Abstract
Background
We tested whether genital herpes simplex virus (HSV) shedding is an appropriate surrogate outcome for the clinical outcome of genital herpes lesions in studies of HSV-2 antiviral interventions.
Methods
We analyzed prospective data from natural history studies and clinical trials of antiviral agents for HSV-2 in which HSV-2–seropositive participants provided self-collected anogenital swab specimens daily over ≥25 days for HSV DNA quantitation by polymerase chain reaction (PCR). Genital recurrences were self-reported.
Results
Among 674 participants, genital HSV shedding was detected on 17% of days, and genital lesions were reported on 10% of days. Within the same session, HSV shedding rates were strongly correlated with lesion rates (ρ = 0.61, P < .0001). The relative reduction in the recurrence rate was 72% (P = .041) for recipients of the antiviral agent pritelivir as compared to recipients of placebo, but it decreased to 21% (P = .75) after adjustment for HSV shedding rate. When evaluating valacyclovir and acyclovir, adjustment for the HSV shedding rate also led to a reduced association of these antivirals with the recurrence rate. Overall, 40%–82% of the antiviral effect on recurrences was explained by its effect on HSV shedding.
Conclusion
HSV genital shedding measured by PCR analysis in swab specimens self-collected daily is an appropriate surrogate outcome for genital herpes lesions because it is in the causal pathway to recurrences.
Keywords: Genital herpes, HSV, recurrence rate, lesion rate, surrogate outcome, shedding rate, HSV shedding, antivirals
Genital herpes recurrence frequency is the standard outcome for demonstrating efficacy of antiviral therapy against herpes simplex virus (HSV). In early phase studies, HSV genital shedding frequency measured by polymerase chain reaction analysis of swab specimens collected daily can serve as a more powerful surrogate outcome for recurrence frequency.
(See the Editorial Commentary by Fife on pages 1689–90.)
Herpes simplex virus type 2 (HSV-2) is estimated to infect 417 million people globally, and 19.2 million new infections occur annually [1]. While most people with HSV-2 infection do not report genital symptoms attributed to the infection, up to 25% experience recurrent genital ulcerations [2, 3]. Despite a lack of recognized symptoms in most infected persons, HSV-2 is the most common cause of genital ulcers worldwide [4]. Currently available therapies that shorten the lesion duration and decrease viral shedding have been on the market for >20 years [5], although novel therapeutic agents, as well as therapeutic vaccines, are in development [6].
Studies that led to the approval of established drugs used clinical end points, such as duration of recurrences or recurrence frequency, both for early assessment of efficacy and in pivotal phase 3 studies [7–10]. However, the use of genital herpes lesions as an end point in designing proof-of-concept studies and dose-ranging studies in early development of anti-HSV therapeutic agents is challenging because genital herpes lesions are highly variable in frequency and duration [11]. Furthermore, the average frequency of recurrences is low, with most symptomatic persons having a median number of 4 recurrences per year [3]. While evaluation of a clinically meaningful end point such as lesions or recurrence rate is currently required prior to approval of new HSV interventions (ie, vaccines and antivirals), the use of HSV detection at mucosal sites, or viral shedding, could be instead used as the surrogate outcome, particularly for early phase evaluations.
A surrogate outcome is a biological or early end point used to substitute for a clinical or late-occurring end point. The surrogate is intended to predict clinical benefit from a therapeutic intervention [12, 13]. Several frameworks have been used to describe the validation of a surrogate [14–19]. The strict, statistical definition of a surrogate requires that the effect of an intervention on the desired outcome be fully explained through the surrogate [20]. However, others have examined continuous rather than absolute measures and sought to determine the degree of surrogacy afforded by particular measures, including partial surrogates and estimation of the proportion of the treatment effect explained by the surrogate [21, 22]. The International Conference of Harmonization Guidelines on Statistical Principles for Clinical Trials has provided a list of characteristics of an effective surrogate, which include (1) biological plausibility, (2) a strong correlation with the clinically meaningful end point, and (3) explanation of the effect of the intervention on the clinical outcome largely through its impact on the surrogate [17, 23–26].
In this article, we evaluate the usefulness of HSV shedding as a surrogate outcome for genital herpes recurrences, the clinical outcomes of interest for use in early phase studies, by examining the following: the association of shedding with clinical outcomes, the consistency of HSV shedding, and whether shedding is in the causal pathway to lesions and recurrences.
