Abstract
As one of the non-selective cation channel gene families, the cyclic nucleotide-gated channel (CNGC) gene family plays a vital role in plant physiological processes that are related to signal pathways, plant development, and environmental stresses. However, genome-wide identification and analysis of the CNGC gene family in maize has not yet been undertaken. In the present study, twelve ZmCNGC genes were identified in the maize genome, which were unevenly distributed on chromosomes 1, 2, 4, 5, 6, 7, and 8. They were classified into five major groups: Groups I, II, III, IVa, and IVb. Phylogenetic analysis showed that gramineous plant CNGC genes expanded unequally during evolution. Group IV CNGC genes emerged first, whereas Groups I and II appeared later. Prediction analysis of cis-acting regulatory elements showed that 137 putative cis-elements were related to hormone-response, abiotic stress, and organ development. Furthermore, 120 protein pairs were predicted to interact with the 12 ZmCNGC proteins and other maize proteins. The expression profiles of the ZmCNGC genes were expressed in tissue-specific patterns. These results provide important information that will increase our understanding of the CNGC gene family in maize and other plants.
Keywords: CNGC, Maize, Genome-wide, Gene family
Introduction
Organism evolution has led to the formation of complex nutrient absorption and transport systems, including ion channels, ion pumps, and carriers. It has been previously shown that these systems respond to endogenous and abiotic stimuli (Saand et al., 2015b). The cyclic nucleotide-gated channel (CNGC) is a Ca2+-permeable cation transport channel, and it has been suggested that it is one of the fundamental mechanisms in organism systems (Yuen & Christopher, 2013; Nawaz et al., 2014). Secondary messengers, such as cyclic nucleotide monophosphates (3′,5′-cAMP and 3′,5′-cGMP) and Ca2+/calmodulin (CaM), can regulate CNGCs by acting as molecular switches. The CNGCs are activated by directly binding cyclic nucleotides and are inhibited when CaM binds to the CaM binding domain (Saand et al., 2015b; Borsics et al., 2007; Defalco et al., 2016; Kaplan, Sherman & Fromm, 2007).
In plants, CNGCs are composed of six transmembrane (TM) domains and one pore region between the fifth and sixth TM domains. The cyclic nucleotide-binding domain (CNBD) is a highly conserved region and has a phosphate-binding cassette (PBC) and a hinge region (Saand et al., 2015b). Unfortunately, although the existence of these domains is necessary for CNGC function, they cannot be used to identify a CNGC protein because other ion transporters such as potassium AKT/KAT channels (Shaker type) also contain both a CNBD and a TM domain (Su et al., 2001; Chérel, 2004). However, previous studies have proposed that a plant CNGC-specific motif, [LIMV0]-X(2)-[GSANCR]-X-[FVIYASCL]-X-G-X(0,1)-X(0,1)-[EDAQGH]-L-[LIVFA]-X-[WRCMLS0]-X-[LMSIQAFT0]-X(7,37)-[SAC]-X(9)-[VTIALMS]-X(0,1)-[EQDN]-[AGSVT]-[FYL]-X-[LIVF], in the PBC and hinge region within the CNBD of CNGC proteins only exists in plant CNGCs and does not occur in other ion transporters (Zelman, Dawe & Berkowitz, 2013; Saand et al., 2015b).
The first plant CNGC was identified in Hordeum vulgare and named HvCBT1 (Schuurink et al., 1998). The use of bioinformatics tools has led to the identification of the CNGC gene family members in Arabidopsis (20), rice (16) and other plants (18 in tomato and 26 in Brassica oleracea) (Bridges, Fraser & Moorhead, 2005; Nawaz et al., 2014; Ward, Mäser & Schroeder, 2009; Zelman et al., 2012; Saand et al., 2015a; Chen et al., 2015; Zelman, Dawe & Berkowitz, 2013; Guo et al., 2017; Kakar et al., 2017).
Previous studies have shown that CNGCs are key components of plant development. Most CNGC s have been characterized by genetic methods. They have been shown to play vital roles related to plant physiological and molecular functions, such as multiple physiological processes involved in signal pathways, plant development, and responses to environmental stresses. For example, Arabidopsis CNGC7 and CNGC8 are essential for male reproductive fertility (Tunc-Ozdemir et al., 2013a); CNGC16 and CNGC18 participate in pollen development (Tunc-Ozdemir et al., 2013b; Frietsch et al., 2007; Gao et al., 2016); AtCNGC2 is involved in jasmonic acid induced apoplastic Ca2+ influx in epidermal cells (Lu et al., 2015; Wang et al., 2017); and Arabidopsis CNGC6, CNGC19, and CNGC20 are involved in abiotic stress response (Kugler et al., 2009; Gao et al., 2012). Arabidopsis CNGC structures have six TM domains and a pore domain. They also possess a cyclic nucleotide-binding domain and CaM-binding domain in the C-terminus. These various domains have diverse functions (Talke et al., 2003; Chin, Moeder & Yoshioka, 2009a; Hua et al., 2003; Köhler & Neuhaus, 2000). For example, AtCNGC2 plays a key role in stress signaling pathways, including changes to the cytosolic free Ca2+ in Arabidopsis. In contrast, CNGC4 is permeable to K+ and Na+, and is activated by both cGMP and cAMP (Balague, 2003; Ali et al., 2007).
In recent years, efforts had been made to study the CNGC gene family in plants. However, there have been few studies on the maize CNGC gene family, even though maize is an important food crop and a source of industrial materials worldwide. This study used maize genome-wide sequence information, research information on Arabidopsis and rice CNGC families, and comprehensive bioinformatics analysis techniques to conduct a genome-wide identification of CNGCs in maize. To the best of our knowledge, this is the first systematic study of CNGC genes in maize and provides the basis for future research on the ZmCNGC gene family.
Materials and Methods
Identification of CNGC genes in the maize genome
A total of 20 Arabidopsis and 16 rice CNGC protein sequences were retrieved from the Arabidopsis Information Resource (TAIR10) database (http://www.arabidopsis.org/) and the Rice Genome Annotation Project (RGAP) database (http://rice.plantbiology.msu.edu/), respectively. This information was then used to identify the CNGC genes in maize. Two methods were utilized to search the maize protein sequences. One used a Hidden Markov Model (HMM) to search against maize protein sequences and the other used the local BLASTP method with a threshold e-value <1e−5. After the searches were conducted, a manual correction was performed to remove any redundancy and proteins without PBC and hinge regions within the CNBD of the CNGC proteins. To further confirm whether the ZmCNGC proteins contained the CNBD domain, the putative ZmCNGC protein sequences were submitted to the SMART (Letunic & Bork, 2018) and NCBI-CDD databases (Marchler-Bauer et al., 2017). The proteins without CNBD domains or with amino acid (aa) numbers below 200 were removed and the ZmCNGCs confirmed. Another 11 gramineae plant CNGCs were identified by applying the same method as that described above.
The PI (theoretical isoelectric point), MW (molecular weight), and GRAVY (grand average of hydropathy) of the ZmCNGCs were predicted by ExPASy (Artimo et al., 2012). CELLO v.2.5 software was used to predict the subcellular location of the ZmCNGCs (Yu, Lin & Hwang, 2010). Information about the chromosome distribution of ZmCNGCs and genetic sequences, such as DNA sequences, CDS, cDNA, and up-stream 1500 base pair (bp) DNA sequences obtained from a BLASTN search of the Ensembl Plant database (Bolser et al., 2016).
