Abstract
Purpose
To develop recommendations for clinical trial reporting that address the unique efficacy, toxicity, and combination and sequencing aspects of immuno-oncology (IO) treatments.
Methods
ASCO and the Society for Immunotherapy of Cancer (SITC) convened a working group that consisted of practicing medical oncologists, immunologists, clinical researchers, biostatisticians, and representatives from industry and government to develop Trial Reporting in Immuno-Oncology (TRIO) recommendations. These recommendations are based on expert consensus, given that existing data to support evidence-based recommendations are limited.
Conclusion
The TRIO recommendations are intended to improve the reporting of IO clinical trials and thus provide more complete evidence on the relative benefits and risks of an IO therapeutic approach. Given the rapid expansion of the number of IO clinical trials and ongoing improvements to the evidence base supporting the use of IO treatments in clinical care, these recommendations will likely need regular revision as the IO field develops.
Introduction
Cancer immunotherapies are treatments in which the primary mechanism of action is the generation of an immune response against cancer. The US Food and Drug Administration has approved multiple cancer immunotherapies for treating different types of cancer. These cancer immunotherapies or immuno-oncology (IO) treatments include but are not limited to cytokines, interferons, and immune checkpoint blocking agents, such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4; ipilimumab), anti-programmed cell death protein 1 (PD-1; nivolumab, pembrolizumab), and anti-programmed death-ligand 1 (PD-L1; atezolizumab, avelumab, durvalumab) agents and genetically modified T-cell therapies (axicabtagene ciloleucel, tisagenlecleucel). Several novel IO treatments are also being explored, such as modified cytokines, cell-based products, oncolytic viruses, CD3 bispecific antibodies, and various vaccine platforms. Clinical trials have been essential to advancing the use of immunotherapies in cancer treatment, and bio-medical journals have been a key mechanism for disseminating this knowledge.
To improve the quality of scientific publications, biomedical journals have adopted guidelines for reporting the design, conduct, analysis, and results of clinical trials (eg, the Consolidated Standards of Reporting Trials [CONSORT]), tumor marker studies (eg, Reporting Recommendations for Tumor Marker Prognostic Studies [REMARK]), and other study designs [1–3]. The principles from CONSORTand REMARK can be applied to IO trials. For example, when novel predictive and prognostic biomarkers are reported in IO trials, investigators should follow the REMARK guidelines, and they should indicate which hypotheses were predefined and which exploratory hypotheses are post hoc. IO therapies, however, use distinct mechanisms of action related to the generation of immune responses and may exhibit unique efficacy and toxicity effects compared with traditional cancer therapies such as chemotherapy. These features of IO therapies may lead to additional considerations for reporting the design, conduct, analysis, and results of IO clinical trials.
In fall 2016, ASCO and the Society for Immunotherapy of Cancer (SITC) convened a working group to develop clinical trial reporting recommendations that address the unique efficacy, toxicity, and combination and sequencing aspects of IO treatments. The working group consisted of practicing medical oncologists, immunologists, clinical researchers, biostatisticians, and representatives of industry and government. It met via a series of conference calls and held an in-person meeting in February 2017.
This ASCO-SITC statement presents 12 specific reporting recommendations—Trial Reporting in Immuno-Oncology (TRIO)— to improve the interpretation and comparison of efficacy and toxicity end points and the combination and sequencing of treatments in IO clinical trials (Table 1). These recommendations are based on expert consensus, given that existing data to support evidence based recommendations are limited. They are, in part, more prescriptive than other reporting guidelines because they recommend that certain analyses be performed and that results be presented to facilitate understanding and comparison across IO trials. As a result, the recommendations are practical, and hopefully they will lead to better trial conduct and relevant data collection. In addition, the recommendations will likely require regular updating as data from IO clinical trials emerge.
Table 1.