METHODS
Study Population and Procedures
Data for these analyses were drawn from 3 cohorts in which participants obtained swab specimens of genital secretions daily. Cohort 1 includes healthy adults followed prospectively for evaluation of the natural history of genital herpes at the University of Washington Virology Research Clinic (Seattle, WA) from 1990 to 2014. We included participants who were HSV-2 seropositive (with or without HSV-1 antibody), were human immunodeficiency virus (HIV) seronegative, had HSV-2 for >1 year, did not use antivirals for at least 7 days prior to study entry, and provided anogenital swab specimens and recorded symptoms in a diary daily for at least 25 days. For studies with >1 swab specimen collected during a day, only the first morning swab specimen was retained. A subset of participants who had >1 genital swabbing session <2 years apart was used to assess the consistency of shedding over time. Because the duration of the swabbing period may influence the correlation between shedding periods and because follow-up varied greatly in this cohort, swabbing and diary reporting during only the first 30 days were retained from each session [27].
Cohort 2 included participants in a proof-of-concept, randomized, parallel-group, double-blind trial of pritelivir, a helicase-primase inhibitor, in healthy persons with HSV-2 infection [28]. Eligible adults were randomly assigned to one of 5 groups: oral pritelivir (5 mg, 25 mg, or 75 mg daily or 400 mg weekly) or placebo administered for 28 days. For this study, we compared the placebo group to participants receiving 75 mg daily, the most effective dose.
Cohort 3 included participants in a randomized, double-blinded, placebo-controlled, 3-period, crossover study of the effect of acyclovir and valacyclovir on genital HSV-2 shedding and lesions [29]. Participants received oral acyclovir 400 mg twice a day, valacyclovir 500 mg twice a day, or placebo twice a day, in random order. Each treatment arm lasted 7 weeks, with a 1-week washout period between arms [29]. All available data were used from cohorts 2 and 3.
The University of Washington Human Subjects Review Committee approved all study protocols, and all participants provided written informed consents.
Demographic and clinical data were collected on standardized forms. Participants were counseled on the clinical signs and symptoms of genital herpes and kept a diary of genital lesions. Participants were taught genital self-examination techniques to detect lesions and to obtain genital swab specimens for HSV detection, as described elsewhere [30, 31]. If a lesion was present, participants collected a separate lesion swab specimen. Swabs were placed in polymerase chain reaction (PCR) transport medium and refrigerated until the subsequent clinic visit.
Laboratory Methods
Blood samples were tested for HSV-1 and HSV-2 antibodies, using the University of Washington Western blot [32]. Swab samples were evaluated for HSV DNA by a validated, quantitative, real-time, fluorescence-based PCR assay [33]. Samples with ≥150 copies/mL of HSV DNA were considered positive [34].
Definitions and Statistical Methods
Shedding was defined by the detection of HSV from the genital area. The shedding rate was calculated as the number of days on which HSV was detected by PCR in specimens from the genital site divided by the total number of days with PCR results. An episode of shedding was defined as 1 or more consecutive days on which HSV was detected, beginning and ending with 2 days on which HSV PCR results were negative. The lesion rate was defined as the number of days on which lesions were detected in the anogenital area divided by the number of days on which diary entries were recorded. Recurrence was defined as a genital lesion episode (ie, 1 or more consecutive days on which an HSV genital lesion was noted by the study participant or study clinician), and the recurrence rate was defined as the number of recurrences divided by the number of days on which diary entries were recorded. Associations between shedding rates and lesion rates were determined using Pearson correlation coefficients.
For cohorts 2 and 3, Poisson regression with a robust error variance was used to determine the relative risk of developing lesions during treatment, compared with that during placebo receipt, with adjustment only for the predetermined stratification variables sex and study site. We used both lesion frequency and recurrence rate as outcomes in separate models. Next, we compared those findings to a similar model in which we included shedding rate as an additional predictor of lesion rate and recurrence rate. The extent to which treatment continues to affect the outcome in the adjusted model demonstrates the extent to which its influence is independent of shedding. The difference in the predictive ability of treatment between these 2 models was used to compute the proportion of the treatment effect (PTE) explained by shedding.
The PTE of antivirals on the genital lesion rate and recurrence rate explained by genital HSV shedding was estimated using the following rationale proposed by Freedman et al [21]: , where βT is the estimated unadjusted treatment effect or the coefficient of treatment in the model prior to including shedding as a predictor variable. βTA is the estimated treatment effect or coefficient of treatment after adjustment for the surrogate outcome, which in this case is HSV shedding. Of note, the coefficients (βs) used in computing PTE are derived from log-linear models of outcome rates, whereas the relative risks have been exponentiated (eβ) using the model coefficients (βs), so the PTE does not correspond exactly to a reduction in relative risk.