Multiple alignments, phylogenetic analysis and gene duplication analysis
Multiple sequence alignments were performed using the T-COFFEE method (Di Tommaso et al., 2011) and visualized by ESPript using the default program setting (Robert & Gouet, 2014). A maximum likelihood (ML) phylogenetic tree was constructed using the MEGA 7 software program with 1,000 bootstrap replications and the Jones-Taylor-Thornton model (Kumar, Stecher & Tamura, 2016). To further validate the accuracy of the ML tree, an un-rooted phylogenetic tree was constructed with 1,000 bootstrap replications using the MEGA 7 software program and was based on full-length protein sequence alignments. The ML analysis of 12 gramineae plants was performed using the IQTree program with the LG + G4 and state frequencies were determined from the amino acid matrix and other default parameters (Lam-Tung et al., 2015). The tree was visualized by Evolview online (He et al., 2016). Segmental duplication between maize genes and the synteny block between maize and Sorghum, and rice and Brachypodium were obtained from the Plant Genome Duplication database (Lee et al., 2013). The substitution rates (Ka/Ks) for duplication events were calculated by the DnaSP v5 software program (Librado & Rozas, 2009), and the divergence times (Mya) were calculated using the formula: Mya = Ks ∕2λ × 10−6, where λ = 6.5 × 10−9 (Lynch & Conery, 2000).
Gene structure and conserved motif analyses
The gene structures (exon-intron) of the ZmCNGC genes were determined by the Gene Structure Display Server (Hu et al., 2015) using the CDS and DNA sequences from the ZmCNGC genes. The conserved motifs of the ZmCNGC proteins were subjected to the MEME Suite web server (Bailey et al., 2015) with the maximum number of motifs set at 10 and an optimum width range for the motifs of 6–200 aa.
Cis-acting regulatory elements and the prediction of protein–protein interaction in ZmCNGCs
The up-stream 1,500 bp DNA sequences in the ZmCNGC genes were used to locate cis-acting regulatory elements by the ‘Signal Scan Search’ programs in the NEW PLACE database (Higo et al., 1999). The interactions between ZmCNGCs and other maize proteins were predicted using the STRING 10 online program (Szklarczyk et al., 2017) and visualized by the Cytoscape v3.4.0 software program (Shannon et al., 2003).
ZmCNGC gene expression profiles and network interaction analysis
To understand the expression of ZmCNGC genes in different tissues, two high-throughput datasets for of maize were obtained from the Expression Atlas datasets (https://www.ebi.ac.uk/gxa/home/) under accession numbers E-MTAB-3826 and E-MTAB-439. These data were used to analyze the expression of ZmCNGC genes in six different tissues (ear, embryo, endosperm, pollen, root, and tassel) and at different developmental stages (embryo, endosperm, and seed). The FPKM values were used to calculate the ZmCNGC genes expressions and these were visualized by OmicShare tools, which is a free online platform for data analysis (http://www.omicshare.com/tools).
Results
Identification of the CNGC genes in maize
A total of 20 Arabidopsis and 16 rice CNGC protein sequences which had been BLAST aligned against maize protein sequences, were used to obtain an overview of the CNGC genes in maize. After BLAST alignment, 18 putative ZmCNGC genes were identified in the maize genome. The 18 putative ZmCNGC genes were confirmed by using SMART and NCBI CDD to determine whether they contained the CNGC-specific domains (CNBD and TM). After removing redundant genes, 12 ZmCNGC genes were finally identified, which was lower than the number of CNGC genes in rice and Arabidopsis (Paterson, Bowers & Chapman, 2004; Yu et al., 2005). The predicted ZmCNGC genes were designated as ZmCNGC1 to ZmCNGC12 based on their family classification (Table 1). To further confirm the existence of the ZmCNGCs, we identified all the expressed sequence tags (ESTs) that had aligned to the ZmCNGC genes by using the BLASTN program provided by the NCBI. The results demonstrated that only ZmCNGC3 had showed no EST hits, whereas the other ZmCNGCs had more than 13 representative matches to ESTs. Five of them were located on chromosome 5, whereas the others were unevenly located on chromosomes 1, 2, 4, 6, 7, and 8. The characteristic features of these 12 ZmCNGC genes are listed in Table 1. The ZmCNGC protein lengths ranged from 326 to 745 aa with an average of 612 aa. The molecular weights of these proteins ranged from 38.63 kDa (ZmCNGC2) to 85.52 kDa (ZmCNGC8) and the calculated pI values ranged from 8.92 (ZmCNGC4) to 9.75 (ZmCNGC12). Subcellular localization prediction analysis showed that all the ZmCNGCs were localized in the plasma membrane except for ZmCNGC3 which was localized in the nuclear fraction. These results are similar to Arabidopsis (Lemtiri-Chlieh & Berkowitz, 2004).
Table 1. Characteristic features of ZmCNGC genes in maize.
| Group | Gene ID | Gene name | Chra | Startb | endc | Length (aa)d | MW (Da)e | pIf | GRAVYg | Localizationh | ESTi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | ZmCNGC1 | GRMZM2G148118 | 4 | 230194909 | 230205383 | 701 | 79914.66 | 9.18 | −0.046 | PlasmaMembrane | 17 |
| ZmCNGC2 | GRMZM2G129375 | 6 | 109824359 | 109825921 | 626 | 38405.22 | 9.17 | −0.484 | PlasmaMembrane | 23 | |
| ZmCNGC3 | GRMZM2G066269 | 4 | 230382717 | 230384596 | 329 | 38632.18 | 9.59 | −0.52 | Nuclear | 0 | |
| II | ZmCNGC4 | GRMZM2G023037 | 2 | 5966501 | 5978989 | 723 | 82856.52 | 8.92 | −0.114 | PlasmaMembrane | 29 |
| ZmCNGC5 | GRMZM2G077828 | 5 | 17453069 | 17455739 | 699 | 80110.81 | 9.42 | −0.091 | PlasmaMembrane | 15 | |
| III | ZmCNGC6 | GRMZM2G005791 | 5 | 218834435 | 218840917 | 700 | 80057.39 | 8.97 | −0.078 | PlasmaMembrane | 31 |
| ZmCNGC7 | GRMZM2G068904 | 5 | 191609046 | 191611784 | 689 | 80038.95 | 9.8 | −0.117 | PlasmaMembrane | 15 | |
| ZmCNGC8 | GRMZM2G135651 | 7 | 150652512 | 150657060 | 739 | 85523.26 | 9.33 | −0.148 | PlasmaMembrane | 61 | |
| IVa | ZmCNGC9 | GRMZM2G141642 | 5 | 217119224 | 217125465 | 463 | 53303.64 | 9.48 | −0.059 | PlasmaMembrane | 13 |
| IVb | ZmCNGC10 | GRMZM5G858887 | 5 | 6938133 | 6943695 | 745 | 83672.62 | 9.46 | 0.081 | PlasmaMembrane | 84 |
| ZmCNGC11 | GRMZM2G074317 | 1 | 283401507 | 283408034 | 730 | 81440.82 | 9.43 | 0.067 | PlasmaMembrane | 74 | |
| ZmCNGC12 | GRMZM2G090528 | 8 | 177244867 | 177247281 | 505 | 57042.47 | 9.75 | 0.014 | PlasmaMembrane | 48 |
Notes.
chromosome location.
star of gene location.
end of gene location.
the length of ZmCNGC proteins.
the molecular weight of ZmCNGC proteins.
the Grand average of hydropathicity of ZmCNGC proteins.
the prediction the ZmCNGCs subcellular location.
expressed sequence tags blast results.
Multiple alignments of maize CNGCs and potassium AKT/KAT channel genes
Many ion transporters other than CNGCs also have a CNBD in the C-terminus and a hexa-TM in the N-terminus. For example, potassium AKT/KAT channels (Shaker type) also contain a CNBD and a TM domain. All AKT/ KAT-type channels consist of six TM regions with one P region (Su et al., 2001). Therefore, 11 maize AKT/KAT proteins were obtained from the NCBI resource and aligned with the 12 ZmCNGC protein sequences (File S1). The results showed that the proteins were highly conserved and that all of them contained six TM domains (S1-S6) and a pore region. The PBC and hinge domain were also highly conserved. A ML phylogenetic tree indicated that maize CNGC and AKT/KAT-type channel genes were clustered into two separate sections (File S1).
