Abstract
Recurrent dehydration, such as commonly occurs with manual labor in tropical environments, has been recently shown to result in chronic kidney injury, likely through the effects of hyperosmolarity to activate both vasopressin and aldose reductase-fructokinase pathways. The observation that the latter pathway can be directly engaged by simple sugars (glucose and fructose) leads to the hypothesis that soft drinks (which contain these sugars) might worsen rather than benefit dehydration associated kidney disease. Recurrent dehydration was induced in rats by exposure to heat (36°C) for 1 h/24 h followed by access for 2 h to plain water (W), a 11% fructose-glucose solution (FG, same composition as typical soft drinks), or water sweetened with noncaloric stevia (ST). After 4 wk plasma and urine samples were collected, and kidneys were examined for oxidative stress, inflammation, and injury. Recurrent heat-induced dehydration with ad libitum water repletion resulted in plasma and urinary hyperosmolarity with stimulation of the vasopressin (copeptin) levels and resulted in mild tubular injury and renal oxidative stress. Rehydration with 11% FG solution, despite larger total fluid intake, resulted in greater dehydration (higher osmolarity and copeptin levels) and worse renal injury, with activation of aldose reductase and fructokinase, whereas rehydration with stevia water had opposite effects. In animals that are dehydrated, rehydration acutely with soft drinks worsens dehydration and exacerbates dehydration associated renal damage. These studies emphasize the danger of drinking soft drink-like beverages as an attempt to rehydrate following dehydration.
Keywords: fructokinase, renal injury, vasopressin, uric acid, stevia
a major epidemic of chronic kidney disease is occurring in Central America among workers in the sugarcane fields. To date there have been reported to be over 20,000 deaths. While the etiology is unknown, most studies suggest that recurrent dehydration is a major risk factor (10, 15, 24, 32).
Subjects working in hot environments tend to lose both salt and water, but usually have a greater loss of water, leading to transient hyperosmolarity. Hyperosmolarity activates two major systems: vasopressin release from the posterior pituitary and the aldose reductase enzyme. Vasopressin has been shown to drive low grade renal injury and to exacerbate chronic kidney disease in laboratory animals (8). Likewise, the aldose reductase system converts glucose to sorbitol, which can then be converted to fructose, which is a substrate for fructokinase in the proximal tubule (11). In turn, the metabolism of fructose in the proximal tubule can result in tubular injury and the release of oxidants and inflammatory mediators (11, 28). Indeed, we recently reported that recurrent heat-associated dehydration could lead to chronic kidney disease in mice due to endogenous generation of fructose in the kidney from the aldose reductase pathway, and this was prevented in mice unable to metabolize fructose (fructokinase-knockout mice) (34).
The observation that fructose plays a role in dehydration-associated renal injury raises major concerns that rehydration with sugary beverages that contain fructose might worsen rather than help dehydration-associated renal injury. Furthermore, fructose has a peculiar ability to stimulate vasopressin release in humans that is not observed with other sugars such as glucose (42).
We therefore utilized a model of heat-induced dehydration to test the hypothesis that brief (2 h) rehydration with a soft drink beverage (consisting of 7.15% fructose-3.85% glucose similar to standard soft drinks) might worsen renal injury compared with rehydration with water or stevia containing water. Our studies raise serious concerns for the common practice, especially among adolescents and young adults, to drink soft drinks as a means to quench thirst following an episode of dehydration.
METHODS
Ethical Approval
This investigation was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health, and the Mexican Federal Regulation for animal experimentation and care (NOM-062-ZOO-2001) and for the disposal of biological residues (NOM-087-ECOL-1995).
Reagents
Chemicals were of reagent or higher grade from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Antiurinary neutrophil gelatinase-associated lipocalin (N-GAL), antinephrin, antivasopressin V1a receptor, anti-superoxide dismutase (SOD)-1, anticatalase, and anti-GPx anti-antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). Antifructokinase (KHK) and antialdose reductase were obtained from GeneTex (Irvine, CA), antivasopressin V2 receptor antibody was obtained from Abcam (Cambridge, MA), and anti-β-actin antibody was obtained from Cell Signaling (Danvers, MA). Secondary antibodies conjugated with horseradish peroxidase were from Cell Signaling.
Experimental Protocol
Heat-induced dehydration protocol.
Three groups of male Wistar rats were placed in a 36°C closed environment for 1 h without food and water from Monday to Friday during 4 wk. Thermal exposure is considered a valid model to induce dehydration in rats (2–6, 29–31). After heat-induced dehydration, animals were allowed to rehydrate for 2 h with either tap water (W, n = 6), a sweetened beverage made with an 11% of a fructose-glucose combination, which is a similar composition used in major brands of soft drinks (FG, 7.15% fructose and 3.85% glucose, respectively, n = 7) (10, 12), or water sweetened with the noncaloric edulcorant stevia (ST) (4 g/l water, Svetia, Metco Mexico, n = 7). Pelleted food was provided ad libitum. For the rest of the day and during weekends animals received tap water and food ad libitum. The loss of weight induced by heat and the amount of drinking fluid consumed during rehydration period were recorded daily.
Normal control group (C).
This group consisted of five male Wistar rats of similar body weight and age. They received food and water ad libitum during 4 wk. The amount of water and food consumed were measured daily. Body weight was measured weekly.
