Abstract
In man, central sensitisation (CS) contributes to the pain of osteoarthritis (OA). Dogs with spontaneous OA may also exhibit CS. Electrophysiological reflex measurements are more objective than behavioural assessments, and can be used to evaluate CS in preclinical and clinical studies. It was hypothesised that dogs suffering from OA would exhibit electrophysiological characteristics indicative of CS, associated with reduced diffuse noxious inhibitory controls (DNIC). 117 client owned dogs were recruited to the study. Hindlimb nociceptive withdrawal reflex (NWR) thresholds, stimulus response, and temporal summation characteristics were recorded, during alfaxalone anaesthesia, from 46 OA dogs, 29 OA dogs receiving non-steroidal anti-inflammatory drugs (OANSAID), and 27 breed- and weight-matched control dogs. Efficacy of DNIC was evaluated in 12 control and 11 of the OA dogs, by application of a mechanical conditioning stimulus to the contralateral forelimb.
NWR thresholds were higher in OA compared with control dogs (p = 0.02). Stimulus response characteristics demonstrated an augmented response in OANSAID dogs compared with OA (p < 0.001) and control (p < 0.001) dogs. Temporal summation demonstrated exaggerated C-fibre mediated responses in both OA (p < 0.001) and OANSAID (p = 0.005) groups, compared with control animals. Conditioning stimulus application resulted in inhibition of test reflex responses in both OA and control animals (p < 0.001); control animals demonstrated greater inhibition compared with OA (p = 0.0499). These data provide evidence of neurophysiological changes consistent with CS in dogs with spontaneous OA, and demonstrate that canine OA is associated with reduced DNIC.
Keywords: Central Sensitisation, Diffuse Noxious Inhibitory Controls, Dog, Nociceptive Withdrawal Reflex, Osteoarthritis
1. Introduction
Spontaneous canine osteoarthritis (OA) has been proposed as a model of human OA [39]. In man, in addition to mechanisms local to affected joints, central sensitisation (CS) may exacerbate pain [30]. Some dogs affected by OA respond to centrally acting antihyperalgesic drugs [26] and have altered nociceptive thresholds [21], suggesting CS; however, there is no ‘gold standard’ approach for identifying and quantifying CS in dogs. Therefore, it is currently unknown whether OA in dogs is also associated with CS, yet this information is essential if canine OA is to be used as a valid model of human OA.
The RIII withdrawal response threshold and magnitude, and temporal summation (TS) to repeated stimuli are altered in pain syndromes associated with CS in man, and may be used as objective markers of CS [38]. In dogs, the nociceptive withdrawal reflex (NWR) [6] and TS of the NWR [8] have been suggested as potential biomarkers for CS. We have previously developed methods to evaluate these measures during anaesthesia [19]. There are, presently, no reports of alterations in NWR or TS associated with painful disease in dogs, and the potential for the technique to characterise the state of spinal excitability remains untested.
Diffuse noxious inhibitory controls (DNIC) represent an endogenous supraspinal anti-nociceptive mechanism activated by heterotopic noxious (‘conditioning’) stimulation [5,15]. Efficacy of conditioned pain modulation (CPM) in man (considered the equivalent of DNIC) is a predictor of acute [14] and chronic post-operative [42] pain, and is commonly reduced in chronic pain states, including OA [2]. There are no investigations of DNIC efficacy associated with OA in dogs. CPM may be modulated by cognitive influences [31], which are challenging to control for experimentally. Therefore, it is desirable to develop a non-tissue damaging paradigm, which may be applied to anaesthetised animals.
The primary aim of the studies described here was to compare electrophysiological responses, including temporal summation of C fibre responses, in a cohort of client owned pet dogs suffering spontaneous OA, with a matched group of control pet dogs. Dogs within the OA cohort were divided into those receiving daily NSAIDs to manage OA associated pain (OANSAID) and dogs not receiving drug treatment (OA). We hypothesised that dogs with OA would exhibit electrophysiological characteristics indicative of CS, and that these characteristics would be exaggerated in the OANSAID group compared to the OA group because of the greater pain that was likely experienced by OANSAID dogs despite ongoing NSAID administration.
Our second aim was to develop an effective protocol to evaluate DNIC in dogs. CPM has been elicited by mechanical conditioning stimulation (MCS) [34], therefore we sought to investigate whether MCS would evoke DNIC, and whether DNIC efficacy was decreased by OA. We hypothesised that in control dogs, application of MCS would inhibit the NWR, and that the degree of inhibition would be reduced in dogs affected by OA.
2. Methods
Part i) NWR/TS investigation
2.1. Ethics
The study was conducted under the terms of the Animal (Scientific Procedures) Act, 1986 (as amended, 2013) (A(SP)A) licence number PPL 30/3157, and the experimental protocol was approved by the University of Bristol Animal Welfare and Ethical Review Body.
2.2. Recruitment criteria
Advertisements to recruit participants for the study were posted on social media (Facebook, Twitter), within the local University of Bristol intranet, and within local veterinary practices. For the osteoarthritis (OA) group suitable dogs were 12 kg bodyweight and over, of any age, body condition and sex exhibiting suspected painful uni- or bilateral coxofemoral or stifle degenerative joint disease (DJD) as evidenced by lameness/stiffness/difficulty rising or ascending steps. Dogs with primarily forelimb lameness were excluded. During the study recruitment phase a large proportion of dogs screened were already receiving daily treatment with non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain and the decision was made to recruit these animals and permit them to continue daily NSAID treatment, but to designate them as a separate group (OANSAID) for analysis within the study. This decision was based on the fact that pain and disability were still present in these individuals despite treatment with the NSAID.
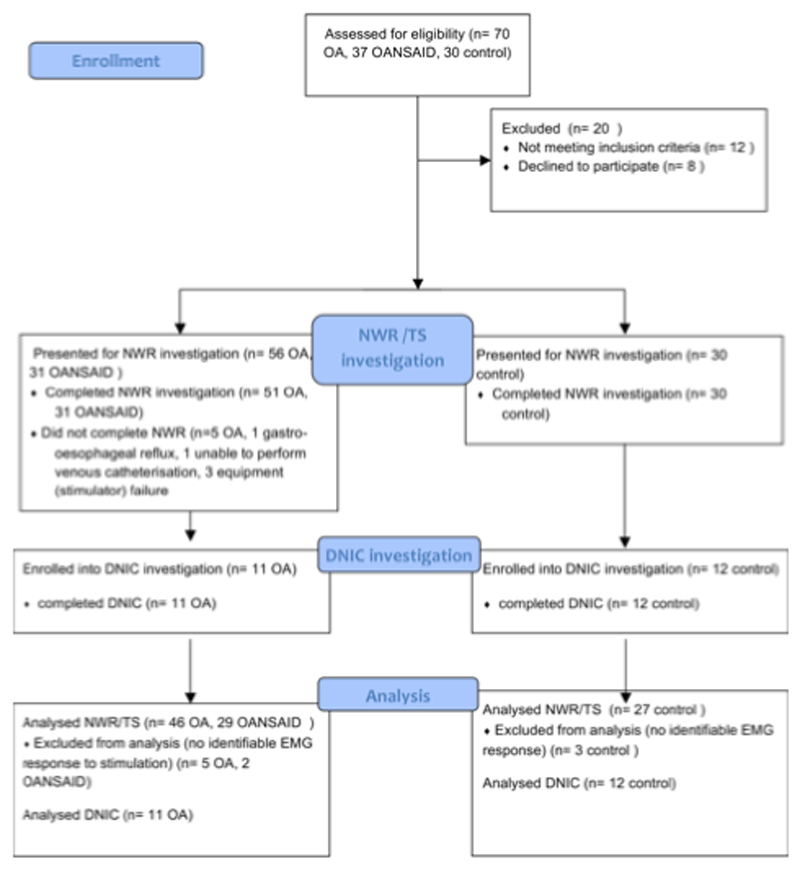
The inclusion criteria for the control group were based on the demographics of a cohort of OA dogs recruited to a separate study at the University of Bristol [16], who recorded a mean (SD) age of 9.5 ± 3 years and weight of 27.5 ± 11.6 kg. For the present study dogs were recruited to the control group that were 6 years old or greater and 12 kg bodyweight and over, exhibiting no evidence of lameness or stiffness and with no other painful condition (e.g. otitis externa) and no previous diagnosis of OA. Figure 1 illustrates the outcomes for all dogs that attended screening and the subsequent numbers that were used at each stage of the study.
Figure 1.
Illustration of the number of animals recruited to each OA category, and attrition through different stages of the study.
