Abstract
Previously, we reported that electroporation-mediated (EP) delivery of the FER gene improved survival in a combined trauma-pneumonia model. The mechanism of this protective effect is unknown. In this paper, we performed a pneumonia model in C57/BL6 mice with 500 CFU of Klebsiella pneumoniae. After inoculation, a plasmid encoding human FER was delivered by EP into the lung (PNA/pFER-EP). Survival of FER-treated vs. controls (PNA; PNA/EP-pcDNA) was recorded. In parallel cohorts, bronchial alveolar lavage (BAL) and lung were harvested at 24 and 72 h with markers of infection measured. FER-EP-treated animals reduced bacterial counts and had better 5-day survival compared to controls (80 vs 20 vs 25%; p<0.05). Pre-treatment resulted in 100% survival. With FER, inflammatory monocytes were quickly recruited into BAL. These cells had increased surface expression for Toll-receptor 2 and 4, and increased phagocytic and myeloperoxidase activity at 24 h. Samples from FER electroporated animals had increased phosphorylation of STAT transcription factors, varied gene expression of IL1β, TNFα, Nrf2, Nlrp3, Cxcl2, HSP90 and increased cytokine production of TNF-α, CCL-2, KC, IFN-γ and IL-1RA. In a follow-up experiment, using Methicillin-Resistant Staphylococcus aureus (MRSA) similar bacterial reduction effects were obtained with FER gene delivery. We conclude that FER overexpression improves survival through STAT activation enhancing innate immunity and accelerating bacterial clearance in the lung. This constitutes a novel mechanism of inflammatory regulation with therapeutic potential in the setting of hospital-acquired pneumonia.
Keywords: FER, pneumonia, electroporation, STAT3, HSP90, IL-1RA
INTRODUCTION
National Vital Statistic Reporting shows that overall mortality rates in the United States are in steady decline. However, death related to trauma and severe infection (pneumonia and sepsis) is increasing in prevalence(1–3). Patients in these categories are highly likely to have encountered a Hospital-associated infection (HAI). Methicillin-resistant Staphylococcus aureus (MRSA) and Klebsiella pneumonia and other Gram-negative bacteria are the most common organisms involved in HAI pneumonia (PNA)(4). Concern about potentially preventable sepsis related deaths from HAIs has prompt different health organizations to declare them as threats to public health and a global priority to be addressed(5).
Complicating the problem of increasing HAIs is the growing multidrug antibiotic resistance within this group, making them harder to treat and contributing to more virulent infections(4, 6–8). To counter, alternative pharmacologic compounds to existing antibiotic classes have been deployed. However, recent clinical reports show bacteria are also developing resistance to these agents, such as in the case of Colistin-resistant Escherchia coli and Carbapenem-resistant Klebsiella pneumoniae(9, 10). This warrants the investigation of new strategies to fight infectious diseases.
A novel and different approach is the use of transient short-term gene therapy to mobilize an immunological response against infection(11). Genomic inference and high through put analyses are currently identifying potential therapeutic targets. A recent Genome-wide association study (GWAS) published by Rautanen et al(12) found that FER, an evolutionary conserved non-receptor cytosolic tyrosine kinase, was associated with increased survival in patients with pneumonia and sepsis, regardless of age of subjects. In a proof of concept experiment, our laboratory using electroporation-mediated delivery of human FER gene (EP-hFER), found that its overexpression was protective and improved survival (80% vs 20%, p < 0.001) in a murine model of combined lung contusion and. pneumonia(13). However, neither of these studies was designed to understand the mechanisms by which FER conferred this protective immune response.
Based on our prior work, we found that transient FER overexpression was able to accelerate Gram-negative bacteria clearance from contused lungs. Thus, we hypothesize that this effect is related to an enhancement of the lung’s local immune response. In the following study we examine the role of FER gene overexpression through a model of primary Klebsiella pneumonia. Our data shows active involvement of FER in the recruitment and activation of inflammatory monocytes and macrophages, with corresponding modifications of known signaling transduction pathways that enhance bacterial clearance and therefore improve survival. Additionally, we show the presence of FER in human lungs with gross inflammation. These findings indicate a novel mechanism of inflammatory modulation within the lung. FER gene up regulation represents a promising avenue of research and therapeutic potential towards the resolution of severe bacterial pneumonia, complementary to traditional antibiotic therapy.