Poisson regression was also used to assess the impact of reported recurrences in the previous year on subsequently observed shedding rates. Statistical analyses were performed with SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Cohort 1: Characteristics of Natural History Study Participants
Six hundred and seventy-four persons contributed 764 sessions for this analysis (Table 1). The median age was 39 years (range, 19–76 years); 413 (61%) were women. Most participants (78%) were white. Three hundred and sixty-two persons (54%) were HSV-2 seropositive only, whereas 312 (46%) were both HSV-1 and HSV-2 seropositive (Table 1). Among 674 participants with a history of primary infection, the median time since acquisition at the first session was 10 years (range, 1–44 years). The median number of reported recurrences in the year preceding enrollment was 3 (range, 0–28 recurrences). Overall, 19962 swab specimens were included in the analysis. The subset of 90 participants with 2 swabbing sessions within 2 years had baseline characteristics similar to those of the entire cohort (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | Cohort 1: Natural History (n = 674) |
Multiple Sessions <2 y Apart (n = 90) |
Cohort 2: Pritelivir Triala Participants (n = 59) |
Cohort 3: ACV/VAL Trial Participants (n = 69) |
|---|---|---|---|---|
| Age, y | 39 (19–76) | 45 (21–66) | 42 (20–67) | 32 (21–67) |
| Male sex | 261 (39) | 38 (42) | 18 (31) | 27 (39) |
| Race | ||||
| White | 527 (78) | 72 (80) | 41 (69) | 66 (96) |
| Other | 112 (17) | 18 (20) | 18 (31) | 3 (4) |
| Not provided | 35 (5) | 0 (0) | 0 (0) | 0 (0) |
| Sexual preference | ||||
| Heterosexual | 442 (66) | 50 (56) | Unknown | Unknown |
| MSM or WSW | 118 (18) | 23 (25) | Unknown | Unknown |
| Not provided | 114 (17) | 17 (19) | Unknown | Unknown |
| HSV antibody status | ||||
| HSV-2 only | 362 (54) | 37 (41) | 35 (59) | 39 (57) |
| HSV-1 and 2 | 312 (46) | 53 (59) | 24 (41) | 30 (43) |
| Time since acquisition | ||||
| <1 year | 0 (0) | 0 (0) | 0 (0) | 29 (42) |
| ≥1 year | 507 (75) | 70 (78) | 56 (95) | 39 (57) |
| Unknown | 167 (25) | 20 (22) | 3 (5) | 1 (1) |
| No. of years since acquisitionb | 10 (1–44) | 12 (1–35) | 15 (1–40) | 2 (0–21) |
| No. of annual recurrences | 3 (0–28) | 5 (0–12) | Unknown | 6 (0–36) |
| No. of swabbing daysc | 30 (25–30) | 30 (25–30) | 28 (8–29) | 56 (39–63) |
| No. of PCR-positive swabs/no. of swab specimens (%)c | 3306/19962 (16.6) | 453/2627 (17.2) | 138/833 (16.6) | 1315/3272 (40.2) |
| No. of days with lesions/ no. of days with diary entries (%)c | 1985/19962 (9.9) | 359/2627 (13.7) | 75/836 (9.0) | 758/3430 (22.1) |
Data are no. (%) of participants or median value (range), unless otherwise indicated.
Abbreviations: ACV, acyclovir; HSV, herpes simplex virus; MSM, men who have sex with men; PCR, polymerase chain reaction; VAL, valacyclovir; WSW, women who have sex with women.
aWe included 2 study arms: placebo and 75 mg daily.
bData were missing for some participants.
cDuring first session or while receiving placebo.
Association Between Recurrences, Lesion Rates, and Genital HSV Shedding
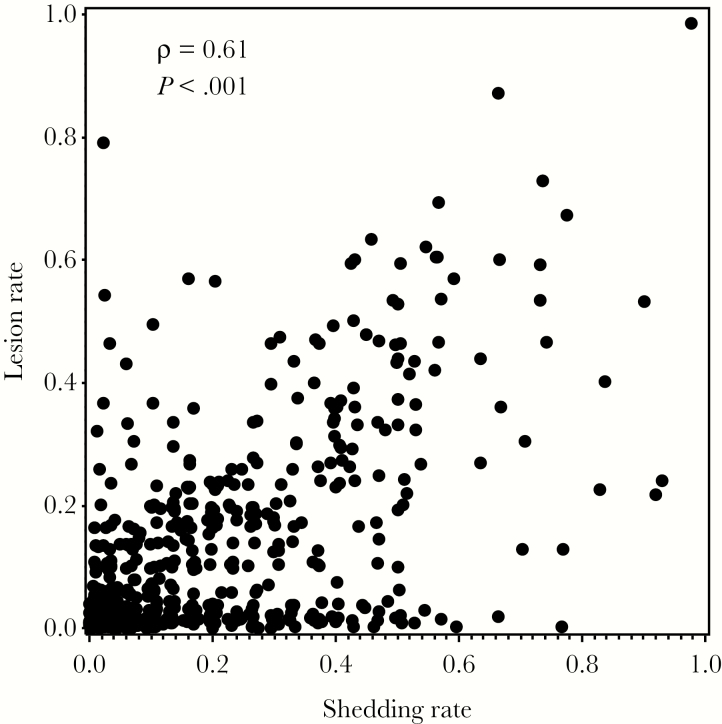
In the natural history cohort, HSV was detected on 17% of days (3306 of 19962 swabs) and in 69% of the participants. Within the same swabbing session, HSV shedding rates were strongly correlated with lesion rates (ρ = 0.61, P < .0001; Figure 1). Participants who reported no recurrences in the preceding year had a shedding rate of 13%, whereas participants who reported having 12 recurrences in the preceding year had a shedding rate of 26%. For each additional reported recurrence, the predicted shedding rate increased by 6% on a relative (not absolute) scale (95% confidence interval [CI], 4%–8%; P < .0001).