2.3. Study protocol
Owners of eligible dogs were asked to attend a screening appointment, at which the purpose and procedures of the study were explained verbally and in writing, and signed consent to participate was obtained prior to any study procedures being performed. Dogs underwent physical and musculoskeletal examination by a veterinarian (JRH). Body condition score (1, emaciated – 9, morbidly obese [24]) was assessed by manual palpation. Any dogs with identifiable co-morbidities which would have an increased risks associated with general anaesthesia, or dogs with neurological dysfunction evidenced by weak or absent conscious proprioception, were excluded from the study. Microchip details were confirmed as a means of permanently identifying participating dogs to comply with the terms of the A(SP)A. Owners were asked to complete the ACVS Canine Orthopaedic Index [9], the Helsinki Chronic Pain Index (HCPI) [17], the Liverpool Osteoarthritis in Dogs (LOAD) questionnaire, the Canine Brief Pain Inventory (CBPI) [10], and the Sleep and Night time Restlessness Evaluation (SNORE) [22]. Jugular blood samples were obtained and submitted for routine biochemistry and haematology prior to scheduling general anaesthesia.
2.4. Musculoskeletal examination (appendix 1)
Scores for lameness (0-10) and mobility (0-3) were assigned by a veterinarian (JRH), according to the criteria shown in appendix 1.
Examination of each joint was performed and individual appendicular joints were scored from 0 (not affected) to 3 (severely affected) for the criteria “range of motion”, “pain on extension or flexion”, “crepitus”, “effusion” and “thickening”. The sum of the joint disease scores produced an overall OA score between 0 and 192, while the sum of the pain scores for each joint produced an overall joint pain score between 0 and 48.
2.5. General anaesthesia
Seven days after the initial screening appointment dogs were admitted to the Wellcome Comparative Anaesthesia Research Laboratory, Langford, Bristol in order to undergo radiography and NWR testing under general anaesthesia.
On admission, confirmation that dogs had had food withheld for 8 hours was sought from owners and a veterinary examination was repeated.
Acepromazine (ACP 2mg/ml solution, Elanco Animal Health, Basingstoke, UK) was administered intramuscularly (0.03mg kg-1) and dogs were left undisturbed for 30 minutes, following which a cephalic venous catheter was placed. Insufficient sedation to permit intravenous catheterisation warranted exclusion from the study.
Alfaxalone (Alfaxan, Jurox (UK) Ltd, Crawley, UK) (1-2 mg kg-1) was administered intravenously over a period of 60 seconds until orotracheal intubation was possible. Oxygen was delivered via a circle breathing system and anaesthesia maintained with a constant rate infusion of alfaxalone (0.1 mg kg-1 min-1) during radiography, reducing to 0.09 mg kg-1 min-1 for NWR testing. Body temperature was monitored every 30 minutes and supported with insulated electric blankets. Following NWR testing alfaxalone infusion was discontinued and the dogs constantly monitored until they were discharged to the owner once able to walk and having eaten. All dogs not ordinarily receiving NSAIDs were treated with meloxicam (Metacam 5mg/ml solution, Boehringer Ingelheim, Bracknell, UK) (0.2 mg kg-1) to treat any pain caused by positioning for radiography or NWR recording.
2.6. Radiography
Lateral and cranial-caudal views of the elbows and stifles; lateral views of the lumbosacral junction; and ventrodorsal views of the pelvis and coxofemoral joints were obtained in the Bristol Veterinary School imaging suite. Each of these seven joints was assessed for severity of radiographic osteoarthritis by two investigators (ME, BDXL) who were unaware of the OA group classification of the dogs. The investigators assigned scores from 0 (no radiographic signs of osteoarthritis) to 10 (severe radiographic osteoarthritis) for each joint and a thus a global score for each dog out of 70 was recorded. The investigators performing NWR testing remained unaware of the results of the radiographs.
2.7. Nociceptive Withdrawal Reflex testing
Dogs were positioned in left lateral recumbency with the right pelvic limb resting on a sandbag, perpendicular to the table top. Paired stimulating electrodes (disposable subdermal needle electrode 12 x 0.40 mm, Natus Neurology Inc. Middleton, WI, USA) were placed 10mm apart subdermally into the plantar aspect of digit 3 of the right hindlimb; paired recording electrodes were placed 20mm apart into the body of the right cranial tibial muscle, and a ground electrode placed subcutaneously dorsal to the dorsal spinous process of L6. As previously described [23] the recorded signal was processed via a differential amplifier (DAM50, World Precision Instruments, Herts, UK) which applied a bandpass filter from 10 - 1kHz and gain of 1000, and was subsequently captured in Labchart 8 software (AD instruments, Oxford, UK) following conversion by an analogue to digital converter with a sampling frequency of 1kHz (Powerlab 4/35, AD instruments, Oxford, UK).
2.8. EMG threshold
Electrical stimuli were delivered via the toe electrodes using a constant current stimulator from an isolated 100 V source (Stimulus isolator FE180, AD instruments, Oxford, UK).
The threshold current at which a single 1ms square wave stimulating pulse would evoke a visually discernable cranial tibial (CT) EMG response (a response greater than the baseline amplitude) was identified by increasing current stepwise from 0 to a maximum of 10mA in 0.5mA increments. Following a response, the current was decreased by 0.1mA increments until the response was no longer elicited. This up and down adjustment was continued until 3 stable readings for threshold were obtained at 60 second intervals.
2.9. Stimulus Response Curve
One stimulus event comprised five 1ms stimuli (Train-of-5, To5 [23]), which were delivered at a frequency of 100 Hz. An EMG stimulus response determination was performed by triggering To5 events at 60-second intervals using currents of 0.1 (baseline), 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 mA. The complete series of stimulating currents were applied in the same ascending order on a second occasion following a five-minute interval.
2.10. Temporal Summation
A stimulus sequence of 8x 1ms 10mA stimuli delivered at a rate of 1Hz was repeated 3 times at five-minute intervals.
2.11. EMG analysis
Post recording, a 10Hz high pass digital filter was applied to the EMG traces, to further decrease movement artefact. The primary outcome measure for the study was the integral of the rectified EMG response which was extracted for each stimulus within each pre-defined time window. The EMG response was designated as early (representing an A-fibre response 0-100ms) or late (C-fibre; 100-500ms) latency, time locked to the start of the stimulus train [23]. Although the late response may also contain components of supraspinal origin, this differentiation was based on previous work in dogs [8] where conduction velocity of the different nerve fiber types and the length of the afferent distance were used to calculate latency ranges for the different (A and C fiber) responses. Baseline activity in the absence of any electrically-evoked response (the 0.1mA stimulus for the stimulus response experiments and from within a 2s period prior to application of the first of the eight stimuli for temporal summation experiments) was subtracted from each measurement.
2.12. Statistical methods
Recordings of NWR data were visually examined by two investigators (JRH, JH) and where no identifiable response could be appreciated to a stimulation protocol the data for that protocol for the individual dog were excluded from the analysis. Sex distribution data were analysed using Chi squared tests. Comparisons of mean or median measures at single time points (e.g. body weight, lameness, owner completed metrology instruments) between the three groups were performed using one-way ANOVA or Kruskal-Wallis tests followed by Tukey (or Dunn’s) post-hoc testing if applicable. The hierarchical structure of the data comprising the stimulus response and temporal summation data was accounted for by employing multilevel modelling within the MLwiN statistics package [35]. In the case of the stimulus response data, no transformation of the outcome variable was necessary as the residuals from the analyses showed appropriate normality and homoscedasticity. It was necessary to apply natural log transformation to the temporal summation data to meet the assumptions of the statistical models. Data analysed using parametric tests are presented as mean (95% confidence interval (CI)) and the results of non-parametric testing are presented as median (25-75% interquartile range). The final multilevel, general linear models took the form of equations which described the effect of the statistically significant predictor variables on the outcome measures. The parameter estimates from the analyses are presented below and the models are represented as graphs.
2.13. Power calculation
A power calculation for the overarching project, based on preliminary data using von Frey mechanical threshold data, indicated a total of 68 dogs, evenly divided between OA and control groups, would be required for a power of 90%, at an alpha of 0.05 to detect a difference between control and OA dogs. However, this calculation assumed uniformity within the OA group, whereas we suspected that the OA group would be heterogenous, based on data from human OA patients and laboratory animal models of OA. In humans, up to 70% of OA patients have at least one somatosensory abnormality [41]. Based on this, we estimated that recruiting 100 OA dogs would give us an appropriate cohort of central sensitisation (CS) negative dogs (i.e. approximately the same number as control dogs), and a cohort of CS positive dogs that may be as large as 70.
Part ii) DNIC investigation
2.14. Animals
Following completion of the NWR/TS protocol described above, some dogs underwent DNIC investigations during the same anaesthetic period (see figure 1).
