Abstract
New pain treatments are in demand due to the pervasive nature of pain conditions. Hyperbaric oxygen (HBO2) has shown potential in treating pain in both clinical and preclinical settings, although the mechanism of this effect is still unknown. The aim of this study was to investigate whether the major inhibitory neurotransmitter γ-aminobutyric acid (GABA) is involved in HBO2-induced antinociception in the central nervous system. To accomplish this goal, pharmacological interactions between GABA drugs and HBO2 were investigated using the behavioral acetic acid abdominal constriction test. Western blotting was used to quantify protein changes that might occur as a result of the interactions. GABAA but not GABAB receptor antagonists dose-dependently reduced HBO2 antinociception, while antagonism of the GABA reuptake transporter enhanced this effect. Western blot results showed an interaction between the pain stimulus and HBO2 on expression of the phosphorylated β3 subunit of the GABAA receptor at S408/409 in homogenates of the lumbar but not thoracic spinal cord. A significant interaction was also found in neuronal nitric oxide synthase (nNOS) expression in the lumbar but not thoracic spinal cord. These findings support the notion that GABA may be involved in HBO2-induced antinociception at the GABAA receptor but indicate that more study will be needed to understand the intricacies of this interaction.
Keywords: Hyperbaric oxygen, GABA receptors, Nitric oxide synthase, Spinal cord, Antinociception
1. Introduction
HBO2 therapy is the use of 100% oxygen (O2) at higher-than-atmospheric pressures for limited periods of time (generally 60–90 min) to achieve beneficial clinical outcomes. HBO2 therapy is approved by the U.S. Food and Drug Administration (FDA) for only limited clinical indications, which include decompression sickness, carbon monoxide poisoning, wound healing and delayed radiation injury (Weaver, 2014). The use of HBO2 to treat chronic pain is the subject of ongoing research in both clinical and preclinical research. Clinically, HBO2 treatment is reportedly effective in a variety of off-label applications, including some chronic pain conditions such as complex regional pain syndrome (formerly reflex sympathetic dystrophy syndrome) (Katznelson et al., 2016; Kiralp et al., 2004; Peach, 1995), fibromyalgia syndrome (Efrati et al., 2015; Yildiz et al., 2004), migraine or cluster headache (Di Sabato et al., 1993; Myers and Myers, 1995; Wilson et al., 1998), myofascial pain syndrome (Kiralp et al., 2009) and idiopathic trigeminal neuralgia (Gu et al., 2012). In the preclinical setting, HBO2 has been shown to produce long-lasting pain relief in experimental animals (Chung et al., 2010; Gibbons et al., 2013; Warren et al., 1979). However, the mechanism through which HBO2 produces these effects is not, at present, fully characterized.
Research in our laboratory has focused on elucidating the mechanism of antinociceptive action of HBO2. We have reported that an 11-min HBO2 treatment significantly reduced glacial acetic acid-induced abdominal constrictions in mice, an animal model of acute pain (Ohgami et al., 2009; Quock et al., 2011). We also found that repeated daily 60-min HBO2 treatments for 4 days induced an unparalleled biphasic antinociceptive response that consisted of 1) an early phase that lasted at least 6 h after the HBO2 treatment before dissipating; and 2) a late phase that emerged about 18 h after the early phase and lasted for up to 3 weeks (Chung et al., 2010). The acute antinociceptive response of mice to HBO2 is dependent on nitric oxide (NO) (Ohgami et al., 2009). Centrally administered inhibitors of neuronal nitric oxide synthase (nNOS) and antagonists of opioid receptors are able to block HBO2-induced antinociception in both acute and long-lasting antinociceptive models, indicating a centrally active pathway mediating HBO2-induced antinociception (Chung et al., 2010; Gibbons et al., 2013; Ohgami et al., 2009; Quock et al., 2011; Zelinski et al., 2009). NO can act as a signaling molecule in the central nervous system and is associated with diverse behaviors including learning, memory and sleeping, as well as sensory and motor function (Garthwaite, 2008). NO has also been shown to function similarly to a neurotransmitter in the CNS depending upon the circuit (Garthwaite, 2008). NO may be produced both pre- and post-synaptically and has been shown to both depolarize and hyperpolarize nerve cells (Garthwaite, 2008). Finally, NO can act as both a neurotransmitter and a neuromodulator by modulating the release of several other neurotransmitters, including GABA (Kuriyama and Ohkuma, 1995).
γ-Aminobutyric acid (GABA) neurons are widely distributed in the central nervous system, including supraspinal and spinal sites involved in transmission of afferent pain signals and descending pain-modulating pathways (Enna and McCarson, 2006). Drugs that activate GABA receptors have been found to produce antinociception in rodents (Krogsgaard-Larsen et al., 2004; Sawynok, 1987). Moreover, GABA receptors have been implicated in mediating stress-induced antinociception (Tokuyama et al., 1992) as well as the antinociceptive effects of drugs such as benzodiazepines (Kunchandy and Kulkarni, 1987), delta opioid peptides and heroin (Rady and Fujimoto, 1996, 1995).
NO has been shown to be necessary for long-term potentiation (LTP) of GABAergic neurons in the amygdala (Lange et al., 2012), the hippocampus (Zhuo et al., 1993), the ventral tegmental area (VTA) (Nugent et al., 2009), and the spinal cord (Fenselau et al., 2011). NO is also known to increase GABA release from presynaptic cells by increasing calcium in the presynaptic cells as well as through the sodium-dependent carrier GABA uptake system (Kuriyama and Ohkuma, 1995). HBO2-induced antinociception has been shown to be mediated by a NO-sGC-cGMP-PKG pathway (Ding et al., 2017; Quock et al., 2011). The same signaling pathway is involved in NO enhancement of GABA release in the basolateral complex of the amygdala (Lange et al., 2012).
The objective of the present investigation was to assess the possible involvement of GABAergic mechanisms in the acute antinociceptive effect of HBO2 in a mouse model of acute pain and to provide neuroanatomical evidence for interactions of brainstem nociceptive GABAergic pathways with HBO2. It is likely that HBO2-induced acute antinociception is partially mediated by GABA transmission in the spinal cord. HBO2 can potentially increase NO, which, in turn, increases GABA activity, which contributes to the acute antinociceptive effect of HBO2.
2. Results
2.1. Drug interaction results
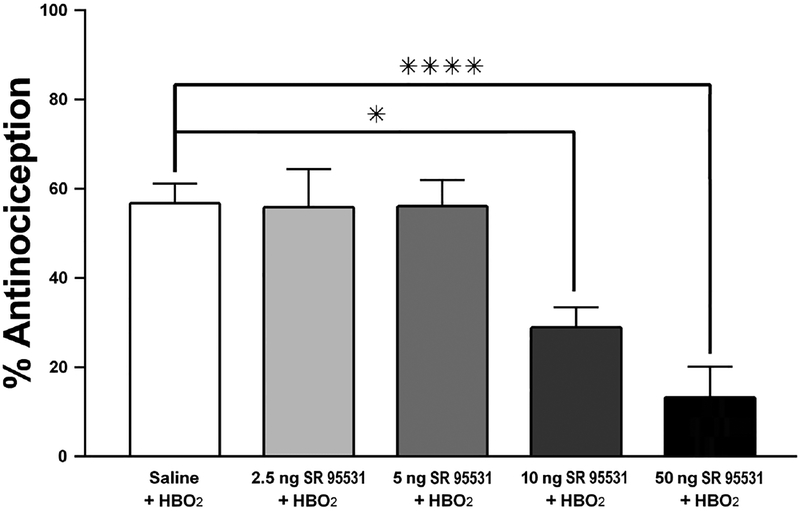
Intrathecal pretreatment with the non-competitive GABAA antagonist SR 95531 dose-dependently decreased the percent antinociception observed while under HBO2. A one-way between-subjects ANOVA revealed a main effect of SR 95531 on HBO2-induced antinociception [F(4,47) = 10.395, P < 0.0001]. A Dunnett’s post hoc test was conducted to determine which doses of SR 95531 significantly affected HBO2-induced antinociception. Animals pretreated with saline vehicle exhibited 56.70 ± 4.4% antinociceptive response to HBO2. Mice pretreated with 2.5 and 5.0 ng SR 95531 exhibited an antinociceptive effect that held steady at 55.8± 8.6% and 56.1± 6.0%, respectively. The 10-ng dose of SR 95531 decreased HBO2-induced antinociception to 28.9 ± 4.6% (P < 0.01). Furthermore, a 50-ng pretreatment dose further reduced the antinociception to 13.2 ± 6.9% (P < 0.0001). Fig. 2 depicts the dose-related antagonism of HBO2-induced antinociception by 10 and 50 ng SR 95531 when administered directly into the spinal cord.
