Abstract
The vocal behavior of birds is remarkable for its diversity, and songs can feature elaborate characteristics such as long duration, rapid temporal pattern, and broad frequency range. The respiratory system plays a central role in generating the complex song patterns that must be integrated with its life-sustaining functions. Here, we explore how precise coordination between the neural circuits for breathing and singing is fundamental to production of these remarkable behaviors.
Birds such as the grasshopper warbler (Locustella naevia) or European nightjar (Caprimulgus europaeus) sing songs that consist of a rapid trill (25-28 syllables/s) that can last 30 s to over 1 min (11, 33). To a listener, these vocalizations sound like uninterrupted sounds, and one wonders how small birds can sustain song for so long. The rapid rate with which repeated sounds are produced during trills is equally remarkable (e.g., Refs. 65, 68). Species such as the Brewer's sparrow, Spizella breweri, and nightingale, Luscinia megarhynchos (Goller F, Todt D, Hultsch H, unpublished observations) can generate trill rates of up to 80–100 syllables/s. These two impressive temporal features of song, long duration and high trill rate, likely play a critical role in broadcasting the male's genotypic or phenotypic quality, which is used by females to select their mate (51, 67). This impressive temporal performance also poses significant questions about the physiological systems that produce them. Why do birds not run out of air during long songs, and how, for example, does the respiratory system contribute to the generation of such rapid song rhythms? To answer these questions, it is important to understand some of the features of the avian respiratory system and its interaction with the vocal production system.
Coordinating Breathing With Singing
Unlike mammals, the bird lung consists of rigid tubes, and air is moved through these by an elaborate air sac system that acts as a bellows (reviewed in Ref. 57). Pressure in the air sac system provides the force for ventilation of the lungs. We can experimentally monitor this pressure in one of the air sacs during song and therefore show how respiration changes during sound production. Typically, respiratory pressures are below 1 cmH2O during normal breathing and increase as much as 5- to 30-fold during phonation (e.g., Refs. 53, 65). This elevated pressure is accompanied by substantially increased effort of expiratory and inspiratory muscles (74), given that, without a mammalian-type diaphragm, expiration cannot be driven by passive recoil action. Both respiratory phases are therefore always actively driven by contraction of the respective muscles.
Air sac pressure measurements show how the elaborate temporal aspects of song are generated. During singing, birds typically produce sound with an expiratory airstream, and the available air supply in the air sac system therefore limits duration of continuous sound production. Many birds generate their songs by alternating expiratory pulses with short (12–15 ms), deep inspirations, called minibreaths, which enable them to sing songs of long duration (e.g., Ref. 32). These minibreaths require interruptions in sound production to permit refilling of the air sacs through inhalation. In many cases, however, these switches between respiratory phases can occur very rapidly during trills, with the highest minibreath rate measured in the Waterslager canary (Serinus canaria) at ∼30.s−1. Typically, however, minibreath rate is not much above 20.s−1 in wild bird species for which data are available (32, 53, 64, 65). Many species also produce trill rates that are much too fast (80–100.s−1) for producing minibreaths, and these trills are generated with a sustained expiratory pulse whose amplitude is, in part, actively modulated by rapid bursting of expiratory muscles. Here, sound pulses coincide with peaks in air sac pressure and silent periods with minima in this modulation. Because trill duration is limited by the air supply, temporal features of song, such as maximal trill rate, have been implicated as indicators of male fitness and are thought, therefore, to be under strong sexual selection (e.g., Refs. 51, 67). The respiratory constraints involved in producing the song therefore must be an important factor in the evolution of song features (FIGURE 1).
FIGURE 1.
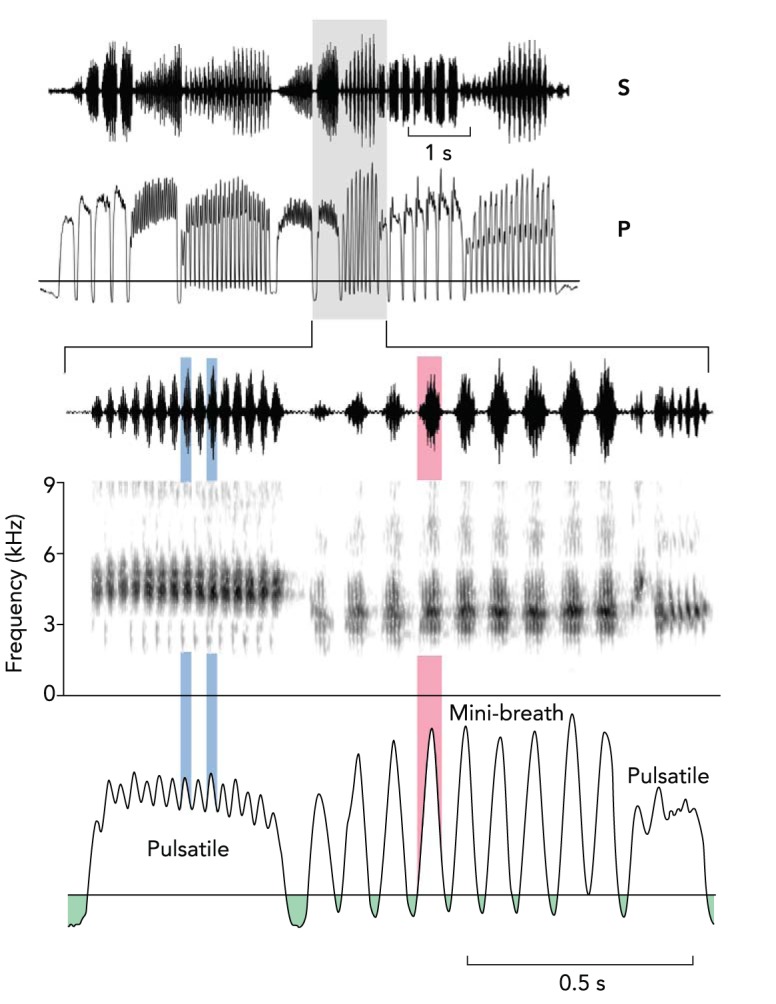
Song of the Brewer's sparrow (Spizella breweri) illustrates different respiratory mechanisms of trill production
Data represent simultaneously recorded subsyringeal air sac pressure (P) and sound (S). Air sac pressure patterns of a longer segment of song (top) indicate that respiratory activity is characteristic of the different syllable types and drives the observed changes in song rhythm. Two trill types of this song are shown in more detail below with spectrographic representation (gray area). A very high trill rate emerges from a pulsatile respiratory pattern (left), where air sac pressure is still modulated during the sustained expiration. Sound pulses generated during the maxima and minima coincide with silent periods in the trill. A second trill type is produced with mini-breaths (right). Mini-breaths ensure that air supply is replenished during silent inter-syllable intervals, but at the cost of trill rates that are lower than those generated with pulsatile respiration. Figure is modified from Ref. 28a with permission from Bentham Science Publishers.