2.15. DNIC protocol
Five minutes after the final TS experiment, the DNIC protocol began by recording EMG responses in the CT muscle to test stimuli delivered at twice the individually determined threshold current (2xThr) at a rate of 1Hz for 100 seconds. This occasion was denoted ‘pre-DNIC’. An identical test stimulation protocol (2xThr, 1Hz, 100 seconds) was repeated on three more occasions at five minute intervals; however, during occasions two (‘DNIC 1’) and three (‘DNIC 2’), the effect on CT responses of an additional mechanical conditioning stimulus, comprising a bulldog clip applied for 20 sec to the 3rd digit of the contralateral forelimb, was assessed (figure 2). The fourth and final occasion (‘post DNIC’) was a repeat of the pre-DNIC stimulating protocol, without the addition of a conditioning stimulus.
Figure 2.
During anaesthesia, a bulldog clip conditioning stimulus was applied for 20 seconds to the third digit of the left cranial limb, whilst electrical test stimuli were delivered to the right pelvic limb.
Measurement of the force delivered by the bulldog clip at a jaw separation of 11 mm (mean jaw opening measured during the application to the digit) was achieved using a Loadcell 50N gauge (Mecmesin, Slinfold, West Sussex, UK). The force recorded by the gauge at 11mm separation was 33.4 N, but this was also found to be consistent over the range of jaw opening from 2-12mm. Examination of the site of application following the DNIC protocol, and 7 days later, demonstrated no evidence of immediate or delayed ongoing pain or tissue damage.
2.16. Statistical methods
Sex distribution data were assessed using Fisher’s exact test. Comparisons of weight and owner completed metrology instrument scores between the two groups were performed using Student’s t-test or Mann-Whitney U test. The hierarchical structure of the DNIC testing data was accounted for within the statistical analysis by employing general linear modelling within a multilevel modelling framework using the MLwiN statistics package [42]. Predictor variables were retained within the model based upon a Wald test at α ≤ 0.05. It was necessary to apply a natural log transformation to the EMG magnitude data, to meet the assumptions of the tests with regards to normality and homoscedasticity of residuals. The pre-DNIC occasion was denoted as the reference occasion for comparisons within the model. Data subject to parametric tests are presented as mean (95% confidence interval (CI)) and results subject to non-parametric testing are presented as median (25-75% interquartile range).
2.17. Power calculation
A power calculation was performed for the overarching project; however, the DNIC investigation was performed in order to develop an effective but non-tissue damaging model for evaluating DNIC in dogs, and to provide pilot data for ongoing investigations, hence a power calculation was not performed specifically with regard to the primary outcome measure (magnitude of EMG response) reported here.
3. Results
Part i) NWR/TS investigation
3.1. Demographics
Data were analysed from 27 control, 46 OA, and 29 OANSAID dogs. Breed and sex distribution are shown in table 1. There was no significant difference in sex distribution, and breed distribution appeared to be visually well matched between groups. Weight and body condition scores were not different between groups (table 1). Dogs in the control group were younger than dogs in both the OA and OANSAID groups (table 1). The duration of NSAID treatment in the OANSAID group was variable between individuals, but animals had been receiving daily NSAIDs for at least 3 months prior to recruitment to the study.
Table 1.
Demographics M Male, Mn Male neuter, F Female, Fn Female neuter. Superscript letters indicate groupings within the data, shared superscripts indicate no significant difference between groups on post-hoc testing, differing superscripts indicate a difference with a p– value of less than 0.05 on post-hoc testing. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Breed | Control (n = 27) | OA (n = 46) | OANSAID (n = 29) | p |
|---|---|---|---|---|
| Border collie | 7 | 10 | 5 | - |
| Labrador | 5 | 8 | 11 | - |
| Retriever | 3 | 3 | 1 | - |
| Lurcher | 3 | 2 | 0 | - |
| Spaniel | 1 | 5 | 3 | - |
| Other | 8 | 18 | 9 | - |
| Sex | ||||
| M | 3 | 3 | 3 | 0.61 |
| Mn | 7 | 18 | 14 | 0.61 |
| F | 1 | 3 | 2 | 0.61 |
| Fn | 16 | 22 | 10 | 0.61 |
| Weight (kg) | 22.8 (95%CI 20.5-25.0) | 26.8 (95%CI 23.6-29.9) | 28.7 (95%CI 24.8-32.6) | 0.0563 |
| Body condition score (1-9) | 5 (4-6) | 5 (5-6) | 5 (4-6) | 0.19 |
| Age (years) | 7.8 (95%CI 7.3-8.4)a |
9.8 (95%CI 9.2-10.3)b |
9.6 (95%CI 8.5-10.6)b |
< 0.001*** |
3.2. Veterinary assessment
Degree of lameness, mobility score, total osteoarthritis score, and total joint pain score were all significantly higher in OA and OANSAID groups compared with controls (table 2); however, there were no differences between OA and OANSAID groups with regard to these measures.
Table 2.
Musculoskeletal examination, owner completed metrology instrument and radiographic severity data. Superscript letters indicate groupings within the data, shared superscripts indicate no significant difference between groups on post-hoc testing, differing superscripts indicate a difference with a p– value of less than 0.05 on post-hoc testing. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Control | OA | OANSAID | p | |
|---|---|---|---|---|
| Lameness (0-10) | 0 (0-0)a | 3 (1-3)b | 3 (2-3)b,c | <0.001*** |
| Mobility (0-3) | 0 (0-0)a | 1 (1-1)b | 1 (1-1)b,c | <0.001*** |
| OA score (0-192) | 0 (0-2)a | 10 (7-16)b | 14 (9-19)b,c | <0.001*** |
| Joint pain score (0-48) | 0 (0-0)a | 4 (2-4)b | 4 (3-5)b,c | <0.001*** |
| CBPI pain (0-10) | 0 (0-0.0625)a | 1.75 (0-3.5)b | 3.375 (1.813-4.688)c | <0.001*** |
| CBPI function (0-10) | 0 (0-0.833)a | 1.167 (0.1667-4.50)b | 2.833 (1.50-5.042)b,c | <0.001*** |
| HCPI (0-44) | 3 (0-8.25)a | 14 (8-22)b | 20.5 (15.25-21.75)b,c | <0.001*** |
| ACVS stiffness (0-16) | 0 (0-0.25)a | 5 (2-8)b | 8 (5-9)b,c | <0.001*** |
| ACVS function (0-16) | 0 (0-0.25)a | 5 (1-8)b | 8 (6-12)c | <0.001*** |
| ACVS gait (0-20) | 0.5 (0-2.25)a | 7 (2-11)b | 9 (7-11.75)b,c | <0.001*** |
| ACVS QoL (0-12) | 0 (0-1)a | 3 (1-5)b | 4.5 (2.6)b,c | <0.001*** |
| LOAD (0-52) | 2 (0-5)a | 14 (9-23)b | 18.5 (12-23)b,c | <0.001*** |
| Radiographic OA score (0-70) | 3 (1-10)a | 14 (8.25-24.75)b | 20 (8-26)b,c | <0.001*** |
3.3. Owner completed clinical metrology instruments (CMI)
Questionnaire data were analysed by subsection if the questionnaire was constructed in a section format. Owner attributed scores for all of the questionnaire subsections were significantly higher (more dysfunction/pain) in OA and OANSAID animals compared with controls. Additionally, the CBPI pain and ACVS function subsections were significantly higher in OANSAID compared with OA animals (table 2), indicating that dogs receiving NSAID therapy experienced greater pain and greater dysfunction (e.g. reduced mobility) than dogs with OA that were not receiving NSAID treatment.
3.4. Radiographic scores
Radiographic osteoarthritis severity was significantly higher in both OA and OANSAID animals compared with controls, but was not significantly different between OA and OANSAID animals (table 2).
