Abstract
The adequate stimuli and molecular receptors for muscle metaboreceptors and nociceptors are still under investigation. We used calcium imaging of cultured primary sensory dorsal root ganglion (DRG) neurons from C57Bl/6 mice to determine candidates for metabolites that could be the adequate stimuli and receptors that could detect these stimuli. Retrograde DiI labeling determined that some of these neurons innervated skeletal muscle. We found that combinations of protons, ATP, and lactate were much more effective than individually applied compounds for activating rapid calcium increases in muscle-innervating dorsal root ganglion neurons. Antagonists for P2X, ASIC, and TRPV1 receptors suggested that these three receptors act together to detect protons, ATP, and lactate when presented together in physiologically relevant concentrations. Two populations of muscle-innervating DRG neurons were found. One responded to low metabolite levels (likely nonnoxious) and used ASIC3, P2X5, and TRPV1 as molecular receptors to detect these metabolites. The other responded to high levels of metabolites (likely noxious) and used ASIC3, P2X4, and TRPV1 as their molecular receptors. We conclude that a combination of ASIC, P2X5 and/or P2X4, and TRPV1 are the molecular receptors used to detect metabolites by muscle-innervating sensory neurons. We further conclude that the adequate stimuli for muscle metaboreceptors and nociceptors are combinations of protons, ATP, and lactate.
INTRODUCTION
In muscle, nociceptive afferent neurons are mostly group III and group IV that correspond in conduction velocity and size to cutaneous Aδ and C afferents, respectively (see reviews by Graven-Nielsen and Mense 2001; Kaufman et al. 2002). Most previous examinations of response properties of afferent neurons innervating skeletal muscle have used mechanical stimuli to evoke responses (Adreani et al. 1997; Bove and Light 1995; Diehl et al. 1993; Hayes et al. 2006; Hoheisel et al. 2004, 2005; Kaufman et al. 2002; Mense and Meyer 1985). Equally important are chemical stimuli, since these are critical for understanding fatigue and pain states induced by various levels of exercise in working muscles. Chemical mediators liberated by muscle contraction include lactic acid, adenosine 5′-triphosphate (ATP), and increasing protons. Previous investigations tested these on muscle afferents along with nonphysiological chemicals such as hypertonic saline, acid saline, and capsaicin. However, to activate significant numbers of muscle-innervating primary afferent neurons, the concentrations of these chemicals were often at levels that are rarely present in skeletal muscle. Nevertheless, these investigations suggested key roles for the molecular receptor types ASICs (acid-sensing ion channels), P2X (purinergic type 2X), and TRPV (transient receptor potential of the vanilloid type) in mediating muscle pain induced by metabolites (e.g., Adreani and Kaufman 1998; Gao et al. 2007; Hoheisel et al. 2004; Kaufman and Rybicki 1987; Leffler et al. 2006; Reinohl et al. 2003; Rotto and Kaufman 1988).
The mismatch between data that strongly suggest roles for these molecular receptors, and the poor activation of muscle sensory neurons by physiological or pathophysiological concentrations of known agonists for these receptors points to our need for better understanding of the factors involved. These include 1) which normally occurring metabolites are the adequate stimuli for muscle nociceptors and 2) what mechanisms do these nociceptors use to detect these substances?
Recent reports suggest a resolution to this enigma. Molecular receptors found on cardiac and skeletal muscle afferent neurons do not respond to a single metabolite in the physiological range, but rather to a combination of two or more factors produced by muscle contraction (Naves and McCleskey 2005; Spelta et al. 2004). Further work suggests that at least two different molecular receptors cooperate to enhance the sensitivity of detection for protons and that these two receptors colocalize on cardiac and skeletal muscle afferent endings. The cooperation between these molecular receptors apparently allows the sensory nerve endings to precisely detect and quantify the amounts of muscle metabolites produced by contraction (Connor et al. 2005; Immke and McCleskey 2001, 2003; Molliver et al. 2005; Sutherland et al. 2001; Yagi et al. 2006).
In addition to muscle pain, muscle group III and group IV primary afferent neurons are very important as the afferent arm of sympathetic reflexes evoked by muscle contraction (see review by Kaufman and Hayes 2002). Primary afferent neurons detecting metabolites produced by contracting muscle were shown to evoke sympathetic reflexes >70 yr ago (Alam and Smirk 1937). In these initial observations, it was already clear that afferent receptors within the muscle were responsible for detecting muscle metabolites. Later experiments confirmed that small-diameter primary afferent fibers carried the message to the CNS, causing these reflexes (Coote et al. 1971; McCloskey and Mitchell 1972). Even early experiments indicated that these reflexes were accompanied by the sensation of “tiredness or pressure” from the fatiguing muscle when lower levels of metabolites were present, and sensations of muscle pain were reported only when higher levels of metabolites accumulated (Alam and Smirk 1937). These experiments suggested that primary afferent endings in muscle could detect and code muscle work using both mechanical and metabolic information. These “work receptors” were later defined as “ergoreceptors,” that is sensory endings encoding muscle work, a subset of which could be “metaboreceptors,” sensory endings that encode muscle metabolite levels produced by muscle work (Kniffki et al. 1981).
Despite the early suggestion that muscle afferents specific for detecting nonnociceptive metabolic information might exist, ergoreceptors and metaboreceptors have only rarely been recorded (Adreani and Kaufman 1998; Kaufman et al. 1983, 1984; Kniffki et al. 1978; Mense and Stahnke 1983; Rotto and Kaufman 1988; Thimm and Baum 1987). It remains controversial as to whether specific populations of muscle sensory afferents for nociception versus innocuous metaboreception exist (Kniffki et al. 1978, 1981; Mense 1996; Mense and Stahnke 1983).
Combinations of metabolites using two or more cooperating molecular receptors to enhance responses might also apply for metaboreceptors as well as nociceptors innervating skeletal muscle. Knowing the proper combination of metabolites might allow better characterization of muscle afferents so that the potential existence of specific classes of metaboreceptors versus nociceptors might be easier to determine.
Therefore to determine the likely combination of metabolites detected by muscle afferents we used calcium imaging of acutely dissociated, cultured dorsal root ganglion neurons, some identified as projecting to muscle, and metabolites applied in a manner that mimics the increases found in normally exercising, ischemic, and injured muscles. We further used selective antagonists for the likely molecular receptors involved to determine which molecular receptors are involved in the detection of these metabolites.
METHODS
All procedures in this investigation comply with the rules and regulations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the IACUC of the University of Utah Health Sciences Center.
Harvesting/culturing cells/loading cells
Thirty-nine 3-wk-old (four wells of DRG neurons plated from each mouse), C57Bl/6 mice were anesthetized with isoflurane and euthanized by cervical dislocation. Dorsal root ganglia (DRGs) from lumbar regions were removed into Hank's balanced salt solution, treated with trypsin, and washed with MEM (Invitrogen 51200-038) containing 10% fetal bovine serum (Hyclone), 2.4% glucose, 1% glutamax (Invitrogen 35050-061), and 1% penicillin/streptomycin (Atlanta Biological B2110). DRGs were triturated, preplated, and incubated at 37°C for 1 h to reduce nonneuronal cells. Neurons were gently swirled and centrifuged (110 g, 5 min). Cells were removed, resuspended, and 30 μL plated onto the four center wells of 24-well plates coated with poly-l-lysine and laminin. A 0.5-mm-thick silicone ring with a 4-mm-diameter opening sealed to the surface of each well reduced the plating area in each well. After 1 h, wells were filled with 37°C culture medium with 10 ng/mL GDNF (glial-derived neurotrophic factor; Preprotech 450-10). Sixteen to 23 h after plating, cultures were loaded with Fura-2 AM (F-1221; Molecular Probes) fluorescent dye for 40–60 min, then washed with pH 7.4 oxygenated observation medium containing no additional ATP (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM citrate, 10 mM glucose, 10 mM MES, 10 mM HEPES). The final well volume was 500 μL.
Choice and concentrations of metabolites
Because the afferent receptors in muscle have been reported to be on the outside of blood vessels supplying muscle (Molliver et al. 2005), we used values obtained for muscle interstitium from experiments using cat, rat, and human for muscle contraction evoked by mild to moderate to extreme exercise.
PROTONS (PH).
We used values between 7.6 and 6.2 spanning the range found in skeletal and cardiac muscle in mammals from above resting values to those found with injury and disease (Bangsbo et al. 1993, 1996; Liu et al. 2007; Pan et al. 1999; Sinoway et al. 1989; Street et al. 2001; Yagi et al. 2006).
LACTATE.
We used 1–50 mM lactate, a range of values that spans from rest to ischemia in muscles of humans and rats and mice (Bangsbo et al. 1993, 1996; Mohr et al. 2007).
ATP.
Opposite to what might be expected, ATP concentrations in muscle interstitium increase from 300 nM at rest to 5 μM with ischemic muscle contraction (Hellsten et al. 1998; Li et al. 2003, 2005). We used these levels in our experiments.
ATP receptors, particularly many P2X receptor subtypes, demonstrate considerable if not complete desensitization within 1 min of agonist binding. To allow for physiological levels of desensitization of possible P2X receptors we applied freshly prepared ATP at 300 nM for ≥30 s before any further increases in ATP combined with other metabolites, to establish resting conditions in DRG neurons in these experiments. Freshly prepared ATP was necessary because we determined that adenosine diphosphate (ADP) was not capable of enhancing metabolite responses. Thus although 1 μM concentrations of ATP were capable of enhancing metabolite responses when freshly prepared, solutions kept at room temperature for >20 min were ineffective, presumably because some of the ATP had hydrolyzed to ADP. Taking all of this information together, we used protons, lactate, and ATP in combinations as shown in Table 1.
TABLE 1.
Concentrations of metabolites used in experiments
| pH | 7.6 | 7.4 | 7.3 | 7.2 | 7.0 | 6.8 | 6.6 | 6.4 | 6.2 |
|---|---|---|---|---|---|---|---|---|---|
| Lactate | 1 mM | 1 mM | 5 mM | 10 mM | 15 mM | 20 mM | 50 mM | 50 mM | 50 mM |
| ATP | 300 nM | 300 nM | 400 nM | 500 nM | 1 μM | 2 μM | 5 μM | 5 μM | 5 μM |
We also used pH 6.0 and 4.0 without additional increases in metabolites to confirm that our imaging experiments could replicate recent reports using patch-clamp techniques (Leffler et al. 2006).
Combinations of metabolites applied to mimic naturally occurring conditions
When applied in an escalating series to mimic increases in metabolites that occur with muscle exercise and ischemic muscle exercise, we used the metabolite values shown in Table 1. The values associated with pH 7.6 are at or below those normally found at rest in the mouse. The values at 7.4 approximate those found at rest. Generally, the low metabolite values associated with pH >7.0 are likely to be nonnoxious, whereas higher metabolite values at pH ≤7.0 may be associated with pain. These values should bracket the physiological range of metabolites found in skeletal muscle, with the last two values (those associated with 6.4 and 6.2) being higher than the physiological range (Bangsbo et al. 1993, 2001; Li et al. 2003, 2005; Reeh and Kress 2001; Steinhagen et al. 1976).
In initial experiments we also used the same concentrations of metabolites as in Table 1, but returned to a “resting” value of pH 7.4, 1 mM lactate, and 300 nM ATP after each application of increasing metabolites to determine the amount of adaptation that occurred due to increasing escalation of metabolite values. In still other experiments, we applied the indicated metabolites in reverse order.
Antagonists
ASIC ANTAGONISTS.
