1.
Characteristics of included patients with valid SAMHD1 data
| Total | MDACC | Singapore | P | |
|---|---|---|---|---|
| Number of included patients | 189 | 87 | 102 | |
| Age (years), median (range) | 52 (18–80) | 56 (18–79) | 48 (19–80) | 0.054a |
| SAMHD1 percent positive blasts, median (range) | 30 (1–100) | 40 (3–100) | 25 (1–95) | 0.021a |
| Male sex, n (%) | 103 (54.5) | 50 (57.5) | 53 (52.0) | 0.47d |
| Cytogenetic risk group | <0.0001b | |||
| Favorable, n (%) | 12 (6.3) | 0 (0.0) | 12 (11.8) | |
| Intermediate, n (%) | 157 (83.1) | 87 (100.0) | 70 (68.6) | |
| Poor, n (%) | 19 (10.1) | 0 (0.0) | 19 (18.6) | |
| N/A, n (%) | 1 (0.5) | 0 (0.0) | 1 (1.0) | |
| Response | <0.0001b | |||
| Complete response, n (%) | 106 (56.1) | 74 (85.1) | 32 (31.4) | |
| No complete response, n (%) | 68 (36.0) | 11 (12.6) | 57 (55.9) | |
| N/A, n (%) | 15 (7.9) | 2 (2.3) | 13 (12.7) | |
| FLT3-ITD mutation | 0.56b | |||
| Positive, n (%) | 45 (23.8) | 32 (36.8) | 13 (12.7) | |
| Negative, n (%) | 84 (44.4) | 54 (62.1) | 30 (29.4) | |
| N/A, n (%) | 60 (31.7) | 1 (1.1) | 59 (57.8) | |
| FLT3-TKD mutation | 0.17b | |||
| Positive, n (%) | 11 (5.8) | 10 (11.5) | 1 (1.0) | |
| Negative, n (%) | 115 (60.8) | 76 (87.4) | 39 (38.2) | |
| N/A, n (%) | 63 (33.3) | 1 (1.1) | 62 (60.8) | |
| NPM1 mutation | 0.0075b | |||
| Positive, n (%) | 44 (23.3) | 38 (43.7) | 6 (5.9) | |
| Negative, n (%) | 60 (31.7) | 37 (42.5) | 23 (22.5) | |
| N/A, n (%) | 85 (45.0) | 12 (13.8) | 73 (71.6) | |
| IDH mutation | ||||
| Positive, n (%) | 11 (5.8) | 0 (0.0) | 11 (10.8) | |
| Negative, n (%) | 37 (19.6) | 0 (0.0) | 37 (36.3) | |
| N/A, n (%) | 141 (74.6) | 87 (100.0) | 54 (52.9) | |
| DNMT3A mutation | ||||
| Positive, n (%) | 10 (5.3) | 0 (0.0) | 10 (9.8) | |
| Negative, n (%) | 39 (20.6) | 0 (0.0) | 39 (38.2) | |
| N/A, n (%) | 140 (74.1) | 87 (100.0) | 53 (52.0) | |
| Induction treatment | <0.0001c | |||
| High-dose AraC | 89 (47.1) | 87 (100.0) | 2 (2.0) | |
| Low-dose AraC | 55 (29.1) | 0 (0.0) | 55 (53.9) | |
| Other/N/A, n (%) | 45 (23.8) | 0 (0.0) | 45 (44.1) | |
| Consolidation treatment | <0.0001d | |||
| High-dose AraC | 119 (63.0) | 87 (100.0) | 32 (31.4) | |
| Other/N/A, n (%) | 70 (37.0) | 0 (0.0) | 70 (68.6) | |
| Clofarabine | ||||
| Yes, n (%) | 23 (12.2) | 23 (26.4) | 0 (0.0) | |
| No, n (%) | 64 (33.9) | 64 (73.6) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
| Fludarabine | ||||
| Yes, n (%) | 25 (13.2) | 25 (28.7) | 0 (0.0) | |
| No, n (%) | 62 (32.8) | 62 (71.3) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
| Cladribine | ||||
| Yes, n (%) | 2 (1.1) | 2 (2.3) | 0 (0.0) | |
| No, n (%) | 85 (45.0) | 85 (97.7) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
| Sorafenib | ||||
| Yes, n (%) | 6 (3.2) | 6 (6.9) | 0 (0.0) | |
| No, n (%) | 81 (42.9) | 81 (93.1) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
| Gemtuzumab-ozogamicin | ||||
| Yes, n (%) | 6 (3.2) | 6 (6.9) | 0 (0.0) | |
| No, n (%) | 81 (42.9) | 81 (93.1) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
| Vorinostat (SAHA) | ||||
| Yes, n (%) | 12 (6.3) | 12 (13.8) | 0 (0.0) | |
| No, n (%) | 75 (39.7) | 75 (86.2) | 0 (0.0) | |
| N/A, n (%) | 102 (54.0) | 0 (0.0) | 102 (100.0) | |
aMann–Whitney U-test
bFisher’s exact test (excluding N/A category)
cChi-square test
dFisher’s exact test
Therapy
Treatment was as follows: 144 patients received ara-C-based induction therapy, four received non-ara-C containing induction therapy, and 41 lacked clinical data on the induction regimen used. Among the 189 patients in this study group with evaluable SAMHD1 data, 66 underwent allogeneic stem cell transplantation (allo-SCT). Signed informed consents (MDACC) or waiver of consent (NUH) were obtained before all procedures and before the administration of all investigational therapy according to local practice guidelines.