Trial reporting in immuno-oncology (TRIO) standards
| Reporting Standards | |
|---|---|
| Efficacy reporting standards | |
| 1. Report the criteria used to evaluate response to therapy and the rationale for the chosen criteria. | |
| 2. Include spider plots or swimmer plots in efficacy descriptions to better report kinetics of response (Figs 1 and 2). | |
| 3. Report how disease control rate is defined and how its components are assessed. | |
| 4. Report criteria that allow patients to continue treatment beyond disease progression. | |
| 5. Report the number (proportion) of patients who are treated beyond progression, treatment beyond progression duration, emergence of new toxicity, and efficacy after initial progression. | |
| 6. Report progression-free survival and overall survival using Kaplan-Meier analyses. | |
| Toxicity reporting standards | |
| 7. Differentiate between the clinical diagnoses of IO toxicity and the specific symptoms that led to the diagnoses. | |
| 8. If the prespecified clinical diagnoses used in data collection belong to categories such as “immune-related adverse events” or “adverse events of special interest,” report how these terms are defined and why these categories were selected for trial reporting | |
| 9. Report all toxicity by specific grade. | |
| 10. Report clinical interventions used to manage IO toxicity (Table 2). | |
| 11. Report time of onset and duration of IO toxicity (Table 2). | |
| Combination or sequencing of immunotherapies reporting standard | |
| 12. Report the scientific hypothesis for the combination or sequence on the basis of preclinical and/or clinical data as well as the rationale for the selection of the particular dose(s) and sequence of agents. |
Standards 1 to 5 and 7 to 11 are unique to immuno-oncology (IO) therapies
Efficacy reporting standards for IO trials
Immunotherapies can exhibit unusual patterns of response because of the temporal aspects of the generation of immunity, the dynamic nature of the anticancer immune response, and the impact of tumor inflammation on tumor measurements. Specifically, there may be lag time before efficacy of IO therapies becomes evident, presumably because of the time required to activate an anticancer immune response. Once active, the anticancer immune response can fluctuate over time based on several host intrinsic and extrinsic factors [4]. It is also possible that tumor inflammation makes some tumors seem to be larger on imaging scans after the initial target measurement, which complicates response assessment [5]. To capture these atypical patterns of response, various immune-related response criteria have been proposed to define efficacy in IO clinical trials [5–9]. Because these criteria are not yet fully validated, the reporting recommendations in this section are intended to address the heterogeneous methods currently used to report efficacy across IO clinical trials and to ensure that investigators report the information necessary to enable objective assessment of antitumor efficacy.
Defining antitumor activity
Report the criteria used to evaluate response to therapy and the rationale for the selected criteria
Response Evaluation Criteria in Solid Tumors (RECIST) are used to evaluate antitumor activity of oncology drugs in most clinical trials, including most IO trials [10]. These criteria were developed for trials of conventional chemotherapy in patients with solid tumors and declare progression when existing tumors increase in size by a certain amount (eg, ≥ 20% in tumor measurements) or if new tumors develop. Emerging evidence on IO therapies suggests that RECIST may underestimate the benefit of some IO approaches by declaring progression too soon in patients who ultimately benefit from treatment [11, 12]. Several iterations of IO-specific response criteria have been proposed in selected disease settings (ie, immune-related RC [irRC], irRECIST, iRECIST, imRECIST) [5–9]. These criteria have important differences, such as whether the tumor burden is calculated as the sum of the longest diameters (unidimensional) or the sum of the product of perpendicular diameters (bidimensional). None of the IO-specific response criteria have been uniformly adopted across all IO trials [13]. While they are being validated, investigators should specify which criteria were used, whether they were prespecified, and the rationale for their selection. To provide comparisons with prior clinical trials, consideration should be given to continuing to report responses according to standard RECIST criteria in parallel with the IO-specific response criteria.
