Abstract
STUDY QUESTION
Does loss of DMRT1 in human fetal testis alter testicular development and result in testicular dysgenesis?
SUMMARY ANSWER
DMRT1 repression in human fetal testis alters the expression of key testicular and ovarian determining genes, and leads to focal testicular dysgenesis.
WHAT IS KNOWN ALREADY
Testicular dysgenesis syndrome (TDS) is associated with common testicular disorders in young men, but its etiology is unknown. DMRT1 has been shown to play a role in the regulation of sex differentiation in the vertebrate gonad. Downregulation of DMRT1 in male mice results in trans-differentiation of Sertoli cells into granulosa (FOXL2+) cells resulting in an ovarian gonadal phenotype.
STUDY DESIGN, SIZE, DURATION
To determine the effect of DMRT1 repression on human fetal testes, we developed a novel system for genetic manipulation, which utilizes a Lentivral delivered miRNA during short-term in vitro culture (2 weeks). A long-term (4–6 weeks) ex vivo xenograft model was used to determine the subsequent effects of DMRT1 repression on testicular development and maintenance. We included first and second-trimester testis tissue (8–20 weeks gestation; n = 12) in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human fetal testes were cultured in vitro and exposed to either of two DMRT1 miRNAs (miR536, miR641), or to scrambled control miRNA, for 24 h. This was followed by a further 14 days of culture (n = 3–4), or xenografting (n = 5) into immunocompromised mice for 4–6 weeks. Tissues were analyzed by histology, immunohistochemistry, immunofluorescence and quantitative RT-PCR. Endpoints included histological evaluation of seminiferous cord integrity, mRNA expression of testicular, ovarian and germ cell genes, and assessment of cell number and protein expression for proliferation, apoptosis and pluripotency factors. Statistical analysis was performed using a linear mixed effect model.
MAIN RESULTS AND THE ROLE OF CHANCE
DMRT1 repression (miR536/miR641) resulted in a loss of DMRT1 protein expression in a sub-population of Sertoli cells of first trimester (8–11 weeks gestation) human fetal testis; however, this did not affect the completion of seminiferous cord formation or morphological appearance. In second-trimester testis (12–20 weeks gestation), DMRT1 repression (miR536/miR641) resulted in disruption of seminiferous cords with absence of DMRT1 protein expression in Sertoli (SOX9+) cells. No differences in proliferation (Ki67+) were observed and apoptotic cells (CC3+) were rare. Expression of the Sertoli cell associated gene, SOX8, was significantly reduced (miR536, 34% reduction, P = 0.031; miR641 36% reduction, P = 0.026), whilst SOX9 expression was unaffected. Changes in expression of AMH (miR536, 100% increase, P = 0.033), CYP26B1 (miR641, 38% reduction, P = 0.05) and PTGDS (miR642, 30% reduction, P = 0.0076) were also observed. Amongst granulosa cell associated genes, there was a significant downregulation in R-spondin 1 expression (miR536, 76% reduction, P < 0.0001; miR641, 49% reduction, P = 0.046); however, there were no changes in expression of the granulosa cell marker, FOXL2. Analysis of germ cell associated genes demonstrated a significant increase in the expression of the pluripotency gene OCT4 (miR536, 233%, P < 0.001). We used the xenograft system to investigate the longer-term effects of seminiferous cord disruption via DMRT1 repression. As was evident in vitro for second-trimester samples, DMRT1 repression resulted in focal testicular dysgenesis similar to that described in adults with TDS. These dysgenetic areas were devoid of germ cells, whilst expression of FOXL2 within the dysgenetic areas, indicated trans-differentiation from a male (Sertoli cell) to female (granulosa cell) phenotype.
LIMITATIONS, REASONS FOR CAUTION
Human fetal testis tissue is a limited resource; however, we were able to demonstrate significant effects of DMRT1 repression on the expression of germ and somatic cell genes, in addition to the induction of focal testicular dysgenesis, using these limited samples. In vitro culture may not reflect all aspects of human fetal testis development and function; however, the concurrent use of the xenograft model which represents a more physiological system supports the validity of the in vitro findings.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings have important implications for understanding the role of DMRT1 in human testis development and in the origin of testicular dysgenesis. In addition, we provide validation of a novel system that can be used to determine the effects of repression of genes that have been implicated in gonadal development and associated human reproductive disorders.
STUDY FUNDING/COMPETING INTEREST(S)
This project was funded by a Wellcome Trust Intermediate Clinical Fellowship (Grant No. 098522) awarded to RTM. LBS was supported by MRC Programme Grant MR/N002970/1. RAA was supported by MRC Programme Grant G1100357/1. RMS was supported by MRC Programme Grant G33253. This work was undertaken in the MRC Centre for Reproductive Health which is funded by the MRC Centre grant MR/N022556/1. The funding bodies had no input into the conduct of the research or the production of this manuscript. The authors have declared no conflicts of interest.
Keywords: human fetal testis, gonadal development, DMRT1, testicular dysgenesis, sex differentiation, miRNA
Introduction
Development of the mammalian testis and ovary from the bi-potential gonad occurs through sex-specific differentiation of somatic cell populations which support the development of the gonad into a testis or an ovary. Key to this process is the differentiation of a population of somatic precursor cells into either Sertoli cells (testis) or pre-granulosa cells (ovary). In males, this process is primarily directed by the presence of a Y-chromosome, and subsequent gonadal development is under the control of several signaling cascades working in concert to drive cell lineage specification in the testis (Stevant et al., 2018): In females, specific cascades are also involved in determining ovarian development (Pannetier et al., 2016). Once testicular somatic cell populations are established, testosterone production from fetal Leydig cells is initiated to induce masculinization of the fetus. Failure of normal sex-determination or perturbed hormone production during fetal gonadal development in humans can result in disorders of sex development (DSD) (Jørgensen et al., 2015a; Hersmus et al., 2017) and is also implicated in the development of testicular dysgenesis syndrome (TDS; cryptorchidism, hypospadias, testicular cancer and low sperm counts; Skakkebaek et al., 2016; van den Driesche et al., 2017).
Our earlier understanding of gonadal somatic cell development was that, once specified, Sertoli and granulosa cell populations were fixed. However, it has recently been demonstrated in mice that loss of expression of key genes in postnatal life can re-programme Sertoli cells into granulosa cells and vice-versa, demonstrating a plasticity of gonadal fate long after normal formation of a testis or ovary (Uhlenhaut et al., 2009; Matson et al., 2011). DMRT1 (Doublesex and mab-3 related transcription factor 1) is a conserved autosomal gene expressed by both the Sertoli and germ cells in the developing mammalian testis (Raymond et al., 1998, 1999a). DMRT1 has been shown to play a role in the regulation of sex determination in the vertebrate gonad (Matson and Zarkower. 2012) and also during the mitosis to meiosis switch in germ cells (Matson et al., 2010, Jorgensen et al., 2012). In mice, DMRT1 expression in Sertoli cells represses ovarian determining genes such as FOXL2 via the retinoic acid pathway (Minkina et al., 2014). Conversely, downregulation of DMRT1 in males during prepuberty or adulthood results in upregulation of FOXL2 and trans-differentiation of Sertoli cells into granulosa-like cells, resulting in an ovarian gonadal phenotype, including estrogen production (Matson et al., 2010). Studies using DMRT1 null mice showed that despite the lack of DMRT1 expression, mice are born phenotypically male and it is not until after birth that they undergo sex reversal (Raymond et al., 2000; Matson et al., 2011). These studies indicate that, in contrast to DMRT1 homologs in other vertebrates, mammalian DMRT1 is not involved in the initial sex determination, but is instead required for maintaining male gonadal fate (Matson and Zarkower. 2012). In humans, mutations and/or deletions in the short arm of Chromosome 9, where DMRT1 is located, can result in varying degrees of sex reversal (Hoo et al., 1989; Bennett et al., 1993; Vinci et al., 2007) and 46,XY individuals with DMRT1 mutations may present with DSD or features of TDS (Murphy et al., 2015).
Much of our understanding of mammalian gonadal development is derived from studies in mice. However, in humans, the genes and signaling networks that control specification of the fetal testis and whether there is plasticity in somatic cell differentiation are not well established. Research into the human relevance of findings from rodent studies has been hampered by the lack of dynamic experimental model systems that can determine the effect of genetic manipulation on subsequent development in the human fetal gonad.
