Abstract
Vitamin C (ascorbate) is maintained at high levels in most immune cells and can affect many aspects of the immune response. Intracellular levels generally respond to variations in plasma ascorbate availability, and a combination of inadequate intake and increased turnover during severe stress can result in low plasma ascorbate status. Intracellular ascorbate supports essential functions and, in particular, acts as an enzyme cofactor for Fe- or Cu-containing oxygenases. Newly discovered enzymes in this family regulate cell metabolism and epigenetics, and dysregulation of their activity can affect cell phenotype, growth and survival pathways, and stem cell phenotype. This brief overview details some of the recent advances in our understanding of how ascorbate availability can affect the hydroxylases controlling the hypoxic response and the DNA and histone demethylases. These processes play important roles in the regulation of the immune system, altering cell survival pathways, metabolism and functions.
Keywords: ascorbate, demethylation, hydroxylases, hypoxia inducible factors, vitamin C
Background
Humans have an absolute requirement for vitamin C (ascorbate) as part of their diet, and deficiency due to inadequate intake is associated with a plethora of symptoms, reflecting the diverse functions attributed to the vitamin [1–4]. There is widespread belief that ascorbate supports the immune system, and claims along this line are frequently encountered, including on commercially available dietary supplements. Since its discovery more than 80 years ago, the function of ascorbate in the immune system has been the subject of much research and more than a little controversy. One of the main drivers of this interest is that leukocytes accumulate the vitamin to high intracellular concentrations, signalling an important role for it in these cells [5–9].
Additionally, in the last 15 years, the many cofactor functions of ascorbate have come to the fore. It is well established that ascorbate is a specific cofactor for many biosynthetic enzymes including dopamine β-hydroxylase, which converts dopamine to norepinephrine, and the collagen prolyl and lysyl hydroxylases which form cross-links to stabilise the tertiary structure of collagen [10–13]. More recently, however, it has become apparent that ascorbate is also a cofactor for newly characterised hydroxylases that regulate gene transcription and cell signalling pathways [14,15]. These hydroxylases belong to the family of Fe-containing 2-oxoglutarate-dependent dioxygenases; members of this family are widespread throughout biology, and include enzymes involved in biosynthesis, post-translational protein modification and the oxidative demethylation of methylcytosine and methylated histone residues [16–20]. Examples of these enzymes include N-trimethyllysine hydroxylase and γ-butyrobetaine dioxygenase that synthesise carnitine [16], and the prolyl, lysyl and arginine hydroxylases that modify collagen and the alpha regulatory subunit of the hypoxia-inducible factors [19,20].
This short review will focus on the cell signalling and gene regulatory (cofactor) actions of vitamin C and their potential roles in regulating the immune system. The contributions of ascorbate as an antioxidant in immune cells have been well reviewed by others [21–27] and will not be discussed here. We will consider the functional effects of ascorbate on cells of both the innate and adaptive immune responses that particularly reflect the cofactor activity of ascorbate and include a discussion of the role of vitamin C on immune cells in cancer.
Ascorbate levels in immune cells
The optimal concentration of ascorbate required for its cofactor activity supporting the Fe- and Cu-containing enzymes in vitro is in the mM range [28], similar to the intracellular levels measured in many cell types [1,29,30]. The ascorbate content of immune cells is also in this range and reflects plasma availability. Intracellular ascorbate concentrations in circulating lymphocytes, monocytes and neutrophils have been reported to be ∼3.5, ∼3 and ∼1.5 mM, respectively, when plasma levels are at least 50 µM, reflecting the status in healthy individuals consuming ≥100 mg ascorbate daily [8,31]. However, when plasma levels fall below 50 µM, immune cell ascorbate content decreases, with intracellular concentrations at ∼1.5, 1.2 and 0.5 mM in lymphocytes, monocytes and neutrophils, respectively, when plasma levels are ≤20 µM [8,31]. Plasma levels below 23 µM represent a state of hypovitaminosis C and are commonly seen in individuals with low fresh fruit and vegetable intake [32–36]. In addition, there is substantial evidence that plasma and cellular ascorbate levels are depressed in conditions of active inflammation [37–40] and in cancer patients [41–43], including patients with haematological cancers [44–50]. Severely depleted plasma levels of ≤20 µM are commonly reported, particularly in very ill patient populations [37,40,44]. Ascorbate loss during illness is thought to reflect increased turnover due to oxidative and metabolic stress [51,52]. This variable availability of ascorbate may modulate ascorbate-dependent enzyme reactions and thereby affect immune cell function.
The cellular ascorbate content referred to above applies to mature circulating white blood cells. A recent report indicated that haematopoietic and multipotent stem cells and haematopoietic progenitor cells in the bone marrow contain 2- to 20-fold more ascorbate than differentiated cells and that increased ascorbate content correlated with increased expression of the specific ascorbate transporter, SVCT2 [53]. This information suggests an essential role for ascorbate in bone marrow stem cell differentiation. Evidence for this is accumulating, with recent reports of ascorbate-mediated regulation of epigenetic programming and differentiation in bone marrow stem cells and particularly in myeloid leukaemia cells containing mutations in TET2 or IDH1 [54,55]. For more in-depth information, the reader is referred to recent reviews of this interesting and fast-developing field of research [56,57].
The role of ascorbate in the hypoxic response and implications for immune cell function
The hydroxylase enzymes that regulate the activity of the hypoxia-inducible factors (HIF)s require ascorbate for optimal activity [28,29]. The HIFs are controlled by hydroxylation of proline and asparagine residues on the regulatory alpha subunit and, in response to changes in oxygen availability, they direct the transcription of hundreds of genes via the hypoxia response element [58–61]. The dependence of the hydroxylases on ascorbate as a cofactor has been demonstrated in cell-free systems [28,61,62], with other reducing agents such as glutathione being very much less effective as a recycler of the hydroxylase active site Fe2+ [28,63–65]. Depleted intracellular ascorbate levels have been shown to contribute to the up-regulation of HIF activation, particularly under conditions of mild or moderate hypoxia [29,66].
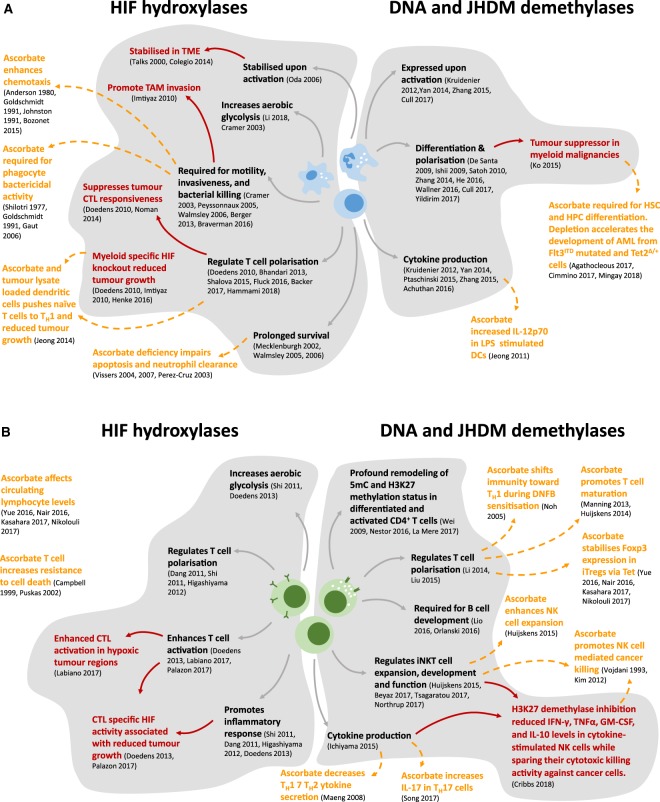
The interaction between ascorbate and the HIFs is relevant to the function of immune cells in both inflammation and cancer. Inflammatory sites are known to be under hypoxic stress, potentially as a consequence of the increased oxidative metabolism of inflammatory cells [67–69]. Growing tumours are also well characterised as being hypoxic tissues due to rapid proliferation and outgrowth of the established blood supply [70,71]. The resulting up-regulation of the HIFs is instrumental in the activation of glycolysis, angiogenesis, resistance to chemotherapy and the promotion of a stem cell phenotype, thereby promoting tumour growth and metastasis [59,72,73]. At inflammatory sites and in tumour tissue, the hypoxic environment affects immune cell function and, given the interdependence between the activation of the HIFs and cellular ascorbate [14,29,74–78], we propose that many effects of ascorbate on immune cell function are likely to reflect the regulation of HIF-mediated functions. Figure 1 shows a summary of the interactions that are discussed in the sections below.