Measurements
At the end of the 4-wk study period, systolic blood pressure was measured, and urine was collected for 18 h (overnight) in metabolic cages. Food was not provided during the urine collection. Rats were then euthanized by anesthesia with isoflurane and exsanguination. A blood sample was collected and centrifuged. Plasma and urine samples were frozen until further analyses. Both kidneys were perfusion washed with cold phosphate-buffered saline, and the right kidney was excised and divided into cortex and medulla, frozen in liquid nitrogen, and stored until further processing. The left kidney was fixed by perfusion with 4% paraformaldehyde for histology.
Blood and Urine Analyses
Plasma and urine osmolality was measured using a freezing point depression osmometer (Advanced Instruments, Norwood, MA). Plasma and urine creatinine were measured by a validated enzymatic method (22) and creatinine clearance was calculated. Plasma and urine urea nitrogen concentrations were analyzed by autoanalyzer (Instrumentation Laboratory, Bedford, MA). Sodium concentration was analyzed by flame photometry. Fructose was measured by a colorimetric assay (18), and uric acid was measured with a fluorometric kit (Amplex Red, Life Technologies, Carlsbad, CA). Plasma copeptin was extracted using Sep-pack C18 cartridges (Waters, Milford, MA) and then measured by a rat-specific competitive enzyme immunoassay (Peninsula Laboratories, San Carlos, CA).
Solute-free water reabsorption (TcH2O) during the 18 h of urine collection was calculated accordingly to the following formula: TcH2O = osmolar excretion − urine volume = (Uosm/Posm) × V − V, where Uosm and Posm are the urine and plasma osmolality, respectively, and V the urine volume collected in 18 h.
Blood Pressure
Systolic blood pressure (SBP) was measured in conscious rats by a validated volume-based tail-cuff method (17) at the end of the follow-up.
Evaluation of Markers of Tubular Damage
For the determination of N-acetyl-β-d-glucosaminidase (NAG) activity in urine samples, 4-nitrophenyl-N-acetyl-β-d-glucosaminide was used as a substrate.
Neutrophil gelatinase-associated lipocalin (NGAL) expression, a sensitive marker of renal proximal tubule damage (25), was evaluated by Western blotting. Renal cortex proteins were extracted using a mitogen-activated protein (MAP) kinase lysis buffer, as previously described (33), and incubated with a primary antibody against NGAL (Santa Cruz Biotechnology) at 4°C overnight, using β-actin antibody (Cell Signaling) as load control.
Renal Cortex Content of Fructose and Uric Acid
Fructose was extracted from cortical renal tissue by perchloric acid precipitation, and its concentration was measured by the anthrone-based colorimetric method (18). Uric acid (UA) is a by product of fructose catabolism in tissues expressing KHK and is associated with its detrimental effects (21). Therefore, tissue UA was measured both as a marker of renal damage and also as a surrogate of fructose increased metabolism. UA was extracted as previously described (11). UA was measured using Amplex Red assay kit (Life Technologies). Fructose and UA concentrations were normalized by protein concentration.
Renal Cortex Markers of Oxidative Stress
Tissue was homogenized in phosphate buffer containing a cocktail of protease inhibitors. Protein carbonyls and lipid peroxidation (4-hydroxynonenal, 4-HNE) were measured using previously published methods (27, 39) and normalized by protein concentration.
NOX4, catalase, glutathione peroxidase, and superoxide dismutase-1 protein expression.
Renal cortex proteins were extracted using a MAP kinase lysis buffer, as previously described (22). Each of the following primary antibodies were incubated at 4°C overnight: anti-NOX4 (GeneTex, Irvine, CA), anticatalase, -GPx, and anti-SOD-1. Protein loading was controlled with and anti-β-actin antibody (Cell Signaling). Chemiluminescence was captured using Clarity horseradish peroxidase chemiluminescence kit (Bio-Rad, Hercules, CA) and exposure of membranes over X-ray film inside a standard developing cassette. Film was developed manually and exposure was repeated varying the time as needed for optimal detection; thereafter the film was scanned. Blots were recorded, and densitometry was performed using the Image Studio Lite Software (Licor, Lincoln, NE).
Aldose reductase, KHK, and vasopressin V1a and V2 receptors protein expression by Western blot analysis.
Renal cortex proteins were extracted using a MAP kinase lysis buffer, as previously described (33). Each of the following primary antibodies were incubated at 4°C overnight: anti-KHK (GeneTex, Irvine, CA), antialdose reductase (GeneTex), antivasopressin V1a receptor (Santa Cruz Biotechnology), antivasopressin V2 receptor (Abcam), and anti-β-actin antibody (Cell Signaling). Chemiluminescence was recorded and quantified using the Image Studio Lite Software (Biotech, Lincoln, NE).
Histological Analysis
Fixed renal tissue was embedded in paraffin and processed accordingly. The evaluation was performed blinded. Sections were stained with periodic acid-Schiff's stain (PAS). Glomerular changes (glomerulosclerosis or hypoperfusion as evidenced by wrinkling and collapse of the glomeruli) were qualitatively evaluated. Tubulointerstitial cellular infiltration was studied in PAS-stained sections taking advantage that the nucleus of inflammatory cells is stained in dark blue. The number of inflammatory cells were quantified in 20 nonoverlapping fields at ×400 magnification and expressed as positive cells in 20 fields.