Fig. 2.
Influence of i.t. pretreatment with a GABAA receptor antagonist (SR 95531)on the acute (11-min) antinociceptive effect of HBO2 in the glacial acetic acid-induced abdominal constriction test. Each column represents the percent antinociceptive response ± S.E.M. of at least 10 mice per group. Significance of difference: *, P < 0.05 and ****, P < 0.0001, compared to the saline-pretreated control group.
A one-way ANOVA was conducted to compare the effects of SR 95531 at room air with a vehicle pretreatment at room air. This analysis was not statistically significant [F (2,30) = 0.7261, P > 0.05], i.e., they were not significantly different from saline vehicle pretreatment at room air. This indicates that while the 10- and 50-ng doses of SR 95531 are able to antagonize the antinociceptive effect of HBO2, they have no appreciable effect on their own under room air.
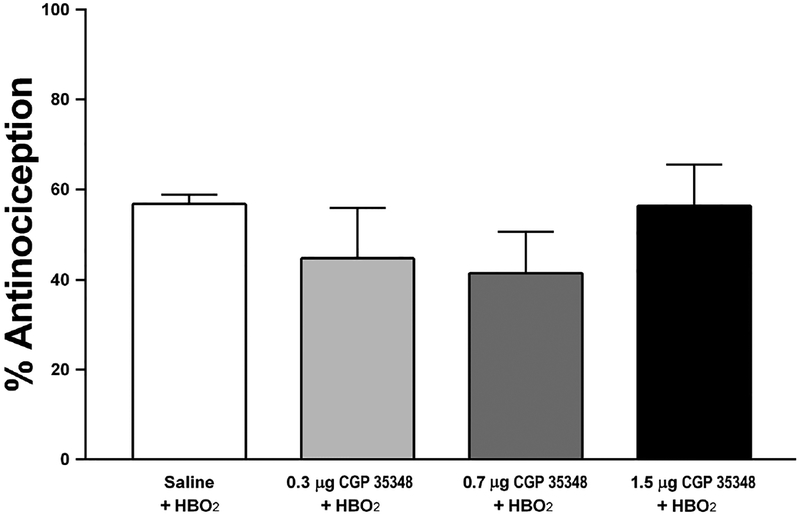
Intrathecal pretreatment with the selective GABAB receptor antagonist CGP 35348 failed to affect the antinociceptive effect of HBO2 at all doses tested [F (3,38) = 0.854, P > 0.05]. Because no effect was found, no multiple comparisons tests were run on CGP 35348. Pretreatment with the highest dose of CGP35348 had no effect on the abdominal constriction test under room air conditions [t(22) = 0.27, p>0.05]. These results indicate that the GABAB receptor is not involved in the acute antinociceptive effect of HBO2 induced by an 11-min treatment of HBO2. Results of this experiment are shown in Fig. 3.
Fig. 3.
Influence of i.t. pretreatment with a GABAB receptor antagonist (CGP 35348) on the acute (11-min) antinociceptive effect of HBO2 in the glacial acetic acid-induced abdominal constriction test. Each column represents the percent antinociceptive response ± S.E.M. of at least 10 mice per group. There were no statistically significant differences among the groups.
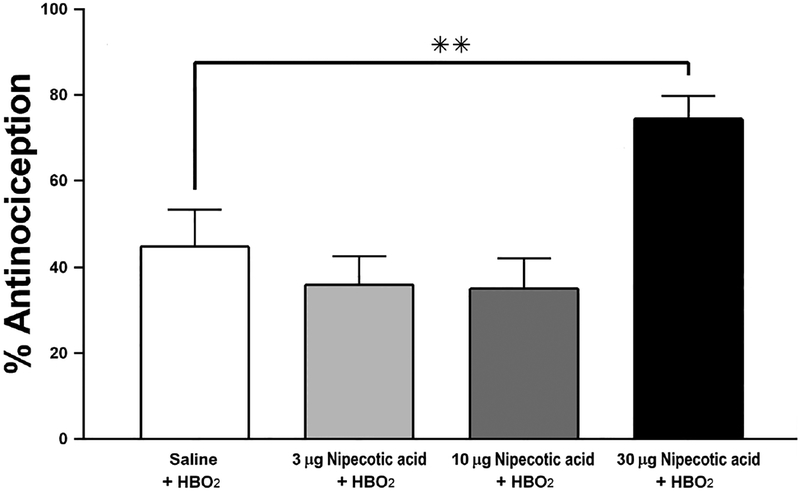
Intrathecal pretreatment with the GABA uptake inhibitor nipecotic acid increased HBO2-induced antinociception only at the highest dose used (30 μg). A one-way ANOVA identified an effect of nipecotic acid on HBO2-induced antinociception [F(3,49) = 6.974, P < 0.005] (Fig. 4). Multiple comparisons analysis revealed a significant difference between the effects of the vehicle vs. 30 μg of nipecotic acid on HBO2- induced antinociception (P < 0.05). The 30-μg dose of nipecotic acid increased the antinociceptive effect to 75.5 ± 5.2%, compared to the response of the saline vehicle-pretreated group, 44.6 ± 8.7 (Fig. 4). Microinjections of 10 μg and 30 μg nipecotic acid microinjections failed to cause significant change from saline vehicle [F(2,46)=0.968 p>0.05]. These results indicate that nipecotic acid, a GABA reuptake inhibitor, can increase the antinociceptive effect of HBO2 if the dose is sufficiently high enough.
Fig. 4.
Influence of i.t. pretreatment with a GABA uptake inhibitor (nipecotic acid) on the acute (11-min) antinociceptive effect of HBO2 in the glacial acetic acid-induced abdominal constriction test. Each column represents the percent antinociceptive response ± S.E.M. of at least 10 mice per group. Significance of difference: **, P < 0.01, compared to the saline-pretreated control group.
2.2. Western immunoblotting results
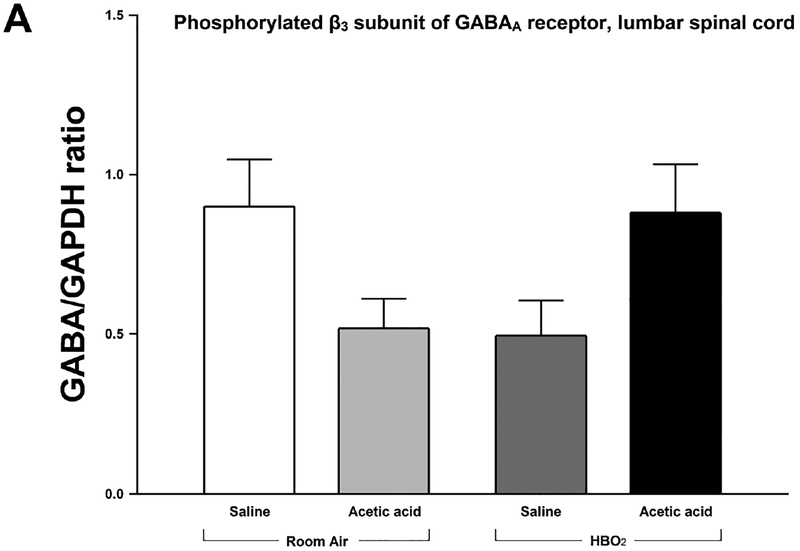
Densitometric analysis of immunoblots of the GABAA receptor phosphorylated at the β3 subunits showed no significant changes in expression of the phosphorylated β3 subunit. A two-way between-subjects ANOVA found a significant interaction between HBO2 and glacial acetic acid [F(1,20) = 8.782, P < 0.05]. However, no main effects of either HBO2 [F(1,20) = 0.027, P > 0.05] or glacial acetic acid [F(1,20) = 0.00017 p>0.05] were found. Expression of the phosphorylated β3 subunit of the GABAA receptor decrease in response to either HBO2 or glacial acetic acid. However, when an animal is administered glacial acetic acid and exposed to HBO2, expression significantly increases in the lumbar region of the spinal cord. This indicates a significant interaction between the presence of the nociceptive stimulus, in this case, glacial acetic acid, and HBO2 on phosphorylation of the β3 subunit of the GABAA receptor in the lumbar spinal cord (Figs. 5A and 5B).
