Abstract
Background:
A perennial challenge in systemic cytotoxic cancer therapy is to eradicate primary tumors and metastatic disease while sparing normal tissue from off-target effects of chemotherapy. Anthracyclines such as doxorubicin are effective chemotherapeutic agents for which dosing is limited by development of cardiotoxicity. Our published evidence shows that targeting CD47 enhances radiation-induced growth delay of tumors while remarkably protecting soft tissues. The protection of cell viability observed with CD47 is mediated autonomously by activation of protective autophagy. However, whether CD47 protects cancer cells from cytotoxic chemotherapy is unknown.
Methods:
We tested the effect of CD47 blockade on cancer cell survival using a 2-dimensional high-throughput cell proliferation assay in 4T1 breast cancer cell lines. To evaluate blockade of CD47 in combination with chemotherapy in vivo we employed the 4T1 breast cancer model and examined tumor and cardiac tissue viability as well as autophagic flux.
Results:
Our high-throughput screen revealed that blockade of CD47 does not interfere with the cytotoxic activity of anthracyclines against 4T1 breast cancer cells. Targeting CD47 enhanced the effect of doxorubicin chemotherapy in vivo by reducing tumor growth and metastatic spread by activation of an anti-tumor innate immune response. Moreover, systemic suppression of CD47 protected cardiac tissue viability and function in mice treated with doxorubicin.
Conclusions:
Our experiments indicate that the protective effects observed with CD47 blockade are mediated through upregulation of autophagic flux. However, absence of CD47 in did not elicit a protective effect but it enhanced macrophage-mediated cancer cell cytolysis. Therefore, the differential responses observed with CD47 blockade are due to autonomous activation of protective autophagy in normal tissue and enhancement immune cytotoxicity against cancer cells.
Keywords: CD47, Autophagy, Cytoprotection, Breast Cancer, Cardio-oncology
Background
Due to advances in early cancer diagnosis and improvements in anti-neoplastic drugs, 20 million cancer survivors are expected by the year 2024 in the United States alone [1]. This is a positive milestone, but that success comes with serious adverse side effects causing secondary pathologies and limiting patient quality of life after anti-cancer therapy. Therefore, we need new drugs and combinatorial strategies that decrease or prevent off target effects but do not interfere with the anti-cancer effects of cytotoxic therapy. Breast cancer patients, particularly those diagnosed with triple-negative breast cancer (TNBC), which respond only to systemic cytotoxic chemotherapy, are susceptible to off-target effects of chemotherapeutic agents including anthracycline-based drugs. We previously demonstrated that blockade of CD47 sensitizes tumors to death associated with local ionizing radiation, but protects bone marrow and soft tissues [2, 3]. A hepatocellular carcinoma xenograft model suggested similar additive effects of CD47 blockade with doxorubicin chemotherapy [4] and another study found that doxorubicin treatment increases CD47 expression in TNBC [5].
CD47 is a widely expressed receptor that controls cell fate via two major functions: (1) when it interacts with SIRPα on phagocytic cells, a “don’t eat me” signal results that limits phagocytic activity. (2) On binding to TSP1, CD47 transduces signals that alter cellular calcium, cyclic nucleotide, integrin, growth factor signaling and control cell, viability and resistance to stress [6]. This latter function is fundamental to understanding why targeting CD47 could provide therapeutic benefits in disease such as cardiovascular diseases and cancer. This duality in survival effects makes CD47 an ideal target for cancer therapy. However, the mechanisms resulting in the selective protection of nonmalignant tissues are not completely understood. Deficiency of CD47 protects T lymphocytes and primary endothelial cells from ionizing radiation through the activation of protective autophagy[3, 7]. Our group and others have attributed the anti-tumor effects of CD47 to enhancement of innate and adaptive immune system actions against the tumor [8],[9]. However, potential cell-autonomous differences in the cytoprotective effects of CD47 blockade in normal versus cancer cells has not been examined, and is a critical question to address as agents targeting CD47 progress in human clinical trials.
We implemented a fully automated high throughput screen using a 2-dimensional cell proliferation assay, where 1912 approved or experimental drugs were tested against 4T1 breast cancer cells with or without CD47 antisense morpholino treatment. The objective was to determine that blocking CD47 would not interfere with the oncologic efficacy of cytotoxic cancer therapy. Our data shows that compounds in the anthracycline family fit this paradigm. Anthracyclines are used to treat several cancer types and are the standard approach for adjuvant treatment of breast cancer because they result in improved outcomes compared to other modalities [10–13]. However, high cumulative doses of anthracyclines increase the risk of congestive heart failure by up to 26% [1]. Furthermore, although initial damage is acute, symptomatic manifestations occur long after chemotherapy.
In this study we report that the cytoprotective effect of blocking CD47 extends to cardiac tissue. Our data shows that blockade of CD47 in combination with anthracycline treatment reduced primary 4T1 tumor growth and prevented metastatic spread to the lungs. Moreover, cardiac tissue of mice administered with anthracycline alone showed increased cell death. However, heart tissue from mice administered the combination treatment showed protection from anthracycline mediated death. Our data suggest that the protective effect of CD47 are due to an increase in autophagy whereas the anti-tumor effects are associated to stimulation of macrophage mediated cancer cell cytotoxicity. As shown previously, CD47 blockade increased protective autophagy hearts deficient in CD47 treated with anthracycline just as we had observed before in our previous studies in T cells [7]. The sensitization of tumors to anthracycline therapy was associated with increases in danger associated proteins HMBG1 and Calreticulin. Overall, our data shows that CD47 generally protects against chemotherapymediated cytotoxicity in normal cells, but this protective effect is lost in cancer cells and thus not compromising the oncologic efficacy of cytotoxic chemotherapy.
Methods:
Cell Culture:
4T1 mouse breast cancer cells were a gift from Dr. Patricia Steeg [National Cancer Institute (NCI, National Institutes of Health (NIH) Bethesda, Maryland) H9C2 rat cardiac myoblast cells and Raw 264.7 murine macrophage cell lines were obtained from ATCC (CRL1446, TIB-71) respectively. All cell lines were cultured in DMEM supplemented with 10% FBS penicillin/streptomycin, and glutamine kept at 37˚C and 5% CO2.
Quantitative High-Throughput Screen (qHTS).
4T1 breast cancer cells, were seeded in 1536-well plates at 500 cells per well, in 50 μl of medium. Anti-sense CD47 morpholino was administered at a dose of 10 μM and was incubated in the flask for 48 h prior to the screen. Compounds were added at multiple doses ranging from .8 nM to 46 μM. Cell viability was assessed after 48 hrs incubation at 37°C by adding 30 μl of CellTiter-Glo reagent (Promega) and measuring luminescence (RLU) after a 15 m incubation at 25 °C, with ViewLux (PerkinElmer) [14].
qHTS data analysis.