Statistical Analysis
Values are expressed as means ± SD. One-way ANOVA determined significant differences between groups. When the ANOVA P value was <0.05, posttest comparisons were made using Sidak's multiple-comparison test assuming an α[PF (per family of tests)] = 0.05. Each computed P value was adjusted to account for six multiple comparisons per family. The possible relationship between variables was tested by correlation analysis. Statistical analysis was performed with Prism version 6.05 (Graph Pad Software, San Diego, CA).
RESULTS
Dehydration Protocol
The central hypothesis of this study was that rehydration with fructose/glucose concentrations similar to that observed in soft drinks might accelerate heat-induced dehydration renal injury. Heat-induced dehydration was performed by exposure of rats daily to heat (1 h at 36°C) followed by 2 h of ad libitum rehydration with either water of a fructose-glucose (FG) solution similar in composition to that present in soft drinks. The rest of the 24-h period all groups only received water for fluid intake. Because rats drank more FG solution than regular water, we also included a group given stevia water, as rats drank the same amount of stevia water as FG water thus providing a control for fluid intake following the dehydration procedure. These three groups were than compared with normal control rats that were not exposed to heat.
Effects on Weight and Fluid Intake
Heat-induced dehydration resulted in equivalent mean daily body weight loss among the three groups (water, stevia, and FG) at the end of the heat period (Table 1). During the 2-h ad libitum rehydration period, rats administered FG and stevia water drank more fluid (∼30%) compared with water alone. However, total 24-h water intake was similar among the three groups (Table 1), although it was higher than the normal control group.
Table 1.
Fluid consumption, plasma and urine parameters, and systolic blood pressure
| Heat-Induced Dehydration |
||||
|---|---|---|---|---|
| Parameter | Normal Control (n = 5) | W (n = 6) | FG (n = 7) | S (n = 7) |
| Beverage | ||||
| Mean fluid drunk during rehydration, ml/2 h | 9 ± 0.1 | 12 ± 1.1B | 11 ± 0.9B | |
| Mean BW loss after HD, % BW | 1.7 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.1 | |
| Mean total fluid consumption, ml/24 h | 24 ± 0.3 | 33 ± 1.0A | 34 ± 0.9A | 33 ± 1.3A |
| Plasma | ||||
| Urea, mg/dl | 20 ± 2 | 22 ± 3 | 24 ± 2.1 | 21 ± 1.5 |
| Creatinine, mg/dl | 0.85 ± 0.12 | 0.93 ± 0.07 | 1.73 ± 0.13A,B | 0.98 ± 0.08C |
| Fructose, μg/ml | 0.89 ± 0.14 | 0.80 ± 0.20 | 1.14 ± 0.04A,B | 1.10 ± 0.04B |
| UA, mg/dl | 0.28 ± 0.15 | 0.50 ± 0.19 | 0.50±.07 | 0.13 ± 0.05B,C |
| Urine | ||||
| Urine flow, μl/min | 15 ± 6 | 28 ± 6A | 19 ± 4B | 9 ± 3B,C |
| Urea, mg/18 h | 186 ± 24 | 437 ± 131A | 652 ± 106A,B | 141 ± 60BC |
| Creatinine, mg/18 h | 11 ± 2 | 10 ± 2 | 11 ± 1 | 11.9 ± 2 |
| Fructose, μg/18 h | 21 ± 9 | 41 ± 11A | 30 ± 7 | 13 ± 5B,C |
| UNa, meq/18 h | 0.29 ± 0.13 | 1.81 ± 0.32A | 4.91 ± 1.21A,B | 0.32 ± 0.12B,C |
| CrCl, ml/min | 1.24 ± 0.1 | 1.01 ± 0.13 | 0.60 ± 0.06A,B | 1.12 ± 0.15C |
| FENa, % | 0.12 ± 0.05 | 0.87 ± 0.16A | 3.6 ± 1.1A,B | 0.15 ± 0.05C |
| SBP, mmHg | 116 ± 7 | 143 ± 3A | 139 ± 7A | 120 ± 7B,C |
Values are means ± SD: n, number of rats.
BW, body weight; SBP, systolic blood pressure; CrCl, creatinine clearance; UNa, sodium excretion.
FENa, fractional sodium excretion.
Statistical comparisons: A vs. normal control. B vs. Water. C vs. FG.
