Abstract
The serine/threonine protein kinase mechanistic target of rapamycin (mTOR) has been implicated in the regulation of an array of cellular functions including protein and lipid synthesis, proliferation, cell size and survival. Here, we describe the role of mTOR during haemopoiesis within the context of mTORC1 and mTORC2, the distinct complexes in which it functions. The use of conditional transgenic mouse models specifically targeting individual mTOR signalling components, together with selective inhibitors, have generated a significant body of research emphasising the critical roles played by mTOR, and individual mTOR complexes, in haemopoietic lineage commitment and development. This review will describe the profound role of mTOR in embryogenesis and haemopoiesis, underscoring the importance of mTORC1 at the early stages of haemopoietic cell development, through modulation of stem cell potentiation and self-renewal, and erythroid and B cell lineage commitment. Furthermore, the relatively discrete role of mTORC2 in haemopoiesis will be explored during T cell development and B cell maturation. Collectively, this review aims to highlight the functional diversity of mTOR signalling and underline the importance of this pathway in haemopoiesis.
Keywords: haemopoiesis, intracellular signalling, mechanistic target of rapamycin
Introduction
Mechanistic target of rapamycin (mTOR) is a serine/threonine protein kinase which was initially identified due to its ability to be inhibited by rapamycin, an antifungal macrolide first characterised in Streptomyces hygroscopicus. Purification and identification of mTOR in mammals subsequently revealed that it regulates a plethora of biological functions, including protein and lipid synthesis, mitochondrial function, autophagy and cytoskeleton organisation, contributing towards proliferation and cell survival [1]. However, the mechanisms regulated by mTOR signalling in specific cell contexts are still not fully understood.
The mTOR-mediated signalling pathway
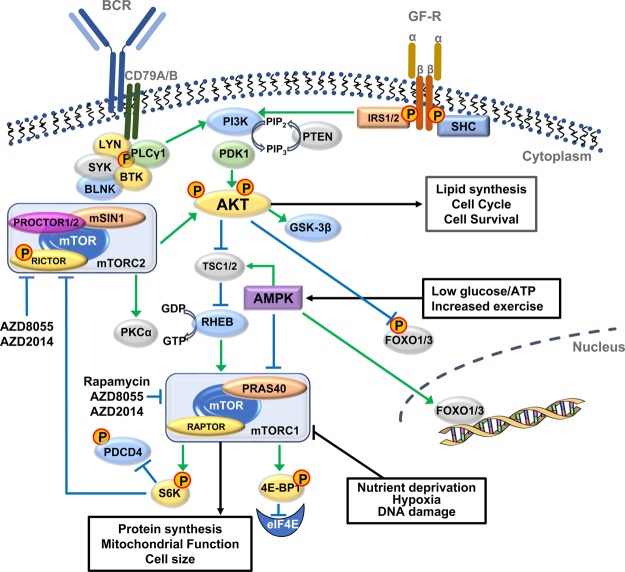
mTOR is activated by a variety of upstream mediators including growth factor (GF) receptors (e.g. insulin/insulin-like GF, tumour necrosis factor receptors), and nutrients such as glucose and amino acids (AAs). mTOR belongs to the phosphoinositide 3-kinase (PI3K)-related kinase (PIKK) family and forms two distinct complexes — mTORC1 and mTORC2. These complexes share the proteins mTOR, GβL, DEPTOR and Tti1/Tel2. The subunits which make the respective complexes unique are RAPTOR (rapamycin TOR sensitive) and PRAS40 contained within mTORC1, and RICTOR (rapamycin TOR insensitive), mSIN1 and PROCTOR1/2 specific to mTORC2 [1]. As suggested by the names, RAPTOR and RICTOR define the sensitivity of mTORC1 and mTORC2 to rapamycin [2]. Rapamycin interacts with FK506-binding proteins, such as FKBP12, with high affinity and the resultant complex interacts with mTOR in the context of mTORC1 to allosterically inhibit mTORC1 activity [3,4]. PI3K, an upstream mediator of mTOR, can be activated through GF receptor activation, leading to PI3K binding to insulin receptor substrate (IRS) proteins and conversion of phosphatidylinositol-4,5-phosphate (PIP2) into phosphatidylinositol-3,4,5-phosphate (PIP3) (Figure 1). Phosphatase and tensin homolog (PTEN), a tumour suppressor, reduces PIP3 accumulation, while PI3K promotes the activation of 3-phosphoinositide-dependent protein kinase 1 (PDK1), which then phosphorylates and activates AKT [5]. AKT then activates mTORC1 by inhibiting tuberous sclerosis complex (TSC)1/2. TSC proteins form a heterodimer and inhibit Ras homolog enriched in brain (RHEB), a positive regulator of mTORC1, which binds to mTORC1 causing conformational changes in the protein and activation [5].
Figure 1. Diagram of the AKT/mTOR Signalling Pathway.
Summary of downstream signalling from the BCR and growth factor receptors (GF-R) is shown. Activation of these receptors results in phosphorylation and activation of Akt, which leads to the activation of mTORC1 (PRAS40, RAPTOR), thereby initiating cell processes such as protein synthesis and proliferation. The downstream target of mTORC1, S6K negatively regulates mTORC2 (PROTOR1/2, mSIN1, RICTOR), which is responsible for the activation of Akt. This creates a negative feedback loop, which regulates this pathway. mTORC1 and mTORC2 share the subunits mTOR, GβL, DEPTOR and Tti1/Tel2 (not shown). Akt negatively phosphorylates FOXO1/3, which regulates the cell cycle. Kinases such as AMPK are activated in stress responses and inhibit the mTORC1 pathway. Allosteric inhibitors such as rapamycin and other rapalogs partially inhibit mTORC1 activity, whereas ATP competitive inhibitors such as AZD2014 are pan mTOR inhibitors. All inhibitory and non-inhibitory signalling is represented in blue and green, respectively. PLC, phospholipase C; BLNK, B cell linker; BTK, Bruton tyrosine kinase.
mTORC1 signalling
RAPTOR binds mTOR to enhance its activity, as indicated by the finding that RAPTOR inhibition, using RNAi, leads to decreased mTOR activity [6]. In a low-energy state (low ATP:AMP ratio), AMP-protein kinase (AMPK), a conserved energy sensor, is activated, leading to TSC2 phosphorylation and the subsequent inhibition of mTORC1 activity [7]. However, in a high-energy state, the mTORC1 pathway is activated, promoting protein synthesis, lipogenesis, and mitochondrial biogenesis and function [8–11]. Indeed, mTORC1 plays a pivotal role in mitochondrial oxidative function through regulation of the transcription factor yin-yang1 (YY1), which subsequently controls gene expression of mitochondrial transcriptional regulators including PGC-1α [10]. Furthermore, mTORC1 controls mitochondrial biogenesis and respiration through phosphorylation/inhibition of the eukaryotic initiation factor 4E (eIF-4E) binding proteins (4E-BPs) and regulation of the translation of nucleus-encoded mitochondrial-related mRNAs, which in turn increases ATP generation in the cell [11]. In the absence of mTORC1 activity, 4EBP1 is hypophosphorylated and interacts with the mRNA cap-binding protein eIF4E, inhibiting translation of cap-dependent proteins. Upon mTOR activation, hyperphosphorylation of 4E-BP1 releases eIF4E, enabling its association with eIF-4A (RNA helicase) and the scaffolding protein eIF-4G to form the eIF-4F complex (Figure 1). The mTORC1-eIF4E pathway is up-regulated in most cancers and thus represents an attractive therapeutic target [12].