In addition to restoring air supply during long songs, respiratory patterns during song also maintain gas exchange. In the few investigated species with varying song rhythms, oxygen uptake is not diminished even during long songs (e.g., Refs. 24, 48). However, pronounced and song-duration-dependent apneic periods after song (24, 32) suggest that the rapid switching between respiratory phases may result in increased carbon dioxide excretion, as is seen in other hyperventilatory situations. These observations suggest that song behavior must be integrated into the various homeostatic control mechanisms (gas exchange, acid-base balance) of respiration, which include the well characterized sensory feedback systems of the lung, air sacs, and putative sensory mechanisms of the avian vocal organ, known as the syrinx (FIGURE 2). However, the fine control of gas exchange and air supply during song via feedback mechanisms is largely unexplored.
FIGURE 2.
Schematic of experimental approach and recorded parameters
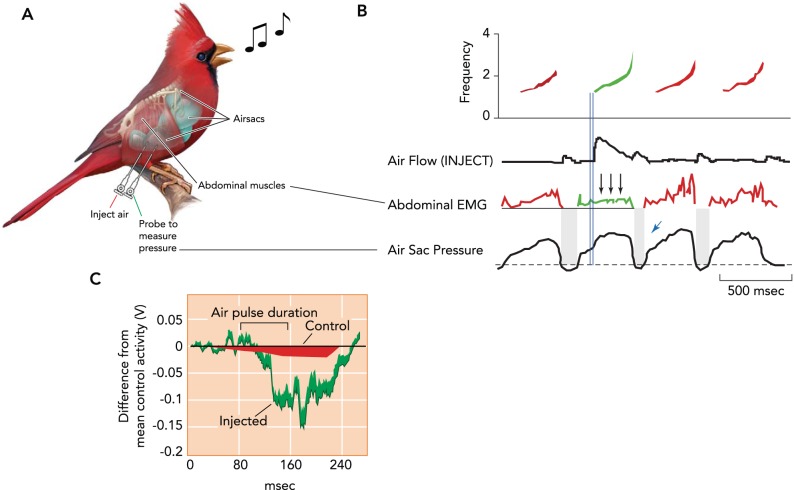
Northern cardinals (Cardinalis cardinalis) show a compensatory response in expiratory muscles when a supplementary air pulse is injected into the air sac system during singing. A: schematic of the experimental approach and recorded parameters. Subsyringeal pressure (air sac pressure) is measured via cannulae (green line; cannula to measure pressure) implanted in the air sacs, and abdominal muscle (M. obliquus externus abdominus) activation is measured by electrodes placed in the muscle (Abominal EMG). Although the cardinal is singing, air is injected directly into the air sac through a second cannula (red line; Inject Air). B: when an air pulse is injected into a thoracic air sac at the beginning of a syllable (second of four syllables; shown in green), activation of the expiratory muscles is reduced during the remainder of the syllable (three arrows). Injection flow (onset illustrated by the double blue lines) is depicted in the trace below the spectrogram, with control syllables in red and the stimulated syllable in green. Abdominal muscle activation is shown as the rectified and integrated amplitude of the recorded EMG. The small blue arrow depicts the smaller than normal airflow and duration of the minibreath following injection of the air puff. C: quantification showing the reduction in relative mean amplitude of abdominal muscle activation when air was injected (green) compared with uninjected control syllables (red). The decrease in activation occurs with a delay (∼50 ms) after the onset of injection. Figure is modified from Ref. 66 with permission from Proceedings of the National Academy of Sciences USA.
Generating Complex Songs Requires Precise Vocal-Respiratory Coordination
Although the motor systems for ventilation produce the airstream required for inducing self-sustained oscillations of the vibrating tissues (labia or membranes), the syringeal motor systems provide additional regulatory mechanisms for control of oscillatory behavior. The sound-generating tissues of the syrinx constitute a valve that can be used to regulate airflow during vocal and non-vocal behaviors. In fact, muscle activity during expiration is required to prevent the labia from engaging in oscillation. In some songbirds, denervation of the syringeal muscles causes wheezing or even complete closure of the syringeal lumen (e.g., Refs. 53, 54).