Include spider plots or swimmer plots in efficacy descriptions to better report kinetics of response for nonrandomized trials
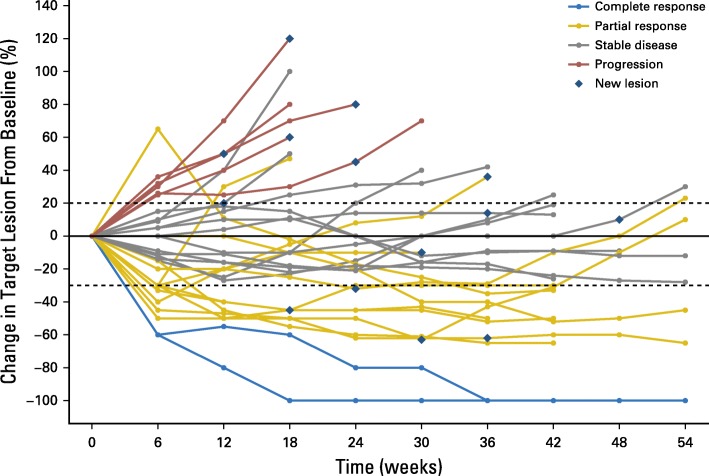
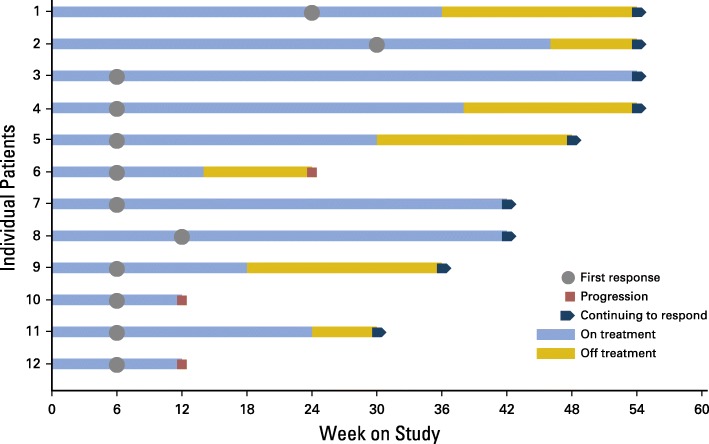
Most IO trials display efficacy as a waterfall plot. However, waterfall plots are inherently limited because only the patients’ best overall response is reported. They do not capture the temporal relationship of response after treatment is administered (ie, the timing of patients’ response and whether the response occurred during therapy or after discontinuation of therapy) nor do they provide any information about the duration of response. Thus, in addition to waterfall plots, investigators should report the kinetics of change in tumor burden using spider plots or swimmer plots to display more meaningful information about efficacy over time.
Spider plots (Fig. 1) typically illustrate change in tumor burden over time within individual patients and usually include symbols indicating when new lesions appear. The major limitations of spider plots are that their interpretation is difficult when the number of patients is large and the representation of patients with progressive disease versus those who have complete response plus partial response plus stable disease is imbalanced. Specifically, the loss of data from the patients who have progressive disease may lead to misrepresentation of benefit for the overall population [14]. Therefore, they are most useful in reporting smaller trials or selected populations of interest within larger trials. Swimmer plots (Fig 2) typically depict the treatment course of individual patients and may show how long patients receive treatment, time off treatment, and duration of response or other relevant clinical events such as time of disease progression [15].
Fig. 1.
Hypothetical example of a spider plot showing tumor growth or shrinkage from baseline in a cohort of patients. Patients are often color coded to correspond to their best objective response. By displaying index lesion tumor burden over time, spider plots clearly illustrate tumor burden changes over time. They can demonstrate a favorable antitumor response in index lesions by showing their decrease, even in patients determined to have a best response of progressive disease as defined by the presence of a new lesion
Fig. 2.
Hypothetical example of a swimmer plot showing time of objective response in relationship to duration of treatment and time of treatment cessation. Symbols along each bar and at the end of each bar could be used to represent various relevant clinical events, such as disease progression or start of a new anticancer therapy. Swimmer plots provide useful information about responses, which may start after cessation of immunotherapy, and about the potential persistence of these responses even without ongoing treatment. Continuation of response despite immunotherapy discontinuation is an important efficacy metric
Report how disease control rate is defined and how its components are assessed
Investigators often collect and report nonstandardized end points in clinical trials using terms such as “disease control” and “clinical benefit.” These terms may include patients with objective response (complete and partial) and patients with stable disease, usually for a prespecified minimum number of months (eg, 6 months). When such composite end points are reported, they should be clearly defined, and the frequency and method of assessment of their components should be clearly described. For time-to-event end points, investigators should provide a clear definition of baseline (time 0) as well as what occurrences are considered censored and what are considered events.
Although the importance of clearly defining time-to-event end points is not unique to IO trials, it is especially relevant in the current IO trial landscape because of the vast number of nonrandomized IO trials investigating new agents alone and in various combinations. The controversial issue surrounds patients who are determined to have stable disease by standard imaging criteria. For example, a patient with stable disease for 6 months on an experimental IO therapy may have met a trial’s clinical benefit or disease control designation, but if that patient had a cancer with an indolent underlying disease biology, there may have been no significant drug benefit from the intervention. Nonrandomized studies are inherently limited in demonstrating true clinical benefit or disease control of an experimental approach because of the absence of a comparator arm, which is necessary to interpret time-to-event end points.