In the present study, we describe a novel system for genetic manipulation in the human fetal testis, which utilizes short-term in vitro culture followed by long-term xenografting to determine the effects of gene repression on subsequent testicular development and maintenance. Using this system, we show that DMRT1 can be successfully repressed in Sertoli cells of first and second-trimester fetal testes with resulting effects on the expression of key testicular and ovarian determining genes, and leading to focal testicular dysgenesis in the human fetal testis.
Materials and Methods
Experimental design
Given the evidence from rodent studies for effects of DMRT1 repression on testicular development, we aimed to determine whether experimentally-induced repression of DMRT1 results in aberrant development of the human fetal testis. Effects of DMRT1 repression was studied in human fetal testes taken from different stages of pregnancy using hanging drop culture (first and second-trimester testes) and a xenograft system (second-trimester testes). Samples were exposed to scrambled miRNA (control) or either of two miRNAs that targeted different regions of the human DMRT1 gene. Endpoints evaluated included histological evaluation of seminiferous cord formation and maintenance, mRNA expression levels of testicular, ovarian and germ cell genes known to be important in gonad development and assessment of cell number, proliferation status and of pluripotency marker expression. Inclusion criteria and measured endpoints were defined before the start of the study. For human fetal gonad experiments, the sample sizes (n = 4–8) were based on those required to achieve statistical significance in previous studies using the same methodology (van den Driesche et al., 2015). The study ended once the required number of experiments had been conducted, and data were analyzed after the cessation of the study. For fluorescence immunohistochemistry, all samples from the same experiment were stained in the same run. Cell counts were determined with the investigator blind to the treatment group. To compare the effects of treatment versus vehicle in xenografts for each individual human fetal testis, we grafted tissue from each fetus into replicate host mice (n = 3–6) and randomly allocated these mice to receive tissue that had been exposed to either scrambled miRNA, miR536 or miR641.
Ethics statement and sample collection
Ethical approval for receipt and use of human fetal tissues was obtained from the South East Scotland Research Ethics Committee (LREC08/S1101/1) and NRES committee North East – Newcastle and North Tyneside 1 (08/H0906/21 + 5) and written and informed consent was provided by the pregnant women. First- and second-trimester human fetal testes (8.5–20 weeks gestation; n = 12) were obtained after medical termination of pregnancy as described previously (Mitchell et al., 2008). None of the terminations were due to fetal abnormalities. Gestation was determined by ultrasound scan and subsequent direct measurement of foot length. The sex of first trimester testes was confirmed by PCR for the male-specific gene SRY. To permit subsequent gene expression analysis, where possible a small sample of gonad tissue was removed, snap frozen and stored at −70°C until RNA extraction. The remainder was placed immediately into medium containing Leibovitz L-15 with added glutamine, 10% fetal bovine serum, 1% penicillin/streptomycin and 1% non-essential amino acids (all Sigma-Aldrich).
For studies involving animals, specific approval, including ethical approval, was given by the UK Home Office and all procedures were carried out in accordance with the Animal (Scientific procedures) Act 1986.
Viral transduction of HEK293T cells
Recombined vectors pLent6.2_cppt_ CMV_mCherry_miR-NEG_control containing sequences encoding scrambled miRNA (negative control), and pLent6.2_cppt_CMV_mCherry_rDMRT1_miR536 and miR641, two miRNA targeted against human DMRT1, were created and transfected into HEK293T cells to produce lentiviral particles Lv-cppt-CMV-mCherry-miR-NEG control (2.7 × 106 TU/ml), Lv-cppt-CMV-mCherry-rDMRT1-miR536 (7 × 106 TU/ml) and miR641 (5.1 × 106 TU/ml). Briefly, DMRT1-miRNA oligonucleotides were designed using the Invitrogen BLOCK-iT™ RNAi designer web programme to identify DNA sequences within rat DMRT1 (transcript variant 1 accession no. NM_000044), labeled according to their position from the translational start site. These and the non-targeting sequence for the Scrambled miRNA are shown in Supplementary Table S1. Targeting sequences were initially designed based on rat DMRT1 with subsequent confirmation of similarity for the human DMRT1 sequence. Rat DMRT1 (rDMRT1) miRNA and Scrambled miRNA oligo constructs were each cloned into a pcDNA6.2_GW_EmGFP_miR Gateway plasmid (Invitrogen) using the BLOCK-iT™ Pol II miR RNAi Expression Vector Kit (Invitrogen, K4935-00) according to the manufacturer’s protocols, and the inserts were verified by DNA sequencing before recombining into pLent6.2 vectors. HEK293T cells were transfected with the pLent6.2 vectors to produce the Lentivirus particles using the BLOCK-iT™ Lentiviral Pol II miR RNAi Expression System (Invitrogen, K4937-00).
Viral transduction of tissue in a ‘hanging drop’ culture system
Following fetal gonadal dissection, the testis was cut into small pieces (~1 mm3). Tissue pieces were placed in ‘hanging drop’ culture in medium spiked with the appropriate virus preparation in a total volume of 30μl/tissue piece, for 24 h at 37°C under 5% CO2 (Jørgensen et al., 2015b). Tissue was then either used for xenografting or transferred to fresh culture medium containing alpha MEM (Lonza), 10% fetal bovine serum, 1% penicillin/streptomycin, 1% non-essential amino acids, 2 mM l-glutamine, 2 mM sodium pyruvate and 1% insulin transferrin selenium (ITS) (all Sigma-Aldrich). The latter tissue was then maintained in culture for 13 days, with medium changed every 2 days.
Human tissue xenografting
Animals
Castrate CD1-nude (host) mice were used for xenografting studies. Castration was performed by scrotal incision under anesthesia (inhalation of isoflurane) at least two weeks prior to xenografting. For 3 days post-castration, the mice were administered an analgesic (Carprofen, Pfizer) in their drinking water.
Human tissue and xenografting procedure
As previously described (Mitchell et al., 2010), second-trimester human fetal testes (n = 5) were grafted subcutaneously into host CD1-nude mice using a 13-gauge cancer implant needle (Popper and sons). For each fetus, 4–6 pieces (~1 mm3) of testis tissue was inserted under the dorsal skin of the host mouse, and grafts were distributed evenly along either side of the midline. A total of 3–6 mice were grafted per fetus, and the mice were maintained for 4–6 weeks post grafting before retrieval of the tissue.
Xenograft retrieval
Host mice were killed by cervical dislocation and the retrieved xenografts were fixed in Bouin’s fluid (Clin-Tech). Post fixation, samples were paraffin embedded, sectioned and assessed by H&E or used for immunohistochemistry as below.
Immunohistochemistry
Immunohistochemistry was carried out using standard immunohistochemical and immunofluorescent techniques as previously described (Fisher et al., 2003; Mitchell et al., 2008). Primary antibodies are described in Supplementary Table SII. Images were captured either using an Olympus Provis AX70 light microscope or in the case of fluorescent images a Zeiss LSM 510 Axio Observer Z1 confocal laser microscope (Carl Zeiss Ltd.). All images were compiled using Zen (Blue edition, Carl Zeiss Ltd.), Photoshop 9.0 (Adobe Systems Inc.). The total number of proliferative (Ki67) or apoptotic (Cleaved Caspase 3) positive stained cells was quantified and expressed relative to the total tissue section area for all treatment groups (n = 4–6) in first trimester hanging drop cultures.
Gene expression analysis
Total RNA was extracted from human fetal gonads using the RNeasy Micro Kit with on-column DNase digestion (Qiagen, UK), as per the manufacturer’s instructions. Concentration and purity of the extracted RNA was assessed using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific inc). cDNA was prepared using the maxima first strand cDNA synthesis kit (Thermo Fisher Scientific inc). Gene expression using specific human primers (Supplementary Table SIII) was determined using SYBR Green PCR Master Mix (Qiagen) to carry out Quantitative real time PCR (qRT-PCR) on the ABI 7900HT (Applied Biosystems). ABI7900HT runs consisted of a hot start at 95°C (3 min) followed by 40 cycles of 95°C (5 sec) and 65°C (15 sec). Melt curve analysis was performed as described previously (Bayne et al., 2004) to confirm specific PCR products. Standard curve analysis, with increasing dilutions (from 1:10 to 1:640) of control cDNA were used to confirm the efficiency of the PCR amplification. All slopes of the standard curves were close to −3.3 (equivalent to 100% PCR efficiency or two-fold amplification per cycle), allowing quantification using the 2−ΔΔCt method (Bayne et al., 2004). Expression levels were normalized to RPS20 and RPS29 (both validated for use with human testis tissues) and also to SOX9 (Sertoli cells) expressed as a ratio with control tissue set to 1 (Svingen et al., 2014).