Activity of the hits from the qHTS screen was analyzed as shown previously[15] [14]. Normalized data were Normalized fitted to 4-parameter concentration response curves (CRC) using a grid-based algorithm. Each CRC was then assigned a heuristic curve response class (CRC). Curve classes of −1.1, −1.2, −2.1 and −2.2 are considered highest quality hits; Classes of −1.3, −1.4, −2.3, −2.4 and −3 are inconclusive hits; and curve class values of 4 are inactive compounds. Additional parameters obtained from the qHTS fitting procedure and used for hit selection were the Maximum Response, which is the % activity at the maximum concentration of compound tested (46 μM) and the AC50, which is obtained from the curve fit. Differential activity of each compound between the wild type and knock-down CD47 conditions in each cell line was determined by calculating a difference in the maximum response or logAC50 for each compound, between the two CD47 conditions, in each cell.
In vivo 4T1 orthotopic breast cancer model:
Female Balb/c mice were injected with 4T1 cells (1 ×105 cells) into the mammary fat pad to induce tumor growth using a 26 gauge needle. Once tumors reached an average of 100 mm3 mice (n=8) were randomized, and one group received weekly IP injections of antisense CD47 morpholino (CD47 M) at a volume of 500μL. Groups of control or morpholino-treated mice received 100 μL saline or doxorubicin (DOX) intravenously 48 h after each CD47 M injection. Tumor size was measured every third day using a caliper, and wet weight of the tumors was determined at the end of the study.
LC3 Staining:
H9C2 cardiac myoblast cells were transfected with a GFP-LC3 construct as shown previously [16] and treated 24 h after transfection. Images were sequentially acquired with Zeiss AIM software on a Zeiss LSM 510 confocal system and quantified blindly using Zen 2.3 sp1 software.
Histology:
Tissue arrays from developed from tissues of human breast cancer patient sections analyzed in the Laboratory of Pathology National Cancer Institute, under approved protocol by the Institutional Review Board of the National Cancer Institute were immunostained as shown previously[9] using 1:250 B6H12 antibody. Immunoreactivity was examined through light microscopy. Mouse lungs were harvested at the end of the study and subjected to Hematoxylin and Eosin stain to visualize tissue structure. Tumor lesions were quantified blindly by a pathologist under light microscopy.
Cytotoxicity Assays:
4T1 cells and H9C2 cells were plated at a density of 10,000 per well in a 96-well plate. 4T1 and H9C2 were transfected using 1 μM Endoporter and 10 μM CD47 morpholino. Twenty-four hours after transfection and plating, cells were treated with 1 μg/mL DOX and were incubated for 48 h. Cytotoxicity was measured by LDH release detected using a colorimetric activity assay (Invitrogen) and spectrophotometer (Benchmark plus microplate spectrophotometer, Bio-Rad).
Real-Time Measurement of Macrophage Mediated Cytotoxicity.
4T1 breast cancer target cells were seeded into 16-well plates. Cell growth was dynamically monitored using xCelligence system (ACEA Biosciences) for 24 h. RAW 264.7 macrophages cells were activated for 24h at 37°C with 10 ng/ml of LPS in complete medium and also used at an effector/target ration ratio of 5:1. After addition of effector cells, measurements were automatically collected by the analyzer. Cell mediated cytolysis was calculated using xCELLigence software set to collect impendence data (reported as cell index).
Statistical Analysis:
Data are presented as the mean ± standard error of the mean (SEM). Statistical differences were evaluated by Student’s t test or one way analysis of variance (ANOVA) followed by Tukey post hoc test. Animal studies were evaluated by repeated measures ANOVA followed by Tukey post hoc test. The criterion for statistical significance was set at p < 0.05.
Results:
Screening of anti-sense CD47 morpholino activity in combination with cytotoxic drugs.
High CD47 expression is associated with decreased survival in many cancers including breast[17]. Analysis of TCGA breast cancers classified based on RNAseq data using the established PAM50 panel of 50 genes[18, 19] shows that CD47 mRNA is elevated in basal-like breast cancers relative to HER2 and luminal A and B subtypes (Figure 1A). Our examination of human breast cancer tissue sections shows that CD47 expression is elevated in cancerous lesions of patients diagnosed with invasive breast cancer when compared to normal breast tissue (Supplemental figure 1A).
Figure 1. CD47 does not interfere with oncologic efficacy of anthracyclines.
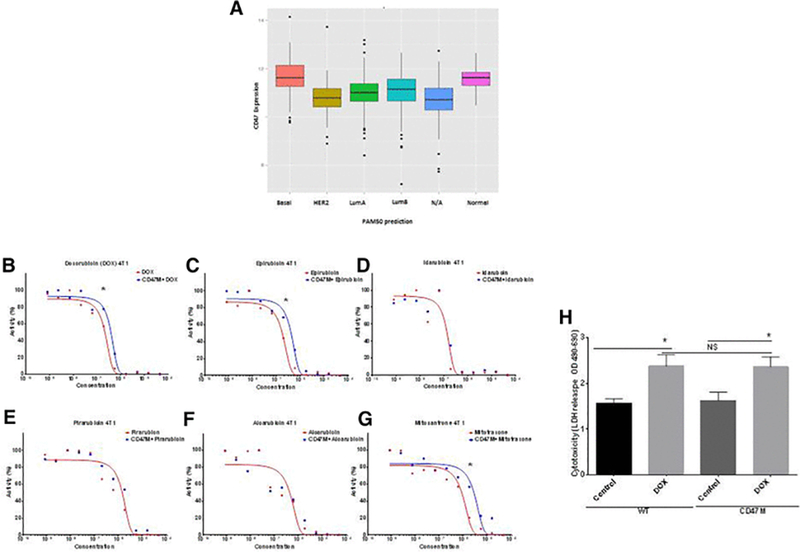
(A) CD47 mRNA expression in TCGA breast cancers classified based on expression of the PAM50 panel of genes (B-G) High throughput screen measuring dose-responses for anthracycline effects on cell viability with or without CD47 blockade in 4T1 breast cancer cells. Twenty four hours after plating cells were subjected to automated robotic high throughput screen using by CellTiter-Glo® as endpoint analysis. Curve regression analysis of data for anthracycline family compounds fitting concentration-response curves as (log(inhibitor) vs. normalized response) (*p<0.01). (H) Measurement of DOX cytotoxicity in 4T1 breast cancer cells by LDH release (*p<0.05)
We have previously shown that blockade of CD47 enhances radiation tumor growth delay while protecting normal tissue from death from ionizing radiation[9, 20]. Thus we wanted to know if CD47 blockade would also protect breast cancer cells from death associated with cytotoxic chemotherapy treatment and whether this additive activity extends to syngeneic tumors. Using a cell proliferation assay in a 1536-well microplate high throughput screening format, we screened a comprehensive library of approved and late stage development oncology drugs in combination with a CD47 antisense morpholino to find combinatorial strategies that are more cytotoxic against breast cancer cells. We used the 4T1 breast cancer cell line to identify whether blockade of CD47 would protect cancer cells from cytotoxic chemotherapy. The endpoint for the high throughput screen was cell viability assessed by measurement of cellular ATP using
CellTiter Glo reagent. Compounds were tested at eleven doses ranging from 0.8 nM to 46 μM. Our data showed blocking CD47 decreased the anti-growth activity of doxorubicin (DOX) chemotherapy less than 2-fold (Figure 1B). Moreover, as observed in Figures 1 B-G, blockade of CD47 generally did not result in significant protection of breast cancer cells from cytotoxicity mediated by treatment with other members of the anthracycline family of drugs. In the 4T1 screen 3 out of 6 drug treatments showed a modest increase in IC50 with CD47 blockade a (Figures 1B, 1C and 1G, Table I). Treatment with DOX showed a 1.4 increase in IC50 (Figure 1B *p<0.01), epirubicin a 2.0 fold IC50 increase (Figure 1C, *p<0.01) and Mitotraxone a 3.0 fold increase IC50 increase (Figure 1G, *p<0.01). However t-test analysis of the Hill Slope value was not significant (differences between means, 963720 ± 1.692e+006, p= 0.5995).