MATERIALS AND METHODS
Mouse model of Klebsiella pneumoniae Bacterial Pneumonia (PNA)
Female C57BL/6 wild-type mice, 18-20 g weight (Charles River Laboratories, Wilmington, MA, USA) were used for Klebsiella bacterial infection experiments. Mice were housed under specific pathogen-free conditions and were allowed a 1-week acclimation period to their new surroundings prior to use. All experiments were performed in accordance with National Institutes of Health guidelines for care and use of animals. Approval for all experimental work was obtained from the University of Michigan Committee on Use and Care of Animals (UCUCA) Protocol PRO00006392 and the Institutional Biosafety Committee (IBC) Protocol IBCA00000315.
To induce bacterial pneumonia, we administered Klebsiella pneumoniae, strain 43816, serotype 2 (American Type Culture Collection, Manassas, VA, USA) at 1h before or after electroporation. We followed our previously reported methodology of PNA, which produces consistent and significant mortality (80% at 7 days)(13–16). A concentration of 500 CFUs of bacteria, achieved by serial dilution in a 30 μl of inoculum corresponding to LD50 for this particular strain of Klebsiella in C57/Bl6 mice, was used. The administration of the bacterial suspension was performed via deep oral hypopharynx injection under isoflurane anesthesia. Animals were allowed to recover spontaneously and transported back to Biosafety Level-2 (BSL2) housing where animal health and survival data were recorded every 8 h.
Recombinant plasmid DNA
pSG5-FER (human FER tyrosine kinase) plasmid was purchased from Addgene (Cambridge, MA, USA) and propagated in E. coli. Plasmids were harvested using QIAGEN Giga-prep kits (Valencia, CA, USA) as per manufacturer’s instructions. The original pSG5-FER plasmid was a gift from Nora Heisterkamp deposited in Addgene DNA repository (Addgene plasmid # 30191). The expression cassette encodes human FER (hFER), which is a non-receptor tyrosine kinase located on human chromosome 5q21 (Gene ID 2241; HPRD 01491; NM_005246). An empty plasmid -pcDNA3.1 (Promega, Madison, WI, USA) containing similar backbone structure without the FER expression open reading frame was used as a control. All plasmids of DNA were stored in 10 mM Tris–1 mM EDTA buffer (pH 8.0) at − 20 °C until the day of gene delivery. Luciferase plasmid PGL-4 (Promega, Madison, WI, USA) with expression being driven by SV40 promoter as in pSG5-FER plasmid was used for reporter gene assays. Protocols for the use of recombinant DNA technology required prior approval from the University of Michigan Institutional Biosafety Committee (IBC) Protocol IBCA00000315.
EP-mediated delivery of genes into mouse lungs
EP-mediate transfer of FER gene was performed as described by Dolgachev et al(13). Briefly, 150 μg of DNA suspended in 100 mM NaCl was delivered to the lungs via hypopharyngeal drop in anesthetized animals. After several regular breaths, metal plated caliper electrodes were placed under each forelimb assuring contact with an electro-conductive gel. Using a BTX Harvard Apparatus ECM 830 generator (Holliston, MA, USA), eight square wave EP 200 V cm−1 pulses of 10 ms duration and 1 s apart were given. The electroporation of pcDNA 3.1 empty vector was used as a control for the effects from non-specific activation due to exposure to DNA and electroporation. Animals were allowed to recover and then kept under BSL2 containment. For survival curve experiments two groups received treatment, 1 h before and 1 h after bacterial challenge. Aside from survival curves, all other experiments were performed with animals receiving plasmid DNA electroporation only after bacterial challenge. For the analysis of gene transfer efficiency a parallel cohort of electroporation experiments was performed using luciferase reporter gene in the presence or absence of Klebsiella pneumonia, at a 6 h time line if insult was present. Animals were sacrificed at 24 h and harvested lungs were processed as in Dean et al(17) to assess for luciferase activity.