mTORC1 also phosphorylates/activates S6 Kinase 1 (S6K1) at Thr389, which was initially thought to play a role in protein/ribosomal biogenesis by activating 40S ribosomal protein. However, it is now appreciated that S6K1 is important in many mechanisms, together with S6K2, including transcription, cell proliferation, apoptosis and potential mRNA splicing [13]. S6K phosphorylates programmed cell death 4 protein (PDCD4) at Ser67, targeting it for proteasomal degradation [14]. PDCD4 is a tumour suppressor responsible for inhibiting eIF4E. Moreover, a recent study in a colorectal cancer model shows that S6K phosphorylates and inhibits elongation factor-2 kinase (EF2K), in turn relieving EF2K inhibition of EF2 and thus elongation of nascent polypeptide chains [15]. S6K also plays a role in actin organisation by the direct binding to F-actin. A role in cytoskeletal rearrangement is also seen as S6K activates Rho family members regulating this — Cdc42 and Rac1 and their downstream target PAK1. As deletion of S6K leads to a decrease in activation of the Rho family members, cytoskeletal organisation and migration in ovarian cancer cells, S6K is a promising target for enabling reduction in tumour progression [16]. An additional target of S6K1 is RICTOR [17], which, when phosphorylated at Thr1135, leads to mTORC2 inhibition, establishing a negative feedback loop between mTORC1 and mTORC2 [18].
mTORC2 signalling
AKT can be considered the hub of the PI3K pathway as its downstream signalling leads to mechanisms controlling a multitude of diverse functions within the cell. AKT is activated upon phosphorylation of two key sites, Thr308 by PDK1 and Ser473 by mTORC2, via GF receptor activation [19]. The majority of mTORC2 functions occur through AKT regulation, including activation of mTORC1, placing AKT both upstream of and downstream from mTOR regulation. During low-energy conditions, AMPK activation leads to an up-regulation of mTORC2 activity and modulation of downstream targets [20]. Such downstream targets include the Forkhead Box O (FOXO) family of transcription factors, which when phosphorylated by AKT leads to an inhibition of their function. FOXOs play an important role in the repression of cell proliferation and survival, but in certain cell contexts can also play a role in tumorigenesis [21]. FOXOs regulate apoptosis in distinct ways, repressing apoptosis through the down-regulation of the pro-apoptotic BCL2 family member Bim, or promoting apoptosis through transcriptional up-regulation of the FAS ligand [22,23]. mTORC2-FOXO1 signalling also regulates innate immune responses as RICTOR deletion leads to attenuated AKT signalling, thereby increasing nuclear FOXO1, resulting in hyper-inflammatory responses via toll-like receptor 4 (TLR4) [24]. mTORC2 can localise at the mitochondria-associated endoplasmic reticulum membranes (MAMs) in a GF-dependent manner, and RICTOR deletion disrupts AKT-dependent phosphorylation of mitochondria-associated proteins. These events lead to a reduction in mitochondrial function, increasing mitochondrial membrane potential and affecting energy metabolism and cell survival, thereby demonstrating a vital role of mTORC2 signalling in mitochondrial physiology [25]. The importance of mTORC2 in AKT activation was highlighted by a recent study demonstrating that deletion of the AKT-binding site within the mTORC2 component mSIN1 greatly reduced AKTS473 phosphorylation, rendering it unable to phosphorylate FOXO1/3a, while other targets such as glycogen synthase kinase 3 (GSK3) and mTORC1 were unaffected [26,27]. These findings suggest that mTORC2 activation is important for AKT-mediated cell survival mechanisms, but not for mTORC1 mechanisms.
Additional targets of mTORC2 include protein kinase C-alpha (PKCα) as mTORC2 inactivation reduced PKCα phosphorylation [28], which is responsible for functions including cell proliferation, differentiation, motility, apoptosis and inflammation [29]. mTORC2 also regulates growth and ion transport by phosphorylating the hydrophobic motif of serum and glucocorticoid-induced protein kinase 1 (SGK1) [30]. SGK1 inhibition induces autophagy, apoptosis and cell cycle arrest in the G2/M phase in prostate cancer cell lines, at least in part through an mTOR-FOXO3a-mediated pathway [31]. SGK1 also regulates TH2 differentiation and negatively regulates interferon gamma (IFNγ) production, thereby highlighting the importance of mTORC2 in T cell effector function [32]. mTORC2 has also shown to play a role in cytoskeletal organisation by activating RhoA GTPases [33].
mTOR in embryogenesis
The mTOR complexes are essential for cell survival and growth, and studies generating knockout (KO) mice established that mTOR kinase and individual complexes mTORC1/2 were essential for normal embryogenesis [34,35]. A homozygous KO of mTOR (mTOR−/−) resulted in the death of mouse embryos soon after implantation (E5.5–6.5). Despite normal blastocyst development, the embryo did not develop further due to limited proliferation and survival signalling. Nevertheless, mTOR+/− mice developed fertile and normal embryos. Similarly, Raptor−/− embryos die during early development (E7), whereas the Rictor−/− mice survived slightly longer (E10.5) [35]. These studies indicate mTOR function is mediated mainly through mTORC1 during early embryogenesis, but both mTORC1/2 play critical roles.
Role of mTOR signalling in haemopoiesis
Haemopoiesis initially occurs in the yolk sac and in two waves, the first of which is known as ‘primitive’ haemopoiesis. At this stage de novo haemangioblasts are generated and produce large quantities of erythrocytes to promote increased oxygenation, accommodating rapid growth. During the second wave of haemopoiesis or definitive haemopoiesis, haemopoietic stem cells (HSCs) appear in the aorta–gonad–mesonephros region around E10 [36]. From E11, HSCs migrate to and colonise the foetal liver (FL) and subsequently the bone marrow (BM) with waves of repopulating HSCs that provide a continuous source of mature haemopoietic lineage cells during the adult lifespan. The nature of the HSCs differ depending on the micro-environmental niche, with HSCs in the BM being more quiescent than those in the FL [37,38]. HSC differentiation into multipotent progenitor (MPP) cells occurs mainly in the FL prior to migration into specific haemopoietic organs, such as the thymus, for further lineage differentiation. MPPs give rise to oligopotent common myeloid or lymphoid progenitors (CMPs or CLPs). CMPs further give rise to megakaryocyte-erythroid progenitors and granulocyte–macrophage progenitors, while CLPs give rise to lymphoid lineage cells [39]. Targeted deletion of mTORC1 and/or mTORC2 in mouse models demonstrate a critical role for the mTOR pathway in haemopoiesis, and highlight the importance of the individual mTOR-containing complexes at specific stages of HSC homeostasis and haemopoietic lineage commitment and maturation, as discussed below.