Describing clinical events after disease progression
Report criteria that allow patients to continue treatment beyond disease progression
Historically, cancer clinical trials have required cessation of experimental therapy upon determination of disease progression. IO clinical trials, however, often allow patients to continue therapy beyond objective determination of progressive disease because of the recognition of a phenomenon known as pseudo-progression. Pseudo-progression is an event that can occur with IO therapies that denotes the appearance of new lesions (usually with shrinkage of baseline index tumor burden) or an initial increase in index lesion(s) with subsequent index lesion response by clinical or radiographic assessment [5, 7, 11, 12, 16].
Common criteria for treating beyond progression are that patients have stable or improved clinical condition, no severe laboratory abnormalities or adverse events associated with IO therapy, and lack of clinically significant additional progression on confirmatory subsequent imaging. However, there are no universally accepted criteria for defining what may happen in an IO clinical trial after disease progression. Thus, the specific criteria used in a trial to permit treatment beyond progression should be reported. This information should include the timing of the required confirmatory imaging. Investigators should also report whether alternative treatments were discussed with the patients and whether patients signed a consent form at the time of initial documentation of disease progression.
Report the number (proportion) of patients who are treated beyond progression and the treatment beyond progression duration, emergence of new toxicity, and efficacy after initial progression
It is critical to distinguish patients with pseudo-progression from true progression to avoid exposing the latter group to ineffective treatment that may be associated with adverse events [17]. It is also important to capture the incidence of pseudo-progression [18]. Therefore, investigators should report the overall number of patients who are treated beyond progression and their outcomes. Specifically, this information should include the number of patients treated beyond progression who experienced tumor response but ultimately had confirmed progression on a subsequent scan, the median duration of time patients were treated beyond initial progression before treatment was discontinued as a result of progression; and new toxicities that arose or existing toxicities that worsened while patients were treated beyond initial progression.
Reporting progression-free survival and overall survival
Report progression-free survival and overall survival using Kaplan-Meier analyses
Investigators should report progression free survival and overall survival using Kaplan-Meier analyses in IO trials. The landmark methodology may be used for the correlation of progression-free survival or overall survival with response status [19]. By using this method to evaluate outcomes, patients who die early do not prejudicially influence the analysis of a postdiagnosis end point. Two noticeable aspects of the Kaplan Meier survival curves in IO trials are the presence of delayed separation of curves resulting from delayed clinical effect in the IO arm and presence of nonzero tail probabilities because of a higher proportion of patients experiencing sustained tumor control or prolonged survival in the IO arm. These two aspects of the survival curves could potentially lead to underestimation of trial duration and loss of power to detect treatment effects [20]. Any statistical test used to compare survival curves between treatment arms should be described in detail; the unique characteristics of IO Kaplan-Meier curves have led to varied recommendations concerning tests that differently weight survival differences early versus late in the time course [21, 22].
Investigators should also report whether patients were censored atthe time they started a new therapy. The date of earliest evidence of disease progression should be reported as the date of disease progression for patients who continued treatment beyond progression but were confirmed to have true progression on a subsequent assessment.
Toxicity reporting standards for IO trials
Toxicities that patients experience from IO therapies (IO toxicity) differ from those with other cancer therapies, such as chemotherapy and molecularly targeted therapy. Immune check-point blockade, for example, is associated with organ-specific adverse events, such as hepatitis, colitis, dermatitis, pneumonitis, and endocrinopathies such as hypophysitis [23]. The adverse events of IO therapies can last longer or be more transient than adverse events from other treatment modalities. There is also variability in the onset of toxicity; for example, IO toxicity may begin after treatment cessation [24, 25]. Managing these toxicities requires interventions unique to the immune-based mechanism of action for IO therapies, including those related to chimeric antigen receptor T-cell therapies [26–28]. Nonetheless, most toxicities from immunotherapies are reversible with immune suppression that uses corticosteroids and/or other immunosuppressive agents such as mycophenolate mofetil and tumor necrosis factor-alpha antagonists (eg, infliximab) [29].
Despite the critical importance of knowing the safety profiles of cancer treatments, there is evidence that toxicity reporting is suboptimal in clinical trials [30–33], including IO trials [34]. Accurate and timely toxicity reporting in IO trials would provide critical details about the occurrence and management of IO toxicities. Thus, the reporting recommendations in this section are intended to ensure that investigators describe all captured toxicities, especially those unique to IO therapies, to enable better characterization of their safety profile. Some of the recommendations may require collecting information that is not standard in all clinical trials. However, the hope is that proposing these recommendations will inspire improved methods of collecting data in future clinical trials.