Time point selection
Based on our observed survival curves, we found that mortality occurs usually after 72 h from bacterial inoculation. Thus in order to better-characterized markers of gene expression, infection and inflammation, we selected an early (24 h) and late (72 h) time points throughout our experiments.
Quantitative bacterial culture assessments
At specific time points as defined above, animals were euthanized by anesthetic overdose and blood sample was obtained via cardiac puncture using a sterile 18-gauge needle. To measure bacteremia, 100 μl of undiluted blood was plated onto 5% sheep blood agar plates (Thermo Fisher Scientific, Remel Products, Lenexa, KS, USA). Plates were incubated overnight at 37 °C and CFUs per ml of blood were counted after 16 h. All lung lobes, from these same animals, were harvested via mid-line thoracotomy and homogenized in room temperature in 1 ml of phosphate-buffered saline (PBS). A 100 μl aliquot of this homogenate was used and counted in a similar process similar to that described for bacterial blood quantification, normalized to the total lung homogenate.
Bronchial Alveolar Lavage (BAL) fluid collection
In separate experiments, right after euthanasia, the trachea was dissected and canulated with a blunt 18-ga needle. Lungs were lavage with ice cold 1.5 ml of sterile phosphate buffered saline (PBS) using several 1 ml syringes. BAL fluid was centrifuged at 400 g for 8 min at 4 °C. Following centrifugation, supernatant was separated from precipitated cells, and stored frozen at − 80 °C until use. Spun down cells were re-suspended in appropriate buffer for further assays.
Cytospins BAL cell staining
Cytospins were performed by loading 100 μl from spun down BAL into each cuvette and spinning against glass slides at 350 g for 5 min. Slides were fixed with Diff-Quik (Baxter, Detroit, MI, USA) based on Wright - Giemsa stain for microscopic examination.
Flow cytometry
Flow-cytometric analysis of at least 105 cells per sample was performed using a BD LSR II Flow Cytometer (BD Biosciences) following protocol as described in Dolgachev et al (2012)17 and using the following antibodies Gr-1-PE,CD11c-APC-Cy7, F4/80-AF488, CD11b-PE-Cy7, CD206-APC, phospho-STAT1 and phospho-STAT6 (BioLegend and BD Biosciences, San Jose, CA, USA). Obtained data were plotted and analyzed using the FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
Whole-lung extracts and nuclear fractions were run on 12.5% SDS-polyacrylamide gels, with detailed protocol for sample processing as described in our previous publications(18, 19). Polyvinylidene difluoride membranes were probed sequentially with rabbit anti-STAT3 or anti-phospho-STAT3 (1:2000); followed by horseradish peroxidase-conjugated anti-rabbit IgG antibodies (1:3000) (AbCAM, Cambridge, MA, USA). We detected actin as a loading control using mouse anti-actin (1:2000) (AbCAM) followed by secondary as described above. Proteins were visualized by chemiluminiscense (Super Signal West Pico chemiluminescent substrate, Pierce, Rockford, IL, USA) using a Kodak photo imager. In our previous report(13) we found that the peak of transgene expression of FER by EP delivery was at 24 h weaning off by 72 h, thus we performed lung sample collection using these time points for our Western Blot analysis.
In Vitro Phagocytosis Assay
Phagocytosis assays were performed as described elsewhere(18). Briefly, BAL alveolar macrophages (AMϕ) were isolated from BAL and plated at a concentration of 2×105 cells/well and cultured overnight in Dulbecco’s Modified Eagle Medium. Wells were aspirated and replaced with 50 μL serum-free medium. AMϕ were then incubated with fluorescein isothiocyanate (FITC)-labeled, heat-killed Klebsiella pneumoniae. Phagocytosis of FITC-labeled bacteria was measured after quenching of extracellular non-ingested bacteria with trypan blue.