Unless the clinical condition precluded intensive chemotherapy, patients were treated with ara-C and anthracycline-based therapies. Low-dose ara-C treatment was 100–200 mg/m2. Patients treated at MDACC were all enrolled in clinical trials NCT00542971 (n = 5), NCT00656617 (n = 15), NCT00422591 (n = 1), NCT01025154 (n = 4), NCT01019317 (n = 25), NCT01289457 (n = 45), and NCT02115295 (n = 3) that—in addition to anthracyclines and ara-C—evaluated the addition of other antimetabolites, such as clofarabine, fludarabine, and cladribine, as well as tyrosine kinase inhibitors (sorafenib), histone deacetylase inhibitors (vorinostat/SAHA), and anti-CD33 antibodies (gemtuzumab-ozogamicin). CR was defined as absence of disease for at least 1 month as determined by physical examination, lack of blasts in the peripheral blood and blast counts below 5% in bone marrow aspirate smears. Partial remission (PR) was defined as at least a 50% decrease in the percentage of blasts in bone marrow aspirate smears to 5–25% of cellularity. Primary treatment failure was defined as failure to achieve CR during initial therapy. Relapse was defined as progression occurring at least 1 month after CR or PR.
Construction of tissue microarrays (TMA) and immunohistochemical methods
All tissue samples were diagnostic bone marrow biopsy specimens obtained prior to induction therapy. The TMA were constructed using duplicate tumor cores selected from representative areas rich in blasts in each specimen. In addition, full tissue sections from five reactive bone marrow specimens as well as sections from formalin-fixed, paraffin-embedded cell blocks prepared from AML cell lines (Supplementary Methods) were included as controls. All immunohistochemical analyses were performed in the same research laboratory (Karolinska Institutet) using identical protocols for all TMAs, control bone marrow specimens and cell blocks after antigen retrieval as described previously16. A polyclonal rabbit anti-SAMHD1 antibody (cat. no. A303-691A, Bethyl Laboratories, San Antonio, TX, USA) and an automated detection system (Ventana Medical Systems, Roche, Basel, Switzerland) were utilized. The specificity of the polyclonal antibody was previously tested by Western blot analysis in AML cell lines and by immunohistochemistry using paraffin-embedded cell blocks as shown in Suppl. Figure 1. In addition to immunohistochemical detection of SAMHD1, double immunostainings were performed on reactive bone marrow specimens using four combinations of antibodies including SAMHD1/MPO, SAMHD1/GPA, SAMHD1/CD61, and SAMHD1/CD34 (Cat. no. 760-2659 for MPO, 760-4257 for GPA, 760-4249 for CD61, 790-2927 for CD34, all from Ventana, Roche Diagnostics Scandinavia, Stockholm, Sweden) in order to assess SAMHD1 expression in normal hematopoietic cells. In selected CD34+ AML cases, SAMHD1/CD34 double immunostaining was performed as well. SAMHD1 expression was restricted to the nuclei of blasts and positivity was defined as any level of unequivocal staining. Evaluation of the immunostained slides was blinded to any clinical data. At least 500 blasts were counted in each case, and the level of SAMHD1 expression was defined as the percentage of positive blasts. Based on visual inspection of the frequency distribution of the proportions of SAMHD1-positive AML blasts (histogram) in both the MDACC and NUH databases, two cutoffs were arbitrarily chosen to define a low (<25%) versus high (>75%) level of SAMHD1 expression as described below. Survival analysis also included the intermediate group with a percentage of SAMHD1-positive blasts between 25 and 75%.