Figure 1.
Comparison between shedding rates and lesion rates within same swabbing session.
Consistency of Genital HSV Shedding
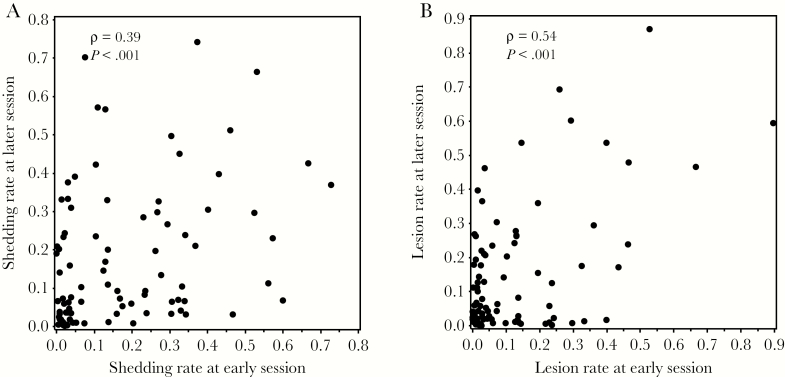
To assess the consistency of HSV shedding within persons over time, we analyzed results for 90 persons who had multiple sessions within 2 years [11]. We limited the interval between the sessions to 2 years because shedding may decline with longer follow-up [11]. These sessions were a median of 6 months apart (range, 1 month–2 years). In these 90 pairs of sessions, the HSV shedding rate was consistent within individuals (ρ = 0.39, P < .0001; Figure 2A).
Figure 2.
A, Comparison between shedding rates within individuals at an early session and a later session. B, Comparison between lesion rates within individuals at an early session and a later session.
Genital Lesions
Overall, genital lesions were reported in 42% of participants and on 10% of days. For the same 90 persons who contributed multiple swabbing and diary sessions, we compared lesions rates between the 2 sessions. There was a strong correlation between the rate of genital lesions at an earlier and later session (ρ = 0.54; P < .0001; Figure 2B). Viral shedding at an earlier session was also strongly correlated with the lesion rate at a subsequent session (ρ = 0.41, P < .0001).
Cohort 2: Effect of Pritelivir on HSV Shedding and on Clinical Outcomes
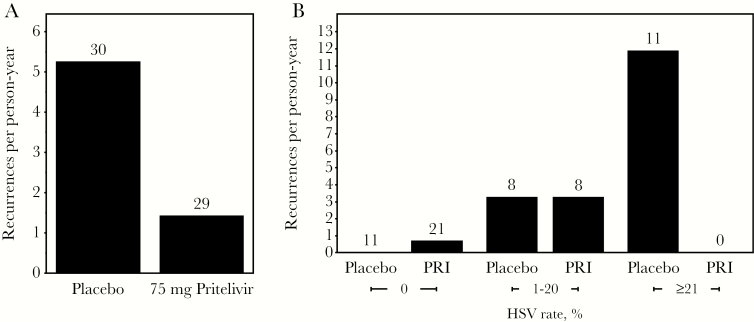
Thirty participants receiving placebo shed HSV on 16.6% of days (138 of 833), and 29 participants receiving the most effective dose of pritelivir (75 mg daily) shed HSV on 2.1% of days (16 of 766). The recurrence rate was 5.4 cases per person-year in the placebo group and 1.4 cases per person-year in the pritelivir group (Figure 3A) [28]. After adjustment for sex and study site, the risk ratio for HSV recurrence while receiving pritelivir versus placebo was 0.28 (95% CI, .08–.95; P = .041; Table 2). When the HSV shedding rate, the candidate surrogate outcome, was added to the model, the risk ratio for recurrence increased to 0.79 and was no longer statistically significant (95% CI, .19–3.32; P = .75; Table 2). Comparison of the effect of pritelivir on the recurrence rate between these 2 models yielded a PTE of 0.82: 82% of the effect of pritelivir on the recurrence frequency was explained by its effect on HSV shedding. Figure 3B demonstrates that the annualized recurrence rates are similar by treatment arm for persons within the same shedding rate category.