Rehydration With Soft Drinks is Associated With Evidence for Persistent Dehydration Despite Normal Renal Function
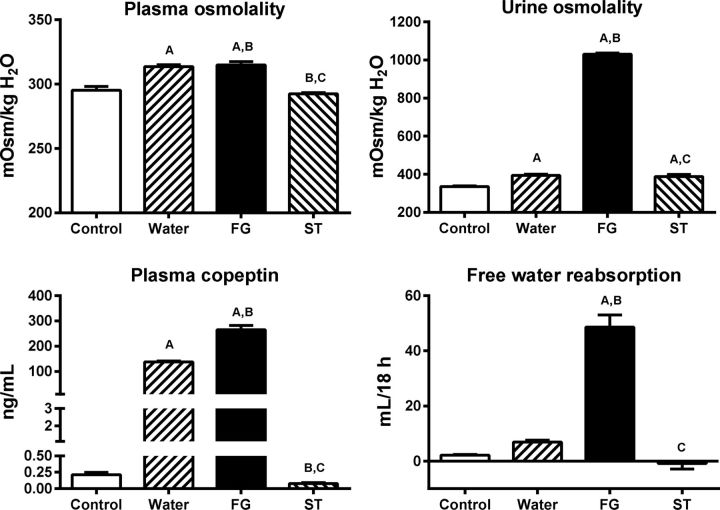
Rats underwent blood testing in the morning after having ample time to be rehydrated. Despite daily rehydration, rats that had been exposed to daily heat showed higher mean levels of plasma and urine osmolarity with higher plasma copeptin levels compared with normal control rats (Fig. 1). Nevertheless, rats hydrated with FG showed significantly higher plasma and urine osmolality and higher copeptin levels and higher free water reabsorption by the kidneys despite greater fluid intake during the 2-h rehydration period (Fig. 1). In contrast, rehydration with stevia water was associated with lower plasma osmolarity and lower copeptin levels than that observed with water alone.
Fig. 1.
Markers of vasopressin stimulation caused by mild dehydration induced by heat exposure in animals rehydrated for 2 h with water (W), an 11% fructose-glucose beverage (FG), or water sweetened with the noncaloric edulcorant stevia (ST). Statistical comparisons: A vs. normal control; B vs. water C vs. FG.
Heat-induced dehydration raised absolute sodium urine excretion and sodium fractional excretion; this effect was enhanced in FG-rehydrated group. In contrast, in rats rehydrated with stevia the rise in sodium excretion, both absolute and fractional, was prevented (Table 1).
Heat-induced dehydration alone also increased blood pressure compared with normal control rats. Rehydration with FG did not further raise blood pressure. Stevia rehydration was associated with lower blood pressure than the group rehydrated with water (Table 1).
In summary, recurrent and transient heat-induced dehydration results in some persistent evidence for dehydration (elevations in plasma and urine osmolarity, elevated copeptin levels) and this is significantly worse in rats rehydrated with FG solutions.
Renal Injury Induced by Dehydration is Worsened by Rehydration With Soft Drink
No differences in plasma urea were observed in these four groups as measured in the fasting morning blood samples. However, rehydration with FG solutions to heat-exposed rats significantly increased plasma creatinine and decreased Cr clearance (Table 1).
We evaluated two markers of proximal tubule damage: NAG urine excretion and the expression of NGAL. In rats that received FG as rehydration fluid there were significant increments in the excretion of NAG in urine and in NGAL renal cortex expression. Rehydration with stevia prevented those changes (Fig. 2A).
Fig. 2.
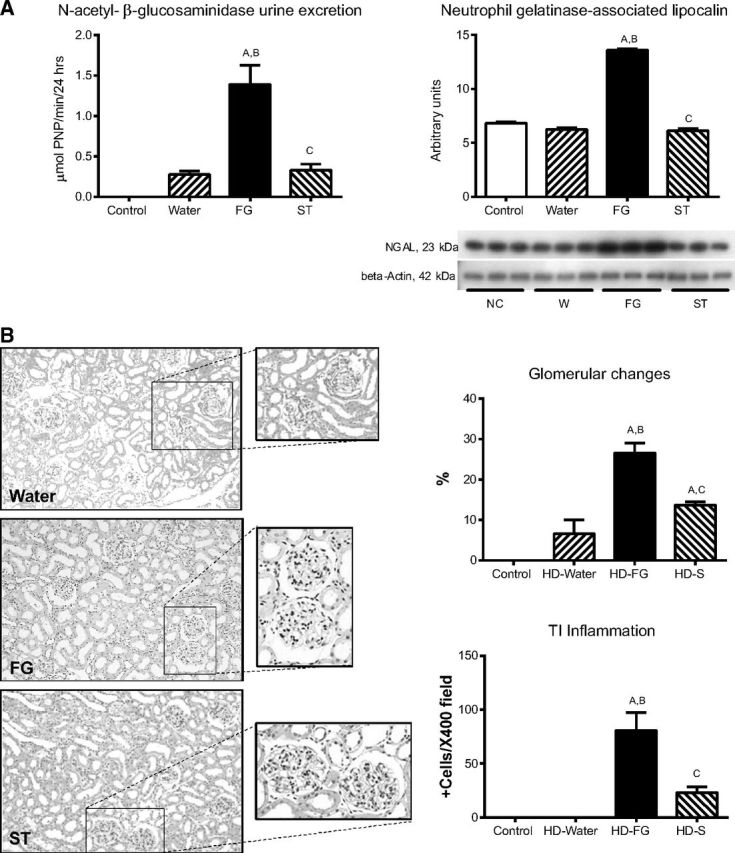
Markers of tubular injury in mildly dehydrated animals (A) and glomerular changes (B) evaluated as percentage of glomeruli affected by hypoperfusion and glomerulosclerosis as well as tubulointerstitial inflammation (Peryodic acid Schiff's staining, ×400 magnification) in the various studied groups. Statistical comparisons: A vs. normal control; B vs. water C vs. FG.