Haemopoietic stem cells
Conditional knockout (cKO) mouse models of PTEN and TSC1, upstream negative regulators of mTORC1 in HSCs, revealed an increase in short-term HSC cycling and a concomitant decline in long-term HSC (LT-HSC) quiescence and self-renewal through constitutive activation of mTORC1 [40–42]. TSC1−/− in HSCs led to an elevation in mitochondrial biogenesis, resulting in increased reactive oxygen species (ROS) production, driving HSCs from quiescence to rapid cell cycling, thereby reducing their self-renewal capacity [43]. These studies identify the role of mTOR in regulating HSC cycling through modulation of ROS levels. Interestingly, similar findings were reported in mTOR cKO mice, in which BrdU labelling revealed rapid cell cycling of HSCs leading to a loss of quiescence and defective HSC engraftment and repopulation upon transplantation into NSG mice [44]. Recent studies establish cross-talk between the ERK and mTOR signalling pathways, with ERK activity regulating mTORC1 activation, thus limiting its strength to promote HSC cycling in favour of quiescence. Indeed, HSCs derived from MEK1 cKO mice exhibit exhaustion due to increased mTORC1-mediated ROS production, resulting in increased mitochondrial damage [45]. Collectively, these studies identify the importance of precise mTOR regulation during HSC maintenance and haemopoiesis.
The Wnt-signalling pathway can activate the mTOR pathway by inhibiting GSK3-mediated phosphorylation of TSC2 independently of β-catenin transcription. GSK3 inhibits mTOR activation by phosphorylating TSC2 in a manner co-ordinated by AMPK [46]. Knockdown (KD) of Gsk3 initially resulted in an increase in Lin−Sca1+cKit+ (LSK) populations due to an activation of both Wnt-signalling (β-catenin-dependent) and mTOR pathways. However, long-term disruption of GSK3 expression/activity led to a depletion of HSC populations due to mTOR activation, which could be reversed through modulation of mTORC1 and β-catenin [47]. Inhibition of the mTOR pathway together with activation of the Wnt-β-catenin pathway led to increased LT-HSC number and the potential to culture HSCs ex vivo in a cytokine-free environment, highlighting the importance of appropriate regulation of the Wnt and mTOR signalling pathways in stem cell renewal [48].
Reduction in signalling downstream of mTOR enhances HSC self-renewal and repopulating properties: mice lacking S6K1 [49] or mice treated with rapamycin [50] exhibit an increased life- and health span compared with controls due to an increase in repopulating LT-HSCs. Collectively, these findings suggest that the loss in mTOR function during haemopoiesis primarily represents a loss of mTORC1 activity, with mTORC2 not playing a key role in HSCs.
Haemopoietic stem/progenitor cell
Assessing the role of mTOR on haemopoietic lineage commitment using an mTOR cKO model in adult mice revealed significant aberrations in the development of haemopoietic lineage populations, resulting in a reduction in splenic weight and size. Closer analysis revealed that mTOR disruption led to pancytopenia, including a block in erythrocyte development at the pro-erythroblast stage, and anaemia [44]. The decline in haemopoietic lineage commitment was accompanied by increased apoptosis and decreased Mcl-1 expression. Within BM haemopoietic progenitor populations, there was a skew towards CMPs and a decrease in CLPs in mTOR-deficient mice. While there was an increase in the LSK population, the colony-forming ability of these LSKs was impaired. There was also an attenuation in S6K and 4E-BP1 phosphorylation/activation and increased phosphorylation of AKT, implicating an aberration in the S6K-mediated negative feedback loop regulation of mTORC2 activity [16,44].
The mTORC1 cKO model (Raptor-Mx1-cre) exhibited an increased LSK population in the spleen in addition to the BM, indicative of extramedullary haemopoiesis. The LSKs were arrested at the G1 phase of the cell cycle compared with controls, suggesting a reduction in cell division [51]. Metabolite analysis of LSKs revealed an increase in intermediates used in lipid metabolism, and in AMP and NADP involved in redox homeostasis, and a decrease in nitrogen metabolism [51]. LSK−CD48−CD150+ cells derived from the Raptor−/− BM failed to engraft into recipient mice. While these cells were able to home to the BM, they localised further from osteoblast cells, indicating a role for mTORC1 in the integration of niche signals. Raptor−/− HSCs also possessed regenerative and self-renewal aberrations compared with controls and those cells that ‘escaped deletion’. A compound Rictor/Raptor cKO in adult mice exhibited similar results as Raptor−/− mice in haemopoiesis; however, this mouse model did not develop BM failure and retained the deletion of alleles of Raptor and Rictor for almost one year after KO induction [51]. These results suggest that the mTOR pathway is not essential for survival and haemopoietic maintenance in adult mice but is essential for haemopoiesis initiation during embryonic development.
Genetic targeting studies to ablate mTORC2 function in haemopoiesis revealed a more subtle role compared with mTORC1. Studies in Rictor cKO (Rictor-Mx1-cre) mice indicated that mTORC2 does not play a significant role in HSCs and progenitor populations [51,52]. However, Magee et al. elegantly demonstrated that after PTEN deletion, which activates the mTOR-signalling pathway, deletion of Rictor abrogates leukaemogenesis and HSC depletion in adult, but not neonatal, mice [53], highlighting that the PTEN-mTORC2 signalling axis has a role in activating these processes in a temporally dependent manner.
Red blood cells
The mTOR cKO model revealed a block in erythropoiesis at the pro-erythroblast stage, highlighting the importance of mTOR signalling in RBC (red blood cell) development [44]. Interestingly, results from this model are similar to that observed in the Tsc1 cKO mouse, which exhibited a reduction in erythrocytes in the BM [42], through activation of mTORC1-mediated signalling. These studies indicate that a complex regulation of mTOR signalling is required for appropriate RBC development. Analysis of the importance of mTOR function in erythropoiesis revealed that loss of FOXO3 in erythroblasts results in an overactivation of the mTOR pathway, thereby compromising erythroid maturation [54]. FOXO3 regulates GATA-1 expression and represses Exosc8 expression, which are both involved in erythroid maturation [55]. Additionally, ectopic expression of microRNA9 disrupts erythropoiesis via the suppression of FOXO3-mediated pathways, causing an increase in ROS due to the down-regulation in ROS-scavenging enzymes [56].