Statistics
To take into account the inter-individual variation between fetuses, the results were analyzed using a linear mixed effects model in R (www.r-project.org). Testicular pieces were randomly allocated to receive either the scrambled miRNA virus or one of the DMRT1-miRNA viral constructs. Significance was set at P < 0.05 and no outliers were excluded.
Results
DMRT1 repression does not affect seminiferous cord formation in the first trimester human fetal testis
First trimester human fetal testis tissue was exposed to lentiviral particles containing sequences that encoded either a scrambled miRNA (control) or one of two miRNAs targeted against DMRT1 (miR536 and miR641). Using the hanging drop culture system, testis pieces were exposed to their respective miRNA for 24 h, followed by maintenance in standard culture medium for a further 13 days. Initial histological assessment of the tissue was carried out to assess survival and cellular development, which demonstrated normal gross morphology after culture (Fig. 1A). Cell counts for proliferating (Ki67+) and/or apoptotic (Cleaved Caspase 3+) cells showed no significant difference between the control and DMRT1-targeted miRNA samples (Supplementary Fig. S1A).
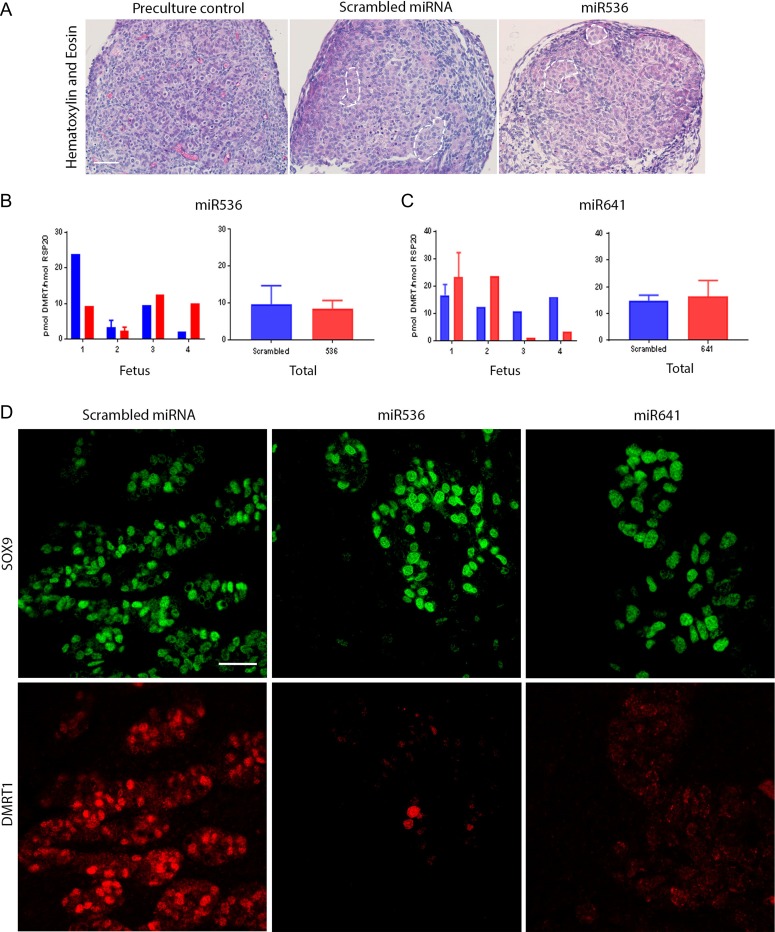
Figure 1.
DMRT1 knockdown does not affect seminiferous cord formation in the first trimester human fetal testis.(A) H + E staining of pre-culture control (left panel) and after culture for 13 days following an initial 24 h lentiviral exposure to scrambled miRNA (middle panel) or miR536 (right panel). Seminiferous cords are delineated by broken white lines. DMRT1 mRNA expression in (B) miR536- and (C) miR641-exposed tissues compared to scrambled miRNA controls (n = 4 for each DMRT1-miRNA exposure group). (D) Immunofluorescence analysis of SOX9 (green) and DMRT1 (red) expression in Sertoli cells in fetal testis tissue following transduction with either scrambled miRNA (left panel), miR536 (middle panel) or miR641 (right panel) lentiviral constructs. Scale bars: (A) 20 μM and (D) 50 μM. Graphs show mean ± SEM. Data analyzed using a linear mixed effects model in R.
DMRT1 gene expression in the first trimester fetal testis tissue, after culture, was quantified using RT-qPCR (Fig. 1B,C). Due to considerable inter-individual variation between fetuses, no significant difference in DMRT1 gene expression was observed in tissues exposed to either miR536 or miR641 when compared to scrambled miRNA-exposed control tissue. Given that miRNA-induced changes in gene expression are not always detectable at the transcript level, we used double immunofluorescence to determine DMRT1 protein expression. Sertoli cells (SOX9+) devoid of DMRT1 protein expression were frequently observed in both the miR536 and miR641-exposed tissue, indicating effective repression, whilst in comparison DMRT1 expression was present in all of the Sertoli cells of scrambled miRNA controls (Fig. 1D).
Seminiferous cords had not yet formed in the first trimester controls; however, cord formation could be clearly identified in all of the tissues exposed to the scrambled control and also in those in which partial repression of DMRT1 expression had been achieved, indicating that DMRT1 is not required for seminiferous cord formation (Fig. 1A).
Repression of DMRT1 in second-trimester human fetal testis alters expression of genes involved in testicular and ovarian development
To investigate the effects of DMRT1 repression in second-trimester fetal testis cultures, we used the same approach as described above for first trimester tissues. In control tissues, seminiferous cords had already formed and cord structure was largely maintained in the tissue over the culture period for both scrambled and DMRT1-miRNA (Fig. 2A). Similar to first trimester tissue, proliferation (Ki67+ cells) was observed in a large proportion of cells within the testis, with no discernible difference between DMRT1 repression or control tissue. Apoptotic (Cleaved Caspase 3+) cells were rarely identified (Supplementary Fig. S1B). These results are consistent with previously published work using non-exposed human fetal gonadal tissue under similar culture conditions (Jørgensen et al., 2015b).
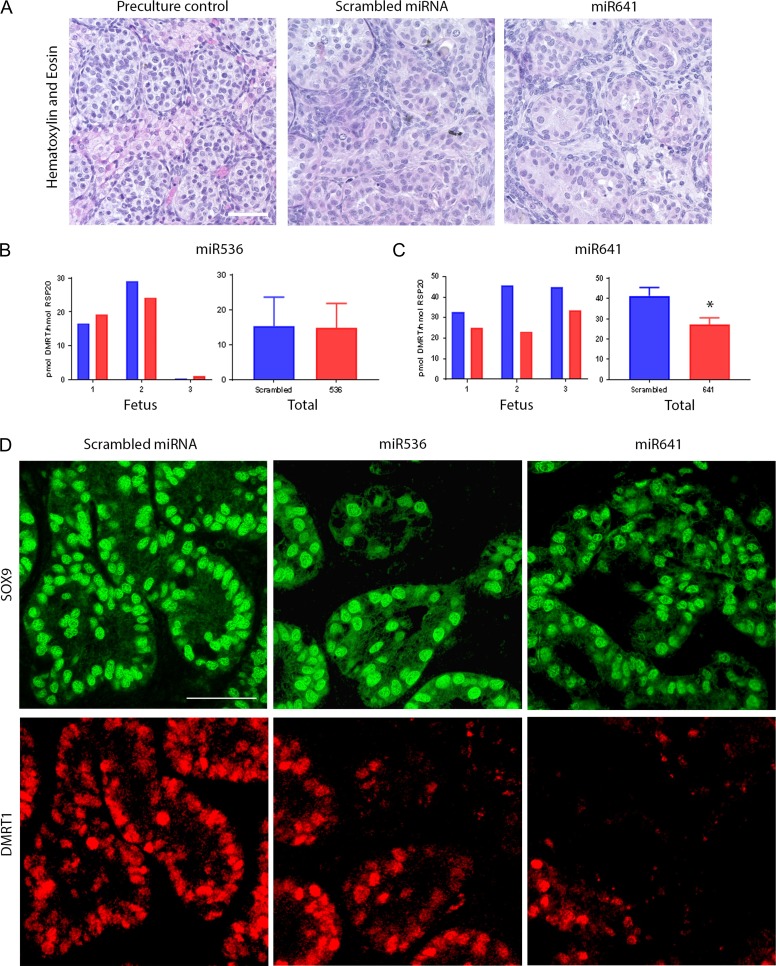
Figure 2.