Quantification of Soluble Mediators by Enzyme-Linked Immunosorbent Assay (ELISA)
Saved BAL fluid was centrifuged at 400g for 5 min at 4°C. Supernatants were used for ELISA. Samples were stored at −80°C until use. IL-1RA (IL-1 receptor antagonist), TNF-[alpha], CCL2 (C-C Motif Chemokine Ligand 2), C-X-C motif chemokine 10 (CXCL10) also known as Interferon gamma-induced protein 10 (IP-10), RAGE, the receptor for advanced glycation end products, and keratinocyte-derived chemokine (KC) were measured in BAL supernatant using commercially available ELISA (R&D Systems, Inc, Minneapolis, Minn). These cytokines and chemokines reflect markers of inflammation found to be altered in our previous studies of pneumonia. Myeloperoxidase (MPO) levels were determined using an ELISA kit following manufacturer’s instructions (Hycult Biotech, Plymouth Meeting, PA) using previously collected BAL cells pellets homogenized in 1 ml of sterile phosphate buffered saline (1×), in the presence of protease inhibitors cocktail (Roche). All plates were read using a microplate reader (Biotek Instruments, Winooski, VT) at 450-and 540-nm with concentrations calculated using a 6-point standard curve performed in duplicate.
Real-time PCR (TaqMan)
In parallel experiments, from control and experimental group animals under same injury conditions, treatments and time points as described above, after euthanasia the chest cavity was opened. After cannulating the heart, lung circulation was perfused blood free with ice-cold PBS before their removal from chest cavity. RNA was isolated from the upper right lobes of lung using TRIzol (Ambion, Invitrogen, Carlsbad, CA, USA) and from BAL cells using Qiashredder columns (QIAGEN) as per manufacturer’s instructions. Levels of mRNA for IL1b, NLRP3, TNF alpha, Cxcl2, Nrf2 Hsp90 transcripts were assessed using quantitative PCR analysis (TaqMan) with pre-developed primers and probe sets (Applied Biosystems, Carlsbad, CA, USA). Quantification of the genes of interest was normalized to GAPDH and expressed as fold increases over the naive control for each treatment at each time point.
Mouse model of Methicillin-resistant Staphylococcus aureus (MRSA) Bacterial Pneumonia (PNA)
Female A/J mice with reported susceptibility to MRSA, 18 to 20 g weight, (Charles River Laboratories, Wilmington, MA, USA) were used for MRSA bacterial infection experiment. A non-lethal concentration of 107 CFU of MRSA (ATCC catalog number 4012) was used to induce pneumonia. Procedures for inoculation and electroporation were performed in similar fashion as preceding Klebsiella experiments. After recovery, animals were house in a BSL2 facility. Animals were sacrificed at 24 h post-infection; BAL was collected to perform flow cytometry on cell populations as described above. Lung parenchyma was divided for quantitative culture, TRIzol (Ambion, Invitrogen, Carlsbad, CA, USA) RNA extraction and finally collagenase tissue digest (Sigma, St. Louis, MO) to perform additional flow cytometry following methods as described in Taddonio et al(20).
Human Lungs
Human lungs were obtained during multi-organ procurement for transplant donation, under an experimental protocol from Gift of Life of Michigan. These lungs had been rejected for transplant due to suspected infection. After all allocated organs had been removed; a 1-cm3 sample of tissue was excised from best and worst areas of the explanted lungs. These samples were further divided and placed in separate containers to be preserved in 10% PBS-formalin, OCT-media and liquid nitrogen for further analysis. A portion of frozen tissues was later processed in TRIzol for RNA isolation and subject to RT-PCR as above.
Histopathological examination and staining
OCT-embedded human lungs were processed at the University of Michigan histopathology core. Fixed 4-μm thick frozen sections were stained using α-FER (Abcam, cat#AB191060) and secondary α-rabbit- Alexa-594 (Invitrogen-Molecular Probes) following manufacturer instructions. Representative pictures were taken at × 600 magnification using Tx2 Nikon microscope (Nikon Instruments Inc., Melville, NY, USA).