Table I:
LoglC50 of WT and CD47 (−) treated with anthracyclines
| 4T1 Breast Cancer Calls Screen Logic50±4Std Error | ||
|---|---|---|
| Drug | WT (Log IC50) | CD47 (·) LogIC50) |
| Doxonabicin | 258±025 × 1−7 | 3 89±0 37 × 10−7 |
| Efwubicin | 1 69±0 18× 1−7 | 3 98±043 × 10−7 |
| Pirarubicin | 1 02±0 19 × 10−6 | 1 74±0 25 × 10−6 |
| Alcarubicin | 3 83±1 03 × 10−7 | 4 35±1 33 × 10−7 |
| idarubicin | 1 I8±0 24 × 10−7 | 1 69±0 21 × 10−7 |
| Mftotraxone | 8 14±0 17 × 10−7 | 2 62±0 56 × 10−6 |
To corroborate that blockade of CD47 would not protect cells from anthracycline cytotoxicity we examined cell cytotoxicity by measuring LDH release (Figure 1H) from 4T1 cells treated with DOX in the presence or absence of anti-sense morpholino. DOX treatment increased breast cancer cell cytotoxicity in the presence or absence of CD47 blockade (*p<0.05). A similar result was obtained when treating MDA-MB-231 cells (Supplemental Figure 1B). Thus, our data indicates that blockade of CD47 does not directly limit the oncologic efficacy of anthracyclines.
CD47 is over expressed in human invasive breast cancer, and its blockade enhances anthracycline mediated tumor cytotoxicity in a murine model of breast cancer.
We chose DOX as the lead compound for our studies since it is widely used in the clinic particularly in patients presenting invasive breast cancer and triple negative breast cancer patients[21]. Moreover, clinical use of anthracyclines is limited due to its off target cytotoxic effects particularly in the cardiovascular system. We implanted 1×105 4T1 breast cancer cells in the fat pads of Balb/c mice and treated them with saline, DOX, CD47 morpholino (CD47M) or CD47M with DOX. Treatment with CD47M was done two days prior to each treatment with DOX and was repeated weekly for 30 days (Figure 2A). As observed in Figure 2B and 2C, tumors of mice treated with saline tripled in size. DOX treatment caused the expected reduction in tumor volume and weight, but the combination of CD47 blockade and DOX further reduced tumor mass by 50% (Figure 2B). We confirmed knockdown of CD47 with antisense morpholino by western blot using the CD47 antibody miap301 (Supplemental Figure 1C). Therefore, blockade of CD47 enhances the effects of DOX to reduce breast tumor growth in a syngeneic tumor model, indicating that CD47 potentiates anthracycline-mediated breast tumor therapy. The 4T1 model of breast cancer is known to develop lung metastasis after cells are injected orthotopically in the host mouse [22]. At the end of the study we performed histopathological analysis of lung sections and observed that lungs of animals administered the combination treatment had a significant over 70% (*p<0.01) reduction of metastatic lesions when compared to lungs of animals treated with saline (Figure 2D & 2E, 4.2 ± .75 saline vs. 1.125 ± 0.6391 CD47M+ Dox n=8). DOX alone caused over 50% reduction in metastasis when compared with saline alone (4.2 ± .75 saline vs 2.000 ± 0.4629 doxorubicin alone, n=8). CD47M monotherapy reduced metastasis by 46% when compared to saline alone. Notably only 25% of mice treated with the combination treatment developed metastatic lesions in the lungs when compared to 100% of mice treated with saline and 75% of both CD47M and DOX monotherapies (Figure 2E). This indicates that combination treatment of CD47M and DOX reduces metastatic spread, which suggests using this approach could improve clinical outcomes of breast cancer patients.
Figure 2. Blockade of CD47 enhances anti-tumor effects of DOX chemotherapy in vivo.
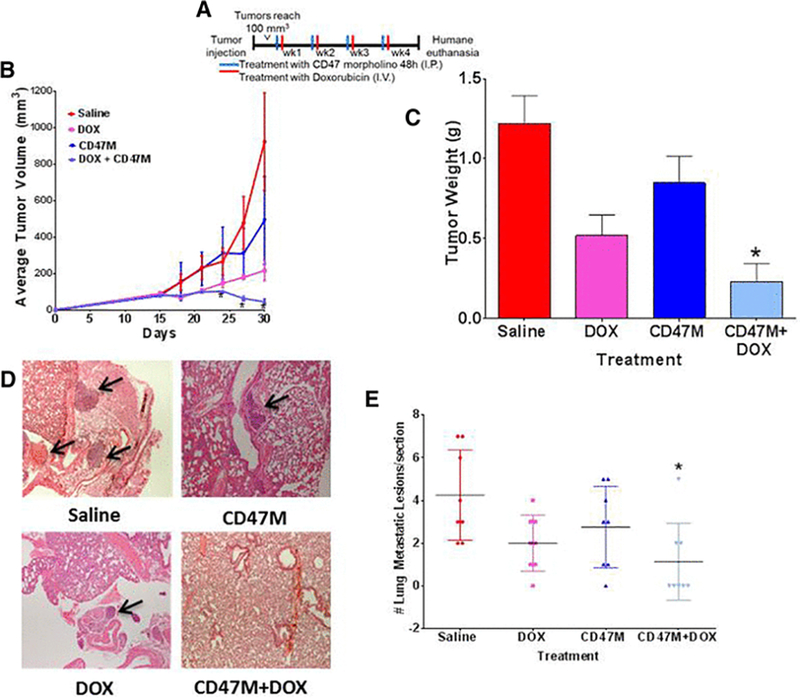
(A) Schedule of treatment. (B) Balb/c mice were injected in the mammary fat pad with 4T1 mouse breast cancer cells. Tumor volume was measured with a caliper every three days and was calculated by the formula: volume = W2 × L/2, where W = shortest diameter and L = longest diameter (n=8, *p<0.001 repeated measures ANOVA, followed by Tukeys multiple comparison test. (C) Tumors were excised, and weights were recorded at the end of the study. (D, E). After 30 days animals were euthanized, and lungs were excised. Tissue sections were paraffin embedded and stained with Hematoxylin and Eosin to determine tissue structure. Tumor lesions were counted by the pathologist under bright field microscopy N=8, *p<0.05.