Statistical analysis
EFS was measured (number of days) from the beginning of treatment to death, relapse, or last follow-up. OS was measured (number of days) from the beginning of treatment to the time of last follow-up or death from any cause. As the present study investigated effects of SAMHD1 on induction and consolidation treatment, patients receiving allogeneic stem cell transplantation (allo-SCT) were censored on the date of transplant. Survival was visualized using Kaplan–Meier curves, and compared between groups of patients using the log-rank test. Cox proportional hazards models were fitted in order to evaluate the percentage of SAMHD1 positive blasts as a continuous predictor, and to adjust for other covariates. Interaction terms between SAMHD1 and clofarabine, and fludarabine, were tested but found non-significant, and were thus not included in the final models. Associations between SAMHD1 percent positive blasts and presenting clinical and laboratory features were evaluated using Spearman’s rank-correlation coefficient and the Mann–Whitney U-test, for continuous and dichotomous variables, respectively. Kruskal–Wallis test was used to test differences in percentage of SAMHD1 positive blasts between the three risk groups in the Singapore cohort. Presenting clinical and laboratory features were compared between the two patient cohorts from MDACC and NUH, respectively, using the Mann–Whitney U-test for continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables, as appropriate. All statistical analyses were performed using R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Expression of SAMHD1 in reactive bone marrows and AML blasts
In reactive bone marrows (free of malignancy), double immunohistochemistry demonstrated that normal hematopoietic stem cells and progenitors (CD34+), cells belonging to the myeloid/erythroid lineage (MPO+ or GPA+) as well as megakaryocytes (CD61+) were all negative for SAMHD1 expression (Fig. 1). In AML, SAMHD1 was detected predominantly in the nuclei of the blasts (Fig. 2a, c). In the entire study group, the median and mean percentage of the SAMHD1-positive blasts were 30% and 42%, respectively, and ranged from 1 to 100%. The percentage of SAMHD1-positive blasts differed significantly between the two cohorts (median 40% in MDACC vs. 25% in NUH, P = 0.021), but not between risk groups of the NUH cohort (P = 0.5266). The distribution of SAMHD1-positive blasts at diagnosis is shown as a histogram in Fig. 2f. Based on visual inspection of the distribution of these data, a 25% cutoff was chosen arbitrarily to define low SAMHD1 levels and a 75% cutoff to define high SAMHD1 levels. The associations between the levels of SAMHD1 (% positive AML blasts) and presenting clinical and laboratory features of the entire patient group are shown in Table 2. As a continuous variable, the percentage of SAMHD1-positive blasts was significantly associated with serum LDH levels and the white blood count, but not with blast count, CD34 expression in the blast population as assessed by flow cytometry and mutational status of FLT3, NPM1, IDH, or DNMT3A (Table 2). Importantly, no correlation between the percentage of SAMHD1-positive blasts at diagnosis and the subsequently administered ara-C dose was observed (Table 2).
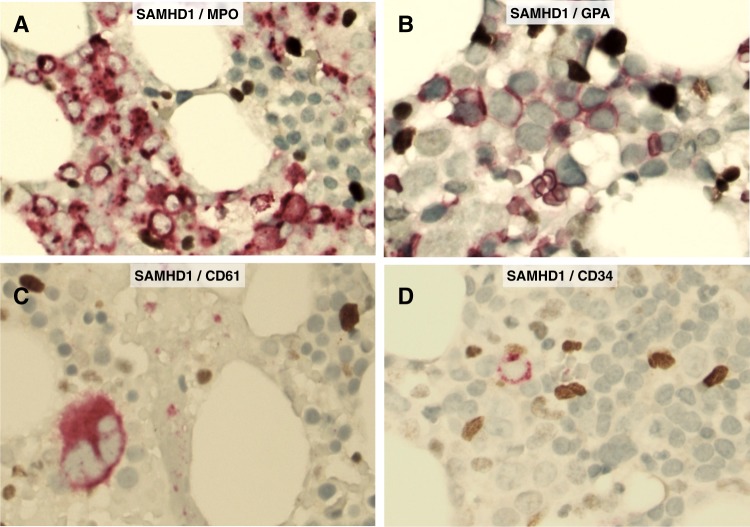
Fig. 1. Expression of SAMHD1 protein in normal bone marrow.