We also evaluated whether renal structural damage was already present. Dehydrated rats with FG rehydration had significantly more glomerular hypoperfusion and glomerulosclerosis compared with water and stevia rehydration (Fig. 2B). Mild tubulointerstitial inflammation was also observed in the group that received FG.
These data document that heat-induced dehydration is associated with both structural and urinary biomarker evidence of renal injury and this is worsened with hydration using FG solutions.
Potential Mechanisms for Renal Injury: Aldose Reductase-Fructokinase-Uric Acid Pathway and the Vasopressin Pathways
We also measured uric acid and fructose as both may be induced by dehydration [by renal retention of uric acid coupled with increased generation of both fructose and uric acid via the aldose reductase pathway (34)]. As expected, heat-induced dehydration was associated with a numerical rise in plasma uric acid and a significant rise in urine fructose, although no change in plasma fructose from normal controls was observed (Table 1). In contrast, rehydration with FG was associated with similar rise in plasma uric acid as control heat-dehydrated rats, but with higher plasma levels, despite that urine levels were found similar to water rehydrated rats. Stevia-treated rats showed lower plasma uric acid and comparable levels of plasma and urine fructose as control rats.
In renal cortex of rats that received water as rehydration fluid, concentrations of fructose and uric acid were not different compared with normal control rats (Fig. 3). On the contrary, rats that received FG during rehydration period, a significant increment in the concentration of fructose and uric acid were observed. In stevia-rehydrated groups, fructose and uric acid renal concentrations remained comparable to water rehydrated and control groups (Fig. 3).
Fig. 3.
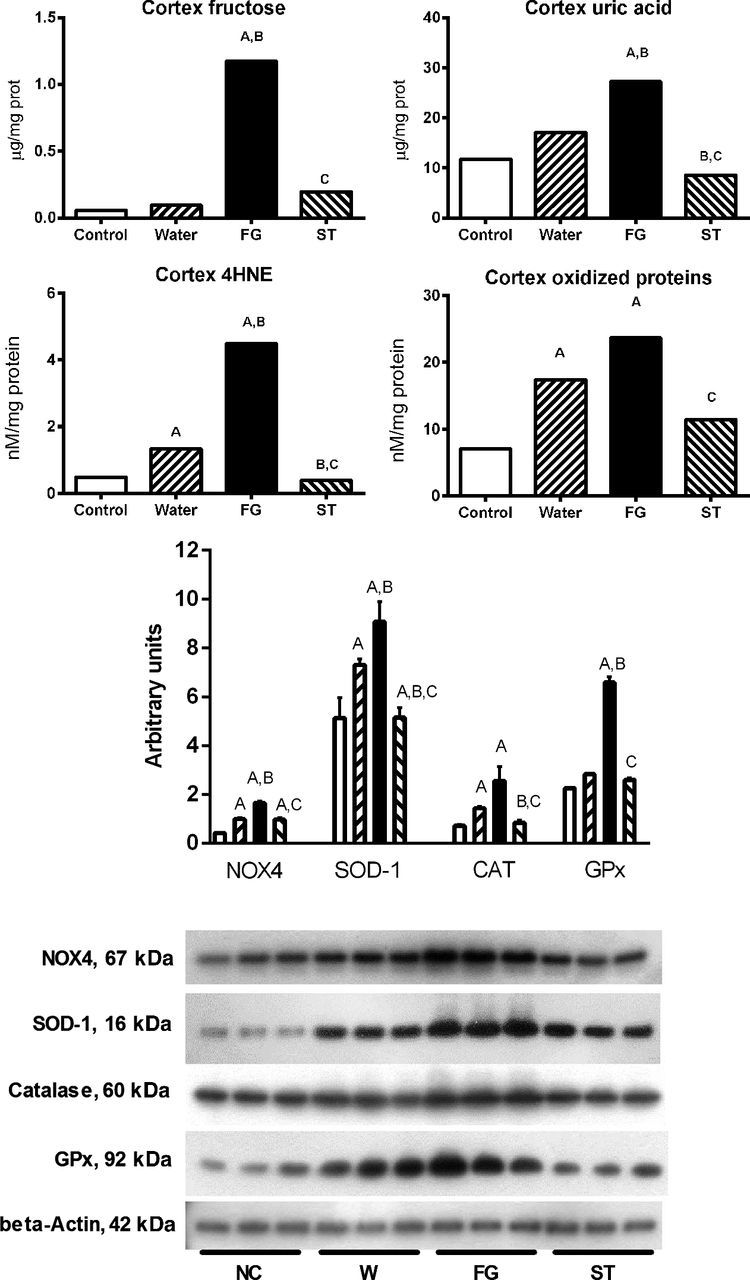
Renal cortex fructose and uric acid and markers of oxidative stress: lipid peroxidation (4-hydroxynonenal, 4-HNE), protein oxidation, NOX-4, superoxide dismutase-1 (SOD-1), catalase, and glutathione peroxidase (GPx) protein expression in mildly dehydrated animals induced by heat exposure in animals rehydrated for 2 h with water (W), an 11% fructose-glucose beverage (FG), and water sweetened with the noncaloric edulcorant stevia (ST). Statistical comparisons: A vs. normal control; B vs. water; C vs. FG.