Syringeal control of airflow modulates the expiratory airstream, and this control can range from on- and offset of sound production to fine control of oscillation amplitude. Combined syringeal and respiratory activity therefore contributes to the fine control of acoustic parameters of sound. The best-documented feature is control of the fundamental frequency of sound (e.g., Refs. 21, 28). Pressurization of the air sacs during phonation generates a pressure differential across the syrinx and also from the interclavicular air sac into the trachea. These pressure gradients cause a displacement of the vibrating tissues into the lumen and in the direction of airflow, which affects their tension. In some suboscine species (e.g., tyrannid flycatchers), the fundamental frequency of sounds is very closely correlated to the driving pressure, and frequency is not directly controlled by syringeal muscles (4). In contrast, syringeal muscles in oscine songbirds regulate the tension of the vibrating tissues and thus provide the main frequency control mechanism (reviewed Ref. 28). However, even in these birds, pressure fluctuations exert forces on the labia and thus influence tension slightly. For example, if a bird sings the same sound with higher amplitude, i.e., with increased driving pressure, this increase will slightly elevate the tension of the labia. This raises the question of whether songbirds compensate for pressure difference-induced changes in tension by adjusting direct control of tension by syringeal muscles. In two species, fluctuations in driving pressure between renditions of the same syllable are positively correlated with corresponding variations in fundamental frequency [e.g., canary (2); zebra finch, Taeniopygia guttata (Goller F, unpublished observations)], suggesting that birds do not compensate for the slight variation in pressure. This interpretation is supported by experimental manipulation of air sac pressure during song (5) and by the magnitude of frequency changes, because it corresponds to that of purely pressure-driven changes as revealed after denervation of the syringeal muscles [i.e., when muscular control of sound frequency is not possible (20, 53)]. To what degree this is true in other species is unknown.
Sensory Feedback and Its Role in Maintaining a Stable Song Output
Elaborate respiratory activity sets the coarse temporal pattern of the bird's song while maintaining gas exchange and air supply. Respiratory activity also contributes to the syringeal control of acoustic parameters. This central role of respiration during song production requires intricate physiological integration into vital functions as well as delicate coordination with syringeal motor systems. Sensory feedback mechanisms must play a critical role in achieving this coordination, especially given the importance of the need to maintain a stable and steady song when it is intended for a female during the reproductive season.
In addition to the feedback channels typical for most motor systems, vocal production generates auditory feedback. This form of feedback has been studied extensively in the context of singing in large part because it is necessary for learning the song (45). Additionally, because its perturbation can lead to pronounced degradation of vocal output (7, 12, 25, 35, 47), it likely also plays a role in maintaining song stability. Interestingly, the effect of auditory feedback perturbation is not immediate, and, depending on how feedback is manipulated, degradation of the song motor pattern becomes apparent only after a significant delay. Initial work suggested this delay was weeks to months (reviewed in Ref. 13), but recent improvements in auditory feedback perturbation have shown that it is possible to alter overall song temporal patterns within a few days (13, 25) and acoustic properties within several hours (7, 25, 69). Despite its role in maintaining song stability, there is as yet no clear evidence that auditory feedback plays a role in immediate online adaptive modifications of motor output. This conclusion is strengthened, at least in the zebra finch, by recent work showing that auditory feedback perturbations do not alter the stereotyped cortical neural activation patterns responsible for generating song (31, 36, 70). This does not rule out, however, changes that might be induced by different feedback perturbations. If auditory feedback is not used for moment-to-moment correction of song, then instead it may inform the motor system of persistent errors in vocal output.
In contrast to auditory feedback, sensory inputs from the vocal-respiratory periphery are likely to have a more direct and immediate influence on song output. During singing, receptors in the airways and the vocal-respiratory apparatus become engaged, and the activation of their respective sensory systems forms a critical additional feedback system for vocal production. One class of sensory activation involves pressure sensors in the trachea and larynx, where airflow can vary significantly during phonation. This information is relayed primarily by the somatosensory system and has not been studied in songbirds. Its importance is highlighted, however, by studies in mammals where manipulation of this sensory system can significantly alter vocal output and speech in humans (reviewed in Ref. 59). A second class of sensory receptors is specifically associated with the vocal-respiratory apparatus and includes stretch and pressure receptors in the lungs and air sacs as well as Pco2-sensing chemosensory receptors in the lungs (reviewed in Ref. 57). Although little is known regarding the nature of the specific receptors in birds, this class of sensory information is relayed by the viscerosensory system and therefore carried by the vagus nerve to the respiratory brain stem via the nucleus of the tractus solitarius (NTS).
Viscerosensory feedback is intimately linked to the vital function of breathing, and its perturbation results in moment-to-moment compensation of normal eupneic breathing (37). Similar compensation seems to occur during vocal-driven respiration. The current view is that viscerosensory feedback loops can shape the breathing pattern on a cycle-by-cycle basis by directly modulating the respiratory pattern generating circuits in the brain stem (44). In mammals, the best-known example of this class of modulation is the Hering-Breuer reflex, where activation of stretch receptors in the lungs will cause premature termination of the inspiratory phase during both eupneic breathing (15) and vocal production (46). Although viscerosensory feedback is critical in both birds and mammals, some of the specific sensors are different. For example, because lung volume remains relatively fixed in birds, termination of inspiration appears instead to be controlled by intrapulmonary CO2 receptors innervated by vagal afferents, and respiratory pressure is monitored by stretch receptors in the air sacs, which undergo massive expansion during inspiration (reviewed in Ref. 57). Proprioceptive signals from respiratory muscles also seem to play a key role in providing feedback regarding the expansion and contraction of the thoracoabdominal cavity given that both expiration and inspiration are always actively controlled in birds.