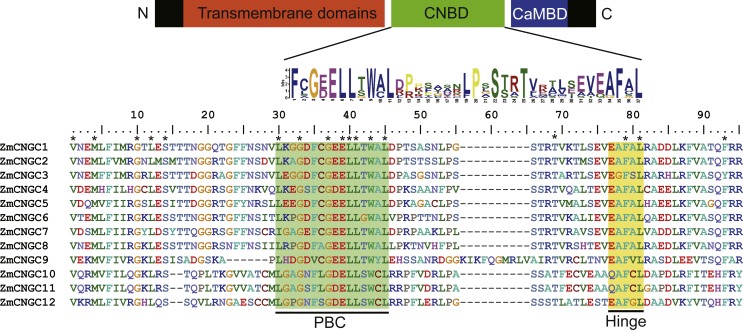
The CNBD is a gene structural feature element in plant CNGCs that contains the PBC and hinge region (Diller et al., 2001). Figure 1 illustrates that within the maize PBCs, a conserved phenylalanine (F), a stabilizing glycine (G) and an acidic residue (D or E), and two aliphatic leucines (L) were 100% conserved inside the PBCs. Additionally, aromatic phenylalanine (F) and leucine (L) were 100% conserved within the hinge region. These two conserved regions occurred between the CNBD and CaMBD regions. Based on corresponding alignments with other plants, a stringent motif (L-X(2)-G-[ED]-ELL-[TSG]-W-[ACY]-L-X(10,20)-[SA]-X-T-X(7)-[EQ]-[AG]-F-X-L) which was recognized in the 12 maize CNGCs that included the PBC and hinge domain, was found to be consistent with other plant species (Saand et al., 2015b; Nawaz et al., 2014). The maize, rice, and Arabidopsis CNGCs were also aligned. The results showed that no positions were specific to the maize CNGC consensus, which suggested that the PBCs and hinge domain were highly conserved among plants (File S2).
Figure 1. The maize CNGC-specific motif spans the putative PBC and the hinge within the CNBD of the 12 ZmCNGCs.
The diagram at the top represents three regions of plant CNGCs: the six transmembrane domains (TM), a CNBD containing a PBC and the hinge, and CaMBD. The maize CNGC-specific amino acid motif is shown below the cartoon. The asterisk indicate 100% identity among the 12 ZmCNGCs. Below is the alignment of CNBD domains of 12 ZmCNGCs. Residues in blue and yellow highlighted indicate the PBC and hinge domain, respectively.
Phylogenetic and duplication analyses of ZmCNGCs
Large phylogenetic trees with minimal homologous characters increase the likelihood of confounding relationships between different species. Therefore, we constructed a ML phylogenetic tree based on the alignment of CNGC proteins from 12 gramineae plants. The tree included 24 CNGC proteins in Aegilops tauschii, 16 in Brachypodium distachyon, 20 in Hordeum vulgare, 23 in Leersia perrieri, 21 in Nicotiana attenuata, 16 in Oryza sativa, 28 in Setaria italic, 13 in Sorghum bicolor, 79 in Triticum aestivum, 21 in Triticum urartu, and 12 maize CNGCs identified by this study (Table S1). The phylogenetic tree (File S4), was used to cluster these plant CNGC proteins into six groups, which were named Groups I, II, III, IV, IVa, and IVb, all of which had significant bootstrap values. The results showed that the CNGC proteins from B. distachyon, O. sativa, S. bicolor, and maize did not cluster in Group IV, and the N. attenuata CNGCs did not cluster in Group I. Additionally, the number in each group was unevenly distributed. Group IV was the largest with 86 genes, followed by 69 in Group III, 44 in Group II, 33 in Group IVb, 25 in Group I, and 16 in Group IVa. These data demonstrate that gramineae plant CNGC gene expansion appeared to have occurred unequally during evolution.
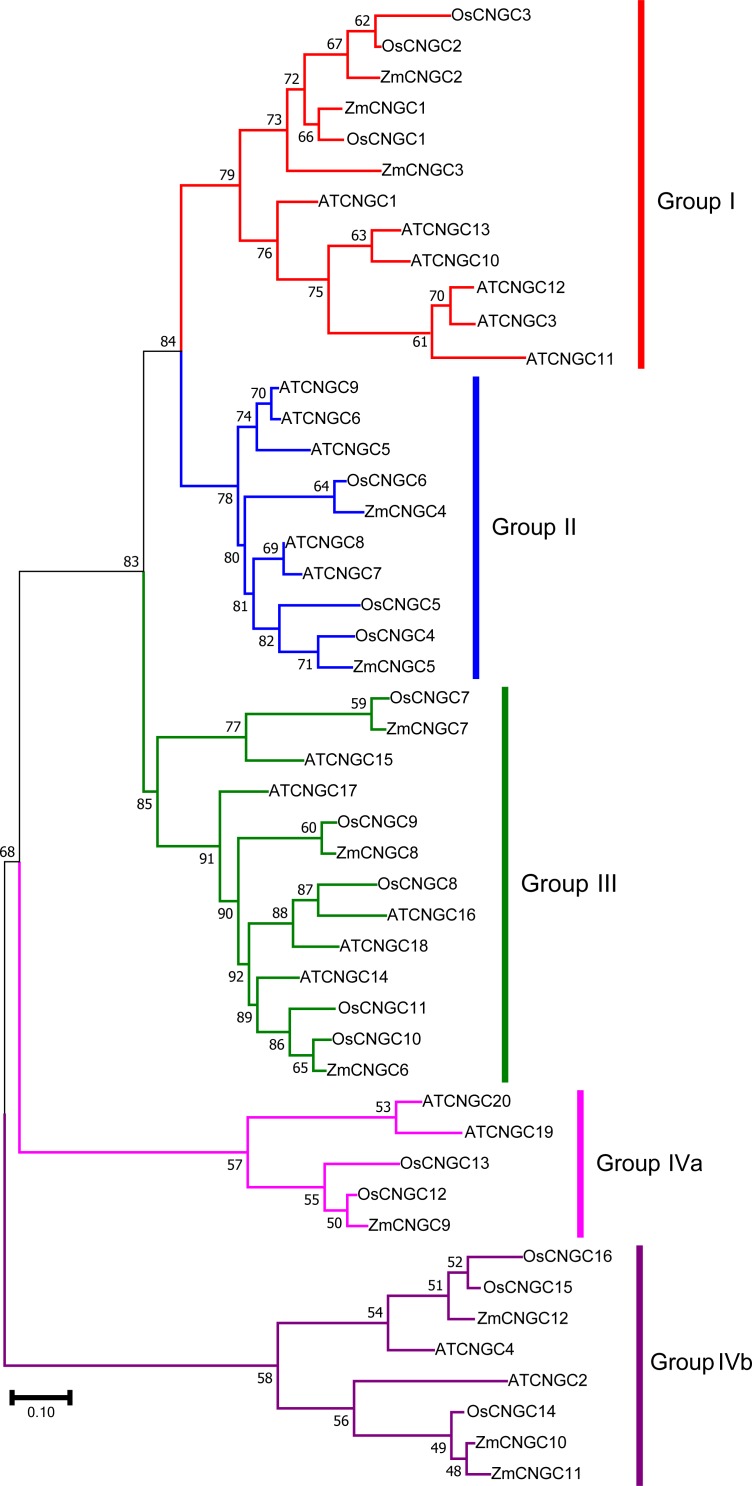
To better understand the evolutionary relationship among CNGC proteins, a ML phylogenetic tree was created based on the full-length protein alignments of 12 ZmCNGC s, 19 AtCNGCs and 16 OsCNGCs (Nawaz et al., 2014; Maser et al., 2001). The phylogenetic tree shows that the 47 CNGC proteins could be classified into five groups with significant bootstrap values (Fig. 2). These were named Groups I, II, III, IVa, and IVb. This was consistent with what has been previously reported for flowering plant CNGCs (Saand et al., 2015b). Group I contained three maize CNGC genes (ZmCNGC1, ZmCNGC2, and ZmCNGC3), five Arabidopsis, and two rice CNGC genes; Group II contained two maize (ZmCNGC4 and ZmCNGC5), five Arabidopsis, and three rice CNGC genes. Group III contained three maize CNGC genes (ZmCNGC6, ZmCNGC7, and ZmCNGC8); and Group IV contained five rice and four Arabidopsis CNGC genes. The maize genes in Group IV were separated into two groups, Group IVa contained ZmCNGC9 and Group IVb contained three (ZmCNGC10, ZmCNGC11, and ZmCNGC12).