Figure 1. A summary of the recently reported effects of ascorbate-dependent processes in immune cells.
(A) Effects on myeloid cells and (B) lymphoid cells. Effects shown in black font represent a reported role of HIF, TET or Jumonji demethylases, text in red indicates a reported effect of HIF, TET or Jumonji in the context of cancer and orange text indicates an effect of ascorbate on immune cells. The inter-relationships between these are indicated by arrows. References from the Figure: Achuthan 2016 [135]; Agathocleous 2017 [53]; Anderson 1980 [164]; Backer 2017 [88] Berger 2013 [101]; Beyaz 2017 [162]; Bhandari 2013 [165]; Bozonet 2015 [108]; Braverman 2016 [86]; Campbell 1999 [166]; Cimmino 2017 [142]; Colegio 2014 [90]; Cramer 2003 [84]; Cribbs 2018 [163]; Cull 2017 [131]; Dang 2011 [112]; De Santa 2009 [137]; Doedens 2010 [93]; [113]; Fluck 2016 [115]; Gaut 2006 [107]; Goldschmidt 1991 [106]; Hammami 2018 [116]; He 2016 [167]; Henke 2016 [94]; Higashiyama 2012 [114]; Huijskens 2014,2015 [122,123]; Ichiyama 2015 [148]; Imtiyaz 2010 [87]; Ishii 2009 [139]; Jeong 2011,2014 [95,96]; Johnston 1991 [168]; Kasahara 2017 [158]; Kim 2012 [169]; Ko 2015 [170]; Kruidenier 2012 [141]; LaMere 2017 [151]; Labiano 2017 [119]; Li 2014 [153]; Li 2018 [83]; Lio 2016 [171]; Liu 2015 [154]; Maeng 2008 [172]; Manning 2013 [124]; Mecklenburgh 2002 [98]; Mingay 2018 [55]; Nair 2016 [156]; Nestor 2016 [147]; Nikolouli 2017 [157]; Noh 2005 [173]; Noman 2014 [92]; Northrup 2017 [161]; Oda 2006 [82]; Orlanski 2016 [174]; Palazon 2017 [117]; Perez-Cruz 2003 [103]; Peyssonnaux 2005 [85]; Ptaschinksi 2015 [146]; Puskas 2002 [175]; Satoh 2010 [134]; Shalova 2015 [89]; Shi 2011 [111]; Shilotri 1977 [176]; Song 2017 [159]; Talks 2000 [177]; Tsagaratou 2017 [160]; Vissers 2004,2007 [178,179]; Vojdani 1993 [180]; Wallner 2016 [132]; Walmsley 2005,2006 [99,100]; Wei 2009 [152]; Yan 2014 [140]; Yildirim 2017 [136]; Yue 2016 [155]; Zhang 2014,2015 [133,145].
Effects of HIFs and ascorbate on immune cells
Immune cells undergo dramatic metabolic changes following activation, and increased aerobic glycolytic activity and fatty acid oxidation have been observed [79,80]. These metabolic changes, once thought to be a consequence of cell activation, are now being re-examined as a mechanism for phenotype switching, termed metabolic reprogramming (reviewed in ref. [81]). Central to this switch are the HIF proteins which not only up-regulate the glycolytic machinery but also direct the inflammatory and immune response (reviewed in ref. [80]).
Monocytes/macrophages
The high ascorbate concentrations in monocytes [31] may be related to their dependency on HIF for many essential functions. HIF-1 has been shown to be activated in monocytes following activation with phorbol esters [82] and pathogenic stimuli [83–86], even under non-hypoxic conditions. That HIF activation is an integral part of monocyte function is indicated by the demonstrations that HIF-1α or HIF-2α deletion in myeloid cells caused profound impairment of cell aggregation, motility, invasiveness and bacterial killing [84–86], resulting in decreased bacterial resistance and failure to restrict systemic spread of a localised infection [85–87]. HIF-1/2 appears to be important for monocyte-mediated host defence; HIF-1 activation has been shown to contribute to disease progression in colitis and myeloid HIF-1α knockout shifts the balance to an anti-inflammatory phenotype resulting in a less severe inflammation [88]. The sepsis-related host immunosuppressive monocyte phenotype has also been shown to be mediated by chronic HIF-1α expression, resulting in supressed pro-inflammatory cytokine expression and increased ability to induce Treg cell polarisation [89].
In cancer, activation of HIF-1/2 in monocytes has been implicated in the development and phenotype of tumour-associated macrophages [87,90]. This is associated with an increased M2-like gene profile, increased expression of immunosuppressive and pro-tumour proteins such as arginase 1, iNOS and VEGF, as well as induction of PD-L1 expression [87,90–92]. These changes lead to greater monocyte/macrophage tumour invasion [87] and tumour cytotoxic T-cell suppression [92,93]. Interestingly, a macrophage-targeted HIF-1α and HIF-2α knockout resulted in delayed tumour progression in models of breast tumour, fibrosarcoma and colitis-associated colon carcinoma [87,93,94].
The potential complexity of ascorbate engagement with immune cells in the tumour microenvironment is well demonstrated by the observations that dendritic cells treated with ascorbate secreted increased levels of IL-12p70 after activation with LPS and induced more Th1 cytokine and IFN-γ, but less Th2-cytokine, IL-5 expression in naive T cells [95]. Ascorbate-treated dendritic cells also increased the frequency of IFN-γ + T cells when co-cultured with both CD4+ and CD8+ T cells and demonstrated an improved anti-tumour effect [96].
Neutrophils
Neutrophils are short-lived cells that are the first responders to an inflammatory challenge. Their recruitment to, and clearance from, inflammatory sites is dependent on the regulation of cell death and survival pathways [97]. It appears that HIF-1 and ascorbate are intimately involved in determining neutrophil cell fate. Hypoxia has been shown to prolong neutrophil survival via activation of HIF-1 and its downstream pathways [98–100]. HIF-1 activation also enhanced overall neutrophil antibacterial function as demonstrated by increased susceptibility to bacterial keratitis in mice when HIF-1 was inhibited [101]. This was supported by findings of delayed rates of apoptosis and enhanced bacterial phagocytosis under normoxic conditions in neutrophils from patients with a monoallelic mutation of von Hippel Lindau protein who exhibit a ‘partial hypoxic’ phenotype [99]. These results suggest that a functional hypoxic response supports neutrophil function at hypoxic inflammatory sites in vivo. A similar anti-apoptotic phenotype in ascorbate-deficient neutrophils was shown to be associated with HIF-1 activation under normoxic conditions [102]. Recognition of aged neutrophils by macrophages was also reported and neutrophil clearance from an inflammatory site was delayed in deficient cells [102]. Interestingly, increasing neutrophil ascorbate content was found to inhibit neutrophil Fas-induced cell death [103] as well as the rates of neutrophil and monocyte apoptosis in patients with sepsis [104]. Also, in the ascorbate-dependent Gulo−/− mouse, a high ascorbate diet was found to increase circulating granulocyte and monocyte numbers [105].
Not all effects of ascorbate on neutrophil function will be HIF-related. Severe ascorbate deficiency has been shown to impair neutrophil bactericidal ability towards phagocytosed pathogens following infection with actimoycetes and K pneumoniae [106,107], possibly as a result of altered oxidative capacity. Neutrophils from individuals with suboptimal circulating ascorbate levels showed a modest increase in neutrophil chemotaxis and oxidative burst ex vivo following supplementation to restore vitamin C status to healthy levels [108]. Vitamin C deficiency also increased the generation of neutrophil extracellular traps (NETs) in the Gulo−/− mouse [109].
T cells
Differentiation of CD4 T cells dictates the type of inflammatory response occurring via the development of different T-helpers and iTreg subsets and their corresponding effector function [80,110]. Therefore, depending on the nature of the insult or source of inflammation, the prevailing ratio and species of T cells could alter the outcome. HIF-1 appears to play an important, although unresolved, role in T-cell differentiation. For example, HIF-1α T-cell-targeted knockout protected mice from autoimmune neuro-inflammation and was associated with a shift from TH17 to Treg response, possibly by increasing glycolysis [111–113], while the opposite was observed in irritable bowel disease where T-cell HIF-1α knockout increased TH1 and TH17 leading to severe colonic inflammation [114]. HIF-1α-mediated myeloid- and dendritic cell-driven differentiation of T cells also greatly affected the inflammatory outcome; HIF-1α knockout in myeloid cells resulted in lesser TH17 prevalence and decreased inflammation [88]. In dendritic cells, HIF-1 knockout resulted in impaired Treg development and increased inflammation [115], and HIF-1-mediated events were reported to limit Th1 cell development by preventing IL-12 production and to exacerbate Leishmania infections [116].