The need to maintain a stable robust song during courtship makes it essential that small perturbations in the vocal respiratory system should elicit a near immediate compensatory response. In a now classic experiment, Suthers and colleagues used the Northern Cardinal (Cardinalis cardinalis) to show the precision of such compensation by injecting air through a small cannula in the air sac during singing and showing that these brief perturbations caused little to no noticeable effect on the acoustic properties of the song or the recorded subsyringeal pressure (66). Instead, these perturbations caused a near instantaneous (30- to 60-ms lag) compensatory decrease in EMG activity of the abdominal expiratory muscles. This observation suggests the existence of a rapid sensory-mediated modification of expiratory drive to maintain appropriate subsyringeal pressure and vocal output (FIGURE 2). Although the specific role of the vagus nerve was not tested directly in this experiment, its role is implied by evidence that unilateral vagotomy will cause immediate and significant perturbation to vocal output in both songbirds (42) and mammals (17). Therefore, unlike auditory feedback perturbations, which take time to modify song output, transient perturbations of vocal respiratory afferents directly engage the vocal motor systems to produce immediate compensatory changes necessary for maintaining a stable song output.
Neural Circuit for Vocal-Respiratory Control
Much of the work on vocal control in songbirds has focused on the nature of the neural commands generated by higher-order forebrain areas. Although this work has led to exciting findings on the nature of the abstract code generated by these “cortical” song circuits, it also has implied a more passive role for the brain stem circuits responsible for generating the vocal-respiratory pattern and processing viscerosensory feedback. An alternative view of song control, however, is one where the vocal-respiratory brain stem plays a much more active role in song production. This perspective paints the brain stem as a dynamic system that integrates descending “cortical” inputs with viscerosensory feedback to produce the vocal respiratory patterns that give rise to song (9) (FIGURE 3).
FIGURE 3.
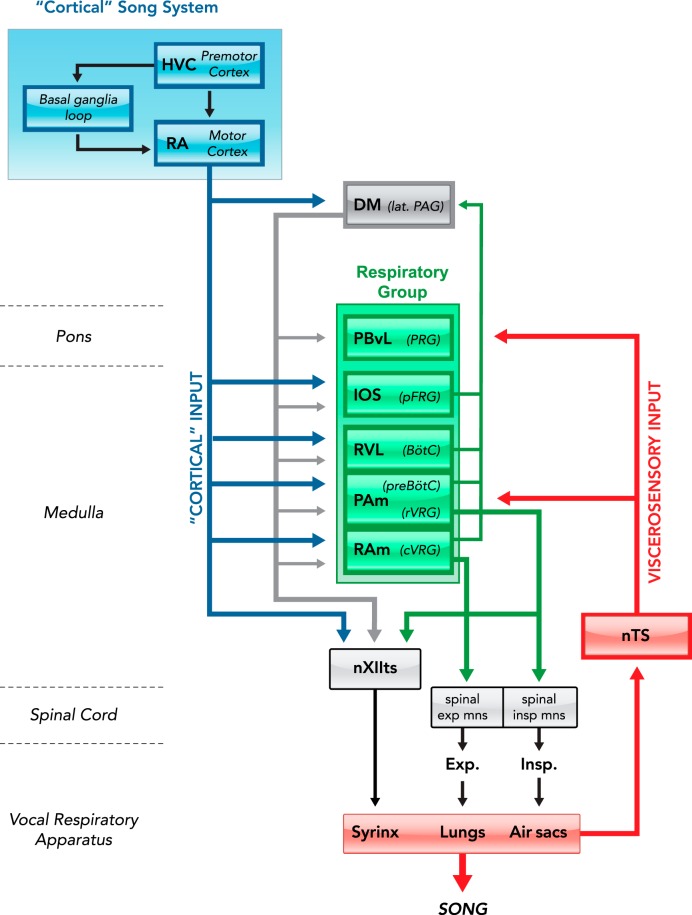
Schematic of the avian vocal control circuit highlighting the prominent position of the respiratory circuit (green) and its primary inputs from the “cortical” song system (blue) and viscerosensory afferents from the vocal respiratory apparatus (red)
For clarity, interconnectivity between the various divisions of the respiratory group is not shown. Anatomical names for each structure are placed to the left in bold, with likely mammalian homologs indicated in italics. RA, robust nucleus of the arcopallium; Uva, nucleus uvaeformis; DM, dorsomedial nucleus of the intercollicular complex; PBvl, ventrolateral part of the parabrachial nucleus; IOS, nucleus infra-olivarus superior; RVL, ventrolateral nucleus of the rostral medulla; PAm, nucleus parambigualis; nTS, nucleus of the solitary tract; RAm, nucleus retroambigualis; XIIts, tracheosyringeal part of the hypoglossal nucleus; Insp, inspiratory motoneurons at the level of spinal cord segment 14 (lower brachial); Exp, expiratory motoneurons at the level of spinal cord segment 19 (lower thoracic); PAG, periacqueductal gray; PRG, pontine respiratory group; pFRG, para-facial respiratory group; BötC, Bötzinger complex; preBötC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group.
During singing, many different systems become engaged (e.g., postural, beak gape, breathing, etc.). Because sound production itself can be remarkably well modeled by activation of the syringeal vocal musculature and the respiratory system, with the addition of some degree of upper vocal tract filtering (6, 9), much of the focus on neural control of song has been restricted to the neural circuitry that controls syringeal activation and breathing. The syringeal vocal motor system is made up of motoneurons in the tracheo-syringeal portion of the hypoglossal nucleus (nXIIts), which in oscine songbirds innervate the (at least) six bilaterally present syringeal muscle pairs (18). Based on hodological and recent molecular evidence, this motoneuron pool is somatic in origin and therefore fundamentally different from the laryngeal motoneurons of branchial origin that innervate the mammalian vocal organ (1). In songbirds, nXIIts is myotopically organized with specific motoneuron pools innervating the different muscles of the syrinx. It is also strongly innervated by medullary respiratory areas (as evidenced by the strong respiratory phase-locking observed under anesthesia) as well as the dorsal medial nucleus of the intercollicular complex (DM), a likely homolog to the lateral PAG (33), an area known to coordinate the respiratory pattern during vocalization in mammals (62). In addition to these inputs from the brain stem, the nXIIts also receives a robust “cortical” input from the ventral portion of forebrain nucleus RA (robust nucleus of the arcopallium), which might act as the functional equivalent of layer 5 of motor cortex.