3.5. NWR recordings
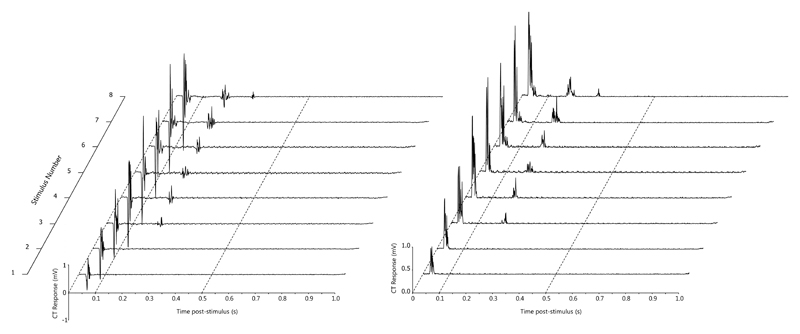
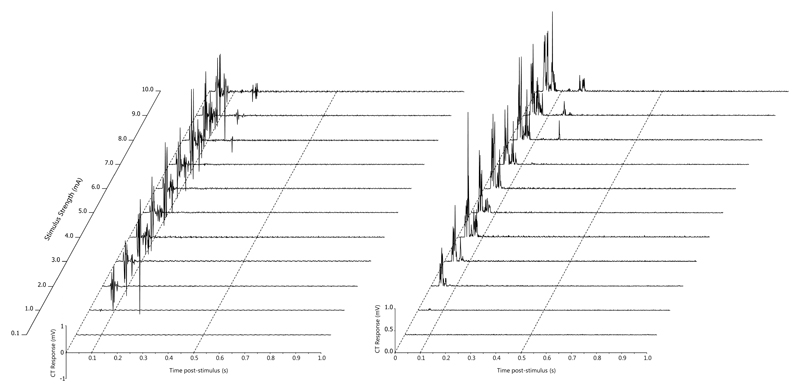
The early phase of the NWR could be reliably and repeatedly elicited in the cranial tibial muscle during the multiple trials at each stimulus intensity. Examples of raw traces obtained during NWR recording are provided (Figures 3 and 4).
Figure 3.
An example of temporal summation in the cranial tibial muscle (recorded from dog 71). The top channel is a stimulus marker channel, with a train of 8 1ms 10 mA stimuli delivered at a frequency of 1 Hz. The lower channel shows the early and late responses in the cranial tibial muscle. The time base is 0.2s/division.
Figure 4.
An example of the electrical stimulus response curve recorded from the cranial tibial muscle in dog 98. The top channel is the stimulus marker channel, with each single line representing five 1 ms stimuli delivered at a frequency of 100 Hz. Eleven stimuli were delivered with a 60 second interval between them starting at 0.1 mA (baseline), 1 mA and increasing in 1 mA increments through to 10 mA. The middle channel shows the early responses in the cranial tibial muscle and the lower channel shows the rectified EMG response in the cranial tibial muscle. The time base is 0.2s/division
3.6. Electrical threshold
The threshold current to elicit an EMG response was significantly lower in control (2.3 (95%CI 1.8 – 2.9mA)) compared with OA dogs (3.8 (95%CI 3.0 – 4.6mA) (F2,93 = 3.859, p = 0.02) but neither group was different from OANSAID (3.2 (95%CI 2.4 – 3.9mA) which had an intermediate value.
3.7. Stimulus response (table 3)
Table 3.
Effect size estimates and p- values for the general linear model which was fitted to the stimulus response (early) data. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Response magnitude (mV.s) | S.E. | Conf Int 2.5% | Conf Int 97.5% | p-value | |
|---|---|---|---|---|---|
| intercept | -0.001230 | 0.003234 | -0.007569 | 0.005109 | 0.704 |
| weight | 0.000018 | 0.000113 | -0.000204 | 0.000240 | 0.873 |
| OA | 0.000753 | 0.002481 | -0.004110 | 0.005615 | 0.762 |
| OANSAID | 0.000353 | 0.002782 | -0.005100 | 0.005806 | 0.899 |
| mA | 0.004864 | 0.000540 | 0.003807 | 0.005922 | <0.001*** |
| mA2 | -0.000170 | 0.000052 | -0.000271 | -0.000069 | 0.001** |
| weight.mA | -0.000094 | 0.000019 | -0.000132 | -0.000056 | <0.001*** |
| weight.mA2 | 0.000004 | 0.000002 | 0.000001 | 0.000008 | 0.026* |
| OA.mA | -0.000092 | 0.000119 | -0.000325 | 0.000141 | 0.440 |
| OANSAID.mA | 0.000759 | 0.000134 | 0.000497 | 0.001021 | <0.001*** |
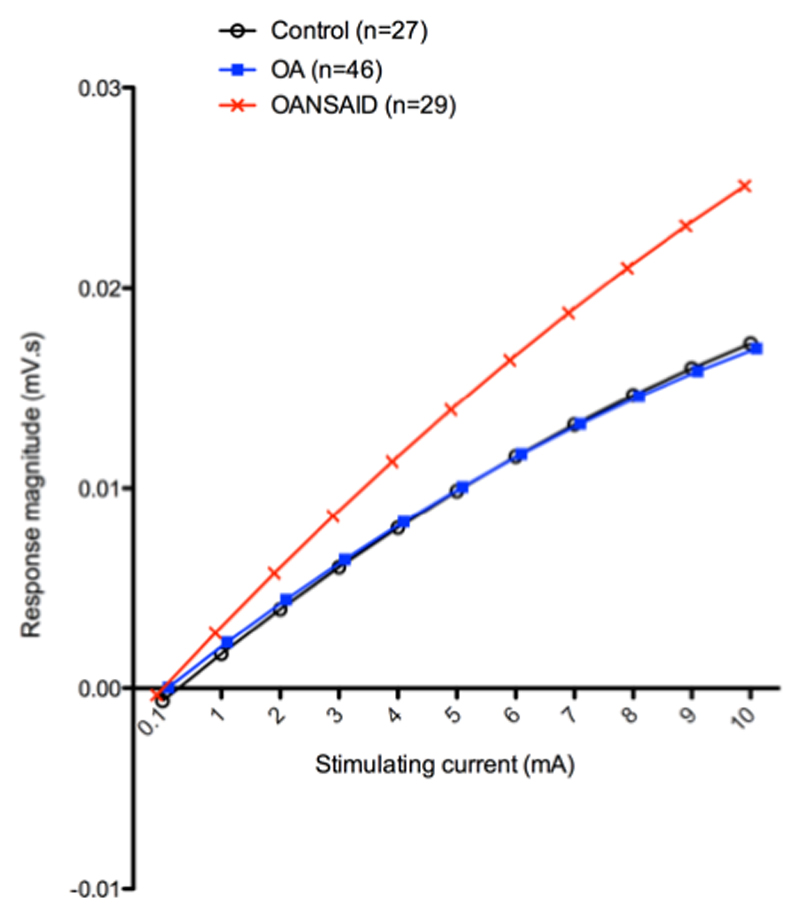
Only the early component of the response was analysed, as the late response was absent in the majority of recordings. The parameter estimates of those predictor variables significantly associated with the response are presented in table 3. The final model, containing only the significant terms, demonstrated that the magnitude of the measured response increased as a curvilinear function of the stimulating current (mA). There was a significant negative interaction between bodyweight and stimulating current (weight.mA; p < 0.001); larger animals demonstrated a lesser increase in response magnitude with increasing current compared with smaller animals. There was a significant positive interaction between the OANSAID category and stimulating current (OANSAID.mA) compared with control (p < 0.001) and OA category (p < 0.001) animals; OANSAID category animals demonstrated increased magnitude responses at a given stimulating current, compared with both control and OA category animals. These relationships are shown graphically in figure 5, at a fixed weight of 25 kg.
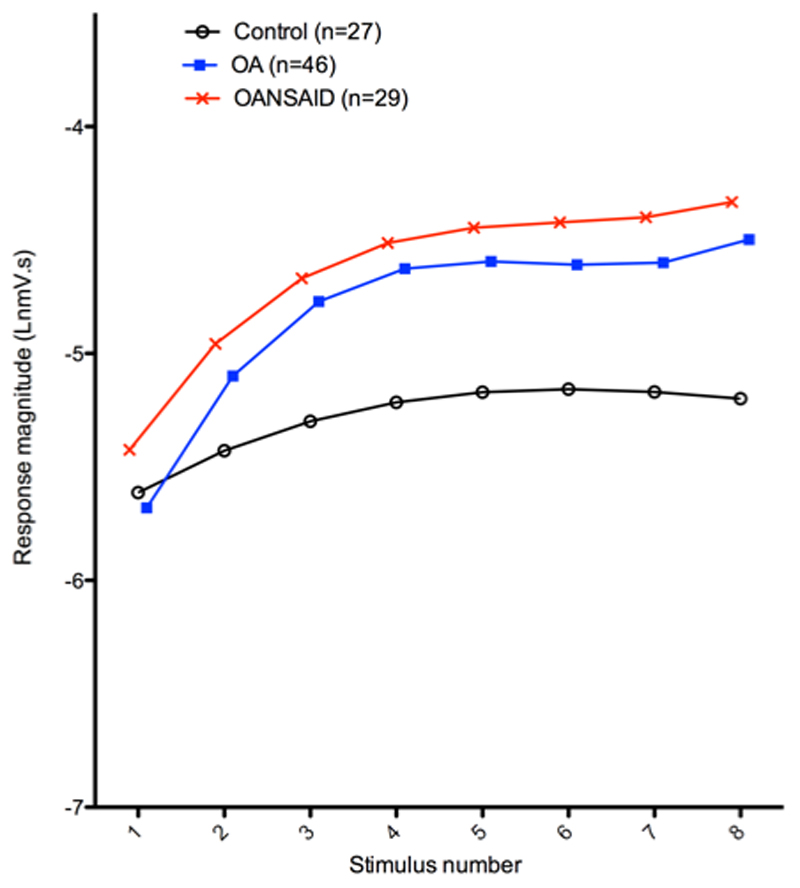
Figure 5.