Apart from T-cell differentiation, HIF-1 has also been shown to affect T-cell activation and function. HIF activation enhanced the expression of effector molecules, co-stimulatory receptors, activation and inhibitory receptors, and key transcriptional regulators of effector and memory cell differentiation [113,117]. However, this was in contrast with a previous report showing higher levels of pro-inflammatory cytokines, stronger antibacterial effects and much better survival of septic mice with T-cell targeted deletion of HIF-1α [118].
In cancer, HIF-1 activation is associated with expression of CD69 (a marker of activated T cells) on cytotoxic T lymphocytes (CTLs) in hypoxic regions of tumour, suggesting a pro-tumour killing role for HIF-1α [119]. This is supported by two studies, showing that T-cell HIF-1 activation significantly delayed tumour growth [113] and, conversely, accelerated tumour progression in the presence of HIF-1α knockout CTLs [117] in a murine model of ectopic B16 melanoma.
There have been many studies that have suggested that ascorbate influences lymphocyte differentiation, including early studies that indicated that increased circulating lymphocytes were associated with ascorbate availability [120,121]. High ascorbate supplementation for one-year also significantly increased all circulating leukocytes, including lymphocytes, in the SMP30KO ascorbate-dependent mice [105]. Ascorbate was required for the progression of mouse bone marrow-derived progenitor cells into functional T-lymphocytes and also increased the NK cell population in vitro [122–124]. Many of these effects show a significant correlation with the regulation of the TET and Jumonji demethylases and epigenetic changes, rather than with the expression of HIF-1. This topic is discussed in the following section.
Ascorbate and the regulation of epigenetics in immune cells
In mammals, one of the most widespread epigenetic modifications is DNA cytosine methylation which can be actively reversed by the TET enzymes that catalyse the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [125]. Ascorbate availability enhances TET activity [126,127] through its cofactor function, likely maintaining the active site Fe2+ of these dioxygenases [128]. Although other reducing agents could reduce Fe3+ and promote TET activity in a cell free system, ascorbate was shown to be the most efficient [128] and glutathione was incapable of increasing murine embryonic TET activity compared with equimolar ascorbate [126,127]. The Jumonji C domain-containing histone demethylases (JHDMs) are also members of the Fe- and 2-oxoglutarate-dependent dioxygenase family and similarly to TETs, full enzyme activity of JHDMs occurs in the presence of ascorbate [129,130]. The JHDMs are the third and largest class of demethylase enzymes, capable of removing all three histone lysine-methylation states through oxidative reactions [130].
Monoytes/macrophages
Epigenetic regulation plays an important role in macrophage differentiation, with rapid TET-dependent demethylation observed in colony-stimulating factor-1-differentiated human monocytes [131,132]. TET2 transcription was further induced by LPS but not by IL-4 stimulation [131]. The genes affected by TET-mediated demethylation are part of ten consolidated pathways related to the regulation of actin cytoskeleton, phagocytosis and the innate immune system [132], and in macrophages, TET2 is thought to restrain the inflammatory response by up-regulating expression of genes involved in dampening Toll-like receptor 4 signalling [131]. This notion is supported by a report showing TET2 represses IL-6 production during LPS-induced inflammation and that TET2 knockout exacerbates the expression of macrophage pro-inflammatory molecules such as IL-6, MCP-1 and MCP-3 in response to LPS stimulation, resulting in an enhanced inflammatory response [133].
The JHDM enzyme JMJD3 is expressed in monocytes/macrophages and is inducible by differentiating factors [134–136] as well as by pathogenic [137,138] and damage-associated molecules [139,140]. Although JMJD3 has been shown to affect gene expression in macrophages, the role of JMJD3 in macrophage function is still unclear. For example, 70% of macrophage–LPS–inducible genes were found to be JMJD3 targets but only a few hundred genes, including inducible inflammatory genes, were moderately affected by JMJD3 deletion [137]. However, Kruidenier et al. [141] demonstrated a drastic drop in LPS-induced cytokine expression using a specific JMJD3 inhibitor and siRNA, among them TNF-α. In contrast, Satoh et al. showed no effect on M1 cytokine secretion following LPS stimulation in JMJD3 knockout macrophages including TNF-α [134]. Contradictions aside, two studies looking at macrophage response to parasitic infection have associated JMJD3 demethylation activity with acquisition of an M2 phenotype, demonstrated by up-regulation of M2 proteins such as Arg1, Ym1, Fizz1, MR and iNOS [134,139]. Two other studies have associated JMJD3 activity with an M1 macrophage phenotype following serum amyloid A stimulation [140] and in arthritis [135] resulting in induction of pro-inflammatory cytokines.
Epigenetic processes regulated by the demethylases are associated with leukaemogenesis and ascorbate availability has been closely linked to this phenomenon. As mentioned above, haematopoietic stem and progenitor cells (HSPCs) accumulate high intracellular concentrations of ascorbate, and this is essential for HSPC differentiation via support of TET2 activity [53]. TET2 inhibition in HSPCs by ascorbate depletion retards differentiation and increases HPSC frequency. TET2 mutations are also known to co-operate with FLT3ITD mutations to cause acute myeloid leukaemia [53]. Ascorbate depletion coupled with FLT3ITD mutations was adequate for leukaemogenesis [53]. It appears then, that ascorbate accumulation within HSCs promotes TET function in vivo, limiting HSPC frequency and suppressing leukaemogenesis. These findings were corroborated in part by another group that described the use of ascorbate as a combination therapy for treating leukaemia [142]. Patients with leukaemia often have low plasma ascorbate levels [44,47–50] and the capacity for ascorbate to influence the epigenetic drivers of some leukaemias has led to conjecture that increased ascorbate supply may provide clinical benefit to some individuals with leukaemia. Two recent publications have provided support for this hypothesis [143,144].
Dendritic cells
DNA demethylation changes occur during the development of monocytes into immature DCs and mature DCs [145]. TET2 represses late-phase expression of dendritic cell pro-inflammatory molecules such as IL-6, MCP-1 and MCP-3 in response to LPS stimulation and TET2 knockout results in a greater degree of inflammatory response in mice challenged with LPS and colitis [133]. KDM5B acts to repress type I IFN and other innate cytokines in DCs to promote an altered immune response following RSV infection that contributes to the development of chronic disease [146].
T cells
Widespread DNA methylation remodelling has been reported at genes and cell-specific enhancers with known T-cell function during human CD4+ T differentiation [147,148], and TET2 was reported to be the critical DNA demethylase involved in the differentiation of TH1 and TH17 cells, leading to activation of effector cytokine gene expression [148]. TET2 has also been shown to regulate CD8+ T-cell fate, particularly in formation of memory CD8+ T cells [149]. Prolonged antigen stimulation in peptide immunotherapy is associated with demethylation of conserved regions of PD-1 promoter, possibly via TET, leading to sustained PD-1 expression in CD4+ effector T cells [150].
Profound demethylation of histone H3K27 is observed after activation in CD4+ T cells and corresponds to pathways crucial to T-cell function, including T-cell activation and the regulation of the JAK/STAT pathways [151,152]. Deletion of the histone demethylase JMJD3 was found to regulate gene expression resulting in TH2 and TH17 differentiation and inhibiting TH1 and Treg cell differentiation via altered methylation status of H3K27 and/or H3K4 [153,154].
Recent studies focusing on the role of ascorbate in T-cell differentiation and function suggest close alignment with epigenetic regulation and demethylase activity. Initial work showed ascorbate to be required for the progression of mouse bone marrow-derived progenitor cells into functional T-lymphocytes in vitro and in vivo by a JMJC-mediated process [123,124]. Subsequent studies reported ascorbate-mediated stabilisation of Foxp3 expression in TGF-β-induced Tregs by TET enzymes [155,156]. Also, ascorbate enhanced alloantigen-induced Treg suppressive capacity in skin allograft and GVHD in mice was attributed to the stabilisation of Foxp3 expression, presumably via demethylation of Foxp3 and other Treg-specific epigenetic genes [157,158]. Apart from Tregs, ascorbate has also been implicated in the maintenance of TH17 phenotype by increasing IL-17 expression in TH17-differentiated T cells via reduced trimethylation of histone H3 lysine 9 (H3K9me3) in the regulatory elements of the IL-17 locus [159].
