Abstract
Passive heat therapy (repeated hot tub or sauna use) reduces cardiovascular risk, but its effects on the mechanisms underlying improvements in microvascular function have yet to be studied. We investigated the effects of heat therapy on microvascular function and whether improvements were related to changes in nitric oxide (NO) bioavailability using cutaneous microdialysis. Eighteen young, sedentary, otherwise healthy subjects participated in 8 wk of heat therapy (hot water immersion to maintain rectal temperature ≥38.5°C for 60 min/session; n = 9) or thermoneutral water immersion (sham, n = 9), and participated in experiments before and after the 8-wk intervention in which forearm cutaneous hyperemia to 39°C local heating was assessed at three microdialysis sites receiving 1) Lactated Ringer's (Control), 2) Nω-nitro-l-arginine (l-NNA; nonspecific NO synthase inhibitor), and 3) 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol), a superoxide dismutase mimetic. The arm used for microdialysis experiments remained out of the water at all times. Data are means ± SE cutaneous vascular conductance (CVC = laser Doppler flux/mean arterial pressure), presented as percent maximal CVC (% CVCmax). Heat therapy increased local heating plateau from 42 ± 6 to 53 ± 6% CVCmax (P < 0.001) and increased NO-dependent dilation (difference in plateau between Control and l-NNA sites) from 26 ± 6 to 38 ± 4% CVCmax (P < 0.01), while no changes were observed in the sham group. When data were pooled across all subjects at 0 wk, Tempol had no effect on the local heating response (P = 0.53 vs. Control). There were no changes at the Tempol site across interventions (P = 0.58). Passive heat therapy improves cutaneous microvascular function by improving NO-dependent dilation, which may have clinical implications.
Keywords: laser Doppler flowmetry, hot water immersion, oxidative stress, microdialysis
NEW & NOTEWORTHY
We showed for the first time that passive heat therapy improves cutaneous microvascular function in humans via improved nitric oxide (NO)-dependent dilation. This is the first study to investigate the mechanisms underlying improvements in vascular health associated with heat therapy. Our data add to the currently limited but strong evidence suggesting heat therapy could be a powerful novel tool for improving cardiovascular health, particularly in disease states characterized by impaired vascular function secondary to reduced NO bioavailability.
passive heat therapy is a novel intervention for improving cardiovascular health that may prove to be a viable alternative for a variety of patient populations, particularly those who are unable to exercise at an extent great enough to induce cardioprotective effects. Short-term (2-3 wk) sauna therapy has been shown to improve some aspects of conduit vessel function in patients with elevated cardiovascular risk (25, 30). Recently, we reported that 8 wk of heat therapy improves conduit vessel endothelial-dependent dilation and arterial stiffness in young, sedentary individuals (47). However, microvascular dysfunction often precedes dysfunction in the conduit vessels (1). Furthermore, microvascular dysfunction is attributable to impaired nitric oxide (NO) signaling in the majority of cardiovascular-related diseases. Therefore, developing novel strategies to specifically target this important vascular bed is of critical importance. Few studies to date have investigated the effects of heat therapy on microvascular function in humans, and none have investigated the molecular mechanisms that underscore potential adaptions.
In humans, the cutaneous circulation offers an ideal site to study microvascular function. The accessibility of the skin allows the molecular pathways to be studied easily and relatively noninvasively using cutaneous microdialysis. A commonly used test of cutaneous microvascular function is thermal hyperemia, in which the skin is locally heated to 39–42°C, producing a biphasic vasodilator response (9, 19, 33, 35). Importantly, thermal hyperemia has been shown to be impaired under a variety of disease conditions (3, 36, 42, 44) and to mirror microvascular dysfunction in other microvascular beds. For example, thermal hyperemia is substantially impaired in diseases such as end-stage renal disease (31), essential hypertension (40), type II diabetes mellitus (11), and coronary artery disease (2), all of which are characterized by impaired microvascular function independent of changes in the cutaneous circulation. Furthermore, the secondary plateau phase is primarily dependent on NO (9, 29, 35). Given that the majority of disease states are characterized by impaired NO-dependent dilation and that the skin offers an accessible means of investigating the underlying mechanisms of microvascular function, thermal hyperemia is an ideal test for assessing the potential therapeutic effects of heat therapy on the microvasculature.
Only one study has so far investigated the effects of chronic passive heating on cutaneous thermal hyperemia. Carter et al. (7) showed that 8 wk of lower limb heating sufficient to raise body core temperature by 0.5–1.0°C increased the cutaneous forearm response to a local thermal stimulus. However, a few limitations to this study warrant further investigation. For example, maximal vasodilation at the skin sites were not obtained, a commonly performed practice to account for limitations in the laser Doppler flowmetry technique (12). In this setting, recording maximal skin blood flow is necessary to determine whether any observed changes were due to a structural or functional improvement. Additionally, to test microvascular function, a slow local heating protocol to 42°C was utilized, which has been shown in microdialysis studies to be only ∼30% NO dependent (9) and to be confounded by axon reflex involvement (24). Furthermore, the underlying mechanisms were not investigated, so it is unknown whether the improvements in skin blood flow were due to improved NO bioavailability.