Illustration of the mean curves predicted by the general linear model for stimulus response of dogs within differing OA categories, assuming a weight of 25kg. Each data point for the control animals is based on 27 dogs, for the OA group it is based on 46 dogs and for the OANSAID group it is based on 29 dogs. For each animal the mean response to the two repetitions of the stimulus response curve was averaged prior to analysis. The Y axis represents the natural logarithm of the magnitude of the EMG response and the X axis shows the magnitude of the stimulating current.
3.8. Temporal summation early (A-fibre) response (table 4)
Table 4.
Effect size estimates and p- values for the general linear model which was fitted to the temporal summation data. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| temporal summation early response (lnmV.s) | S.E. | Conf Int 2.5% | Conf Int 97.5% | p-value | Temporal summation late response (lnmV.s) | S.E. | Conf Int 2.5% | Conf Int 97.5% | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| intercept | -4.900 | 0.341 | -5.569 | -4.231 | <0.001*** | -7.142 | 0.700 | -8.513 | -5.771 | <0.001* |
| weight | -0.040 | 0.012 | -0.064 | -0.017 | 0.001** | 0.017 | 0.015 | -0.012 | 0.046 | 0.246 |
| OA | -0.722 | 0.348 | -1.404 | -0.040 | 0.038* | |||||
| OANSAID | -0.254 | 0.362 | -0.964 | 0.456 | 0.483 | |||||
| Occasion 2 | -0.022 | 0.019 | -0.059 | 0.016 | 0.265 | -0.058 | 0.026 | -0.109 | -0.007 | 0.026* |
| Occasion 3 | -0.048 | 0.019 | -0.086 | -0.010 | 0.013* | -0.120 | 0.026 | -0.171 | -0.069 | <0.001*** |
| Stimulus number | 1.084 | 0.145 | 0.800 | 1.369 | <0.001*** | 2.401 | 0.373 | 1.670 | 3.132 | <0.001*** |
| Stimulus number2 | -0.243 | 0.036 | -0.314 | -0.171 | <0.001*** | -0.474 | 0.094 | -0.658 | -0.291 | <0.001*** |
| Stimulus number3 | 0.016 | 0.003 | 0.011 | 0.021 | <0.001*** | 0.030 | 0.007 | 0.016 | 0.043 | <0.001*** |
| weight.Stimulus number | -0.014 | 0.005 | -0.024 | -0.003 | 0.009** | -0.037 | 0.008 | -0.053 | -0.022 | <0.001*** |
| weight.Stimulus number2 | 0.004 | 0.001 | 0.001 | 0.006 | 0.006** | 0.008 | 0.002 | 0.004 | 0.012 | <0.001*** |
| weight.Stimulus number3 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009** | -0.001 | 0.000 | -0.001 | 0.000 | <0.001*** |
| OA.Stimulus number | - | - | - | 0.805 | 0.186 | 0.442 | 1.169 | <0.001*** | ||
| OANSAID.Stimulus number | - | - | - | 0.540 | 0.193 | 0.161 | 0.919 | 0.005** | ||
| OA.Stimulus number2 | - | - | - | -0.160 | 0.047 | -0.251 | -0.069 | 0.001** | ||
| OANSAID.Stimulus number2 | - | - | - | -0.100 | 0.048 | -0.195 | -0.005 | 0.039* | ||
| OA.Stimulus number^3 | - | - | - | 0.010 | 0.003 | 0.004 | 0.017 | 0.003** | ||
| OANSAID.Stimulus number^3 | - | - | - | 0.006 | 0.004 | -0.001 | 0.013 | 0.079 | ||
| age | - | - | - | 0.088 | 0.067 | -0.043 | 0.218 | 0.187 | ||
| age.Stimulus number | - | - | - | -0.121 | 0.036 | -0.190 | -0.051 | 0.001** | ||
| age.Stimulus number2 | - | - | - | 0.024 | 0.009 | 0.007 | 0.042 | 0.006** | ||
| age.Stimulus number3 | - | - | - | -0.002 | 0.001 | -0.003 | 0.000 | 0.016* | ||
The magnitude of A-fibre responses increased with increasing stimulus number from 1-8 within each repetition of the protocol (temporal summation) (p < 0.001), but was reduced on the third (final) occasion of the temporal summation (train of 8) protocol, compared with the first (p = 0.013). Higher weight animals demonstrated reduced magnitude responses to stimulation (p = 0.001), and lesser increases in magnitude of response with increasing stimulus number (weight.stimulus number interaction) (p = 0.009). OA and OANSAID animals did not differ from control.
3.9. Temporal summation late (C-fibre) response (table 4)
The temporal summation protocol consistently elicited late responses. The magnitude of the late (C-fibre) response increased with increasing stimulus number from 1-8 within each repetition of the protocol (temporal summation) (p < 0.001) but was decreased on both the second and third occasion of repeating the protocol (train of 8) compared with the first trial. Higher weight animals demonstrated lesser increases in magnitude of response with increasing stimulus number (weight.stimulus number interaction; p < 0.001), and older animals also demonstrated lesser increases in magnitude of response with increasing stimulus number (age.stimulus number interaction; p = 0.001). Both OA (OA.stimulus number interaction; p < 0.001) and OANSAID (OANSAID.stimulus number interaction; p = 0.005) category animals demonstrated larger increases in magnitude of response with increasing stimulus number compared with control animals (figure 6) but there were no differences between the OA and OANSAID groups.
Figure 6.
Illustration of the mean curves predicted by the general linear model for the first occasion temporal summation late response for dogs within differing OA categories, assuming a weight of 25kg and age of 9 years. The Y axis represents the natural logarithm of the magnitude of the EMG response and the X axis shows stimulus number.
Part ii) DNIC investigation
3.10. Demographics
Data were analysed from 12 control and 11 OA dogs (none receiving NSAIDs). The sex distribution between the groups was not different, and the distribution of breeds appeared well matched on visual inspection (table 5). OA dogs were significantly older than control dogs (table 5). Groups were not different in terms of weight; however, body condition score was higher in OA (6, 5-7) compared with control dogs (5, 4.25-5.75, p = 0.047).
Table 5.
Demographic data * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Breed | Control (n=12) | OA (n=11) | p |
|---|---|---|---|
| Labrador | 6 | 3 | - |
| Collie | 2 | 1 | - |
| Retriever | 2 | 2 | - |
| Lurcher | 2 | 1 | - |
| German Shepherd | 0 | 1 | - |
| Rottweiler | 0 | 2 | - |
| Spaniel | 0 | 1 | - |
| Sex | |||
| Male neuter | 6 | 5 | 1.0 |
| Female neuter | 6 | 6 | 1.0 |
| Weight | 23.8 (95%CI 21.6-26.1) | 31.3 (95% CI 23.2-39.4) | 0.053 |
| Age | 7.5 (95%CI 6.9-8.2) | 9.8 (95%CI 8.5-11.1) | 0.002** |
| Body condition score (0-9) | 5 (4.25-5.75) | 6 (5-7) | 0.047* |
3.11. Veterinary musculoskeletal and gait assessments
Degree of lameness, mobility impairment, OA burden and joint pain burden were all increased in OA compared with control dogs (table 6).
Table 6.