The avian respiratory system is organized as a rostrocaudal column from the pons to the caudal medulla that shows an overall organization that is strikingly similar to that of the mammalian respiratory system (41, 73). In birds, as in mammals, the more caudal part of this medullary “ventral respiratory group” contains the pre-motoneuron pool that innervates expiratory motoneurons (n. retroambigualis; RAm), whereas the region immediately rostral to it (n. parambigualis; PAm) contains the pre-motoneurons that drive inspiration. Based on many decades of rigorous characterization, the prevailing view in mammals (and probably birds as well) is that the “ventral respiratory group” contains all of the intrinsic neural properties sufficient to generate the intrinsic rhythms for both inspiration and expiration (reviewed in Ref. 22). Interestingly, the most rostral part of PAm contains neurons with characteristics similar to those observed in the mammalian pre-Bötzinger complex, the mammalian pattern-generating network for inspiration (41). In addition to its abilities to generate the intrinsic rhythm for breathing, the respiratory brain stem can also reconfigure its rhythmic output in response to afferent feedback (52, 58) and thereby produce the respiratory patterns that are consistent with the animal's needs.
Despite major advances in our understanding of the neural mechanisms that underlie respiratory pattern generation, the majority of experiments in mammals, even those investigating stimulus-induced vocalization (61), have been performed in brain stem preparations that maximize the ability to study the pattern-generating circuitry in isolation. As a consequence, the general role of cortical input in modulating the respiratory system, especially in the context of vocal production, remains poorly understood. In songbirds, by contrast, the majority of studies have focused on a telencephalic neural circuit, known as the “song system,” and on its role in vocal production and learning (reviewed in Ref. 13). Anatomically, the majority of areas in the respiratory column, including RAm and PAm, receive robust inputs from RA as well as a strong input from DM, which itself also is strongly innervated by RA (reviewed in Ref. 57). Given these properties, we suggest that the avian vocal control circuit provides a unique system for investigating how the respiratory network integrates “cortical” inputs from RA with afferent inputs from the lungs, air sacs, and respiratory musculature to generate complex vocal respiratory patterns such as those observed during singing.
Forebrain-Brain Stem Coupling for the Control of Vocalization
A unique advantage of studying song in species such as the zebra finch, which sings a highly stereotyped song, is the ability to correlate with great temporal precision recorded single-unit neural activity to the specific acoustic features of the song. Recordings from nucleus HVC, a pre-motor area that provides the primary motor input to RA, suggest a rather unusual and abstract neural code with individual projection neurons (to RA) only producing a single short (∼8 ms) burst of action potentials during a given repeated stereotyped sequence of syllables (29, 40, 49), which in the zebra finch is known as a song motif. The temporal precision of this neural code is remarkable because neurons are active at the exact same time in the song from one rendition to the next. Given the one-to-many projection pattern from HVC to RA, neural recordings in RA exhibit an equally temporally precise neural pattern, but one that is much less sparse (14). Projection neurons in RA, rather than firing a single burst during a given song motif, produce multiple bursts with a temporal precision that is equal to that observed in HVC. It is therefore this temporally precise pattern of bursts that is presumably decoded and used by the brain stem respiratory and syringeal networks to drive vocal output (FIGURE 4).
FIGURE 4.
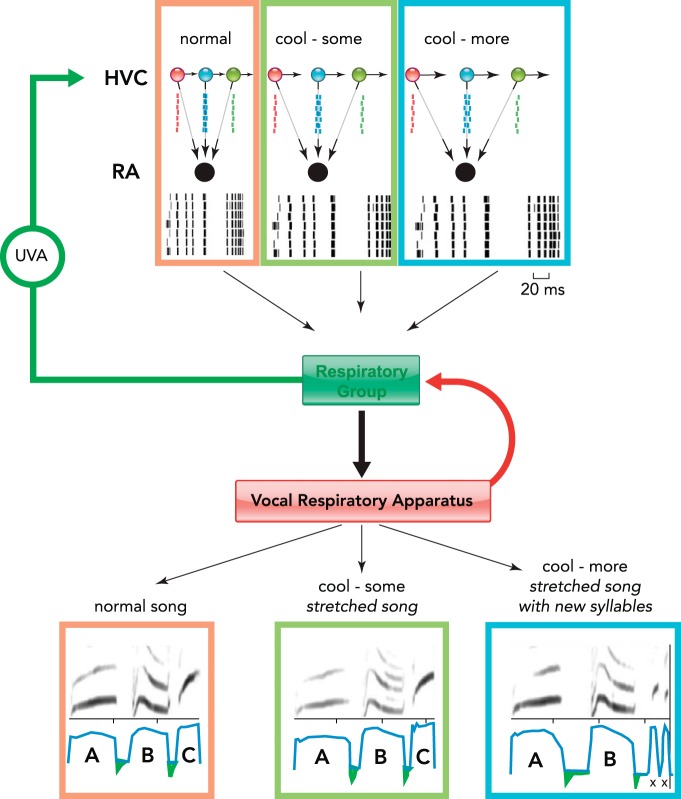
A model for transforming a temporal sparse code into respiratory patterns for song
Top: different neurons in HVC (red, blue, green) become sequentially active with each, only producing a single precisely timed burst of action potentials. Because of the many-to-one projection pattern, neurons in RA (black) receive many inputs that produce a temporally precise but complex code that is then transmitted to the respiratory system. The respiratory system then converts this code into the respiratory patterns necessary for the production of song syllables A-B-C (bottom left). In the process, the respiratory system, which receives sensory input from the vocal-respiratory apparatus, relays viscerosensory information back to HVC via the thalamic nucleus uvaeformis (UVA). Perturbation of HVC activity by cooling (green boxes), which slows the transition between sparsely firing neurons, results in a slowed temporally precise code in RA. This input gets transformed by the respiratory system into slightly stretched expiratory pulses (syllables A-B-C). Further cooling of HVC (blue boxes) results in an even more slowed neural code, which gets translated by the respiratory system as even more elongated syllables A and B. In the case of syllable C, however, the respiratory system transforms the slowed code for C into two new, shorter syllables (X). Spike rasters used to represent RA were recorded from an adult male zebra finch and were generously provided by Kyler J. Brown and Daniel Margoliash. Spectrograms of stretched syllables are the same as those in Fig. 2 in Ref. 27, where HVC in the canary was cooled to varying levels.
