These studies reveal novel NKG2D-dependent pathways on mouse pulmonary dendritic cells (DCs) required for normal DC responses towards Toll-like receptor ligands. Further, we demonstrate that DC–NKG2D interactions play critical roles in host defenses against respiratory syncytial virus infection
Keywords: dendritic cells, respiratory syncytial virus, NKG2D, interleukin 12, dendritic cell regulation, inflammation
Abstract
Background
Respiratory syncytial virus (RSV) is a common cause of respiratory tract infection in vulnerable populations. Natural killer (NK) cells and dendritic cells (DC) are important for the effector functions of both cell types following infection.
Methods
Wild-type and NKG2D-deficient mice were infected with RSV. Lung pathology was assessed by histology. Dendritic cell function and phenotype were evaluated by enzyme-linked immunosorbent assay and flow cytometry. The expression of NKG2D ligands on lung and lymph node DCs was measured by immunostaining and flow cytometry. Adoptive transfer experiments were performed to assess the importance of NKG2D-dependent DC function in RSV infection.
Results
NKG2D-deficient mice exhibited greater lung pathology, marked by the accumulation of DCs following RSV infection. Dendritic cells isolated from NKG2D-deficient mice had impaired responses toward Toll-like receptor ligands. Dendritic cells expressed NKG2D ligands on their surface, which was further increased in NKG2D-deficient mice and during RSV infection. Adoptive transfer of DCs isolated from wild-type mice into the airways of NKG2D-deficient mice ameliorated the enhanced inflammation in NKG2D-deficient mice after RSV infection.
Conclusion
NKG2D-dependent interactions with DCs control the phenotype and function of DCs and play a critical role in pulmonary host defenses against RSV infection.
Respiratory syncytial virus (RSV) is a common cause of respiratory tract infection in young, elderly, and immunocompromised patients. It is estimated that there are 33.8 million new cases and 199000 RSV-related deaths worldwide each year [1]. In susceptible populations, the RSV infection can spread to the lower respiratory tract, causing bronchiolitis and pneumonia associated with airway epithelial cell destruction and sloughing, mucus secretion, and pulmonary immune-cell infiltration [2, 3]. Currently, there are no safe and effective RSV vaccines, and therapeutic options are limited. The incomplete understanding of RSV infection-related pathogenesis, combined with the urgent need to identify safe and effective therapeutic targets, underscores the need to investigate the host response during RSV infection.
Natural killer (NK) cells are important effector cells in controlling RSV infection, although the mechanisms are not fully understood [4, 5]. NKG2D (KLRK1, killer cell lectin like receptor K1, CD314) is a well-characterized activating receptor expressed on virtually all NK cells [6], most NK T cells, and subpopulations of γδ T cells [7]. Additionally, all human CD8+ αβ T cells express NKG2D, whereas mice express NKG2D only on activated and memory type αβ CD8+ T cells [8]. Effector cells of both the innate and adaptive immune system use NKG2D in the surveillance of inflammation and cancers, as well as in some autoimmune processes and transplantation reactions. NKG2D recognizes multiple ligands, including MICA, MICB, and the UL-16 binding proteins in humans (ULBP1-6) and the UL16-binding protein-like transcript 1 (Mult1), retinoic acid–inducible early transcripts (Rae1), and H60 in mice [9]. NKG2D ligands exhibit little or no surface expression under homeostatic conditions but are induced under conditions of stress, including cellular transformation, DNA damage, infection, and other cellular stresses on several cell types [10].
As the most efficient antigen presentation cells, dendritic cells (DCs) play a pivotal role in the onset and regulation of innate and adaptive immune responses [11], with NK cell and DC cross-talk being a critical mechanism for effective antiviral immunity [12]. Multiple studies have shown the importance of DCs and NK cells in RSV infection [4, 13]. Human DCs express NKG2D ligands after in vitro stimulation with viral mimetics and RSV [5]. However, the interactions between NK cells and DCs and their roles in lung pathogenesis in response to RSV infection are not well defined. Therefore, we sought to examine the role of NKG2D and DCs in the response to RSV infection in our mouse model.
MATERIALS AND METHODS
Mice
C57BL/6J mice and OT-1 mice (female, aged 8–10 wk) were purchased from the Jackson Laboratory (Bar Harbor, ME). NKG2D-deficient (Klrk1−/−) mice were generated and maintained as described [14]. Both male and female mice (aged 6–8 wk) were included in the study. All mice were housed in the University of Cincinnati animal care facilities, and experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of Cincinnati Medical Center.
Respiratory Syncytial Virus and Infection
Respiratory syncytial virus strain A2 was passaged in Vero cells (ATCC; Manassas, VA) cultured in serum-free-media (SFM4MegaVir; Hyclone; Logan, UT). Mice were infected intranasally with 1 × 106 PFU or control vehicle in 50 µL of phosphate-buffered saline (PBS). Mice were monitored and body weight changes were recorded daily after infection. Viral load was determined by reverse transcription–polymerase chain reaction of lung RNA as described [15].