As activation of fructokinase-uric acid pathway is associated with increased oxidative stress via activation of NOX4 (23, 35, 37, 44), we also evaluated lipid peroxidation and protein oxidation in renal cortex (Fig. 3). Heat-dehydrated rats that received water as rehydration fluid showed a slight but significant increment in lipid peroxidation and protein oxidation in renal cortex (Fig. 3). The increased content of fructose and uric acid induced by rehydration with FG was associated with a significant augmentation in oxidative stress as noted by a further increase in lipid peroxidation and protein oxidation. Stevia rehydration was associated with lower oxidative stress compared with FG groups. In agreement with the increment in oxidative stress observed in dehydrated rats rehydrated with FG beverage, we also observed significant overexpression of NOX4 as well as the antioxidant enzymes catalase, glutathione peroxidase (GPx), and SOD-1 in the kidneys (Fig. 3).
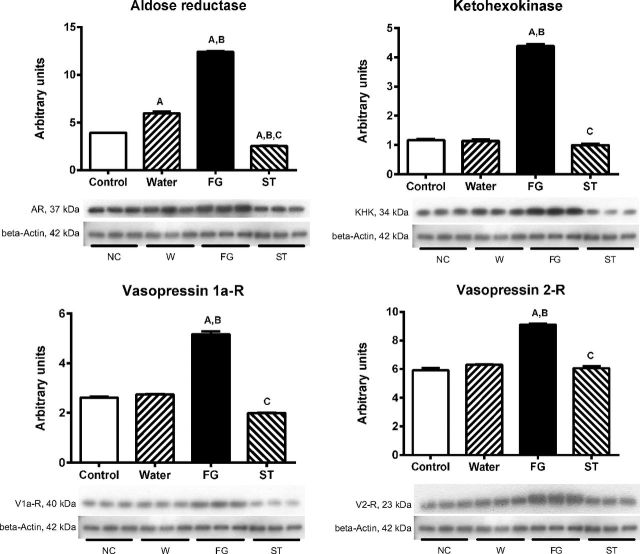
We also evaluated the renal cortex expression of the enzymes involved in fructose-uric acid pathway and vasopressin receptors (Fig. 4). Rats that the received water as rehydration fluid showed a slight but significant increment in the expression of aldose reductase in renal cortex. In parallel to the further increment in cortex fructose, uric acid and oxidative stress, the group of dehydrated animals that received FG for rehydration showed a significant increment in the expression of fructokinase, vasopressin receptors 1a and 2, and a further increase in the expression of aldose reductase. In contrast, rats that received stevia did not showed those changes (Fig. 4).
Fig. 4.
Renal cortex aldose-reductase, fructokinase (KHK), vasopressin V1a and V2 receptors expressions in mildly dehydrated animals induced by heat exposure in animals rehydrated for 2 h with water (W), an 11% fructose-glucose beverage (FG), and water sweetened with the noncaloric edulcorant stevia (ST). Statistical comparisons: A vs. normal control; B vs. water; C vs. FG.
DISCUSSION
In this study, we tested the hypothesis that the type of rehydration solution might influence renal outcomes associated with recurrent mild dehydration. Specifically, we hypothesized that short-term rehydration with sugary beverages containing fructose might have adverse effects on the kidney. To test this hypothesis, we performed studies in a model of thermal dehydration and compared water, stevia-containing water, and a solution of fructose-glucose that is similar in composition to standard soft drinks. The present study shows that short-term rehydration with a FG sugary beverage after a mild dehydration stimulates the two systems that have been implicated in kidney injury, i.e., vasopressin (1) and aldose reductase-fructokinase activities (34). After rehydration with a fructose-rich beverage, we observed a greater renal oxidative stress and mild renal injury (glomerular and tubular alterations). In contrast, rehydration with plain water or with the noncaloric edulcorant stevia did not produce such deleterious effects.
Fructose is a substrate for fructokinase that is present in the proximal tubule, and a 60% fructose diet in rats can induce modest tubular injury (11, 28). Fructose infusion in humans can also stimulate vasopressin release, whereas an equimolar solution of glucose does not (42). Our basic hypothesis was that recurrent stimulation of these pathways might induce renal disease and that hydration with fructose-containing solutions could increase vasopressin release and provide a substrate for fructokinase that might lead to further renal damage.
The type of rehydration fluid had significant effects on most of the outcomes. The administration of FG but not that of ST as rehydration fluid resulted in a further and significant increment in plasma copeptin. Moreover, rats continued to show signs of dehydration with higher urine osmolality and increased free water reabsorption, likely due in part to increased urinary sodium excretion observed in the FG-rehydrated rats. The evidence for worse dehydration despite increased fluid intake compared with the water-only group was striking. The consequence was also a greater renal oxidative stress, renal tubule injury, and subtle inflammatory infiltration. An additional marker of renal impairment in fructose-rehydrated rats was a significant fall in creatinine clearance. This finding suggests that glomerular filtration was reduced in this condition. However, it cannot be excluded that the observed differences in the creatinine clearances are due to different renal handling of creatinine by organic anion and/or cation transporters (16, 40).
An effect of a beverage containing simple sugars as replacement fluid was a significant increase in the accumulation of fructose and uric acid in the renal cortex. In previous studies, we and others have reported that tissue accumulation of uric acid is associated with augmented oxidative stress and damage (12, 26, 35). The results of the present studies are in agreement with those previous works.