Recent studies show that mTORC1 plays a critical role in RBC commitment, growth, proliferation and homeostasis. Knight et al. [57] demonstrated that mTORC1 activity is regulated by dietary iron, and a loss or overexpression of mTORC1 in HSCs leads to microcytic or macrocytic anaemia, respectively, with a loss of proliferation in RBC progenitors. Furthermore, treatment of mice with the ATP competitive mTOR inhibitor MLN0128, and subsequently with phenylhydrazine to induce haemolysis, was shown to be lethal, demonstrating the reliance of the mTOR pathway in RBC development [57]. Zhang et al. have shown that the heme-regulated eIF2α kinase (HRI)-activating transcription factor 4 (ATF4) pathway, which regulates heme uptake for haemoglobin production and stress response genes, suppresses mTORC1 activity in iron deficiency anaemia. The HRI-ATF4 pathway promoted RBC progenitor differentiation, and pharmacological inhibition of mTORC1 rescued RBC counts and haemoglobin content in the blood [58]. mTOR also plays a role in micro-environmental homeostasis associated with RBC development, through regulation of neutral essential AA (NEAA) uptake into cells during erythropoiesis for haemoglobin production. mTORC1/4EBP1 signalling regulates Lat3, a transporter of NEAAs in RBCs [59].
Myeloid lineage cells
cKOs of Mtor or Raptor in HSCs lead to a significant accumulation of the CD11b+Gr1− population [60]. mTOR cKO mice on a SCID background exhibited reduced monocyte/macrophage populations in in vitro and in vivo assays. However, removal of mTOR expression specifically in myeloid cells (Mtor-Lyzs-cre) revealed normal levels of monocyte/macrophage populations, suggesting that mTOR plays a role during lineage commitment, but not during survival and maturation [61]. Following mTOR deficiency, a decrease in expression of the M-CSF receptor, CD115 was noted, which may result in decreased monocyte/macrophage populations due to overactive STAT5 and down-regulation of IRF8 [61]. M-CSF promotes mTORC1 activation, which further promotes CD115 expression, and expression of PU.1 and IRF8 to promote myelopoiesis. In the absence of mTORC1 activity there is a block in glucose uptake and lipid metabolism, thereby abrogating myeloid differentiation and generating an impaired immune response to bacterial infection [62]. Constitutive activation of mTORC1 in TSC KO BM-derived macrophages (BMDMs) attenuated AKT signalling through the negative feedback loop via mTORC2, leading to a defect in IL4-induced M2 polarisation. These BMDMs produce more pro-inflammatory responses compared with controls, suggesting an important role of mTORC1 in the regulation of inflammation [63]. Additionally, it has recently been shown that Raptor cKO, but not granulocyte-specific KO (Raptor-lyz2-cre) mice exhibit a significant increase and accumulation of innate myelo-lymphoblastoid effector cells (IMLECs). This suggests that IMLEC accumulation driven by Raptor deficiency occurs earlier in development, caused by reduced expression of Myb in CMPs [64].
A myeloid lineage-specific KO model of Rictor (lysM-cre) revealed a significant decrease in monocytes, while the neutrophil population was unaffected. BM monocytes and peritoneal macrophages displayed decreased proliferation and increased susceptibility to pro-apoptotic stimuli. Furthermore, stimulation of TLR4 on Rictor−/− macrophages with LPS potentiated a pro-inflammatory response, with cells skewing towards an M1 phenotype and down-regulating IL10 expression, suggesting that mTORC2 signalling is a negative regulator of TLR signalling in macrophages [65,66]. Interestingly, the inflammatory response observed in Rictor−/− cells was reversed by the inactivation of Raptor, indicating that mTORC1 regulates inflammatory responses in macrophages [65].
Mice with reduced/absent mTOR activity/expression also exhibit a reduction in neutrophils in peripheral blood [44,67]. Neutrophils release antimicrobial cargos, which are carried on chromatin fibres called neutrophil extracellular traps (NETs). This first line of defence by neutrophils is known as NETosis, and requires autophagic activity. Treatment of neutrophils with mTOR inhibitors WYE-354 or rapamycin led to an increased release of NETs upon stimulation of neutrophils with a bacterial-derived stimulant, formyl-Met-Leu-Phe (fMLP), and an elevation in autophagosome formation [68]. These studies suggest mTOR signalling plays an important role in neutrophil activation. However, a decrease in NET formation in neutrophils treated with LPS in the presence of mTOR inhibition has also been reported, due to reduced HIF1-α translation, therefore further clarification is required [69]. Inhibition of mTORC2 activity through RICTOR KD severely abrogates chemotaxis in neutrophils through the inhibition of polarisation and directed migration induced by chemoattractants. The chemoattractants are unable to generate cAMP, thereby perturbing the cAMP/RhoA signalling axis [70]. This pathway appears to be regulated by PKCβII (downstream from mTORC2) as PRKCB KD leads to a similar disruption in chemotaxis as with mTORC2 inhibition. There is a significant decrease in PKCβII expression with RICTOR KD which disrupts subsequent translocation of PKCβII to the membrane to bind chemoattractant-induced adenylyl cyclase 9 and activate chemotaxis [71].
Ablation of mTORC1, in vitro via mTOR inhibitor torin-1 or in vivo in myeloid-specific transgenic mouse models, revealed an increase in eosinophil differentiation in the presence of IL-5, a cytokine involved in the recruitment of eosinophils in the presence of allergy. This increase was partially related to an up-regulation of GATA1 with mTOR inhibition. Interestingly, mTOR deletion in myeloid cells promotes eosinophil development but disrupts neutrophil development [72].
B lymphocytes
B cell development comprises several phenotypic stages enabling B cell lineage commitment/maturation from HSCs initially in the FL during embryogenesis and then in the BM. Recombination of heavy and light immunoglobulin (Ig) chain genes form a platform for B cell development: comprising the rearrangement of the variable (V), diversity (D) and joining (J) gene segments of the Ig heavy chain initially, followed by VJ rearrangement of the Ig light chain genes. B cells that generate functional rearrangements express a B cell receptor (BCR) and can be stimulated to produce antibodies that recognise specific antigens [73]. Immature B cells, expressing surface-bound IgM, migrate into the spleen where they mature into naïve, follicular or marginal zone (MZ) B cells via transitional phases (T1–T3). Follicular 1 cells (B220+IgDloCD21loIgMhi) form the bulk of the circulating population, whereas follicular 2 cells (B220+IgDhiCD21hiIgMhi) form one-third of the circulating population and are considered to be more primitive [74]. Further migration into the lymph nodes as memory B cells or plasma cells is also observed.