Cell Isolation
Purification of DCs from lung and mediastinal lymph nodes (mLNs) was performed as previously described [16]. Briefly, mice were killed with an intraperitoneal injection of sodium pentobarbital. Lung vasculature was perfused with 10 mL of PBS containing 0.6 mM ethylenediaminetetraacetic acid (EDTA) via the right atrium. Single-cell suspensions of mLNs were obtained by passing the nodes through a 100-μm nylon mesh. Lung single-cell suspensions were obtained by using a gentle MACS Dissociator (Miltenyi Biotec Ltd; Woking, UK) after incubating harvested lung tissues with 100 mg/mL of collagenase type IV and 20000 U/mL of DNases (Sigma Aldrich Company Ltd, Gillingham, UK) as described [17]. After red blood cell lysis, CD11c+ cells were isolated by positive selection using CD11c magnetic MicroBeads beads following manufacturer’s protocol (Miltenyi Biotec GmbH; Germany). T cells from OT-I mice were isolated from spleens following the manufacturer’s protocol using the EasySep Mouse T Cell isolation kit (Stemcell).
Lung Lavage, Fixation, and Pathology
Bronchoalveolar lavage (BAL) fluid for cytokine analysis was collected by instilling the lungs once with 1.0 mL of calcium- and magnesium-free PBS containing 5 mM EDTA via a tracheal cannula. In separate mice, lungs were lavaged 3 times to obtain BAL for cell enumeration. Bronchoalveolar lavage cells were centrifuged and resuspended at 5 × 105 cell/mL. The cells were then directly used for flow cytometry and cytospin staining. Cytospin cell preparations were obtained with 300 µL of BAL cells centrifuged at 300 × g for 5 minutes on cytospin slides (Newcomer Supply; Middleton, WI). Mouse lungs were inflation-fixed with buffered formalin and embedded in paraffin for histological analysis as previously described [18]. Three noncontiguous tissue sections were stained with hematoxylin and eosin, and the number of infiltrated inflammatory cells was assessed by light microscopy. The pathology scoring was similar to that previously described [19]. Specifically, “1” represented cell count within the normal range; “2” represented mild immune cell infiltration and accumulation; “3” represented moderate, multifocal aggregation; and “4” represented high cellularity and aggregation.
Flow Cytometry
Dendritic cells were incubated with anti-CD16/CD32 for 30 minutes, then incubated with specific monoclonal antibodies at 4°C for 30 minutes in PBS containing 1% bovine serum albumin (BSA). Cells were washed twice with PBS containing 0.1% sodium azide and 0.5% BSA and immediately analyzed by flow cytometry (Attune, Thermofisher). All antibodies were obtained from Ebioscience; (PE-cyanine 7 anti-mouse CD11c [N418], APC-efluor 780 anti-mouse CD11b [M1/70], APC anti-mouse CD86 [GL1]); BD Bioscience [PerCP-Cy 5.5 Anti-mouse CD103 {M290}]); and APC anti-mouse Rae1 pan specific [186109], PE anti-mouse Mult-1 [237104]; R&D Systems). Data were analyzed using Flowjo software (v10) and FCS Express V5 (De Novo Software). Cells were first gated on lymphocytes, and doublets were excluded by gating on single cells. CD11c cells were then gated, and macrophages were discriminated from DCs based on cellular autofluorescence. Dendritic cells were gated as CD11c+, MHCII+, and autofluorescence mid/low cells. Subsets of DC and CD86 expression were then further gated from DC populations. The compensation matrix was determined by using UltraComp eBeads (eBioscience). Appropriate controls were used to set gates for the above populations.
Immunohistochemistry
Immunofluorescent staining was performed on cytospin preparations by double indirect immunofluorescence method as previously descripted [20]. Briefly, slides were blocked with serum and then incubated overnight at 4°C with biotin anti-mouse CD11c (1:500; eBioscience [N418]) and either APC anti-mouse pan-specific Rae1 (1:500; R&D Clone 186107) or anti-mouse Mult-1 (1:500; R&D Clone 237104). Binding was detected following 24 hours of incubation with streptavidin 488 (1:500; Vector Labs). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (1:2000; Sigma-Aldrich). Images were captured using a Zeiss LSM710 inverted confocal microscope at the Live Microscopy Core, Department of Molecular and Cellular Physiology, University of Cincinnati. Negative controls were included in each experiment.
Adoptive Transfers
Dendritic cells (> 90% purity) from wild-type and Klrk1−/− mice were isolated from lung as described above. A total of 1.5 × 106 DCs in 50 µL of PBS or vehicle only were transferred intranasally to recipient mice 16 hours before the RSV challenge.
Dendritic Cell Stimulation
Freshly isolated DCs were maintained in complete medium (Roswell Park Memorial Insittute medium containing 10% fetal bovine serum, 2 mM of L-glutamine, 1mM of sodium pyruvate, 0.1 mM of nonessential amino acids, 50 µM of β-mercaptoethanol, and 1000 U/mL of Pen/Strep). Toll-like receptor (TLR) ligand activation was assessed using 1 × 105 freshly isolated DCs seeded in 100 uL of DC media in 96-well round-bottom plates. Dendritic cells were rested for 1 hour followed by a preincubation with 20 ng/mL of interferon γ (IFN-γ; PeproTech) for 2 hours. The DCs were then treated for 16 hours with 1 µg/mL of polyinosinic:polycytidylic acid (InvivoGen), 1 µg/mL of lipopolysaccharide (Sigma Aldrich), 100 ng/mL of ssRNA40 (InvivoGen) or 50 ng/mL of CpG oligodeoxynucleotides 1826 (InvivoGen). Interleukin 12 (IL-12) p40 was measured by enzyme-linked immunosorbent assay (ThermoFisher). To assess antigen presentation capacity, freshly isolated DCs were pulsed with 100 ng/mL of ovalbumin (OVA) peptide SIINFEKL (InvivoGen) or irrelevant peptide for 1 hour, then cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-1 T cells at a 1:5 ratio for 5 days. The OT-1 T-cell proliferation index was then measured by flow cytometry [21].