Amiloride antagonizes all ASIC receptor responses to low pH. However, Yagi et al. (2006) recently demonstrated that ASIC3 responses to moderate pH levels (pH 7.0) are actually enhanced by amiloride. APETx2, a peptide extracted from sea anemone, can selectively inhibit ASIC3 and ASIC3 heteromers (Chagot et al. 2005; Diochot et al. 2004). This peptide was not available at the time of these experiments. We used A-317567 (provided, synthesized, and characterized by Dr. Jon Rainier and Dr. Zhuqing Liu) as an effective antagonist in the experiments reported here. A-317567 is an antagonist of all ASICs with IC50 values of 2.0, 9.1, and 9.5 μM for ASIC1, ASIC2, and ASIC3, respectively (Dube et al. 2005). A-317567 caused no decrements in calcium responses evoked by capsaicin, ATP, or KCl. If applied for >20 min A-317567 caused some decrements in these responses.
P2X ANTAGONISTS.
Pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) has the same IC50 at P2X3 and P2X5 receptors (0.2 μM) (Gever et al. 2006) and has an IC50 value of 25 μM at mouse P2X4 receptors (Jones et al. 2000; Khakh et al. 2001). Trinitrophenyl-adenosine triphosphate (TNP-ATP) has an IC50 value of 0.31 nM at P2X3 receptors, 0.5 μM at P2X5 receptors (Wildman et al. 2002), and 16 μM at P2X4 receptors (Khakh et al. 2001).
TRPV1 ANTAGONISTS.
Capsazepine, the most used TRPV1 antagonist, has little inhibitory action on pH responses of TRPV1 receptors in mouse (Correll et al. 2004). In these investigations, we used JYL-1433 and LJO-328 (provided, synthesized, and characterized by Dr. J. Lee), which are potent full antagonists of TRPV1 (Lee et al. 2003; Reilly et al. 2005; Wang et al. 2002).
Preparation of antagonists.
TNP-ATP and PPADS were dissolved in observation media to a concentration of 5 mM, quick-frozen, and stored at −20°C. JYL-1422, LJO-328, A-317567, and a selected stereoisomer of A-317567 were each dissolved in 5% DMSO/95% water and a few microliters of 1 M HCl to a concentration of 10 mM, then quick frozen and stored at −20°C. Aliquots of antagonists were thawed and diluted to the appropriate concentrations just prior to use in the experiments. The strong buffers maintained the pH of these antagonists when mixed with metabolites.
DiI labeling.
In some experiments, neurons were identified as innervating muscle by prior muscle labeling with 1,1′-di-octadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Molecular Probes). Twelve 7- to 9-day-old mice were anesthetized by cooling. Using a dissecting microscope, hindlimb muscles (both heads of the gastrocnemius, plantaris, tibialis anterior, biceps femoris, and in some mice, semitendinosus) were exposed through a small skin incision and each muscle was injected with 0.05 μL of DiI diluted in dimethyl formamide, using glass micropipettes with tips broken to 10- to 25-μm diameter attached to Hamilton syringes. Any DiI leaking to the muscle surface was immediately absorbed with cotton-tip applicators. The incision was closed with cyanoacrylate. The DRGs from the labeled mice were harvested at 2–3 wk of age (1–2 wk after DiI labeling). DiI-labeled DRG neurons were imaged and outlined immediately before each experiment.
Similar strategies have been used for patch-clamp investigations of the properties of retrogradely labeled neurons (Connor et al. 2005; Jiang et al. 2006; Molliver et al. 2005; Rau et al. 2007; Yagi et al. 2006).
Imaging procedure.
All experiments were performed at room temperature (20–25°C) to allow correspondence with most previous experiments using calcium imaging and patch clamp of DRG neurons.
Cells were imaged using the Meta Imaging Series Metafluor program (Universal Imaging). All cells identifiable as neurons based on their round shape, and clear, single nucleus were selected from a phase image of the field. At the end of each experiment, we applied 200 nM capsaicin, followed by a wash, and then applied 50 mM KCl. Capsaicin activation indicated the presence of TRPV1 receptors on neurons; KCl ensured that recorded objects were active neurons and established the maximal calcium response in each neuron. For some neurons the administration of capsaicin abolished the potential for responding to KCl; thus a response either to capsaicin or to KCl indicated viability of neurons and only viable neurons were included in analyses.
For each field an average of 151 ± 10 cells were tracked in real time. For each cell the ratio of fluorescent intensities was computed (340 nm/380 nm, normalized to a maximum of 1.0, minimum of 0.0) and recorded in a data table as well as displayed in a graph. Figures 1 and 2 show traces from these graphs. For each experiment about 30 baseline images were acquired before the addition of drug or metabolite changes, after which 600–1,000 fluorescent images were acquired (2 s between each image) to record the effect of metabolite and drug applications.
FIG. 1.
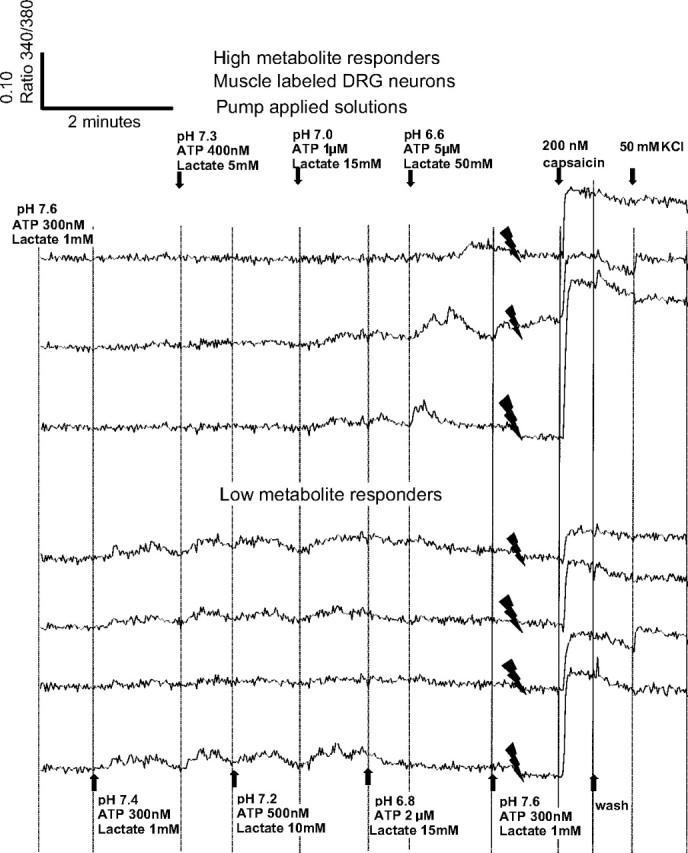
Responses of individual dorsal root ganglion (DRG) neurons to increases in pump-applied metabolites, with responses to 200 nM capsaicin and 50 mM KCl added at the end of each trace. Solid downward-pointing spike-like symbols indicate where a portion of the trace stopped, then started to allow the capsaicin and KCl responses to be included on this figure. In all, 17 DRG neurons were retrogradely labeled from skeletal muscle with DiI in this well, and all 7 of these that responded to metabolite increases are shown here. To allow for larger font size, the amounts of metabolites applied are split between top and bottom, even though the same metabolites are applied at the same time to the neurons depicted on both top and bottom. Arrows and vertical lines indicate when metabolite changes were made; wash = pH 7.4, 1 mM lactate, with 0 adenosine triphosphate (ATP). Vertical scale is the ratio of intensity of light emitted at 340 nm/380 nm. Maximum possible calcium responses caused by application of either capsaicin or KCl are at the right of each trace.
FIG. 2.
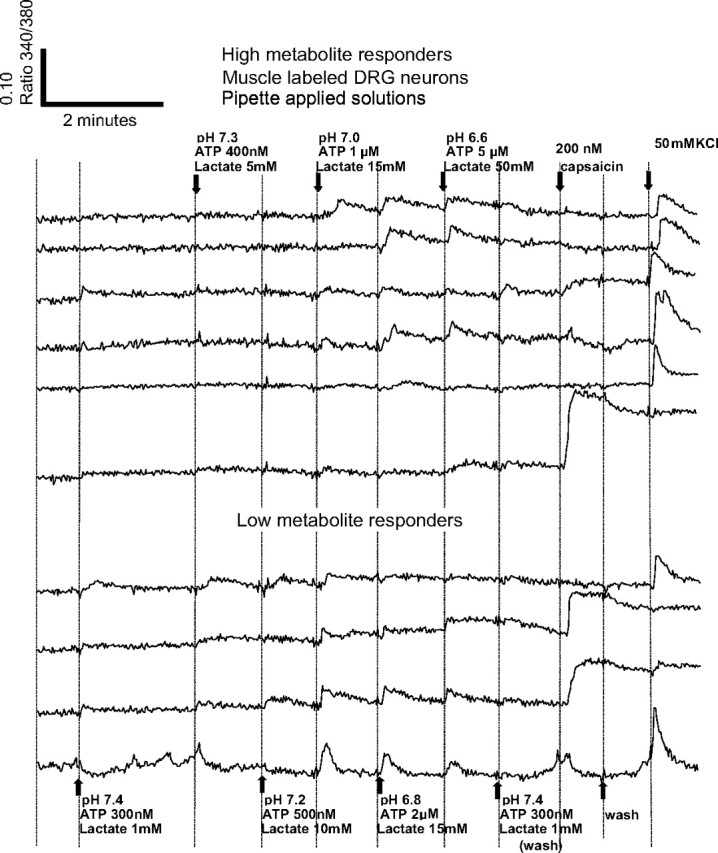
Responses of individual DRG neurons to changes in metabolites, with their responses to 200 nM capsaicin and 50 mM KCl included at the end of each trace. Pipetting artifacts were reduced or omitted. Note apparent metabolite responses at pH 7.4 caused by application 300 nM ATP, given that no other metabolites were increased during this application. These ATP-induced artifacts were not seen in Fig. 1 because ATP responses had adapted before the application of pH 7.4. Shown here are all 10 of the metabolite-responsive DRG neurons labeled with DiI from the hindlimb muscles. Twenty-two DRG neurons were labeled with DiI in this well but 12 of these did not respond to metabolite increases. All other labels as in Fig. 1.
Drug/metabolite application
The metabolite or metabolite/drug solutions were applied via a custom-designed peristaltic pump apparatus. In other experiments, metabolites and drugs were delivered using two pipettes: one pulling solution out of the well immediately followed by the other pumping solution back into the well. In using the two-pipette method, clear artifacts were visible on the real-time graphs that correlated directly to the changing volumes in the well during the pipetting process, which also served as an indicator of the exact time the metabolite levels changed.
The two-pipette method also served as a control for the pump data, omitting the effects of the flow of solutions across the well, for the potential for release of absorbed substances from the walls of the tubing and for the possible pressure changes that may have occurred while actually switching from one solution to the next. Both techniques produced similar results, so we combined data except where noted.
For all of the data presented here, each well was used for only one metabolite series.
Analysis
Off-line data from each run were loaded into an Excel spreadsheet for further analysis. This analysis subtracted baseline variations, computed baseline means and SDs, and compared them with means and SDs during each metabolite addition. We defined responses as increases of Fura ratios >2SDs above the baseline. The baseline was 30–60 s of imaging prior to the addition of the metabolites. Figures 1 and 2 show typical responses with both pump and pipette application of metabolites.
For statistical analyses, we used a standard, one-way ANOVA to compare the responses to the doses, with α < 0.01. Means were compared only if the ANOVA was significant. For multiple means compared with controls, Dunnett's test was used with α < 0.05. To compare means to each other, a Tukey–Kramer honestly significant difference analysis was used with α < 0.05. Throughout the text and figures, values are expressed as means ± SE.