Dehydration tended to increase systemic blood pressure in W and FG- but not in ST-rehydrated rats. This effect may be a consequence of increased renal oxidative stress in those groups (41). Whether this adverse influence of the rehydration fluid on SBP participated in kidney damage deserves further investigation.
We have previously shown that recurrent exposure to heat can induce renal injury through a fructokinase-dependent mechanism. In that study, the dehydration procedure was severe (34). We have also shown that a 60% fructose diet induces renal damage in the course of 8 wk (36). Moreover, rats that received a similar FG beverage ad libitum developed mild renal damage; however, those animals ingested ∼80 ml of this fluid per day (39) in contrast to the present study in which rats had a limited consumption of this sweetened beverage (12 ml/day). It was also observed that rats that received a similar daily amount of fructose/glucose beverage (14 ml/day) but without dehydration did not develop renal alterations (data not shown). Therefore, the power of the current study is that the renal injury was found even with very mild recurrent dehydration when short-term rehydration with fructose-containing beverages was provided.
An interesting finding in this study was that dehydration was associated with increased urinary sodium excretion and that this was worsened by the rehydration with FG. Although volume contraction is known to cause a prerenal state with a decrease in fractional excretion of sodium, dehydration-induced hyperosmolarity can cause a mild natriuresis (known as dehydration natriuresis) (13). The signaling mechanisms (Sgk1 and TonEBP) involved in causing dehydration-induced natriuresis are the same as those known to stimulate the aldose reductase-fructokinase pathway and thus may account for the potentiation of this mechanism with the FG solutions (13, 43). It was also observed a significant increase in urinary urea excretion in FG-rehydrated animals. We do not have a definite explanation for this effect; however, a mechanism that might contribute to increased urea excretion could be an overall increase in proteolytic activity induced by dehydration (9). Thus cellular dehydration is believed to be a driving force behind the severe protein wasting observed within the liver and skeletal muscles of extremely ill patients (19).
There is evidence that water intake is decreasing in the general population (20). Taking into account that, at present, a common practice is to rehydrate with sugary beverages when the threshold of mild dehydration has been reached (a situation in which vasopressin secretion is already increased), these findings might provide evidence for a pathophysiological mechanism that partially explain the association between sugary beverages consumption and renal damage (7, 14, 38).
Finally, stevia solution used as rehydration fluid prevented the rise in vasopressin secretion and preserved plasma and urine osmolality to normal levels. In addition, stevia-rehydrated animals had normal blood pressure and no evidence of renal tubule damage. It is not possible to know if this protective effect is due to a significantly higher volume ingested for rehydration in the stevia groups than in the plain water groups or if it is an effect induced by stevia itself.
In conclusion, this study shows that short-term rehydration with fructose-containing beverages in rats undergoing mild recurrent dehydration results in enhanced renal injury in association with greater stimulation of the vasopressin and polyol-fructokinase pathways. The simultaneous triggering of both systems was associated with increased urinary concentration, oxidative stress, renal injury, and systemic hypertension. On the other hand, increased ingestion of fluids devoid of simple sugars (plain water or stevia solution) prevented the stimulation of vasopressin induced by mild dehydration.
Perspectives and Significance
This study is relevant to the epidemic of chronic kidney disease (CKD) in Central America, in which sugarcane and other workers are developing CDKs associated with recurrent heat-associated dehydration. However, it may also be an important factor in the high frequency of CKD that is occurring in hot climates such as Mexico and the southern United States. Further studies investigating the mechanisms involved in this injurious process are warranted in the future.
GRANTS
This study was funded by CONACyT Mexico No 133232 and No 155604, and INC Ignacio Chavez own funds allocated for research. R. J. Johnson also has funding from Amway and is on the Scientific Board of Amway. L.-G. Sánchez-Lozada, R. J. Johnson, and M. A Lanaspa have grants from Danone, National Institutes of Health, and the Department of Defense. Parts of the results of this paper were presented at the ASN Renal Week 2014, Philadelphia, PA. This paper represents a contribution from the Colorado Climate and Health Consortium.
DISCLOSURES
R. J. Johnson and M. A Lanaspa are listed as inventors on patent applications related to blocking fructose metabolism as a means to prevent acute and chronic kidney disease. R. J. Johnson, C. Roncal-Jiménez and L.-G. Sánchez-Lozada are members of Colorado Research Partners. F. E. García-Arroyo, M. Cristóbal, A. S. Arellano-Buendia, H. Osorio, E. Tapia, V. Soto, L. Bankir, and M. Madero have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
F.E.G.-A., R.J.J., and L.-G.S.-L. conception and design of research; F.E.G.-A., M.C., A.S.A.-B., H.O., and V.S. performed experiments; F.E.G.-A., M.C., A.S.A.-B., H.O., E.T., V.S., M.A.L., C.A.R.-J., L.B., R.J.J., and L.-G.S.-L. analyzed data; F.E.G.-A., M.C., A.S.A.-B., H.O., E.T., V.S., M.M., M.A.L., C.A.R.-J., L.B., R.J.J., and L.-G.S.-L. interpreted results of experiments; F.E.G.-A., M.C., A.S.A.-B., H.O., E.T., V.S., M.M., M.A.L., C.A.R.-J., L.B., R.J.J., and L.-G.S.-L. edited and revised manuscript; F.E.G.-A., R.J.J., and L.-G.S.-L. approved final version of manuscript; L.B., R.J.J., and L.-G.S.-L. drafted manuscript; R.J.J. and L.-G.S.-L. prepared figures.