NK cells
Many recent studies have demonstrated the impact of TET- and JHDM-mediated demethylation on NKT cell development, proliferation and function [160–162]. Interestingly, inhibition of the H3K27 demethylase reduced IFN-γ, TNF-α, GM-CSF and IL-10 levels in cytokine-stimulated NK cells while sparing their cytotoxic killing activity against cancer cells [163].
Summary
The demonstrated dependency of the Fe-containing 2-oxoglutarate-dependent dioxygenase family on ascorbate availability and the involvement of members of this family of enzymes on many immune cell functions provide a rational basis for the belief that ascorbate supports the immune system. Ascorbate availability will influence HIF activation and immune cell function in hypoxic inflammatory and tumour environments, affecting the resolution of inflammation and potentially tumour survival in as yet unknown ways. There is also an impressive amount of information emerging that highlights the impact of the TET DNA demethylases and some histone demethylases on epigenetic remodelling of immune cells. These enzymes have also been shown to be highly responsive to ascorbate, and new insights into ascorbate function in immunity will no doubt continue to emerge.
Abbreviations
- CTLs
cytotoxic T lymphocytes
- HIFs
hypoxia-inducible factors
- HSPCs
haematopoietic stem and progenitor cells
- JHDMs
Jumonji C domain-containing histone demethylases
- NETs
neutrophil extracellular traps
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Banhegyi G., Benedetti A., Margittai E., Marcolongo P., Fulceri R., Nemeth C.E. et al. (2014) Subcellular compartmentation of ascorbate and its variation in disease states. Biochim. Biophys. Acta 1843, 1909–1916 10.1016/j.bbamcr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 2.Sauberlich, H.E. (1997) A history of scurvy and vitamin C In Vitamin C in health and disease, 1st edn. (Packer L. and Fuchs J., eds), pp. 1–24, Marcel Dekker, Inc., New York [Google Scholar]
- 3.Frei B., Birlouez-Aragon I. and Lykkesfeldt J. (2012) Authors’ perspective: what is the optimum intake of vitamin C in humans? Crit. Rev. Food Sci. Nutr. 52, 815–829 10.1080/10408398.2011.649149 [DOI] [PubMed] [Google Scholar]
- 4.Grosso G., Bei R., Mistretta A., Marventano S., Calabrese G., Masuelli L. et al. (2013) Effects of vitamin C on health: a review of evidence. Front. Biosci. 18, 1017–1029 10.2741/4160 [DOI] [PubMed] [Google Scholar]
- 5.Bergsten P., Amitai G., Kehrl J., Dhariwal K.R., Klein H.G. and Levine M. (1990) Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J. Biol. Chem. 265, 2584–2587 PMID: [PubMed] [Google Scholar]
- 6.Bergsten P., Yu R., Kehrl J. and Levine M. (1995) Ascorbic acid transport and distribution in human B lymphocytes. Arch. Biochem. Biophys. 317, 208–214 10.1006/abbi.1995.1155 [DOI] [PubMed] [Google Scholar]
- 7.Levine M., Dhariwal K.R., Welch R.W., Wang Y. and Park J.B. (1995) Determination of optimal vitamin C requirements in humans. Am. J. Clin. Nutr. 62, 1347S–1356S 10.1093/ajcn/62.6.1347S [DOI] [PubMed] [Google Scholar]
- 8.Levine M., Wang Y., Padayatty S.J. and Morrow J. (2001) A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl Acad. Sci. USA 98, 9842–9846 10.1073/pnas.171318198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Russo T.A., Kwon O., Chanock S., Rumsey S.C. and Levine M. (1997) Ascorbate recycling in human neutrophils: induction by bacteria. Proc. Natl Acad. Sci. USA 94, 13816–13819 10.1073/pnas.94.25.13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterkofsky B. (1991) Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 54, 1135S–1140S 10.1093/ajcn/54.6.1135s [DOI] [PubMed] [Google Scholar]
- 11.Pihlajaniemi T., Myllylä R. and Kivirikko K.I. (1991) Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 13, S2–S7 10.1016/0168-8278(91)90002-S [DOI] [PubMed] [Google Scholar]
- 12.Levine M., Morita K., Heldman E. and Pollard H.B. (1985) Ascorbic acid regulation of norepinephrine biosynthesis in isolated chromaffin granules from bovine adrenal medulla. J. Biol. Chem. 260, 15598–15603 [PubMed] [Google Scholar]
- 13.Englard S. and Seifter S. (1986) The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 6, 365–406 10.1146/annurev.nu.06.070186.002053 [DOI] [PubMed] [Google Scholar]
- 14.Kuiper C. and Vissers M.C. (2014) Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Front. Oncol. 4, 359 10.3389/fonc.2014.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loenarz C. and Schofield C.J. (2011) Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem. Sci. 36, 7–18 10.1016/j.tibs.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Nelson P.J., Pruitt R.E., Henderson L.L., Jenness R. and Henderson L.M. (1981) Effect of ascorbic acid deficiency on the in vivo synthesis of carnitine. Biochim. Biophys. Acta 672, 123–127 10.1016/0304-4165(81)90286-5 [DOI] [PubMed] [Google Scholar]
- 17.Young J.I., Züchner S. and Wang G. (2015) Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 35, 545–564 10.1146/annurev-nutr-071714-034228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camarena V. and Wang G. (2016) The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 73, 1645–1658 10.1007/s00018-016-2145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stolze I.P., Mole D.R. and Ratcliffe P.J. (2006) Regulation of HIF: prolyl hydroxylases. Novartis Found Symp. 272, 15–25; discussion 25–36 PMID: [PubMed] [Google Scholar]
- 20.Vissers M.C., Kuiper C. and Dachs G.U. (2014) Regulation of the 2-oxoglutarate-dependent dioxygenases and implications for cancer. Biochem. Soc. Trans. 42, 945–951 10.1042/BST20140118 [DOI] [PubMed] [Google Scholar]
- 21.Bendich A. (1990) Antioxidant vitamins and their functions in immune responses. Adv. Exp. Med. Biol. 262, 35–55 10.1007/978-1-4613-0553-8_4 [DOI] [PubMed] [Google Scholar]
- 22.Mangge H., Becker K., Fuchs D. and Gostner J.M. (2014) Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 6, 462–477 10.4330/wjc.v6.i6.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meydani S.N., Meydani M. and Blumberg J.B. (1990) Antioxidants and the aging immune response. Adv. Exp. Med. Biol. 262, 57–67 10.1007/978-1-4613-0553-8_5 [DOI] [PubMed] [Google Scholar]
- 24.Pohanka M., Pejchal J., Snopkova S., Havlickova K., Karasova J.Z., Bostik P. et al. (2012) Ascorbic acid: an old player with a broad impact on body physiology including oxidative stress suppression and immunomodulation: a review. Mini-Rev. Med. Chem. 12, 35–43 10.2174/138955712798868986 [DOI] [PubMed] [Google Scholar]
- 25.Puertollano M.A., Puertollano E., de Cienfuegos G.A. and de Pablo M.A. (2011) Dietary antioxidants: immunity and host defense. Curr. Top. Med. Chem. 11, 1752–1766 10.2174/156802611796235107 [DOI] [PubMed] [Google Scholar]
- 26.Ginter E., Simko V. and Panakova V. (2014) Antioxidants in health and disease. Bratisl. Lek. Listy. 115, 603–606 PMID: [DOI] [PubMed] [Google Scholar]
- 27.Maggini S., Wenzlaff S. and Hornig D. (2010) Essential role of vitamin C and zinc in child immunity and health. J. Int. Med. Res. 38, 386–414 10.1177/147323001003800203 [DOI] [PubMed] [Google Scholar]
- 28.Flashman E., Davies S.L., Yeoh K.K. and Schofield C.J. (2010) Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 427, 135–142 10.1042/BJ20091609 [DOI] [PubMed] [Google Scholar]
- 29.Kuiper C., Dachs G.U., Currie M.J. and Vissers M.C. (2014) Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 69, 308–317 10.1016/j.freeradbiomed.2014.01.033 [DOI] [PubMed] [Google Scholar]
- 30.Banhegyi G., Braun L., Csala M., Puskas F. and Mandl J. (1997) Ascorbate metabolism and its regulation in animals. Free Radic. Biol. Med. 23, 793–803 10.1016/S0891-5849(97)00062-2 [DOI] [PubMed] [Google Scholar]
- 31.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R. et al. (1996) Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl Acad. Sci. U.S.A. 93, 3704–3709 10.1073/pnas.93.8.3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleicher R.L., Carroll M.D., Ford E.S. and Lacher D.A. (2009) Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 90, 1252–1263 10.3945/ajcn.2008.27016 [DOI] [PubMed] [Google Scholar]