Double immunohistochemical staining shows that SAMHD1 protein is not detected in hematopoietic stem cells and progenitors (CD34+), cells of the myeloid/erythroid lineage (MPO+ or GPA+), and megakaryocytes (CD61+). Small reactive T-lymphocytes and macrophages are strongly positive for SAMHD1, thus serving as internal positive controls in each experimental setting (original magnification ×400; SAMHD1 in dark brown; MPO, GPA, CD61 and CD34 in red)
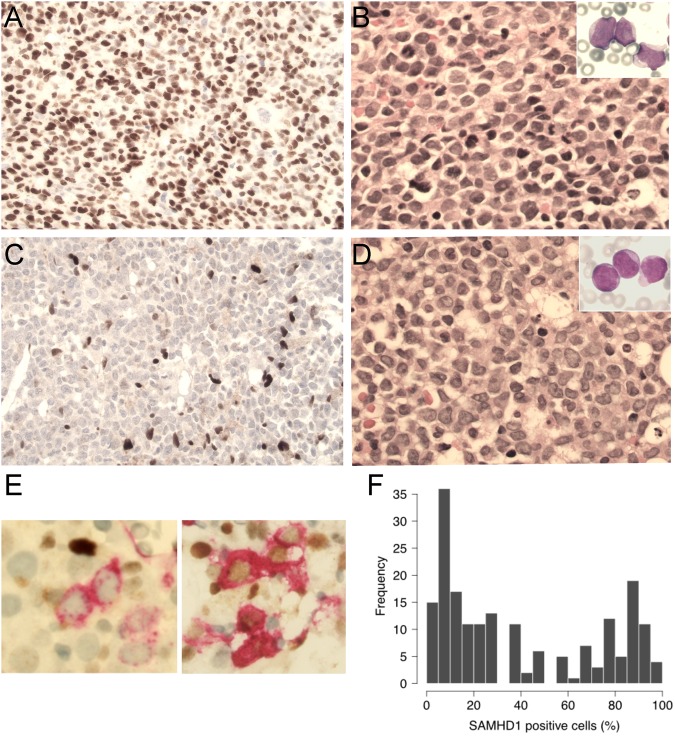
Fig. 2. Expression of SAMHD1 protein in AML blasts.
a, b. SAMHD1 is predominantly expressed in the nucleus of AML blasts. A representative AML case with high SAMHD1 expression (>75%) (a). Morphology of blasts is shown by routine hematoxylin-eosin (H&E) stain of bone marrow section (b) and by May-Grünwald Giemsa (MGG) stain of bone marrow smear (b, inset) (original magnification ×400 for a, b (and ×600 for b, inset). c, d. A representative case of AML with low SAMHD1 expression (<25%) (c). Morphology of blasts is shown by H&E stain of bone marrow section (d) and by MGG stain of bone marrow smear (d, inset) (original magnification ×400 for c, d and ×600 for d, inset). e Examples of double immunostainings (SAMHD1/CD34) in two CD34+ AML cases, one with SAMHD1-/CD34+ blasts (left) and another with SAMHD1+/CD34+ blasts (right). (original magnification ×600; SAMHD1 in dark brown; CD34 in red). f Histogram showing the distribution of SAMHD1 expression level (% of positive blasts) in TMAs of AML patients
Table 2.
Association of SAMHD1 protein levels (% positive AML blasts) with patient characteristics at diagnosis
| Spearman correlation coefficient | P | |
|---|---|---|
| Age (years) | 0.081 | 0.27 |
| PB blast count | 0.10 | 0.34 |
| BM blast count | −0.0061 | 0.94 |
| WBC | 0.230 | 0.034 |
| Platelets | −0.15 | 0.17 |
| Hemoglobin | 0.091 | 0.40 |
| LDH | 0.36 | 0.00056 |
| CD34 | −0.10 | 0.37 |
| FLT3-ITD mutation status | 0.91a | |
| FLT3-TKD mutation status | 0.20a | |
| NPM1 mutation status | 0.36a | |
| IDH mutation status | 0.10a | |
| DNMT3A mutation status | 0.27a | |
| Gender | 0.63a | |
| AraC doseb | 0.40a | |
aMann–Whitney U-test
b1.0 g × 5 days vs 1.5 g × 4 days
Association of SAMHD1 expression levels with achievement of CR
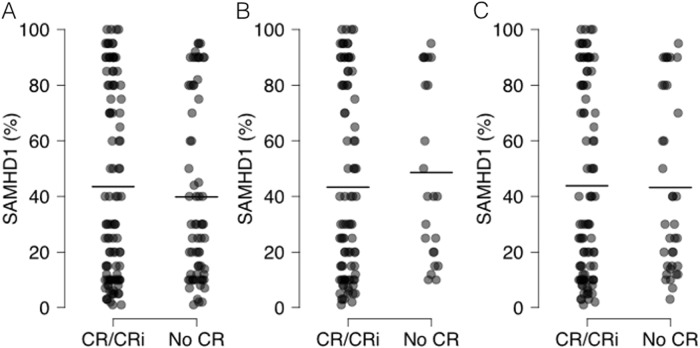
Following induction chemotherapy, 101 (53.4%) patients achieved CR, 6 (3.2%) patients achieved CR without platelet recovery (CRi) or PR, and 82 (43.4%) patients did not achieve CR or had no evaluable CR data (Table 1). CR rates differed significantly between the two cohorts (85.1% in MDACC vs. 31.4% in NUH, P < 0.0001). Importantly, SAMHD1 expression levels did not correlate with response to induction therapy either in the entire study group of AML (P = 0.7583, Mann–Whitney U test) or in the subgroup analyses of only patients treated with HDAC during consolidation (P = 0.3203, Mann–Whitney U test). This is particularly important, as patients in the MDACC cohort all received HDAC during induction therapy. In addition, no differences for CR were found when analyzing patients for SAMHD1 expression that received ara-C-based treatment as an induction therapy either at NUH (P = 0.1241, Mann–Whitney U test) or at MDACC (P = 0.7659, Mann–Whitney U test), separately (Fig. 3).