Statistical methods
A priori sample size for each experimental survival curve group (N=10) was calculated based on observed results in our previous publication(13) using G*power 3.1 (Franz Faul, Edgar Erdfelder, Albert-Georg Lang, and Axel Buchner) where observed survival was 85% for experimental group and 20% for control group, accepting a probability of α error of 0.05 and a power of 0.8. Electroporation experiments were performed three times assigning littermates under same condition and output measurement as per institutional guidelines. No samples were excluded for output measures analysis. All statistical analysis and graphs were performed using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Results are presented as mean values ± the SEM unless otherwise noted. Continuous variables were analyzed using an unpaired two-tailed Student’s t-test for samples less than 5. Groups greater than 5 samples and multiple groups were compared by one-way ANOVA with Tukey’s multiple comparison test used for post hoc analysis or two-way ANOVA with Tukey’s posttests, when two time points were assessed. Survival curves were generated using the Kaplan–Meier method. The log-rank (Mantel–Cox) Test was used to compare the survival data between experimental groups. Statistical significance was defined as a p-value of 0.05.
RESULTS
Electroporation-mediated delivery of human FER (pFER) gene was associated with increased survival following Klebsiella pneumoniae pneumonia (PNA) challenge.
Our baseline model of primary PNA induced by Klebsiella pneumoniae had a very high mortality rate, with a median survival of 3.5 days and resulted 5-day-survival of 20%. Using electroporation-mediated delivery of FER gene prior to PNA we were able to achieve a protective effect with 100% survival of infected animals (Figure 1). In a more clinically relevant model in which electroporation was conducted after bacteria inoculation (PNA/EP-pFER) we were able to obtain a significant (80%, p < 0.05) survival rate. Negligible benefit was found with the electroporation of the empty vector plasmid (pcDNA 3.1) or electroporation alone (EP-saline) which had survival rates of < 25% by day 5.
Figure 1. Electroporation-mediated (EP) delivery of FER gene was associated with increased survival following Klebsiella pneumonia (PNA) challenge.

500 CFU of Klebsiella pneumoniae was given to C57/Bl6 female mice. 150 μg of plasmid DNA containing gene encoding human tyrosine kinase FER was given either one hour prior (solid triangle) or after (solid square) insult and animals were electroporated using a BTX ECM 830 generator receiving eight 200 V cm−1square wave pulses,10 ms duration and 1 second apart. Sham electroporated with saline solution (EP-saline) and EP of empty vector (pcDNA 3.1) served as controls. Both protective and therapeutic strategies of EP-mediated FER gene transfer showed improved survival over controls. N=10 mice per group. Significance placed in graph. Log-rank (Mantel-Cox) test.
Report gene transgene expression in the lung is not inhibited by established PNA
We asked if electroporation mediated delivery could be affected by the presence of diffuse inflammation in the lung caused by Klebsiella pneumoniae infection (Figure 2). A plasmid containing luciferase reporter gene was electroporated in the presence or absence of infection (EP after PNA). In this particular model, the presence of diffuse inflammation increased transgene expression 4 fold over the naïve uninjured electroporated control (p < 0.001). As with inflammation there are more cellular infiltrates, the availability of targets to express delivered genes is increased, thus contributing with the increased efficiency.
Figure 2. Electrogene transfer after pneumonia infection has increase expression of reporter gene.

Electroporation-mediated delivery of a plasmid containing luciferase reporter gene (PGL4) driven by SV40 was performed in the presence or absence of pneumonia 6 h after inoculum. Lungs were harvest at 24 h and homogenates were assessed for luciferase activity. N=4. Significance placed in graph. One-way ANOVA with Tukey’s multiple comparison test (*** p < 0.001).
FER gene therapy significantly reduces Klebsiella bacterial counts in circulating blood and in the lungs.