Differentiate between the clinical diagnoses of IO toxicity and the specific symptoms that led to the diagnoses
Clinical diagnoses of IO toxicity often describe a constellation of specific signs and symptoms that afflict a patient. For example, a patient receiving immunotherapy may have individual symptoms such as headache and fatigue. The clinical diagnosis for these symptoms, however, may be immunotherapy-related hypophysitis. Similarly, a patient who reports abdominal pain and diarrhea may ultimately be diagnosed with colitis. Both symptoms and diagnoses are reported in clinical trials, often describing the same event and resulting in confusion regarding the precise incidence of particular toxicities [35]. To avoid this confusion, investigators should report the clinical diagnoses of IO toxicity separately from the raw data of associated symptoms. They should also report the clinical evaluations that were used to determine a diagnosis of IO toxicity. The National Cancer Institute’s (NCI’s) Common Terminology Criteria for Adverse Events (CTCAE) is the current standard for toxicity reporting in clinical trials. To better reflect clinical diagnoses of IO toxicities, such as hypophysitis, the NCI recently updated to CTCAE v5.0 to clarify the symptoms and data required for the diagnosis of an IO toxicity [36]. Future updates will likely be necessary as knowledge of IO toxicities increases.
If the prespecified clinical diagnoses used in data collection belong to categories such as immune-related adverse events or adverse events of special interest, report how these terms are defined and why these categories were selected for trial reporting
The terms “immune-related adverse events” (irAE) or “adverse events of special interest” (AEOSI) are often used to describe clinical diagnoses of IO toxicity (eg, hypophysitis, colitis, hepatitis). Many clinical trials distinguish adverse events belonging to these categories from other toxicities and separately report them. When investigators use predetermined categories of toxicities, such as irAE or AEOSI, they should explain how and why certain toxicities were selected for inclusion within these categories and what criteria were met to attribute a toxicity to one of these terms [37].
Report all toxicity by specific grade
Clinical management of IO therapies would be better informed by increased granularity of toxicity grading data. Investigators usually report all grade toxicity and grade 3 to 4 toxicity separately to highlight the incidence of serious (grade 3 to 4) toxicities relative to the total number of toxicities observed. Given the nature of IO toxicities, investigators should report the specific grade of each toxicity collected in a clinical trial. There are clinically meaningful differences between grade 1 and 2 toxicities as well as between grade 3 and 4 toxicities. For example, grade 1 pneumonitis is defined in the CTCAE as asymptomatic and based on clinical or diagnostic observations, whereas grade 2 pneumonitis indicates symptoms interfering with instrumental activities of daily living for which medical intervention is indicated. Grade 3 pneumonitis is associated with oxygen initiation, whereas grade 4 toxicity is life threatening and accompanied by intubation. Grade 2 or higher immune-related pneumonitis usually requires that IO treatment be withheld and that steroids be initiated. When only grade 3 to 4 toxicity is reported in IO trials, grade 2 events are not clearly captured despite being clinically relevant. Reporting toxicity by each specific grade (ie, separate columns in tables for grades 1 to 4 adverse events) will optimize the value of the available information on the toxicity profile of IO therapies.
Report clinical interventions used to manage IO toxicity
Table 2 illustrates a proposed format for summarizing the clinical consequences and management of adverse events (ie, dose delays, use of immunosuppression) in IO trials, providing a comprehensive description of the toxicity profile not otherwise captured by standard CTCAE criteria. Several retrospective studies have reanalyzed immunotherapy toxicity data from clinical trials with the goal of capturing the clinical management associated with immunotherapy toxicity, including the use of various forms of immunosuppression and rates of emergency room visits and hospital admissions for toxicity management [38, 39]. However, investigators should ideally report this information for at least the most common toxicities in the initial publications of prospective IO clinical trials. Investigators should also comment in the discussion section of manuscripts on whether the reported toxicity management in the clinical trial adhered to consensus management guidelines, at least for unexpected toxicities or those that significantly affected the patients. Professional organizations such as the National Comprehensive Cancer Network, SITC, and ASCO have published guidelines for generally managing IO toxicities [26, 27]. There are also specific toxicity management guidelines for chimeric antigen receptor T-cell and other adoptive transfer cell therapies, given their highly unusual toxicities [28].