Fig. 3. Expression of SAMHD1 in AML blasts in the groups of patients that achieved CR/CRi or not.
The black lines indicate the mean percentage of SAMHD1 positive AML blasts. a All patients; b patients that received HDAC consolidation therapy; c patients that received ara-C-containing induction therapy
Prognostic significance of SAMHD1 expression levels in patients with AML treated with ara-C containing regimens
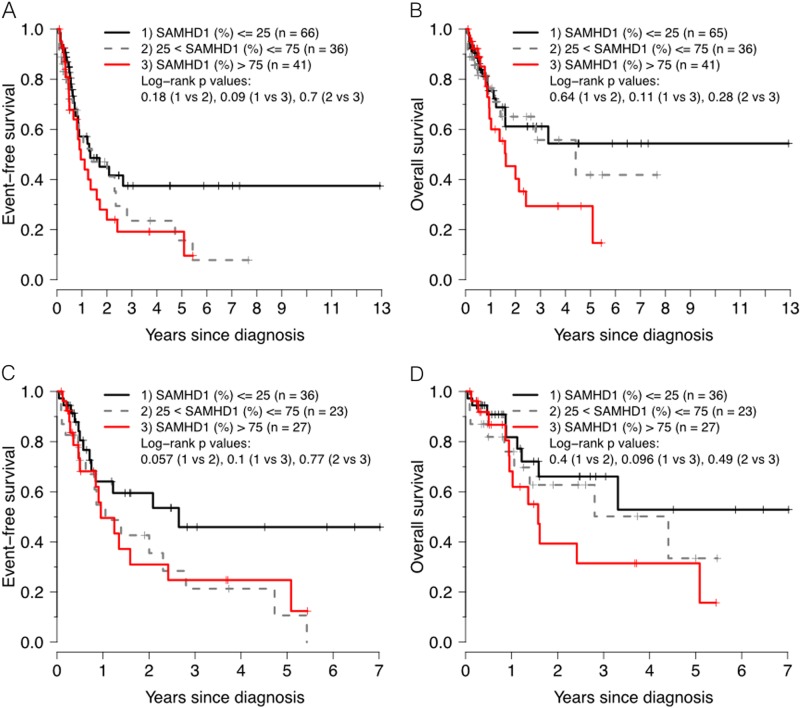
In the group of patients (n = 143) with available SAMHD1 and survival data receiving ara-C-based chemotherapy as an induction therapy, irrespective of their subsequent consolidation regimen, the 5-year EFS for patients with low versus high SAMHD1 levels was 37% versus 19%, respectively (P = 0.0896 by log-rank test) (Fig. 4a) after a median follow up of 36 months for survivors. Analyzing SAMHD1 expression as a continuous variable by multivariate Cox proportional hazards model revealed a hazard ratio of 1.008 for 1% increments (P = 0.022), and significance was maintained when adjusting for age, gender, risk group and FLT3-ITD status (P = 0.04), but not when adjusting for age, gender, risk group, and NPM1 status (Table 3). Similarly, the 5-year OS was 54 and 29%, for low versus high SAMHD1 levels, respectively (P = 0.107) (Fig. 4b) with a hazard ratio of 1.009 for 1% increments of SAMHD1 expression (P = 0.044), even though significance was lost in the adjusted analyses (P = 0.159 and P = 0.266) (Table 3).