NO bioavailability and cutaneous thermal hyperemia can also be limited by oxidative stress (33). The effects of oxidative stress can by investigated via microdialysis using 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol), a superoxide dismutase mimetic. While Tempol has been shown to have no effect in young, recreationally active subjects when locally heating to 42°C (33), this protocol elicits vasodilation to ∼85–95% of maximal, and so a ceiling effect may have been observed. The effects of Tempol have not yet been investigated using local heating to 39°C, which reaches a plateau of ∼40–60% of maximal (9), allowing the effects of pharmacological agents and/or interventions which may increase observation of vasodilation. Additionally, Tempol has not been investigated in sedentary subjects or following long-term heat therapy. It has been reported that short-term heat acclimation (≤14 days) can increase total peroxide concentration and oxidative stress index (28). However, heat shock proteins, which are elevated following heat acclimation (32), can increase expression of antioxidative enzymes, including superoxide dismutase (10).
Therefore, we investigated the effects of 8 wk of passive heat therapy on cutaneous microvascular function, consisting of four to five sessions per wk in which rectal temperature (Tre) was maintained at ≥38.5°C for 60 min per session throughout the 8 wk. The arm used for microdialysis studies was kept out of the water the entire time so that adaptations would be induced by systemic increases in body core temperature rather than increases in local skin temperature. We chose to study sedentary (but otherwise healthy) subjects to investigate the mechanisms of how heat therapy affects microvascular function without the confounding factors of a disease state, while still providing translatability of our data by studying a population with elevated vascular risk. To assess cutaneous microvascular function, we utilized rapid local heating to 39°C, a thermal hyperemia protocol which is ∼80% dependent on NO (9). Specifically, we hypothesized that, compared with a sham group (thermoneutral water immersion), 8 wk of heat therapy would improve the overall plateau response of 39°C cutaneous thermal hyperemia by improving NO-dependent dilation [investigated by delivering a NO synthase (NOS) inhibitor via microdialysis]. Second, we hypothesized that Tempol would augment thermal hyperemia in sedentary subjects prior to heat therapy, and that Tempol-mediated dilation (the difference between Control and Tempol microdialysis sites, indicative of oxidative stress) would decrease with 8 wk of heat therapy.
METHODS
Subjects
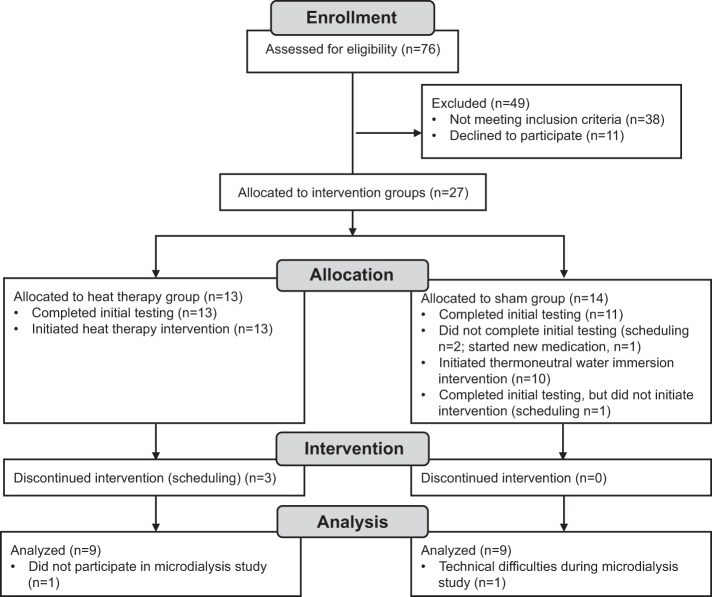
Of 76 subjects assessed for eligibility, 27 were enrolled in the study (Fig. 1). All were young (18–30 yr), sedentary (<2 h aerobic exercise per wk), nonsmokers. Subjects underwent a health screening and were excluded if they had any cardiovascular-related diseases, took any prescription medications, with the exception of contraceptives, or had previously had any heat-related illness. All subjects provided oral and written informed consent as set forth by the Declaration of Helsinki. Studies were approved by the Institutional Review Board at the University of Oregon.
Fig. 1.
Progression through the phases of the study.
The study was registered as a clinical trial at clinicaltrials.gov (NCT02518399; https://www.clinicaltrials.gov/ct2/show/NCT02518399?term=heat+therapy&rank=1). Included in this study were the same subjects as those in which we measured macrovascular function (on separate experimental days), as published previously (47).
Intervention
Upon enrollment, subjects were assigned by investigators to participate in either 8 wk of heat therapy or thermoneutral water immersion (sham group). Due to the smaller cohort size, subjects were not randomly assigned to groups, but were instead assigned by investigators to match sex, age, body mass index (BMI), and time of year across the groups. Of the 27 subjects initially enrolled, 24 completed the initial (0 wk) testing, 23 initiated participation in the intervention, and 20 completed the full 8 wk intervention. Of these 20, data were analyzed from 18 (n = 9 in each group). One subject elected not to participate in the microdialysis experiments, and we excluded data from one subject in which we experienced technical difficulties (nonphysiological responses across the sites, most likely due to a heater malfunction). Although outside temperature varied over the course of the study, similar numbers of subjects participated in the heat therapy and sham groups in each season. Subjects were instructed to maintain any regular physical activity (such as walking to school or work) consistently across the 8 wk and to avoid hot tub or sauna use outside of the lab. No subjects traveled to warmer climates at least 2 mo prior to or during the 8-wk intervention.