Table 2.
Reporting of clinical consequences of toxicity
| Patients Who Experience Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse Event | Dose Delay (No. and Proportion of patients | Dose Discontinuationa No. and proportion patients) | Timing of Toxicity Onset (median and range)b | Use of High-Dosec Steroids (No. and proportion of patients | Duration of High-Dose Steroid Use (median and range | Duration of Dose Taperingd (median and range) | Additional Immune- Suppressing Agents (No. and proportion of patients who required escalation beyond steroids; specify drugs) | Time to Resolution of Toxicitye (median and range, percent of patients with unresolved toxicity) | Emergency Center Visit/ Hospitalization (No. and proportion of patients) |
| Adverse event 1 | |||||||||
| (e.g., colitis) | |||||||||
| Adverse event 2 | |||||||||
| Adverse event 3 | |||||||||
| Adverse event 4 | |||||||||
aDefined as the inability to continue on the protocol; may include irreversible toxicity and toxicity resulting in ineligibility for subsequent treatment
bDays from cycle 1, day 1 to time of onset (include cycle, day and period from initiation of treatment)
cDefined as at least 40 mg prednisone equivalents per day
dIf the protocol required collecting this information
eDefine specifically if “resolution” refers to return to grade 1 or 0 (indicate whether this includes patients who are on steroids to manage adverse events)
Report time of onset and duration of IO toxicity
In addition to capturing the clinical management of adverse events resulting from IO therapies, Table 2 also captures the timing of toxicity onset and of toxicity resolution, which enables determination of duration of toxicity. Because IO toxicity can be long lasting [24, 25], reporting the duration of toxicity is arguably as clinically important as severity (grade) of toxicity. These data would aid clinical management and provide information that is useful to the design and interpretation of subsequent combinatorial IO trials. For example, if drug X as a single agent is not known to cause transaminitis and drug Y is not known to cause transaminitis until, on average, 6 weeks after treatment initiation, significant transaminitis occurring within 2 weeks of therapy in a combination study of drug X plus drug Y could raise the possibility of synergistic toxicity. To clearly illustrate this information, investigators could enhance swimmer plots to capture time of toxicity onset and duration of toxicity relative to when a therapy was given or develop new plots with greater informational content [40].
Combination or sequencing of immunotherapies reporting standard
An increasing number of clinical trials are testing immunotherapies in binary, ternary, and more complex combinations and sequences. These studies are evaluating IO therapies with each other and with targeted therapies, chemotherapy, and radiation therapy [41]. There are already several Food and Drug Administration–approved IO combination treatments [42]. The rationale for conducting combination and sequencing trials is that the use of multiple treatments may broaden and/or deepen the antitumor activity of IO drugs. The combination of nivolumab and ipilimumab, for instance, has a 58% response rate in patients with metastatic melanoma, which is greater than the response rate of either therapy alone [43, 44]. However, combining therapies also increases the riskof added or novel toxicity. The rate of grade 3 to 4 toxicity in patients receiving nivolumab plus ipilimumab is close to 60%, which is higher than that in patients receiving either drug as a single agent [43–45]. The goal of the reporting standard in this section is to ensure that the justification for combining or sequencing treatments in IO trials is adequately reported, including biologic and/or clinical evidence for contribution of the individual components.
Report the scientific hypothesis for the combination or sequence, as well as the rationale for the selection of the particular dose(s) and sequence of agents
Investigators should report preclinical and/or clinical data that support the hypothesis that a combination or sequencing of therapies in an IO trial is likely to have additive or synergistic antitumor activity and a favorable therapeutic index [17]. They should also provide a rationale for the selection of the particular dose and sequence of agents. This information can be illustrated in a figure that captures the dose and sequence of the agents administered, dose level, administration frequency, length of dosing interruption, duration of treatment of each agent, and whether the treatments are administered sequentially or concurrently. Many IO therapies have only a modest dose-response relationship and have the potential for prolonged biologic effects after discontinuation of treatment. Determining the dose for IO combinations is further complicated by the fact that nonstandard doses or schedules of the individual agents may be necessary. Some IO therapies may have limited benefit when used alone but high potential for benefit when used in combination [46].