Figure 2. Phylogenetic relationships among the ZmCNGCs, OsCNGCs and AtCNGCs.
The multiple alignment was performed by ClustalX program. MEGA 7.0 was used to create maximum likelihood (ML) under the Jones-Taylor-Thornton (JTT) model. The tree was constructed with 1,000 bootstrap replications. Each group identified is indicated on the right.
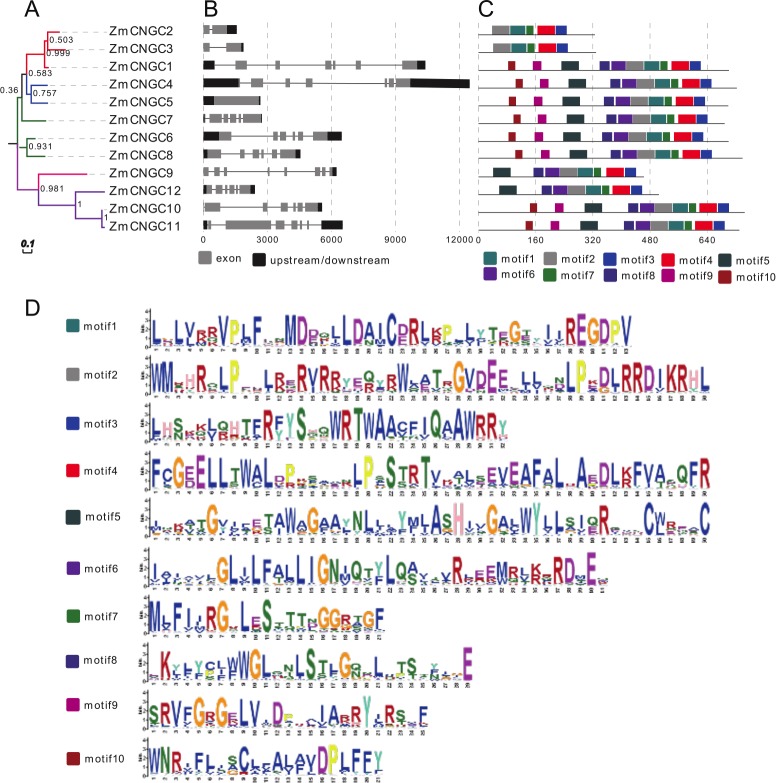
The phylogenetic tree also showed that the maize CNGC genes could also be grouped into five groups (Fig. 3A). Consistent with other plant CNGC genes, Group IVa contained only one or two gene members and formed the smallest group (Saand et al., 2015b). The results suggested that during the evolution of CNGCs, Group IV CNGC genes may have emerged first whereas Groups I and II CNGC genes appeared later. Furthermore, the tree topology produced by the neighbor joining analyses was the same as the ML tree in Fig. 2, and it contained all the groups (File S3).
Figure 3. Phylogenetic relationships (A), motif compositions (B), gene structure (C), and (D) motif logo of ZmCNGCs.
The maximum likelihood (ML) tree under the Jones-Taylor-Thornton (JTT) model was constructed with 1,000 bootstrap replications using MEGA7 based on the full-length protein sequence. The exon-intron structures of these genes were graphically displayed by the Gene Structure Display Server using the CDS and genome sequence of ZmCNGC genes. The protein sequences of ZmCNGC genes were used to predict the conserved motifs by using the MEME Suite web server.
Additionally, only one segmental duplication gene pair, ZmCNGC10-ZmCNGC11, was formed in the maize genome (Table 2). The evolutionary process between maize CNGCs and other gramineae plants was further explored by investigating the genome synteny among Sorghum, rice, and Brachypodium. The results showed that there were two, two and one ZmCNGC genes that showed syntenic bias towards particular Sorghum, rice, and Brachypodium chromosomes, respectively (Table 2). In addition, Ka/Ks was used to evaluate their specific positions under positive selection pressure after duplication (Mayrose et al., 2007). Ka/Ks values that =1, <1 or >1 indicate neutral, purifying, and positive selection, respectively (Lynch & Conery, 2000). The Ka/Ks value of each gene pair was calculated and the Ka/Ks value for all gene pairs was less than 1, which suggested that these genes had evolved under strong purifying selection. Furthermore, the results indicated that the divergence of maize CNGCs from other gramineae plants did not occur evenly.
Table 2. The Ka/Ks ratios and estimated divergence time for orthologous CNGC proteins between maize and other Gramineae plants.
| Gene ID | Gene ID | Ka | Ks | Ka/Ks | Mya |
|---|---|---|---|---|---|
| ZmCNGC11 | ZmCNGC10 | 0.0258 | 0.1829 | 0.141061 | 14.06923 |
| ZmCNGC5 | Sobic.001G155100 | 0.5298 | 0.8917 | 0.594146 | 68.59231 |
| ZmCNGC12 | Sobic.009G188800 | 0.095 | 0.4878 | 0.194752 | 37.52308 |
| ZmCNGC12 | Sobic.003G317700 | 0.0306 | 0.0993 | 0.308157 | 7.638462 |
| ZmCNGC5 | LOC_Os03g44440 | 0.7415 | 1.2253 | 0.605158 | 94.25385 |
| ZmCNGC12 | LOC_Os05g42250 | 0.1187 | 0.4278 | 0.277466 | 32.90769 |
| ZmCNGC12 | LOC_Os01g57370 | 0.073 | 0.3017 | 0.241962 | 23.20769 |
| ZmCNGC5 | Bradi1g13740 | 0.7912 | 1.1721 | 0.675028 | 90.16154 |
ZmCNGC gene structures and the conserved motif analyses
Gene structure analysis could improve our understanding of gene function and evolution. The number of introns ranged from 0 to 7 (Fig. 3B), which was different from rice and Arabidopsis CNGCs. In rice, the OsCNGC s introns numbers ranged from 1 to 11, whereas Arabidopsis CNGCs ranged from 4 to 10 (Nawaz et al., 2014). The Group IVa and IVb ZmCNGCs had distinct gene structures compared to those of the other groups, with more introns at different phases and lengths, which is consistent with most flowering plant species Group IV CNGC genes (Saand et al., 2015b).
The motif-based recognition of proteins can improve understanding of their the evolutionary history (Seoighe & Gehring, 2004). Ten putative motifs were characterized and named as motif1 to motif10 in the ZmCNGCs. The relative positions of the motifs in the five groups were found to have various patterns (Figs. 3C and 3D), for example, all the ZmCNGCs contained motif1, motif2, motif3, and motif4, which meant they presented typical ZmCNGC domains. Motif3 was a combination of the calmodulin binding (CaMB) domain and the IQ domain (QWRTWAA[CV]FIQ[AL]AW[RH]RY), and motif4 was the cyclic nucleotide-binding (CNB) domain, which was located in the C-terminal. Furthermore, motif10, 9, 5, 8 (or 6), 2, and 1 were the motif logos of the transmembrane domains. They represented the S1, S2, S3, S4, S5, and S6 regions of the transmembrane domain in the N-terminal. Motif7 represented the ion transport protein (ITP) domain. In addition, 10 of the ZmCNGCs, but not ZmCNGC2 and ZmCNGC3, possessed motif 5 and 6 that are associated with ion transport (Nawaz et al., 2014). Although other motifs have not been reported in plants or animals, they undertake important functions within the organism.