RESULTS
Responses of DRG neurons to protons
In initial experiments, we applied metabolites individually and returned to control levels after each application of increased metabolites. In most of the experiments reported here, we applied increasing metabolites in a more physiological manner. Thus the DRG neurons were initially in a medium that contained the approximate levels of three metabolites (ATP, lactate, and protons) found under resting conditions in vivo. We then changed the medium in the well to the next lower pH level and corresponding increased lactate and ATP levels. Thus metabolites increased much the way they do with increasing muscle work. Figures 1 and 2 show typical responses to increasing metabolites in this fashion. We refer to the decrease in pH as “increasing protons” in the rest of this report, and include “increasing protons” as one of the metabolites. The other two are lactate and ATP.
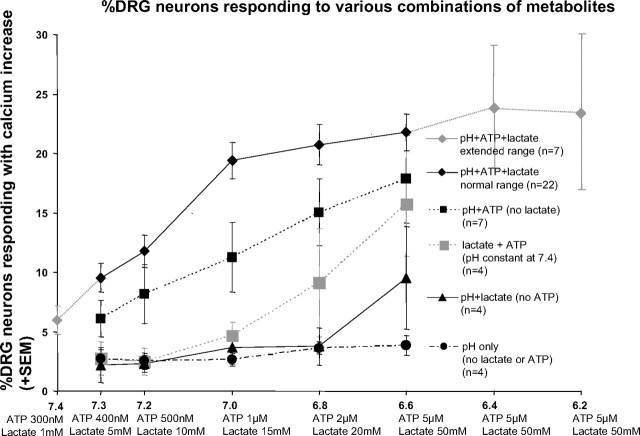
Very few DRG neurons responded to pH values >6.0 without lactate and ATP present
Less than 4% of DRG neurons responded with increases in intracellular free calcium when protons alone were increased to physiologically relevant values in a series of steps (pH 7.4, 7.3, 7.2, 7.0, 6.8, 6.6) (see Fig. 3, filled circles; Fig. 4, far left bar). Previous reports using whole cell patch-clamp techniques had similar findings (Jiang et al. 2006; Yagi et al. 2006).
FIG. 3.
Percentage of DRG neurons with increases in intracellular calcium evoked by the metabolite levels indicated on the x-axis. The combinations of metabolites contributing to each of the curves are indicated in the legend on right. For example, the filled squares represent experiments in which protons and ATP were varied, but lactate was 0. For each series of points, the metabolites were administered in the increasing order shown. n = the number of wells under each condition; in each well an average of 152 ± 9 (SE) active DRG neurons were imaged. In all, 5,307 DRG active neurons were analyzed for this figure.
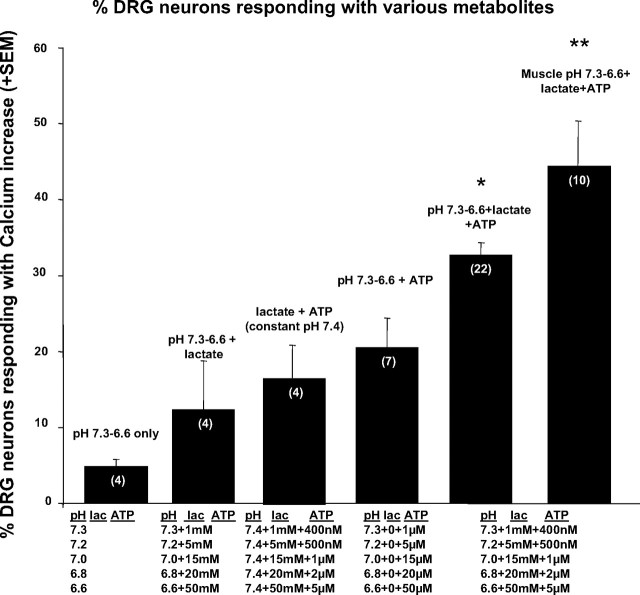
FIG. 4.
Total percentage of DRG neurons responding with a rapid calcium increase to the application of increasing metabolite combinations as applied to wells in Fig. 3. To calculate the percentages represented by each bar, all of the DRG neurons responding at any of the 5 concentrations of metabolites presented in increasing amounts shown beneath the bar were counted. As shown in Figs. 1 and 2, because many neurons responded at more than one, but not all doses of metabolites, the total percentage of responding neurons here will be higher than any single percentage in Fig. 3. Wells measured for both the 5th and 6th bars were combinations of all 3 metabolites indicated under both bars. The 6th bar represents the total percentage of active neurons labeled from muscle that responded to increases of all 3 metabolites. Numbers of wells tested for each condition are indicated in parentheses within each bar. Statistical analysis in this and other figures used the number of wells as the sample size. ANOVA for all groups: F = 11.96, P < 0.0001. Dunnett's method compared all other means to that of all neurons responding to pH 7.4–6.6/lacate/ATP applications. * indicates mean greater than all means to the left of this bar (P < 0.05). ** indicates mean greater than all other means (P < 0.01).
However, when the pH was reduced directly from 7.4 to 6.0 with no lactate, or ATP present, 9% of the DRG neurons responded [compare with 18% reported by Leffler et al. (2006) using patch-clamp techniques]. When lowered directly from pH 7.4 to pH 4.0, 27% of the DRG neurons responded. If only neurons <25 μM in diameter were included in this analysis, 35% of the active neurons responded at pH 4. This compares with 41% of DRG neurons in the mouse that responded to pH 4 with current increases when whole cell patch clamp was used and small and medium cells (comparable to neurons <25 μM) were selected as reported by Leffler et al. (2006). Thus altering protons alone produced results similar to previous experiments using patch clamp of mouse DRG neurons.
Responses to lactate
Lactate alone (1–50 mM) activated few DRG neurons, but lactate enhanced proton-induced responses
Lactate alone (1–50 mM, data not shown) caused no increases in DRG neuron responses at pH 7.4. Similarly, protons at pH 7.3–6.8 combined with lactate at 5–15 mM activated <4% of DRG neurons (see Fig. 3, triangles). However, pH 6.6 and lactate at 50 mM activated 9.5% of DRG neurons, with a total of >10% of DRG neurons activated if all levels of metabolites were included (see Fig. 4, second bar from left). This moderate enhancement of pH responsiveness by lactate was consistent with patch-clamp experiments reported previously (Yagi et al. 2006).
Responses to ATP
High levels of ATP alone or with lactate present activated DRG neurons independently of pH. However, physiological levels of ATP presented alone activated few DRG neurons
ATP at 5 μM, with lactate present and pH held constant at 7.4, activated ≤30% of DRG neurons if applied to cells naïve to ATP (data not shown). As has been suggested by other investigators (Gao et al. 2007), lower pH (6.8) slightly reduced responses of DRG neurons to ATP (data not shown). However, ATP applied at lower doses (300 nM) and maintained for more than a few seconds prevented most of the calcium increases to ATP applied at higher doses, presumably through desensitization. Thus an increasing, graded series of ATP applications (300 nM, 400 nM, 500 nM, 1 μM, 2 μM, 5 μM), activated <10% of DRG neurons when 2 μM was reached, and about 16% of DRG neurons when going from 2 to 5 μM (see Fig. 3, gray squares; Fig. 4, third bar from the left).
Responses to combinations of metabolites
DRG responses to ATP combined with “increasing protons” were more than additive
As noted previously (Naves and McCleskey 2005; Spelta et al. 2004), ATP appeared to act synergistically with protons to enhance responses of DRG neurons. This “more than additive” effect was most apparent at moderate metabolite concentrations. Thus as can be seen in Fig. 3, at pH 7.0/ATP 1 μM, the percentage of DRG neurons responding to increasing protons with the addition of ATP was 11.2. This percentage was more than that of DRG neurons responding to pH 7.0 protons alone (2.7) plus the percentage of DRG neurons responding to ATP with lactate (4.7) = 7.4.
Combinations of physiological levels of ATP, lactate, and protons produced by working muscles significantly increased intracellular free calcium in one third of mouse DRG neurons in culture
Although individual or combinations of two of the metabolites activated relatively few DRG neurons, increasing the combination of all three metabolites within the physiological range of working skeletal muscle activated 32.4 ± 1.6%. The combination of all three metabolites activated more DRG neurons than any single metabolite activated and more than combinations of any two metabolites activated (ANOVA, F = 11.96, df = 5:31, P < 0.0001, Dunnett's test for DRG with all three metabolites vs. all other conditions, P < 0.05). Figures 3 and 4 clearly show these relationships.
The total number of DRG neurons that responded to any of the metabolite combinations cannot be determined from Fig. 3 because many DRG neurons responded to several of the doses of metabolite combinations, although most did not respond to all doses of metabolites (see examples of individual DRG responses in Figs. 1 and 2). Figure 4 shows the total percentages of DRG neurons that responded to specific combinations of any dose of metabolites. Metabolite combinations are listed above the bars. We performed statistical analyses only on the data shown in Fig. 4, not on the repeated measures shown in Fig. 3.
A higher percentage of DRG neurons innervating skeletal muscle responded to increasing metabolites than in the overall population of DRG neurons
Forty-four percent of DRG neurons retrogradely labeled from skeletal muscle responded to the combination of all three metabolites (see Fig. 4). This percentage (44.2 ± 6.3%, range 32–89%) was higher than the percentage of all DRG neurons responding to the combination of all three metabolites (32.4 ± 1.6%, range 26–49%).
A larger range of metabolites indicated that DRG neurons responded at resting metabolite values and that DRG neurons showed nearly flat metabolite dose–response relationships above pH 7.0, ATP 1 μM, and lactate 15 mM
Figure 3 (top curve, “pH+ATP+lactate extended range,” depicted with gray diamonds and lines) shows the percentage of DRG neurons activated at each increased metabolite level over a larger range. Note that the percentage of neurons responding increased until pH 7.0/lactate 15 mM/ATP 1 μM. More extreme metabolite increases to pH 6.4/50 mM lactate/5 μM ATP and further to pH 6.2/50 mM lactate/5 μM ATP caused only small (statistically nonsignificant) additional increases in the percentage of responsive neurons. Overall, these data indicated that the responses to increasing metabolites were flat above pH 7.0. Thus if the entire population of DRG neurons were lumped together, they unambiguously encoded increases in metabolites only up to pH 7.0/15 mM lactate/ 1 μM ATP.
However, when collecting these data, we noted that responses of DRG neurons were heterogeneous. As Figs. 1 and 2 show, some neurons (labeled “Low metabolite responders” in these figures) began responding near the resting values of metabolites: pH 7.4/1 mM lactate/300 nM ATP. “Low metabolite responders” increased their responses only up to pH 7.0/15 mM lactate/1 μM ATP, then showed a decrease in response as metabolite values increased further. Other neurons (labeled “High metabolite responders”) began to respond with metabolites only near pH 7 in these figures and increased their responses to metabolites at pH 6.8 and 6.6, but sometimes decreased responses at higher metabolite levels.
From the preceding observations, we hypothesized at least two populations of DRG neurons according to their thresholds and maximum responses to metabolite levels
These observations prompted us to divide the DRGs into two groups. One group, “High metabolite responders,” included those that discriminated metabolite values higher than pH 7.0/ATP 1 μM/lactate 15 mM (see top panels in Figs. 1 and 2 for examples). The other group, “Low metabolite responders,” included those capable of detecting metabolite values pH 7.0/ATP 1 μM/lactate 15 mM or lower (see examples in Figs. 1 and 2, bottom panels). We assumed that a sensory neuron could unambiguously signal increasing metabolites only if they increased their calcium responses to further metabolite increases.
Figure 5 indicates the percentage of DRG neurons responding to progressive increases of metabolites (lactate, ATP, and protons) when divided into low metabolite and high metabolite responding types for all DRG neurons (Fig. 5A) and those retrogradely labeled from skeletal muscle (Fig. 5B). Note that these two populations of DRG neurons have different peak percentages of responding neurons. The low metabolite responding population had a peak at pH 7.0 and the high metabolite responding population had a peak at pH 6.4. For DRGs that were retrogradely labeled from hindlimb skeletal muscle (Fig. 5B) the high metabolite peak was at pH 6.6, but this was not statistically different from the value at 6.4. The characterization as low or high metabolite responsive was relatively stable. After a complete series of metabolites had been tested, and the well was returned to resting values (pH 7.4, ATP 300 nM, lactate 1 mM) for 5 min, applying another series of metabolites did not shift neurons from one classification to another. In other words, “low metabolite responders” did not become “high metabolite responders” or vice versa (data not shown). However, some desensitization was evident because 10–15% of the DRG neurons that responded during the first series failed to respond to the second series of metabolites.