Blockade of CD47 enhances macrophage mediated cytotoxicity of breast cancer cell.
The increased efficacy of combining DOX with CD47M in vivo contrasts with the weak cell-autonomous effects observed in vitro and suggested that a non-cell autonomous immune mechanism is involved. Since blockade of CD47 enhances macrophage-mediated cancer cell killing, we tested whether pre-treatment with DOX modulated macrophage cytolytic capacity. We plated 4T1 breast cancer cells in 16-well plates and performed dynamic monitoring of RAW 264.7 mouse macrophage-mediated cytotoxicity of 4T1 breast cancer cells by measuring cell impedance using the ACEA xCelligence system (Figure 3A). The ACEA system measures killing of adherent target tumor cells by a decrease in surface impedance, and is presented as a normalized cell index. Cell index is derived from the measured electrical impedance and is a quantitative measure of the number of viable adherent target cells in each well[23]. Therefore, a decreased cell index indicates loss of viable target tumor cells. This is an established quantitative real-time method for effector cell mediated cytotoxicity against adherent target cells[9, 23]. We pretreated 4T1 breast cancer cells with anti-CD47 morpholino for 24h and DOX for 4h the following day then proceeded to remove the media and washed twice with new media and plated a suspension of macrophages at a 1:5 effector target ratio. As observed in figure 3A DOX pre-treatment reduced cell viability as expected from control (Figures 3B-C). Pre-treatment with DOX enhanced macrophage mediated cytotoxicity when compared to effector target pairs alone. Blockade of CD47 enhanced macrophage mediated killing over untreated effector target pairs around 15h (Figure 3B) but no difference was observed at 24h (Figure 3C). However, combination of CD47 blockade and DOX pre-treatment significantly enhanced macrophage mediated killing of cancer cells by reducing cell viability 5-fold (*p<0.001).
Figure 3. Blockade of CD47 enhances macrophage mediated clearance of breast cancer cells.
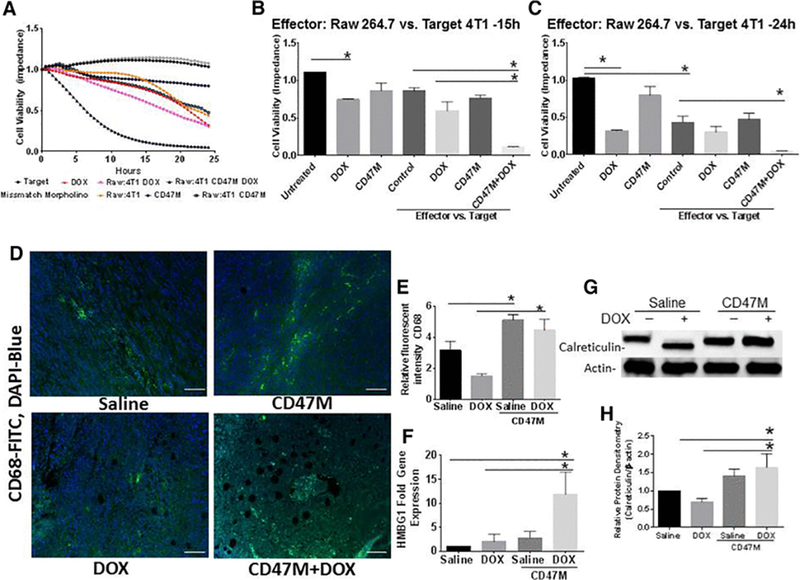
(A) Macrophage mediated cytotoxicity was measured in 16-well plates equipped with electrodes to measure cell impedance, cell cytotoxicity was counted as changes in cells index (impedance) when cells migrated to bottom plate equipped with electrodes N=4 *p<0.05 (B ) cell viability at 15h and (C) 24 h post treatment (*p<0.01). (D) Tumor tissue sections where stained with a fluorescent antibody to CD68 (FITC-green) to detect tissue infiltrating macrophages and DAPI to denote nuclei (blue), (E) sections were quantified by examining fluorescence of CD68/DAPI over area of section at 20x under multispectral fluorescent imaging (Scale bar = 50 μm). (F) HMGB1 expression was assessed by RT-PCR in tumor extracts (*p<0.05), (G) calreticulin gene expression was assessed in lysates from in vivo tumor studies by Western Blot hybridization and (H) was quantified by densitometry (*p<0.05).
This suggested that the enhancement observed in tumor killing in vivo by CD47 blockade is mediated in part by the macrophage mediated cytotoxicity against cancer cells. To determine whether CD47 regulated macrophage infiltration in vivo we stained tumor tissue sections with a fluorescent antibody to CD68 and DAPI to denote nuclei (Figure 3D). Our data shows that blocking CD47 in the presence or absence of DOX treatment increases macrophage infiltration by over 40% (*p<0.05) when compared to DOX treatment alone (Figures 3D, 3E). DOX treatment showed an approximately 50% (*p<0.05) decrease in macrophage infiltration when compared to saline treated animals (Figures 3D, 3E). The increase in macrophage infiltration was significant with CD47 therapy alone when compared to saline treated animals (Figures 3D, 3E, *p<0.05). Assessment of HMBG1, which facilitates macrophage mediated cell clearance, was increased by over 10-fold in the combination treatment when compared to control (Figure 3F). Single DOX and CD47M treatments increased HMBG1 2-fold (*p<0.05) and 3-fold (*p<0.05), respectively. Moreover, examination of calreticulin protein expression which facilitates the clearance of cancer cells by engaging its receptor on phagocytic cells [24] was increased by 30% (*p<0.05) with CD47 treatment alone or and over 50% (*p<0.05) with combination with DOX in tumor lysates from in vivo treated mice(Figure 3G-3H). This suggests that the enhanced reduction in tumor growth with the DOX and anti-sense CD47 treatment is mediated in part by activation of an immunogenic cell death pathway.
Blockade of CD47 prevents anthracycline-mediated cardiac cell and tissue toxicity.
Anthracyclines are used to treat several cancer types and are the standard approach for adjuvant treatment of breast cancer because they result in improved outcomes compared to other modalities [10–13]. However, high cumulative doses of anthracyclines compromise cardiovascular tissue causing the onset of congestive heart failure in patients[1]. To determine whether targeting CD47 may protect cardiac tissue from acute anthracycline associated death, we excised hearts of mice after one round of weekly DOX treatment in the presence or absence of prior treatment with CD47M. Sections were paraffin-embedded and subjected to TUNEL staining to detect cell death and were stained with wheat germ agglutinin to demark cardiomyocytes. Hearts of mice treated with DOX showed over a 15-fold increase in TUNEL staining when compared to saline treated mice (Figure 4A, 4B). CD47 blockade in combination with DOX prevented the increase in TUNEL positive nuclei when compared to DOX alone (Figure 4A, 4B).