Prediction of cis-acting regulatory elements and protein-protein interactions that involve ZmCNGC proteins
Cis-acting regulatory elements are important molecular switches that are associated with the transcriptional regulation of genes when environmental stresses are encountered (Nakashima, Ito & Yamaguchi-Shinozaki, 2009). To better understand the possible biological processes undertaken by the ZmCNGCs involved, 1.5 kb sequences upstream of the ZmCNGC gene genomic sequences were used to identify cis-regulatory elements. A total of 137 different putative cis-elements were found to be associated with the identified ZmCNGC genes and only 12, including CACTFTPPCA1, EBOXBNNAPA, DOFCOREZM, MYCCONSENSUSAT, CAATBOX1, GTGANTG10, WRKY71OS, GT1CONSENSUS, ROOTMOTIFTAPOX1, POLLEN1LELAT52, MYBCORE, and OSE2ROOTNODULE, were in the promoter region of the ZmCNGC genes (Table S2), which was highly consistent with rice CNGCs. Additionally, five cis-elements were gene-specific. ACGTCBOX, TATABOX3, CTRMCAMV35S, and HDZIP2ATATHB2 were unique to ZmCNGC6, ZmCNGC7, ZmCNGC8, and ZmCNGC11, respectively. Additionally, some cis-elements were involved in responses to different abiotic/biotic stimuli, including hormones (e.g., abscisic acid, auxin, ethylene), stress (e.g., drought, temperatures, disease) and development (e.g., mesophyll specific, tissue specific) responses, which indicated that these ZmCNGC genes might be involved in regulating diverse stress responses.
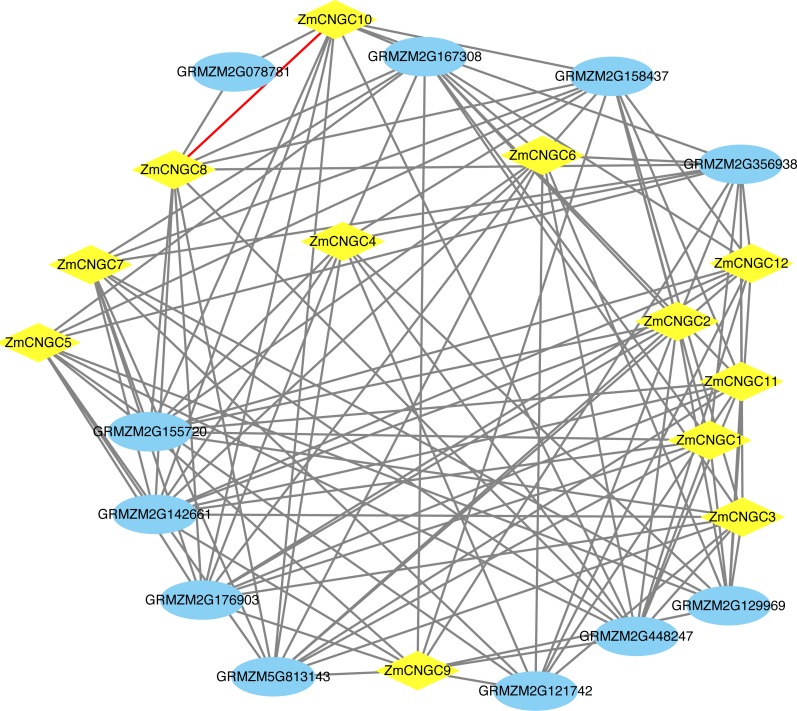
The ZmCNGC protein-protein interactions were predicted in order to gain a better understanding of ZmCNGC protein roles in the plant. A total of 120 protein pairs were predicted to interact between the 12 ZmCNGC proteins and the other 11 maize proteins, and ZmCNGC8 was found to interact with ZmCNGC10 by prediction analysis (Fig. 4 and Table S3). We also used genes in Arabidopsis that were homologous to the ZmCNGC genes to predict the protein-protein interactions. The results showed that three were validated by the experimental data (Table S3).
Figure 4. The protein–protein interaction network of ZmCNGC genes in maize.
The protein–protein interaction network were constructed based on STRING database.
Expression profiles of ZmCNGC genes in different tissues
The physiological function of ZmCNGC genes was investigated using transcriptome sequencing which evaluated the tissue-specific expression levels of ZmCNGC genes in different tissues (see Materials and Methods section). The expression levels among the ZmCNGC genes are shown in Fig. 5A and Table S4 suggested that their expression levels were tissue-specific. For example, ZmCNGC5 was specifically expressed in pollen compared to other tissues, which implied that it played a particular role in pollen physiological development, whereas ZmCNGC2, ZmCNGC4, ZmCNGC6, and ZmCNGC8 had higher expression levels in the roots than in the other tissues, which suggested that they had important roles in root growth and development. All Group IVb ZmCNGC genes, including ZmCNGC10, ZmCNGC11, and ZmCNGC12, were relatively highly expressed in the embryo, which implied that these genes played crucial roles in the growth and development of the maize embryo.
Figure 5. Expression profiles of ZmCNGCs in six different tissues and different development stages in embryo, endosperm and seed after pollination, respectively.
(A) Expression profile in six different tissues, including ear, embryo, endosperm, pollen, root and tassel. Expression profile in (B) embryo, (C) endosperm and (D) seed of some days after pollination.
We also evaluated the expression of several ZmCNGC genes in the embryo, endosperm, and seed for several days after pollination. ZmCNGC3, ZmCNGC5 and ZmCNGC7 were not detected or had no expression in any of the tissues. However, ZmCNGC5 was expressed in pollen (Figs. 5A, 5B, 5C, and 5D). Cis-acting regulatory elements analysis showed that only ZmCNGC5 and ZmCNGC7 did not contain CANBNNAPA, which is the element required for embryo and endosperm-specific transcription (Ellerström et al., 1996). This might be the reason why they did not show any expression in these tissues. The embryo specific-expression gene ZmCNGC10 gradually increased over time in the embryo (Fig. 5B). ZmCNGC8 was highly expressed in the embryo, endosperm, and seeds after pollination, and the Group IVb gene showed a similar expression pattern.
Discussion
Features and evolution of plant CNGC family genes
Plant CNGC family genes are characterized by the presence of a CNBD in the C-terminal and a hexa-TM in the N-terminal (Saand et al., 2015b). A total of 12 ZmCNGC genes were identified after a BLAST search against the maize genome protein sequences. Among them, ZmCNGC3 showed no ESTs and was not expressed in all the tissues, which suggested that it was a non-expressed pseudogene. In Arabidopsis, AtCNGC16 (AT3G48010) had no EST alignments and might also be a pseudogene (Maser et al., 2001). Many other ion transporters also possess these domains. For example, potassium AKT/KAT channels (Shaker type) contain both a CNBD domain and a TM domain. All AKT/ KAT-type channels consist of six transmembrane (TM) regions with one P region (Su et al., 2001). The ML phylogenetic tree showed that the maize CNGC and the AKT/KAT-type channel genes were clustered into two separate sections (File S1). Previous studies have shown that the CNGC-specific motif with a PBC and a hinge domain (L-X(2)-G-[ED]-ELL-[TSG]-W-[ACY]-L-X(10,20)-[SA]-X-T-X(7)-[EQ]-[AG]-F-X-L) only exists in maize plant CNGCs and does not occur in the ion transporters found in other plants, such as rice, Arabidopsis, and tomato (Saand et al., 2015b; Nawaz et al., 2014; Zelman, Dawe & Berkowitz, 2013). In this study, 12 ZmCNGCs were identified and the PBCs and hinge domains were highly conserved after alignment, which further confirmed the previous hypothesis (Saand et al., 2015b). The conserved motif analysis showed that the motif3 (QWRTWAA[CV]FIQ[AL]AW[RH]RY) pattern was the IQ domain among these 12 ZmCNGCs. The IQ domain is conserved among plant CNGCs and enhances the changeable Ca2+-dependent channel control mechanisms in plants (Fischer et al., 2013). The results from this study showed that all CNGC proteins contained the IQ motif, which suggested that they bind CaM in a Ca2+-dependent manner. Notably, motif4 is the sequence logo of the CNBD domain, which is conserved in most plants and animals (Jackson, Marshall & Accili, 2007).