FIG. 5.
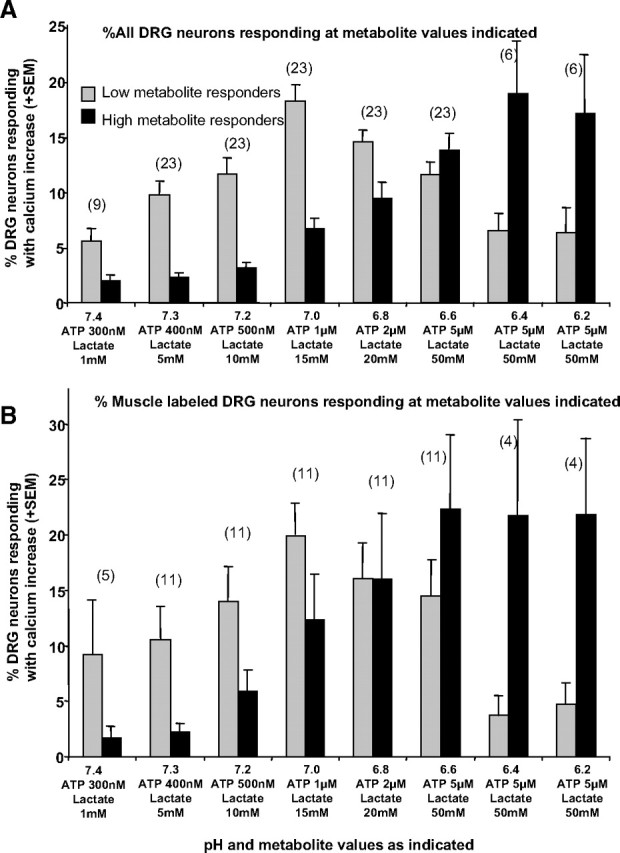
Graph of percentage of responding DRG neurons divided into high and low metabolite responders, respectively. A: graph is for all DRG neurons. B: graph includes only DRG neurons retrogradely labeled from hindlimb muscles. The numbers in parentheses indicate the number of wells analyzed. Each well in the top graph averaged 123 ± 8 neurons. Each well in the bottom graph averaged 28 ± 4 active DiI-labeled neurons.
Our data indicated approximately equal numbers of low metabolite versus high metabolite responding DRG neurons. For the 44% of DRG neurons retrogradely labeled from skeletal muscle and activated by metabolites, 46.5% responded best to low metabolite levels, whereas 53.5% responded best to high metabolite levels. For all DRG neurons responding to metabolites, 54.9% responded best to low metabolite levels, whereas 45.1% responded best to high metabolite levels.
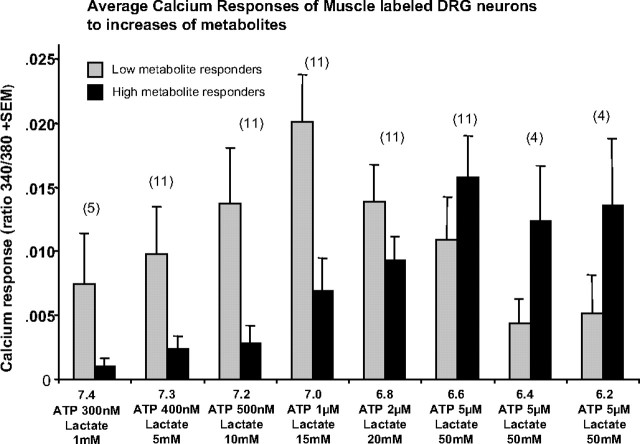
Figure 6 shows the magnitude of the calcium responses of muscle-innervating DRG neurons. These responses are very similar to the percentage of responding DRG neurons for the low metabolite versus high metabolite groups (data shown only for muscle-labeled DRGs in Fig. 6).
FIG. 6.
Graph of calcium responses of DRG neurons retrogradely labeled from skeletal muscle to the application of the indicated metabolites for each bar. DRG neurons were divided into those that increased responses up to metabolites associated with pH 7.0, then decreased calcium responses to higher levels of metabolites (Low metabolite responders) and those that increased calcium responses to metabolites associated with pH levels <7.0 (High metabolite responders). Only neurons that showed a significant calcium increase above baseline were used in this analysis.
Although the drop off in responses (past pH 7.0 for low metabolite responders and pH 6.6 for high metabolite responders) could have been interpreted as “ceiling” effects, the much higher calcium responses evoked by capsaicin and/or KCl suggested otherwise (see Figs. 1 and 2).
Receptor antagonist effects on responses to increasing metabolites
A selective ASIC antagonist blocked all responses of DRG neurons to increased metabolites
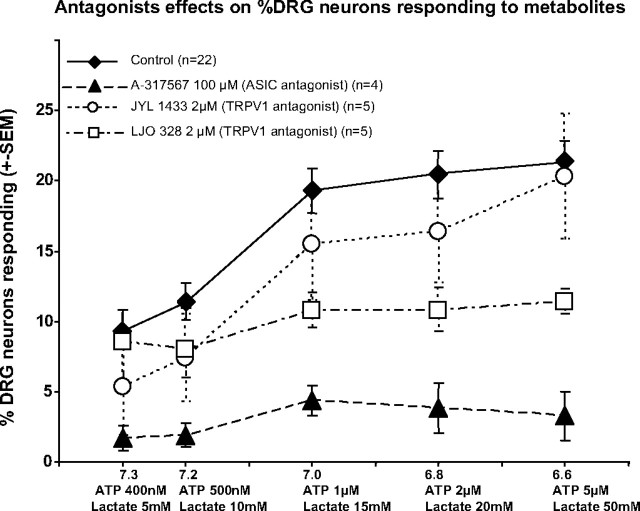
To determine the likely molecular receptors mediating the responses to increased metabolites, we used antagonists selective for the receptors previously hypothesized by other investigators as mediators of metabolite responses in sensory neurons innervating muscle. ASIC3 (also known as DRASIC) may be a mediator of the pH responsiveness of DRG neurons (Molliver et al. 2005; Naves and McCleskey 2005). We found that 100 μM A-317567 [a selective antagonist for ASIC1–ASIC3 (Dube et al. 2005)] reduced the responses to metabolites to 23% of the control level (P < 0.0001) as shown in Fig. 7 (filled triangles). A-317567 at 200 μM blocked all responses to metabolites (data not shown). As confirmation of selectivity, A-317567 (applied for ≤10 min) at any dose did not decrease responses to capsaicin, ATP, or KCl (data not shown).
FIG. 7.
Effects of antagonists to acid-sensing ion channels (ASICs; A-317567: filled triangles) and transient receptor potential of the vanilloid type 1 (TRPV1; JYL-1433 and LJO-328: open circles and squares, respectively) at indicated doses on responses to increased metabolites as presented in previous graphs. Controls (filled diamonds) received only the metabolite increases indicated on the x-axis. For the antagonist applications, each well averaged 108 ± 5 neurons imaged for calcium increases. Statistical analyses used wells as the sample size.
The TRPV1 antagonist LJO-328 at 2 μM blocked some of the DRG neuron responses, as shown in Fig. 7 (squares) (P < 0.01, Dunnett's test vs. controls). DRGs labeled from muscle were similarly affected (data not shown). TRPV1 antagonist inhibition of responses to metabolites appeared to affect both low and high metabolite responding neurons. As evidence for the effectiveness and selectivity of these antagonists, the 2 μM doses of LJO-328 and JYL-1433 abolished nearly all responses to capsaicin (<2% responded), but responses to KCl application remained normal.
P2X antagonists suggested involvement of more than one P2X subtype on the responses of DRG neurons to increases in metabolites
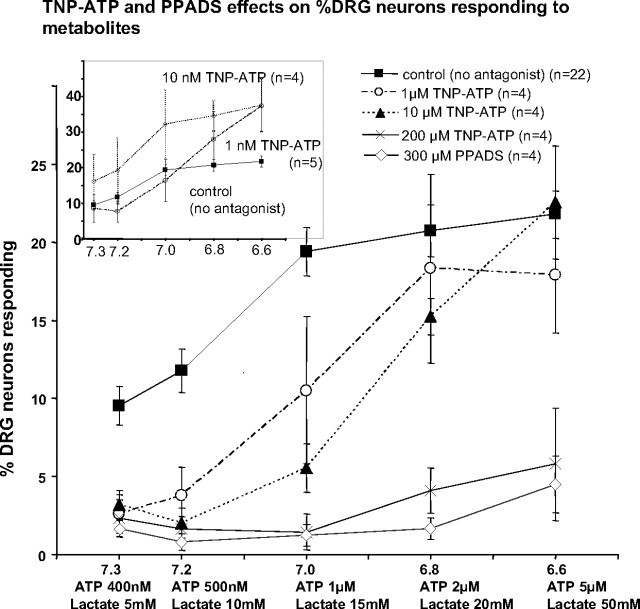
At 10 nM, TNP-ATP, a potent P2X1 and P2X3 antagonist, increased responses to metabolites (see inset in Fig. 8) (P < 0.05, Dunnett's test). The 10 nM dose was well above the IC50 value of P2X3 (0.3 nM), but below the IC50 value for both P2X4 and P2X5 (16 and 0.5 μM, respectively).
FIG. 8.
Effects of the purinergic type 2X (P2X) antagonists trinitrophenyl-adenosine triphosphate (TNP-ATP) and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) at various doses on responses of DRG neurons to increases in metabolites. Note that very low concentrations of TNP-ATP (10 nM) facilitated responses (inset graph), whereas concentrations of TNP-ATP that antagonize P2X5 (1 μM) reduced low metabolite responses, and concentrations that block all P2X receptors including P2X4 (200 μM) blocked nearly all responses to metabolites.
At 1 and 10 μM, however, TNP-ATP reduced responses of DRG neurons evoked by increasing metabolites between pH 7.3 and 7.0 (see Fig. 8, triangles) (P < 0.05, Dunnett's test). As shown in Fig. 8, TNP-ATP applied at 200 μM abolished most responses to increasing metabolites. However, capsaicin and KCl responses were unaffected by any dose of TNP-ATP (data not shown), indicating specificity of TNP-ATP. PPADS at 300 μM (capable of blocking P2X3, P2X4, and P2X5) eliminated most responses to increasing metabolites (Fig. 8, open diamonds).