In conclusion, the 12 reporting recommendations presented in this statement are intended to improve the reporting of IO clinical trials and thus provide more complete evidence on the relative benefits and risks of an IO therapeutic approach. Although some of these recommendations may be relevant for other types of cancer clinical trials, the goal of these recommendations is to address the unique efficacy, toxicity, and combination and sequencing characteristics of IO treatments. Given the rapid expansion of the number of IO clinical trials and ongoing improvements to the evidence base that supports the use of IO treatments in clinical care, these recommendations will likely need regular revision as the IO field develops. This iterative process will be critical to ensuring that IO clinical trial data are interpreted appropriately and are ultimately useful to improving the care of patients with cancer.
Acknowledgments
ASCO and SITC thank the following individuals for participating in the immunotherapy working group: Jeff Allen (Friends of Cancer Research), Donald Berry (MD Anderson Cancer Center), Lajos Pusztai (Yale Cancer Center), Marc Theoret (US Food and Drug Administration), Lauren Wood (National Cancer Institute), Jeffrey S. Weber (Perlmutter Cancer Center), and Cassian Yee (MD Anderson Cancer Center). We also thank the following ASCO staff for their contributions to the project: Caroline Schenkel, Nicole Jenkins, and Suanna S. Bruinooge.
This article is co-published in the journals Journal of Clinical Oncology and Journal for Immuno Therapy of Cancer 10.1200/JCO.18.00145 or https://doi.org/[insert DOI/JITC]
Authors’ contributions
Conception and design: All authors. Administrative support: LAL. Provision of study materials or patients: AMT, LMM. Collection and assembly of data: AMT, LAL, DC, JMK, ES, KFM. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Competing interests
Disclosures provided by the authors are available with this article at jco.org.
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Apostolia M. Tsimberidou
Consulting or Advisory Role: Roche
Research Funding: EMD Serono (Inst), Baxter (Inst), Foundation Medicine (Inst), Onyx Pharmaceuticals (Inst), Bayer HealthCare Pharmaceuticals (Inst), Boston Biomedical (Inst), Placon Therapeutics (Inst), Immatics Biotechnologies (Inst), Karus Therapeutics (Inst), Tvardi Therapeutics (Inst), OBI Pharma (Inst)
Laura A. Levit
No relationship to disclose
Richard L. Schilsky
Research Funding:
AstraZeneca (Inst), Bayer AG (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Eli Lilly (Inst), Merck (Inst), Pfizer (Inst)
Steven D. Averbuch
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb, Foundation Medicine
Daniel Chen
No relationship to disclose
John M. Kirkwood
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma, Merck, Roche, Amgen, Immunocore
Research Funding: Merck (Inst), Prometheus Laboratories (Inst)
Lisa M. McShane
No relationship to disclose
Elad Sharon No relationship to disclose
Kathryn F. Mileham
Consulting or Advisory Role: AstraZeneca
Speakers’ Bureau: Merck
Research Funding: Celgene
Michael A. Postow
Honoraria: Bristol-Myers Squibb, Merck
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma, NewLink Genetics, Incyte, Merck
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Array BioPharma (Inst), Infinity Pharmaceuticals (Inst), Rgenix (Inst)
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 2.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat Clin Pract Oncol. 2005;2:416–422. doi: 10.1038/ncponc0252. [DOI] [PubMed] [Google Scholar]
- 3.Transparent and accurate reporting increases reliability, utility, and impact of your research: Reporting guidelines and the EQUATOR Network. https://www.ncbi.nlm.nih.gov/pubmed/?term=Transparent+and+accurate+reporting+increases+reliability%2C+utility%2C+and+impact+of+your+research%3A+reporting+guidelines+and+the+EQUATOR+Network. [DOI] [PMC free article] [PubMed]
- 4.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer. 2016;4(30). [DOI] [PMC free article] [PubMed]
- 8.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–858. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Policy Forum, Board on Health Care Services, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine . Policy issues in the clinical development and use of immunotherapy for cancer treatment. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 11.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver JA, Hazarika M, Mulkey F, et al. Patients withmelanomatreatedwithananti-PD-1antibodybeyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229–239. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anagnostou V, Yarchoan M, Hansen AR, et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23:4959–4969. doi: 10.1158/1078-0432.CCR-16-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LoRusso PM, Anderson AB, Boerner SA, et al. Making the investigational oncology pipeline more efficient and effective: are we headed in the right direction? Clin Cancer Res. 2010;16:5956–5962. doi: 10.1158/1078-0432.CCR-10-1279. [DOI] [PubMed] [Google Scholar]