Subjects reported to the laboratory four to five times per week for heat therapy or thermoneutral water immersion sessions (36 sessions total across the 8 wk). All subjects provided a first-morning urine sample prior to each session to ensure euhydration [urine specific gravity (USG) <1.02]. If USG was >1.02, subjects drank 5 ml/kg water prior to entering the tub. Subjects were instrumented with a sterile rectal thermistor (YSI Series 400; Yellow Spring Instruments, Yellow Springs, OH) inserted ∼10 cm past the anal sphincter (Tre) and a heart rate monitor chest strap (Polar Electro Inc., Lake Success, NY).
For heat therapy, subjects were immersed up to the shoulder (except for the arm used for microdialysis experiments) in a 40.5°C hot tub until Tre reached 38.5°C, which took ∼25–35 min. Once target Tre was reached, subjects sat up such that the water reached waist level for another 60 min, or until the total time of hot water immersion reached 90 min, whichever came first. While the subject was sitting up, Tre was maintained between 38.5 and 39.0°C. This heating protocol was selected because 60 min spent at 38.5°C has been previously shown to be the most effective passive hyperthermia protocol for inducing classical markers of heat acclimation (14) and because 38.5°C is considered to be the threshold temperature required to induce detectable increases in heat shock protein (Hsp) expression (43).
Subjects in the sham group were immersed to the shoulder in a 36°C tub for 30 min and then sat up for another 60 min (90 min total). This protocol was selected to match hydrostatic pressures experienced by the heat therapy group. A water temperature of 36°C was selected to match weighted mean body temperature, ensuring minimal transfer of heat between the water and subjects. No changes in Tre >0.2°C were observed in any sham subjects.
Experimental Measures
Prior to and following the 8 wk of heat therapy or thermoneutral water immersion, subjects reported to the lab having refrained from all over-the-counter medications, including vitamins and supplements for 24 h, alcohol and caffeine for at least 12 h, food for at least 4 h, and exercise for at least 24 h. Female subjects provided a negative pregnancy test prior to studies, measured using urine human chorionic ganadotropin. Studies were held at the same time of day within subjects and the poststudy was held at least 48 h after the last water immersion session to capture the chronic effects of heat therapy. Female subjects taking hormonal contraceptives (n = 10) were studied during the active phase. We did not control for menstrual phase in naturally menstruating females (n = 2), but the phase they were in was consistent between the pre- and poststudies. Because this investigation was part of a larger study in which subjects reported to the lab every 2 wk, it was not possible to always study women under low hormone conditions. We chose this approach to control for hormone status across experimental sessions within subjects as best as possible. Studies were held on separate days from macrovascular function testing (47), separated by at least 24 h.
Upon arrival at the laboratory, subject height and weight were obtained. Subjects then rested semisupine, and three microdialysis fibers [MD2000, 30-kDa cutoff membrane (Bioanalytical Systems, West Lafeyette, IN) or CMA 31 Linear Probe, 55-kDa cutoff membrane (CMA Microdialysis, Kista, Sweden)] were placed at least 5 cm apart in the ventral skin of the nondominant forearm. Fibers were introduced with a 25-gauge needle, which was inserted ∼1 mm below the surface of the skin with entry and exit sites ∼2.5 cm apart. Fibers were then threaded through the lumen of the needle and the needle was removed, leaving the fiber in place under the skin. Fibers were secured with tape and infused with Lactated Ringer's solution at a rate of 2.0 μl/min (CMA 102 Microdialysis Pump; CMA Microdialysis) until the start of study drugs. Due to manufacturing issues, we had to switch to a different microdialysis fiber manufacturer midway through the study. However, each subject received fibers from the same manufacturer pre- and poststudy. All drugs delivered have molecular weights well below the membrane cutoffs, the concentrations of drugs delivered were high enough to cause maximal inhibition, and the infusion rate was low enough to allow for equilibration with the tissue. Therefore, this switch should not have impacted our results.
A period of at least 60 min was allowed for the trauma associated with needle insertion to subside. During this time, subjects were instrumented with local heaters (SH02 Skin Heater/Temperature Monitor; Moor Instruments, Axminster, United Kingdom) over each microdialysis site. Laser Doppler flowmeter probes (MoorLab; Moor Instruments) were seated in the center of each local heater, flush with the skin to measure red blood cell flux, an index of skin blood flow. A fourth local heater with laser Doppler probe was placed at a fourth site that did not have a microdialysis fiber. A blood pressure cuff was placed on the brachium of the opposite arm (Datex Ohmeda CardioCap; GE Medical Systems, Tampa, FL).
After flux had returned to baseline values, local heaters were set to 33°C and a 5-min baseline was recorded. After this, microdialysis fibers were randomly assigned to receive: 1) Lactated Ringer's (sham site), 2) 10 mM Nω-nitro-l-arginine (l-NNA; Sigma-Aldrich, St. Louis, MO), a nonspecific NO synthase inhibitor, or 3) 10 μM Tempol (EMD Millipore Chemicals, Billerica, MA), a superoxide dismutase mimetic, to reduce oxidative stress. All drugs were dissolved in Lactated Ringer's solution. Concentrations of each drug were selected based on previous studies as the minimum concentrations capable of producing maximal inhibition (33, 34). To confirm efficacy of this concentration of Tempol, we performed additional pilot data in subjects with chronic spinal cord injury (SCI), which is known to be associated with elevated oxidative stress (26) (see DISCUSSION). Drugs were delivered for 60 min, after which a second postdrug 5-min baseline was recorded, immediately prior to the start of local heating.