The results showed that the CNGC family in 12 gramineae plants at various evolutionary nodes is adequate for analyzing the phylogeny and evolution of the CNGC gene family in gramineae plants. In Table S1 shows that 4 out of 12 plants under analysis contained <20 CNGC genes. These were B. distachyon (16), O. sativa (16), S. bicolor (13) and maize (12). The ML tree for the 273 CNGCs clearly indicated that gramineae CNGCs clustered into six groups (I, II, III, IV, IVa, and IVb) with significant bootstrap values; Groups IV, IVa and IVb had particularly high bootstrap support values, but group IV CNGCs did not cluster with B. distachyon, O. sativa, S. bicolor, and maize CNGCs. This suggested that there are missing CNGCs that need to be identified or there was duplication during evolution. Davidson, Guthrie & Lipsick (2013) showed that gene duplication can lead to the generation of significant numbers of novel genes. Therefore, these results imply that duplication events play a principal role in gene evolution. Phylogenetic, gene structure, and conserved motifs analyses were used to classify the 12 ZmCNGC s into five groups with significant bootstrap values. Among these five groups, Group IV ZmCNGC s emerged first and had more introns than the genes in the other groups.
CNGC genes play an important role in plant development
The CNGCs are involved in the regulation of plant growth and development (Chin, Moeder & Yoshioka, 2009b). This study focused on the role that maize CNGCs play in different tissues by investigating its expression in plant embryo, endosperm and seed expression. The results showed that some ZmCNGC genes have tissue-specific expression. For example, ZmCNGC2, ZmCNGC4, ZmCNGC6 and ZmCNGC8 were highly expressed in the roots; ZmCNGC5 was specifically expressed in pollen; and all Group IVb genes were expressed in the embryo. Group IVb genes are the evolutionary ancestors of CNGC genes and were mainly expressed in the embryo and seeds after pollination, which suggested that Group IVb genes play a significant role in embryo development. In addition, all Group IVb genes were linked to gene duplication and they had a similar expression pattern in the different tissues, which indicated that their function was to enhance adaptability during gene evolution.
Most previous studies have shown that CNGC genes are related to pollen development and responses to environmental stimuli. For example, Arabidopsis CNGC16 is critical for pollen fertility under heat and drought stress (Tunc-Ozdemir et al., 2013b), and CNGC18 has been shown to play a role in pollen tube tip growth (Frietsch et al., 2007). In rice, OsCNGC13 promotes the seed-setting rate by facilitating pollen tube growth in stylar tissues (Xu et al., 2017). ZmCNGC1 and ZmCNGC5, two homologous genes of CNGC16 and CNGC18, are mainly expressed in pollen, which indicates that they are predominantly involved in pollen development. Previous studies have shown that AtCNGC3 is mainly expressed in the embryo, leaves, and roots, and ZmCNGC4 expression level is similar to that of AtCNGC3 which was highly expressed during plant development (Kaplan, Sherman & Fromm, 2007). These results imply that these genes play crucial roles during the growth and development of maize.
Conclusion
A total of 12 CNGC genes were identified in maize via bioinformatics. The results were based on the presence of a plant CNGC-specific motif that spanned the PBCs and hinge domain in the CNBD of CNGC proteins. Phylogenetic analyses showed that Group IV ZmCNGCs emerged first and had more introns than the other ZmCNGC groups, whereas Groups I and II evolved later. Phylogenetic analysis of 12 gramineae plants revealed that some CNGCs are probably missing or have been duplicated during evolution. Significantly, the ZmCNGC genes had diversity in gene structures, protein lengths and sizes. We modified a maize stringent motif (L-X(2)-G-[ED]-ELL-[TSG]-W-[ACY]-L-X(10,20)-[SA]-X-T-X(7)-[EQ]-[AG]-F-X-L) that contained a PBC and a hinge domain to better characterize classification. Expression profiles of the ZmCNGC genes were tissue-specific and were related to pollen development. This study provides important information about plant CNGCs during gene evolution.
Supplemental Information
The AKT and CNGC indicated maize AKT/KAT channel genes and CNGC genes. S1–S6 indicated six transmembrane (TM) regions with one P region of AKT/KAT or CNGC proteins.
Residues in highlighted indicate most conserved among these CNGCs.
The multiple alignment was performed by ClustalX program. MEGA 7.0 was used to create the neighbor joining (NJ) under the poisson model. The tree was constructed with 1,000 bootstrap replications.
The multiple alignment was performed by ClustalX program. IQTREE was used to create the Maximum likelihood (ML) with JTT+LG4 model. The tree was constructed with 1,000 bootstrap replications.
Table S1. The GNGC information of 12 gramineae plants.
Table S2. Numbers of known stress-related elements in the promoter regions of ZmCNGCs.
Table S3. The protein–protein interaction information of maize CNGC protein with other maize proteins.
Table S4. The FPKM data of ZmCNGC genes in different tissues.
The conserved motifs of ZmCNGC proteins were using the MEME Suite web server (http://meme-suite.org/index.html) with the maximum number of motif sets at 10 and optimum width of motifs from 6 to 200 amino acids.
Including DNA, CDS, Cdna, protein sequences of ZmCNGCS, and protein sequences of maize AKT/KAT channel genes and other plant CNGC gens.
Acknowledgments
We extend our gratitude to Dr. Lihe Yu for providing valuable suggestions.
Funding Statement
This work was supported by the Natural Science Foundation of Heilongjiang Province, China (B2016010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Lidong Hao, Email: haolidong1987@163.com.
Xiuli Qiao, Email: shxynxhld@163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Lidong Hao conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xiuli Qiao conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The information of chromosome distribution of ZmCNGCs and the sequences including DNA sequences, CDS, cDNA, up-stream 1,500 bps of ZmCNGC genes were obtained from BLASTN (the Ensembl Plant database, http://plants.ensembl.org/index.html): accession numbers GRMZM2G148118, GRMZM2G129375, GRMZM2G066269, GRMZM2G023037, GRMZM2G077828, GRMZM2G005791, GRMZM2G068904, GRMZM2G135651, GRMZM2G141642, GRMZM5G858887, GRMZM2G074317, and GRMZM2G090528.
13 maize AKT/KAT genes were downloaded from Ensembl Plant database with gene ID: C234152.1_FGT002,GRMZM2G020859,GRMZM2G081666,GRMZM2G178356, GRMZM2G310569,GRMZM2G156255,GRMZM2G093313, GRMZM2G022915, GRMZM2G171279,GRMZM2G028258,GRMZM2G342375,GRMZM2G428859, GRMZM5G838773,GRMZM2G095861.
The 20 Arabidopsis and 16 rice CNGC protein sequences were obtained from TAIR10 (http://www.arabidopsis.org) and RGAP (http://rice.plantbiology.msu.edu/) respectively. Arabidopsis asscession numbers: AT5G53130, AT3G48010,AT2G23980, AT2G24610, AT5G54250, AT2G28260, AT5G15410, AT1G15990, AT4G30560, AT5G57940, AT3G17700, AT1G01340, AT1G19780, AT2G46450, AT4G30360, AT5G14870, AT4G01010, AT3G17690, AT2G46430, AT2G46440, LOC_Os06g33570, LOC_Os06g33610, LOC_Os03g44440, LOC_Os12g28260, LOC_Os04g55080, LOC_Os02g41710, LOC_Os12g06570, LOC_Os09g38580, LOC_Os02g54760, LOC_Os06g08850, LOC_Os02g53340, LOC_Os06g10580, LOC_Os03g55100, LOC_Os01g57370, LOC_Os05g42250.