Figure 4. Blockade of CD47 protects cardiac myocytes and cardiac tissue from doxorubicin mediated toxicity.
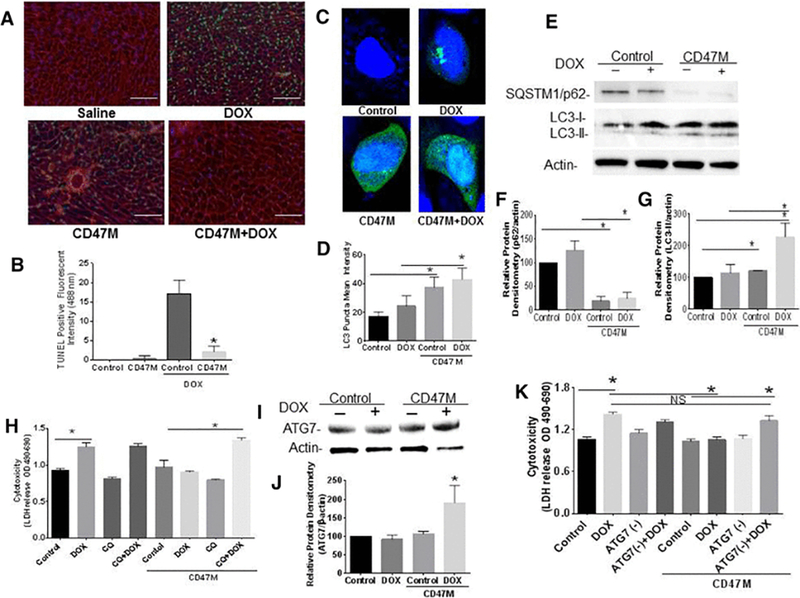
(A) Hearts of mice were excised after doxorubicin treatment, sections were paraffin embedded and tissue was stained with TUNEL and WGA (red) to detect apoptotic cardiac cells (40x, Scale bar= 100μm). Autophagic flux was measured by expression of SQSTM1/p62 and LC3 lipidation by Western Blot hybridization (B) and quantified (C, D n=4 *p<0.05). H9C2 cardiac myoblasts were treated with CD47M and or DOX and transfected with LC3-GFP LC3 puncta was determined by confocal microscopy (E) and quantified using ZEN 2.3 SP1 software (F). H9C2 cardiac myoblast cells were plated and left untreated or administered CD47 Morpholino (CD47 M); 48 h after cells were treated with or without DOX in the presence or absence of 25 μM chloroquine cells were incubated 48 h and LDH release was measured to determine cell cytotoxicity n=4 *p<0.001 (G).
Since we have previously shown that blockade of CD47 increases autophagy as a mechanism to prevent death from ionizing radiation[16] we transfected H9C2 rat cardiac myoblasts with anti-sense morpholino to CD47 and treated with DOX to determine autophagy is regulated as a protective mechanism from cytotoxicity associated with DOX. Transfection of GFP-LC3 construct into cardiac myoblast shows that LC3 puncta is increased by over 50% (*p<0.001) with CD47 treatment in the presence or absence of DOX (Figure 4C, 4D). Suggesting that autophagy is upregulated as a consequence of DOX treatment. Moreover, DOX treatment did not change accumulation of SQSTM1/p62 in cardiac cells when compared to control and showed a reduction in LC3 lipidation when compared to the combination treatment. (Figure. 4E-4G). Blockade of CD47 reduced the expression SQSTM1/p62 alone by 80% (*p<0.01) (Figure 4E, 4F and increased LC3 lipidation by 20 % over control (Figure 4E, 4G), however combination treatment reduced SQSTM1/p62 degradation by almost 75% and increased LC3 lipidation 3-fold (*p<0.01) (Figure 4E-4G). This data indicates that that the protective effect of CD47 blockade against DOX associated cytotoxicity is associated with an increase in autophagic flux.
To determine whether autophagy is necessary for the protective effect against death associated with DOX we cultured cardiac myoblasts in complete medium and treated with the autophagy inhibitor chloroquine (CQ). CQ elevates lysosomal pH, resulting in the inhibition autophagosome lysosome fusion[25]. Cardiac cells were treated with CQ 1h prior to DOX treatment and cell viability was measured by LDH release. DOX increased cytotoxicity in cardiac myoblasts (Figure 4H *p<0.001). However, CD47 blockade prevented the anthracycline-mediated cytotoxicity of cardiac myoblasts, demonstrating that the protective effect or CD47 blockade is cell autonomous. However, the protective effect of CD47 blockade from DOX associated cell cytotoxicity was lost following treatment with CQ. Thus, the observed chemo-protection from DOX treatment requires an autophagy mechanism.
We have previously demonstrated that the cytoprotection conferred by CD47 blockade in nonmalignant cells is mediated by the upregulation of Autophagy Related Genes (ATG’s)[7]. Our data shows that targeting CD47 in combination with DOX increased ATG7 gene expression by over 50% (*p<0.05) when compared to the rest of the treatment groups (Figure 4I, 4J). This serves as a further indication that autophagy is regulated as a protective mechanism with blockade of CD47. siRNA mediated silencing of ATG7 did not affect cardiac cell cytotoxicity associated DOX treatment. However, silencing of ATG7 reversed the protective effect of CD47 blockade against DOX cardiac cell cytotoxicity as it increased cytotoxicity over control and the combination of DOX and CD47M conditions (*p<0.05, Figure 4K). This indicates that the cytoprotective effect of CD47 against DOX cardiac cell cytotoxicity is mediated by upregulation of the autophagy pathway.
Deficiency of CD47 preserves cardiac function after treatment with DOX.
The heart has restricted regenerative capability; cumulative doses of anthracyclines surpass the threshold of damage that triggers ventricular remodeling, causing cardiac injury and limiting function [26]. Since fibrosis can accumulate in cardiac tissue limiting contractility and function we measured cardiac fibrosis as measured by picrosirus red staining (Figure 5A). Examination of tissue sections showed that there is a 5-fold increase (*p<0.01) in Fibrosis after DOX treatment (Figure 5B) over saline. However, treatment with CD47 prevented the increase in fibrosis after DOX treatment. Therefore, we predicted that CD47 blockade will improve cardiac function inhibiting collagen deposition.
Figure 5. Deficiency of CD47 prevents DOX associated cardiac fibrosis and preserves cardiovascular function.