The local heaters were increased to a temperature of 39°C at a rate of 0.1°C/s and maintained at this temperature until skin blood flux reached a stable plateau for at least 5 min, which took ∼30–45 min. Once a stable plateau in skin blood flux was reached, the local heaters were further increased to 43.5°C at a rate of 0.1°C/s and all fibers were infused with 56 mM sodium nitroprusside (US Pharmacopeia, Rockville, MD) to obtain maximal flux.
Data Analysis
Data were digitized and recorded to a computer using data acquisition software (Windaq; Dataq Instruments, Akron, OH). The local heating response was characterized by initial peak, nadir, and plateau. The initial peak was determined as the highest 30-s period of flux values occurring within the first 5 min of local heating. Nadir was determined as the lowest 30-s period of flux values occurring within 5 min following the initial peak. Plateau was averaged over the last 5 min of stable flux values prior to moving on to obtaining maximal flux. All flux values were then converted to cutaneous vascular conductance (CVC = flux/mean arterial pressure) and normalized to a percentage of maximal CVC (% CVCmax). There were no differences between CVC at the Lactated Ringer's site and the site which had no fiber (paired t-test across all 0 wk data: P = 0.49) and so data were averaged (Control). NO-dependent dilation was calculated as the difference in normalized plateau CVC between the Control and l-NNA sites. Tempol-mediated dilation was calculated as the difference in normalized plateau CVC between the Tempol and Control sites.
Statistics
Subject demographic data (age, height, weight, BMI) were compared across groups using Student's unpaired t-test. Mean arterial blood pressure was compared across groups and across time (0 vs. 8 wk) using two-way mixed design ANOVA with a between factor of group and a within factor of week.
Effects of the drugs using data pooled across all subjects who completed 0 wk testing.
Data were pooled across 22 subjects who completed initial (0 wk) testing. Data from two subjects were excluded due to the same reasons previously described. Changes in normalized CVC were compared using two-way repeated measures ANOVA with factors of drug site (Control, l-NNA, and Tempol) and phase into local heating (baseline, peak, nadir, and plateau). Comparisons across paired variables were made with Bonferroni's post hoc test. Maximal CVC was compared across drug sites using one-way repeated measures ANOVA. Pre- to postdrug baseline was compared at each site using Student's paired two-tailed t-test.
Effects of the interventions.
Changes in normalized CVC at the Control site (e.g., the normal response) were compared using three-way mixed design ANOVA with a between factor of group and within/paired factors of week (0 vs. 8) and phase into local heating (baseline, peak, nadir, and plateau). Changes in NO-dependent dilation (difference in plateau between Control and l-NNA sites) and Tempol-mediated dilation (difference in plateau between Control and Tempol sites) were compared using two-way mixed design ANOVA with a between factor of group and a within/paired factor of week. CVCmax was compared using three-way mixed design ANOVA with between factors of group and drug site and a within factor of week. For all analyses, when significant main effects or interactions were detected, comparisons across paired variables (within group) were made with Bonferroni's post hoc test, and comparisons across groups were made using Student's unpaired two-tailed t-test.
For all analyses, the level of significance was set at α = 0.05. Demographic data and data characterizing the heat therapy responses are presented as means ± SD. All other data are means ± SE.
RESULTS
Subject demographic data are presented in Table 1. Subjects were well matched across the two groups for sex, age, height, weight, and BMI (P > 0.64 between groups for all).
Table 1.
Subject demographics
| All Subjects (n = 22) | Heat Therapy Group (n = 9) | Sham Group (n = 9) | |
|---|---|---|---|
| Females, n | 12 | 5 | 5 |
| Age, yr | 22 ± 3 | 22 ± 3 | 22 ± 3 |
| Height, cm | 172 ± 10 | 174 ± 10 | 172 ± 10 |
| Weight, kg | 66 ± 9 | 68 ± 9 | 66 ± 10 |
| BMI, kg/m2 | 22.4 ± 1.7 | 22.6 ± 1.8 | 22.2 ± 2.0 |
| Mean arterial pressure, mmHg | |||
| 0 wk | 84 ± 7 | 83 ± 7 | 83 ± 7 |
| 8 wk | — | 81 ± 6 | 85 ± 5 |
Values are means ± SD.
BMI, body mass index.
Subjects tolerated heat therapy well. There were a few reports of light-headedness, but these typically only occurred within the first one to five sessions. Subjects became heat acclimated across the 8 wk of heat therapy, as indicated by a reduction in resting Tre (P = 0.004) and an increase in mean whole body sweat rate (P < 0.001; Table 2). There were no changes in heart rate across the 8 wk, either prior to entering the hot tub (P = 0.16) or during heat therapy sessions (P = 0.39). Subjects in the sham group experienced no change in Tre or heart rate during thermoneutral water immersion sessions and did not sweat (Table 2).
Table 2.