Statistics
In each group, 4–8 mice were used, and at least 3 independent experiments were conducted. Unless otherwise indicated, data are expressed as mean ± standard errors of the means (SEMs). Student’s t tests and analysis of variance were used for statistical analyses. P < .05 was considered statistically significant.
RESULTS
Increased Severity of Respiratory Syncytial Virus–Induced Lung Pathology in NKG2D-Deficient Mice
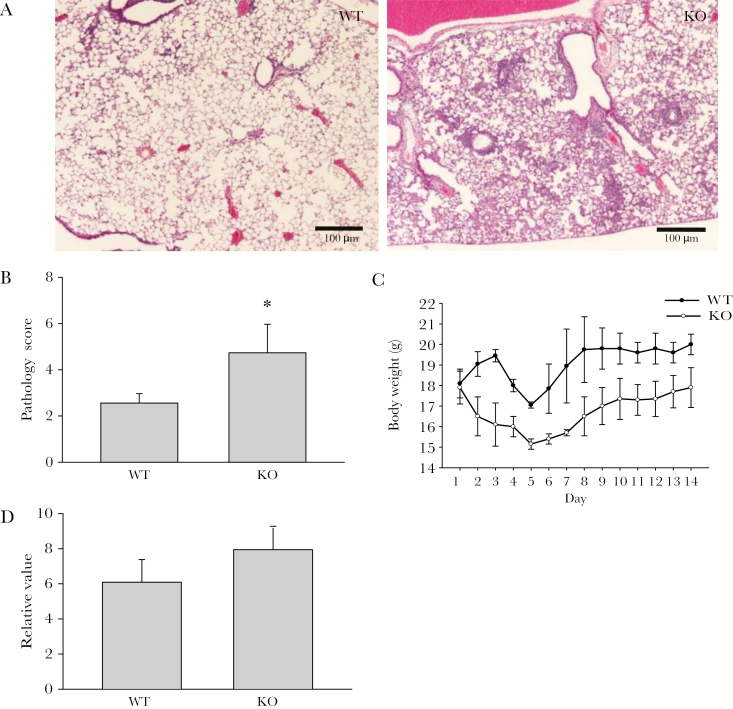
We first examined lung inflammation, weight loss, and viral clearance in NKG2D- deficient and wild-type (WT) mice following RSV infection. The B6 mice are semipermissive to RSV strain A2, and the infection induced only mild lung pathology [22, 23]. However, we found that the bronchiolitis and alveolitis induced by A2 in B6 NKG2D-deficient mice were severe compared with the B6 control mice (Figure 1A). There was also increased inflammatory cell infiltration (Figure 1B) and weight loss in the infected NKG2D-deficient mice compared with WT mice (Figure 1C). The inter-mouse strain difference in RSV titer in lung homogenates was not different after 5 days of incubation, but there was a trend toward higher titer in NKG2D-deficient mice (Figure 1D). Also, pro-inflammatory cytokines such IL-12 and interleukin 6 were increased in the BAL of NKG2D-deficient mice after RSV infection compared with WT mice (data not shown). These data suggest that NKG2D performs a critical function in the immune response to RSV infection.
Figure 1.
NKG2D deficiency augments respiratory syncytial virus (RSV)–induced lung pathology. Mice were challenged intranasally with saline or RSV. A, Representative hematoxylin and eosin lung histology from wild-type and NKG2D-deficient mice 5 days after RSV infection. Scale bar, 100 μm. n = 6. B, Mouse lungs were harvested for pulmonary histopathology examination 5 days after RSV infection. The pathology features were scored as described in the Materials and Methods. Data are shown as the mean ± SEM from at least 5 independent experiments. *P < .05. C, Daily weight changes of control and RSV-infected mice were measured individually after infection. Data are means ± SEM. n = 3 for each group. D, Relative RSV titer in lung was measured by polymerase chain reaction 5 days after infection. n = 8. *P < .05. Abbreviations: KO, knockout; WT, wild-type.