The existence of the sparse firing pattern in HVC gave rise to the hypothesis that song is functionally coded by the sequential activation of small populations of similarly active neurons that only produce a single ∼8-ms burst per motif (23, 26). This pattern of activation suggested an organization reminiscent of computational models known as synfire chains (6). Based on this idea, it was hypothesized that slowing transition latency between different links (i.e., cell groups) in the chain should slow down the song. In a clever experiment, Long and Fee tested this idea by placing a Peltier device directly on top of HVC, cooling it by just a few degrees (38). Cooling HVC had a direct effect on the stereotyped song of the zebra finch, causing it to slow down and stretch in a quasi-linear fashion. Although these findings are consistent with a synfire chain model, which assumes continuous sequential activation throughout the motif, we should mention that an alternative model has been suggested in which HVC neurons are proposed to be active predominantly at critical points where a change in peripheral motor control is required (6).
Together with other findings (39, 72), these experiments helped consolidate a top-down view of song motor control where the neural code in HVC determined both the acoustic and the temporal features of song. It also suggested that downstream areas, including the respiratory areas, played a less active role in song production, which was at odds with the known dynamic nature of the respiratory control system (44, 63). Interestingly, further investigations, using cooling of HVC while also monitoring changes in air sac pressure to more carefully quantify temporal transitions between expiration and inspiration, revealed that, although cooling affected both phases of breathing, it was less pronounced during the inspiratory minibreath. Specifically, the first half of the inspiratory phase showed no stretching in most syllables (8). These new findings suggest that forebrain motor commands determine syllable duration, whereas the control of inspiratory minibreaths is more complex, with influences likely from cortex, respiratory brain stem, and viscerosensory afferents.
How neural commands generated in HVC might shape the acoustic and temporal characteristics of individual syllables is not understood and is likely quite complex. A case in point is the quite different outcome that is obtained from similar HVC cooling experiments when performed in the canary, a different songbird species with a more complex syntax but acoustically simpler syllables (27). Here, mild cooling of HVC stretches individual syllables in a similar linear fashion to what is observed in the zebra finch. Cooling beyond a certain point, however, causes the syllable to eventually “break” and become transformed into two novel and very stable syllables of shorter duration (FIGURE 4). These findings suggest that a more integrated view of song control is necessary, one in which the nonlinear properties of the brain stem respiratory circuit could contribute to the formation of the observed respiratory pattern of song, rather than simply being driven passively by “cortical” motor commands (30, 56). Consistent with the idea that dynamic properties within the respiratory control system can transform higher-order neural commands into complex vocal respiratory patterns (60, 61), rhythmic stimulation of RA can cause stable entrainment of the respiratory rhythm in relatively complex but predictable subharmonic patterns. If the stimulus strength is sufficiently high, it can eventually lead to the production of long-duration expiratory pulses followed by minibreaths that resemble those observed during singing (43).
Do Respiratory Circuits Help Shape the “Cortical” Motor Commands They Learn to Decipher?
The nonlinear transformation between cortical motor commands and the respiratory circuit required to produce the stereotyped spectrotemporal patterns of song suggests that it might be a transformation that needs to be learned and one that forms a critical part of song ontogeny. In support of this idea, respiratory patterns in juvenile birds during subsong, the songbird equivalent to human babbling, are much more poorly synchronized with phonation than they are in the adult bird (64, 71). Where this learning occurs is not known, but it likely is spread across multiple levels of the vocal-respiratory system, given that perturbations at both forebrain and brain stem levels can influence vocal-respiratory coupling (71) and respiratory entrainment (19).
The existence in songbirds of feedback loops that link respiratory brain stem areas, such as PAm, back to the forebrain song control nucleus HVC suggests that the respiratory brain stem might itself also influence the generation of motor commands in the forebrain (10, 56). These feedback loops form a critical component of the song motor system, given that interruption of the PAm to HVC pathway completely disrupts singing (16). Although the presence of these feedback loops for normal song production is unequivocal, the nature of the information they carry is less clear. One possibility is that these loops carry motor-related information in the form of an efference copy of the intended motor commands for the inspiratory motoneurons that could be used to update an impending motor plan in HVC or act as a timing signal for synchronizing HVC with inspiratory onset (3, 55). Alternatively, feedback could be sensory in nature, relaying viscerosensory information from the air sacs and lungs, which enter PAm via the nTS, back to HVC. How such feedback would modify forebrain song motor commands is presently not clear, given recent findings that sustained decreases in air sac pressure fail to modify the motor code in HVC (70). In the end, these feedback circuits might well carry both motor- and sensory-related information. Either way, the bottom-up connections from the respiratory brain stem to HVC close a loop that creates a recurrent motor circuit where no single structure lies at the top of a hierarchy and where each forms a key link to the generation of song (3, 55).