The importance of mTOR signalling during B cell development is evident from a study analysing mice in which mTOR expression is reduced [68]. A reduction in progenitor B cells was observed in these mice, with a block between large pre-B cells (B220+CD24+CD43+) and small pre-B cells (B220+CD24+CD43−). Within the spleen, an increased number of mature B cells (B220+IgDhighCD21+IgM−) and decreased T1 and T2 transitional B cells were observed compared with wild-type mice [68]. Deletion of the TSC1 complex in B cells (CD19-Cre) renders mTOR constitutively active, resulting in a partial block in B cell maturation as indicated by an elevation in T1 and T2 transitional B cells, and a depletion of MZ B cells [75].
Assessing mTORC1 signals more directly, Raptor cKO mice exhibit a significant decrease in B cell generation, due to an early block in lineage commitment [60,76]. For this reason, the effect of Raptor ablation on B cells was analysed using B cell-specific models (Mb1-Cre), which resulted in a profound block at the pre-B cell stage abrogating B cell maturation, proliferation, germinal centre reaction and antibody production [77]. A cKO of Raptor specifically in B cells (hCD20-Tam-Cre) resulted in a decrease in germinal centre B cells and nascent antibody-secreting plasma cells and the elimination of germinal centres, resulting in a decline in serum antibodies [77]. These studies illustrate the importance of mTORC1 at multiple stages of B cell maturation, and highlights the critical role played by mTOR in mounting an appropriate humoral immune response.
Rictor KO models revealed a role for mTORC2 during B cell maturation, resulting in a decrease in mature B cells [78]. Studies demonstrate an increase in early B cell populations including the pro-B, pre-B and immature B cells in Rictor cKO mice, characterised by elevated FOXO1 and RAG1 expression and a subsequent reduction in mature splenic B cells [52]. However, HSCs isolated from SIN1−/− mice reconstituted haemopoietic lineages, suggesting a minimal role of mTORC2 in B cell development during early stages. These mice exhibited increased IL7 production and RAG1/2 expression at the pro-B stage and fewer IgM+ immature B cells, suggesting a role for mTORC2 after B lineage commitment [79]. mTORC2 has also been shown to play an important role in B cell survival, as Rictor−/− mice display increased caspase-3 and PARP expression, together with increased cell death and a decrease in B-cell activating factor (BAFF) expression in mature B cells [58]. Lee et al. proposed that mTORC2 regulates canonical and non-canonical NFκB signalling pathways responsible for mature B cell maintenance and survival [78]. Interestingly, Rictor−/− mice possess increased CIP2A binding to PP2A, leading to increased c-myc phosphorylation and expression, and decreased E2F1 expression, which leads to apoptosis [80].
T lymphocytes
T cells develop in the thymus, undergoing rigorous positive and negative selection processes at the CD4+CD8+ double positive (DP) stage of development, to generate a pool of T cells that recognise foreign peptides in the context of self MHC-I (CD8+-cytotoxic T cells; Tc) or MHC-II (CD4+-helper T cells; Th). Analysis of mTORC1 signalling inhibition during T cell development showed that rapamycin treatment and Raptor deletion in cKO mice resulted in reduced thymic cellularity and a decrease in the proportion of DP cells, coupled with a concomitant increase in CD4−CD8− double negative (DN) cells [81]. Within the DN population, rapamycin blocked T cell development at the DN3 stage, probably prior to the proliferative burst associated with β-selection, while development was arrested at the DN1–DN2 transition in Raptor−/− mice both in vitro and in vivo. This block was associated with a reduction in proliferation due to an instability of cyclinD/CDK6 complexes. Similar results were noted with an mTOR cKO mouse model, while Rictor cKO mice exhibited a block in proliferation at the DN3 stage of development [82,83], suggesting that mTORC1 plays a critical role, which is distinct from mTORC2, during the early stages of T cell development.
The thymic micro-environment plays a critical role in enabling the appropriate development of nascent T cells, particularly thymic epithelial cells (TECs). Selective Rictor−/− in TECs results in a reduction in thymic mass and cellularity of TECs, and decreased generation of specific T cell lineages: TCRαβ, TCRγδ, invariant NKT [84] and regulatory T cells, thereby revealing an important role of mTORC2 in thymopoiesis and T cell lineage generation [85].
In the periphery, reduced mTOR expression/activity decreased T cell numbers, T cell activation and proliferation [68]. Furthermore, TSC1 ablation drove naïve T cells from quiescence to a poor immune response, altering the cell size and cycling [86]. mTORC1 activation, through TSC2 deletion, led to increased, terminally differentiated effector CD8+ T cell formation not capable of conforming to a memory T cell phenotype. However, mice deficient in mTORC1 activity through deletion of RHEB, led to loss of effector CD8+ T cell formation with no change in memory T cell expression, suggesting a role of mTORC1 in the differentiation of specific T cell subsets [87]. Indeed, mTORC1 is involved in Th1 and Th17 differentiation from naïve CD4+ T cells, as deletion of RHEB blocks Th1/Th17 differentiation, but not Th2 differentiation in vivo and in vitro [88]. mTORC1-mediated signalling plays a critical role in CD4+ T cell proliferation, by enhancing PPARγ activity, which in turn activates fatty acid metabolism, thus enabling the metabolic reprogramming required to activate CD4+ T cells [89].
Th cells require mTORC2 for differentiation as ablation of mTORC2 in T cells led to impaired Th1 and Th2 differentiation, which could be reversed by activating AKT and PKCθ, respectively [90]. However, mTORC2 is mainly considered to regulate Th2 differentiation, as Rictor KO in CD4+ T cells specifically led to the generation of Th1 and Th17 cells but not Th2 cells [91]. mTORC2 controls CD8+ T cell differentiation in a FOXO1-dependent manner as ablation of mTORC2 led to an increase in memory precursor effector cells and not in the short-lived effector cells driven by Eomes and Tcf1 up-regulation caused by FOXO1 [85,92]. Recently, Velde and Murray demonstrated that mTORC2 plays an important role in micro-environment sensing in CD4+ T cells. In a normal setting, CD4+ T cells require essential and non-essential AAs to undergo cell division. Limiting arginine and leucine resulted in cell cycle disruption, which could be bypassed in the absence of Rictor. This resulted in cells initiating the cell cycle regardless of limiting AAs, thus bypassing micro-environmental sensing [93].
Conclusion
Since the discovery of mTOR nearly 25 years ago, considerable data have been generated regarding its cellular functions. Through mTORC1 and mTORC2, the mTOR signalling pathway plays a critical role in protein and lipid biosynthesis, cell survival, migration, cell development and cell cycling. This is achieved through precise regulation of mTOR activity, which are tightly controlled through cross-talk with additional signalling pathways such as the ERK/MAPK and Wnt-signalling cascades. Here, we have highlighted the importance of mTOR in haemopoiesis, discussing the essential nature of mTORC1 during embryogenesis and haemopoiesis, regulating HSC self-renewal capacity, long-term potentiation and lineage commitment. On the other hand, genetic studies have revealed a more subtle role for mTORC2, establishing key roles in cell survival and later stages of haemopoietic cell lineage development, and supporting the haemopoietic niche. Further dissection of the physiological roles of mTOR as it resides in these complexes will provide fundamental information to assist in the development of therapeutic compounds to target specific mTOR functions.