Altered Dendritic Cell Phenotype in NKG2D-Deficient Mice Following Respiratory Syncytial Virus Infection
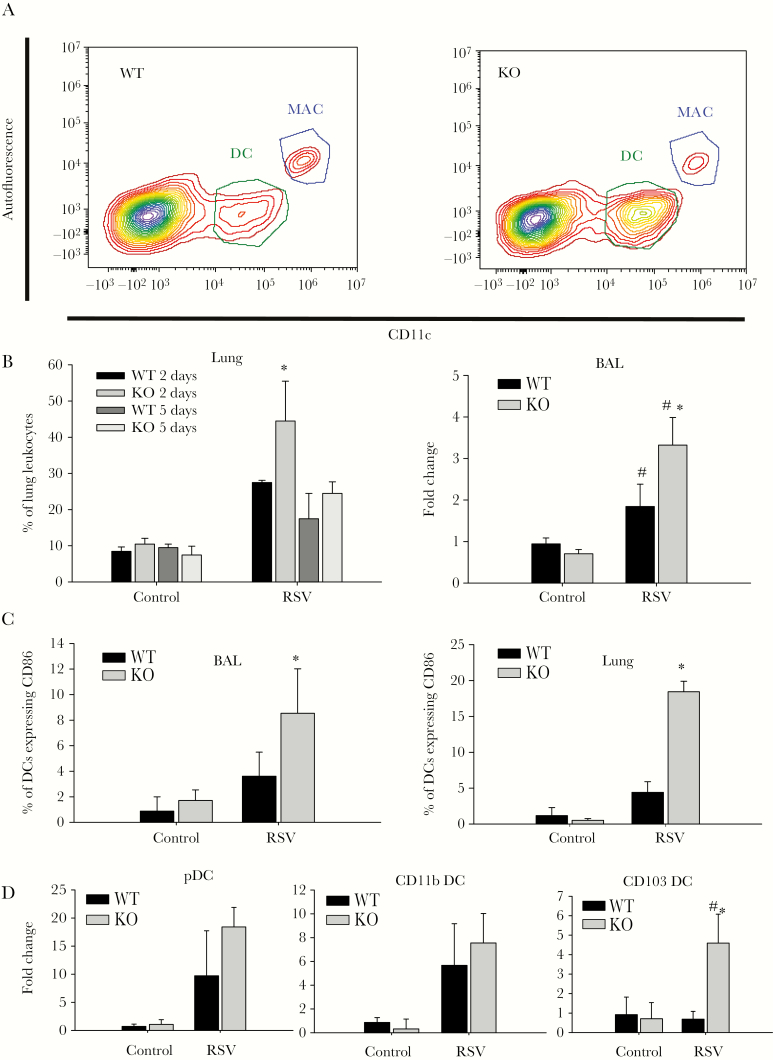
Dendritic cells play a critical role in RSV infection because they impart specific effector functions to cells mediating both the innate and adaptive immune response. Following intranasal RSV infection, NKG2D-deficient mice exhibited increased whole lung DC accumulation and BAL DC accumulation (Figure 2B) compared with WT mice, with a peak difference at day 2 after infection (Figure 2A). A more detailed examination of the DC populations revealed that there is a larger subpopulation of mature DCs in the BAL and lung of NKG2D-deficient mice (Figure 2C). The lung leukocyte phenotypic analysis revealed that the increased numbers of DCs in NKG2D-deicient mice are mainly plasmacytoid DCs (pDC) (PDCA-1+, CD11c+, CD11b−, CD103−) and CD103 DCs (CD11c+, CD103+, CD11b−) (Figure 2D). The discrepancy of DC composition and increased number of DCs in NKG2D-deficient mice that we observed demonstrate that the NKG2D receptor also plays an important role in regulating DC biology during RSV infection.
Figure 2.
Altered pulmonary dendritic cell (DC) phenotype after respiratory syncytial virus (RSV) infection in NKG2D-deficient mice. Mice were challenged intranasally with saline or RSV. Pulmonary DCs were isolated from lung tissue. A, Representative flow cytometry plot for DC population in lung after RSV challenge 2 days after infection. Dendritic cells were gated as CD11chigh autofluorescencelow. Alveolar macrophage (AM) were gated as F4/80+, CD11chigh autofluorescencehigh. B, Percentage of DCs in lung single-cell homogenate of early infection (2 days after infection) and late infection (5 days after infection) and the fold change of DC number compared with wild-type control mice was assessed in bronchoalveolar lavage (BAL) fluid of mice 2 days after infection by flow cytometry. Data are shown as the mean ± SEM of at least 5 independent experiments. *P < .05. C, CD86 expression on DC populations in BAL and lung were measured by flow cytometry. Data are shown as the mean ± SEM of at least 3 independent experiments. *P < .05. D, Dendritic cell subpopulations in BAL were measured by flow cytometry. Plasmacytoid DCs are gated as CD11c+, PDCA1+, CD11b−, CD103−; CD11b DCs are gated as CD11c+, CD11b+, and CD103−; CD103 DCs are gated as CD11C+, CD103+, and CD11b−. Data are shown as the mean ± SEM of at least 3 independent experiments. Each experiment consisted of at least 4 mice. *P < .05. Abbreviations: BAL, bronchoalveolar lavage; DC, dendritic cell; KO, knockout; MAC, macrophage; pDC, plasmacytoid dendritic cell; RSV, respiratory syncytial virus; WT, wild-type.