Heat therapy
| Heat Therapy (n = 10) |
Sham (n = 10) |
|||
|---|---|---|---|---|
| 0 wk | 8 wk | 0 wk | 8 wk | |
| Rectal temperature, °C | ||||
| Resting | 37.3 ± 0.4 | 36.9 ± 0.4*† | 37.3 ± 0.3 | 37.2 ± 0.3 |
| Peak | 39.0 ± 0.1† | 38.8 ± 0.1*† | 37.4 ± 0.2 | 37.3 ± 0.3 |
| Heart rate, beats/min | ||||
| Initial | 83 ± 11 | 79 ± 9 | 80 ± 17 | 81 ± 11 |
| Peak | 124 ± 10† | 122 ± 12† | 85 ± 11 | 80 ± 8 |
| Mean whole body sweat rate, l/h | 0.54 ± 0.20† | 1.29 ± 0.40*† | 0.03 ± 0.04 | 0.02 ± 0.04 |
Values are means ± SD. Rectal temperature and heart rate immediately prior to and during water immersion sessions. Mean whole body sweat rate was calculated as body weight loss across the 90-min sessions, after correcting for fluid intake, and normalized for time.
P < 0.05 vs. 0 wk within group;
P < 0.05 vs. sham group at the same time point.
Local heating of the skin elicited a biphasic vasodilator response, consisting of an initial peak and plateau with a brief nadir in between the two phases (Fig. 2). When data were pooled across all subjects who completed the initial 0 wk testing (n = 22), average plateau in the Control site was 43 ± 3% CVCmax, which is similar to what we have reported previously (9). There was a significant interaction between drug site and phase into local heating (P < 0.001). l-NNA significantly attenuated initial peak, nadir, and plateau compared with both the Control and Tempol sites (P < 0.001 for all; Fig. 1), such that NO accounted for 78 ± 4% of the overall plateau increase in CVC above baseline. There was no effect of Tempol on any phase of the local heating response compared with the Control site (P = 0.53).
Fig. 2.
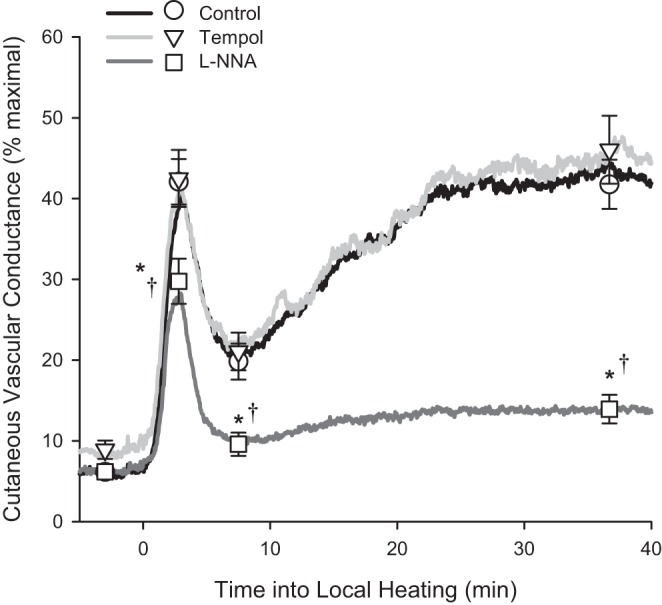
Average normalized cutaneous vascular conductance across time into local heating in all subjects who completed 0 wk testing (n = 22) at three microdialysis sites receiving 1) Control (Lactated Ringer's solution), 2) 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol), and 3) Nω-nitro-l-arginine (l-NNA). Data over time were averaged across subjects at 4 Hz (solid lines). Baseline, initial peak, nadir, and plateau were analyzed for each subject and presented as means ± SE. Note, for initial peak, the symbols do not line up exactly with the solid lines since they were analyzed at the subject's true peak cutaneous vascular conductance (CVC), rather than at a certain time point into local heating. *P < 0.05 vs. Control. †P < 0.05 vs. Tempol.
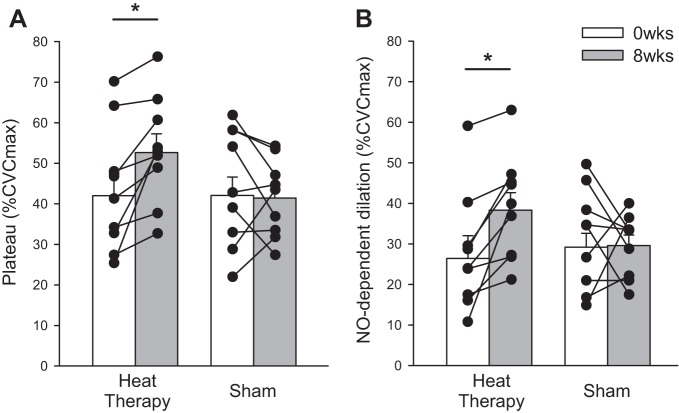
Heat therapy significantly improved the local heating response (group × week × phase of local heating: P = 0.04). Within the heat therapy group, initial peak (P < 0.001), nadir (P = 0.03), and plateau (P < 0.001) were all significantly elevated after 8 wk of heat therapy, whereas no changes were observed in the sham group (week × phase within group: P = 0.22; Fig. 3A and Table 3). Heat therapy significantly improved NO-dependent dilation, the difference between the Control and l-NNA sites (group × week: P = 0.049; 0 vs. 8 wk within heat therapy group: P < 0.01), whereas no change in NO-dependent dilation was observed in the sham group (P = 0.93; Fig. 2B). No changes were observed in Tempol-mediated dilation, the difference between the Tempol and Control sites, across heat therapy or thermoneutral water immersion (P = 0.58; Table 3).