DMRT1 expression is lost in Sertoli cells of the second-trimester human fetal testis after in vitro exposure to miR536 and miR641. H + E staining of pre-culture control (left panel) and after culture for 13 days following an initial 24 h lentiviral exposure to scrambled miRNA (middle panel) or miR641 (right panel). Normal histological appearance of the testicular tissue is demonstrated. DMRT1 mRNA expression in (B) miRNA536- and (C) miRNA641-exposed tissues compared to scrambled miRNA controls (n = 3 for each DMRT1-miRNA treatment group, *P < 0.05). (D) Immunofluorescence analysis of SOX9 (green) and DMRT1 (red) expression in Sertoli cells in fetal testis tissue following transduction with either scrambled miRNA (left panels), miR536 (middle panels) or miR641 (right panels) lentiviral constructs. Scale bar (A) 20 μM and (D) 50 μM. Graphs show mean ± SEM. Data analyzed using a linear mixed effects model in R.
Analysis of the mRNA expression levels of DMRT1 in the tissue at the end of the culture period was quantified using RT-qPCR (Fig. 2B,C). Again, no significant difference in DMRT1 mRNA was observed for miR536-exposed tissue compared with scrambled miRNA-exposed controls (Fig. 2B); however, there was a significant reduction in DMRT1 gene expression (34%, P = 0.018) in tissue exposed to miR641 virus compared to scrambled controls (Fig. 2C). Similar to first trimester tissue, there was clear inter-individual variation between fetuses. Using fluorescence immunohistochemistry, we observed a loss of DMRT1 protein expression in Sertoli (SOX9+) cells in testes exposed to either miR536 or miR641 (Fig. 2D).
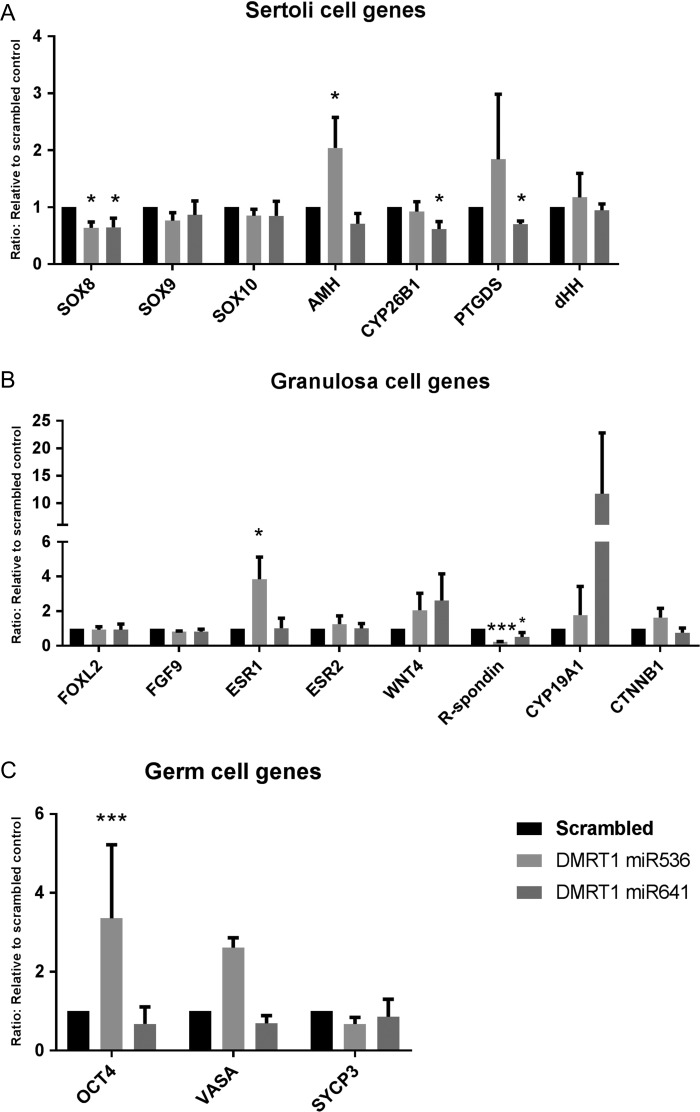
We investigated the expression of a panel of Sertoli, granulosa and germ cell genes shown previously to be affected by changes in DMRT1 expression in rodent studies. Second-trimester testis tissue exposed to miR536, miR641 or scrambled control virus and cultured as above was assessed. For Sertoli cell associated genes, a significant reduction in SOX8 expression was demonstrated following exposure to either miR536 (34%, P = 0.031) or miR641 (36%, P = 0.026), whilst expression of SOX9 was unaffected (Fig. 3A). A significant increase in AMH expression (100%, P = 0.033) in the miR536-exposed tissue and downregulation of CYP26B1 (38%, P = 0.05) and PTGDS (30%, P = 0.0076) in the miR641-exposed samples was also observed. Investigation of granulosa cell associated genes revealed a significant downregulation in R-spondin 1 expression in tissue transduced with either miR536 (76% reduction, P < 0.0001) or miR641 (49% reduction, P = 0.046) DMRT1-targeted viruses. In contrast, upregulation of ESR1 (283%, P = 0.019) was seen in response to miR536 transduction (Fig. 3B). There were no changes in expression of the granulosa cell marker, FOXL2. Analysis of germ cell associated genes demonstrated a significant increase in the expression of the pluripotency gene OCT4 (233%, P < 0.001) in the miR536-exposed tissue compared to the scrambled control (Fig. 3C).
Figure 3.
DMRT1 knockdown in second-trimester human fetal testis alters expression of genes involved in testicular and ovarian development. RT-qPCR analysis of genes associated with development of (A) Sertoli (B) granulosa and (C) germ cells in second-trimester human fetal testes tissue culture for 13 days after an initial 24 h exposure to scrambled miRNA, miR536 or miR641. Expression was normalized to RPS29 (n = 2–3 per treatment group, *P < 0.05, **P < 0.01, ***P < 0.001). Graphs show mean ± SEM. Data analyzed using a linear mixed effects model in R.
DMRT1 repression results in ‘focal dysgenesis’ in second-trimester human fetal testes
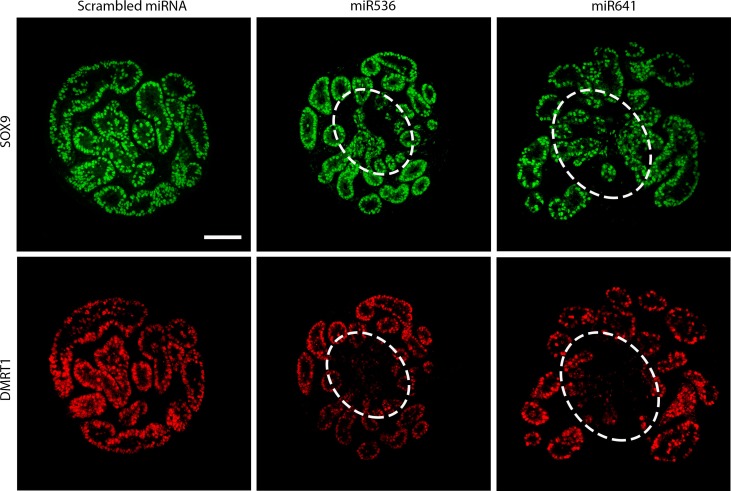
We further investigated the morphology of first and second-trimester human fetal testis tissues in which we had identified loss of DMRT1 in Sertoli cells (DMRT1−/SOX9+) after miRNA repression. We used SOX9 to identify Sertoli cells, based on our earlier finding of unchanged mRNA expression (Fig. 3). Morphology of first trimester tissues was similar to the corresponding (scrambled) controls (Fig. 1A). However, in second-trimester human fetal testes, we observed areas of disrupted seminiferous cord structure in regions where DMRT1 expression had been lost in Sertoli cells (Fig. 4).
Figure 4.
DMRT1 knockdown in vitro results in disruption of seminiferous cords in second-trimester human fetal testis tisse. Immunofluorescence analysis of the expression of SOX9 (Sertoli cells; green) and DMRT1 (Sertoli and germ cells; red) in fetal second-trimester testis tissue cultured for 13 days following an initial 24 h transduction with either scrambled miRNA, miR536 or miR641 lentiviral constructs. Regions of disruption of seminiferous cords can be identified (broken white line) where DMRT1 expression has been reduced but SOX9 expression has been retained. Scale bar: 100 μM.