NKG2D Ligand Expression in Lymph Node and Pulmonary Dendritic Cells
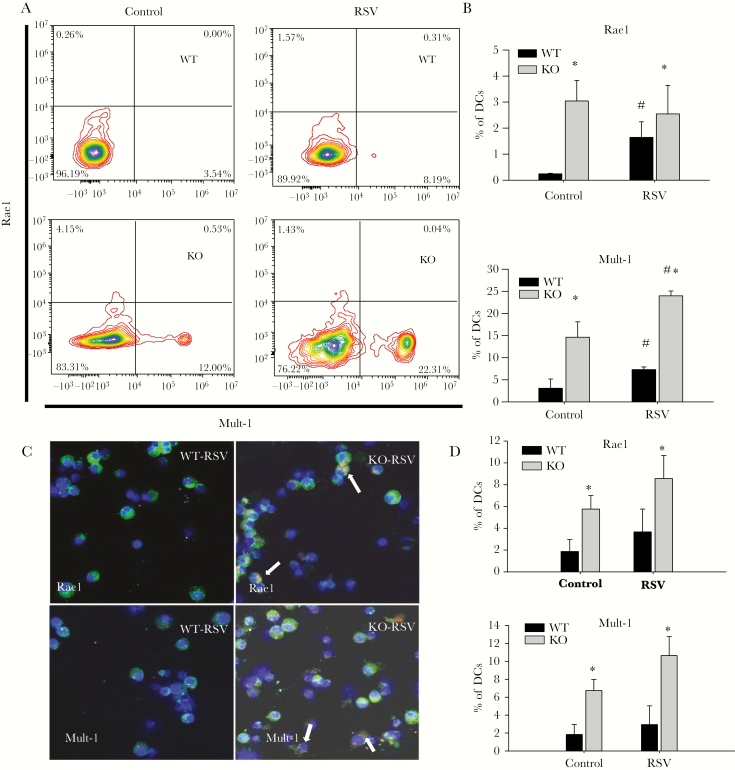
Multiple reports have demonstrated the presence of NKG2D ligands on human DCs in naive or inflammatory states [5, 24]. However, mouse DC expression of NKG2D ligands has not been reported. We isolated mLNs from naive and RSV-infected mice and measured the NKG2D ligands Rae1 and Mult-1 on DCs by flow cytometry. We found 2 populations of DCs that express either Rae1 or Mult-1 in mLNs, and the percentages of these subpopulations increased following RSV infection (Figure 3A and 3B). The NKG2D ligand expressing DC subpopulations appears to be distinct based on the lack of coexpression of either assayed NKG2D ligand. The percentages of these subpopulations of DCs were higher in both naive and RSV-infected NKG2D-deficient mice compared with WT (Figure 3A and 3B). Lung DCs exhibited the same expression patterns of NKG2D ligands. The number of DCs expressing Rae1 or Mult-1 increased in NKG2D knockout (KO) mice compared with WT mice and further increased following RSV infection (Figure 3C and 3D). The expression of NKG2D ligands is independent of DC maturity because further gating strategies showed Rae1 and Mult-1 expression can be found on both mature and immature DCs (data not shown).
Figure 3.
Increased expression of NKG2D ligands on dendritic cells (DCs) in NKG2D-deficient mice, which is enhanced during respiratory syncytial virus (RSV) infection. A and B, Rae1 and Mult-1 expression on mediastinal lymph node (mLN) DCs were measured by flow cytometry 2 days after RSV infection. Single lung cell suspension was prepared from mice at 2 days after RSV infection and stained with Rae1 and Mult-1 (C and D). Arrows point to cells that are positive for CD11c and Rae1 or Mult-1 staining (Blue: 4′,6-diamidino-2-phenylindole; Green: CD11c; Red: Rae 1/Mult-1). Data are representative of 4 independent experiments with n = 6–8 mice. *P < .05 compared to KO control. #P < .05 compared to WT RSV. Abbreviations: KO, knockout; RSV, respiratory syncytial virus; WT, wild-type.
Hyporesponsive Dendritic Cells in NKG2D-Deficient Mice Following Toll-like Receptor Activation
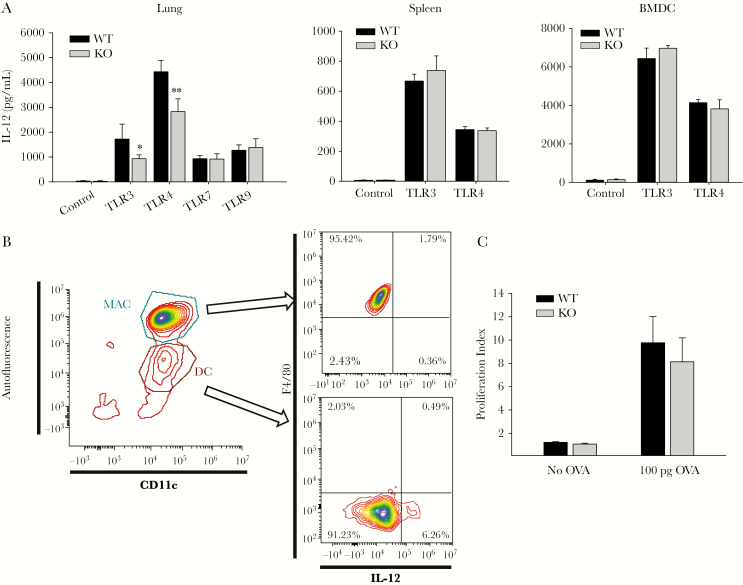
The difference in surface expression of NKG2D ligands on DCs prompted us to examine their potential functional implications by assessing TLR ligand stimulation and antigen presentation capabilities. Interleukin 12 is a critical pro-inflammatory cytokine secreted by DCs and is also important in activating NK cells [25]. Therefore, we assessed IL-12 secretion as an indicator of DC responsiveness. Interestingly, the responses to TLR3 and TLR4 ligands were lower in DCs isolated from the lungs of NKG2D-deficient mice compared with WT mice. In, contrast, there were no detectable differences in lung DC responsiveness to TLR7 and TLR9 ligands (Figure 4A). Impaired responsiveness toward TLR3 and TLR4 ligands was not observed in splenic DCs or bone marrow−derived DCs (Figure 4A) isolated from NKG2D-deficient mice, which suggests a unique role for the pulmonary microenvironment in modulating DC phenotype. To confirm that the IL-12 p40 was produced by DCs but not pulmonary macrophages that may be contaminating the DC isolates, we measured intracellular IL-12 expression level and found that, indeed, most of the IL-12 was produced by DCs (Figure 4B).
Figure 4.