- 33.Carr A.C., Bozonet S.M., Pullar J.M., Simcock J.W. and Vissers M.C. (2013) Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 97, 800–807 10.3945/ajcn.112.053207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr A.C., Bozonet S.M., Pullar J.M., Simcock J.W. and Vissers M.C. (2013) A randomized steady-state bioavailability study of synthetic versus natural (kiwifruit-derived) vitamin C. Nutrients 5, 3684–3695 10.3390/nu5093684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr A.C., Bozonet S.M., Pullar J.M. and Vissers M.C. (2013) Mood improvement in young adult males following supplementation with gold kiwifruit, a high-vitamin C food. J. Nutr. Sci. 2, e24 10.1017/jns.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr A.C., Bozonet S.M. and Vissers M.C. (2013) A randomised cross-over pharmacokinetic bioavailability study of synthetic versus kiwifruit-derived vitamin C. Nutrients 5, 4451–4461 10.3390/nu5114451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonham M.J., Abu-Zidan F.M., Simovic M.O., Sluis K.B., Wilkinson A., Winterbourn C.C. et al. (1999) Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br. J. Surg. 86, 1296–1301 10.1046/j.1365-2168.1999.01182.x [DOI] [PubMed] [Google Scholar]
- 38.Jiang Q., Lykkesfeldt J., Shigenaga M.K., Shigeno E.T., Christen S. and Ames B.N. (2002) γ-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic. Biol. Med. 33, 1534–1542 10.1016/S0891-5849(02)01091-2 [DOI] [PubMed] [Google Scholar]
- 39.Bakaev V.V. and Duntau A.P. (2004) Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int. J. Tuber. Lung Dis. 8, 263–266 PMID: [PubMed] [Google Scholar]
- 40.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J. and Shaw G.M. (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 21, 300 10.1186/s13054-017-1891-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu T.K., Raven R.W., Dickerson J.W. and Williams D.C. (1974) Leucocyte ascorbic acid and urinary hydroxyproline levels in patients bearing breast cancer with skeletal metastases. Eur. J. Cancer 10, 507–511 10.1016/0014-2964(74)90074-7 [DOI] [PubMed] [Google Scholar]
- 42.Anthony H.M. and Schorah C.J. (1982) Severe hypovitaminosis C in lung-cancer patients: the utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br. J. Cancer 46, 354–367 10.1038/bjc.1982.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayland C.R., Bennett M.I. and Allan K. (2005) Vitamin C deficiency in cancer patients. Palliat. Med. 19, 17–20 10.1191/0269216305pm970oa [DOI] [PubMed] [Google Scholar]
- 44.Huijskens M.J., Wodzig W.K., Walczak M., Germeraad W.T. and Bos G.M. (2016) Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 6, 8–10 10.1016/j.rinim.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abou-Seif M.A., Rabia A. and Nasr M. (2000) Antioxidant status, erythrocyte membrane lipid peroxidation and osmotic fragility in malignant lymphoma patients. Clin. Chem. Lab. Med. 38, 737–742 [DOI] [PubMed] [Google Scholar]
- 46.Blair C.K., Roesler M., Xie Y., Gamis A.S., Olshan A.F., Heerema N.A. et al. (2008) Vitamin supplement use among children with Down's syndrome and risk of leukaemia: a Children's Oncology Group (COG) study. Paediatr. Perinat. Epidemiol. 22, 288–295 10.1111/j.1365-3016.2008.00928.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy D.D., Tucker K.L., Ladas E.D., Rheingold S.R., Blumberg J. and Kelly K.M. (2004) Low antioxidant vitamin intakes are associated with increases in adverse effects of chemotherapy in children with acute lymphoblastic leukemia. Am. J. Clin. Nutr. 79, 1029–1036 10.1093/ajcn/79.6.1029 [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa K. (2000) Effect of chemotherapy on ascorbate and ascorbyl radical in cerebrospinal fluid and serum of acute lymphoblastic leukemia. Cell. Mol. Biol. 46, 1375–1381 PMID: [PubMed] [Google Scholar]
- 49.Neyestani T.R., Fereydouni Z., Hejazi S., Salehi-Nasab F., Nateghifard F., Maddah M. et al. (2007) Vitamin C status in Iranian children with acute lymphoblastic leukemia: evidence for increased utilization. J. Pediatr. Gastroenterol. Nutr. 45, 141–144 10.1097/MPG.0b013e31804c5047 [DOI] [PubMed] [Google Scholar]
- 50.Sharma A., Tripathi M., Satyam A. and Kumar L. (2009) Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 50, 809–815 10.1080/10428190902802323 [DOI] [PubMed] [Google Scholar]
- 51.Evans-Olders R., Eintracht S. and Hoffer L.J. (2010) Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 26, 1070–1074 10.1016/j.nut.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 52.Gan R., Eintracht S. and Hoffer L.J. (2008) Vitamin C deficiency in a university teaching hospital. J. Am. Coll. Nutr. 27, 428–433 10.1080/07315724.2008.10719721 [DOI] [PubMed] [Google Scholar]
- 53.Agathocleous M., Meacham C.E., Burgess R.J., Piskounova E., Zhao Z., Crane G.M. et al. (2017) Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549, 476–481 10.1038/nature23876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mastrangelo D., Pelosi E., Castelli G., Lo-Coco F. and Testa U. (2018) Mechanisms of anti-cancer effects of ascorbate: cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 69, 57–64 10.1016/j.bcmd.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 55.Mingay M., Chaturvedi A., Bilenky M., Cao Q., Jackson L., Hui T. et al. (2018) Vitamin C-induced epigenomic remodelling in IDH1 mutant acute myeloid leukaemia. Leukemia 32, 11–20 10.1038/leu.2017.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillberg L., Orskov A.D., Liu M., Harslof L.B.S., Jones P.A. and Gronbaek K. (2018) Vitamin C—a new player in regulation of the cancer epigenome. Semin. Cancer Biol. 51, 59–67 10.1016/j.semcancer.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 57.Vissers M.C.M. and Das A.B. (2018) Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front. Physiol. 9, 809 10.3389/fphys.2018.00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaelin W.G.J. and Ratcliffe P.J. (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 59.Ratcliffe P.J. (2013) Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J. Physiol. 591(Pt 8), 2027–2042 10.1113/jphysiol.2013.251470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenza G.L. (2010) HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 20, 51–56 10.1016/j.gde.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozer A. and Bruick R.K. (2007) Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat. Chem. Biol. 3, 144–153 10.1038/nchembio863 [DOI] [PubMed] [Google Scholar]
- 62.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J. et al. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 10.1126/science.1059796 [DOI] [PubMed] [Google Scholar]
- 63.Hirsila M., Koivunen P., Gunzler V., Kivirikko K.I. and Myllyharju J. (2003) Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 278, 30772–30780 10.1074/jbc.M304982200 [DOI] [PubMed] [Google Scholar]
- 64.Nytko K.J., Maeda N., Schlafli P., Spielmann P., Wenger R.H. and Stiehl D.P. (2011) Vitamin C is dispensable for oxygen sensing in vivo. Blood 117, 5485–5493 10.1182/blood-2010-09-307637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nytko K.J., Spielmann P., Camenisch G., Wenger R.H. and Stiehl D.P. (2007) Regulated function of the prolyl-4-hydroxylase domain (PHD) oxygen sensor proteins. Antioxid. Redox Signal. 9, 1329–1338 10.1089/ars.2007.1683 [DOI] [PubMed] [Google Scholar]
- 66.Campbell E.J., Vissers M.C. and Dachs G.U. (2016) Ascorbate availability affects tumor implantation-take rate and increases tumor rejection in Gulo−/− mice. Hypoxia 4, 41–52 10.2147/HP.S103088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colgan S.P., Campbell E.L. and Kominsky D.J. (2016) Hypoxia and mucosal inflammation. Annu. Rev. Pathol. 11, 77–100 10.1146/annurev-pathol-012615-044231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whyte M.K. and Walmsley S.R. (2014) The regulation of pulmonary inflammation by the hypoxia-inducible factor-hydroxylase oxygen-sensing pathway. Ann. Am. Thorac. Soc. 11, S271–S276 10.1513/AnnalsATS.201403-108AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell E.L., Bruyninckx W.J., Kelly C.J., Glover L.E., McNamee E.N., Bowers B.E. et al. (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 10.1016/j.immuni.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adam J., Yang M., Soga T. and Pollard P.J. (2014) Rare insights into cancer biology. Oncogene 33, 2547–2556 10.1038/onc.2013.222 [DOI] [PubMed] [Google Scholar]
- 71.Brahimi-Horn M.C., Chiche J. and Pouysségur J. (2007) Hypoxia and cancer. J. Mol. Med. 85, 1301–1307 10.1007/s00109-007-0281-3 [DOI] [PubMed] [Google Scholar]