Fig. 5.
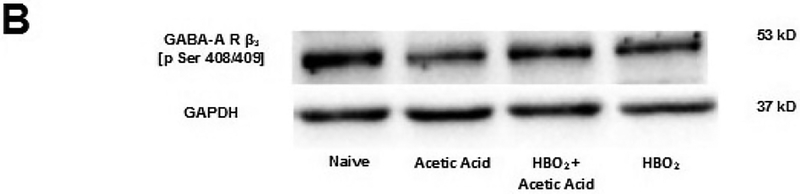
(A) Effect of glacial acetic acid and HBO2 treatment on the optical density of phosphorylated β3 subunit of the GABAA receptor in the lumbar section of the spinal cord. Each column represents the ratio compared to naïve of GABAA β3 Pser408/409 to GAPDH ratios of 6 western blots representing 2 separate samples per group. No significant differences were found. (B) Representative example of typical western blot of GABAA β3 Pser408/409 and GAPDH controls.
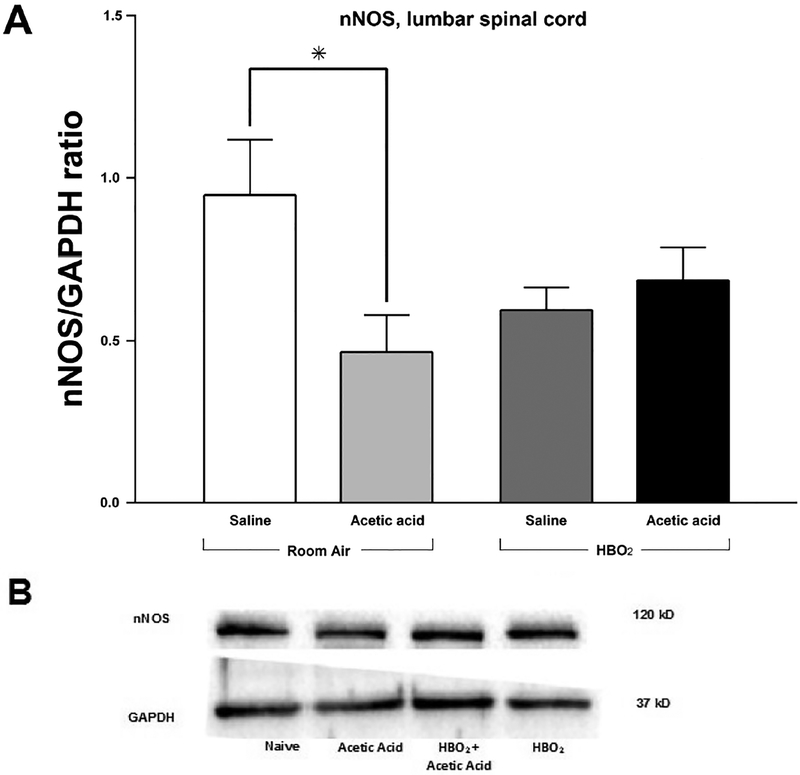
Densitometric analysis indicates a reduction in expression of nNOS 11 min following injection with glacial acetic acid in the lumbar spinal cord when compared to animals injected with saline and sacrificed at the same time (Figs. 6A and 6B). A twoway ANOVA was run on the densitometric analysis data and revealed a significant interaction between HBO2 and glacial acetic acid as measured by nNOS expression [F(1,20) = 5.748, P < 0.05]. No main effects were found for either glacial acetic acid [F(1,20) = 2.67, P > 0.05] or HBO2 [F(1,20) = 0.297, P > 0.05]. This indicates that nNOS expression decreased in response to HBO2 when saline, rather than glacial acetic acid, was administered. nNOS expression also decreased in response to glacial acetic acid, but this effect alone was not significant. This would mean that in the absence of a noxious stimulus, HBO2 would decrease nNOS expression. However, when HBO2 was administered to animals after glacial acetic acid, nNOS expression increased in the lumbar region of the spinal cord.
Fig. 6.
(A) Effect of glacial acetic acid and HBO2 treatment on optical density of neuronal nitric oxide synthase (nNOS) in the lumbar section of the spinal cord. Glacial acetic acid causes a decrease in optical density that HBO2 is able to reverse. Each column represents the ratio compared to naïve of nNOS to GAPDH ratios of 6 western blots representing at least 2 separate samples. Significance of difference: *, P<0.05. (B) Representative example of western blot of lumbar spinal cord showing expression of nNOS and GAPDH.
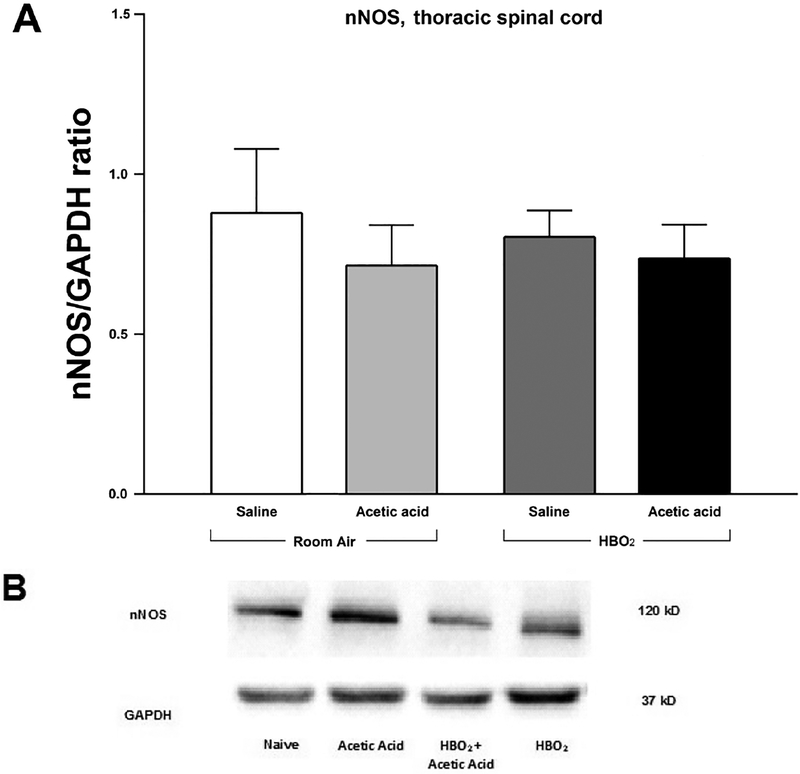
Analysis of the expression of the phosphorylated β3 subunit of the GABAA receptor showed an interaction between HBO2 and glacial acetic acid. A two-way between-subjects ANOVA found no significant interaction [F(1,20) = 1.060, P > 0.05]. There was no main effect of HBO2[F(1,20) = 3.161, P > 0.05], and there was also no main effect of glacial acetic acid [F(1,20) = 0.47, P > 0.05]. This indicates that neither HBO2 nor glacial acetic acid significantly affected expression of the β3 subunit of the GABAA receptor in the thoracic region of the spinal cord and that there was no significant interaction between the two at this level of the spinal cord.
A two-way between-subjects ANOVA revealed no interaction between HBO2 and glacial acetic acid in expression of nNOS in the thoracic spinal cord [F(1,20) = 0.146, P > 0.05]. There was also no main effect of either HBO2 [F(1,20) = 0.05, P > 0.05], or glacial acetic acid [F(1,20) = 0.77, P > 0.05] in density of nNOS as compared to density of GAPDH across the different conditions of animal exposure. This indicates that neither HBO2 nor glacial acetic acid affected nNOS expression in the thoracic region of the spinal cord.