Figure 3.
A, Effect of pritelivir on the recurrence rate. The bar height is the annualized recurrence rate, and the number of persons contributing to the calculation is shown above each bar. B, Effect of pritelivir (PRI) on the recurrence rate for specified categories of herpes simplex virus (HSV) shedding rate. Numbers of persons for each category are shown at the top of each bar. No persons shed HSV on ≥21% of days while receiving PRI.
Table 2.
Calculation of the Treatment Effect Explained by Shedding
| Treatment, Outcome, Model | βa | RRb for Treatment (95% CI) | P | PTEc |
|---|---|---|---|---|
| Pritelivir | ||||
| Lesion | ||||
| Treatment only | -2.05 | 0.13 (.02–.70) | .018 | |
| Treatment and shedding rate | -0.54 | 0.58 (.18–1.91) | .37 | 74% |
| Recurrence | ||||
| Treatment only | -1.28 | 0.28 (.08–.95) | .041 | |
| Treatment and shedding rate | -0.23 | 0.79 (.19–3.32) | .75 | 82% |
| Acyclovir | ||||
| Lesion | ||||
| Treatment only | -1.85 | 0.16 (.09–.27) | <.0001 | |
| Treatment and shedding rate | -0.62 | 0.54 (.30–.98) | .042 | 67% |
| Recurrence | ||||
| Treatment only | -1.76 | 0.17 (.09–.32) | <.0001 | |
| Treatment and shedding rate | -1.03 | 0.35 (.18–.71) | .0041 | 41% |
| Valacyclovir | ||||
| Lesion | ||||
| Treatment only | -1.86 | 0.16 (.09–.27) | <.0001 | |
| Treatment and shedding rate | -0.87 | 0.42 (.21–.86) | .018 | 53% |
| Recurrence | ||||
| Treatment only | -1.64 | 0.19 (.13–.30) | <.0001 | |
| Treatment and shedding rate | -0.98 | 0.37 (.23–.61) | .0001 | 40% |
The calculation involved comparing a model where treatment is the only predictor to an adjusted model that also includes the shedding rate. Results of trials including pritelivir, acyclovir, or plus valacyclovir are shown.
Abbreviation: CI, confidence interval.
aCoefficient for treatment from model.
bThe relative risk (RR) is computed as eβ.
cThe proportion of the treatment effect (PTE) is computed as the percentage reduction in β when the shedding rate is added.
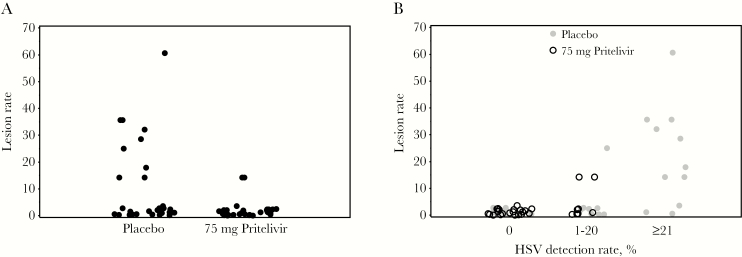
The lesion rate in the pritelivir clinical trial was 9.0% (75 of 836 days) in the placebo group and 1.2% (9 of 775 days) in the pritelivir group (Figure 4A) [28]. After adjustment for sex and study site, the relative risk for lesions during pritelivir as compared to placebo administration was 0.13 (95% CI, .02–.70; P = .018) [28]. When the shedding rate was additionally included as a predictor of the lesion rate, the risk ratio for pritelivir increased to 0.58 (95% CI, .18–1.91; P = .37; Table 2). Comparison of the treatment effect between these 2 models yielded an estimated PTE of 0.74: 74% of the effect of pritelivir on the genital lesion frequency was due to its effect on HSV shedding. Figure 4B demonstrates the role of shedding within the pathway toward lesions, with lesion rates appearing similar for each treatment arm within the same shedding rate category. Additionally, in this adjusted model, for each 10% increase in the shedding rate, the lesion rate doubled (RR, 2.1; 95% CI, 1.6–2.6; P < .0001).
Figure 4.
A, Effect of pritelivir on lesion rate. Each dot represents the lesion rate for an individual on that arm of study. B, Effect of pritelivir on the lesion rate for each category of HSV shedding. Since no persons shed herpes simplex virus (HSV) on ≥21% of days while receiving pritelivir, there are no open black circles at the right of the figure.