- 72.Potter C. and Harris A.L. (2004) Hypoxia inducible carbonic anhydrase IX, marker of tumour: hypoxia, survival pathway and therapy target. Cell Cycle 3, 159–162 10.4161/cc.3.2.618 [DOI] [PubMed] [Google Scholar]
- 73.Semenza G.L. (2015) Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors. Clin. Sci. 129, 1037–1045 10.1042/CS20150451 [DOI] [PubMed] [Google Scholar]
- 74.Karaczyn A., Ivanov S., Reynolds M., Zhitkovich A., Kasprzak K.S. and Salnikow K. (2006) Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel. J. Cell. Biochem. 97, 1025–1035 10.1002/jcb.20705 [DOI] [PubMed] [Google Scholar]
- 75.Knowles H.J., Mole D.R., Ratcliffe P.J. and Harris A.L. (2006) Normoxic stabilization of hypoxia-inducible factor-1α by modulation of the labile iron pool in differentiating U937 macrophages: effect of natural resistance-associated macrophage protein 1. Cancer Res. 66, 2600–2607 10.1158/0008-5472.CAN-05-2351 [DOI] [PubMed] [Google Scholar]
- 76.Knowles H.J., Raval R.R., Harris A.L. and Ratcliffe P.J. (2003) Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 63, 1764–1768 PMID: [PubMed] [Google Scholar]
- 77.Kuiper C., Dachs G.U., Munn D., Currie M.J., Robinson B.A., Pearson J.F. et al. (2014) Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible factor-1 activation in human colorectal cancer. Front. Oncol. 4, 10 10.3389/fonc.2014.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuiper C., Molenaar I.G., Dachs G.U., Currie M.J., Sykes P.H. and Vissers M.C. (2010) Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 70, 5749–5758 10.1158/0008-5472.CAN-10-0263 [DOI] [PubMed] [Google Scholar]
- 79.Pearce E.L. and Pearce E.J. (2013) Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 10.1016/j.immuni.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corcoran S.E. and O'Neill L.A. (2016) HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 126, 3699–3707 10.1172/JCI84431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palsson-McDermott E.M. and O'Neill L.A. (2013) The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays 35, 965–973 10.1002/bies.201300084 [DOI] [PubMed] [Google Scholar]
- 82.Oda T., Hirota K., Nishi K., Takabuchi S., Oda S., Yamada H. et al. (2006) Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am. J. Physiol. Cell Physiol. 291, C104–C113 10.1152/ajpcell.00614.2005 [DOI] [PubMed] [Google Scholar]
- 83.Li C., Wang Y., Li Y., Yu Q., Jin X., Wang X. et al. (2018) HIF1α-dependent glycolysis promotes macrophage functional activities in protecting against bacterial and fungal infection. Sci. Rep. 8, 3603 10.1038/s41598-018-22039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cramer T., Yamanishi Y., Clausen B.E., Forster I., Pawlinski R., Mackman N. et al. (2003) HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 10.1016/S0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peyssonnaux C., Datta V., Cramer T., Doedens A., Theodorakis E.A., Gallo R.L. et al. (2005) HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 115, 1806–1815 10.1172/JCI23865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braverman J., Sogi K.M., Benjamin D., Nomura D.K. and Stanley S.A. (2016) HIF-1α is an essential mediator of IFN-γ-dependent immunity to Mycobacterium tuberculosis. J. Immunol. 197, 1287–1297 10.4049/jimmunol.1600266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imtiyaz H.Z., Williams E.P., Hickey M.M., Patel S.A., Durham A.C., Yuan L.J. et al. (2010) Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 120, 2699–2714 10.1172/JCI39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Backer V., Cheung F.Y., Siveke J.T., Fandrey J. and Winning S. (2017) Knockdown of myeloid cell hypoxia-inducible factor-1α ameliorates the acute pathology in DSS-induced colitis. PLoS ONE 12, e0190074 10.1371/journal.pone.0190074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shalova I.N., Lim J.Y., Chittezhath M., Zinkernagel A.S., Beasley F., Hernandez-Jimenez E. et al. (2015) Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 42, 484–498 10.1016/j.immuni.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 90.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V. et al. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corzo C.A., Condamine T., Lu L., Cotter M.J., Youn J.I., Cheng P. et al. (2010) HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noman M.Z., Desantis G., Janji B., Hasmim M., Karray S., Dessen P. et al. (2014) PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doedens A.L., Stockmann C., Rubinstein M.P., Liao D., Zhang N., DeNardo D.G. et al. (2010) Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res. 70, 7465–7475 10.1158/0008-5472.CAN-10-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henke N., Ferreiros N., Geisslinger G., Ding M.G., Essler S., Fuhrmann D.C. et al. (2016) Loss of HIF-1α in macrophages attenuates AhR/ARNT-mediated tumorigenesis in a PAH-driven tumor model. Oncotarget 7, 25915–25929 10.18632/oncotarget.8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeong Y.J., Hong S.W., Kim J.H., Jin D.H., Kang J.S., Lee W.J. et al. (2011) Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell. Immunol. 266, 192–199 10.1016/j.cellimm.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 96.Jeong Y.J., Kim J.H., Hong J.M., Kang J.S., Kim H.R., Lee W.J. et al. (2014) Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8+ memory T cell production capacity of these cells in vivo. Immunobiology 219, 554–564 10.1016/j.imbio.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 97.Maianski N.A., Maianski A.N., Kuijpers T.W. and Roos D. (2004) Apoptosis of neutrophils. Acta Haematol. 111, 56–66 10.1159/000074486 [DOI] [PubMed] [Google Scholar]
- 98.Mecklenburgh K.I., Walmsley S.R., Cowburn A.S., Wiesener M., Reed B.J., Upton P.D. et al. (2002) Involvement of a ferroprotein sensor in hypoxia-mediated inhibition of neutrophil apoptosis. Blood 100, 3008–3016 10.1182/blood-2002-02-0454 [DOI] [PubMed] [Google Scholar]
- 99.Walmsley S.R., Cowburn A.S., Clatworthy M.R., Morrell N.W., Roper E.C., Singleton V. et al. (2006) Neutrophils from patients with heterozygous germline mutations in the von Hippel Lindau protein (pVHL) display delayed apoptosis and enhanced bacterial phagocytosis. Blood 108, 3176–3178 10.1182/blood-2006-04-018796 [DOI] [PubMed] [Google Scholar]
- 100.Walmsley S.R., Print C., Farahi N., Peyssonnaux C., Johnson R.S., Cramer T. et al. (2005) Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 201, 105–115 10.1084/jem.20040624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berger E.A., McClellan S.A., Vistisen K.S. and Hazlett L.D. (2013) HIF-1α is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog. 9, e1003457 10.1371/journal.ppat.1003457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vissers M.C., Winterbourn C.C. and Hunt J.S. (1984) Degradation of glomerular basement membrane by human neutrophils in vitro. Biochim. Biophys. Acta 804, 154–160 10.1016/0167-4889(84)90144-7 [DOI] [PubMed] [Google Scholar]
- 103.Perez-Cruz I., Carcamo J.M. and Golde D.W. (2003) Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 102, 336–343 10.1182/blood-2002-11-3559 [DOI] [PubMed] [Google Scholar]
- 104.Ferron-Celma I., Mansilla A., Hassan L., Garcia-Navarro A., Comino A.M., Bueno P. et al. (2009) Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 153, 224–230 10.1016/j.jss.2008.04.024 [DOI] [PubMed] [Google Scholar]
- 105.Uchio R., Hirose Y., Murosaki S., Yamamoto Y. and Ishigami A. (2015) High dietary intake of vitamin C suppresses age-related thymic atrophy and contributes to the maintenance of immune cells in vitamin C-deficient senescence marker protein-30 knockout mice. Br. J. Nutr. 113, 603–609 10.1017/S0007114514003857 [DOI] [PubMed] [Google Scholar]
- 106.Goldschmidt M.C. (1991) Reduced bactericidal activity in neutrophils from scorbutic animals and the effect of ascorbic acid on these target bacteria in vivo and in vitro. Am. J. Clin. Nutr. 54, 1214S–1220S 10.1093/ajcn/54.6.1214s [DOI] [PubMed] [Google Scholar]