Acknowledgements
Our thanks are expressed to Dr Jodie Hay for critically reviewing the manuscript.
Abbreviations
- AAs
amino acids
- AMPK
AMP-protein kinase
- BCR
B cell receptor
- BM
bone marrow
- BMDMs
BM-derived macrophages
- cKO
conditional knockout
- CLPs
common lymphoid progenitors
- CMPs
common myeloid progenitors
- DN
double negative
- DP
double positive
- FL
foetal liver
- FOXO
Forkhead Box O
- GF
growth factor
- GSK3
glycogen synthase kinase 3
- HSCs
haemopoietic stem cells
- IMLECs
innate myelo-lymphoblastoid effector cells
- KD
knockdown
- KO
knockout
- LSK
Lin−Sca1+cKit+
- MPP
multipotent progenitor
- mTOR
mechanistic target of rapamycin
- MZ
marginal zone
- NETs
neutrophil extracellular traps
- PDK1
3-phosphoinositide-dependent protein kinase 1
- PI3K
phosphoinositide 3-kinase
- PIP2
phosphatidylinositol-4,5-phosphate
- PIP3
phosphatidylinositol-3,4,5-phosphate
- PKCα
protein kinase C-alpha
- PTEN
phosphatase and tensin homolog
- RBC
red blood cell
- RHEB
Ras homolog enriched in brain
- ROS
reactive oxygen species
- S6K1
S6 Kinase 1
- SGK1
serum and glucocorticoid-induced protein kinase 1
- TECs
thymic epithelial cells
- TLR4
toll-like receptor 4
Funding
This review was supported by a project grant from Bloodwise (15041) awarded to A.M.M. and O.J.S.; N.M. is funded by a MRC-DTP PhD studentship.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Laplante M. and Sabatini D.M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wullschleger S., Loewith R. and Hall M.N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 3.Zoncu R., Sabatini D.M. and Efeyan A. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballou L.M. and Lin R.Z. (2008) Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 1, 27–36 10.1007/s12154-008-0003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay N. and Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 10.1101/gad.1212704 [DOI] [PubMed] [Google Scholar]
- 6.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S. et al. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]
- 7.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S. et al. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M. and Sabatini D.M. (2009) An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19, R1046–R1052 10.1016/j.cub.2009.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin G., Liang Y., Wang Y., Yang Y., Yang M., Cen X. et al. (2017) mTOR complex 1 signalling regulates the balance between lipid synthesis and oxidation in hypoxia lymphocytes. Biosci. Rep. 37, BSR20160479 10.1042/BSR20160479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham J.T., Rodgers J.T., Arlow D.H., Vazquez F., Mootha V.K. and Puigserver P. (2007) mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450, 736–740 10.1038/nature06322 [DOI] [PubMed] [Google Scholar]
- 11.Morita M., Gravel S.P., Chénard V., Sikström K., Zheng L., Alain T. et al. (2013) mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 18, 698–711 10.1016/j.cmet.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui N. and Sonenberg N. (2015) Signalling to eIF4E in cancer. Biochem. Soc. Trans. 43, 763–772 10.1042/BST20150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavares M.R., Pavan I.C.B., Amaral C.L., Meneguello L., Luchessi A.D. and Simabuco F.M. (2015) The S6K protein family in health and disease. Life Sci. 131, 1–10 10.1016/j.lfs.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Dorrello N.V., Peschiaroli A., Guardavaccaro D., Colburn N.H., Sherman N.E. and Pagano M. (2006) S6K1- and ßTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314, 467–471 10.1126/science.1130276 [DOI] [PubMed] [Google Scholar]
- 15.Faller W.J., Jackson T.J., Knight J.R., Ridgway R.A., Jamieson T., Karim S.A. et al. (2015) mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature 517, 497–500 10.1038/nature13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ip C.K.M., Cheung A.N.Y., Ngan H.Y.S. and Wong A.S.T. (2011) P70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 30, 2420–2432 10.1038/onc.2010.615 [DOI] [PubMed] [Google Scholar]
- 17.Julien L.-A., Carriere A., Moreau J. and Roux P.P. (2010) mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell Biol. 30, 908–921 10.1128/MCB.00601-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treins C., Warne P.H., Magnuson M.A., Pende M. and Downward J. (2010) Rictor is a novel target of p70 S6 kinase-1. Oncogene 29, 1003–1016 10.1038/onc.2009.401 [DOI] [PubMed] [Google Scholar]
- 19.Vadlakonda L., Dash A., Pasupuleti M., Anil Kumar K. and Reddanna P. (2013) The paradox of Akt-mTOR interactions. Front. Oncol. 3, 165 10.3389/fonc.2013.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M., Kong Q., Hua H., Yin Y., Wang J., Luo T. et al. (2016) AMPK-mediated up-regulation of mTORC2 and MCL-1 compromises the anti-cancer effects of aspirin. Oncotarget 7, 16349–16361 10.18632/oncotarget.7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam E.W.-F., Brosens J.J., Gomes A.R. and Koo C.-Y. (2013) Forkhead box proteins: tuning forks for transcriptional harmony. Nat. Rev. Cancer 13, 482–495 10.1038/nrc3539 [DOI] [PubMed] [Google Scholar]
- 22.Gilley J., Coffer P.J. and Ham J. (2003) FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 162, 613–622 10.1083/jcb.200303026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S. et al. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- 24.Brown J., Wang H., Suttles J., Graves D.T. and Martin M. (2011) Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates toll-like receptor 4-mediated inflammatory response via FoxO1. J. Biol. Chem. 286, 44295–44305 10.1074/jbc.M111.258053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N. and Hall M.N. (2013) mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl Acad. Sci. U.S.A. 110, 12526–12534 10.1073/pnas.1302455110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao C.-A., Ortiz-Vega S., Sun Y.-Y., Chien C.-T., Chuang J.-H. and Lin Y. (2017) Association of mSin1 with mTORC2 Ras and Akt reveals a crucial domain on mSin1 involved in Akt phosphorylation. Oncotarget 8, 63392–63404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y. et al. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 10.1016/j.cell.2006.08.033 [DOI] [PubMed] [Google Scholar]
- 28.Ikenoue T., Inoki K., Yang Q., Zhou X. and Guan K.-L. (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 27, 1919–1931 10.1038/emboj.2008.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konopatskaya O. and Poole A.W. (2010) Protein kinase Cα: disease regulator and therapeutic target. Trends Pharmacol. Sci. 31, 8–14 10.1016/j.tips.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Martínez J.M. and Alessi D.R. (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 10.1042/BJ20081668 [DOI] [PubMed] [Google Scholar]