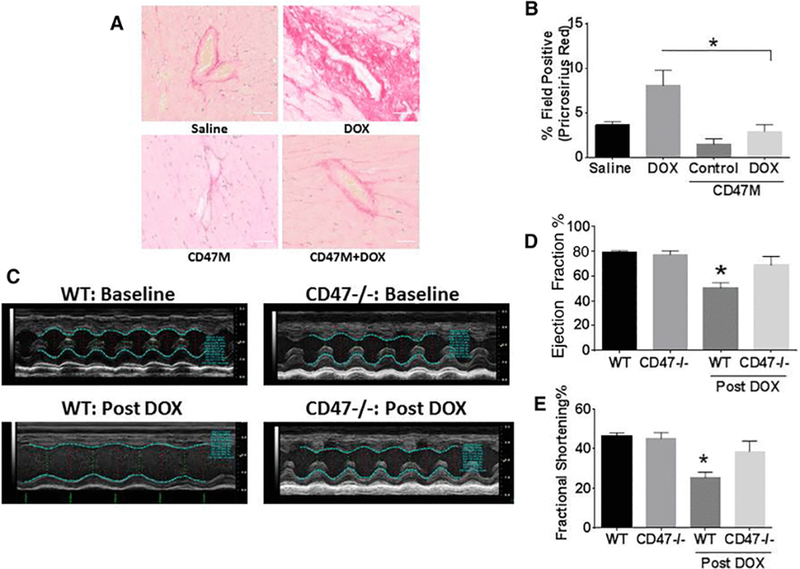
Hearts of mice were excised at the end of the study and were paraffin embedded. Sections were stained with pricosirius red (A) and quantified using mantra inform pathology suite software (B) n=5 *p<0.05 (Scale bar= 50μm). Cardiovascular function was assessed using in vivo ultrasound with Vevo LAZR Photoacoustic Imaging System (C-E).
To test this we injected WT and Cd47−/− mice with DOX I.V. and measured cardiovascular function using in vivo ultrasound with the Vevo LAZR system. M-Mode tracing with in in vivo ultrasound (Figure 5C) shows no difference in cardiac function as measured by ejection fraction and fractional shortening between untreated WT and Cd47−/− mice (Figure 5C top panels, 5D, 5E). However, treatment with DOX significantly reduced the ejection fraction by almost 40% and fractional shortening by 50% (*p<0.05). On the other hand, deficiency of CD47 preserved cardiac function after DOX treatment. These results indicate that CD47 blockade could be used as an adjuvant to prevent cardiac dysfunction in cancer patients receiving anthracycline chemotherapy and improve long-term cancer patient quality of life.
Discussion
One of the many challenges in cancer therapy is to maximally suppress tumor growth while preserving the integrity of normal tissue. We have previously demonstrated that blockade of CD47 enhances radiation growth delay in several syngeneic tumor models. In the same study we showed that blockade of CD47 spared bone marrow and soft tissues from death caused by IR [2]. Further studies show the protective effect was mediated by an increase in protective autophagy [3, 16]. A screen performed with 4T1 breast cancer cells demonstrated that blockade of CD47 did not interfere with the anticancer activity of most compounds (Figure 1B-1H). Since we have shown previously in several models that blockade of CD47 is protective against cytotoxic stress, our goal was to rigorously show that absence of CD47 would not interfere with the oncologic efficacy of chemotherapeutic compounds. Our results demonstrate that the anthracycline family of compounds fit these criteria. Our data confirms that DOX treatment is cytotoxic to cardiac myoblasts, but this toxicity is decreased by suppressing CD47, thus suggesting that targeting CD47 in combination with cytotoxic therapy may decrease the off target effects of chemotherapy. Because elevated CD47 expression on tumor cells is associated with increased cancer progression by limiting innate and adaptive antitumor immunity, targeting this receptor may also enhance the antitumor effects of chemotherapy by protecting tumor-associated immune cells but not the tumor cells.
Clinical studies generally show that increased expression of CD47 is associated with poor prognosis in several types of cancer, including breast cancer [27, 28]. A study with over 700 patients demonstrated that patients with high expression of CD47 in the bone marrow cells had worse disease free survival (DFS) when compared to those with low levels of CD47 expression[29]. The expression profile also correlated with SIRPα expression. However, the same study showed that CD47 was an independent prognostic factor, and increased expression was associated with reduced overall survival and DFS [30]. Prior data using RNAseq shows that CD47 expression is elevated in TNBC when compared to other breast cancer subtypes [31] Consistent with previous publications our new data indicates that expression of CD47 is elevated in basal-like breast cancer, and clinical samples of invasive breast cancer. This suggests a link between CD47 expression and poor prognosis of breast cancer patients. Preclinical studies focus on how elevated CD47 expression allows cancer cells to escape innate immunosurveillance [32–34]. This reflects the normal homeostatic function of CD47 as a counter-receptor for SIRPα (Fig. 3) that blocks phagocytosis [35]. On the other hand, CD47 also serves as a signaling receptor for the matricellular protein thrombospondin-1 [36, 37]. This interaction regulates responses to stress, tissue perfusion, and angiogenesis. Relevant to cancer therapy, the thrombospondin-1 peptide 4N1, binds to CD47, and downregulates caspase-3 protecting thyroid carcinoma cells against cytotoxic agents including DOX [38]. Thus, elevated expression of CD47 could cause resistance to chemotherapy, while also suggesting that inhibition of CD47 could be a pharmacological target to sensitize cancer cells to death.
Our published studies identified autophagy as a major target for CD47 signaling, to preserve cell and tissue viability during stress [3, 16]. Our data shows that autophagic flux is increased in cardiac cells deficient of CD47 after DOX treatment. Upregulation of autophagy has been demonstrated to protect against DOX associated cardiomyocyte cytotoxicity [39]. Moreover, DOX treatment of cardiomyocytes inhibits the autophagic flux, causing the accumulation of autophagosomes[40]. Consistent with these findings, DOX treatment causes accumulation of autophagosomes in cardiac myoblast evident by the clump pattern observed in figure 4C. which has been associated with accumulation of autophagosomes previously[41]. The inhibition of autophagic flux by DOX also explains why targeting with either CQ or knockdown with ATG7 (Figures 4H, 4K) does not further sensitize cardiac cells to death, which could be expected in the treatment of cancer cells.
Autophagy signaling can have divergent effects on tumors. Inhibition of autophagy can sensitize cancer cells to chemotherapy[42]. However, the promotion of autophagy can also promote cancer cell death, particularly in in vivo models, whereas a recent study reported that autophagy inhibition increased the efficacy of inhibiting the CD47/SIRPα interaction in a glioblastoma xenograft model [43]. However, whether CD47 blockade regulates autophagy in cancer cells is unknown. Our data shows that blockade of CD47 in 4T1 and MDA-MB-231 breast cancer cells does not interfere with DOX associated cytotoxicity and thus not limiting its oncologic efficacy (Figures 1B-H and supplemental Figure 1A). Our data (not shown) suggests that the canonical pathway of autophagy is not regulated in cancer cells by blockade of CD47. MDA-MB-231 cells express high levels of LC3-II [25], suggesting that levels of autophagic flux may not play a cytoprotective role in these cancer cells.