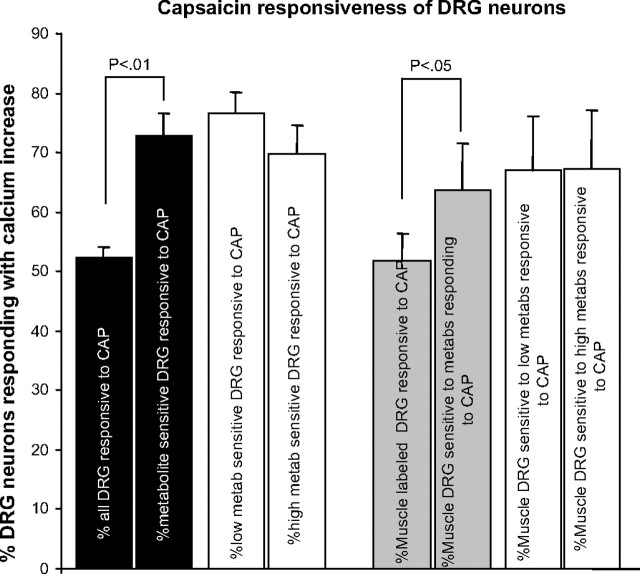
Capsaicin responses of DRG neurons responding to metabolite pH values
As shown in Fig. 9, when all DRG neurons were included, 50.9 ± 4.2% responded to 200 nM capsaicin. This is higher than previous reports of capsaicin responses in DRG neurons in the mouse using patch-clamp techniques [36% reported by Leffler et al. (2006) and 20% reported by Lin et al. (2008)]. Significantly more metabolite-responding DRG neurons also responded to capsaicin (71.9 ± 4.2%, P < 0.01, overall ANOVA, P < 0.0005) (see Fig. 9). For DRG neurons retrogradely labeled from muscle, as with all DRG neurons, more metabolite-responsive neurons also responded to capsaicin (all muscle-labeled neurons, 48.8 ± 5.8% vs. muscle-labeled metabolite responders, 63.4 ± 5.8%, P < 0.05; see Fig. 9, right).
FIG. 9.
Percentage of DRG neurons responding with rapid calcium increase to application of 200 nM capsaicin. Black-filled bars are data from all DRG neurons. Gray-filled bars are from DRG neurons retrogradely labeled from hindlimb muscles. Unfilled bars further divide all DRG neurons (left) and muscle-labeled DRG neurons (right) into those responsive to low metabolites and high metabolites that also respond to capsaicin. Error bars are +SE. Statistical analysis was ANOVA followed by t-test, α <0.05.
Size of responsive DRG neurons
Other groups using whole cell recordings suggested that all sizes of DRG neurons respond to pH decreases, but especially intermediate size neurons (Lin et al. 2008; Molliver et al. 2005). Our data are consistent with these observations.
Table 2 contains the mean diameters, SEs, ranges, and comparisons for a sample of 1,104 active, cultured mouse DRG neurons. Table 2 shows that, although metabolite-responsive neurons were of all sizes, they were most commonly smaller neurons with an average diameter of 20.8 μm compared with 22.2 μm for all active DRG neurons. Metabolite-responsive neurons were about the same size as capsaicin-responsive neurons. Low metabolite-responsive neurons were not different in size from high metabolite-responsive neurons (P > 0.64).
TABLE 2.
Diameters of DRG neurons
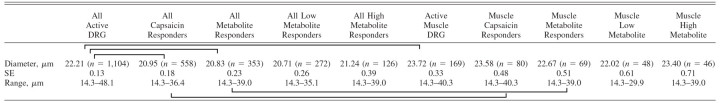 |
Bars connecting means indicate significant differences using Tukey–Kramer test, α < 0.05.
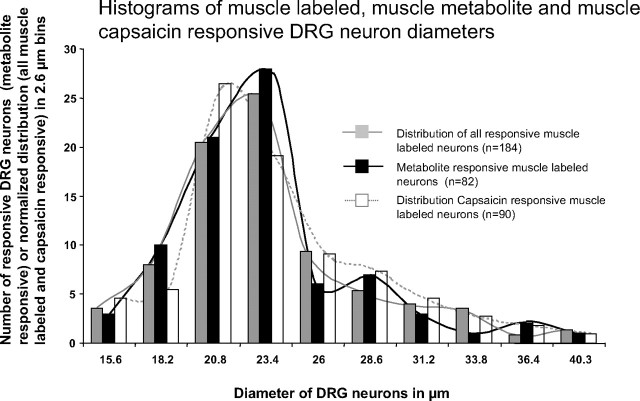
Muscle-innervating DRG neurons, however, were larger on average than all DRG neurons (average diameter 23.7 vs. 22.2 μm). More surprising, but consistent with findings in rats, the diameters of metabolite-responsive, muscle-labeled DRG neurons were larger on average than the diameters of metabolite-responsive neurons from the overall DRG neuron populations. Interestingly, the diameters of capsaicin-responsive, muscle-innervating DRG neurons were also larger than the diameters of capsaicin-responsive neurons from the overall DRG population. Figure 10 shows histograms of muscle-labeled DRG neurons demonstrating the smaller number of very large DRG neurons responding to metabolites, compared with all muscle-innervating DRG neurons. Figure 10 also shows that muscle-labeled metabolite-responsive neurons included many sizes and shows that capsaicin-responding neurons had a similar size distribution to muscle-labeled, metabolite-responding neurons.
FIG. 10.
Histograms of diameters of muscle-labeled DRG neurons responsive to increasing metabolites. Gray bars: diameter histogram generated from 184 active muscle-labeled DRG neurons and scaled to the number of metabolite-responsive neurons. Black bars: diameter histogram of 82 metabolite-responsive muscle-labeled DRG neurons. Open bars: diameter histogram generated from 90 capsaicin-responsive, muscle-labeled DRG neurons and scaled to the number of metabolite-responsive neurons. Note the overabundance of small diameters and decreased abundance of large diameters in the metabolite-responsive DRG neurons compared with all active muscle-labeled DRG neurons. Smoothed lines connecting the histogram bars were added to aid in observing distribution differences.
Overall, these data suggest that most metabolite-responding DRG neurons innervating muscle were among the smallest neurons innervating muscle and therefore most of these DRG neurons likely had unmyelinated or thinly myelinated axons characteristic of group III and group IV neurons. However, the muscle-innervating, metabolite-responding DRG neurons were larger on average than metabolite-responding DRG neurons that did not innervate muscle.
DISCUSSION
Methodology
CALCIUM IMAGING OF CULTURED DORSAL ROOT GANGLION NEURONS.
In general, molecular receptor clusters determined from cultured DRG neurons have proven to be similar to those found in specific types of sensory receptor endings (Lawson 1996; Leffler et al. 2006; Petruska and Mendell 2004; Petruska et al. 2000b; Price et al. 2001; Rau et al. 2005, 2007; Sluka et al. 2003). In spite of the advantages of sample size and access to the membrane surface, for a variety of reasons the responses of DRG neurons may differ from the responses of receptor endings in the tissues they normally innervate.
We used imaging of increased intracellular calcium as an indicator that the DRG neurons were more likely to activate action potentials, not as an indicator of other calcium-mediated cellular processes. Rapid increases in calcium may not reflect an increased likelihood of action potential generation in some circumstances. However, we and others have shown that data obtained with calcium imaging relate well to data derived using patch-clamp techniques (e.g., Hjerling-Leffler et al. 2007; Jiang et al. 2006; Leffler et al. 2006; Lu et al. 2006; Munns et al. 2007; Smith et al. 2004). However, for these and other reasons, it will be important to verify the findings reported here using in vitro and in vivo recordings from intact sensory fibers innervating muscle to determine the responses of their receptor endings in situ.
SPECIES DIFFERENCES: RAT VERSUS MOUSE.
Many previous investigations of pH and metabolite responses of muscle-innervating DRG neurons have used dogs, cats, and rats (Adreani and Kaufman 1998; Darques et al. 1998; Hayes et al. 2007; Immke and McCleskey 2001; Kaufman and Rybicki 1987; Rotto and Kaufman 1988; Sinoway et al. 1993; Sutherland et al. 2001; Yagi et al. 2006). The exceptions have been experiments examining the specific molecular receptors that mediate the pH responses where mice were used because of the availability of functional targeted gene deletions (Benson et al. 2002; Hjerling-Leffler et al. 2007; Leffler et al. 2006; Price et al. 2001; Sluka et al. 2003, 2007; Xie et al. 2003).
These previous experiments have indicated some possible differences in responses of DRG neurons from rat versus mouse. First, capsaicin-sensitive neurons were fewer in mouse than those in rat (Leffler et al. 2006). Our experiments, however, indicate nearly the same proportion of capsaicin-sensitive neurons in mouse as in rat. The larger number of capsaicin-responsive neurons in our experiments may have resulted from lower levels of presented capsaicin (higher levels may cause very rapid desensitization) or from enhanced excitability because of metabolite presentations before capsaicin presentations. Second, the proton response of DRG neurons in mouse was less prevalent and smaller in magnitude compared with responses from rat DRG neurons (Leffler et al. 2006). Our experiments confirm this potential difference. Third, P2X receptor subtypes differ in rat versus mouse. For example, Jones et al. (2000) found that, although ATP has similar effects in these two species, a common agonist (α,β-meATP) and a common antagonist (PPADS) were ineffective for the rat P2X4 receptor, but were effective for human and mouse P2X4 receptors. We found that PPADS was effective on mouse P2X receptors.
Main findings
1)Combinations of increasing protons, lactate, and ATP activated more DRG neurons than individual or pairs of metabolites
Increasing protons alone (pH from 7.4 to 6.6) was ineffective in activating DRG neurons as determined by calcium imaging in our experiments. Increasing lactate alone from 1 to 50 mM activated very few mouse DRG neurons if the pH was constant at 7.4. Similarly, Rotto and Kaufman (1988) previously showed that buffered sodium lactate alone had little or no effect on group III and group IV muscle afferents in the cat. ATP alone at 5 μM induced calcium responses in nearly 30% of DRG neurons (independent of pH between 7.4 and 6.6) when applied to naïve cultures. Others have reported similar excitatory effects of ATP on DRG neurons (Burnstock and Wood 1996; Chen et al. 1995; Cook et al. 1997; Hu and Li 1996; Jahr and Jessell 1983; Kindig et al. 2007; Rang et al. 1991; Zimmermann 1994). However, if neurons were exposed to the amounts of ATP found in resting muscle interstitium, then ATP was gradually increased in amounts similar to those found during muscle contractions in vivo (Li et al. 2003, 2005), <11% of DRG neurons were activated, and then only at concentrations >2 μM. Previous investigations have also found that individually applied ATP, lactate, or protons can activate muscle afferents at high concentrations, but are relatively ineffective at lower concentrations (Graham et al. 1986; Hanna and Kaufman 2004; Hoheisel et al. 2004; Kaufman and Rybicki 1987; Kindig et al. 2006; Li and Sinoway 2002; Reinohl et al. 2003; Rotto and Kaufman 1988; Rybicki et al. 1985; Sinoway et al. 1993; Thimm and Baum 1987). Thus the metabolites lactate, protons, and ATP evoked few responses in DRG neurons when applied individually in the normal, physiological ranges. However, protons or ATP, individually applied, can evoke responses at the high concentrations caused by vascular and muscle injury and may play an active role in detecting traumatic muscle damage.
The effects of individually applied metabolites contrasted sharply with the excitatory effects of the combination of all three metabolites (lactate, ATP, and protons). The combination of all three metabolites, even at modest levels (pH 7.0, ATP 1 μM, lactate 15 mM) activated >20% of all DRG neurons and >30% of DRG neurons innervating skeletal muscle. Higher amounts of all three metabolites activated 30% of all DRG neurons and nearly 50% of those innervating muscle. This synergy of metabolite effects was predicted by previous reports (Immke and McCleskey 2001, 2003; Naves and McCleskey 2005; Yagi et al. 2006). These previous investigations demonstrated that lactate, presumably by chelating calcium, enhanced the responses of DRG neurons to protons in the physiological range. They also demonstrated that ATP acting via a P2X receptor enhanced ASIC3 responses to protons. Thus it appears that all three of the metabolites—protons, lactate, and ATP—play a role in the response of DRG neurons to the concentrations of metabolites that are normally produced by contraction of skeletal muscle.
It is very interesting that active muscle contraction rather uniquely produces increases in this combination of metabolites. The metaboreceptor requirement for this specific combination of three metabolites reduces the chances of muscle afferent responses to proton changes not caused by muscle contraction, such as metabolic or respiratory acidosis and alkalosis.