Musculoskeletal examination, owner completed metrology instrument, radiographic scoring and nociceptive withdrawal reflex (NWR) data in dogs undergoing the DNIC protocol. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Control | OA | p | |
|---|---|---|---|
| Lameness (0-10) | 0 (0-0) | 3 (3-3) | <0.001*** |
| Mobility (0-3) | 0 (0-0) | 1 (1-2) | <0.001*** |
| OA score (0-192) | 0 (0-2) | 9 (6-12) | <0.001*** |
| Joint pain score (0-48) | 0 (0-0) | 4 (2-5) | <0.001*** |
| CBPI pain (0-10) | 0 (0-0) | 1.125 (0-2.69) | 0.0085** |
| CBPI function (0-10) | 0 (0-0) | 2.375 (0-6.938) | 0.0022** |
| HCPI (0-44) | 1 (0-1.75) | 15.5 (3.5-20.5) | 0.0026** |
| ACVS stiffness (0-16) | 0 (0-0) | 5.5 (0-7) | 0.0029** |
| ACVS function (0-16) | 0 (0-0) | 4 (0-8.75) | 0.0076** |
| ACVS gait (0-20) | 0 (0-0) | 5 (2.25-11.5) | 0.0022** |
| ACVS QoL (0-12) | 0 (0-0.75) | 3 (0-6.25) | 0.0076** |
| LOAD (0-52) | 2.5 (0-3) | 15.5 (5-25) | 0.0042** |
| SNoRE | 13.5 (10.5-18.5) | 15.5 (14-25.25) | 0.21 |
| Radiographic OA score (0-70) | 2 (0.25-3) | 20 (16-28) | <0.001*** |
| Number of joints radiographically affected | 1 (0.25-2) | 5 (2-6) | <0.001*** |
| NWR threshold | 1.9 (95%CI 1.4-2.5) | 3.8 (95%CI 2.4-5.2) | 0.013* |
3.12. Owner completed clinical metrology instruments (CMI)
The CBPI, HCPI, ACVS COI, and LOAD were all rated significantly higher by owners of OA compared with control dogs (table 6) but there was no significant difference in scores for the SNoRE questionnaire.
3.13. Radiography
Significantly more radiographic signs of osteoarthritis were identified in dogs in the OA compared with control group, and significantly more of the seven joints assessed demonstrated radiographic signs of OA in OA compared with control dogs (table 6).
3.14. NWR threshold
The threshold current required to elicit a NWR was significantly higher in OA (3.8 (95% CI 2.4-5.2 mA)) compared with control dogs (1.9 (95% CI 1.4-2.5 mA), p = 0.013) (table 6).
3.15. DNIC efficacy
The 2xThr stimulation did not elicit consistent late responses, therefore only the early (0-100ms) latency response was analysed [19].
The final, significant general linear model which described the magnitude of the early response took the form of an equation, the parameter estimates of which and p- values associated with the predictor variables within the model are presented in table 7. The predictor variables and their relationship with the magnitude of the response are described below. Time and age were considered continuous scale variables. Each occasion of DNIC testing (pre, DNIC 1, DNIC 2, post) was considered a categorical variable, as was OA status (OA/control). Figure 7 shows the effect of mechanical “conditioning” stimulation of the forepaw on electrically evoked “test” EMG reflexes in the cranial tibial muscle of the contralateral hindlimb.
Table 7.
Parameter estimates, se, 95% CIs and p- values for the general linear model fitted to the stimulus response (early) data (ln(mV.s)). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
| Predictor variable | Parameter estimate | S.E. | Conf Int 2.5% | Conf Int 97.5% | p-value |
|---|---|---|---|---|---|
| Fixed Effects | |||||
| cons | -5.420132 | 0.265083 | -5.939685 | -4.90058 | <0.001*** |
| DNIC1 | -0.158861 | 0.095373 | -0.345789 | 0.028067 | 0.048* |
| DNIC2 | -0.508912 | 0.095373 | -0.695839 | -0.321984 | <0.001*** |
| Post DNIC | -0.433574 | 0.100943 | -0.631419 | -0.235729 | <0.001*** |
| Time | -0.009741 | 0.006579 | -0.022635 | 0.003153 | 0.069 |
| Time2 | -0.000054 | 0.000418 | -0.000873 | 0.000764 | 0.448 |
| Time3 | 0.000005 | 0.000007 | -0.00001 | 0.000019 | 0.265 |
| OA | -0.183357 | 0.381486 | -0.931054 | 0.564341 | 0.315 |
| OA.DNIC1 | 0.181664 | 0.127397 | -0.068029 | 0.431357 | 0.077 |
| OA.DNIC2 | 0.349945 | 0.127397 | 0.100251 | 0.599638 | 0.003** |
| OA.postDNIC | 0.271047 | 0.131377 | 0.013553 | 0.528541 | 0.020** |
| Time.DNIC1 | -0.055631 | 0.009303 | -0.073866 | -0.037397 | <0.001*** |
| Time2.DNIC1 | 0.003449 | 0.000591 | 0.002291 | 0.004607 | <0.001*** |
| Time3.DNIC1 | -0.000052 | 0.00001 | -0.000072 | -0.000032 | <0.001*** |
| Time.DNIC2 | -0.05043 | 0.009303 | -0.068664 | -0.032195 | <0.001*** |
| Time2.DNIC2 | 0.00353 | 0.000591 | 0.002372 | 0.004688 | <0.001*** |
| Time3.DNIC2 | -0.000057 | 0.00001 | -0.000077 | -0.000037 | <0.001*** |
| Time.postDNIC | 0.000054 | 0.009522 | -0.01861 | 0.018717 | 0.497 |
| Time2.postDNIC | 0.000264 | 0.000605 | -0.000922 | 0.001449 | 0.331 |
| Time3.postDNIC | -0.000006 | 0.00001 | -0.000026 | 0.000015 | 0.299 |
Figure 7.
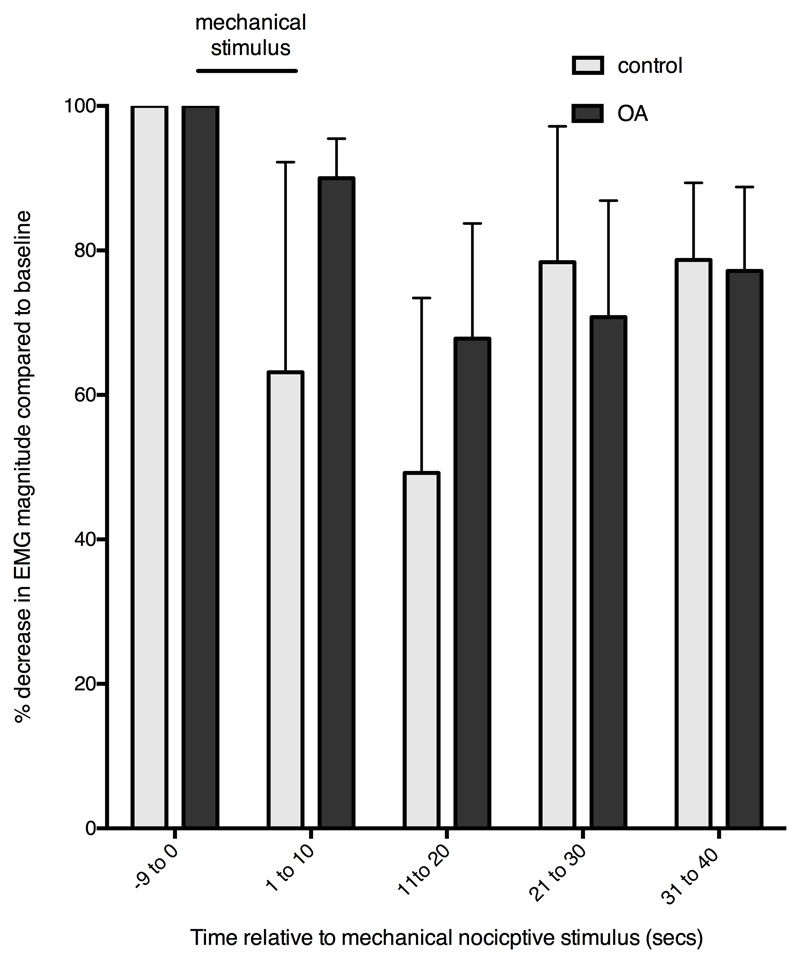
Effect of mechanical “conditioning” stimulation of the forepaw on electrically evoked “test” EMG reflexes in the cranial tibial muscle of the contralateral hindlimb. Clip was applied at time 0 for 20 secs. In the control group EMG responses to the test stimulus were reduced (greater % reduction in EMG) during clip application, indicating antinociception and a DNIC effect. When all time points were considered DNIC in the OA group (n = 11) was significantly less compared to control animals (n = 12) (P=0.016). Responses are medians, errors are 75th percentiles.
3.15.1. Stability of response magnitude within occasion
Time alone did not account for a significant variation in magnitude within a test occasion (p = 0.069).
3.15.2. Stability of response magnitude between occasions
Between different test occasions, response magnitude was decreased in DNIC 1 and 2, and in the post DNIC state, compared with the original pre DNIC occasion (p = 0.048, <0.001, and < 0.001 respectively), indicating a decreasing magnitude of response with repeated occasions of the stimulating protocol.