Previous reports suggest DOX treatment increases autophagy signaling [44, 45]. However, this may be context dependent; DOX may have different cell viability effects in various cancer or normal cell types[40]. Duality of autophagic signaling is well known[46]. Autophagy is known as program cell death III as formation of autophagosomes were associated with apoptosis [47] and autophagy may supplement metabolic signaling to promote survival induce drug resistance [46, 48]; Therefore these observations may be in part of the complexities of interpreting autophagy. Our goal here is to determine the regulation of autophagy at the time we observe cell death in the heart which was 24h after DOX treatment as shown by TUNEL staining (Figure 4A, 4B). While we can’t rule out that other genes of the autophagy pathway are not regulated by CD47 in cancer, the canonical protective autophagy pathways seem to be regulated differently between malignant and non-transformed cells.
The differential sensitivity of normal and cancer cells to CD47 blockade may be influenced by many factors including metabolism differences in tissues, levels of ROS, inflammatory environment among others. However, the sensitization of cancer cells to chemotherapy by CD47 is due in part to the well-established effects of this receptor on anti-tumor immunosurveillance. Our data shows that pretreatment with DOX and CD47 blockade significantly enhanced macrophage mediated killing of breast cancer cells (Figures 4A-4C). We have shown recently that sensitization of breast cancer cells to anti-estrogen therapy by targeting the Unfolded Protein Response (UPR) pathway is due in part to a reduction in CD47 expression and increase of the “eat me signals” calreticulin and high molecular group box 1 (HMGB1)[49, 50]. Therefore, regulation of these proteins in the absence of CD47 could account for the enhancement in the reduction of tumor growth over DOX alone.
DOX has been demonstrated to increase the release of “damage associated molecular patterns” (DAMPs) such as HMGB1, which stimulated immune cell clearance of B16 melanoma cells[51, 52] Consistent with these results we observed increased levels of HMBG1 in DOX treated tumors when compared to saline treated controls, which could account for the enhanced macrophage-mediated cytotoxicity observed with DOX-pretreatment in vitro. Furthermore, blockade of CD47 in combination with DOX resulted in a 5-fold increase in HMBG1 over DOX alone, suggesting increased activation of an anti-tumor immune response over DOX consistent with the increased macrophage infiltration in the combination treatment (Figure 3D).
The clearance of cancer cells by CD47 blockade is limited by the expression of “eat me” signals such as calreticulin. Calreticulin facilitates clearance of cell by engaging its counter receptor LDL-receptor-related protein (LRP) on the phagocytic cell. Our data shows that in vivo blockade of CD47 resulted in an increase in calreticulin that was not impaired with DOX treatment. Dendritic cell vaccination resulted in clearance of refractory lymphomas, which was associated with a decrease in CD47 and increase in calreticulin, HMGB1[53]. This strategy increase immunogenicity of tumors. Our data shows for the first time that targeting CD47 in combination with chemotherapy causes a similar regulation in breast cancer, indicating that the blockade of CD47 in combination with DOX results in activation of an immunogenic cells death pathway that results in enhanced tumor ablation in vivo. This combination treatment may also result in enhanced immunogenicity of breast cancer, which has been a limitation in observing meaningful benefits of immunotherapy in clinical treatment of breast cancer[54]. The immune microenvironment is complex and other immune cells contribute to anti-tumor immunosurvillance. Us and others have shown that CD47 enhances adaptive immune responses against cancer cells[9, 55], therefore the enhancement in tumor killing with CD47 blockade could be due to stimulation of other immune cell types.
CD47 was recently demonstrated to be under the transcriptional control of HIF-1 [56]. The low oxygen tension and the harsh conditions of the tumor microenvironment and expression levels of CD47 may be responsible for signaling effects in tumor and protection from cytotoxicity in tissues distant from the tumor. Cardiac tissue from our tumor bearing animals treated with DOX showed increased cell death, but this was inhibited when DOX was administered in combination with anti-sense CD47. The microenvironment in the heart is rich in oxygen and displays a different metabolic demand than the tumor. Therefore, the interplay of oxygen tension and metabolism may regulate CD47 expression as well as differential responses or normal and malignant tissues to cytotoxic therapy. Further studies are warranted to determine if hypoxia and metabolism may play a role in the autonomous regulation of these differential responses to CD47 targeting.
Conclusions:
Overall our studies indicate that blockade of CD47 enhances anti-tumor responses to chemotherapy while preserving viability and function of the cardiovascular system. Since agents targeting CD47 are currently in clinical trials, these experiments suggest new combinatorial strategies to improve responses of patients with invasive and triple negative breast cancer and the further examination of anti-CD47 therapy as an adjuvant to prevent secondary cardiovascular pathologies that arise as a consequence of chemotherapy in cancer survivors.
Supplementary Material
(A) immunohistochemical staining of human mammary gland (top) and human invasive breast cancer (representative of 5 cases) with human antibody B6H12 (brown stain). (B) LDH release assay in MDA-MB-231 cells. (C)Efficiency of CD47 knock down using anti-sense morpholino treatment in tumors N=5, *p<0.05.
Acknowledgements
We would like to acknowledge the statistical and editorial assistance of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by NCATS, National Institutes of Health, through Grant Award Number UL1TR001420.
Funding:
This work was supported by the NCI Career Development Award-K22 1K22CA181274–01A1 (DSP), Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30CA012197.(DSP, KLC), the Intramural Research Program of the NIH/NCI (DDR) and The National Center for Advancing Translational Sciences (NCATS) (MF, CJT).