3. Discussion
The purpose of this study was to investigate a possible role for spinal cord GABA in a model of HBO2-induced acute antinociception using a combination of pharmacological, behavioral and molecular biology methods. The GABAA antagonist SR 95531 attenuated HBO2-induced acute antinociception in a dose-dependent manner at very low doses (nanomolar range), thus implicating GABAA receptors in HBO2-induced antinociception. We were also able to enhance HBO2-induced antinociception by pretreating with nipecotic acid, an inhibitor of the GABA reuptake transporter (Johnston et al., 1979). This indicates that the synaptic levels of GABA are positively correlated with the magnitude of the HBO2-induced acute antinociceptive effect.
A role for the GABAB receptor was discounted as the doses of CGP 35348 used failed to affect the antinociceptive effect of HBO2. CGP 35348 alone also produced no change in abdominal constrictions. We conclude that spinal GABAB receptors are not involved in HBO2-induced acute antinociception in mice, although it is possible that the doses used were too low to influence the antinociceptive effect of HBO2. However, CGP 35348 doses comparable to ours administered into the mouse lateral cerebral ventricle antagonized (±)-baclofen-induced antinociception in the abdominal constriction test (Malcangio et al., 1991). It is possible that this dose is not high enough when administered into the thecal space compared to ventricular space. Unpublished data from our lab indicates that this might not be the case as intracerebroventricular administration of the same doses of CGP35348 also failed to block HBO2. This evidence supports the supposition that antagonism of the GABAB receptor does not affect antinociception under HBO2.
Previously, we demonstrated that the antinociceptive effect of an 11-min HBO2 treatment was modulated in part by both NO and opioid mechanisms (Heeman et al., 2013; Ohgami et al., 2009; Quock et al., 2011). Our current results indicate that acetic acid decreases nNOS expression in the lumbar spinal cord and this effect is not as prevalent in animals treated with HBO2. In another study, it was observed that a 60-min treatment of HBO2 increased levels of NO oxidation products in the spinal cord of adult rats (Ohgami et al., 2008). Furthermore, the involvement of NO was verified by dose-related antagonism of HBO2-induced antinociception by i.t. administered nitric oxide synthase (NOS) inhibitors and attenuation of the HBO2 effect by using nNOS knockout mice and antisense oligodeoxynucleotides to nNOS to knock down NOS (Ohgami et al., 2009). Our current results indicate that GABA acting at the GABAA receptor in the spinal cord may also be involved in the HBO2 induced antinociception. Our current results do not conclusively link HBO2 effects on nNOS to effects HBO2 may have on GABA although previous studies have indicated that nNOS and may influence GABAergic signaling. GABA localization studies indicate the presence of many GABAergic interneurons in the spinal cord, especially in the superficial lamina (Sardella et al., 2011). In particular, co-localized nNOS- and GABA-immunoreactive neurons have been found in lamina I and lamina II, and nNOS was found in terminal boutons containing vesicular GABA transporters (Sardella et al., 2011). Therefore, this is a possible pathway in which GABA may interact with the NO-sGC-cGMP pathway, which has been implicated in HBO2-induced antinociception (Ding et al., 2017; Quock et al., 2011).
Isoflurane administration during i.t. procedures can complicate the interpretation of our results since isoflurane has been shown to have both neuroprotective and neurotoxic affects (Jiang et al., 2017). Isoflurane pretreatment has been implicated as having protective effects in ischemia in animal models (Jiang et al., 2017). In animal models of neural injury in neonatal rats, isoflurane was shown to decrease the hypoxia-related increase in the release of amino acid neurotransmitters while elevating levels of GABA (Zhao et al., 2016). However, in the above experiment, animals were exposed to varying concentrations of isoflurane for a prolonged period — 6 hours (Zhao et al., 2016). In our experiments, animals were exposed to isoflurane at a concentration of 2.5% isoflurane for no more than 5 min at a time. In addition, all our animals receiving i.t. injections received isoflurane so any effect of isoflurane on GABA concentrations would be consistent across all animals receiving i.t. injections.
It should be noted, however, there are other possible explanations for these results. A previous study using a chronic constriction injury (CCI) model of neuropathic pain found that HBO2 was effective at decreasing CCI induced allodynia (Fu et al., 2017). Furthermore, while CCI increased apoptosis of GABAergic neurons in the dorsal horn, HBO2 administration inhibited this effect which protects the balance between excitation and inhibition in the spinal cord (Fu et al., 2017). The results of this paper implicate a neuroprotective effect of HBO2 on GABAergic neurons in the dorsal horn of the spinal cord in relief of neuropathic pain (Fu et al., 2017). While our experiments did not investigate neuroprotective effects of HBO2, they do implicate GABA as having a role in HBO2-induced antinociception which is in agreement with the prior experiment. HBO2-induced antinociception could be caused by interactions between the opioidergic and GABAergic pathways that are induced by changes cause by HBO2. Still other possibilities could include supraspinal serotonergic and/or noradrenergic pathways that recruit local GABAergic inhibitory neurons in the spinal cord (Millan, 2002). These data indicate that the pathways involved in HBO2-induced antinociception are complex and interactive. More research is needed to distinguish exactly how various pathways interact in HBO2 induced antinociception.
The western blotting results indicate an interaction between the noxious stimulant for abdominal constrictions, glacial acetic acid, and HBO2 in expression of the phosphorylated β3 subunit of the GABAA receptor in the lumbar but not thoracic spinal cord. Glacial acetic acid and HBO2 independently decreased expression of the pβ3 subunit of the GABAA receptor; however, in the presence of both, expression of the GABAA receptor subunit was restored to control levels. Isoflurane was not given to animals involved in western blotting eliminating the possibility that elevated GABA levels related to isoflurane exposure could explain these results (Zhao et al., 2016). This could indicate that, in the presence of an acute pain stimulus, expression is reduced, but this decrease is reversed by HBO2. In contrast to the GABAA receptor, HBO2 did not fully restore nNOS expression in the spinal cord to control levels. In the presence of noxious stimulation, nNOS expression in the spinal cord is significantly decreased. HBO2 did slightly increase expression of nNOS in the lumbar spinal cord in the presence of pain, but this effect was not statistically significant. HBO2 alone itself decreased nNOS expression compared to control, and this is not significantly affected by the presence or absence of noxious stimulation, although the reduction in nNOS in the lumbar spinal cord was not as great as that in the presence of pain. These results indicate that, in the lumbar spinal cord, HBO2 increases the expression of the phosphorylated β3 subunit of the GABAA receptor. We focused on this receptor because phosphorylation at this site could indicate increased activity of the receptor (McDonald et al., 1998). Thus, restoration of phosphorylation of this site by HBO2 and painful stimuli could indicate that GABA activity at the GABAA receptor in lumbar spinal cord is restored when both HBO2 is producing antinociception. However, this effect is not dependent upon nNOS pathways because, while nNOS expression is also decreased when pain is present, it is not restored by HBO2.
We hypothesize that HBO2 increases GABA activity at the GABAA receptor via a nitric oxide dependent mechanism. Blotting for the phosphorylated β3 subunit of the GABAA receptor should theoretically be increased when there is more GABA activity at the receptor (McDonald et al., 1998). We expected that more phosphorylation at the GABAA receptor would occur after a pain stimulus due to increased activation of inhibitory post-synaptic processes at the lumbar level of the spinal cord. An increase in phosphorylation at the β3 subunit has been linked to both increased and decreased receptor function of the GABAA receptor (Brandon et al., 2000; McDonald et al., 1998). This effect may be partially caused by the protein kinase that phosphorylated the subunit. Specifically, a major site of regulation on the β3 subunit of the GABAA receptor is the serine 408/409 phosphorylation site. PKA can differentially regulate phosphorylation to either increase activation or decrease activation based on whether both serines 408 and 409 are phosphorylated (potentiation of GABAA activity) or whether just 409 is phosphorylated (inhibition of GABAA receptor activity) (McDonald et al., 1998). Our antibody was specific for phosphorylation of both serine 408 and 409 so it is reasonable to infer that a decrease in expression can be linked to inhibition of activity and an increase (or restoration) can be linked to potentiation of GABAA receptor activity. Our results support the conclusion that glacial acetic acid and HBO2 separately inhibit GABAA receptor activity. However, when combined, HBO2 reversed the decreased activity. These results support our behavioral finding that blockade of the GABAA receptor reduces antinociceptive activity of HBO2 in the acetic acid test.