References
- Ali et al. (2007).Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, Bodman SV, Berkowitz GA. Death don’t have no mercy and neither does calcium: arabidopsis cyclic nucleotide gated channel2 and innate immunity. The Plant Cell. 2007;19(3):1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artimo et al. (2012).Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, De Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research. 2012;40(Web Server issue):W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey et al. (2015).Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Research. 2015;43(W1):W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balague (2003).Balague C. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. The Plant Cell Online. 2003;15(2):365–379. doi: 10.1105/tpc.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser et al. (2016).Bolser D, Staines DM, Pritchard E, Kersey P. Ensembl plants: integrating tools for visualizing, mining, and analyzing plant genomics data. Methods in Molecular Biology. 2016;1374:115–140. doi: 10.1007/978-1-4939-3167-5_6. [DOI] [PubMed] [Google Scholar]
- Borsics et al. (2007).Borsics T, Webb D, Andemeondzighi C, Staehelin LA, Christopher DA. The cyclic nucleotide-gated calmodulin-binding channel AtCNGC10 localizes to the plasma membrane and influences numerous growth responses and starch accumulation in Arabidopsis thaliana. Planta. 2007;225(3):563–573. doi: 10.1007/s00425-006-0372-3. [DOI] [PubMed] [Google Scholar]
- Bridges, Fraser & Moorhead (2005).Bridges D, Fraser ME, Moorhead GB. Cyclic nucleotide binding proteins in the Arabidopsis thaliana and Oryza sativa genomes. BMC Bioinformatics. 2005;6(1):1–12. doi: 10.1186/1471-2105-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen J, Yin H, Gu J, Li L, Liu Z, Jiang X, Zhou H, Wei S, Zhang S, Wu J. Genomic characterization, phylogenetic comparison and differential expression of the cyclic nucleotide-gated channels gene family in pear (Pyrus bretchneideri Rehd.) Genomics. 2015;105(1):39–52. doi: 10.1016/j.ygeno.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Chérel (2004).Chérel I. Regulation of K+ channel activities in plants: from physiological to molecular aspects. Journal of Experimental Botany. 2004;55(396):337–351. doi: 10.1093/jxb/erh028. [DOI] [PubMed] [Google Scholar]
- Chin, Moeder & Yoshioka(2009a).Chin K, Moeder W, Yoshioka KY. Biological roles of cyclic-nucleotide-gated ion channels in plants: what we know and don’t know about this 20 member ion channel family. Botany-Botanique. 2009a;87(7):668–677. doi: 10.1139/B08-147. [DOI] [Google Scholar]
- Chin, Moeder & Yoshioka(2009b).Chin KC, Moeder WM, Yoshioka KY. Biological roles of cyclic-nucleotide-gated ion channels in plants: what we know and don’t know about this 20 member ion channel family. Botany-Botanique. 2009b;87(7):668–677. doi: 10.1139/B08-147. [DOI] [Google Scholar]
- Davidson, Guthrie & Lipsick (2013).Davidson CJ, Guthrie EE, Lipsick JS. Duplication and maintenance of the Myb genes of vertebrate animals. Biology Open. 2013;2(2):101–110. doi: 10.1242/bio.20123152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defalco et al. (2016).Defalco TA, Marshall CB, Munro K, Kang HG, Moeder W, Ikura M, Snedden WA, Yoshioka K. Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis cyclic nucleotide-gated channel12. The Plant Cell. 2016;28(7):1738–1751. doi: 10.1105/tpc.15.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso et al. (2011).Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Research. 2011;39(Web Server issue):W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller et al. (2001).Diller TC, Madhusudan, Xuong NH, Taylor SS. Molecular basis for regulatory subunit diversity in cAMP-dependent protein kinase—crystal structure of the type IIbeta regulatory subunit. Structure. 2001;9(1):73–82. doi: 10.1016/S0969-2126(00)00556-6. [DOI] [PubMed] [Google Scholar]
- Ellerström et al. (1996).Ellerström M, Stålberg K, Ezcurra I, Rask L. Functional dissection of a napin gene promoter: identification of promoter elements required for embryo and endosperm-specific transcription. Plant Molecular Biology. 1996;32(6):1019–1027. doi: 10.1007/BF00041385. [DOI] [PubMed] [Google Scholar]
- Fischer et al. (2013).Fischer C, Kugler A, Hoth S, Dietrich P. An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant and Cell Physiology. 2013;54(4):573–584. doi: 10.1093/pcp/pct021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch et al. (2007).Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(36):14531–14536. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2012).Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant Journal for Cell & Molecular Biology. 2012;70(6):1056–1069. doi: 10.1111/j.1365-313X.2012.04969.x. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2016).Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(11):3096–3101. doi: 10.1073/pnas.1524629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2017).Guo S, Zuo Y, Zhang Y, Wu C, Su W, Jin W, Yu H, An Y, Li Q. Large-scale transcriptome comparison of sunflower genes responsive to Verticillium dahliae. BMC Genomics. 2017;18(1):42. doi: 10.1186/s12864-016-3386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2016).He Z, Zhang H, Gao S, Lercher MJ, Chen WH, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Research. 2016;44(Web Server issue):W236–W241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo et al. (1999).Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research. 1999;27(1):297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2015).Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua et al. (2003).Hua BG, Mercier RW, Zielinski RE, Berkowitz GA. Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiology & Biochemistry. 2003;41(11–12):945–954. doi: 10.1016/j.plaphy.2003.07.006. [DOI] [Google Scholar]
- Jackson, Marshall & Accili (2007).Jackson HA, Marshall CR, Accili EA. Evolution and structural diversification of hyperpolarization-activated cyclic nucleotide-gated channel genes. Physiological Genomics. 2007;29(3):231–245. doi: 10.1152/physiolgenomics.00142.2006. [DOI] [PubMed] [Google Scholar]
- Kakar et al. (2017).Kakar KU, Nawaz Z, Kakar K, Ali E, Almoneafy AA, Ullah R, Ren XL, Shu QY. Comprehensive genomic analysis of the CNGCgene family in Brassica oleracea: novel insights into synteny, structures, and transcript profiles. BMC Genomics. 2017;18(1):869. doi: 10.1186/s12864-017-4244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, Sherman & Fromm (2007).Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Letters. 2007;581(12):2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Köhler & Neuhaus (2000).Köhler C, Neuhaus G. Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Letters. 2000;471(2–3):133–136. doi: 10.1016/S0014-5793(00)01383-1. [DOI] [PubMed] [Google Scholar]
- Kugler et al. (2009).Kugler A, Köhler B, Palme K, Wolff P, Dietrich P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biology. 2009;9(1):140. doi: 10.1186/1471-2229-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology & Evolution. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam-Tung et al. (2015).Lam-Tung N, Schmidt HA, Arndt VH, Quang MB. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology & Evolution. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2013).Lee TH, Tang H, Wang X, Paterson AH. PGDD: a database of gene and genome duplication in plants. Nucleic Acids Research. 2013;41(Database issue):1152–1158. doi: 10.1093/nar/gks1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh & Berkowitz (2004).Lemtiri-Chlieh F, Berkowitz GA. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. The Journal of Biological Chemistry. 2004;279(34):35306–35312. doi: 10.1074/jbc.M400311200. [DOI] [PubMed] [Google Scholar]
- Letunic & Bork (2018).Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Research. 2018;46(D1):D493–d496. doi: 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2015).Lu M, Zhang Y, Tang S, Pan J, Yu Y, Han J, Li Y, Du X, Nan Z, Sun Q. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. Journal of Experimental Botany. 2015;67(3):809–819. doi: 10.1093/jxb/erv500. [DOI] [PubMed] [Google Scholar]
- Lynch & Conery (2000).Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290(5494):1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer et al. (2017).Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Research. 2017;45(D1):D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser et al. (2001).Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology. 2001;126(4):1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrose et al. (2007).Mayrose I, Doron-Faigenboim A, Bacharach E, Pupko T. Towards realistic codon models: among site variability and dependency of synonymous and non-synonymous rates. Bioinformatics. 2007;23(13):i319–i327. doi: 10.1093/bioinformatics/btm176. [DOI] [PubMed] [Google Scholar]