Fig. 3.
A: local heating plateau. B: nitric oxide (NO)-dependent dilation [difference in plateau between microdialysis sites receiving Lactated Ringer's (Control) and l-NNA] in subjects who participated in 8 wk of heat therapy or thermoneutral water immersion (sham). Heat therapy increased both plateau and NO-dependent dilation in every subject, as indicated by the individual data. Data are means ± SE. *P < 0.05 0 vs. 8 wk within group.
Table 3.
Initial peak, nadir, and maximal cutaneous vascular conductance
| Heat Therapy (n = 9) |
Sham (n = 9) |
||||
|---|---|---|---|---|---|
| All Subjects, (n = 22) | 0 wk | 8 wk | 0 wk | 8 wk | |
| Initial peak, % CVCmax | 42 ± 3 | 44 ± 4 | 59 ± 4* | 39 ± 4 | 45 ± 5 |
| Nadir, % CVCmax | 20 ± 2 | 21 ± 4 | 26 ± 4* | 17 ± 3 | 19 ± 3 |
| Tempol-mediated dilation | 4.0 ± 3.3 | 0.2 ± 6.1 | −4.3 ± 7.6 | 5.6 ± 5.2 | 0.0 ± 2.4 |
| CVCmax, mV/mmHg·100 | |||||
| Control | 253 ± 15 | 302 ± 26 | 285 ± 33 | 214 ± 14 | 271 ± 12 |
| l-NNA | 198 ± 14 | 185 ± 22 | 218 ± 41 | 197 ± 15 | 235 ± 31 |
| Tempol | 241 ± 32 | 199 ± 26 | 339 ± 46* | 214 ± 18 | 180 ± 16 |
Values are means ± SE.
CVC, cutaneous vascular conductance; CVCmax, maximal CVC; l-NNA, Nω-nitro-l-arginine; Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl. Tempol-mediated dilation, difference in plateau CVC between the Control and Tempol microdialysis sites.
P < 0.05 vs. 0 wk within group.
CVCmax and Baseline
There were no significant effects of the drugs on CVCmax within the pooled 0 wk data (P = 0.20; Table 3). Across the intervention, there was a significant interaction between group, week, and drug site (P = 0.02), but the only significant difference in paired variables that was found was the increase in CVCmax at the Tempol site in the heat therapy group (P = 0.01). l-NNA significantly decreased baseline CVC (P = 0.02), and Tempol significantly increased baseline CVC (P = 0.03; Table 4).
Table 4.
Baseline drug effects
| Predrug Baseline | Postdrug Baseline | P | |
|---|---|---|---|
| Control | 5.5 ± 0.4 | 6.1 ± 0.5 | 0.13 |
| l-NNA | 7.8 ± 0.9 | 6.2 ± 0.9* | 0.02 |
| Tempol | 7.4 ± 0.8 | 8.5 ± 1.1* | 0.03 |
Values are means ± SE. Baseline data pooled across all subjects completed 0 wk testing (n = 22).
P < 0.05 from predrug baseline.
DISCUSSION
This is the first study to investigate the molecular mechanisms by which passive heat therapy improves microvascular function. Our major finding is that passive heat therapy improves cutaneous thermal hyperemia via improved NO bioavailabilty. We observed no change in NO-dependent dilation in our sham subjects and no change in the sites receiving Tempol in either group, demonstrating that the observed findings in the experimental group were due to the heat therapy intervention through improved NO-dependent dilation.
Local heating of the skin produces a biphasic vasodilator response, consisting of an initial peak occurring within the first 5 min into heating, and a secondary plateau, which is achieved after ∼20–40 min into heating. The initial peak is a sensory nerve axon reflex which elicits dilation via both NO and endothelial-derived hyperpolarizing factors (EDHFs) (6, 24, 35), most likely initiated via transient receptor potential vanilloid type-1 channels (46) located on the sensory nerves, which are thought to release calcitonin gene-related peptide and/or substance P (45). The plateau phase is heavily dependent on NO (29, 35) and also EDHFs to varying extents, depending on the temperature used (6, 9).
Heat Therapy
Heat therapy increased all phases of the local heating response, indicating improved microvascular function. Furthermore, these effects were NO dependent, because no changes in the NOS inhibited site were observed. No changes were observed in the sham group, suggesting that improvements in microvascular function in the heat therapy group were the result of the heat exposure itself and not due to possible hemodynamic changes secondary to changes in hydrostatic pressure or visitation to the lab environment.