Glacial acetic acid caused a decrease in nNOS expression that was partially prevented by HBO2. This supports earlier findings from our lab that blocking NO could antagonize the antinociceptive effect of HBO2 because NO release being at levels seen prior to the pain stimulus would be required for antinociception under HBO2 (Ohgami et al., 2009; Quock et al., 2011). We expected HBO2 to further increase this level and that this would coincide with an increase in nNOS levels. Similarly, we expected nNOS expression to be increased after HBO2. While nNOS expression was higher under HBO2 when the animals were exposed to glacial acetic acid, levels of expression were decreased, although not significantly, compared to control. In neuropathic and inflammatory pain models, NO is hypothesized to play a role in central sensitization (Wu et al., 2001). However, NO has also been found to have an antinociceptive role in pain (Schmidtko et al., 2009).
Our results could indicate that, while HBO2 does prevent or decrease expression of the phosphorylated β3 subunit of the GABAA receptor in the presence of a noxious stimulus, this effect does not coincide with an increase in nNOS above normal levels. Therefore, we cannot conclude that the effects of antinociceptive effects of HBO2 involve a pathway involving both nNOS and GABA. However, it should be important to note that we only blotted for one subunit of the GABAA receptor and our results might not hold for phosphorylation at any of the other receptor subunits. In addition, we may have a low density of β3 subunits in the lumbar spinal cord. This is not likely because immunohistochemical studies have found that β3 subunits are widespread in the dorsal horn of the spinal cord, although not in the motor neurons (Bohlhalter et al., 1996). We also did not examine whether either of the other two NOS isoforms—endothelial NOS (eNOS) or inducible NOS (iNOS)—might increase under these same conditions. If so, this might compensate for the lower amounts of available NO from nNOS. Indeed, prior studies with knockout mice indicate that this is likely as inhibition of any one of the three isoforms can result in a compensatory increase in the other two isoforms (Boettger et al., 2007). It is also possible that we did not wait sufficiently long for major changes in nNOS expression to occur. Since nNOS is post-transcriptionally regulated, it may take longer than the time elapsed in this study for significant changes to occur so we may not be collecting samples at the time of maximum change. Indeed, changes in nNOS levels and NO activity were generally seen at later time points in previous studies. The maximum time varies, but other studies have found it to be at least 30 min after injections of acetic acid (Larson et al., 2000; Shi et al., 2005; Wu et al., 2001). Animals in this study were sacrificed immediately after decompression and 30 min did not elapse from the time of the initial injection to the time of sacrifice.
In the thoracic spinal cord, neither glacial acetic acid nor HBO2 had an effect on nNOS expression or expression of the phosphorylated β3 subunit of the GABAA receptor. This result was not unexpected due to the preponderance of nociceptive signaling in the lumbar versus thoracic spinal cord. In addition, the acetic acid test is not necessarily a valid model of visceral pain due to specificity problems with the model; for example, non-analgesics have been found to have antinociceptive activity according to the model, and the abdominal constrictions include the muscles of the abdominal wall rather than just the organs of the viscera (Langford and Mogil, 2011; Ness, 1999). Despite this criticism, many laboratories use the acetic acid test as a model of visceral pain because it has been a very powerful predictive tool and has a unique ability to detect weaker antinociceptive drugs (Langford and Mogil, 2011). Indeed, some labs use the acetic acid test as an assay of chemical nociception rather than a model of visceral pain (Lariviere et al., 2002; Spindola et al., 2012). Our study found that an injection of glacial acetic acid into the peritoneal cavity had no effect on protein expression in the spinal cord. In other pain models such as the formalin and capsaicin models, nNOS expression has been shown to increase in response to noxious insult in the spinal cord (Shi et al., 2005; Wu et al., 2001).
4. Conclusions
We found evidence that the GABAA receptor is involved in HBO2-induced antinociception because HBO2-induced acute antinociception was antagonized in a dose-dependent manner by the selective competitive GABAA antagonist SR 95531. Preventing GABA reuptake using the GAT inhibitor nipecotic acid increased the antinociceptive response to an 11-min treatment of HBO2. The GABAB antagonist CGP 35348 failed to antagonize HBO2-induced antinociception at the doses used. HBO2 did restore expression of the phosphorylated β3 subunit of the GABAA receptor in the presence of acetic acid. It is possible that the antinociceptive effects of HBO2 are caused by preventing or restoring activity of GABAergic inhibitory interneurons in the lumbar region of the spinal cord. We were unable to conclusively link this effect to NO system because HBO2 failed to restore nNOS expression in the presence of acetic acid or by itself.
5. Experimental Procedure
5.1. Animals
A total of 221 Male NIH Swiss mice, 24–26 g, were used in these experiments (Harlan Laboratories, Indianapolis, IN). Animals were group-housed (4 per cage) in standard mouse cages (11.5 cm × 16.5 cm × 28.0 cm) and kept under standard laboratory conditions (22 ± 1°C room temperature, 33% humidity) on a 12-h light cycle from 0600 to 01800 h. All animals were housed in the Animal Resource Unit and Wegner Hall Vivarium of Washington State University, which are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals received food and water ad libitum. Mice were kept in the holding room for four days after arrival in the facility for acclimation prior to experimentation. All procedures were approved by the Washington State University Institutional Animals Care and Use Committee (IACUC) and subject to post-approval review and carried out in accordance with The Guide for the Care and Use of Laboratory Animals, 8th Edition (National Academies Press, Washington, DC, 2011). All measures to minimize pain or discomfort were taken by the investigators.
5.2. HBO2 treatment
A single cage of four mice was placed in a B-11 research hyperbaric chamber (Reimers Systems Inc., Lorton, VA) following i.p. injection with glacial acetic acid. The B-11 research chamber consists of a cylindrical clear acrylic chamber (27.9 cm diameter × 55.9 cm L). The chamber was ventilated with 100% oxygen (O2), U.S.P. (AL Compressed Gases Inc., Spokane, WA) at a flow rate of 20 L/min. The pressure inside the chamber was increased to the desired pressure of 3.5 atmospheres absolute (ATA) over a period of about two min. The mice breathed normally during the HBO2 treatment. The pressure was held at 3.5 ATA for about three min prior to the start of the 6-min observation period. After completion of the HBO2 treatment, animals were decompressed over the next 2–3 min.