In addition to the metabolites used in the present experiments, several other metabolites closely associated with muscle injury, but also shown to increase with muscle contraction, may enhance the activation of small-diameter-fiber afferents from muscle. These include bradykinin, potassium, and arachidonic acid metabolites such as prostaglandin E2 and various cytokines (Cui et al. 2007; Hoheisel et al. 2005; Kaufman and Rybicki 1987; Kaufman et al. 1983; Mense 1977; Mense and Schmidt 1974; Rotto and Kaufman 1988). Most of these substances activate muscle afferent fibers at relatively high concentrations, although the actual effective dose at the nerve endings may be in the physiological range. It is interesting that nonsteroidal antiinflammatory compounds that have been used to suggest prostaglandin involvement can directly block ASIC3 receptors (Lin et al. 2008; Voilley et al. 2001). Potassium has an effect on muscle afferents at relatively low concentrations. However, Kaufman and Rybicki (1987) suggested that K+ application produced rapidly adapting responses that would not be capable of encoding the sustained discharge observed with the increases in metabolites caused by muscle contraction. Although it is unknown whether these metabolites enhance responses of muscle afferents under normal conditions, it is likely that some or all of these agents may play a role in the enhancement of sensitivity of group III and group IV afferents under a variety of conditions such as muscle ischemia, traumatic muscle injury, and metabolic insults caused by toxins, infections, and disease. Interestingly, the direct effects of increased oxidative metabolism (low oxygen and/or high carbon dioxide levels) have not been shown to increase the responses of group III and group IV muscle afferents (Wenk and McCleskey 2006).
2)Our results suggest at least two populations of DRG neurons: one responding to higher metabolites, the other to lower metabolites
Previous reports have suggested the existence of at least two populations of metaboreceptive group III and group IV muscle afferents. One population should detect innocuous levels of metabolites and contribute to the sympathetic pressor response evoked by active muscle contraction, but should not contribute to muscle pain. The other population should detect noxious levels of metabolites and contribute to muscle pain (Kniffki et al. 1978, 1981; Mense 1993, 1996; Mense and Stahnke 1983). Both populations could contribute to sympathetic reflexes and to increasing perceptions of fatigue. Even one of the earliest descriptions of the exercise pressor reflex in human subjects indicated that increasing levels of metabolites evoked innocuous perceptions associated with the exercise pressor response preceding the perception of pain (Alam and Smirk 1937). These results suggest that a population of innocuous receptors distinct from nociceptors may exist that can detect increases in muscle-metabolite levels before painful levels are reached.
Our present investigation cannot determine what concentrations of metabolites are selective for pain as opposed to the exercise pressor response; indeed, there may be at least some overlap in effective concentrations. However, we did use the three most likely metabolites in combinations produced by contracting muscles that should span the range from resting values to the values observed with maximal muscle pain. Using this combination of three metabolites, we demonstrated at least two populations of DRG neurons innervating skeletal muscle in the mouse.
Low metabolite-responsive DRG neurons (46.5% of metabolite-responsive neurons).
One population of DRG neurons labeled from skeletal muscle was maximally activated at metabolite values of pH 7.0, lactate 15 mM, and ATP 1 μM. These metabolite values have been reported with heavy, but not necessarily painful muscle exercise (Gibala et al. 2002). In this population of DRG neurons, when higher levels of metabolites were applied, both the percentage of responding DRG neurons decreased and the magnitude of calcium responses decreased. With still higher levels of metabolites, the responses in this population plateaued or increased slightly. However, this increase never reached the maximum response observed at pH 7.0, lactate 15 mM, and ATP 1 μM.
Low metabolite-responsive DRG neurons were sensitive to resting, or lower than resting, values of metabolites (pH 7.4, lactate 1 mM, ATP 300 nM). The sensitivity to increased metabolites and the observed response profile seem to mirror the pressor response evoked by sustained muscle contraction. This sensitivity would also be adequate to mediate the sense of “tiredness” and/or “pressure” described by many subjects with the initiation and maintenance of sustained muscle contraction (Alam and Smirk 1937). Adreani et al. (1997) and Adreani and Kaufman (1998) observed that 16 of 19 group IV muscle afferents (in cats) responded to levels of exercise that were consistent with nonpainful conditions in human subjects. Interestingly, 9 of these 19 increased their response when the muscle was made ischemic, raising the metabolite levels to potentially painful levels. This could indicate that about half of the group IV neurons they recorded were low metabolite responsive, whereas the other half were high metabolite responsive, proportions consistent with our findings in mice (see also reviews by Kaufman and Hayes 2002; Kaufman et al. 2002).
High metabolite-responsive DRG neurons (53.5% of metabolite-responsive neurons).
A second population of DRG neurons labeled from skeletal muscle was maximally activated at metabolite values of pH 6.6, lactate 50 mM, and ATP 5 μM. The proportion of responding neurons increased steadily to the maximum value noted earlier (see Fig. 5). This population would seem well suited to detect large increases in metabolites between pH values of 7.0 and 6.6. These values have been associated with ischemic, contracting muscle that is often painful (Alam and Smirk 1937; Bangsbo et al. 1993, 2001; Li et al. 2003, 2005; Reeh and Kress 2001; Steinhagen et al. 1976). Our separation of these two populations of DRG neurons was empirical, based on our initial observations of responses of cultured neurons to increases in the three metabolites used in these experiments. Whether these two populations actually contribute differentially to different perceptions and/or differently to the exercise pressor reflex is unknown.
The present findings apply only to the chemical responsiveness of these afferent fibers. It is likely that at least some of the neurons in these populations will also have mechanical responsiveness as has been determined by previous investigators (Adreani and Kaufman 1998; Berberich et al. 1988; Haouzi et al. 1999; Kaufman et al. 1984; Mense and Meyer 1985). Any selective function of these afferents will depend on the specificity of the central connections they establish, which can be determined only in intact animals.
Both of the populations of DRG neurons described here (“low and high metabolite responders”), by virtue of their responsiveness to capsaicin and protons, could belong to the “class 8a” defined by Jiang et al. (2006) and Petruska et al. (2000b). These reports suggested that class 8a afferents innervate specific types of tissues, such as blood vessels, that are distributed throughout various organs and tissues of the body. Muscle-innervating afferents may be one type of class 8a afferents, since group III and group IV muscle afferents are most often associated with neurovascular bundles (Molliver et al. 2005). Further in vivo experiments are necessary to determine whether muscle afferents in situ respond to the metabolite combinations and utilize the same molecular receptors reported here.
3)Selective antagonists suggest the synergy of three molecular receptor types in mediating DRG responses to increasing metabolites (protons, lactic acid, and ATP)
ASIC antagonist: A-317567.
A-317567 at 100 μM, a potentially selective ASIC antagonist (Dube et al. 2005), blocked about 80% of DRG neuron responses to metabolites increased as high as pH 6.6, lactate 50 mM, and ATP 5 μM. At a concentration of 200 μM, A-317567 blocked all responses to metabolites; these same concentrations of A-317567 did not block DRG neuron capsaicin responses, KCl responses, or responses to 5 μM ATP applied to naïve DRG neurons. The blockade by A-317567 suggests that at least one of the subtypes of ASIC1–ASIC3 is necessary for all responses of DRG neurons to metabolites ≤pH 6.6, lactate 50 mM, and ATP 5 μM. However, because the amounts of A-317567 necessary to block all metabolite responses were quite high (almost tenfold the highest IC50 of the three ASICs), the present results cannot determine the most likely ASIC receptor(s) involved. Hayes et al. (2007) also recently provided evidence that ASICs on muscle sensory afferents play a key role in detecting metabolites generated by muscle contraction in cats, using the nonspecific antagonist amiloride.
Other investigators have also shown that ASIC receptors are likely to be involved in the responses of mouse and rat primary sensory neurons to increasing protons (Habelt et al. 2000; Krishtal 2003; Leffler et al. 2006; Lin et al. 2008; Price et al. 2001; Reeh and Kress 2001; Sluka et al. 2003; Waldmann and Lazdunski 1998; Wemmie et al. 2006). However, in most of these experiments, the pH values used to activate sensory neurons were lower than those found in living skeletal muscle. Several recent reports (Immke and McCleskey 2001; Naves and McCleskey 2005; Spelta et al. 2004; Sutherland et al. 2001; Yagi et al. 2006) have suggested that ASIC3 may be the molecular detector of pH in cardiac and skeletal muscle. When combined with lactate and ATP, these receptors in heterologous expression systems have a sustained response to lowering pH that mimics the sustained response of DRG neurons. However, other investigators (Benson et al. 2002; Hesselager et al. 2004) demonstrated that splice-variant heteromers of ASIC1 and ASIC2 can also respond to pH in a manner that is consistent with responses of DRG neurons. Benson et al. (2002) also showed that mice lacking any one of the ASIC receptor subtypes had DRG neurons that still responded to pH, although in a somewhat different fashion. Interestingly, using rats, Molliver et al. (2005) demonstrated that 51% of small DRG neurons (<25 μM), retrogradely labeled from muscle, expressed ASIC3 as determined by immunohistochemistry. This is very similar to the 44% of muscle-labeled neurons we found responsive to metabolites in mouse. Thus previous experiments combined with our data suggest that ASIC receptors are likely to be major mediators of responses to increased metabolites produced by contracting muscles.
P2X antagonists: TNP-ATP and PPADS.
Our experiments indicate that doses of TNP-ATP that are effective at P2X3 receptors (10 nM) may increase responses of DRG neurons to increasing metabolites. Doses of TNP-ATP effective at P2X5 receptors (1 and 10 μM) eliminated most responses to low metabolites, but had little effect on metabolite responses associated with pH ≥6.8. Doses of TNP-ATP and PPADS effective at all P2X receptors, including P2X4 (200–300 μM), eliminated nearly all responses to increasing metabolites (see Fig. 8). These data suggest that P2X3 receptors do not enhance the response of DRG neurons to increasing metabolites. However, P2X5 receptors may be necessary for responses to low levels of metabolites, and both P2X5 and P2X4 receptors may be necessary for responses to high levels of metabolites. These results confirm reports by others (Kindig et al. 2006, 2007) that PPADS can block most of the activation of muscle sensory afferents by metabolites produced by muscle contraction. Also consistent with the involvement of P2X4 and P2X5 receptors on DRG neurons, Kobayashi et al. (2005) indicated that P2X4 and P2X5 mRNAs are found in many DRG neurons.
Many other groups have demonstrated that ATP can directly activate sensory neurons. Much of this activation has been attributed to P2X3 and P2X2 receptors and their heteromers (see reviews by Khakh and North 2006; North 2004; Petruska et al. 2000a). These mechanisms may explain direct effects by sudden surges of ATP similar to the levels that tissue injury can cause.
Contrary to what might be expected, ATP concentrations in muscle interstitium are increased during muscle contractions (Li et al. 2003, 2005). The concentrations of ATP present at sensory receptor endings in muscle fascia, even at rest, should be sufficient to activate, and then desensitize, most P2X receptors on nerve endings within skeletal and cardiac muscles. In addition, P2X receptors have been reported to become less sensitive with the increasing proton concentration that occurs with muscle contraction (Gao et al. 2007). From this information, it would seem that most P2X receptors would not add to the generator potential of sensory neurons when metabolites increase with muscle exercise. Thus the direct detection of muscle metabolites by P2X receptors is problematic. In addition, it is problematic that in our experiments, both P2X antagonists and ASIC antagonists applied individually can block virtually all responses to physiological levels of metabolites.
These problems can be resolved by recent experiments demonstrating that ATP is a potent modulator of ASIC3, dramatically enhancing responsiveness to pH in the normal physiological range (Naves and McCleskey 2005; Spelta et al. 2004). This group has also reported cotransfection experiments that demonstrated that only P2X4 and P2X5 (Spelta et al. 2004; E. W. McCleskey, personal communication) are effective in causing this enhancement of ASIC3 responsiveness. This modulation requires ligand binding, but does not require ionic current flow. The modulation is clearly synergistic, in that the currents activated by ASICs and P2X channels individually are far less than the currents activated by ligand binding to both types of channels when they are coexpressed. Precisely how this interaction occurs is unknown. It is thus possible that both ASIC and P2X receptors are necessary to detect normally occurring muscle metabolites in the physiological range.