- Nakashima, Ito & Yamaguchi-Shinozaki (2009).Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology. 2009;149(1):88–95. doi: 10.1104/pp.108.129791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz et al. (2014).Nawaz Z, Kakar KU, Saand MA, Shu QY. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics. 2014;15(1):1–18. doi: 10.1186/1471-2164-15-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, Bowers & Chapman (2004).Paterson AH, Bowers JE, Chapman BA. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9903–9908. doi: 10.1073/pnas.0307901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert & Gouet (2014).Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research. 2014;42(Web Server issue):W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saand et al. (2015a).Saand MA, Xu YP, Li W, Wang JP, Cai XZ. Cyclic nucleotide gated channel gene family in tomato: genome-wide identification and functional analyses in disease resistance. Frontiers in Plant Science. 2015a;6(303):303. doi: 10.3389/fpls.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saand et al. (2015b).Saand MA, Xu YP, Munyampundu JP, Li W, Zhang XR, Cai XZ. Phylogeny and evolution of plant cyclic nucleotide-gated ion channel (CNGC) gene family and functional analyses of tomato CNGCs. DNA Research. 2015b;22(6):471–483. doi: 10.1093/dnares/dsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink et al. (1998).Schuurink RC, Shartzer SF, Fath A, Jones RL. Characterization of a calmodulin-binding transporter from the plasma membrane of, barley, aleurone. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(4):1944–1949. doi: 10.1073/pnas.95.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoighe & Gehring (2004).Seoighe C, Gehring C. Genome duplication led to highly selective expansion of the Arabidopsis thaliana proteome. Trends in Genetics. 2004;20(10):461–464. doi: 10.1016/j.tig.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shannon et al. (2003).Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su et al. (2001).Su H, Golldack D, Katsuhara M, Zhao C, Bohnert HJ. Expression and stress-dependent induction of potassium channel transcripts in the common ice plant. Plant Physiology. 2001;125(2):604–614. doi: 10.1104/pp.125.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk et al. (2017).Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Research. 2017;45(Database issue):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke et al. (2003).Talke IN, Blaudez D, Maathuis FJM, Sanders D. CNGCs: prime targets of plant cyclic nucleotide signalling? Trends in Plant Science. 2003;8(6):286–293. doi: 10.1016/S1360-1385(03)00099-2. [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir et al. (2013a).Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Rui M, Harper JF. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLOS ONE. 2013a;8(2):e55277. doi: 10.1371/journal.pone.0055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozdemir et al. (2013b).Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, Mcdowell S, Miller G. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiology. 2013b;161(2):1010–1020. doi: 10.1104/pp.112.206888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang Y, Kang Y, Ma C, Miao R, Wu C, Long Y, Ge T, Wu Z, Hou X, Zhang J. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiology. 2017;173(2):1342–1354. doi: 10.1104/pp.16.01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, Mäser & Schroeder (2009).Ward JM, Mäser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annual Review of Physiology. 2009;71(71):59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y, Wang Y, Zhang H, Ren Y, Chen J, Wang Y, Zhang X, Guo X, Wu F, Zhu S, Lin Q, Jiang L, Wu C, Wang H, Wan J. OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLOS Genetics. 2017;13(7):e1006906. doi: 10.1371/journal.pgen.1006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Lin & Hwang (2010).Yu CS, Lin CJ, Hwang JK. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Science. 2010;13(5):1402–1406. doi: 10.1110/ps.03479604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2005).Yu J, Wang J, Lin W, Li S, Li H, Zhou J, Ni P, Dong W, Hu S, Zeng C, Zhang J, Zhang Y, Li R, Xu Z, Li S, Li X, Zheng H, Cong L, Lin L, Yin J, Geng J, Li G, Shi J, Liu J, Lv H, Li J, Wang J, Deng Y, Ran L, Shi X, Wang X, Wu Q, Li C, Ren X, Wang J, Wang X, Li D, Liu D, Zhang X, Ji Z, Zhao W, Sun Y, Zhang Z, Bao J, Han Y, Dong L, Ji J, Chen P, Wu S, Liu J, Xiao Y, Bu D, Tan J, Yang L, Ye C, Zhang J, Xu J, Zhou Y, Yu Y, Zhang B, Zhuang S, Wei H, Liu B, Lei M, Yu H, Li Y, Xu H, Wei S, He X, Fang L, Zhang Z, Zhang Y, Huang X, Su Z, Tong W, Li J, Tong Z, Li S, Ye J, Wang L, Fang L, Lei T, Chen C, Chen H, Xu Z, Li H, Huang H, Zhang F, Xu H, Li N, Zhao C, Li S, Dong L, Huang Y, Li L, Xi Y, Qi Q, Li W, Zhang B, Hu W, Zhang Y, Tian X, Jiao Y, Liang X, Jin J, Gao L, Zheng W, Hao B, Liu S, Wang W, Yuan L, Cao M, McDermott J, Samudrala R, Wang J, Wong GK, Yang H. The genomes of oryza sativa: a history of duplications. PLOS Biology. 2005;3(2):e38. doi: 10.1371/journal.pbio.0030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen & Christopher (2013).Yuen CCY, Christopher DA. The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC20, localize to the vacuole membrane in Arabidopsis thaliana. AoB Plants. 2013;5:plt012–plt012. doi: 10.1093/aobpla/plt012. [DOI] [Google Scholar]
- Zelman, Dawe & Berkowitz (2013).Zelman AK, Dawe A, Berkowitz GA. Identification of cyclic nucleotide gated channels using regular expressions. Methods in Molecular Biology. 2013;1016:207–224. doi: 10.1007/978-1-62703-441-8_14. [DOI] [PubMed] [Google Scholar]
- Zelman et al. (2012).Zelman AK, Dawe A, Gehring C, Berkowitz GA. Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Frontiers in Plant Science. 2012;3:95. doi: 10.3389/fpls.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The AKT and CNGC indicated maize AKT/KAT channel genes and CNGC genes. S1–S6 indicated six transmembrane (TM) regions with one P region of AKT/KAT or CNGC proteins.
Residues in highlighted indicate most conserved among these CNGCs.
The multiple alignment was performed by ClustalX program. MEGA 7.0 was used to create the neighbor joining (NJ) under the poisson model. The tree was constructed with 1,000 bootstrap replications.
The multiple alignment was performed by ClustalX program. IQTREE was used to create the Maximum likelihood (ML) with JTT+LG4 model. The tree was constructed with 1,000 bootstrap replications.
Table S1. The GNGC information of 12 gramineae plants.
Table S2. Numbers of known stress-related elements in the promoter regions of ZmCNGCs.
Table S3. The protein–protein interaction information of maize CNGC protein with other maize proteins.
Table S4. The FPKM data of ZmCNGC genes in different tissues.
The conserved motifs of ZmCNGC proteins were using the MEME Suite web server (http://meme-suite.org/index.html) with the maximum number of motif sets at 10 and optimum width of motifs from 6 to 200 amino acids.
Including DNA, CDS, Cdna, protein sequences of ZmCNGCS, and protein sequences of maize AKT/KAT channel genes and other plant CNGC gens.
Data Availability Statement
The following information was supplied regarding data availability:
The information of chromosome distribution of ZmCNGCs and the sequences including DNA sequences, CDS, cDNA, up-stream 1,500 bps of ZmCNGC genes were obtained from BLASTN (the Ensembl Plant database, http://plants.ensembl.org/index.html): accession numbers GRMZM2G148118, GRMZM2G129375, GRMZM2G066269, GRMZM2G023037, GRMZM2G077828, GRMZM2G005791, GRMZM2G068904, GRMZM2G135651, GRMZM2G141642, GRMZM5G858887, GRMZM2G074317, and GRMZM2G090528.
13 maize AKT/KAT genes were downloaded from Ensembl Plant database with gene ID: C234152.1_FGT002,GRMZM2G020859,GRMZM2G081666,GRMZM2G178356, GRMZM2G310569,GRMZM2G156255,GRMZM2G093313, GRMZM2G022915, GRMZM2G171279,GRMZM2G028258,GRMZM2G342375,GRMZM2G428859, GRMZM5G838773,GRMZM2G095861.
The 20 Arabidopsis and 16 rice CNGC protein sequences were obtained from TAIR10 (http://www.arabidopsis.org) and RGAP (http://rice.plantbiology.msu.edu/) respectively. Arabidopsis asscession numbers: AT5G53130, AT3G48010,AT2G23980, AT2G24610, AT5G54250, AT2G28260, AT5G15410, AT1G15990, AT4G30560, AT5G57940, AT3G17700, AT1G01340, AT1G19780, AT2G46450, AT4G30360, AT5G14870, AT4G01010, AT3G17690, AT2G46430, AT2G46440, LOC_Os06g33570, LOC_Os06g33610, LOC_Os03g44440, LOC_Os12g28260, LOC_Os04g55080, LOC_Os02g41710, LOC_Os12g06570, LOC_Os09g38580, LOC_Os02g54760, LOC_Os06g08850, LOC_Os02g53340, LOC_Os06g10580, LOC_Os03g55100, LOC_Os01g57370, LOC_Os05g42250.