- 31.Liu W., Wang X., Liu Z., Wang Y., Yin B., Yu P. et al. (2017) SGK1 inhibition induces autophagy-dependent apoptosis via the mTOR-Foxo3a pathway. Br. J. Cancer 117, 1139–1153 10.1038/bjc.2017.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heikamp E.B., Patel C.H., Collins S., Waickman A., Oh M.-H., Sun I.-H. et al. (2014) The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat. Immunol. 15, 457–464 10.1038/ni.2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goncharova E.A., Goncharov D.A., Li H., Pimtong W., Lu S., Khavin I. et al. (2011) mTORC2 is required for proliferation and survival of TSC2-null cells. Mol. Cell Biol. 31, 2484–2498 10.1128/MCB.01061-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangloff Y.-G., Mueller M., Dann S.G., Svoboda P., Sticker M., Spetz J.-F. et al. (2004) Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell Biol. 24, 9508–9516 10.1128/MCB.24.21.9508-9516.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J. et al. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 10.1016/j.devcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 36.de Bruijn M.F., Speck N.A., Peeters M.C. and Dzierzak E. (2000) Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474 10.1093/emboj/19.11.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orkin S.H. and Zon L.I. (2008) Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 10.1016/j.cell.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palis J., Robertson S., Kennedy M., Wall C. and Keller G. (1999) Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084 PMID: [DOI] [PubMed] [Google Scholar]
- 39.Notta F., Zandi S., Takayama N., Dobson S., Gan O.I., Wilson G. et al. (2016) Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116 10.1126/science.aab2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yilmaz Ö.H, Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H. et al. (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 10.1038/nature04703 [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Grindley J.C., Yin T., Jayasinghe S., He X.C., Ross J.T. et al. (2006) PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 10.1038/nature04747 [DOI] [PubMed] [Google Scholar]
- 42.Gan B., Sahin E., Jiang S., Sanchez-Aguilera A., Scott K.L., Chin L. et al. (2008) mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl Acad. Sci. U.S.A. 105, 19384–19389 10.1073/pnas.0810584105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C., Liu Y., Liu R., Ikenoue T., Guan K.-L., Liu Y. et al. (2008) TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397–2408 10.1084/jem.20081297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo F., Zhang S., Grogg M., Cancelas J.A., Varney M.E., Starczynowski D.T. et al. (2013) Mouse gene targeting reveals an essential role of mTOR in hematopoietic stem cell engraftment and hematopoiesis. Haematologica 98, 1353–1358 10.3324/haematol.2012.080424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumgartner C., Toifi S., Farlik M., Halbritter F., Scheicher R., Fischer I. et al. (2018) An ERK-dependent feedback mechanism prevents hematopoietic stem cell exhaustion. Cell Stem Cell 22, 879–892 10.1016/j.stem.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X. et al. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 10.1016/j.cell.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 47.Huang J., Zhang Y., Bersenev A., O'Brien W.T., Tong W., Emerson S.G. et al. (2009) Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 119, 3519–3529 10.1172/JCI40572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J., Nguyen-McCarty M., Hexner E.O., Danet-Desnoyers G. and Klein P.S. (2012) Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 18, 1778–1785 10.1038/nm.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selman C., Sinclair A., Pedroni S.M.A., Irvine E.E., Michie A.M. and Withers D.J. (2016) Evidence that hematopoietic stem cell function is preserved during aging in long-lived S6K1 mutant mice. Oncotarget 7, 29937–29943 10.18632/oncotarget.8729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo Y., Li L., Zou P., Wang J., Shao L., Zhou D. et al. (2014) Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence. Transplantation 97, 20–29 10.1097/TP.0b013e3182a7fcf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalaitzidis D., Sykes S.M., Wang Z., Punt N., Tang Y., Ragu C. et al. (2012) mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell 11, 429–439 10.1016/j.stem.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Hu T., Hua C., Gu J., Zhang L., Hao S. et al. (2014) Rictor Is required for early B cell development in bone marrow. PLoS ONE 9, e103970 10.1371/journal.pone.0103970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magee J.A., Ikenoue T., Nakada D., Lee J.Y., Guan K. and Morrison S.J. (2012) Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 11, 415–428 10.1016/j.stem.2012.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X., Campreciós G., Rimmelé P., Liang R., Yalcin S. and Mungamuri S.K. (2014) FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am. J. Hematol. 89, 954–963 10.1002/ajh.23786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Wu K., Xiao X., Liao J., Hu Q., Chen H. et al. (2015) Autophagy as a regulatory component of erythropoiesis. Int. J. Mol. Sci. 16, 4083–4094 10.3390/ijms16024083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Li L., Yu C., Senyuk V., Li F., Quigley J.G. et al. (2018) miR-9 upregulation leads to inhibition of erythropoiesis by repressing FoxO3. Sci. Rep. 8, 6519 10.1038/s41598-018-24628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knight Z.A., Schmidt S.F., Birsoy K., Tan K. and Friedman J.M. (2014) A critical role for mTORC1 in erythropoiesis and anemia. eLife 3, e01913 10.7554/eLife.01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang S., Macias-Garcia A., Velazquez J., Paltrinieri E., Kaufman R.J. and Chen J.-J. (2018) HRI coordinates translation by eIF2αP and mTORC1 to mitigate ineffective erythropoiesis in mice during iron deficiency. Blood 131, 450–461 10.1182/blood-2017-08-799908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung J., Bauer D.E., Ghamari A., Nizzi C.P., Deck K.M., Kingsley P.D. et al. (2015) The mTORC1/4E-BP pathway coordinates hemoglobin production with L-leucine availability. Sci. Signal. 8, ra34 10.1126/scisignal.aaa5903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoshii T., Tadokoro Y., Naka K., Ooshio T., Muraguchi T., Sugiyama N. et al. (2012) mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J. Clin. Invest. 122, 2114–2129 10.1172/JCI62279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Shen X., Na N., Chu Z., Su H., Chao S. et al. (2018) mTOR masters monocyte development in bone marrow by decreasing the inhibition of STAT5 on IRF8. Blood 131, 1587–1599 10.1182/blood-2017-04-777128 [DOI] [PubMed] [Google Scholar]