Whole blood and lung tissue were assayed for quantification of colony forming units (CFUs) of Klebsiella pneumoniae normalized to 1 ml of blood and to the total lysate of lung tissue, respectively. FER gene electroporation was better at reducing bacteria counts in circulating blood by 24 h, and maintained this efficiency for the next 72 h (Figure 3). At the later time point, we found that only two of ten animals with FER-EP showed any evidence of bacteremia; whereas in all other groups (PNA only and PNA/EP-empty vector), representative animals were positive for bacteremia and had impaired clearance as measured by CFUs. The separate analysis of homogenized lung tissue revealed that FER treatment was able to significantly decreased CFU’s within the lung, and effectively showed total bacteria clearance without the use of antibiotics from lungs in 4 out of 10 animals by 72 h. The electroporation of the empty plasmid pcDNA3.1 had minimal effect on inhibiting the growth of bacteria in blood or lung tissue, and exhibited no difference when compared to the pneumonia only group (PNA). This indicates that the contributions to bacterial clearance due to non-specific inflammation from electroporation itself or by Toll-like Receptor-9 (TLR-9) activation(21, 22) stimulated by CpG bacterial plasmid DNA exposure were negligible. Timing the increasing bacteremia and lack of bacterial clearance from the lung to the demise of animals in parallel survival curve experiments, it would be apparent that the cause of death is related to sepsis and associated organ failures.
Figure 3. Quantitative bacterial culture from blood and lungs after PNA challenge and treatment with EP-mediated delivery of FER gene.

Number of CFUs of bacteria were counted 16 h after processing on blood agar plates and normalized to 1 ml of blood and to mass of total lung tissue respectively. EP of FER gene was able to contain and avoid systemic dissemination in blood and eliminate infection by 72 h. in the lung. N=10 animals per group per time point. Significance placed in graph. One-way ANOVA with Tukey’s multiple comparison tests (* p < 0.05; ** p < 0.01; *** p < 0.001).
EP-FER enhances early recruitment of inflammatory monocytes and macrophages in Bronchial Alveolar Lavage (BAL) Fluid after Klebsiella sp. Pneumonia
Electroporation mediated delivery of the FER gene resulted in increased numbers of inflammatory cells in the alveolar space upon higher number of total cells in BAL at 24 h post pneumonia induction (Figure 4 – Top Row). The principal leukocyte responsible for this increase is the inflammatory monocyte. We found that these monocytes presented with a predominantly TLR-2+/TLR-4+ phenotype with broad responsiveness capacity (Figure 4 – Middle Row). Other Monocyte phenotypes were also elevated (TLR-2+/TLR-4− and TLR-2−/TLR-4+) but at smaller proportions. Analysis of BAL macrophages after FER electroporation did not reveal major differences in their total numbers; yet did cause relative increases in the TLR-2+/TLR-4+ and TLR-2+/TLR-4− subphenotypes (Figure 4 – Bottom Row). Finally, flow cytometric analysis of neutrophils in FER electroporated animals did not reveal any major differences when comparing treatment and control groups (data not shown). Modified Giemsa stained thick blot Cytospin slides from these BAL specimens revealed leukocytes engulfing bacteria (as indicated by arrows) at higher numbers per high power field (Figure 5). Additionally, PNA and sham electroporation showed a relative increase of necrotic cells, ghost cells and erythrocyte destruction per high power field.
Figure 4. Flow cytometry analysis of bronchial alveolar lavage fluid (BAL) shows robust recruitment of inflammatory monocytes and modest recruitment of macrophages after FER electroporation treatment.