Cohort 3: Effect of Acyclovir and Valacyclovir on HSV Shedding and on Clinical Outcomes
Next, we extended these observations to the crossover study of acyclovir and valacyclovir [29]. Sixty-nine participants shed on 40.2% of days (1316 of 3272) while receiving placebo, on 8.0% of days (256 of 3189) while receiving acyclovir, and on 7.2% (233 of 3254) of days while receiving valacyclovir [29]. This cohort had a relatively higher shedding frequency relative to other cohorts because eligibility criteria required either recent HSV-2 acquisition or at least 6 recurrences in the previous year, whereas the pritelivir study participants could have fewer annual recurrences. The recurrence rate was 10.8 cases per person-year during placebo receipt, 1.9 cases per person-year during acyclovir treatment, and 2.1 cases per person-year during valacyclovir treatment. After adjustment for sex and study site, the relative risk for recurrence with acyclovir as compared to placebo was 0.17 (95% CI, .09–.32; P < .0001) [29]. When the HSV shedding rate was additionally included as a predictor of the recurrence rate, the risk ratio for acyclovir increased to 0.35 (95% CI, .18–.71; P = .0041). When we compared the adjusted and unadjusted models, the estimated PTE was 0.41 (ie, 41% of the reduction in recurrence frequency was due to the effect of acyclovir on HSV shedding). Additionally, for each 10% increase in viral shedding rate, the recurrence rate increased by 21% (relative risk, 1.21; 95% CI, 1.13–1.29; P < .0001).
Furthermore, the lesion rate was 22.1% (758 of 3430 days) while receiving placebo and 3.1% (102 of 3290 days) while receiving acyclovir. The relative risk for the lesion rate increased from 0.16 (95% CI, .09–.27; P < .0001) to 0.54 (95% CI, .30–.98; P = .042) after adjustment for shedding rate. Comparison of model coefficients revealed that 67% of the overall effect in the lesion rate reduction was due to acyclovir’s effect on HSV shedding. The lesion rate also increased by 39% (relative risk, 1.39; 95% CI, 1.23–1.58; P < .0001) for each 10% increase in shedding rate. Similarly, evaluation of valacyclovir versus placebo showed that 53% and 40% of the drug’s effect on the lesion rate and recurrence rate, respectively, were due to valacyclovir’s effect on HSV shedding (Table 2).
DISCUSSION
Our study showed that genital HSV shedding serves as a surrogate outcome for clinical outcomes in studies of antiviral interventions for genital herpes, predicting up to 82% of the effect of the drug on clinical outcome. Several advantages to using HSV shedding as an outcome rather than recurrences are apparent. First, HSV shedding occurs more commonly than HSV genital lesions. While >65% of our study population had HSV-2 DNA detected in their genital swab samples, only about 40% of participants noted genital lesions. This finding is consistent with previous natural history studies that have shown that genital HSV-2 shedding occurs frequently among persons with HSV-2 seropositivity, even in the absence of genital lesions [2, 3, 27]. As such, studies that use shedding as an outcome require relatively fewer participants to achieve similar power and therefore provide potential efficiency in terms of recruitment time and staffing costs (although PCR assays for HSV DNA detection incur additional laboratory costs). Second, we showed that HSV shedding rates were consistent within persons over time. We conclude that HSV shedding is a consistent measure of HSV disease severity, which appears to describe something fundamental about that individual’s infection, akin to the concept of set point, which has been well defined for other chronic viral infections, such as HIV, HCV, and HBV [35, 36]. Third, the association of shedding frequency with lesion frequency and the ability of the shedding rate to predict the future frequency of the lesion rate lends further credibility to the biological connection and the usefulness of shedding to predict a clinical outcome.
As a final step in assessing shedding as an appropriate surrogate outcome, our study also showed that a drug’s effect on genital lesions and recurrences occurs largely through its effect on shedding rates. Previous studies from our research group showed that genital HSV shedding and lesion rates are reduced by acyclovir [29] and in a dose-dependent manner by oral pritelivir [28]. In this study, using lesion and recurrence rates as outcomes, we achieved values of 40%–82% for the PTE explained through shedding; these large values further confirm HSV shedding is a precursor in the causal pathway to genital lesions. Therefore, we feel confident that future interventions targeting reduction in shedding rates would also reduce recurrence and lesion rates. The moderate consistency over varied study designs, classes of drug (nucleoside analogues and helicase-primase inhibitors), and ways of describing the clinical outcome (genital lesion frequency or recurrence frequency) further support our findings.