We believe the mechanisms behind this adaptation are two-fold. Heat stress induces elevations in core temperature and subsequent increases in blood flow, particularly to the skin. Elevations in core temperature upregulate expression of heat shock proteins. In particular, Hsp90 is an essential cofactor for endothelial NOS (eNOS) (38), meaning that changes in Hsp90 expression can alter eNOS activity and NO production, independent of changes in total eNOS protein (18). In the skin, inhibition of Hsp90 with geldanamycin attenuates thermal hyperemia (39), indicating Hsp90 is a primary player in the response. Second, increases in blood flow and shear stress are essential for cutaneous microvascular adaptation. For example, chronic localized arm heating in a water bath improves cutaneous microvascular function, but these improvements are prevented when a blood pressure cuff is used on the upper arm to prevent increases in blood flow above baseline during heating (16). Shear stress can stimulate NO production via phosphorylation of eNOS through a few mechanisms, including through activating the receptor for vascular endothelial growth factor 2 (27) and activating phosphoinositide 3-kinase, which in turn activates protein kinase A and then eNOS (4). Therefore, not only does heat therapy likely result in increased association of eNOS with Hsp90, but the pathways necessary for eNOS activation are also likely upregulated.
Effects of Tempol
In the skin, oxidative stress-mediated impairments in vasodilator responses with primary aging and hypertension can be reversed by administration of the antioxidant ascorbate (21, 22), and by restoring tetrahydrobiopterin levels (41) or inhibiting arginase (20, 22), which breaks down l-arginine, the substrate necessary for conversion of NO by eNOS, and is upregulated by oxidative stress. However, the effects of Tempol, which targets oxidative stress more specifically than antioxidants such as ascorbate via its direct effects on superoxide, have been studied relatively little in the skin, despite being used extensively in other preparations.
When locally heating the skin to 42°C, Tempol has been shown to have no effect in young, recreationally active subjects (33), but to augment the plateau in young smokers (15), patients with chronic kidney disease (13), and young healthy subjects following ANG II infusion (a model of vascular disease) (33), restoring the plateau back to the level of young, healthy individuals. However, in young, healthy subjects, plateau CVC when heating to 42°C reaches ∼85–95% of CVCmax, and so noneffects of Tempol may have been due to a ceiling effect. Heating to 39°C better isolates the NO component compared with the more commonly used protocol heating to 42°C (∼80% NO vs. ∼50–60% NO with rapid local heating to 42°C), and it reaches a plateau of ∼40–60% CVCmax, allowing for the investigation of interventions which may improve NO-dependent dilation, such as heat therapy (9). In the present study, our data show that Tempol also has no effect in young, sedentary, but otherwise healthy subjects when using this local heating protocol, either across all subjects pooled at 0 wk or following heat therapy or thermoneutral water immersion. As such, this establishes these methods for use in future studies in patient populations who have elevated oxidative stress in which heat therapy (or other interventions) may be able to alleviate oxidative stress-associated impairments in endothelial function.
To ensure that the noneffects of Tempol were truly because oxidative stress was minimal in our young, sedentary subjects and not because the drug did not work in our hands, we studied two pilot subjects with chronic (7–8 yr postinjury) complete SCI (lesions at T11 and T12). SCI is associated with elevated oxidative stress (26), which is likely exacerbated due to increases in circulating ANG II (17), which increases superoxide production via NADPH oxidase (8). This population was selected over other patient groups known to have high oxidative stress because they are young (matched to the mean age of our other subjects) and free of comorbidities which might alter the effects of Tempol. In the two SCI subjects (data averaged across the two), plateau reached 53.6% CVCmax in the Control sites. Tempol increased the plateau in both subjects to an average of 61.0% CVCmax, confirming the efficacy of this concentration of Tempol. Additionally, we have previously studied the effects of Tempol in young smokers in our laboratory using the same techniques and concentration of Tempol (15). In those subjects, 10 μM Tempol fully reversed impairments in thermal hyperemia.
CVCmax
Although we observed some trends in CVCmax across drug sites and interventions, only the increase in CVCmax in the Tempol site with heat therapy was statistically significant. Previous studies from our lab and others (13, 15) have observed no effects of Tempol on CVCmax, and since this effect was only observed in one site, we do not believe it was due to heat therapy. More likely, these differences are due to limitations in the laser Doppler flowmetry technique rather than physiological structural changes in the cutaneous microvasculature. For example, microvessel density can vary greatly from site to site, highlighting the importance of normalizing laser Doppler flux values (12).
Conclusions and Perspectives
In the present study, we have demonstrated that passive heat therapy improves cutaneous microvascular function via improved NO-dependent dilation. While we chose to study a relatively healthy population for this first study, we believe our results may be applicable to other clinical conditions. Many disease states (including a sedentary lifestyle) are characterized by impaired endothelial function, particularly in the microvasculature, and the usual cause is impaired NO bioavailability secondary to chronic oxidative stress and inflammation. Although exercise is a potent means of improving vascular function, many patient populations are either unable or unwilling to exercise to an extent necessary for inducing protective adaptation. Our results demonstrate that passive heat therapy may offer a powerful alternative for improving microvascular function and NO bioavailability that could be implemented by a wide range of patient populations. Given that heat therapy was capable of inducing improvements in our young, sedentary subjects, we believe improvements could be even greater in disease states characterized by greater impairments in vascular function.
Importantly, we kept the arm used for microdialysis experiments out of the hot tub to better isolate the systemic effects of heat therapy rather than the effects of chronic elevations in local skin temperature. By doing so, we believe our results may better reflect changes that occurred in other microvascular beds, because the thermal hyperemia response has been shown to reflect globalized microvascular function (5, 23, 37). However, it is important to recognize that we observed improved function in a microvascular bed which experienced large magnitude vasodilation during hot water immersion sessions. Microvascular beds that do not vasodilate to the same extent during heat stress (e.g., skeletal muscle) or at all (e.g., renal or splanchnic circulations) may not have experienced improvements in microvascular function as did the skin. We did show that forearm postocclusive reactive hyperemia is improved following 8 wk of heat therapy (47). While forearm reactive hyperemia is predominantly reflective of microvascular function in skeletal muscle, it does include a skin component.