TRPV1 antagonists.
The TRPV1 antagonist LJO-328 significantly reduced the response of DRG neurons to increasing metabolites. Even though experiments reported here showed that metabolite-responding neurons were more likely to respond to capsaicin (indicating that they very likely had TRPV1 receptors) than nonmetabolite-responding neurons, we did not expect this result at metabolite values associated with pH values from 7.4 to 6.6. This is because previous investigations in mouse have shown that pH values <6.0 are necessary to evoke substantial TRPV1-mediated current from mouse DRG neurons (Leffler et al. 2006) and from heterologous expression of TRPV1 (Caterina et al. 1997; Jordt et al. 2000; Tominaga et al. 1998). In addition, our finding that the selective ASIC antagonist A-317567 blocked all metabolite responsiveness also suggested that ASICs, not TRPV1, contributed to the response to all metabolite levels used in the experiments reported here.
Our data clearly indicate, however, that antagonizing TRPV1 can reduce the responsiveness of DRG neurons to metabolites. Thus either TRPV1 activation sums with activation of ASICs, or TRPV1 activation can enhance the responsiveness of ASICs similar to the way that P2X receptors enhance responses. Gao et al. (2006, 2007) and Li et al. (2004) have also suggested that TRPV1 and ASIC receptors (but not P2X receptors) interact in a way that is not yet understood to activate the afferent neurons responsible for the exercise pressor reflex in rats as well.
Many other investigators have also proposed that TRPV1 receptors could play a role in muscle pain (Hoheisel et al. 2004; Kaufman et al. 1982, 1983; Rau et al. 2007). Interestingly, Kindig et al. (2005) showed that, although capsaicin likely activates the group III and/or group IV afferents that cause a pressor response in cats, blocking these receptors with iodoresinaferatoxin did not block the exercise pressor response evoked by muscle contraction. Kindig et al.'s findings might be explained if the population of muscle-innervating neurons that responded to low levels of metabolites contributed most of the exercise pressor reflex because much of the response of this population to metabolites was maintained when TRPV1 was blocked with the LJO compound. Alternatively, different TRPV1 antagonists may have other effects on the metabolite responsiveness, such that iodoresinaferatoxin may not block metabolite responses, but may still block capsaicin responses.
Thus TRPV1 receptors, in addition to ASIC and P2X receptors, contribute some of the responsiveness of DRG neurons to physiological levels of metabolites, at least in the mouse.
Although the combination of these three molecular receptor types (ASICs, P2Xs, and TRPs) can respond in a fashion that is adequate to explain the normal responses of muscle sensory afferents to the metabolites produced by muscle contraction, it is certain that other molecular receptors on the primary afferent endings also participate in modulating these responses. Thus receptors for various cytokines and arachidonic acid metabolites are likely to sensitize muscle afferent endings under a variety of conditions and may be responsible for enhanced perceptions of fatigue and pain (Hayes et al. 2006; Rotto et al. 1990a,b). In addition, recently discovered G-protein–coupled receptors such as OGR1 (Huang et al. 2007) and proton-inhibiting potassium channels such as TASks (Cooper et al. 2004; Rau et al. 2006) may play a role in detecting and signaling metabolite increases in sensory neuronal endings.
What sensory perceptions are mediated by the responses to metabolites of primary afferents innervating skeletal muscle?
The population of afferents responsive to the high metabolite levels described here could mediate the sensory experience of muscle pain caused by sustained and/or ischemic muscle contraction (Arendt-Nielsen et al. 2003; Graven-Nielsen et al. 2004). However, the sensory experience mediated by DRG neurons responding to low metabolite levels is more puzzling. Alam and Smirk (1937) indicated that muscle contractions, with the circulation occluded, induced a prepain sensory experience of “tiredness or heaviness not causing appreciable discomfort” that coincided with an increase in the mean arterial pressure. This prepain perception occurred well before the onset of muscle ache and pain. Recent reports suggest that this prepain “pressure” sensation is not mediated by cutaneous afferents, but by small-diameter muscle afferents (Graven-Nielsen et al. 2004). Very little is known about the nonpainful sensation that can emanate from muscle and may be associated with, if not mediated by, the same afferent neurons that mediate the exercise pressor reflex.
We suggest that the sensation evoked by nonpainful metabolite accumulation in muscle may be that of muscle “fatigue.” This would be only loosely related to actual “muscle fatigue,” which is the failure of muscle contraction. Several investigators have suggested that small-diameter afferent fibers innervating muscle are at least partially responsible for the inhibition of motor command that causes true muscle fatigue under many conditions (see review by Garland and Kaufman 1995). We suggest that these same afferent fibers engage sensory systems in the brain analogous to pain pathways and are responsible for the perceptual experience of muscle “pressure and tiredness” (Alam and Smirk 1937) that accompany the accumulation of metabolites in muscle. This sensory experience could be the familiar perception of “muscle fatigue.”
Conclusion
The data presented here demonstrate that physiological levels of three metabolites produced by contracting muscles—protons, ATP, and lactate—act together to activate a significant population of DRG neurons that innervate skeletal muscle. No individual or pair of these metabolites has the effectiveness of the proper combination of all three metabolites.
The DRG neurons responding to metabolites are heterogeneous, consisting of at least two populations. One population responds to the levels of metabolites produced by nonpainful muscle contractions and may contribute significantly to the exercise pressor reflex and, perhaps, to the sensory experience of “fatigue.” The other population responds to levels of metabolites that are likely to be associated with stronger, possibly painful muscle contractions, and could contribute to acute muscle pain produced by ischemic muscle contraction or by muscle inflammation and injury.
At least three molecular receptors appear to work in synergy to detect the full range of metabolites produced by muscle contraction and muscle injury. These receptors may be an ASIC receptor (possibly ASIC3), a P2X receptor (possibly P2X5 for moderate metabolite levels and P2X4 for high levels of metabolites), and TRPV1. Other metabolites and receptors may also play a role in detection of metabolites in normal muscle contractions; still others may play a role in the enhancement of responses that can occur following muscle injury, inflammation, and disease.
GRANTS
This work was supported by a Catalyst Award from the Utah Health Sciences Center, a Synergy Grant from the Vice President's office of the University of Utah, and a research grant from the University of Utah, Department of Anesthesiology, all to A. R. Light; and Korea Science and Engineering Foundation Grant R11-2007-107-02001-0 to J. Lee.
Acknowledgments
We thank Dr. Chris Rodesch (Director of the University of Utah Health Sciences Center Imaging Core) for support for calcium imaging and analysis; K. Fitchen, B. Jensen, T. Michael, and C. Peterson for technical assistance; the Undergraduate Research Opportunities Program at University of Utah; and W. Birdsong (from E. McCleskey's lab), R. Radhakrishnan, and R. Chapman for reading previous versions of this manuscript and making comments for improvement.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Adreani et al. 1997.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol : 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- Adreani and Kaufman 1998.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol : 1827–1833, 1998. [DOI] [PubMed] [Google Scholar]
- Alam and Smirk 1937.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol : 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen et al. 2003.Arendt-Nielsen L, Mense S, Graven-Nielsen T. Assessment of muscle pain and hyperalgesia. Experimental and clinical findings. Schmerz : 445–449, 2003. [DOI] [PubMed] [Google Scholar]
- Bangsbo et al. 1993.Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol : 115–133, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo et al. 2001.Bangsbo J, Krustrup P, Gonzalez-Alonso J, Saltin B. ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab : E956–E964, 2001. [DOI] [PubMed] [Google Scholar]
- Bangsbo et al. 1996.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol : 587–596, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson et al. 2002.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA : 2338–2343, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich et al. 1988.Berberich P, Hoheisel U, Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol : 1395–1409, 1988. [DOI] [PubMed] [Google Scholar]
- Bove and Light 1995.Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. J Neurophysiol : 1752–1762, 1995. [DOI] [PubMed] [Google Scholar]
- Burnstock and Wood 1996.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol : 526–532, 1996. [DOI] [PubMed] [Google Scholar]
- Caterina et al. 1997.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature : 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- Chagot et al. 2005.Chagot B, Escoubas P, Diochot S, Bernard C, Lazdunski M, Darbon H. Solution structure of APETx2, a specific peptide inhibitor of ASIC3 proton-gated channels. Protein Sci : 2003–2010, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. 1995.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature : 428–431, 1995. [DOI] [PubMed] [Google Scholar]
- Connor et al. 2005.Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat (Abstract). Mol Pain : 31, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook et al. 1997.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature : 505–508, 1997. [DOI] [PubMed] [Google Scholar]
- Cooper et al. 2004.Cooper BY, Johnson RD, Rau KK. Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience : 209–224, 2004. [DOI] [PubMed] [Google Scholar]
- Coote et al. 1971.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol : 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll et al. 2004.Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. Cloning and pharmacological characterization of mouse TRPV1. Neurosci Lett : 55–60, 2004. [DOI] [PubMed] [Google Scholar]
- Cui et al. 2007.Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman CS, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol : H1861–H1868, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darques et al. 1998.Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett : 109–112, 1998. [DOI] [PubMed] [Google Scholar]
- Diehl et al. 1993.Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res : 431–440, 1993. [DOI] [PubMed] [Google Scholar]
- Diochot et al. 2004.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J : 1516–1525, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube et al. 2005.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain : 88–96, 2005. [DOI] [PubMed] [Google Scholar]
- Gao et al. 2006.Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J Appl Physiol : 421–426, 2006. [DOI] [PubMed] [Google Scholar]
- Gao et al. 2007.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol : 2288–2293, 2007. [DOI] [PubMed] [Google Scholar]
- Garland and Kaufman 1995.Garland SJ, Kaufman MP. Role of muscle afferents in the inhibition of motoneurons during fatigue. Adv Exp Med Biol : 271–278, 1995. [DOI] [PubMed] [Google Scholar]
- Gever et al. 2006.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pfluegers Arch : 513–537, 2006. [DOI] [PubMed] [Google Scholar]
- Gibala et al. 2002.Gibala MJ, Gonzalez-Alonso J, Saltin B. Dissociation between muscle tricarboxylic acid cycle pool size and aerobic energy provision during prolonged exercise in humans. J Physiol : 705–713, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham et al. 1986.Graham R, Jammes Y, Delpierre S, Grimaud C, Roussos C. The effects of ischemia, lactic acid and hypertonic sodium chloride on phrenic afferent discharge during spontaneous diaphragmatic contraction. Neurosci Lett : 257–262, 1986. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen and Mense 2001.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain : 2–10, 2001. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen et al. 2004.Graven-Nielsen T, Mense S, Arendt-Nielsen L. Painful and non-painful pressure sensations from human skeletal muscle. Exp Brain Res : 273–283, 2004. [DOI] [PubMed] [Google Scholar]
- Habelt et al. 2000.Habelt C, Kessler F, Distler C, Kress M, Reeh PW. Interactions of inflammatory mediators and low pH not influenced by capsazepine in rat cutaneous nociceptors. Neuroreport : 973–976, 2000. [DOI] [PubMed] [Google Scholar]
- Hanna and Kaufman 2004.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol : 1166–1169, 2004. [DOI] [PubMed] [Google Scholar]
- Haouzi et al. 1999.Haouzi P, Hill JM, Lewis BK, Kaufman MP. Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol : 545–553, 1999. [DOI] [PubMed] [Google Scholar]
- Hayes et al. 2006.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol : H2239–H2246, 2006. [DOI] [PubMed] [Google Scholar]