To determine the longer-term effects of DMRT1 repression on these areas containing disrupted seminiferous cords, a xenograft approach was used. Second-trimester human fetal testis tissue was transduced as described for the in vitro experiments using miR536, miR641 or scrambled control. After 24 h of viral exposure in culture, the tissue pieces were transplanted into castrate CD1-nude mice hosts and retrieved 4–6 weeks later. Viral uptake and stable transduction was confirmed by immunofluorescent analysis of mCherry expression in the retrieved grafts (Supplementary Fig. S2). The lack of mCherry staining in the peripheral mouse tissue confirmed viral transduction was restricted to the grafted human fetal tissue and provided an internal control for the experimental analysis.
The overall morphological appearance of the majority of the xenografted tissues was similar to that of the pre-graft control from the same fetus, and seminiferous cord structure was largely maintained in tissues exposed to the scrambled miRNA control, or to miR536 or miR641 (Supplementary Fig. S3A). Cell proliferation (Ki67+) was similar between treatment groups, and apoptotic cells (Cleaved Caspase 3+; CC3) were rarely identified, indicating that all of the xenografted tissues remained healthy (Supplementary Fig. S3B).
We performed immunohistochemical co-localization to identify DMRT1 protein expression in Sertoli (SOX9+) cells of xenografted human fetal testis tissues. Co-localization of DMRT1 and SOX9 was observed in all Sertoli cells in all of the control tissues, including pre-graft and scrambled control tissues (Fig. 5A,B), whilst DMRT1+/SOX9− cells (presumed to be germ cells) were also evident. Exposure to either miR536 or miR641 resulted in areas containing SOX9+/DMRT1− cells in which there was a loss of normal seminiferous cord structure, i.e. focal dysgenesis (Fig. 5C,D); such areas were not observed in either untreated or scrambled control grafts from the same fetuses. In total, sections from 4/6 of the miR536 or miR641-exposed second-trimester fetuses exhibited areas of focal dysgenesis, which was apparent in multiple grafts from individual fetuses (Supplementary Fig. S4).
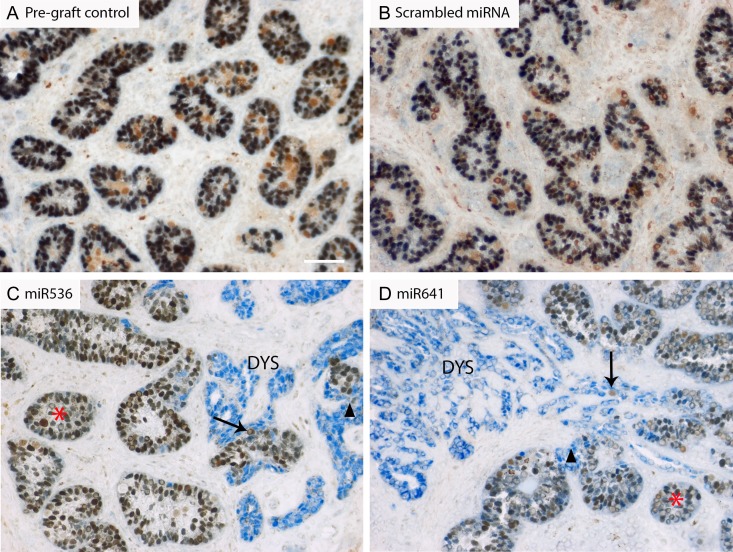
Figure 5.
DMRT1 knockdown results in ‘focal testicular dysgenesis’ in second-trimester human fetal testis xenografts. Double immunohistochemistry for DMRT1 (brown; Sertoli and germ cells) and SOX9 (blue; Sertoli cells) in (A) pre-graft control, or after xenografting for 6 weeks following an initial 24 h transduction period in culture with (B) scrambled miRNA, (C) miR536 or (D) miR641. Red asterisk = normal seminiferous cord structure, black arrowheads show areas of cord breakdown and the absence of DMRT1 protein; black arrows indicate germ cells and DYS identifies focal dysgenetic areas in which there has been a complete breakdown of seminiferous cords. Scale bar: 50 μM.
To analyze the focal dysgenetic areas further, we investigated cellular proliferation, apoptosis, and germ and somatic cell marker expression and compared them to control tissues (Fig. 6). Cellular proliferation (Ki67+) was similar in dysgenetic areas of miR536/miR641-exposed testes compared either to non-dysgenetic areas or to scrambled control tissues, whilst apoptotic (CC3+) cells were infrequently identified across all exposure groups (Fig. 6A and Supplementary Fig. S3B). Leydig cell expression of the steroidogenic enzyme CYP11A1 was seen throughout the interstitial space in scrambled control tissue, consistent with non-exposed controls. However, in the sections of miR536/miR641-exposed tissues containing focal dysgenetic areas, CYP11A1 expression appeared to be reduced in regions surrounding the seminiferous cords with a ‘normal’ appearance and was minimal or absent in the regions surrounding focal dysgenetic areas. Furthermore, within the dysgenetic regions (devoid of DMRT1+ Sertoli cells), CYP11A1 expression was completely absent (Fig. 6B).
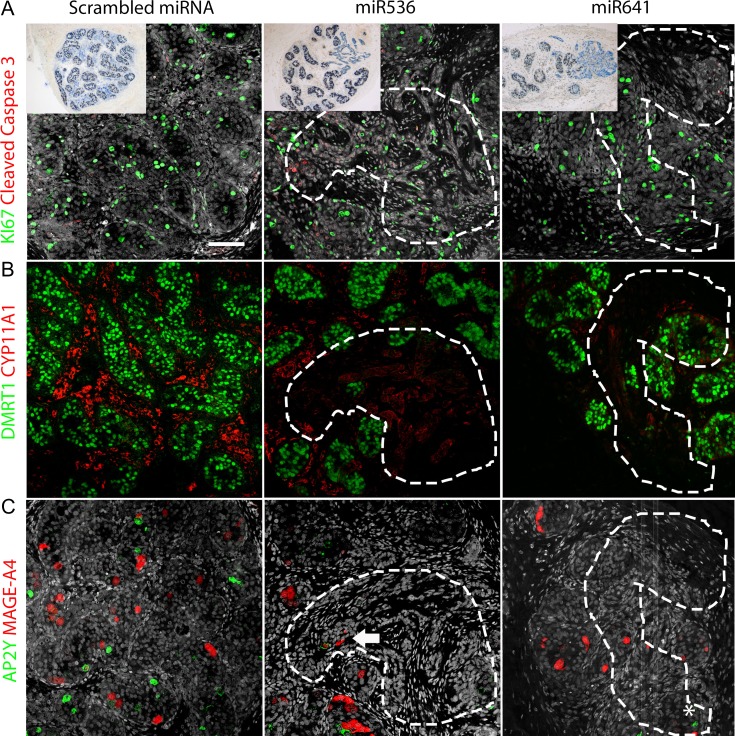
Figure 6.
Germ and somatic cell composition and function is altered in ‘focal dysgenetic’ regions of second-trimester human fetal testis resulting from DMRT1 knockdown. Double immunofluorescence for (A) Ki67 (green; proliferation marker) and Cleaved Caspase 3 (red; apoptotic marker), (B) DMRT1 (green; Sertoli and germ cells) and CYP11A1 (red; Leydig cells), and (C) AP2γ (green; gonocytes) and MAGE-A4 (red; pre-spermatogonia). Nuclear counterstain (gray; DAPI/Hoescht). Focal dysgenetic areas are indicated by dashed white lines. White asterisk highlights a single AP2γ+ germ cell within a dysgenetic area (bottom right panel), White arrow indicates a surviving MAGE-A4+ germ cell within a dysgenetic area. Scale bar: 50 μM.
We investigated the expression of germ cell markers, AP2γ (gonocyte) and MAGE-A4 (pre-spermatogonia) in both scrambled controls and testis tissue with focal DMRT1 repression. In scrambled control tissues, we identified frequent AP2γ+ and MAGE-A4+ germ cells within the seminiferous cords, similar to previous studies in second-trimester human fetal testis tissues (Mitchell et al., 2008). However, in the dysgenetic areas of the miR536/miR641-exposed xenografts, AP2γ+ cells were rarely observed (Fig. 6C). MAGE-A4+ cells were identified in seminiferous tubules on the periphery of the dysgenetic regions, but were rarely identified within the focal dysgenetic areas (Fig. 6C).