5.3. Abdominal constriction test
Antinociception was assessed using the abdominal constriction test (Siegmund et al., 1995). Briefly, mice were injected i.p. with 0.1 ml/10 g body weight of 0.6% glacial acetic acid. After 5 min, the number of abdominal constrictions—lengthwise stretches of the torso with concave arching of the back—were counted for the next 6 min. The experimenter was not blinded to treatment condition; however, some vehicle controls were run simultaneously for direct comparison of drug group and vehicle controls. Calculation of the degree of antinociception was computed using the formula below:
5.4. Intrathecal (i.t.) microinjections
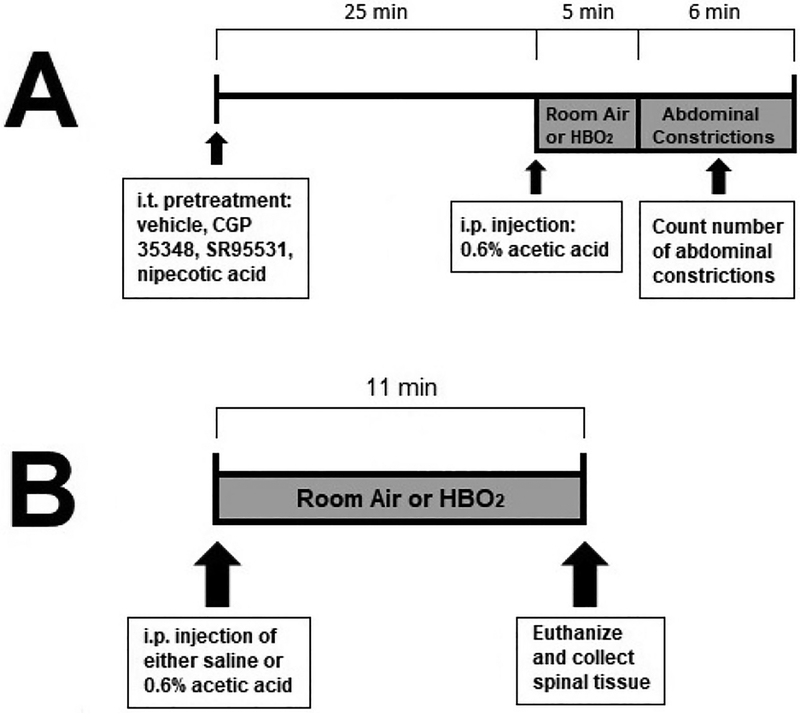
I.t. pretreatments were made using the microinjection technique of Hylden and Wilcox (1980). Briefly, mice were anesthetized with 2% isoflurane in oxygen in an anesthesia chamber or with a nosecone during microinjection. The animal was held by the pelvis with the hind legs extended backward. The microinjection was made through the skin into the spinal cord. A luer-tipped 10-μl microsyringe was fitted with a ½-inch, 30-gauge disposable needle. This needle was then inserted between the lumbar vertebrae below L6. The volume of solution injected i.t. was 5.0 μl delivered over 30 s. Experiments only proceed when the experimenter has achieved >75% accuracy. In addition, experimenters use the hips as an external landmark and hold the hips of the mouse firmly while completing the microinjection. Once the intrathecal space has been penetrated, foot and tail reactions are sought before proceeding with the injection. Intrathecal microinjections occurred 25 min prior to acetic acid injections so that assessment began 30 min following pretreatment. Animals were placed into the hyperbaric chamber immediately following the glacial acetic acid injections. A timeline of these experiments can be seen in figure 1A.
Fig. 1.
Timeline of experiments. (A) depicts the typical time course of i.t. pretreatment experiments to investigate the effect of antagonist pretreatment on the antinociceptive effect of HBO2. Animals were treated with one of four possible treatments: Saline vehicle, SR 95531, CGP 35348, and nipecotic acid, on the antinociceptive effect of HBO2. (B) Depicts the time course of tissue collection for western blot experiments.
5.5. Drugs
Three drugs were used in this study: 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR 95531 – a GABAA receptor antagonist); (3-aminopropyl)-(diethoxymethyl)-phosphinic acid (CGP 35348 – a GABAB receptor antagonist); and (±)-3-piperidine carboxylic acid [(±)-nipecotic acid – a GABA transporter inhibitor]. All three drugs were purchased from Tocris Biosciences (a subsidiary of Bio-Techne, Minneapolis, MN). All drugs were freshly prepared in 0.9% physiological saline prior to administration by i.t. microinjection. The doses were derived from the published literature (Georgiev et al., 1995; Malcangio et al., 1991; Song et al., 1998). Fifty-one mice were randomly assigned to be treated with either saline vehicle or one of four doses of SR95531. Thirty min after the i.t. injection, the animals were injected with acetic acid and immediately exposed to HBO2. An additional 33 mice were given an i.t. injection of either saline vehicle or one of the two highest doses of SR95531. Twenty-nine mice were randomly assigned to be injected with one of three doses of CGP 3534. All of these mice were injected with acetic acid 30 min after i.t. injection and immediately treated with HBO2. An additional 8 mice were given an i.t. injection of either saline vehicle or the highest dose of CGP 35348 30 min prior to room air; these mice were treated with room air and were used as a control. Finally, 53 mice received either saline or a randomly selected dose of nipecotic acid and exposed to HBO2 while undergoing the acetic acid test. An additional 20 mice were given an i.t. injection of the two highest doses of nipecotic acid or vehicle.
5.6. Lysate preparation
A separate group of 24 mice was randomly assigned to receive either saline or acetic acid injections. Immediately following injections, mice were treated with either room air or HBO2. Immediately following cessation of HBO2 treatment (or exposure to room air), animals were sacrificed and spinal cords were removed by forcible injection of saline into the spinal column and recovered from the decapitated end of the column. A time line of these experiments can be seen in figure 1B. Spinal cords were dissected on ice and all but the lumbar and thoracic regions of the spinal cord were discarded. A volume of 1000 μl of ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer, containing commercially available phosphatase and protease inhibitor cocktails (Biotool.com, Houston, TX), was added to a sample of about 5.0 mg of spinal cord. This volume was chosen because of the high concentration of protein. Tissues were homogenized with a manual homogenizer. The homogenate was centrifuged for 20 min at 4°C. The supernatant was transferred to a fresh tube kept on ice, and the pellet was discarded.
5.7. Preparation of samples
Protein concentration of the homogenized spinal cord was determined using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific Pierce, Rockford, IL) and a Synergy Neo Plate reader with Genmate software (BioTek Instruments Inc., Winooski, VT). All protein concentrations were determined by optical density and compared to a standard curve of BSA (bovine serum albumin) in accordance with the BCA kit instructions.
After protein quantification, samples were prepared for western blotting by adding loading buffer to samples. Sixty μg protein were added to each well as follows: Pre-stained protein ladder, control, acetic acid, acetic acid + HBO2, and HBO2 on each gel.
5.8. Western immunoblotting
Protein samples were run on a 10% poly-acrylamide SDS PAGE gel. The gel was inserted into the tank and then run at 95 volts for 120 min. The protein was then transferred to nitrocellulose paper at a current of 350 mA for 60 min. The membranes were blocked in 5% bovine serum albumin (BSA) in a mixture of Tris-buffered saline and Tween 20 (TBS-T) for 60 min at room temperature. After blocking, the nitrocellulose paper was cut at about 120 kDA, 90 kDA and 40 kDA, using the pre-stained protein ladder so that the standard protein concentrations could be compared to the protein of interest. The separate pieces of nitrocellulose were washed briefly with TBS-T.
Following the washing steps, the primary antibodies for phosphorylated GABAA Rβ3 antibody (P1130–4089, PhosphoSolutions, Aurora, CO; NBP-2508, Novus Biologicals, Littleton, CO), nNOS (4231S, Cell Signaling, Danvers, MA), and glyceraldehyde-3-phospahte dehydrogenase (GAPDH) (PA1–988, ThermoFisher Scientific, Grand Island, NY; A300–641A-T, Bethyl Laboratories Inc., Montgomery, TX) were added to the membrane piece containing their corresponding proteins. All primary antibodies were generated in rabbit and diluted to a concentration of 1:1000 in 1% BSA in TBS-T. Primary antibody was allowed to sit on the membrane overnight at 4°C with gentle agitation. The following morning, membrane pieces were washed three times for 10 min in TBS-T. Secondary anti-rabbit antibody (7074S, Cell Signaling, Danvers, MA) conjugated to horseradish peroxidase was applied to each membrane piece for 120 min at room temperature at a concentration of 1:1000 in TBS-T. The membrane pieces were washed again three times at 15 min per wash in TBS-T. Supersignal chemiluminescent substrate (Thermo Scientific) was added to each membrane piece, and the pieces were exposed to fluorescent light using a Chemidoc Imaging System (Biorad, Hercules, CA). Finally, relative expression was analyzed by densitometry using Chemidoc software.
5.9. Statistical analysis of data
Percent changes in antinociception were analyzed using a univariate analysis of variance (ANOVA) test for each drug. Where significant differences were found, a post hoc Dunnett’s multiple comparison test was used to assess HBO2-induced antinociception between groups. For room air controls, univariate ANOVA tests were run for two of the drugs, but one of the antagonists only had two groups at room air so an unpaired t-test was run for this group to make a pairwise comparison. The α-level was set at 0.05 to protect against type I error.
A multivariate ANOVA was used to compare GABA/GAPDH and nNOS GAPDH expression between groups. A post hoc Tukey’s test was used for multiple comparisons between groups.