Dendritic cells (DCs) from NKG2D-deficient mice have impaired responsiveness to Toll-like receptor (TLR) ligands. Dendritic cells were isolated from lung and spleen and derived from bone marrow and treated with 20 ng/mL of interferon γ for 2 hours before being treated with 1 µg/mL of polyinosinic:polycytidylic acid (TLR 3), 1 µg/mL of lipopolysaccharide (LPS) (TLR 4), 100 ng/mL of SSRNA40 (TLR 7), and 50 ng/mL of CpG oligodeoxynucleotides 1826 (TLR 9) for 16 hours. The supernatant was collected, and interleukin 12 (IL-12) p40 was measured by enzyme-linked immunosorbent assay (A). Data are shown as the mean ± SEM of at least 5 independent experiments. *P < .05, ** P < .01. B, Schematic flow cytometry plot of intracellular staining of IL-12 p40 after LPS treatment. Macrophages were gated as CD11c+ autofluorescencehigh, and DCs were gated as CD11c+, autofluorescencelow. C, Freshly isolated DCs were pulsed with 100 ng/mL of OVA peptide for 1 hour, then cultured with carboxyfluorescein succinimidyl ester-labeled OT-1 T cells at 2:10 ratio. The OT-1 T cell proliferation index was measured after 5 days. Data are shown as average proliferative index ± SEM from 4 independent experiments. Abbreviations: BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; IL-12, interleukin 12; KO, knockout; MAC, macrophage; OVA, ovalbumin; TLR, Toll-like receptor; WT, wild-type.
We next assessed the capacity of DCs to induce T-cell proliferation given their critical role as antigen-presenting cells. We explored antigen presentation using a model of ex vivo antigen presentation that uses fluorescently labeled CD8+ T cells (OT-1) isolated from transgenic mice engineered to overexpress T-cell receptors specific for an ovalbumin peptide. OT-1 T cells were then co-cultured with DCs pulsed with the specific ovalbumin peptide. Following 5 days of co-culture, the proliferation index of the T cells was calculated by the dilution of the CFSE fluorescent label by flow cytometry. These data demonstrate that the capacity of DCs to internalize and present antigen to T cells was unaffected in DCs from NKG2D-deficient mice compared with WT mice (Figure 4C).
Amelioration of Enhanced Respiratory Syncytial Virus–Induced Pathology in NKG2D-Deficient Mice by Adoptive Transfer of Wild-Type Dendritic Cells
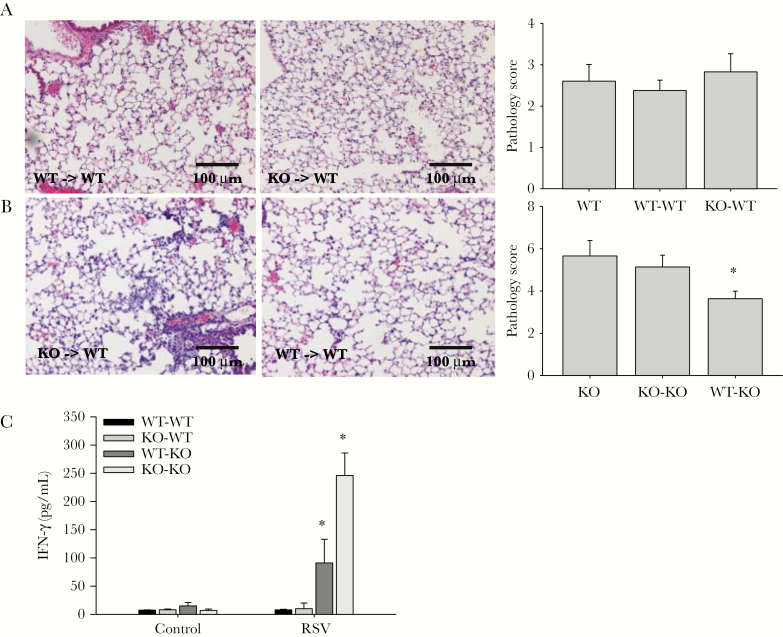
We performed adoptive transfer experiments to directly test whether WT DCs can modulate enhanced lung inflammation in NKG2D-deficient mice. Specifically, we transferred freshly isolated pulmonary DCs from WT or NKG2D-deficient mice into WT or KO recipient mice that then underwent RSV challenge. We examined lung pathology 5 days after infection. These studies demonstrate that adoptive transfer of neither WT DCs nor KO DCs into WT recipient mice altered the disease course and lung pathology (Figure 5A). However, transfer of WT DCs into KO recipient mice alleviated the lung pathology and partially rescued the phenotype displayed in NKG2D-deficient mice (Figure 5B). Furthermore, elaboration of the NK cell pro-inflammatory mediator IFN-γ was lower in NKG2D-deficient recipient mice that received WT DCs (Figure 5C). The amelioration of lung inflammation after adoptive transfer indicates that NKG2D-dependent DC editing is important in regulating immune response during RSV infection.
Figure 5.
Adoptive transfer of wild-type (WT) dendritic cells (DCs) into NKG2D-deficient mice alleviates the respiratory syncytial virus (RSV)–induced pathology. Dendritic cells were isolated from lungs of WT and NKG2D-deficient mice as described in Materials and Methods. Recipient mice received 2 million freshly isolated DCs intranasally in 50 µL of phosphate-buffered saline 24 hours before RSV challenge via the same route. Representative hematoxylin and eosin lung histology from WT (A) and NKG2D-deficient (B) recipient mice who received DCs isolated from WT/knockout (KO) mice 5 days after infection. Scale bar, 100 μm. Lung pathology scores from WT/KO recipient mice were calculated as described (A and B). Data are shown as the mean ± SEM of at least 5 independent experiments. *P < .05. C, Interferon γ level is measured in bronchoalveolar lavage of recipient mice by enzyme-linked immunosorbent assay 5 days after infection. n = 5. *P < .05. Abbreviations: IFN-γ, interferon γ; KO, knockout; RSV, respiratory syncytial virus; WT, wild-type.