Fig. 4.
Kaplan–Meier curves showing EFS and OS of patients treated with ara-C-containing induction treatment: a, b all patients; c, d analysis restricted to intermediate risk group with diploid cytogenetics (MDACC cohort)
Table 3.
Multivariate analysis (Cox proportional hazards model) in the subsets of AML patients with Ara-C induction therapy and HDAC consolidation therapy: OS (HSCT-censored) and EFS (HSCT-censored)
| OS HSCT-censored | EFS HSCT-censored | |||||
|---|---|---|---|---|---|---|
| AC ind.a | HDAC cons.b | AC ind. MDACCc | AC ind. | HDAC cons. | AC ind. MDACC | |
| HRd (95% CI; p-value) | HR (95% CI; p-value) | HR (95% CI; p-value) | HRd (95% CI; p-value) | HR (95% CI; p-value) | HR (95% CI; p-value) | |
| Unadjusted | 1.009 (1.000–1.017; 0.044) | 1.014 (1.004–1.024; 0.007) | 1.011 (1.000–1.022; 0.049) | 1.008 (1.001–1.015; 0.022) | 1.012 (1.004–1.020; 0.003) | 1.009 (1.000–1.017; 0.049) |
| Adjusted for age, gender, risk group, FLT3-TKD | 1.007 (0.997–1.018; 0.159) | 1.010 (0.999–1.022; 0.078) | 1.009 (0.998–1.020; 0.117) | 1.008 (1.000–1.017; 0.040) | 1.011 (1.002–1.020; 0.014) | 1.009 (1.000–1.018; 0.039) |
| Adjusted for age, gender, risk group, NPM1 | 1.006 (0.995–1.017; 0.266) | 1.008 (0.996–1.020; 0.196) | 1.009 (0.997–1.021; 0.147) | 1.008 (0.999–1.017; 0.075) | 1.010 (1.000–1.020; 0.047) | 1.008 (0.998–1.018; 0.102) |
aPatients receiving AraC induction treatment
bPatients receiving high-dose AraC consolidation therapy
cPatients in the M.D. Anderson cohort receiving AraC induction therapy, not adjusted for risk group, due to all patients belonging to the intermediate risk group
dHazard Ratio (HR) for a one-unit increase in SAMHD1 percent positive blasts
As SAMHD1 modulates efficacy of both clofarabine and fludarabine in THP-1 cells [8], we assessed whether SAMHD1 expression would have additional effects in patients receiving clofarabine or fludarabine in addition to HDAC-containing AML therapies by statistically evaluating interactions with SAMHD1. However, no significant interactions were detected (P = 0.755 and P = 0.811, respectively for overall survival) for the limited number of patients treated with clofarabine or fludarabine (n = 23, and n = 25, respectively) in the present study group.
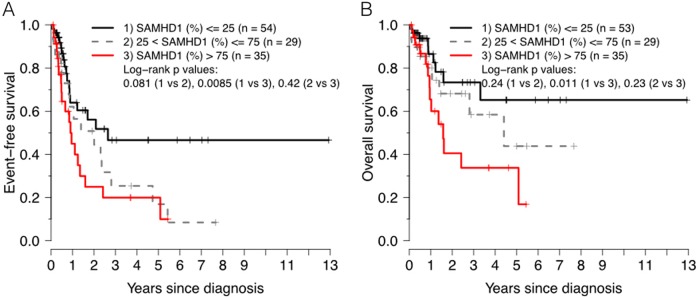
When restricting the analysis to the group receiving HDAC as a consolidation therapy, the 5-year EFS for patients with low versus high SAMHD1 levels was 47% versus 20%, respectively (P = 0.00855 by log-rank test) (Fig. 5a) with a hazard ratio of 1.01 for 1% increments of SAMHD1 expression (P = 0.0032), and significance was maintained in adjusted analyses (P = 0.014 and P = 0.047) (Table 3). The corresponding 5-year OS was 44 and 34%, for low versus high SAMHD1 levels, respectively (P = 0.0114 by log-rank test) (Fig. 5b) with a hazard ratio of 1.01 for 1% increments of SAMHD1 expression (P = 0.0073) (Table 3), even though significance was lost in the adjusted analyses (P = 0.078 and P = 0.196).
Fig. 5.
Kaplan–Meier curves showing event-free (a) and overall (b) survival of patients treated with HDAC consolidation therapy: all patients
Similar results were obtained when the analysis was restricted to the 87 patients in the intermediate risk group with diploid cytogenetics and available SAMHD1 data in the MDACC cohort (Table 3). Importantly, all of these patients were treated with HDAC consolidation regimens.