Looking Forward: Birdsong as a Model for Central Integration of Distributed Respiratory Control
Behavioral evidence suggests strongly that elaborate respiratory “gymnastics” are an important component for generating “sexy” songs (e.g., Refs. 50, 51, 67). The remarkable diversity of vocal behaviors that exist in songbirds offers a rich field for exploration of respiratory dynamics and its integration into vocal motor control. During the evolution of learned vocal behavior, dedicated forebrain neural circuits had to become integrated with the more primitive life-sustaining brain stem circuitry for breathing. As the elaborate, sexually selected respiratory patterns of song evolved in various species, these songs were constrained by the need to maintain gas exchange, respiratory mechanics, and acid-base homeostasis. The need to maintain such a balance thereby put limits on the temporal boundaries that could be achieved in song. How birds balance the opposing needs for generating elaborate temporal patterns while remaining within the physiological constraints of the respiratory system remains one of the challenging questions for future research. Part of this balance is likely in the ability of respiratory circuits to seamlessly integrate “cortical” inputs with feedback from the vocal-respiratory periphery, an area of investigation that has received surprisingly little attention in mammals. Songbirds therefore provide an attractive model not only for investigating descending modulation of respiratory circuits but also in placing these respiratory circuits in roles that might go beyond respiratory control, especially given that these centers have the capacity to modulate “cortical” circuits in a bottom-up fashion.
Footnotes
This work was supported by funds from National Institute on Deafness and Other Communication Distorders Grant DC-006876 (F.G.) and The University of Pennsylvania Research Foundation (M.S.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: M.F.S. and F.G. interpreted results of experiments; M.F.S. and F.G. prepared figures; M.F.S. and F.G. drafted manuscript; M.F.S. and F.G. edited and revised manuscript; M.F.S. and F.G. approved final version of manuscript.
References
- 1.Albersheim-Carter J, Blubaum A, Ballagh IH, Missaghi K, Siuda ER, McMurray G, Bass AH, Dubuc R, Kelley DB, Schmidt MF, Wilson RJ, Gray PA. Testing the evolutionary conservation of vocal motoneurons in vertebrates. Respir Physiol Neurobiol : 2–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso R, Goller F, Mindlin GB. Motor control of sound frequency in birdsong involves the interaction between air sac pressure and labial tension. Phys Rev E Stat Nonlin Soft Matter Phys : 032706, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso RG, Trevisan MA, Amador A, Goller F, Mindlin GB. A circular model for song motor control in Serinus canaria. Front Comp Neurosci : 41, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amador A, Goller F, Mindlin GB. Frequency modulation during song in a suboscine does not require vocal muscles. J Neurophysiol : 2383–2389, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Amador A, Margoliash D. A mechanism for frequency modulation in songbirds shared with humans. J Neurosci : 11136–11144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amador A, Perl YS, Mindlin GB, Margoliash D. Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature : 59–64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA : 12518–12523, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andalman AS, Foerster JN, Fee MS. Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. PLos One : e25461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arneodo EM, Perl YS, Goller F, Mindlin GB. Prosthetic avian vocal organ controlled by a freely behaving bird based on a low dimensional model of the biomechanical periphery. PLoS Comput Biol : e1002546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J Neurosci : 2613–2623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brackenbury JH. Comparison of origin and temporal arrangement of pulsed sounds in songs of grasshopper and sedge warblers, Locustella-Naevia and Acrocephalus-Schoenobaenus. J Zool Lond : 187–206, 1978. [Google Scholar]
- 12.Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nature Rev Neurosci : 31–40, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annu Rev Neurosci : 489–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron : 899–910, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Clark FJ, Voneuler C. Regulation of depth and rate of breathing. J Physiol : 267–295, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman MJ, Vu ET. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J Neurobiol : 70–89, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Davis PJ, Zhang SP, Bandler R. Pulmonary and upper airway afferent influences on the motor pattern of vocalization evoked by excitation of the midbrain periaqueductal gray of the cat. Brain Res : 61–80, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Düring DN, Ziegler A, Thompson CK, Ziegler A, Faber C, Müller J, Scharff C, Elemans CPH. The songbird syrinx morphome: a three-dimensional, high-resolution, interactive morphological map of the zebra finch vocal organ. BMC Biol : 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutschman M, Moerschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol : 4931–4948, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elemans CP, Rasmussen JH, Herbst CT, During DN, Zollinger SA, Brumm H, Srivastava K, Svane N, Ding M, Larsen ON, Sober SJ, Svec JG. Universal mechanisms of sound production and control in birds and mammals. Nat Commun : 8978, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elemans CPH. The singer and the song: the neuromechanics of avian sound production. Curr Opin Neurobiol : 172–178, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol : 423–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. J Neurophysiol : 2038–2057, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Franz M, Goller F. Respiratory patterns and oxygen consumption in singing zebra finches. J Exp Biol : 967–978, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima M, Margoliash D. The effects of delayed auditory feedback revealed by bone conduction microphone in adult zebra finches. Sci Rep : 8800, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibb L, Gentner TQ, Abarbanel HD. Inhibition and recurrent excitation in a computational model of sparse bursting in song nucleus HVC. J Neurophysiol : 1748–1762, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldin MA, Alonso LM, Alliende JA, Goller F, Mindlin GB. Temperature induced syllable breaking unveils nonlinearly interacting timescales in birdsong motor pathway. PLos One : e67814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goller F, Riede T. Integrative physiology of fundamental frequency control in birds. J Physiol (Paris) : 230–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Goller F. Sound production and modification in birds: mechanisms, methodology and open questions. In: Comparative Bioacoustics: An Overview, edited by Brown C, Riede T. Sharjah, United Arab Emirates: Bentham Science. In press. [Google Scholar]