DISCUSSION
The incomplete understanding of immunologic pathogenesis of RSV infection is a major obstacle to the development of effective and safe clinical therapies. This study aims to examine NKG2D-dependent DC functions in murine RSV infection, with an emphasis on the host immune response. Our experiments show that RSV infection–induced pulmonary inflammation is enhanced in NKG2D-deficient mice. We demonstrate that the NKG2D receptor is important in regulating DC accumulation, especially CD103 DCs, during RSV infection. Moreover, we observed subpopulations of DC expressing NKG2D ligands that increase in number in a NKG2D-deficient environment and during RSV infection. Furthermore, DCs isolated from NKG2D-deficient environments have impaired responses toward TLR3 and TLR4 ligands. Finally, lung inflammation in NKG2D-deficient mice after RSV infection was ameliorated after adoptive transfer of WT DCs prior to infection. Collectively, these data demonstrate a role for NKG2D in DC regulation that is important in the immune response to RSV infection.
The NKG2D receptor is a well-characterized activating receptor. Despite its well-known function in regulating the pathogenesis of other virus infections like influenza [16] and human cytomegalovirus infection [26], the importance of the NKG2D receptor in regulating RSV infection has received little attention. Data show that NK cells increased expression of NKG2D in response to RSV infection and secrete IFN-γ, which results in acute lung injury [4]. Our data suggest that there is no difference in viral titer in WT and NKG2D-deficient mice 5 days after RSV challenge, indicating that RSV clearance is independent of the NKG2D receptor ligand effects on DCs and suggests that the exaggerated lung pathology and weight loss induced by RSV infection is likely due to an impaired DC-related host immune response. In addition to DCs, other cells in the lung (such as epithelial cells and alveolar macrophages) may express NKG2D ligands during RSV infection and contribute to the response to and resolution of RSV infection. Future studies will examine the role of non-DC alveolar cells in the recruitment of and modulation of DC phenotype and function.
Dendritic cells play an important role in regulating the immune response against RSV infection, and the impaired antigen uptake and processing of neonatal DCs may explain some of the vulnerability of neonates to RSV [27, 28]. Previous studies indicate lung DCs can be infected with RSV directly [29]. After encounter with RSV, both CD103 DCs and CD11b DCs migrate to mLNs, where they present the antigens to T cells and induce adaptive immune responses [29]. Different subsets of DCs play different roles in RSV infection, with myeloid DCs (mDC) and pDCs modulating the innate immune response [29–31]. The presence of a large number of mDCs often indicates an adverse response to infection. The abnormal accumulation of mDCs in the lung impairs efficient pDC-induced Th-1–based antiviral responses, tipping the immune balance in favor of a Th-2–based response [32]. We observed enhanced RSV-induced lung inflammation in the NKG2D-deficient mice, accompanied by abnormal mDC accumulation. Interestingly, the accumulated mDCs in the NKG2D-deficient mice are composed primarily of CD103+ DCs as opposed to the CD11b DC–predominant inflammation found in WT mice. Whether these 2 subsets of mDCs play different roles in RSV infection has not been well studied. In other infectious diseases, CD103 DCs elicit stronger Th1 and Th17 responses and can induce T cells to secrete large quantities of IFN compared with CD11b DCs [33].
Dendritic cell effector functions are precisely coordinated during infection [34]. We are the first to show murine pulmonary DCs express NKG2D ligands that are further induced in NKG2D-deficient mice and in response to RSV infection. Previous in vitro studies have shown that human DCs express NKG2D ligands after infection with RSV [5] and that NK cells enhance DC responses to Toxoplasma gondii through an NKG2D-dependent mechanism [35]. Transwell experiments demonstrate direct cell–cell contact is required for this regulation and blocking the NKG2D receptor decreases IL-12 secretion from activated DCs when coculturing with NK cells [35]. It has been demonstrated that RSV-induced lung inflammation is increased in IL-12–deficient mice compared with WT mice [36]. In our study, we found that DCs isolated from NKG2D-deficient mice have impaired IL-12 secretion when activated with TLR ligands 3 and 4 but not 7 and 9. Interestingly, this impaired in vitro responsiveness toward TLR ligands by pulmonary DCs isolated from NKG2D-deficient mice was not observed in bone marrow–derived DCs or DCs isolated from spleens, suggesting that unique NKG2D-dependent DC functional subsets reside in the lung. Thus, our data suggest that NKG2D not only is important in mediating DC function during RSV infection but also has an important role in maintaining pulmonary DC homeostasis. Future studies will be required to identify the NKG2D receptor–carrying cells (eg, NK cells, T cells, NK T cells) that modify DC phenotype and function in lung by either direct (editing) or indirect mechanisms.
Adoptive transfer of DCs isolated from WT mice into the NKG2D-deficient mice before the RSV infection ameliorated viral-induced inflammation. The decreased IFN-γ secretion and pathology score demonstrate the protective role of the transplanted “normal” DCs. This rescue of phenotype demonstrates the importance of NKG2D receptor ligand interactions with DCs during an RSV infection. However, it is important to point out the pathology in the recipient mice is still more severe compared with WT mice, emphasizing the possibility that there are likely other cells affected by the absence of NKG2D receptors and that contribute to the pathology. It is interesting to point out that the transfer of DCs isolated from NKG2D-deficient mice into WT mice does not alter the phenotype, which indicates that host DCs have a survival advantage over DCs isolated from NKG2D-deficient mice. Alternatively, there may be enough remaining host DCs to maintain the normal immune response against RSV.