- 15.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoos A, Topalian S, Chen TT, et al: Facilitating the development of immunotherapies: intermediate endpoints for immune checkpoint modulators. Issue brief: conference on Clinical Cancer Research, 2013. https://www.focr.org/sites/default/files/Immunotx%20%20final%2011%204.pdf
- 17.Siu LL, Ivy SP, Dixon EL, et al. Challenges and opportunities in adapting clinical trial design for immunotherapies. Clin Cancer Res. 2017;23:4950–4958. doi: 10.1158/1078-0432.CCR-16-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazandjian D, Keegan P, Suzman DL, et al. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. 2017;44:3–7. doi: 10.1053/j.seminoncol.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 20.Chen Tai-Tsang. Statistical issues and challenges in immuno-oncology. Journal for ImmunoTherapy of Cancer. 2013;1(1):18. doi: 10.1186/2051-1426-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mick R, Chen TT. Statistical challenges in the design of late-stage Cancer immunotherapy studies. Cancer Immunol Res. 2015;3:1292–1298. doi: 10.1158/2326-6066.CIR-15-0260. [DOI] [PubMed] [Google Scholar]
- 22.Huang B. Some statistical considerations in the clinical development of cancer immunotherapies. Pharm Stat. 2018;17:49–60. doi: 10.1002/pst.1835. [DOI] [PubMed] [Google Scholar]
- 23.Postow Michael A. Managing Immune Checkpoint-Blocking Antibody Side Effects. American Society of Clinical Oncology Educational Book. 2015;35:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 24.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 25.Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. 2017;35:3815–3822. doi: 10.1200/JCO.2016.72.1167. [DOI] [PubMed] [Google Scholar]
- 26.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy: assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 30.Péron J, Maillét D, Gan HK, et al. Adherence to CONSORT adverse event reporting guidelines in randomized clinical trials evaluating systemic cancer therapy: a systematic review. J Clin Oncol. 2013;31:3957–3963. doi: 10.1200/JCO.2013.49.3981. [DOI] [PubMed] [Google Scholar]
- 31.Sivendran S, Latif A, McBride RB, et al. Adverse event reporting in cancer clinical trial publications. J Clin Oncol. 2014;32:83–89. doi: 10.1200/JCO.2013.52.2219. [DOI] [PubMed] [Google Scholar]
- 32.Tang E, Ravaud P, Riveros C, et al. Comparison of serious adverse events posted at clinical trials. Gov and published in corresponding journal articles. BMC Med. 2015;13:189. doi: 10.1186/s12916-015-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillet D, Blay JY, You B, et al. The reporting of adverse events in oncology phase III trials: a comparison of the current status versus the expectations of the EORTC members. Ann Oncol. 2016;27:192–198. doi: 10.1093/annonc/mdv485. [DOI] [PubMed] [Google Scholar]
- 34.Chen TW, Razak AR, Bedard PL, et al. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol. 2015;26:1824–1829. doi: 10.1093/annonc/mdv182. [DOI] [PubMed] [Google Scholar]
- 35.Yang A, Baxi S, Korenstein D. ClinicalTrials.gov for facilitating rapid understanding of potential harms of new drugs: the case of checkpoint inhibitors. J Oncol Pract. 2018;14:72–76. doi: 10.1200/JOP.2017.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Cancer Institute, Division of Cancer Treatment and Diagnosis: Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50
- 37.Ioannidis JP, Evans SJ, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 38.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol. 2018;4:98–101. doi: 10.1001/jamaoncol.2017.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horvat TZ, Adel NG, Dang TO, et al. Immunerelated adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering Cancer center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sartor Oliver. Adverse Event Reporting in Clinical Trials: Time to Include Duration as Well as Severity. The Oncologist. 2017;23(1):1–1. doi: 10.1634/theoncologist.2017-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrissey KM, Yuraszeck TM, Li CC, et al. Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin Transl Sci. 2016;9:89–104. doi: 10.1111/cts.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA. 2017;318:1647–1648. doi: 10.1001/jama.2017.14155. [DOI] [PubMed] [Google Scholar]
- 43.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. doi: 10.1186/s40425-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]