Although we did not investigate this in the present study, the skin under the water (e.g., lower torso and legs) presumably received an even greater stimulus for adaptation, because it would have been subject to both the systemic stimuli (e.g., elevated core temperature, exposure to circulating factors upregulated in the blood, and increased shear stress) and to elevations in local temperature. Shear stress may have been even greater in the legs, because vasodilation would likely have been greater given the combined core temperature elevation and local skin temperature elevation. As such, heating the legs may be valuable for improving microvascular health in patient populations such as spinal cord injured and peripheral artery disease patients who suffer from a variety of complications secondary to poor circulation in their legs.
GRANTS
This work was supported by American Heart Association Grant #14PRE20380300, the Eugene and Clarissa Evonuk Memorial Foundation, and the Ken and Kenda Singer Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.E.B. and C.T.M. conception and design of research; V.E.B., T.M.E., M.A.F., and M.J.H. performed experiments; V.E.B. analyzed data; V.E.B., T.M.E., and C.T.M. interpreted results of experiments; V.E.B. prepared figures; V.E.B. drafted manuscript; V.E.B., T.M.E., M.A.F., M.J.H., and C.T.M. edited and revised manuscript; V.E.B., T.M.E., M.A.F., M.J.H., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for their participation in the study and Brett Ely, Alexander Chapman, Kaitlin Livingston, Lindan Comrada, Andrew Jeckell, Alexander Woldt, and Jared Steele for their assistance with data collection.
REFERENCES
- 1.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg : 574–581, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res : 55–59, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud's phenomenon. Arthritis Res Ther : R1103–R1112, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol : H1819–H1828, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brunt VE, Minson CT. Cutaneous thermal hyperemia: more than skin deep. J Appl Physiol : 5–7, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol : 3523–3534, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter HH, Spence AL, Atkinson CL, Pugh CJA, Cable NT, Thijssen DHJ, Naylor LH, Green DJ. Distinct effects of blood flow and temperature on cutaneous microvascular adaptation. Med Sci Sports Exerc : 2113–2121, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Chan SHH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JYH. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res : 772–780, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol : 277–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S, Park KA, Lee HJ, Park MS, Lee JH, Park KC, Kim M, Lee SH, Seo JS, Yoon BW. Expression of Cu/Zn SOD protein is suppressed in hsp 70.1 knockout mice. J Biochem Mol Biol : 111–114, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Colberg SR, Parson HK, Nunnold T, Holton DR, Swain DP, Vinik AI. Change in cutaneous perfusion following 10 weeks of aerobic training in Type 2 diabetes. J Diabetes Complications : 276–283, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci : 503–508, 2006. [DOI] [PubMed] [Google Scholar]
- 13.DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Renal Physiol : F1499–F1506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol : 530–547, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii N, Brunt VE, Minson CT. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. Am J Physiol Heart Circ Physiol : H1507–H1511, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DHJ, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol : 1571–1577, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groothuis JT, Thijssen DHJ, Rongen GA, Deinum J, Danser AHJ, Geurts ACH, Smits P, Hopman MTE. Angiotensin II contributes to the increased baseline leg vascular resistance in spinal cord-injured individuals. J Hypertens : 2094–2101, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Harris MB, Mitchell BM, Sood SG, Webb RC, Venema RC. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol : 795–802, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol : 233–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol : 863–872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol : H1090–H1096, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol : H2965–H2970, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol : 370–372, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol : 811–820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol : 1083–1088, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord : 264–274, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res : 354–363, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kaldur T, Kals J, Ööpik V, Zilmer M, Zilmer K, Eha J, Unt E. Effects of heat acclimation on changes in oxidative stress and inflammation caused by endurance capacity test in the heat. Oxid Med Cell Longev : 107137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol : 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol : 754–759, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int : 157–164, 2006. [DOI] [PubMed] [Google Scholar]
- 32.McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol : R185–R191, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol : 20–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation : 569–579, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol : 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol : 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol : 1239–1246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem : 17621–17624, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol : H232–H236, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension : 935–942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol : 791–797, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension : 767–774, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor NA. Human heat adaptation. Compr Physiol : 325–365, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Van Duijnhoven NTL, Janssen TWJ, Green DJ, Minson CT, Hopman MTE, Thijssen DHJ. Effect of functional electrostimulation on impaired skin vasodilator responses to local heating in spinal cord injury. J Appl Physiol : 1065–1071, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Wallengren J, Ekman R, Sundler F. Occurrence and distribution of neuropeptides in the human skin. An immunocytochemical and immunochemical study on normal skin and blister fluid from inflamed skin. Acta Derm Venereol : 185–192, 1987. [PubMed] [Google Scholar]
- 46.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol : 4317–4326, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness, and blood pressure in sedentary humans. J Physiol. doi: 10.1113/JP272453, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]