- 107.Gaut J.P., Belaaouaj A., Byun J., Roberts L.J. II, Maeda N., Frei B. et al. (2006) Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free Radic. Biol. Med. 40, 1494–1501 10.1016/j.freeradbiomed.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 108.Bozonet S.M., Carr A.C., Pullar J.M. and Vissers M.C. (2015) Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 7, 2574–2588 10.3390/nu7042574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohammed B.M., Fisher B.J., Kraskauskas D., Farkas D., Brophy D.F., Fowler A.A. III et al. (2013) Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients 5, 3131–3151 10.3390/nu5083131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Groux H. and Powrie F. (1999) Regulatory T cells and inflammatory bowel disease. Immunol. Today 20, 442–445 10.1016/S0167-5699(99)01510-8 [DOI] [PubMed] [Google Scholar]
- 111.Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R. et al. (2011) HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y. et al. (2011) Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 10.1016/j.cell.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Doedens A.L., Phan A.T., Stradner M.H., Fujimoto J.K., Nguyen J.V., Yang E. et al. (2013) Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 10.1038/ni.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Higashiyama M., Hokari R., Hozumi H., Kurihara C., Ueda T., Watanabe C. et al. (2012) HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J. Leukoc. Biol. 91, 901–909 10.1189/jlb.1011518 [DOI] [PubMed] [Google Scholar]
- 115.Flück K., Breves G., Fandrey J. and Winning S. (2016) Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 9, 379–390 10.1038/mi.2015.67 [DOI] [PubMed] [Google Scholar]
- 116.Hammami A., Abidin B.M., Heinonen K.M. and Stäger S. (2018) HIF-1α hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci. Rep. 8, 3500 10.1038/s41598-018-21891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palazon A., Tyrakis P.A., Macias D., Velica P., Rundqvist H., Fitzpatrick S. et al. (2017) An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32, 669–683.e5 10.1016/j.ccell.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thiel M., Caldwell C.C., Kreth S., Kuboki S., Chen P., Smith P. et al. (2007) Targeted deletion of HIF-1α gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE 2, e853 10.1371/journal.pone.0000853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Labiano S., Melendez-Rodriguez F., Palazon A., Teijeira A., Garasa S., Etxeberria I. et al. (2017) CD69 is a direct HIF-1α target gene in hypoxia as a mechanism enhancing expression on tumor-infiltrating T lymphocytes. Oncoimmunology 6, e1283468 10.1080/2162402X.2017.1283468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fraser R.C., Pavlovic S., Kurahara C.G., Murata A., Peterson N.S., Taylor K.B. et al. (1980) The effect of variations in vitamin C intake on the cellular immune response of guinea pigs. Am. J. Clin. Nutr. 33, 839–847 10.1093/ajcn/33.4.839 [DOI] [PubMed] [Google Scholar]
- 121.Kennes B., Dumont I., Brohee D., Hubert C. and Neve P. (1983) Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology 29, 305–310 10.1159/000213131 [DOI] [PubMed] [Google Scholar]
- 122.Huijskens M.J., Walczak M., Koller N., Briede J.J., Senden-Gijsbers B.L., Schnijderberg M.C. et al. (2014) Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 96, 1165–1175 10.1189/jlb.1TA0214-121RR [DOI] [PubMed] [Google Scholar]
- 123.Huijskens M.J., Walczak M., Sarkar S., Atrafi F., Senden-Gijsbers B.L., Tilanus M.G. et al. (2015) Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 17, 613–620 10.1016/j.jcyt.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 124.Manning J., Mitchell B., Appadurai D.A., Shakya A., Pierce L.J., Wang H. et al. (2013) Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 19, 2054–2067 10.1089/ars.2012.4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu X. and Zhang Y. (2017) TET-mediated active DNA demethylation: mechanism, function and beyond. Nat. Rev. Genet. 18, 517–534 10.1038/nrg.2017.33 [DOI] [PubMed] [Google Scholar]
- 126.Blaschke K., Ebata K.T., Karimi M.M., Zepeda-Martinez J.A., Goyal P., Mahapatra S. et al. (2013) Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500, 222–226 10.1038/nature12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minor E.A., Court B.L., Young J.I. and Wang G. (2013) Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 288, 13669–13674 10.1074/jbc.C113.464800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hore T.A. (2017) Modulating epigenetic memory through vitamins and TET: implications for regenerative medicine and cancer treatment. Epigenomics 9, 863–871 10.2217/epi-2017-0021 [DOI] [PubMed] [Google Scholar]
- 129.Tsukada Y., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P. et al. (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811–816 10.1038/nature04433 [DOI] [PubMed] [Google Scholar]
- 130.Klose R.J., Kallin E.M. and Zhang Y. (2006) JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 7, 715–727 10.1038/nrg1945 [DOI] [PubMed] [Google Scholar]
- 131.Cull A.H., Snetsinger B., Buckstein R., Wells R.A. and Rauh M.J. (2017) Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 55, 56–70.e13 10.1016/j.exphem.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 132.Wallner S., Schroder C., Leitao E., Berulava T., Haak C., Beisser D. et al. (2016) Epigenetic dynamics of monocyte-to-macrophage differentiation. Epigenetics Chromatin 9, 74 10.1186/s13072-016-0079-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Q., Zhao K., Shen Q., Han Y., Gu Y., Li X. et al. (2015) Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 10.1038/nature15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y. et al. (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 10.1038/ni.1920 [DOI] [PubMed] [Google Scholar]
- 135.Achuthan A., Cook A.D., Lee M.C., Saleh R., Khiew H.W., Chang M.W. et al. (2016) Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J. Clin. Invest. 126, 3453–3466 10.1172/JCI87828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yıldırım-Buharalıoğlu G., Bond M., Sala-Newby G.B., Hindmarch C.C. and Newby A.C. (2017) Regulation of epigenetic modifiers, including KDM6B, by interferon-γ and interleukin-4 in human macrophages. Front. Immunol. 8, 92 10.3389/fimmu.2017.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Santa F., Narang V., Yap Z.H., Tusi B.K., Burgold T., Austenaa L. et al. (2009) Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 28, 3341–3352 10.1038/emboj.2009.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Santa F., Totaro M.G., Prosperini E., Notarbartolo S., Testa G. and Natoli G. (2007) The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130, 1083–1094 10.1016/j.cell.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 139.Ishii M., Wen H., Corsa C.A., Liu T., Coelho A.L., Allen R.M. et al. (2009) Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 114, 3244–3254 10.1182/blood-2009-04-217620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan Q., Sun L., Zhu Z., Wang L., Li S. and Ye R.D. (2014) Jmjd3-mediated epigenetic regulation of inflammatory cytokine gene expression in serum amyloid A-stimulated macrophages. Cell. Signal. 26, 1783–1791 10.1016/j.cellsig.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kruidenier L., Chung C.W., Cheng Z., Liddle J., Che K., Joberty G. et al. (2012) A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 10.1038/nature11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cimmino L., Dolgalev I., Wang Y., Yoshimi A., Martin G.H., Wang J. et al. (2017) Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170, 1079–1095.e20 10.1016/j.cell.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foster M.N., Carr A.C., Antony A., Peng S. and Fitzpatrick M.G. (2018) Intravenous vitamin C administration improved blood cell counts and health-related quality of life of patient with history of relapsed acute myeloid leukaemia. Antioxidants 7, E92 10.3390/antiox7070092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao H., Zhu H., Huang J., Zhu Y., Hong M., Zhu H. et al. (2018) The synergy of vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 66, 1–7 10.1016/j.leukres.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 145.Zhang X., Ulm A., Somineni H.K., Oh S., Weirauch M.T., Zhang H.X. et al. (2014) DNA methylation dynamics during ex vivo differentiation and maturation of human dendritic cells. Epigenetics Chromatin 7, 21 10.1186/1756-8935-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ptaschinski C., Mukherjee S., Moore M.L., Albert M., Helin K., Kunkel S.L. et al. (2015) RSV-induced H3K4 demethylase KDM5B leads to regulation of dendritic cell-derived innate cytokines and exacerbates pathogenesis in vivo. PLoS Pathog. 