- 62.Karmaus P.W.F., Herrada A.A., Guy C., Neale G., Dhungana Y., Long L. et al. (2017) Critical roles of mTORC1 signaling and metabolic reprogramming for M-CSF-mediated myelopoiesis. J. Exp. Med. 214, 2629–2647 10.1084/jem.20161855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Byles V., Covarrubias A.J., Ben-Sahra I., Lamming D.W., Sabatini D.M., Manning B.D. et al. (2013) The TSC-mTOR pathway regulates macrophage polarization. Nat. Commun. 4, 2834 10.1038/ncomms3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang F., Zhang P., Ye P., Lazarski C.A., Wu Q., Bergin I.L. et al. (2017) A population of innate myelolymphoblastoid effector cell expanded by inactivation of mTOR complex 1 in mice. eLife 6, e32497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Babaev V.R., Huang J., Ding L., Zhang Y., May J.M. and Linton M.F. (2018) Loss of Rictor in monocyte/Macrophages suppresses their proliferation and viability reducing atherosclerosis in LDLR null mice. Front. Immunol. 9, 215 10.3389/fimmu.2018.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Festuccia W.T., Pouliot P., Bakan I., Sabatini D.M. and Laplante M. (2014) Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS ONE 9, e95432 10.1371/journal.pone.0095432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S., Readinger J.A., DuBois W., Janka-Junttila M., Robinson R., Pruitt M. et al. (2011) Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood 117, 1228–1238 10.1182/blood-2010-05-287821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Itakura A. and McCarty O.J.T. (2013) Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am. J. Physiol. - Cell Physiol. 305, C348–C354 10.1152/ajpcell.00108.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McInturff A.M., Cody M.J., Elliott E.A., Glenn J.W., Rowley J.W., Rondina M.T. et al. (2012) Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood 120, 3118–3125 10.1182/blood-2012-01-405993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L., Das S., Losert W. and Parent C.A. (2010) mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell 19, 845–857 10.1016/j.devcel.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L., Gritz D. and Parent C.A. (2014) PKCβII acts downstream of chemoattractant receptors and mTORC2 to regulate cAMP production and myosin II activity in neutrophils. Mol. Biol. Cell 25, 1446–1457 10.1091/mbc.e14-01-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu C., Xia L., Li F., Zhou L., Weng Q., Li Z. et al. (2018) mTOR complexes differentially orchestrates eosinophil development in allergy. Sci. Rep. 8, 6883 10.1038/s41598-018-25358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pieper K., Grimbacher B. and Eibel H. (2013) B-cell biology and development. J. Allergy Clin. Immunol. 131, 959–971 10.1016/j.jaci.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 74.Cariappa A., Boboila C., Moran S.T., Liu H., Shi H.N. and Pillai S. (2007) The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J. Immunol. 179, 2270–2281 10.4049/jimmunol.179.4.2270 [DOI] [PubMed] [Google Scholar]
- 75.Benhamron S. and Tirosh B. (2011) Direct activation of mTOR in B lymphocytes confers impairment in B-cell maturation andloss of marginal zone B cells. Eur. J. Immunol. 41, 2390–2396 10.1002/eji.201041336 [DOI] [PubMed] [Google Scholar]
- 76.Iwata T.N., Ramírez J.A., Tsang M., Park H., Margineantu D.H., Hockenbery D.M. et al. (2016) Conditional disruption of raptor reveals an essential role for mTORC1 in B cell development, survival, and metabolism. J. Immunol. 197, 2250–2260 10.4049/jimmunol.1600492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones D.D., Gaudette B.T., Wilmore J.R., Chernova I., Bortnick A., Weiss B.M. et al. (2016) mTOR has distinct functions in generating versus sustaining humoral immunity. J. Clin. Invest. 126, 4250–4261 10.1172/JCI86504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee K., Heffington L., Jellusova J., Nam K.T., Raybuck A., Cho S.H. et al. (2013) Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood 122, 2369–2379 10.1182/blood-2013-01-477505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazorchak A.S., Liu D., Facchinetti V., Di Lorenzo A., Sessa W.C., Schatz D.G. et al. (2010) Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol. Cell 39, 433–443 10.1016/j.molcel.2010.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zou Z., Chen J., Liu A., Zhou X., Song Q., Jia C. et al. (2015) mTORC2 promotes cell survival through c-Myc-dependent up-regulation of E2F1. J. Cell Biol. 211, 105–122 10.1083/jcb.201411128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoshii T., Kasada A., Hatakeyama T., Ohtani M., Tadokoro Y., Naka K. et al. (2014) Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells. Proc. Natl Acad. Sci. U.S.A. 111, 3805–3810 10.1073/pnas.1320265111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang F., Wu Q., Ikenoue T., Guan K.L., Liu Y. and Zheng P. (2012) A critical role for Rictor in T lymphopoiesis. J. Immunol. 189, 1850–1857 10.4049/jimmunol.1201057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K., Nam K.T., Cho S.H., Gudapati P., Hwang Y., Park D.S. et al. (2012) Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J. Exp. Med. 209, 713–728 10.1084/jem.20111470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prevot N., Pyaram K., Bischoff E., Sen J.M., Powell J.D. and Chang C.-H. (2015) Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J. Immunol. 194, 223–230 10.4049/jimmunol.1401985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H.-X., Cheng J.S., Chu S., Qiu Y.-R. and Zhong X.-P. (2016) mTORC2 in thymic epithelial cells controls thymopoiesis and T cell development. J. Immunol. 197, 141–150 10.4049/jimmunol.1502698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang K., Neale G., Green D.R., He W. and Chi H. (2011) Tuberous sclerosis complex 1 (Tsc1) enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897 10.1038/ni.2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollizzi K.N., Patel C.H., Sun I.-H., Oh M.-H., Waickman A.T., Wen J. et al. (2015) mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J. Clin. Invest. 125, 2090–2108 10.1172/JCI77746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waickman A.T. and Powell J.D. (2012) mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 249, 43–58 10.1111/j.1600-065X.2012.01152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Angela M., Endo Y., Asou H.K., Yamamoto T., Tumes D.J., Tokuyama H. et al. (2016) Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat. Commun. 7, 13683 10.1038/ncomms13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee K., Gudapati P., Dragovic S., Spencer C., Joyce S., Killeen N. et al. (2010) Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 10.1016/j.immuni.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delgoffe G.M., Pollizzi K.N., Waickman A.T., Heikamp E., Meyers D.J., Horton M.R. et al. (2011) The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L., Tschumi B.O., Lopez-Mejia I.C., Oberle S.G., Meyer M., Samson G. et al. (2016) Mammalian target of rapamycin complex 2 controls CD8T cell memory differentiation in a Foxo1-dependent manner. Cell Rep. 14, 1206–1217 10.1016/j.celrep.2015.12.095 [DOI] [PubMed] [Google Scholar]
- 93.Van de Velde L.-A. and Murray P.J. (2016) Proliferating helper T cells require Rictor/mTORC2 complex to integrate signals from limiting environmental amino acids. J. Biol. Chem. 291, 25815–25822 10.1074/jbc.C116.763623 [DOI] [PMC free article] [PubMed] [Google Scholar]