3.15.3. Efficacy of DNIC stimulus
There was a significant interaction between time and occasion for DNIC 1 and 2 (p <0.001), but not between time and occasion post DNIC (p = 0.50), demonstrating that the application of the conditioning stimulus was responsible for significantly decreasing the response magnitude during DNIC 1 and 2 compared to the pre-DNIC occasion. The interaction between square and cubic terms of time, and DNIC 1 and 2, were significant, indicating a curvilinear change of response with application of the conditioning stimulus.
3.15.4. Effect of OA status
OA status alone had no significant effect on response magnitude (p = 0.31); however, there was a significant interaction between OA status and occasion during the DNIC 2 (p = 0.003) and post DNIC (p = 0.02) testing, which predicted a higher magnitude of response (i.e. decreased inhibition of response) in OA dogs during these two occasions, compared with control dogs. Inclusion of the overall interaction between OA status and DNIC occasion as a predictor variable significantly improved the model (change in log likelihood = 7.82, df3; p = 0.0499).
3.15.5. Effect of age
The effect of age was tested within models but found to be not significant as either a main effect within the model, nor in interaction with other terms within the models.
4. Discussion
The present studies have shown that several characteristics of the CT NWR were altered in dogs with OA, therefore central neurophysiological changes may play a role in the pathology of OA-associated pain and disability in dogs. DNIC investigations suggest that these central changes may be related in part to less effective descending inhibition of nociceptive stimuli.
In man the RIII (Aδ-fibre mediated) threshold is correlated with the pain threshold [36], and is decreased in painful osteoarthritis states [12]. We anticipated that dogs exhibiting central sensitisation would demonstrate a diminished threshold to elicit a NWR, however our results indicated that threshold current was higher in OA animals compared with controls. The underlying reason for this finding is difficult to explain. The early latency (0-100ms) response elicited by NWR stimulation in our testing paradigm comprises both Aβ- (RII equivalent in man) and Aδ- (RIII equivalent) transmission. The RII response in man is considered non-nociceptive and elicited by sub-pain threshold intensities of stimulation. Central sensitisation may be accompanied by hypoaesthesia to one or more sensory modalities in human subjects [18], therefore it is possible that the greater threshold identified in OA dogs relates to Aβ-mediated hypoaesthesia. Whilst it may have been desirable to further divide the responses by latency into Aβ- or Aδ- mediated, as reported by Bergadano et al (2006) [6], we undertook testing in a mixed population of dogs with a range of weights and conformations, which would have added to the variability in response latency. Visual inspection of pilot data traces revealed that we could only consistently identify an early (A-fibre) and late (C-fibre) response [19]. We could have considered measuring the afferent distance of the conduction pathway in individual animals and using this, together with an estimate of conduction velocity, to calculate more accurately the latency window of the NWR in each individual dog. However our inclusion criteria for the study limited the weight range of the dogs included in the study therefore this was not deemed necessary for the present investigation.
The stimulus response curve demonstrated facilitation of the early response in OANSAID dogs, compared with both control and OA dogs. The amplitude of the RIII response has been shown to correlate with the magnitude of subjective pain in conscious human volunteers [13]; therefore the inference from our data is that OANSAID dogs may exhibit hyperalgesia, compared with dogs in both the OA and control groups. That the OA and OANSAID groups were not different based on veterinarian examination scores and radiographic OA scores was not unexpected – there are no validated veterinarian assessment systems of OA pain, and radiographic evidence of OA is known not to correlated to pain, just as in humans. Although OA and OANSAID groups were comparable with respect to the majority of the clinical metrology instrument (CMI) data, OANSAID were significantly more affected with respect to the CBPI pain and the ACVS description of function subscales, and had higher scores on all the other validated CMIs (LOAD, CBPI function). These data indicate the OANSAID group were more severely affected by OA pain, and suggest that treatment with commonly prescribed veterinary NSAIDs [20] may not prevent or reverse central sensitisation, despite the tentative conclusion from a recent study in humans with OA investigating etoricoxib [1]. The total duration of treatment with NSAIDs in the OANSAID group was not recorded in individual dogs in this study and it is possible that differences in the duration of administration introduced variability into the data. However, all dogs in the OANSAID group had been receiving NSAIDs for at least 3 months prior to recruitment to the study which, from early data in humans [1] would be sufficient time for the NSAID to exhibit an anti-hyperalgesic effect.
Temporal summation data demonstrated no group differences for the early (A-fibre mediated) response, but facilitation of the late (mostly C-fibre) response in OA and OANSAID dogs, compared with controls. The absence of an effect on the early response data is likely due to a significant component being mediated by low threshold A beta fibres. The applied 10mA stimulus, designed as a suprathreshold stimulus, would cause the early response to saturate at this level of stimulation, and therefore differences between groups were minimised. In contrast the higher threshold C-fibre mediated late response displayed the expected increasing magnitude with repeated stimuli and, in alignment with our hypothesis, was augmented in both OA and OANSAID groups compared with the control group. This likely indicates that OA is associated with central sensitisation in dogs. It is also possible that the EMG findings for C fibre mediated responses are due to C fiber sensitisation rather than central sensitisation although it is difficult to make a distinction between these two effects in our data set.
The data produced during the DNIC investigation demonstrate both that MCS elicits quantifiable DNIC in anaesthetised dogs, and that the efficacy of DNIC is compromised in dogs with OA, compared with a control group. A recent meta-analysis concluded that, despite methodological limitations, a number of chronic pain conditions in man, including osteoarthritis, are associated with reduced efficacy of CPM [28]. Reduced net efficacy of nociceptive inhibition may arise through impaired descending anti-nociceptive modulation, or via descending facilitation of nociceptive signalling [3]. We did not probe each of these pathways independently in these clinical cases; however, the magnitude of measured EMG response in this study represents the net effect of balance between inhibitory and facilitatory mechanisms, therefore these data provide evidence that the balance of descending pathways becomes shifted toward pro-nociception in canine OA.
The differences between OA and control groups were only evident on DNIC 2, and then persisted into the post DNIC period. Because previous data on DNIC in dogs using MCS were not available, numbers required to identify significant differences were unknown, however it is clear from our results that the interaction between group and occasion begins to approach significance during DNIC 1 (p = 0.07). Had larger sample sizes been employed we would have had greater power to detect differences between groups, and may have identified a significant difference during DNIC 1. The small sample size is a major limitation of the DNIC investigation and reflected difficulties in establishing the methodology to elicit DNIC in dogs. Only five minutes was allowed to elapse between the temporal summation protocol and the start of the DNIC investigation. This time period was kept deliberately short to avoid prolonging the anaesthesia time for the dogs as far as possible. It is possible that delivery of a supramaximal stimulus during the temporal summation protocol sensitised the nociceptive system so that the nociceptive pathways were not in a naïve state at the start of the DNIC experiment and this may have affected our DNIC results. The optimal time delay between temporal summation and measurement of DNIC is currently unknown.
NWRs are segmental spinal reflexes, subject to supraspinal modulation [11]. Alfaxalone anaesthesia enabled NWR recording in client owned dogs. Whilst alfaxalone increases NWR threshold and decreases magnitude of response to electrical stimulation [19] there is no reason to expect a differential effect of the anaesthetic on control versus OA or OANSAID animals, as alfaxalone is devoid of analgesic activity [40].
With regards to assessment of DNIC, many sedatives and analgesics will interact with descending pro- and anti-nociceptive pathways [27,37] and could alter the measured responses. Acepromazine has been shown not to modulate NWR [7] and, given it is considered to have no anti-nociceptive properties [4], would not be expected to interact with descending modulatory mechanisms. Alfaxalone is a gamma amino butyric acid (GABA) agonist, and DNIC is reportedly unaffected by GABA agonists [23], therefore we consider that the form of anaesthesia employed was appropriate to our investigation.
Although we have identified group level differences in DNIC efficacy, the aim is ultimately to identify individuals in which decreased DNIC efficacy contributes to the pain phenotype, and address this mechanism therapeutically [3]. Determining a normal ‘range’ of DNIC responses in dogs will require study of additional numbers of dogs of a wider demographic, particularly in view of the inconsistently reported gender [33] and age [25] differences associated with CPM in man.
In conclusion, we have demonstrated a number of neurophysiological changes indicative of central sensitisation processes in dogs affected by spontaneous osteoarthritis, consistent with findings in man. However, measurement of electrical thresholds appeared not to be a suitable parameter for central sensitisation using the current methods. The mechanisms involved may encompass both upregulation of nociceptive afferent pathways [26], in addition to alterations in the balance of descending modulatory mechanisms as shown here. Increasingly it appears that the pathophysiological mechanisms of human OA [21] are shared by the spontaneous disease in dogs, further validating canine spontaneous OA as a model for the human disease [32,39] and supporting the use of dogs for mechanistic clinical trials to advance therapeutic development in humans.