Fig. 7.
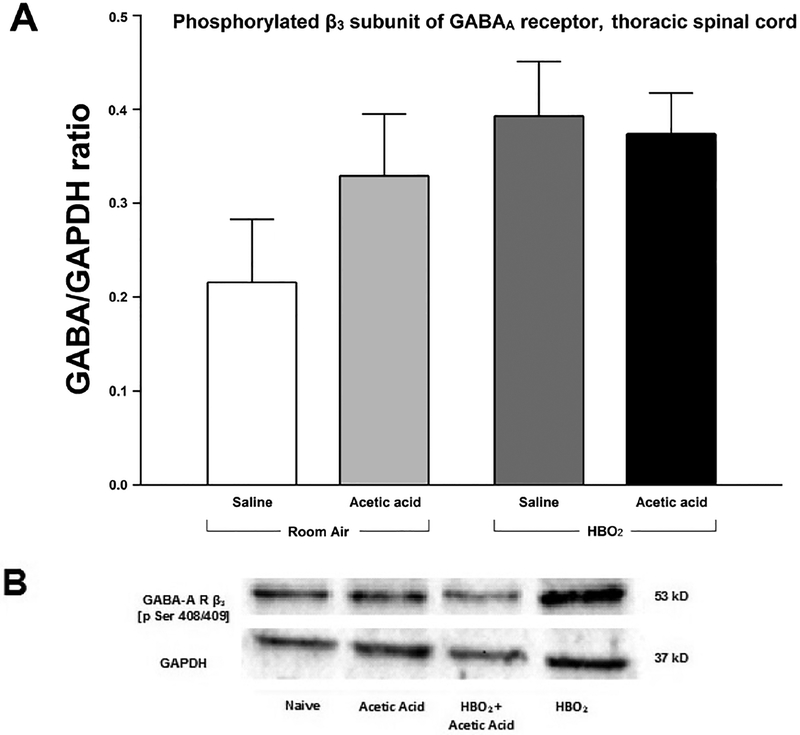
(A) Effect of glacial acetic acid and HBO2 treatment on the optical density of phosphorylated β3 subunit of the GABAA receptor in the thoracic section of the spinal cord. Each column represents the ratio compared to naïve of GABAA β3 Pser408/409 to GAPDH ratios of 6 western blots representing 2 separate samples per group. (B) Representative example of typical western blot of GABAA β3 Pser408/409 and GAPDH.
Fig. 8.
(A) Effect of glacial acetic acid and HBO2 treatment on optical density of neuronal nitric oxide synthase in the lumbar section of the spinal cord. No significant differences were found. Each column represents the ratio compared to naïve of nNOS to GAPDH ratios of 6 western blots representing at least 2 separate samples. (B) Representative example of western blot of lumbar spinal cord showing expression of nNOS and GAPDH.
Highlights.
HBO2-induced antinociception is mediated by spinal GABAA but not GABAB receptors.
Acetic acid reduced expression of nNOS and GABAA β3pSER 408/409.
HBO2 treatment restored nNOS and GABAA β3pSER 408/409 expression to control levels.
Protein expression was altered in lumbar but not thoracic spinal cord.
Acknowledgements
This research was supported by NIH Grant AT-007222, the Allen I. White Distinguished Professorship from Washington State University, the Chico Hyperbaric Center (Chico, California), and the Chinese Scholarship Council (Joint Doctoral Program).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boettger MK, Üceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C, 2007. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur. J. Pain 11, 810–818. 10.1016/j.ejpain.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM, 1996. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J. Neurosci 16, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ, 2000. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem 275, 38856–38862. 10.1074/jbc.M004910200 [DOI] [PubMed] [Google Scholar]
- Chung E, Zelinski LM, Ohgami Y, Shirachi DY, Quock RM, 2010. Hyperbaric oxygen treatment induces a 2-phase antinociceptive response of unusually long duration in mice. J. Pain 11, 847–853. 10.1016/j.jpain.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabato F, Fusco BM, Pelaia P, Giacovazzo M, 1993. Hyperbaric oxygen therapy in cluster headache. Pain 52, 243–245. https://doi.org/0304-3959(93)90137-E [pii] [DOI] [PubMed] [Google Scholar]
- Ding Y, Yao P, Hong T, Han Z, Zhao B, Chen W, 2017. The NO-cGMP-PKG signal transduction pathway is involved in the analgesic effect of early hyperbaric oxygen treatment of neuropathic pain. J. Headache Pain 18, 51 10.1186/s10194-017-0760-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrati S, Golan H, Bechor Y, Faran Y, Daphna-Tekoah S, Sekler G, Fishlev G, Ablin JN, Bergan J, Volkov O, Friedman M, Ben-Jacob E, Buskila D, 2015. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome—a prospective clinical trial. PLoS One 10, 1–25. 10.1371/journal.pone.0127012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE, 2006. The role of GABA in the mediation and perception of pain. Adv. Pharmacol 54, 1–27. 10.1016/S1054-3589(06)54001-3 [DOI] [PubMed] [Google Scholar]
- Fenselau H, Heinke B, Sandkuhler J, 2011. Heterosynaptic long-term potentiation at GABAergic synapses of spinal lamina I neurons. J. Neurosci 31, 17383–17391. 10.1523/JNEUROSCI.3076-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Li F, Thomas S, Yang Z, 2017. Hyperbaric oxygenation alleviates chronic constriction injury (CCI)-induced neuropathic pain and inhibits GABAergic neuron apoptosis in the spinal cord. Scand. J. Pain 17, 330–338. 10.1016/j.sjpain.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Garthwaite J, 2008. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci 27, 2783–2802. 10.1111/j.1460-9568.2008.06285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev VP, Lazarova MB, Kambourova TS, 1995. Further evidence for the interactions between angiotensin II and GABAergic transmission in pentylenetetrazol kindling seizures in mice. Neuropeptides 28, 29–34. 10.1016/0143-4179(95)90071-3 [DOI] [PubMed] [Google Scholar]
- Gibbons CR, Liu S, Zhang Y, Sayre CL, Levitch BR, Moehlmann SB, Shirachi DY, Quock RM, 2013. Involvement of brain opioid receptors in the anti-allodynic effect of hyperbaric oxygen in rats with sciatic nerve crush-induced neuropathic pain. Brain Res 1537, 111–116. 10.1016/j.brainres.2013.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Niu J, Liu WT, Sun Y, Liu SB, Jie Lv Y, Long Dong H, Song X, Xiong LZ, 2012. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur. J. Pain 16, 1094–1105. [DOI] [PubMed] [Google Scholar]
- Heeman JH, Zhang Y, Shirachi DY, Quock RM, 2013. Involvement of spinal cord opioid mechanisms in the acute antinociceptive effect of hyperbaric oxygen in mice. Brain Res. 1540, 42–47. 10.1016/j.brainres.2013.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL, 1980. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol 67, 313–316. 10.1016/0014-2999(80)90515-4 [DOI] [PubMed] [Google Scholar]
- Jiang M, Sun L, Feng D, Yu Z, Gao R, Sun Y, Chen G, 2017. Neuroprotection provided by isoflurane pre-conditioning and post-conditioning. Med. Gas Res 7, 48–55. 10.4103/2045-9912.202910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GAR, Allan RD, Kennedy SMG, Twitchin B, 1979. Systematic study of GABA analogues of restricted conformation, in: Krogsgaard-Larsen P, Scheel-Krüger H, Kofoed H (Eds.), GABA-Neurotransmitter: Pharmacological Biochemical and Pharmacological Aspects. Munksgaard, Copenhagen, pp. 149–164. [Google Scholar]
- Katznelson R, Segal SC, Clarke H, 2016. Successful treatment of lower limb complex regional pain syndrome following three weeks of hyperbaric oxygen therapy. Pain Res. Manag 2016, 3458371 10.1155/2016/3458371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiralp MZ, Uzun G, Dinçer O, Sen A, Yildiz S, Tekin L, Dursun H, 2009. A novel treatment modality for myofascial pain syndrome: hyperbaric oxygen therapy. J. Natl. Med. Assoc 101, 77–80. [DOI] [PubMed] [Google Scholar]
- Kiralp MZ, Yildiz S, Vural D, Keskin I, Ay H, Dursun H, Yildiz Ş, Vural D, Keskin I, Ay H, Dursun H, 2004. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J. Int. Med. Res 32, 258–262. 10.1177/147323000403200304 [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frølund B, Liljefors T, Ebert B, 2004. GABAA agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem. Pharmacol 68, 1573–1580. 10.1016/j.bcp.2004.06.040 [DOI] [PubMed] [Google Scholar]
- Kunchandy J, Kulkarni SK, 1987. Naloxone-sensitive and GABAA receptor mediated analgesic response of benzodiazepines in mice. Methods Find. Exp. Clin. Pharmacol 9, 95–99. [PubMed] [Google Scholar]