Discussion
Here, we report for the first time the expression patterns of SAMHD1 in normal (reactive) bone marrows using an optimized double immunohistochemistry assay that analysed SAMHD1 expression in normal hematopoietic cells of all three lineages including normal CD34+ cells. Using this assay, no SAMHD1 expression was detected in normal MPO+ and GPA+ hematopoetic cells, megakaryocytes and normal CD34+ blasts. By contrast, SAMHD1 was strongly expressed in reactive, mature T-cells and histiocytes in reactive bone marrow specimens. Therefore, expression of SAMHD1 in myeloblasts of AML could be bona fide considered pathologic. Furthermore, this adds important information about expected excess ara-C myelotoxicity in possible clinical trials combining ara-C with putative SAMHD1 inhibitors.
In the present study, SAMHD1 expression as a continuous variable (% positive blasts) significantly correlated with WBC and LDH levels, but not with peripheral or bone marrow blast count and other clinicopathologic parameters. Future studies are needed to elucidate the biological role of SAMHD1 for turnover of AML blasts. Of note, SAMHD1 has anti-proliferative properties in vitro13.
Low expression levels of SAMHD1 protein as detected by immunohistochemistry were not associated with achievement of CR in two independent cohorts of AML patients of different risk groups in the present study, not even in the subgroup of patients that received HDAC as part of their induction therapy. Hence, our results suggest that therapy-limiting properties of SAMHD1 for HDAC therapies only become evident during post-remission consolidation. Notwithstanding, we are currently investigating whether SAMHD1 expression affects the rate of negative minimal residual disease (MRD) following ara-C-based induction courses as MRD significantly correlates with AML relapse. These findings are in accordance with the results of our recent study7 at the RNA level, where we showed, in both adult TCGA AML and the pediatric TARGET AML cohorts treated with ara-C, that SAMHD1 mRNA levels are not lower in patients achieving CR as compared to patients that do not achieve CR. Hence, combined with the current study, no effect of SAMHD1 expression on CR is evident in four independent cohorts of AML patients.
By contrast, using immunohistochemistry, Schneider et al.12 reported a significantly lower expression of SAMHD1 in 112 AML patients who achieved CR as compared to a group of patients who did not achieve CR. Methodological confounders, like the use of a different SAMHD1 antibody (12586-1-AP from Proteintech, Rosemont, IL, USA) may account for this discrepancy8,9,11.
In the group of patients receiving ara-C-based chemotherapy as an induction therapy, irrespective of their subsequent consolidation regimen, Cox regression analyses indicated a significant effect of SAMHD1 expression on survival, however, the statistical power was lost when comparing high vs low expressers dichotomously. These data argue against a threshold effect defining a critical expression of SAMHD1 that confers ara-C resistance, consistent with our in vitro analyses using AML cell lines that showed a continuous, dose-dependent effect of SAMHD1 on ara-C sensitivity7. On the other hand, we showed that the protein levels of SAMHD1 are significantly associated with EFS and OS in patients treated with HDAC consolidation regimens, and these associations retained significance in the multivariate Cox proportional hazards model after adjustment with age, gender, risk group, and presence of FLT3-TKD or NPM1 mutations for EFS, but not OS, as to the loss of power is more pronounced for OS analyses with fewer events as compared to EFS. There were no significant correlations between SAMHD1 expression and these covariates. Larger future studies have to investigate whether SAMHD1 expression levels in specific subgroups of AML, such as the cohort of intermediate risk patients with diploid cytogenetics in the present study, may show stronger effects of SAMHD1 expression on survival.
In conclusion, we have shown that low-level SAMHD1 expression in AML blasts at diagnosis identifies a sizeable patient subset with favorable EFS and OS upon treatment with HDAC-containing consolidation regimens. As such, SAMHD1 expression, which can be assessed conveniently by immunohistochemistry, may represent a novel predictor of outcome in AML patients. Similarly, high SAMHD1 expression promises to be a predicitive biomarker for novel, selective SAMHD1 inhibitors which have been tested in our research laboratory with promising results17 that may lead to new, more efficient combination therapies.