Previous studies in rodents have demonstrated that downregulation of DMRT1 expression in the male gonad can result in male to female sex reversal with trans-differentiation of the Sertoli cells (SOX9+) to granulosa-like cells (FOXL2+) cells (Matson et al., 2011). To investigate the possibility of somatic cell trans-differentiation in the long-term xenografted tissue, we performed immunofluorescent analysis for FOXL2 expression in control and dysgenetic tissue samples. FOXL2 expression was not observed in any of the scrambled control tissues; however, we identified FOXL2 expression in tissue from one of five fetuses from miR536 transduced testes, and this was within a dysgenetic region devoid of DMRT1 expressing cells (Fig. 7).
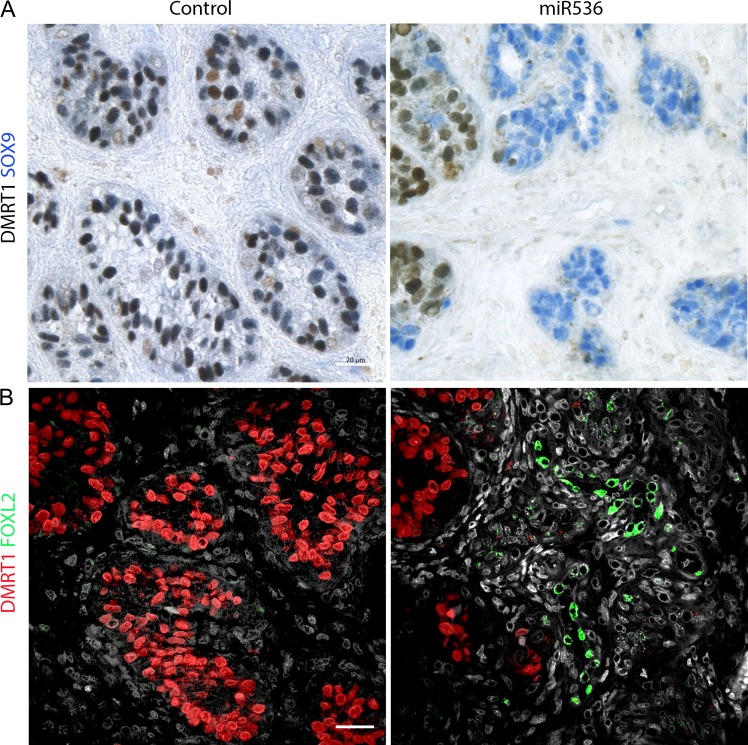
Figure 7.
DMRT1 knockdown results in FOXL2 expression in a region of ‘focal dysgenesis’ in a second-trimester human fetal testis xenograft. FOXL2 protein expression in xenografts of control and DMRT1-miRNA transduced second-trimester human fetal testis tissue. (A) Double immunohistochemistry for SOX9 (Blue) and DMRT1 (brown) identifies regions of loss of DMRT1 with ‘Focal Dysgenesis’. (B) FOXL2 immunoexpression (green) in scattered cells in one fetus in an area devoid of DMRT1 (red) expression following initial transduction with miR536. Scale bar: 20 μM.
Discussion
Impairment of normal development of the human gonad during fetal life may have implications for future reproductive potential and the development of gonadal tumors (Looijenga et al., 2010; Jørgensen et al., 2015a; Hersmus et al., 2017); this has been the foundation for the TDS hypothesis (Skakkebaek et al., 2016). An increasing number of genetic mutations associated with DSD are being described in humans; however, much of our understanding of these mutations and the mechanism(s) by which they result in testicular dysfunction is based on rodent studies. Mutations/deletions in the short arm of Chromosome 9, where DMRT1 is located is strongly associated with DSD and sex-reversal in humans (Raymond et al., 1999b; Ottolenghi et al., 2000; Ounap et al., 2004; Vinci et al., 2007) and a recent study has reported a patient with XY sex-reversal associated with a point mutation in DMRT1 (Murphy et al., 2015). Recent evidence from rodent studies show that inducing DMRT1 mutations, even in postnatal life, can lead to trans-differentiation of the gonad from a testicular to an ovarian phenotype, demonstrating plasticity in gonadal fate throughout life (Matson et al., 2011). In this study, we developed a novel system of lentiviral mediated miRNA manipulation to target a gene of interest in the human fetal testis, which combined in vitro culture with a xenografting approach to investigate the short- and long-term consequences of manipulation. This approach was used to determine the effects of reduced DMRT1 expression on testicular development and assess whether plasticity of gonadal fate exists in humans as in rodents. We show that DMRT1 expression can be repressed in Sertoli cells of the human fetal testis resulting in altered expression of gonad-specific genes and proteins, indicating a move away from a fully differentiated male phenotype, and potentially towards a more ovarian-like phenotype. Moreover, we demonstrate that DMRT1 repression in the human fetal testis results in disruption of prior-formed seminiferous cords with development of focal testicular dysgenesis. A similar disruption of prior-formed seminiferous cords has been reported also in rats after exposure to an environmental chemical (dibutyl phthalate), and shown to result in focal dysgenesis (Lara et al., 2017).
We have validated a dynamic model system for genetic manipulation of the human fetal testis by transducing first and second-trimester testis tissue with miRNAs targeting the DMRT1 gene at two separate regions (miR536 and miR641). This resulted in repression of DMRT1 expression in focal regions of exposed human fetal testis tissue pieces after 14 days of culture. DMRT1 repression was observed at the protein level in both first and second-trimester human fetal testes with either DMRT1-targeted miRNA, although the DMRT1 mRNA transcript level was only reduced in second-trimester tissue transduced with the miR641 transcript. The lack of identifiable transcript alteration in both first and second-trimester tissues with each of the miRNAs is not unusual given that miRNA can result in either translational repression or endonucleolytic activity resulting in mRNA degradation and is dependent on the level of complementarity with the target mRNA (Schmitter et al., 2006). The targeting region of the RT-qPCR primers used to detect repression in relation to the miRNA target site can also influence the ability to identify gene repression at the mRNA level (Holmes et al., 2010). Although we have previously shown that it is possible to inhibit and promote signaling pathways in fetal testis gonad culture using chemical manipulation (Jørgensen et al., 2015b), to the best of our knowledge there are no publications showing successful ‘long-term’ genetic manipulation of the human fetal gonad. We have shown that repression of DMRT1 activity persists over several weeks in this study by using xenografting techniques to extend the experimental time from 14 days (in culture) to 4–6 weeks (via xenografts), after which time SOX9+/DMRT1− cells were still frequently identified in testis tissue exposed to DMRT1-targeted miRNA. The location of the DMRT1 repression appears to relate to the degree of viral penetration within the tissue and the resulting focal areas of dysgenesis are likely to result from proliferation of the DMRT1− Sertoli (SOX9+) cells. The focal distribution of DMRT1 repression and corresponding dysgenesis also provides a useful internal control for identifying specific effects of gene repression within each tissue sample.
It has previously been shown that DMRT1 is important for maintaining the testicular phenotype by preventing Sertoli-to-granulosa cell trans-differentiation in the postnatal mouse testis by inhibiting the expression of key ‘female pathway’ genes whilst promoting the continued expression of ‘male pathway’ promoting genes (Matson et al., 2011; Minkina et al., 2014). To examine potential effects of DMRT1 repression in Sertoli cells on genes known to be linked to maintenance of gonad sex identity, we used RT-qPCR to investigate expression of Sertoli, granulosa and germ cell specific genes. Notably, SOX8 expression was reduced in both the miR536 and miR641-exposed tissues. This is in agreement with a rodent study which elegantly demonstrated that when DMRT1 was knocked out in both prepubertal and adult male mice, it resulted in down- regulation of SOX8 and SOX9 (Matson et al., 2011). Unlike Matson et al. (2011) we did not observe a reduction in SOX9 expression following DMRT1 knockdown in postnatal mice, although this is in keeping with studies involving Dmrt1 deletion in mice, in which Sox9 expression is initially independent of DMRT1 (Matson et al., 2011). It is possible that loss of Sox9 after Dmrt1 ablation is a secondary consequence of the upregulation of ovarian genes(s), such as Foxl2, which does not occur until postnatal life (Matson et al., 2011). Whilst SOX9 is essential for sex determination, it has been shown in some studies to be dispensable for the maintenance of sexual phenotype beyond fetal life (Chaboissier et al., 2004), although this conclusion does not take into consideration the importance of continued expression of DMRT1 or SOX8 (Barrionuevo et al., 2016). It is possible that DMRT1 and SOX9 provide a protection system for the maintenance of sexual phenotype in the male gonad during different stages of development. In contrast, SOX8, remains a potential candidate as a key sexual maintenance gene in the male gonad (Barrionuevo et al., 2009, 2016). Furthermore, mutations in SOX8 have recently been demonstrated in humans with DSD and a lack of testis determination (Portnoi et al., 2018).