- Kuriyama K, Ohkuma S, 1995. Role of nitric oxide in central synaptic transmission: effects on neurotransmitter release. Jpn. J. Pharmacol 69, 1–8. 10.1254/jjp.69.1 [DOI] [PubMed] [Google Scholar]
- Lange MD, Doengi M, Lesting J, Pape HC, Jüngling K, 2012. Heterosynaptic long-term potentiation at interneuron-principal neuron synapses in the amygdala requires nitric oxide signalling. J. Physiol 590, 131–143. 10.1113/jphysiol.2011.221317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Mogil JS, 2011. Pain testing in the laboratory mouse, in: Fish RE, Brown MJ, Danneman PJ, Karas AZ (Eds.), Anesthesia and Analgesia in Laboratory Animals. Academic Press, pp. 549–560. [Google Scholar]
- Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS, 2002. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain 97, 75–86. 10.1016/S0304-3959(01)00492-4 [DOI] [PubMed] [Google Scholar]
- Larson AA, Kovacs KJ, Cooper JC, Kitto KF, 2000. Transient changes in the synthesis of nitric oxide result in long-term as well as short-term changes in acetic acid-induced writhing in mice. Pain 86, 103–111. 10.1016/S0304-3959(00)00236-0 [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ghelardini C, Giotti A, Malmberg-Aiello P, Bartolini A, 1991. CGP 35348, a new GABAB antagonist, prevents antinociception and muscle-relaxant effect induced by baclofen. Br. J. Pharmacol 103, 1303–1308. 10.1111/j.1476-5381.1991.tb09784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG, 1998. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci 1, 23–28. 10.1038/223 [DOI] [PubMed] [Google Scholar]
- Millan MJ, 2002. Descending control of pain. Prog. Neurobiol 66, 355–474. 10.1016/S0301-0082(02)00009-6 [DOI] [PubMed] [Google Scholar]
- Myers DE, Myers RA, 1995. A preliminary report on hyperbaric oxygen in the relief of migraine headache. Headache 35, 197–199. 10.1111/j.1526-4610.1995.hed3504197.x [DOI] [PubMed] [Google Scholar]
- Ness TJ, 1999. Models of visceral nociception. ILAR J 40, 119–128. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA, 2009. PKG and PKA Signaling in LTP at GABAergic synapses. Neuropsychopharmacology 345, 1829–1842. 10.1038/npp.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami Y, Chung E, Shirachi DY, Quock RM, 2008. The effect of hyperbaric oxygen on regional brain and spinal cord levels of nitric oxide metabolites in rat. Brain Res Bull 75, 668–673. 10.1016/j.brainresbull.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Zylstra CC, Quock LP, Chung E, Shirachi DY, Quock RM, 2009. Nitric oxide in hyperbaric oxygen-induced acute antinociception in mice. Neuroreport 20, 1325–1329. 10.1097/WNR.0b013e3283305a49 [DOI] [PubMed] [Google Scholar]
- Peach G, 1995. Hyperbaric oxygen and the reflex sympathetic dystrophy syndrome: a case report. Undersea Hyperb. Med 22, 407–408. [PubMed] [Google Scholar]
- Quock LP, Zhang Y, Chung E, Ohgami Y, Shirachi DY, Quock RM, 2011. The acute antinociceptive effect of HBO2 is mediated by a NO-cyclic GMP-PKG-KATP channel pathway in mice. Brain Res 1368, 102–107. 10.1016/j.brainres.2010.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady JJ, Fujimoto JM, 1996. Supraspinal delta2 opioid agonist analgesia in Swiss-Webster mice involves spinal GABAA receptors. Pharmacol. Biochem. Behav 54, 363–369. 10.1016/0091-3057(95)02150-7 [DOI] [PubMed] [Google Scholar]
- Rady JJ, Fujimoto JM, 1995. Spinal GABA receptors mediate brain delta opioid analgesia in Swiss Webster mice. Pharmacol. Biochem. Behav 51, 655–659. 10.1016/0091-3057(94)00433-J [DOI] [PubMed] [Google Scholar]
- Sardella TCP, Polgár E, Watanabe M, Todd AJ, 2011. A quantitative study of neuronal nitric oxide synthase expression in laminae I-III of the rat spinal dorsal horn. Neuroscience 192, 708–720. 10.1016/j.neuroscience.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, 1987. GABAergic mechanisms of analgesia: an update. Pharmacol. Biochem. Behav 26, 463–474. 10.1016/0091-3057(87)90148-1 [DOI] [PubMed] [Google Scholar]
- Schmidtko A, Tegeder I, Geisslinger G, 2009. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci 32, 339–346. 10.1016/j.tins.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Shi X, Li X, Clark JD, 2005. Formalin injection causes a coordinated spinal cord CO/NO-cGMP signaling system response. Mol. Pain 1, 33 10.1186/1744-8069-1-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund E, Cadmus R, Lu G, 1995. A method for evaluating both non-narcotic and narcotic analgesics. Proc. Soc. Exp. Biol. Med 4, 729–731. [DOI] [PubMed] [Google Scholar]
- Song DK, Suh HW, Huh SO, Jung JS, Ihn BM, Choi IG, Kim YH, 1998. Central GABAA and GABAB receptor modulation of basal and stress-induced plasma interleukin-6 levels in mice. J. Pharmacol. Exp. Ther 287, 144–149. [PubMed] [Google Scholar]
- Spindola HM, Vendramini-Costa DB, Rodrigues MT, Foglio MA, Pilli RA, Carvalho JE, 2012. The antinociceptive activity of harmicine on chemical-induced neurogenic and inflammatory pain models in mice. Pharmacol. Biochem. Behav 102, 133–138. 10.1016/j.pbb.2012.03.030 [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Takahashi M, Kaneto H, 1992. Participation of GABAergic systems in the production of antinociception by various stresses in mice. Jpn. J. Pharmacol 60, 105–110. 10.1254/jjp.60.105 [DOI] [PubMed] [Google Scholar]
- Weaver LK (ed.), 2014. Hyperbaric Oxygen Therapy Indications, 13th Ed. Best Publishing Company, North Palm Beach. [Google Scholar]
- Warren J, Sacksteder MR, Thuning CA, 1979. Therapeutic effect of prolonged hyperbaric oxygen in adjuvant arthritis of the rat. Arthritis Rheum. 22, 334–339. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Foresman BH, Gamber RG, Wright T, 1998. Hyperbaric oxygen in the treatment of migraine with aura. Headache 38, 112–115. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD, 2001. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain 94, 47–58. 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Kiralp MZ, Akin A, Keskin I, Ay H, Dursun H, Çimşit M, 2004. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J. Int. Med. Res 32, 263–267. 10.1177/147323000403200305 [DOI] [PubMed] [Google Scholar]
- Zelinski LM, Ohgami Y, Chung E, Shirachi DY, Quock RM, 2009. A prolonged nitric oxide-dependent, opioid-mediated antinociceptive effect of hyperbaric oxygen in mice. J. Pain 10, 167–172. 10.1016/j.jpain.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D-A, Bi L-Y, Huang Q, Zhang F-M, Han Z-M, 2016. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury by suppressing apoptosis. Brazilian J. Anesthesiol 66, 613–621. 10.1016/j.bjane.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD, 1993. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science 260, 1946–1950. 10.1126/science.8100368 [DOI] [PubMed] [Google Scholar]