Electronic supplementary material
Acknowledgements
This work was supported the Swedish Children’s Cancer Foundation (TJ2017-0021 (to S.G.R.); TJ2016-0040 and PR2016-0044 (to N.H.)), the Swedish Cancer Society (CAN 2017/517 to N.H.), the Stockholm County Council (ALF) (K2892-2016 to N.H.), the Clas Groschinsky Memorial Foundation (M18228 to N.H.), the ìShizu Matsumuraîs donation (2018-01086 to N.H.), the Sigurd och Elsa Goljes Memorial Foundation (LA2018-0038 to N.H.), Karolinska Institutet Foundations (2016-50273 to S.G.R.; and 2016-50756 to N.H.), and the research funds of Radiumhemmet Cancer Foundation (to G.Z.R).
Authors contributions
G.Z.R., N.H.: Study design, data collection, data interpretation, figures, literature search, and writing. I.H.M. (equal contribution as first author): Data analysis, data interpretation, figures, and writing. N.T., S.G.R.: Data collection, data interpretation, and writing. J-I.H., T.S.: Study design, data interpretation, and writing. S-B.N., W.J.C., B.Y., C.H.N.: Data collection, data interpretation, and writing. F.R., M.A., H.M.K., L.J.M.: Study design, data interpretation, and writing. I.X.: Data collection, data interpretation, figures, literature search, and writing. J.D.K.: Study design, data analysis, data interpretation, and writing.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: George Z. Rassidakis, Nikolas Herold, Ida Hed Myrberg.
Contributor Information
George Z. Rassidakis, Phone: +46-851776162, Email: georgios.rassidakis@ki.se
Nikolas Herold, Phone: +46 8 524 861 11, Email: nikolas.herold@ki.se.
Joseph D. Khoury, Phone: +(713) 563-9171, Email: jkhoury@mdanderson.org
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41408-018-0134-z).
References
- 1.de Rooij JD, Zwaan CM, van den Heuvel-Eibrink M. Pediatric AML: from biology to clinical management. J. Clin. Med. 2015;4:127–149. doi: 10.3390/jcm4010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125:767–774. doi: 10.1182/blood-2014-08-551499. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez HF, et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer RJ, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N. Engl. J. Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 5.Plunkett W, et al. Pharmacologically directed ara-C therapy for refractory leukemia. Semin. Oncol. 1985;12(2Suppl 3):20–30. [PubMed] [Google Scholar]
- 6.Rustum YM, Preisler HD. Correlation between leukemic cell retention of 1-beta-D-arabinofuranosylcytosine 5’-triphosphate and response to therapy. Cancer Res. 1979;39:42–49. [PubMed] [Google Scholar]
- 7.Herold N, et al. Targeting SAMHD1 with the Vpx protein to improve cytarabine therapy for hematological malignancies. Nat. Med. 2017;23:256–263. doi: 10.1038/nm.4265. [DOI] [PubMed] [Google Scholar]
- 8.Herold N, et al. SAMHD1 protects cancer cells from various nucleoside-based antimetabolites. Cell Cycle. 2017;16:1029–1038. doi: 10.1080/15384101.2017.1314407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herold N, et al. With me or against me: tumor suppressor and drug resistance activities of SAMHD1. Exp. Hematol. 2017;52:32–39. doi: 10.1016/j.exphem.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Hollenbaugh JA, et al. Substrates and Inhibitors of SAMHD1. PLoS ONE. 2017;12:e0169052. doi: 10.1371/journal.pone.0169052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudd SG, Schaller T, Herold N. SAMHD1 is a barrier to antimetabolite-based cancer therapies. Mol. Cell. Oncol. 2017;4:e1287554. doi: 10.1080/23723556.2017.1287554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider C, et al. SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia. Nat. Med. 2017;23:250–255. doi: 10.1038/nm.4255. [DOI] [PubMed] [Google Scholar]
- 13.Tsesmetzis N., Paulin C. B. J. & Rudd S. G., Herold N. Nucleobase and nucleoside analogues: resistance and re-sensitisation at the level of pharmacokinetics, pharmacodynamics and metabolism. Cancers(Basel). 10, pii: E240 (2018). [DOI] [PMC free article] [PubMed]
- 14.Clifford R, et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 2014;123:1021–1031. doi: 10.1182/blood-2013-04-490847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daddacha W, et al. SAMHD1 promotes DNA end resection to facilitate DNA repair by homologous recombination. Cell Rep. 2017;20:1921–1935. doi: 10.1016/j.celrep.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol. Cancer. 2010;9:292. doi: 10.1186/1476-4598-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold NR, et al. Development of a small molecule inhibitor for SAMHD1 to improve outcome for patients with acute myelogenous leukaemia and relapsed T-lymphoblastic malignancies treated with nucleoside analogues. Pediatr. Blood Cancer. 2017;64(Suppl. 3):S135–S135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.