- 29.Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature : 65–70, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Hamaguchi K, Tanaka M, Mooney R. Distributed recurrent network contributes to temporally precise vocalizations. Neuron : 680–693, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaguchi K, Tschida KA, Yoon I, Donald BR, Mooney R. Auditory synapses to song premotor neurons are gated off during vocalization in zebra finches. Elife : e01833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartley RS, Suthers RA. Airflow and pressure during canary song: evidence for minibreaths. J Comp Physiol A : 15–26, 1989. [Google Scholar]
- 33.Hunter ML. Vocalization during Inhalation in a Nightjar. Condor : 101–103, 1980. [Google Scholar]
- 34.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLos One : e20720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol : 770–783, 1965. [PubMed] [Google Scholar]
- 36.Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol : 4271–4283, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol : 618–627, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature : 189–194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature : 394–399, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch GF, Okubo TS, Hanuschkin A, Hahnloser RH, Fee MS. Rhythmic continuous-time coding in the songbird analog of vocal motor cortex. Neuron : 877–892, 2016. [DOI] [PubMed] [Google Scholar]
- 41.McLean J, Bricault S, Schmidt MF. Characterization of respiratory neurons in the rostral ventrolateral medulla, an area critical for vocal production in songbirds. J Neurophysiol : 948–957, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez JM, Dall'Asen AG, Goller F. Disrupting vagal feedback affects birdsong motor control. J Exp Biol : 4193–4204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez JM, Mindlin GB, Goller F. Interaction between telencephalic signals and respiratory dynamics in songbirds. J Neurophysiol : 2971–2983, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molkov YI, Bacak BJ, Dick TE, Rybak IA. Control of breathing by interacting pontine and pulmonary feedback loops. Front Neural Circuits : 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mooney R. Neural mechanisms for learned birdsong. Learning Memory : 655–669, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Nakazawa K, Shiba K, Satoh I, Yoshida K, Nakajima Y, Konno A. Role of pulmonary afferent inputs in vocal on-switch in the cat. J Neurosci Res : 49–54, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol : 58–66, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Oberweger K, Goller F. The metabolic cost of birdsong production. J Exp Biol : 3379–3388, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Picardo MA, Merel J, Katlowitz KA, Vallentin D, Okobi DE, Benezra SE, Clary RC, Pnevmatikakis EA, Paninski L, Long MA. Population-level representation of a temporal sequence underlying song production in the zebra finch. Neuron : 866–876, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podos J. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes:Emberizidae). Evolution : 537–551, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Podos J, Nowicki editors S. Performance Limits on Birdsong Production. New York: Elsevier, 2004, p. 318–341. [Google Scholar]
- 52.Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci : 1965–1978, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riede T, Goller F. Peripheral mechanisms for vocal production in birds: differences and similarities to human speech and singing. Brain Language : 69–80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riede T, Schilling N, Goller F. The acoustic effect of vocal tract adjustments in zebra finches. J Comp Physiol A Neuroethol Sens Neural Behav Physiol : 57–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt M, Ashmore R, Vu E. Bilateral control and interhemispheric coordination in the avian song motor system. Behav Neurobiol Birdsong : 171–186, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt MF, Ashmore RC. Integrating breathing and singing: forebrain and brainstem mechanisms. In: Neuroscience of Birdsong , edited by Zeigler HP, Marler P. New York: Cambridge Univ. Press, 2008, p. 115–135. [Google Scholar]
- 57.Schmidt MF, Wild JM. The respiratory-vocal system of songbirds: anatomy, physiology, and neural control. Prog Brain Res : 297–335, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JC, Abdala APL, Rybak IA, Paton JFR. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci : 2577–2587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smotherman MS. Sensory feedback control of mammalian vocalizations. Behav Brain Res : 315–326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramanian HH, Arun M, Silburn PA, Holstege G. Motor organization of positive and negative emotional vocalization in the cat midbrain periaqueductal gray. J Comp Neurol : 1540–1557, 2016. [DOI] [PubMed] [Google Scholar]
- 61.Subramanian HH, Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res : 351–384, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Subramanian HH, Holstege G. Periaqueductal Gray Control of Breathing. Adv Exp Med Biol : 353–358, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian HH, Holstege G. Stimulation of the midbrain periaqueductal gray modulates preinspiratory neurons in the ventrolateral medulla in the rat in vivo. J Comp Neurol : 3083–3098, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suthers RA. (Editor). How Birds Sing and Why It Matters. New York: Elsevier, 2004. [Google Scholar]
- 65.Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. Curr Ornithol : 235–288, 1997. [Google Scholar]
- 66.Suthers RA, Goller F, Wild JM. Somatosensory feedback modulates the respiratory motor program of crystallized birdsong. Proc Natl Acad Sci USA : 5680–5685, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suthers RA, Vallet E, Kreutzer M. Bilateral coordination and the motor basis of female preference for sexual signals in canary song. J Exp Biol : 2950–2959, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract, In: Neuroscience of Birdsong Zeigler HP, Marler P. New York: Cambridge Univ. Press, 2008, p.78–98. [Google Scholar]
- 69.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature : 1240–1244, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Vallentin D, Long MA. Motor origin of precise synaptic inputs onto forebrain neurons driving a skilled behavior. J Neurosci : 299–307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veit L, Aronov D, Fee MS. Learning to breathe and sing: development of respiratory-vocal coordination in young songbirds. J Neurophysiol : 1747–1765, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci : 6924–6934, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wild JM. Functional neuroanatomy of the sensorimotor control of singing. Ann NY Acad Sci : 438–462, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Wild JM, Goller F, Suthers RA. Inspiratory muscle activity during bird song. J Neurobiol : 441–453, 1998. [DOI] [PubMed] [Google Scholar]