List of Abbreviations:
- AC
Activity of Maximum Concentration
- CD
Cluster of Differentiation
- CD47
CD47 Deficient
- CD47M
CD47 Morpholino
- CRC
Concentration Response Curves
- DMEM
Dulbecco’s Modified Eagle Medium
- DOX
Doxorubicin
- DAMPs
Damage Associated Molecular Patterns
- HIF1
Hypoxia Inducible Factor 1
- SIRPα
Signal Regulatory Protein alpha
- UPR
Unfolded Protein Response
- WT
Wild Type
Footnotes
Compliance with Ethical Standards:
Conflict of Interest:
The authors of this manuscript have no conflicts of interest to report
Ethical approval:
Experiments in presented in this manuscript comply with the current laws of the United States of America and institutional research integrity policies. Tissue arrays from developed from tissues of human breast cancer patient sections analyzed in the Laboratory of Pathology National Cancer Institute, under approved protocol by the Institutional Review Board of the National Cancer Institute. Animal studies and procedures were approved by the Internal Animal Care and Use Committee (IACUC) of the NIH Intramural Research Program protocol # LP-012 and the Wake Forest IACUC protocol # A16–085. Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Informed consent:
Not applicable
References
- 1.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M: Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc 2014, 89(9):1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD: Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009, 1(3):3ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD: Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep 2013, 3:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo J, Lau EY, So FT, Lu P, Chan VS, Cheung VC, Ching RH, Cheng BY, Ma MK, Ng IO et al. : Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int 2016, 36(5):737–745. [DOI] [PubMed] [Google Scholar]
- 5.Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL: Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 2018, 115(6):E1239E1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto-Pantoja DR, Kaur S, Roberts DD: CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 2015, 50(3):212230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soto Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, AbuAsab M, Wink DA, Tsokos M, Roberts DD: CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy 2012, 8(11):0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, Roberts DD: Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets 2013, 17(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, Berzofsky JA, Roberts DD: CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res 2014, 74(23):6771–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner N, Biganzoli L, Di Leo A: Continued value of adjuvant anthracyclines as treatment for early breast cancer. Lancet Oncol 2015, 16(7):e362–369. [DOI] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P et al. : Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes RC, Bliss JM, Wils J, Morvan F, Espie M, Amadori D, Gambrosier P, Richards M, Aapro M, Villar-Grimalt A et al. : Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. The International Collaborative Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1996, 14(1):35–45. [DOI] [PubMed] [Google Scholar]
- 13.Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, Ewertz M, Jensen BB, Kamby C, Nordenskjold B et al. : Improved outcome from substituting methotrexate with epirubicin: results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007, 43(5):877884. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton DH, Matthews Griner L, Keller JM, Hu X, Southall N, Marugan J, David JM, Ferrer M, Palena C: Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP: Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 2006, 103(31):11473–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, AbuAsab M, Wink DA, Tsokos M, Roberts DD: CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy 2012, 8(11):1628–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD et al. : The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012, 109(17):6662–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z et al. : Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009, 27(8):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG et al. : Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016, 34(10):1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, DeGraff WG, Tsokos M, Wink DA, Isenberg JS, D . RD: Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009, 1:3ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P et al. : Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005, 11(16):5678–5685. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Zhang JJ, Huang XY: Mouse models for tumor metastasis. Methods Mol Biol 2012, 928:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peper JK, Schuster H, Loffler MW, Schmid-Horch B, Rammensee HG, Stevanovic S: An impedance-based cytotoxicity assay for real-time and label-free assessment of Tcell-mediated killing of adherent cells. J Immunol Methods 2014, 405:192–198. [DOI] [PubMed] [Google Scholar]
- 24.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N et al. : Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007, 13(1):54–61. [DOI] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K et al. : Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC: Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010, 53(2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki S, Yokobori T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Miyazaki T, Kato H et al. : CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncology reports 2012, 28(2):465–472. [DOI] [PubMed] [Google Scholar]
- 28.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY et al. : Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2010, 2(63):63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr.,van Rooijen N, Weissman IL: CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138(2):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagahara M, Mimori K, Kataoka A, Ishii H, Tanaka F, Nakagawa T, Sato T, Ono S, Sugihara K, Mori M: Correlated expression of CD47 and SIRPA in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res 2010, 16(18):4625–4635. [DOI] [PubMed] [Google Scholar]
- 31.Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, Manu N, Wu W, Mannan P, Garfield SH et al. : A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 2016, 7(9):10133–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouguermouh S, Van VQ, Martel J, Gautier P, Rubio M, Sarfati M: CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol 2008, 180(12):8073–8082. [DOI] [PubMed] [Google Scholar]
- 33.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H: Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A 2007, 104(12):5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal S, Chao MP, Majeti R, Weissman IL: Macrophages as mediators of tumor immunosurveillance. Trends Immunol 2010, 31(6):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Jr., Chang HY, van de Rijn M et al. : Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009, 106(33):14016–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Pantoja DR, Isenberg JS, Roberts DD: Therapeutic Targeting of CD47 to Modulate Tissue Responses to Ischemia and Radiation. J Genet Syndr Gene Ther 2011, 2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD: Differential interactions of thrombospondin-1, −2, and −4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009, 284(2):11161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rath GM, Schneider C, Dedieu S, Rothhut B, Soula-Rothhut M, Ghoneim C, Sid B, Morjani H, El Btaouri H, Martiny L: The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochimica et biophysica acta 2006, 1763(10):11251134. [DOI] [PubMed] [Google Scholar]
- 39.Dutta D, Xu J, Kim JS, Dunn WA, Jr., Leeuwenburgh C: Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 2013, 9(3):328–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A, Xie M, Jiang N, May H, Kyrychenko V et al. : Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133(17): 1668–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jangamreddy JR, Panigrahi S, Los MJ: Monitoring of autophagy is complicated-salinomycin as an example. Biochimica et biophysica acta 2015, 1853(3):604–610. [DOI] [PubMed] [Google Scholar]
- 42.White E: Role of the metabolic stress responses of apoptosis and autophagy in tumor suppression. Ernst Schering Foundation symposium proceedings 2007(4):2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Chen W, Fan J, Wang S, Xian Z, Luan J, Li Y, Wang Y, Nan Y, Luo M et al. : Disrupting CD47-SIRPalpha axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis 2018, 39(5):689–699. [DOI] [PubMed] [Google Scholar]
- 44.Denton D, Xu T, Kumar S: Autophagy as a pro-death pathway. Immunol Cell Biol 2015, 93(1):35–42. [DOI] [PubMed] [Google Scholar]
- 45.Guo B, Tam A, Santi SA, Parissenti AM: Role of autophagy and lysosomal drug sequestration in acquired resistance to doxorubicin in MCF-7 cells. BMC Cancer 2016, 16(1):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White E, DiPaola RS: The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 2009, 15(17):5308–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Levine B: Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 2015, 22(3):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gewirtz DA: The four faces of autophagy: implications for cancer therapy. Cancer Res 2014, 74(3):647–651. [DOI] [PubMed] [Google Scholar]
- 49.Cook KL, Soto-Pantoja DR: “UPRegulation” of CD47 by the endoplasmic reticulum stress pathway controls anti-tumor immune responses. Biomark Res 2017, 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook KL, Soto-Pantoja DR, Clarke PA, Cruz MI, Zwart A, Warri A, Hilakivi-Clarke L, Roberts DD, Clarke R: Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res 2016, 76(19):5657–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starobinets H, Ye J, Broz M, Barry K, Goldsmith J, Marsh T, Rostker F, Krummel M, Debnath J: Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J Clin Invest 2016, 126(12):4417–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroemer G, Galluzzi L: Autophagy-dependent danger signaling and adaptive immunity to poorly immunogenic tumors. Oncotarget 2017, 8(4):5686–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montico B, Lapenta C, Ravo M, Martorelli D, Muraro E, Zeng B, Comaro E, Spada M, Donati S, Santini SM et al. : Exploiting a new strategy to induce immunogenic cell death to improve dendritic cell-based vaccines for lymphoma immunotherapy. Oncoimmunology 2017, 6(11):e1356964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emens LA: Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res 2018, 24(3):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM: CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015, 21(10):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL: HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015, 112(45):E6215–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) immunohistochemical staining of human mammary gland (top) and human invasive breast cancer (representative of 5 cases) with human antibody B6H12 (brown stain). (B) LDH release assay in MDA-MB-231 cells. (C)Efficiency of CD47 knock down using anti-sense morpholino treatment in tumors N=5, *p<0.05.