11, e1004978 10.1371/journal.ppat.1004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nestor C.E., Lentini A., Hagg Nilsson C., Gawel D.R., Gustafsson M., Mattson L. et al. (2016) 5-Hydroxymethylcytosine remodeling precedes lineage specification during differentiation of human CD4+ T cells. Cell Rep. 16, 559–570 10.1016/j.celrep.2016.05.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ichiyama K., Chen T., Wang X., Yan X., Kim B.S., Tanaka S. et al. (2015) The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 42, 613–626 10.1016/j.immuni.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carty S.A., Gohil M., Banks L.B., Cotton R.M., Johnson M.E., Stelekati E. et al. (2018) The loss of TET2 promotes CD8+ T cell memory differentiation. J. Immunol. 200, 82–91 10.4049/jimmunol.1700559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McPherson R.C., Konkel J.E., Prendergast C.T., Thomson J.P., Ottaviano R., Leech M.D. et al. (2014) Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4+ T cells tolerized by peptide immunotherapy. eLife 3, e03416 10.7554/eLife.03416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LaMere S.A., Thompson R.C., Meng X., Komori H.K., Mark A. and Salomon D.R. (2017) H3k27 methylation dynamics during CD4 T cell activation: regulation of JAK/STAT and IL12RB2 expression by JMJD3. J. Immunol. 199, 3158–3175 10.4049/jimmunol.1700475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z. et al. (2009) Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 10.1016/j.immuni.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Q., Zou J., Wang M., Ding X., Chepelev I., Zhou X. et al. (2014) Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation. Nat. Commun. 5, 5780 10.1038/ncomms6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Z., Cao W., Xu L., Chen X., Zhan Y., Yang Q. et al. (2015) The histone H3 lysine-27 demethylase Jmjd3 plays a critical role in specific regulation of Th17 cell differentiation. J. Mol. Cell Biol. 7, 505–516 10.1093/jmcb/mjv022 [DOI] [PubMed] [Google Scholar]
- 155.Yue X., Trifari S., Aijo T., Tsagaratou A., Pastor W.A., Zepeda-Martinez J.A. et al. (2016) Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 213, 377–397 10.1084/jem.20151438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sasidharan Nair V., Song M.H. and Oh K.I. (2016) Vitamin C facilitates demethylation of the Foxp3 enhancer in a Tet-dependent manner. J. Immunol. 196, 2119–2131 10.4049/jimmunol.1502352 [DOI] [PubMed] [Google Scholar]
- 157.Nikolouli E., Hardtke-Wolenski M., Hapke M., Beckstette M., Geffers R., Floess S. et al. (2017) Alloantigen-induced regulatory T cells generated in presence of vitamin C display enhanced stability of Foxp3 expression and promote skin allograft acceptance. Front. Immunol. 8, 748 10.3389/fimmu.2017.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kasahara H., Kondo T., Nakatsukasa H., Chikuma S., Ito M., Ando M. et al. (2017) Generation of allo-antigen-specific induced Treg stabilized by vitamin C treatment and its application for prevention of acute graft versus host disease model. Int. Immunol. 29, 457–469 10.1093/intimm/dxx060 [DOI] [PubMed] [Google Scholar]
- 159.Song M.H., Nair V.S. and Oh K.I. (2017) Vitamin C enhances the expression of IL17 in a Jmjd2-dependent manner. BMB Rep. 50, 49–54 10.5483/BMBRep.2017.50.1.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsagaratou A., Gonzalez-Avalos E., Rautio S., Scott-Browne J.P., Togher S., Pastor W.A. et al. (2017) TET proteins regulate the lineage specification and TCR-mediated expansion of iNKT cells. Nat. Immunol. 18, 45–53 10.1038/ni.3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Northrup D., Yagi R., Cui K., Proctor W.R., Wang C., Placek K. et al. (2017) Histone demethylases UTX and JMJD3 are required for NKT cell development in mice. Cell Biosci. 7, 25 10.1186/s13578-017-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beyaz S., Kim J.H., Pinello L., Xifaras M.E., Hu Y., Huang J. et al. (2017) The histone demethylase UTX regulates the lineage-specific epigenetic program of invariant natural killer T cells. Nat. Immunol. 18, 184–195 10.1038/ni.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cribbs A., Hookway E.S., Wells G., Lindow M., Obad S., Oerum H. et al. (2018) Inhibition of histone H3K27 demethylases selectively modulates inflammatory phenotypes of natural killer cells. J. Biol. Chem. 293, 2422–2437 10.1074/jbc.RA117.000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Anderson R., Oosthuizen R., Maritz R., Theron A. and Van Rensburg A.J. (1980) The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am. J. Clin. Nutr. 33, 71–76 PMID: [DOI] [PubMed] [Google Scholar]
- 165.Bhandari T., Olson J., Johnson R.S. and Nizet V. (2013) HIF-1alpha influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J. Mol. Med. 91, 1199–1205 10.1007/s00109-013-1052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campbell J.D., Cole M., Bunditrutavorn B. and Vella A.T. (1999) Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis. Cell. Immunol. 194, 1–5 PMID: [DOI] [PubMed] [Google Scholar]
- 167.He C., Larson-Casey J.L., Gu L., Ryan A.J., Murthy S. and Carter A.B. (2016) Cu,Zn-superoxide dismutase-mediated redox regulation of Jumonji domain containing 3 modulates macrophage polarization and pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 55, 58–71 10.1165/rcmb.2015-0183OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnston C.S. and Huang S.N. (1991) Effect of ascorbic acid nutriture on blood histamine and neutrophil chemotaxis in guinea pigs. J. Nutr. 121, 126–130 PMID: [DOI] [PubMed] [Google Scholar]
- 169.Kim J.E., Cho H.S., Yang H.S., Jung D.J., Hong S.W., Hung C.F. et al. (2012) Depletion of ascorbic acid impairs NK cell activity against ovarian cancer in a mouse model. Immunobiology 217, 873–881 10.1016/j.imbio.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 170.Ko M., An J., Pastor W.A., Koralov S.B., Rajewsky K. and Rao A. (2015) TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol. Rev. 263, 6–21 10.1111/imr.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lio C.W., Zhang J., Gonzalez-Avalos E., Hogan P.G., Chang X. and Rao A. (2016) Tet2 and Tet3 cooperate with B-lineage transcription factors to regulate DNA modification and chromatin accessibility. eLife 5 10.7554/eLife.18290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maeng H.G., Lim H., Jeong Y.J., Woo A., Kang J.S., Lee W.J. et al. (2009) Vitamin C enters mouse T cells as dehydroascorbic acid in vitro and does not recapitulate in vivo vitamin C effects. Immunobiology 214, 311–320 10.1016/j.imbio.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 173.Noh K., Lim H., Moon S.K., Kang J.S., Lee W.J., Lee D. et al. (2005) Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol. Lett. 98, 63–72 PMID: [DOI] [PubMed] [Google Scholar]
- 174.Orlanski S., Labi V., Reizel Y., Spiro A., Lichtenstein M., Levin-Klein R. et al. (2016) Tissue-specific DNA demethylation is required for proper B-cell differentiation and function. Proc. Natl. Acad. Sci. U.S.A. 113, 5018–5023 10.1073/pnas.1604365113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Puskas F., Gergely P., Niland B., Banki K. and Perl A. (2002) Differential regulation of hydrogen peroxide and Fas-dependent apoptosis pathways by dehydroascorbate, the oxidized form of vitamin C. Antioxid. Redox Signal. 4, 357–369 PMID: [DOI] [PubMed] [Google Scholar]
- 176.Shilotri P.G. (1977) Phagocytosis and leukocyte enzymes in ascorbic acid deficient guinea pigs. J. Nutr. 107, 1513–1516 PMID: [DOI] [PubMed] [Google Scholar]
- 177.Talks K.L., Turley H., Gatter K.C., Maxwell P.H., Pugh C.W., Ratcliffe P.J. et al. (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411–421 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vissers M.C. and Hampton M.B. (2004) The role of oxidants and vitamin C on neutrophil apoptosis and clearance. Biochem. Soc. Trans. 32, 499–501 10.1042/bst0320499 [DOI] [PubMed] [Google Scholar]
- 179.Vissers M.C. and Wilkie R.P. (2007) Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. J. Leukoc. Biol. 81, 1236–1244 10.1189/jlb.0806541 [DOI] [PubMed] [Google Scholar]
- 180.Vojdani A. and Ghoneum M. (1993) In vivo effect of ascorbic acid on enhancement of human natural killer cell activity. Nutr. Res. 13, 753–764 10.1016/S0271-5317(05)80799-7 [DOI] [Google Scholar]