Unlike surrogates that serve as biomarkers for disease activity or treatment response, genital HSV shedding is also an important indicator for the potential to transmit the infection to sex partners. In HIV infection, several studies have shown an excellent correlation between plasma or genital HIV RNA and the probability of transmission of HIV to sex partners [37–39]. The strength of this association has led to the adoption of viral load measurement for evaluation of community-level provision of antiretroviral therapy and has become a goal in wider implementation of treatment [40]. While viral shedding is required for HSV-2 transmission to either partner or neonate, the relationship between the viral shedding during a given sexual encounter and the risk of transmission is unlikely to ever be empirically verified and can only be modeled [41]. Of note, neither history of genital herpes nor frequency of recurrences predicts HSV-2 transmission; therefore, these clinical manifestations of genital herpes do not qualify as surrogate outcomes for transmission potential [42, 43].
Our study may be limited because recurrence and lesion rates were based on self-reports from participants. Given that lesions can be difficult to detect, their frequency may be underestimated. Thus, the correlation of shedding rates with both lesion rates and recurrence rates may be underestimated. Prior work evaluating outcome mismeasurement has shown that poor sensitivity (as well as poor specificity) often leads to conservative bias in measures of association [44, 45]. Both shedding and clinical outcomes were measured over a 30-day period, and longer observations may lead to greater precision and stronger associations. Therefore, the PTE explained by shedding might increase modestly under longer sampling schemes. Last, differences between cohort 2 and cohort 3 may account for the lower estimated PTE observed for acyclovir and valacylcovir as compared to pritelivir. Cohort 3 included participants who had recent primary genital HSV-2 infection [29].
Despite the potential benefit of a more efficient study design when determining the most effective target dose, regulatory bodies have accepted few surrogate outcomes in infectious diseases as a basis for approving new therapies. For example, CD4+ T-cell count and HIV viremia have been accepted as surrogate outcomes for AIDS in HIV/AIDS therapeutics [16]. The use of sputum culture conversion as a surrogate outcome led to approval of bedaquiline, a novel agent for multidrug-resistant tuberculosis [46, 47]. More recently, Natori et al reported that the CMV load is an appropriate surrogate end point for CMV trials among solid organ transplant recipients [48]. The low acceptance of surrogate outcomes as a basis for approving therapeutic interventions stems from growing concerns that surrogates may not be causally related to the clinical end point and may not capture all the causal pathways to disease, which could lead to unexpected adverse outcomes [13, 24, 49].
To have research value, the HSV shedding rate need not be a perfect surrogate for lesion or recurrence rate, as new candidate treatments initially evaluated with the shedding rate as an outcome may be further evaluated for clinical outcomes, such as the recurrence rate prior to licensure. We note that there was residual association between treatment and clinical outcome for acyclovir and valacyclovir, indicating that shedding does not explain 100% of the effect of treatment on lesions or recurrence. We have shown, however, that shedding can serve as a useful and reliable outcome for early phase studies. In summary, the HSV shedding rate provides an appropriate and clinically informative surrogate outcome for assessing genital HSV recurrence frequency and response to antiviral interventions and would be useful to optimize HSV intervention trials and drug development.
Notes
Presented in part. IDWeek 2015, San Diego, California, 7–11 October 2015.
Financial support. This work was supported by the National Institutes of Health (grants P01 AI-030731 and K24 AI-071113).
Potential conflicts of interest. A. W. is a consultant to AiCuris. C. J. has received research support from Sanofi-Pasteur, AiCuris, Genocea Biosciences, and Vical and is a consultant to Novavax. A. S. M. is a consultant to AiCuris and Immune Design. L. C. has received research support from Sanofi and Immune Design and is a coinventor on several patents associated with the development of an HSV-2 vaccine. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000; 342:844–50. [DOI] [PubMed] [Google Scholar]
- 3. Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011; 305:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mertz KJ, Trees D, Levine WC, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 1998; 178:1795–8. [DOI] [PubMed] [Google Scholar]
- 5. Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004; 10:530–6. [DOI] [PubMed] [Google Scholar]
- 6. Cohen JI. Vaccination to reduce reactivation of herpes simplex virus type 2. J Infect Dis 2017; 215:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryson YJ. Current status and prospects for oral acyclovir treatment of first episode and recurrent genital herpes simplex virus. J Antimicrob Chemother 1983; 12 (Suppl B):61–5. [DOI] [PubMed] [Google Scholar]
- 8. Corey L, Benedetti JK, Critchlow CW, et al. Double-blind controlled trial of topical acyclovir in genital herpes simplex virus infections. Am J Med 1982; 73:326–34. [DOI] [PubMed] [Google Scholar]