REFERENCES
- 1.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol : 223–239, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Barney CC, Folkerts MM. Thermal dehydration-induced thirst in rats: role of body temperature. Am J Physiol Regul Integr Comp Physiol : R557–R564, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Barney CC, Schanhals EM, Grobe JL, Andresen BT, Traver M. Heat acclimation and thirst in rats. Physiol Rep , 2015, e12642. doi: 10.14814/phy2.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barney CC, Smith GL, Folkerts MM. Thermal dehydration-induced thirst in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol : R1302–R1310, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Barney CC, West DR. Control of water intake in thermally dehydrated rats. Physiol Behav : 387–395, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Barney CC, Williams JS, Kuiper DH. Thermal dehydration-induced thirst in rats: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol : R1171–R1175, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int : 609–616, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouby N, Bachmann S, Bichet D, Bankir L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol Renal Fluid Electrolyte Physiol : F973–F979, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts : 345–364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks DR, Ramirez-Rubio O, Amador JJ. CKD in Central America: a hot issue. Am J Kidney Dis : 481–484, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol : 545–553, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens : 269–275, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Grigsby CL, Law CS, Ni X, Nekrep N, Olsen K, Humphreys MH, Gardner DG. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest : 1647–1658, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Kittanamongkolchai W, Erickson SB. Associations of sugar-sweetened and artificially sweetened soda with chronic kidney disease: a systematic review and meta-analysis. Nephrology : 791–797, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Cortez O. Heat stress assesment among workers in a Nicaraguan sugarcane farm. Glob Health Action. 2009, November 11;. doi: 10.3402/gha.v2i0.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int : 519–526, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens : 1288–1291, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Halhoul MN, Kleinberg I. Differential determination of glucose and fructose, and glucose- and fructose-yielding substances with anthrone. Analytical Biochem : 337–343, 1972. [DOI] [PubMed] [Google Scholar]
- 19.Haussinger D, Roth E, Lang F, Gerok W. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet : 1330–1332, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RJ, Kanbay M, Kang DH, Sanchez-Lozada LG, Feig D. Uric acid: a clinically useful marker to distinguish preeclampsia from gestational hypertension. Hypertension : 548–549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin Nutr : 899–906, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int : 74–78, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem : 40732–40744, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramirez-Rubio O, Riefkohl A, Scammell MK, Lopez-Pilarte D, Sanchez JM, Parikh CR, McClean MD. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health : 241–250, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Yang H, Chen H, Zhang M, Ma Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv Med Sci : 133–138, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation : 1356–1364, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina-Jijon E, Tapia E, Zazueta C, El Hafidi M, Zatarain-Barron ZL, Hernandez-Pando R, Medina-Campos ON, Zarco-Marquez G, Torres I, Pedraza-Chaverri J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic Biol Med : 1543–1557, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, Roncal C, Perez-Pozo SE, Johnson RJ, Nakagawa T. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol : F712–F720, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nose H, Morita M, Yawata T, Morimoto T. Recovery of blood volume and osmolality after thermal dehydration in rats. Am J Physiol Regul Integr Comp Physiol : R492–R498, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Nose H, Sugimoto E, Okuno T, Morimoto T. Changes in blood volume and plasma sodium concentration after water intake in rats. Am J Physiol Regul Integr Comp Physiol : R15–R19, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Nose H, Yawata T, Morimoto T. Osmotic factors in restitution from thermal dehydration in rats. Am J Physiol Regul Integr Comp Physiol : R166–R171, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Peraza S, Wesseling C, Aragon A, Leiva R, Garcia-Trabanino RA, Torres C, Jakobsson K, Elinder CG, Hogstedt C. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis : 531–540, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, Andres-Hernando A, Tanabe K, Madero M, Li N, Cicerchi C, Mc Fann K, Sautin YY, Johnson RJ. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism : 1259–1270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, Gonzalez MA, Aragon A, Wesseling C, Sanchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int : 294–302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodriguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol : F1134–F1141, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Lozada LG, Tapia E, Bautista-Garcia P, Soto V, vila-Casado C, Vega-Campos IP, Nakagawa T, Zhao L, Franco M, Johnson RJ. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol : F710–F718, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol : C584–C596, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, Cooper RS. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999–2004. PLos One : e3431, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapia E, Cristobal M, Garcia-Arroyo FE, Soto V, Monroy-Sanchez F, Pacheco U, Lanaspa MA, Roncal-Jimenez CA, Cruz-Robles D, Ishimoto T, Madero M, Johnson RJ, Sanchez-Lozada LG. Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am J Physiol Renal Physiol : F727–F736, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol : F1293–F1299, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol : R913–R935, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res : 379–383, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Woo SK, Lee SD, Kwon HM. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflügers Arch : 579–585, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens : 1234–1242, 2010. [PubMed] [Google Scholar]