Other than SOX8, no other ‘male pathway’ genes were found to be differentially altered after knockdown of DMRT1, although CYP26B1 and PTGDS (Prostaglandin D2 synthase) were both down-regulated in the miR641 transduced samples. CYP26B1 and DMRT1 are both involved in preventing premature initiation of meiosis in rodent fetal testes by repression of retinoic acid (RA) signaling, including inhibiting the expression of the pre-meiosis marker STRA8 (Bowles et al., 2006; Koubova et al., 2006; Matson et al., 2010). In addition, the CYP26B1 expression which is activated by SOX9 in the fetal mouse testis was recently shown to also antagonize the ovarian differentiation pathway possibly through the involvement of DAX1 (Bowles et al., 2018). In terms of genes associated with an ovarian phenotype, the granulosa cell marker R-spondin 1 was down-regulated despite repression of DMRT1, whilst there was no change in FOXL2 expression at a transcript level. However, we did identify FOXL2 protein expression in tissue from one of the xenografted fetuses in an area of the tissue in which DMRT1 was repressed, whilst no expression of FOXL2 was detected in the control xenografts from the same fetus. This might indicate that a longer time-period of DMRT1 repression is required for FOXL2 expression to be initiated, as analysis of xenografts occurred 4–6 weeks later than for the cultured tissues. The rare finding of FOXL2 expression in the xenografted tissue could indicate that there are several pathways or factors working in concert to maintain a gonadal phenotype, a concept that is supported by descriptions of significant inter-individual variation in the occurrence of male to female sex reversal in patients with various forms of XY gonadal dysgenesis (Bagheri-Fam et al., 2015). In addition, the potential for DMRT1 repression to induce FOXL2 expression may be reduced in the xenografts due to the fact that DMRT1 is only lost in a proportion of the Sertoli cells or that focal expression of these FOXL2+ cells may be restricted by a low rate of their proliferation.
A key finding of the study was the demonstration of regions of focal dysgenesis in the second-trimester human fetal testis as a result of DMRT1 repression. Whilst seminiferous cord formation progressed in first trimester human fetal testes in which DMRT1 was repressed, DMRT1 repression in second-trimester tissue, in which the seminiferous cords had already formed, resulted in localized disruption of cord structure after short-term in vitro culture. Long-term maintenance of the testis tissue in xenografts showed further progression of cord breakdown into regions of focal dysgenesis. These findings indicate a role for DMRT1 in the maintenance of testicular phenotype following sex determination and seminiferous cord formation in the human testis. This is in keeping with data from rodent studies where Dmrt1 null XY mice initially develop testes but subsequently undergo male to female sex reversal (Raymond et al., 2000). Whilst the present study suggests that DMRT1 is required to maintain the testicular phenotype during fetal life in humans, studies in Dmrt1fl/fl mutant mice show that trans-differentiation towards a female phenotype does not begin until around postnatal Day 8 (Minkina et al., 2014). Furthermore, the latter authors demonstrated that trans-differentiation was more pronounced in the Dmrt1−/Sox9- double knockout, evidenced by increased numbers of FOXL2+ cells by 14 days and almost complete sex reversal at 5 weeks compared to the Dmrt1- only mutant. In the present study SOX9 expression was unaffected and therefore it is unclear whether simultaneous loss of SOX9 would lead to a more frequent or severe gonadal phenotype. Taken together, these results suggest that, similar to the mouse, DMRT1 is not essential for seminiferous cord formation in the human fetal testis, but is required for subsequent maintenance of the seminiferous cords and for the prevention of trans-differentiation to an ovarian phenotype. In the context of testicular dysgenesis syndrome (TDS) disorders, the present results suggest that the focal dysgenesis which is frequently observed in the testes of patients with reproductive disorders (Skakkebaek et al., 2016), could arise as the result of the breakdown of already formed seminiferous cords, as recently documented in an animal model of TDS (Lara et al., 2017; van den Driesche et al., 2017). Moreover, the fact that DMRT1-miRNA-induced focal dysgenesis was associated with evidence for impaired Leydig cell steroidogenesis, as indicated by reduced CYP11a1 expression, fits with the clinical (Skakkebaek et al., 2016) and rodent experimental (van den Driesche et al., 2017) data, and this is considered to be the underlying cause of some of the TDS disorders.
The present findings have important implications for the study of DSD and TDS. Focal testicular dysgenesis is frequently found in patients with these disorders and there is a strong association between DSD (Y-chromosome+) and testicular germ cell tumors (TGCC; Looijenga et al., 2010; Jørgensen et al., 2015a; Hersmus et al., 2017). Indeed, in humans, the presence of focal dysgenesis has been shown to correlate with the presence of germ cell neoplasia in situ (GCNIS), the precursor to TGCC (Hoei-Hansen et al., 2003; van den Driesche et al., 2017) and genetic variations in or near to DMRT1 have also been associated with TGCC in GWAS studies (Turnbull et al., 2010; Kanetsky et al., 2011). Indeed, DMRT1 is aberrantly expressed in the testicular cancer precursor germ cell neoplasia in situ (GCNIS) as part of a general dysregulation of the mitosis-meiosis switch in these cells (Jorgensen et al., 2013; Sharpe and Mitchell, 2013). Further support for a potential role of DMRT1 in the pathogenesis of TGCC is the present finding that PTGDS gene expression was also down-regulated following DMRT1 repression (miR641) in the human fetal testis. PTGDS expression has been associated with inhibition of cellular proliferation and migration in the human testicular germ cell cancer line NT2 (Wu et al., 2012a; Shyu et al., 2013). GCNIS cells (and gonocytes) express markers including the pluripotency factors OCT4 and AP2γ: we have shown a significant increase in OCT4 gene expression following DMRT1-miRNA (miR536) repression. Furthermore, in xenografts, repression of DMRT1 resulted in focal dysgenesis similar to that described in patients with TGCC (Hoei-Hansen et al., 2003). However, germ cells expressing AP2γ were rarely observed in the dysgenetic regions of xenografted human fetal testes in which DMRT1 had been repressed. Whether the few remaining germ cells within these dysgenetic regions undergo pre-neoplastic change requires further investigation.
More widely, the use of this novel system for genetic manipulation in human fetal testis provides a unique opportunity to determine the role of other genes or gene networks that may be involved in testicular development in health and disease (including DSD and TDS). This may include studies to determine the human-relevance of results from rodent models or those that can investigate the pathogenicity and potential mechanism(s) for mutations identified in patients with these disorders.
In conclusion, the present study has utilized a novel approach for genetic manipulation in the human fetal testis, resulting in the first direct evidence that DMRT1 is required for testicular differentiation in humans. We have shown that DMRT1 repression results in alterations in expression of key genes involved in gonadal development, consistent with DMRT1 preventing trans-differentiation of Sertoli cells to a more granulosa-like cell type in the human testis. Furthermore, we have demonstrated that repression of DMRT1 results in focal dysgenesis in the human fetal testis. These findings relating to DMRT1 effects, coupled with the potential use of this system to investigate the effects of manipulation of other genes, may have important implications for understanding the origins of DSD, TGCC and related disorders in humans.
Supplementary Material
Acknowledgements
We gratefully acknowledge the work of Anne Saunderson, Rosemary Bayne and the staff of the Bruntsfield Suite of the Royal Infirmary of Edinburgh in provision of tissue for these studies and the Human Developmental Biology Resource (www.hdbr.org) for providing some of the fetal material (Joint MRC/Wellcome Trust; Grant No. 099175/Z/12/Z). We also acknowledge William Mungall for assistance with the animal work and tissue collection.
Funding
This project was funded by a Wellcome Intermediate Clinical Fellowship (Grant No: 098522) awarded to RTM. LBS was supported by Medical Research Council Programme Grant MR/N002970/1. RAA was supported by Medical Research Council Programme Grant G1100357/1. RMS was supported by Medical Research Council Programme Grant G33253. This work was undertaken in the Medical Research Council Centre for Reproductive Health which is funded by the Medical Research Council Centre grant MR/N022556/1. The funding bodies had no input into the conduct of the research or the production of this manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author Contributions
R.T.M., J.M. and K.K. conceived and designed the experiments. A.K., J.M., K.K., P.B. and A.J. performed the experiments. R.T.M., A.J., J.M., K.K., L.B.S., R.M.S., R.A.A. and P.B. analyzed the data. R.T.M. contributed reagents/materials/analysis tools. R.T.M., J.M. and K.K. wrote the paper. All authors approved the submitted version.