- 9. Fiddian AP, Halsos AM, Kinge BR, Nilsen AE, Wikstrom K. Oral acyclovir in the treatment of genital herpes. Preliminary report of a multicenter trial. Am J Med 1982; 73:335–7. [DOI] [PubMed] [Google Scholar]
- 10. Nilsen AE, Aasen T, Halsos AM, et al. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet 1982; 2:571–3. [DOI] [PubMed] [Google Scholar]
- 11. Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis 2011; 203:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lesko LJ, Atkinson AJ Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol 2001; 41:347–66. [DOI] [PubMed] [Google Scholar]
- 13. Temple R. Are surrogate markers adequate to assess cardiovascular disease drugs?JAMA 1999; 282:790–5. [DOI] [PubMed] [Google Scholar]
- 14. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431–40. [DOI] [PubMed] [Google Scholar]
- 15. Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 2000; 1:49–67. [DOI] [PubMed] [Google Scholar]
- 16. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled?Ann Intern Med 1996; 125:605–13. [DOI] [PubMed] [Google Scholar]
- 17. Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med 2012; 31:2973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alonso A, Geys H, Molenberghs G, Kenward MG, Vangeneugden T. Validation of surrogate markers in multiple randomized clinical trials with repeated measurements: canonical correlation approach. Biometrics 2004; 60:845–53. [DOI] [PubMed] [Google Scholar]
- 19. Buyse M. Use of meta-analysis for the validation of surrogate endpoints and biomarkers in cancer trials. Cancer J 2009; 15:421–5. [DOI] [PubMed] [Google Scholar]
- 20. Weir CJ, Walley RJ. Statistical evaluation of biomarkers as surrogate endpoints: a literature review. Stat Med 2006; 25:183–203. [DOI] [PubMed] [Google Scholar]
- 21. Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med 1992; 11:167–78. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi F, Kuroki M. A new proportion measure of the treatment effect captured by candidate surrogate endpoints. Stat Med 2014; 33:3338–53. [DOI] [PubMed] [Google Scholar]
- 23. International Conference on Harmonization (ICH) working group of technical requirements for registration of pharmaceuticals for human use. ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials 1998. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed February 2016.
- 24. Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med 2015; 175:1389–98. [DOI] [PubMed] [Google Scholar]
- 25. Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 2005; 24:67–78. [DOI] [PubMed] [Google Scholar]
- 26. Molenberghs G, Burzykowski T, Alonso A, Buyse M. A perspective on surrogate endpoints in controlled clinical trials. Stat Methods Med Res 2004; 13:177–206. [DOI] [PubMed] [Google Scholar]
- 27. Magaret AS, Johnston C, Wald A. Use of the designation “shedder” in mucosal detection of herpes simplex virus DNA involving repeated sampling. Sex Transm Infect 2009; 85:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wald A, Corey L, Timmler B, et al. Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med 2014; 370:201–10. [DOI] [PubMed] [Google Scholar]
- 29. Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 30. Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 1995; 333:770–5. [DOI] [PubMed] [Google Scholar]
- 31. Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996; 124:8–15. [DOI] [PubMed] [Google Scholar]
- 32. Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol 1999; 37:1941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol 2007; 45:1618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004; 104:942–7. [DOI] [PubMed] [Google Scholar]
- 36. Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 2005; 436:946–52. [DOI] [PubMed] [Google Scholar]
- 37. Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 39. Chuachoowong R, Shaffer N, Siriwasin W, et al. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis 2000; 181:99–106. [DOI] [PubMed] [Google Scholar]
- 40. Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 2014; 11:20140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim HN, Wald A, Harris J, Almekinder J, Heitman C, Corey L. Does frequency of genital herpes recurrences predict risk of transmission? Further analysis of the valacyclovir transmission study. Sex Transm Dis 2008; 35:124–8. [DOI] [PubMed] [Google Scholar]
- 43. Wald A, Krantz E, Selke S, Lairson E, Morrow RA, Zeh J. Knowledge of partners’ genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis 2006; 194:42–52. [DOI] [PubMed] [Google Scholar]
- 44. Little RJA, Rubin DB.. Statistical Analysis With Missing Data. Wiley Series in Probability and Mathematical Statistics, Wiley, 1987. [Google Scholar]
- 45. Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol 1997; 146:195–203. [DOI] [PubMed] [Google Scholar]
- 46. Chahine EB, Karaoui LR, Mansour H. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. Ann Pharmacother 2014; 48:107–15. [DOI] [PubMed] [Google Scholar]
- 47. Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360:2397–405. [DOI] [PubMed] [Google Scholar]
- 48. Natori Y, Alghamdi A, Tazari M, et al. ; CMV Consensus Forum. Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis 2018; 66:617–31. [DOI] [PubMed] [Google Scholar]
- 49. Svensson S, Menkes DB, Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Intern Med 2013; 173:611–2. [DOI] [PubMed] [Google Scholar]