Supplementary Material
Summary.
Spinal nociceptive transmission, and descending modulation, were studied in dogs with spontaneous osteoarthritis. Osteoarthritis was associated with augmented reflexes and reduced descending inhibition, suggesting central sensitisation.
Acknowledgements
We are grateful to Lindsey Crane and Liz Carthey, both of the Small Animal Hospital, University of Bristol, for undertaking the radiography procedures.
This study was supported by the BBSRC grant number BB/L00240X/1. The alfaxalone was supplied by Jurox (UK) Limited. JRH, HJ, and MG positions were funded by the BBSRC grant number BB/L00240X/1.
Footnotes
The authors declare no conflict of interest.
References
- [1].Arendt-Nielsen L, Egsgaard LL, Petersen KK. Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain. 2016;157:1634–1644. doi: 10.1097/j.pain.0000000000000562. [DOI] [PubMed] [Google Scholar]
- [2].Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [3].Bannister K, Patel R, Goncalves L, Townson L, Dickenson AH. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain. 2015;156:1803–1811. doi: 10.1097/j.pain.0000000000000240. [DOI] [PubMed] [Google Scholar]
- [4].Barnhart MD, Hubbell JAE, Muir WW. Evaluation of the analgesic properties of acepromazine maleate, oxymorphone, medetomidine and a combination of acepromazine-oxymorphone. Vet Anaes Analg. 2000;27:89–96. doi: 10.1046/j.1467-2995.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- [5].Bars DL, Dickenson AH, Besson J-M. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- [6].Bergadano A, Andersen OK, Arendt-Nielsen L, Schatzmann U, Spadavecchia C. Quantitative assessment of nociceptive processes in conscious dogs by use of the nociceptive withdrawal reflex. Am J Vet Res. 2006;67:882–889. doi: 10.2460/ajvr.67.5.882. [DOI] [PubMed] [Google Scholar]
- [7].Bergadano A, Andersen OK, Arendt-Nielsen L, Spadavecchia C. Modulation of nociceptive withdrawal reflexes evoked by single and repeated nociceptive stimuli in conscious dogs by low-dose acepromazine. Vet Anaes Analg. 2009;36:261–272. doi: 10.1111/j.1467-2995.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- [8].Bergadano A, Andersen OK, Arendt-Nielsen L, Spadavecchia C. Noninvasive assessment of the facilitation of the nociceptive withdrawal reflex by repeated electrical stimulations in conscious dogs. Am J Vet Res. 2007;68:899–907. doi: 10.2460/ajvr.68.8.899. [DOI] [PubMed] [Google Scholar]
- [9].Brown DC. The Canine Orthopedic Index. Step 3: Responsiveness Testing. Vet Surg. 2014;43:247–254. doi: 10.1111/j.1532-950X.2014.12162.x. [DOI] [PubMed] [Google Scholar]
- [10].Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res. 2007;68:631–637. doi: 10.2460/ajvr.68.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clarke RW, Harris J. The organization of motor responses to noxious stimuli. Brain Research Reviews. 2004;46:163–172. doi: 10.1016/j.brainresrev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [12].Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J Pain. 2009;10:1242–1249. doi: 10.1016/j.jpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [13].Dowman R. Spinal and supraspinal correlates of nociception in man. Pain. 1991;45:269–281. doi: 10.1016/0304-3959(91)90051-X. [DOI] [PubMed] [Google Scholar]
- [14].Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M, Drewes AM. Conditioned Pain Modulation and Situational Pain Catastrophizing as Preoperative Predictors of Pain following Chest Wall Surgery: A Prospective Observational Cohort Study. PLoS ONE. 2014;9:e90185. doi: 10.1371/journal.pone.0090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harris J. Involvement of spinal α2 -adrenoceptors in prolonged modulation of hind limb withdrawal reflexes following acute noxious stimulation in the anaesthetised rabbit. Eur J Neurosci. 2016 doi: 10.1111/ejn.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harris LK. Mechanical nociceptive testing in dogs with osteoarthritis. PhD Thesis University of Bristol; 2016. [Google Scholar]
- [17].Hielm-Bjorkman AK, Rita H, Tulamo R-M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res. 2009;70:727–734. doi: 10.2460/ajvr.70.6.727. [DOI] [PubMed] [Google Scholar]
- [18].Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthr Cartil. 2013;21:1236–1242. doi: 10.1016/j.joca.2013.06.023. [DOI] [PubMed] [Google Scholar]
- [19].Hunt J, Murrell J, Knazovicky D, Harris J, Kelly S, Knowles TG, Lascelles BDX. Alfaxalone Anaesthesia Facilitates Electrophysiological Recordings of Nociceptive Withdrawal Reflexes in Dogs (Canis familiaris) PLoS ONE. 2016;11:e0158990. doi: 10.1371/journal.pone.0158990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hunt JR, Dean RS, Davis GND, Murrell JC. An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Vet J. 2015;206:183–190. doi: 10.1016/j.tvjl.2015.07.025. [DOI] [PubMed] [Google Scholar]
- [21].Knazovicky D, Helgeson ES, Case B, Gruen ME, Maixner W, Lascelles BDX. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain. 2016;157:1325–1332. doi: 10.1097/j.pain.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Knazovicky D, Tomas A, Motsinger-Reif A, Lascelles BDX. Initial evaluation of nighttime restlessness in a naturally occurring canine model of osteoarthritis pain. PeerJ. 2015;3:e772. doi: 10.7717/peerj.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kunz M, Scholl KE, Schu U, Lautenbacher S. GABAergic modulation of diffuse noxious inhibitory controls (DNIC): a test by use of lorazepam. Exp Brain Res. 2006;175:363–371. doi: 10.1007/s00221-006-0558-8. [DOI] [PubMed] [Google Scholar]
- [24].Laflamme D. Development and validation of a body condition score system for dogs. Canine practice (Santa Barbara) 1997 [Google Scholar]
- [25].Larivi re M, Goffaux P, Marchand S, Julien N. Changes in Pain Perception and Descending Inhibitory Controls Start at Middle Age in Healthy Adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- [26].Lascelles BDX, Gaynor JS, Smith ES, Roe SC, Marcellin-Little DJ, Davidson G, Boland E, Carr J. Amantadine in a Multimodal Analgesic Regimen for Alleviation of Refractory Osteoarthritis Pain in Dogs. Journal of Veterinary Internal Medicine. 2008;22:53–59. doi: 10.1111/j.1939-1676.2007.0014.x. [DOI] [PubMed] [Google Scholar]
- [27].Lervik A, Haga HA, Ranheim B, Spadavecchia C. The influence of a continuous rate infusion of dexmedetomidine on the nociceptive withdrawal reflex and temporal summation during isoflurane anaesthesia in dogs. Vet Anaes Analg. 2012;39:414–425. doi: 10.1111/j.1467-2995.2012.00713.x. [DOI] [PubMed] [Google Scholar]
- [28].Lewis GN, Rice DA, McNair PJ. Conditioned Pain Modulation in Populations With Chronic Pain: A Systematic Review and Meta-Analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [29].Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. 2014;18:1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- [30].Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nir R-R, Yarnitsky D, Honigman L, Granot M. Cognitive manipulation targeted at decreasing the conditioning pain perception reduces the efficacy of conditioned pain modulation. Pain. 2012;153:170–176. doi: 10.1016/j.pain.2011.10.010. [DOI] [PubMed] [Google Scholar]
- [32].Percie du Sert N, Rice ASC. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171:2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: A systematic review. Pain. 2010 doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- [34].Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [35].Rasbash J, Browne WJ, Healy M, Cameron B. MLwiN Version 3.00. Centre for Multilevel Modelling, University of Bristol; 2017. [Google Scholar]
- [36].Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 2007;128:244–253. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roeckel L-A, Le Coz G-M, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience. 2016;338:160–182. doi: 10.1016/j.neuroscience.2016.06.029. [DOI] [PubMed] [Google Scholar]
- [38].Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [39].Vainio O. Translational animal models using veterinary patients – An example of canine osteoarthritis (OA) Scand J Pain. 2012;3:84–89. doi: 10.1016/j.sjpain.2011.11.007. [DOI] [PubMed] [Google Scholar]
- [40].Winter L, Nadeson R, Tucker AP. Antinociceptive properties of neurosteroids: a comparison of alphadolone and alphaxalone in potentiation of opioid antinociception. Anesth Analg. 2003;97:798–805. doi: 10.1213/01.ANE.0000075835.73967.F3. [DOI] [PubMed] [Google Scholar]
- [41].Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology (Oxford) 2012;51:535–543. doi: 10.1093/rheumatology/ker343. [DOI] [PubMed] [Google Scholar]
- [42].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.