Taken together, our data demonstrate that NKG2D is necessary for the normal control of RSV infection. We speculate that the NKG2D-expressing cells are important in eliminating “unhealthy” DCs that express NKG2D ligands in the lung. In the absence of this surveillance mechanism, DCs accumulate during RSV infection. Thus, although some pulmonary DCs have impaired responses toward TLR ligands individually, the enhanced infiltration of DCs in the lung results in increased levels of pro-inflammatory cytokines in the NKG2D-deficient mice, which redirects the host immune responses, which results in enhanced pathology, such as augmented weight loss and lung inflammation. This newly described DC–NK cell pathway has novel implications for the regulation of DC function and provides insight into developing new potential targets aimed at resolving RSV-induced inflammation.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Financial Support. This work was funded in part by the support from the National Institutes of Health (HL119538 to M. T. B. and U54HL127672 to F. X. M.) and the Veteran’s Administration (I01BX002347 to M. T. B.)
References
- 1. Nair H, Nokes DJ, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dawson-Caswell M, Muncie HL Jr. Respiratory syncytial virus infection in children. Am Fam Physician 2011; 83:141–6. [PubMed] [Google Scholar]
- 3. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol 2012; 86:2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebihara T, Masuda H, Akazawa T et al. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int Immunol 2007; 19:1145–55. [DOI] [PubMed] [Google Scholar]
- 6. Bauer S, Groh V, Wu J et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285:727–9. [DOI] [PubMed] [Google Scholar]
- 7. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3:781–90. [DOI] [PubMed] [Google Scholar]
- 8. Groh V, Smythe K, Dai Z, Spies T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat Immunol 2006; 7:755–62. [DOI] [PubMed] [Google Scholar]
- 9. Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell 2010; 142:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nice TJ, Coscoy L, Raulet DH. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J Exp Med 2009; 206:287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu H, Jakubzick C, Osterburg AR et al. Dendritic cell trafficking and function in rare lung diseases. Am J Respir Cell Mol Biol 2017; 57:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andoniou CE, van Dommelen SL, Voigt V et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol 2005; 6:1011–9. [DOI] [PubMed] [Google Scholar]
- 13. Schwarze J. Lung dendritic cells in respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 2008; 27:S89–91. [DOI] [PubMed] [Google Scholar]
- 14. Guerra N, Tan YX, Joncker NT et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boukhvalova MS, Yim KC, Prince GA, Blanco JC. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real‐time reverse transcription–PCR in vivo: detection of abortive viral replication. Curr Protoc Cell Biol 2010; doi:10.1002/0471143030.cb2606s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wortham BW, Eppert BL, Motz GT et al. NKG2D mediates NK cell hyperresponsiveness and influenza-induced pathologies in a mouse model of chronic obstructive pulmonary disease. J Immunol 2012; 188:4468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wortham BW, Eppert BL, Flury JL, Morgado Garcia S, Borchers MT. TLR and NKG2D signaling pathways mediate CS-induced pulmonary pathologies. PLoS One 2013; 8:e78735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2-/- mice. Am J Respir Crit Care Med 2010; 181:1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J Immunol 2011; 187:3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osterburg AR, Nelson RL, Yaniv BZ et al. NK cell activating receptor ligand expression in lymphangioleiomyomatosis is associated with lung function decline. JCI Insight 2016; 1:e87270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barber MA, Zhang T, Gagne BA, Sentman CL. NK cells negatively regulate antigen presentation and tumor-specific CTLs in a syngeneic lymphoma model. J Immunol 2007; 178:6140–7. [DOI] [PubMed] [Google Scholar]
- 22. Owczarczyk AB, Schaller MA, Reed M, Rasky AJ, Lombard DB, Lukacs NW. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus-induced immune responses. J Immunol 2015; 195:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore ML, Newcomb DC, Parekh VV et al. STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J Immunol 2009; 183:2016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Draghi M, Pashine A, Sanjanwala B et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol 2007; 178:2688–98. [DOI] [PubMed] [Google Scholar]
- 25. Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol 2008; 181:8576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rölle A, Mousavi-Jazi M, Eriksson M et al. Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J Immunol 2003; 171:902–8. [DOI] [PubMed] [Google Scholar]
- 27. Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog 2014; 10:e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jang S, Smit J, Kallal LE, Lukacs NW. Respiratory syncytial virus infection modifies and accelerates pulmonary disease via DC activation and migration. J Leukoc Biol 2013; 94:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J Virol 2009; 83:7235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boogaard I, van Oosten M, van Rijt LS et al. Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells. Immunology 2007; 122:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One 2011; 6:e16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol 2010; 40:1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furuhashi K, Suda T, Hasegawa H et al. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am J Respir Cell Mol Biol 2012; 46:165–72. [DOI] [PubMed] [Google Scholar]
- 34. Moll H. Dendritic cells and host resistance to infection. Cell Microbiol 2003; 5:493–500. [DOI] [PubMed] [Google Scholar]
- 35. Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol 2007; 179:590–6. [DOI] [PubMed] [Google Scholar]
- 36. Wang SZ, Bao YX, Rosenberger CL, Tesfaigzi Y, Stark JM, Harrod KS. IL-12p40 and IL-18 modulate inflammatory and immune responses to respiratory syncytial virus infection. J Immunol 2004; 173:4040–9. [DOI] [PubMed] [Google Scholar]