- Hayes et al. 2007.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol : 1271–1282, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten et al. 1998.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation : 6–8, 1998. [DOI] [PubMed] [Google Scholar]
- Hesselager et al. 2004.Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem : 11006–11015, 2004. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler et al. 2007.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci : 2435–2443, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel et al. 2004.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain : 149–157, 2004. [DOI] [PubMed] [Google Scholar]
- Hoheisel et al. 2005.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain : 168–176, 2005. [DOI] [PubMed] [Google Scholar]
- Hu and Li 1996.Hu HZ, Li ZW. Substance P potentiates ATP-activated currents in rat primary sensory neurons. Brain Res : 163–168, 1996. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2007.Huang CW, Tzeng JN, Chen YJ, Tsai WF, Chen CC, Sun WH. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci : 195–210, 2007. [DOI] [PubMed] [Google Scholar]
- Immke and McCleskey 2001.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci : 869–870, 2001. [DOI] [PubMed] [Google Scholar]
- Immke and McCleskey 2003.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron : 75–84, 2003. [DOI] [PubMed] [Google Scholar]
- Jahr and Jessell 1983.Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurons. Nature : 730–733, 1983. [DOI] [PubMed] [Google Scholar]
- Jiang et al. 2006.Jiang N, Rau KK, Johnson RD, Cooper BY. Proton sensitivity Ca2+ permeability and molecular basis of acid-sensing ion channels expressed in glabrous and hairy skin afferents. J Neurophysiol : 2466–2478, 2006. [DOI] [PubMed] [Google Scholar]
- Jones et al. 2000.Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP. Functional characterization of the P2X(4) receptor orthologues. Br J Pharmacol : 388–394, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt et al. 2000.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA : 8134–8139, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman and Hayes 2002.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res : 429–439, 2002. [DOI] [PubMed] [Google Scholar]
- Kaufman et al. 2002.Kaufman MP, Hayes SG, Adreani CM, Pickar JG. Discharge properties of group III and IV muscle afferents. Adv Exp Med Biol : 25–32, 2002. [DOI] [PubMed] [Google Scholar]
- Kaufman et al. 1982.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res : 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- Kaufman et al. 1983.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol : 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- Kaufman and Rybicki 1987.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res : I60–I65, 1987. [PubMed] [Google Scholar]
- Kaufman et al. 1984.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res : 663–668, 1984. [DOI] [PubMed] [Google Scholar]
- Khakh et al. 2001.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev : 107–118, 2001. [PubMed] [Google Scholar]
- Khakh and North 2006.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature : 527–532, 2006. [DOI] [PubMed] [Google Scholar]
- Kindig et al. 2006.Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol : H1214–H1219, 2006. [DOI] [PubMed] [Google Scholar]
- Kindig et al. 2007.Kindig AE, Hayes SG, Kaufman MP. Purinergic 2 receptor blockade prevents the responses of group IV afferents to post-contraction circulatory occlusion. J Physiol : 301–308, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig et al. 2005.Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol : H1867–H1873, 2005. [DOI] [PubMed] [Google Scholar]
- Kniffki et al. 1978.Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res : 511–522, 1978. [DOI] [PubMed] [Google Scholar]
- Kniffki et al. 1981.Kniffki KD, Mense S, Schmidt RF. Muscle receptors with fine afferent fibers which may evoke circulatory reflexes. Circ Res : I25–I31, 1981. [PubMed] [Google Scholar]
- Kobayashi et al. 2005.Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol : 377–390, 2005. [DOI] [PubMed] [Google Scholar]
- Krishtal 2003.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci : 477–483, 2003. [DOI] [PubMed] [Google Scholar]
- Lawson 1996.Lawson SN. Peptides and cutaneous polymodal nociceptor neurones. Prog Brain Res : 369–385, 1996. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2003.Lee J, Lee J, Kang M, Shin M, Kim JM, Kang SU, Lim JO, Choi HK, Suh YG, Park HG, Oh U, Kim HD, Park YH, Ha HJ, Kim YH, Toth A, Wang Y, Tran R, Pearce LV, Lundberg DJ, Blumberg PM. N-(3-Acycloxy-2-benzylpropyl)-N′-[4-(methylsulfonylamino)benzyl]thiourea analogues: novel potent and high affinity antagonists and partial antagonists of the vanilloid receptor. J Med Chem : 3116–3126, 2003. [DOI] [PubMed] [Google Scholar]
- Leffler et al. 2006.Leffler A, Monter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICs) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience : 699–709, 2006. [DOI] [PubMed] [Google Scholar]
- Li et al. 2003.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol : 577–583, 2003. [DOI] [PubMed] [Google Scholar]
- Li et al. 2005.Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation : 2748–2751, 2005. [DOI] [PubMed] [Google Scholar]
- Li et al. 2004.Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol : 1709–1714, 2004. [DOI] [PubMed] [Google Scholar]
- Li and Sinoway 2002.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol : H2636–H2643, 2002. [DOI] [PubMed] [Google Scholar]
- Lin et al. 2008.Lin YW, Min MY, Lin CC, Chen WN, Wu WL, Yu HM, Chen CC. Identification and characterization of a subset of mouse sensory neurons that express acid-sensing ion channel 3. Neuroscience : 544–557, 2008. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2007.Liu M, Walter GA, Pathare NC, Forster RE, Vandenborne K. A quantitative study of bioenergetics in skeletal muscle lacking carbonic anhydrase III using 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA : 371–376, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. 2006.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol : 169–190, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey and Mitchell 1972.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol : 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense 1977.Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol : 75–88, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense 1993.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain : 241–289, 1993. [DOI] [PubMed] [Google Scholar]
- Mense 1996.Mense S. Group III and IV receptors in skeletal muscle: are they specific or polymodal? Prog Brain Res : 83–100, 1996. [DOI] [PubMed] [Google Scholar]
- Mense and Meyer 1985.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol : 403–417, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense and Schmidt 1974.Mense S, Schmidt RF. Activation of group IV afferent units from muscle by algesic agents. Brain Res : 305–310, 1974. [DOI] [PubMed] [Google Scholar]
- Mense and Stahnke 1983.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol : 383–397, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr et al. 2007.Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J. Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol : R1594–R1602, 2007. [DOI] [PubMed] [Google Scholar]
- Molliver et al. 2005.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons (Abstract). Mol Pain : 35, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns et al. 2007.Munns C, Alqatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium : 331–342, 2007. [DOI] [PubMed] [Google Scholar]
- Naves and McCleskey 2005.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res : 1561–1569, 2005. [DOI] [PubMed] [Google Scholar]
- North 2004.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol : 301–308, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. 1999.Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. J Physiol : 857–866, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska et al. 2000a.Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat : 141–162, 2000a. [DOI] [PubMed] [Google Scholar]
- Petruska and Mendell 2004.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett : 168–171, 2004. [DOI] [PubMed] [Google Scholar]
- Petruska et al. 2000b.Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol : 2365–2379, 2000b. [DOI] [PubMed] [Google Scholar]
- Price et al. 2001.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron : 1071–1083, 2001. [DOI] [PubMed] [Google Scholar]
- Rang et al. 1991.Rang HP, Bevan S, Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull : 534–548, 1991. [DOI] [PubMed] [Google Scholar]
- Rau et al. 2005.Rau KK, Caudle RM, Cooper BY, Johnson RD. Diverse immunocytochemical expression of opioid receptors in electrophysiologically defined cells of rat dorsal root ganglia. J Chem Neuroanat : 255–264, 2005. [DOI] [PubMed] [Google Scholar]
- Rau et al. 2006.Rau KK, Cooper BY, Johnson RD. Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neuroscience : 955–963, 2006. [DOI] [PubMed] [Google Scholar]
- Rau et al. 2007.Rau KK, Jiang N, Johnson RD, Cooper BY. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol : 2651–2662, 2007. [DOI] [PubMed] [Google Scholar]
- Reeh and Kress 2001.Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol : 45–51, 2001. [DOI] [PubMed] [Google Scholar]
- Reilly et al. 2005.Reilly CA, Johansen ME, Lanza DL, Lee J, Lim JO, Yost GS. Calcium-dependent and -independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J Biochem Mol Toxicol : 266–275, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinohl et al. 2003.Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett : 25–28, 2003. [DOI] [PubMed] [Google Scholar]
- Rotto et al. 1990a.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol : H745–H750, 1990a. [DOI] [PubMed] [Google Scholar]
- Rotto and Kaufman 1988.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol : 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- Rotto et al. 1990b.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol : 861–867, 1990b. [DOI] [PubMed] [Google Scholar]
- Rybicki et al. 1985.Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis muscle interstitial potassium concentrations stimulate group III and IV afferents. J Appl Physiol : 936–941, 1985. [DOI] [PubMed] [Google Scholar]
- Sinoway et al. 1989.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol : 429–436, 1989. [DOI] [PubMed] [Google Scholar]
- Sinoway et al. 1993.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol : 1053–1059, 1993. [DOI] [PubMed] [Google Scholar]
- Sluka et al. 2003.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain : 229–239, 2003. [DOI] [PubMed] [Google Scholar]
- Sluka et al. 2007.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain : 102–112, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al. 2004.Smith MP, Beacham D, Ensor E, Koltzenburg M. Cold-sensitive, menthol-insensitive neurons in the murine sympathetic nervous system. Neuroreport : 1399–1403, 2004. [DOI] [PubMed] [Google Scholar]
- Spelta et al. 2004.Spelta V, Fierro L, Naves LA, McCleskey EW. Extracellular ATP enhances ASIC3-like current in ischemia sensing neurons through an electrically quiet ion channel. Program #859.4. 2004 Abstract Viewer and Itinerary Planner. Washington, DC: Society for Neuroscience, 2004. Online.
- Steinhagen et al. 1976.Steinhagen C, Hirche HJ, Nestle HW, Bovenkamp U, Hosselmann I. The interstitial pH of the working gastrocnemius muscle of the dog. Pfluegers Arch : 151–156, 1976. [DOI] [PubMed] [Google Scholar]
- Street et al. 2001.Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol : 993–998, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland et al. 2001.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA : 711–716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm and Baum 1987.Thimm F, Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscle. Pfluegers Arch : 143–152, 1987. [DOI] [PubMed] [Google Scholar]
- Tominaga et al. 1998.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron : 531–543, 1998. [DOI] [PubMed] [Google Scholar]
- Voilley et al. 2001.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci : 8026–8033, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann and Lazdunski 1998.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol : 418–424, 1998. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2002.Wang Y, Szabo T, Welter JD, Toth A, Tran R, Lee J, Kang SU, Suh YG, Blumberg PM, Lee J. High affinity antagonists of the vanilloid receptor. Mol Pharmacol : 947–956, 2002. [DOI] [PubMed] [Google Scholar]
- Wemmie et al. 2006.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci : 578–586, 2006. [DOI] [PubMed] [Google Scholar]
- Wenk and McCleskey 2007.Wenk HN, McCleskey EW. A novel mouse skeletal muscle-nerve preparation and in vitro model of ischemia. J Neurosci Methods : 244–251, 2007. [DOI] [PubMed] [Google Scholar]
- Wildman et al. 2002.Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, King BF. Sensitization by extracellular Ca(2+) of rat P2X(5) receptor and its pharmacological properties compared with rat P2X(1). Mol Pharmacol : 957–966, 2002. [DOI] [PubMed] [Google Scholar]
- Xie et al. 2003.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol : 2459–2465, 2003. [DOI] [PubMed] [Google Scholar]
- Yagi et al. 2006.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res : 501–509, 2006. [DOI] [PubMed] [Google Scholar]
- Zimmermann 1994.Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci : 420–426, 1994. [DOI] [PubMed] [Google Scholar]