References
- Bagheri-Fam S, Ono M, Li L, Zhao L, Ryan J, Lai R, Katsura Y, Rossello FJ, Koopman P, Scherer G et al. FGFR2 mutation in 46,XY sex reversal with craniosynostosis. Hum Mol Genet 2015;24:6699–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 2009;327:301–312. [DOI] [PubMed] [Google Scholar]
- Barrionuevo FJ, Hurtado A, Kim GJ, Real FM, Bakkali M, Kopp JL, Sander M, Scherer G, Burgos M, Jiménez R. Sox9 and Sox8 protect the adult testis from male-to-female genetic reprogramming and complete degeneration. Elife 2016;21:5. pii: e15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne RA, Martins da Silva SJ, Anderson RA. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod 2004;10:373–381. [DOI] [PubMed] [Google Scholar]
- Bennett CP, Docherty Z, Robb SA, Ramani P, Hawkins JR, Grant D. Deletion 9p and sex reversal. J Med Genet 1993;30:518–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Ineson J, Miles K, Spiller CM, Harley VR, Sinclair AH, Koopman P. Retinoic acid antagonizes testis development in mice. Cell Rep 2018;24:1330–1341. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J et al. Retinoid signaling determines germ cell fate in mice. Science 2006;312:596–600. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 2004;131:1891–1901. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod 2003;18:1383–1394. [DOI] [PubMed] [Google Scholar]
- Hersmus R, van Bever Y, Wolffenbuttel KP, Biermann K, Cools M, Looijenga LH. The biology of germ cell tumors in disorders of sex development. Clin Genet 2017;91:292–301. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol 2003;200:370–374. [DOI] [PubMed] [Google Scholar]
- Holmes K, Williams CM, Chapman EA, Cross MJ. Detection of siRNA induced mRNA silencing by RT-qPCR: considerations for experimental design. BMC Res Notes 2010;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo JJ, Salafsky IS, Lin CC, Pinsky L. Possible location of a recessive testis forming gene on 9p24. Am J Hum Genet 1989;45:A78 (Abstract). [Google Scholar]
- Jørgensen A, Lindhardt Johansen M, Juul A, Skakkebaek NE, Main KM, Rajpert-De Meyts E. Pathogenesis of germ cell neoplasia in testicular dysgenesis and disorders of sex development. Semin Cell Dev Biol 2015. a;45:124–137. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Almstrup K, Toft BG, Petersen BL, Rajpert-De Meyts E. Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J Pathol 2013;229:588–598. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Blomberg Jensen M, Graem N, Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod 2012;18:523–534. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Perlman S, Lundvall L, Mitchell RT, Juul A, Rajpert-De Meyts E. Ex vivo culture of human fetal gonads: manipulation of meiosis signalling by retinoic acid treatment disrupts testis development. Hum Reprod 2015. b;30:2351–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D’Andrea K, Vaddi M, Doody DR et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet 2011;20:3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006;103:2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara NLM, Driesche SVD, Macpherson S, Franca LR, Sharpe RM. Dibutyl phthalate induced testicular dysgenesis originates after seminiferous cord formation in rats. Sci Rep 2017;7:2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga LH, Hersmus R, de Leeuw BH, Stoop H, Cools M, Oosterhuis JW, Drop SL, Wolffenbuttel KP. Gonadal tumours and DSD. Best Pract Res Clin Endocrinol Metab 2010;24:291–310. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell 2010;19:612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011;476:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet 2012;13:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkina A, Matson CK, Lindeman RE, Ghyselinck NB, Bardwell VJ, Zarkower D. DMRT1 protects male gonadal cells from retinoid-dependent sexual transdifferentiation. Dev Cell 2014;29:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RT, Cowan G, Morris KD, Anderson RA, Fraser HM, Mckenzie KJ, Wallace WH, Kelnar CJ, Saunders PT, Sharpe RM. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod 2008;23:2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RT, Saunders PT, Childs AJ, Cassidy-Kojima C, Anderson RA, Wallace WH, Kelnar CJ, Sharpe RM. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod 2010;25:2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MW, Lee JK, Rojo S, Gearhart MD, Kurahashi K, Banerjee S, Loeuille GA, Bashamboo A, McElreavey K, Zarkower D et al. An ancient protein-DNA interaction underlying metazoan sex determination. Nat Struct Mol Biol 2015;22:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi C, Veitia R, Quintana-Murci L, Torchard D, Scapoli L, Souleyreau-Therville N, Beckmann J, Fellous M, McElreavey K. The region on 9p associated with 46,XY sex reversal contains several transcripts expressed in the urogenital system and a novel doublesex-related domain. Genomics 2000;64:170–178. [DOI] [PubMed] [Google Scholar]
- Ounap K, Uibo O, Zordania R, Kiho L, Ilus T, Oiglane-Shlik E, Bartsch O. Three patients with 9p deletions including DMRT1 and DMRT2: a girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development. Am J Med Genet A 2004;130A:415–423. [DOI] [PubMed] [Google Scholar]
- Pannetier M, Chassot A. a., Chaboissier MC, Pailhoux E. Involvement of FOXL2 and RSPO1 in ovarian determination, development and maintenance in mammals. Sex Dev 2016;10:167–184. [DOI] [PubMed] [Google Scholar]
- Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ, Eozenou C, Bignon-Topalovic J, Yatsenko SA, Rajkovic A, Reyes-Mugica M et al. Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum Mol Genet 2018;27:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol 1999. a;215:208–220. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev 2000;14:2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Parker ED, Kettlewell JR, Brown LG, Page DC, Kusz K, Jaruzelska J, Reinberg Y, Flejter WL, Bardwell VJ et al. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum Mol Genet 1999. b;8:989–996. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature 1998;391:691–695. [DOI] [PubMed] [Google Scholar]
- Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ, Zavolan M, Svoboda P, Filipowicz W. Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Nucleic Acids Res 2006;34:4801–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Mitchell RT. The downside of ‘inappropriate messaging’: new insight into the development of testicular germ cell tumours in young men? J Pathol 2013;229:497–501. [DOI] [PubMed] [Google Scholar]
- Shyu RY, Wu CC, Wang CH, Tsai TC, Wang LK, Chen ML, Jiang SY, Tsai FM. H-rev107 regulates prostaglandin D2 synthase-mediated suppression of cellular invasion in testicular cancer cells. J Biomed Sci 2013;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jorgensen N, Swan SH, Sapra KJ et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 2016;96:55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stévant I, Papaioannou MD, Nef S. A brief history of sex determination. Mol Cell Endocrinol 2018;468:3–10. [DOI] [PubMed] [Google Scholar]
- Svingen T, Jørgensen A, Rajpert-De Meyts E. Validation of endogenous normalizing genes for expression analyses in adult human testis and germ cell neoplasms. Mol Hum Reprod 2014;20:709–718. [DOI] [PubMed] [Google Scholar]
- Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, Ricketts M, Linger R, Nsengimana J, Deloukas P et al. , UK Testicular Cancer Collaboration . Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet 2010;42:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009;139:1130–1142. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Kilcoyne KR, Wagner I, Rebourcet D, Boyle A, Mitchell R, McKinnell C, Macpherson S, Donat R, Shukla CJ et al. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2017;2:e91204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, Macdonald J, Anderson RA, Johnston ZC, Chetty T, Smith LB, Mckinnell C, Dean A, Homer NZ, Jorgensen A et al. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model. Sci Transl Med 2015;7:288ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci G, Chantot-Bastaraud S, El Houate B, Lortat-Jacob S, Brauner R, McElreavey K. Association of deletion 9p, 46,XY gonadal dysgenesis and autistic spectrum disorder. Mol Hum Reprod 2007;13:685–689. [DOI] [PubMed] [Google Scholar]
- Wu CC, Shyu RY, Wang CH, Tsai TC, Wang LK, Chen ML, Jiang SY, Tsai FM. Involvement of the prostaglandin D2 signal pathway in retinoid-inducible gene 1 (RIG1)-mediated suppression of cell invasion in testis cancer cells. Biochim Biophys Acta 2012. a;1823:2227–2236. [DOI] [PubMed] [Google Scholar]
- Wu YC, Ling TY, Lu SH, Kuo HC, Ho HN, Yeh SD, Shen CN, Huang YH. Chemotherapeutic sensitivity of testicular germ cell tumors under hypoxic conditions is negatively regulated by SENP1-controlled sumoylation of OCT4. Cancer Res 2012. b;72:4963–4973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.