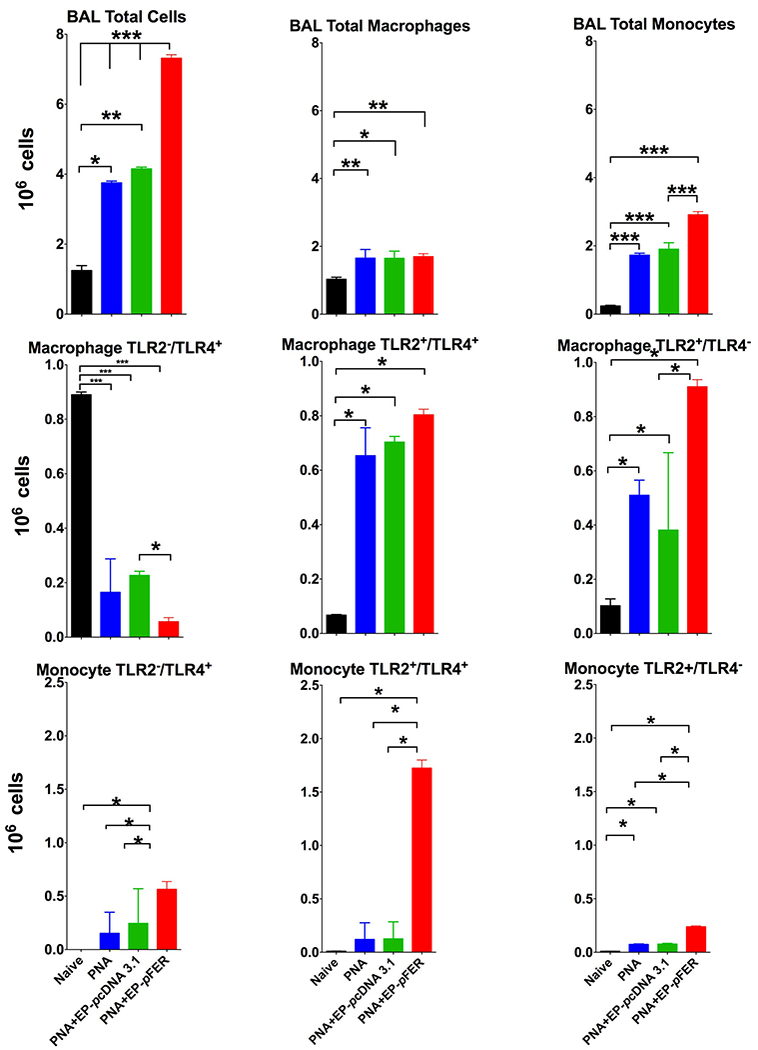
BAL fluid was recovered and cells were stained for Gr-1, F4/80, CD11b and CD206. Top Row Total number of BAL cells were higher in the FER treated group compared to controls (PNA-only, PNA EP-saline) being the inflammatory monocytes the main driver in this difference. Middle Row. Cell sorting of macrophages showing with concomitant TLR-2 and TLR-4 staining incremented by FER treatment showed increased proportion of TLR2+/TLR4+ and TLR2+/TLR4− cells. Bottom Row Cell sorting of inflammatory monocytes with further staining for Toll-like receptors 2 and 4 showed a very significant increase of TLR2+/TLR4+ as compared to other phenotypes. N=10. One-way ANOVA with Tukey’s multiple comparison tests (* p < 0.05; ** p < 0.01; *** p < 0.001).
Figure 5. Representative Cytospin slides with modified Giemsa staining from BAL samples obtained as in Figure 4.

Significant more numbers of leukocytes are seen in the PNA FER-treated group compared to Pneumonia only (PNA) or sham (PNA + EP-saline). Relatively more bacteria are seen engulfed in the FER electroporated group, whereas increased foamy cells and ghost cells are seen in control groups, respectively.
Electroporation mediated delivery of FER gene increases phagocytosis activity and Myeloperoxidase (MPO) in lung inflammatory cells.
Using an in vitro quantitative fluorescence-based phagocytosis assay we found that EP mediated gene transfer of FER gene confirmed our observations of Giemsa stain Cytospins of BAL cells, in which much larger numbers of macrophage-like cells engulfing bacteria were found. Better phagocytic activity per field was present within the treatment group (Figure 6A). This proportional difference in activity was independent from the presence of antibodies (opsonins) in the buffer solution. Additionally, total lung lysates from FER treated animals showed an increased concentration of MPO inferring an increased bacterial clearance capacity from newly recruited cells in the lung (Figure 6B). By 72 h this increased MPO activity was able to wean down to basal levels. Minimal MPO concentrations were found in non-FER-EP groups. This is a surprising find, as neutrophils can be observed in thick blot Cytospins from PNA-only animals, yet there was no significant increase of MPO concentration. This indicates in this model, that bacterial clearance is a predominantly dependent on the relative abundance of activated macrophage and monocytes.
Figure 6. Phagocytosis and Myeloperoxidase (MPO) Assay of BAL recovered leukocytes after PNA and electroporation treatment.
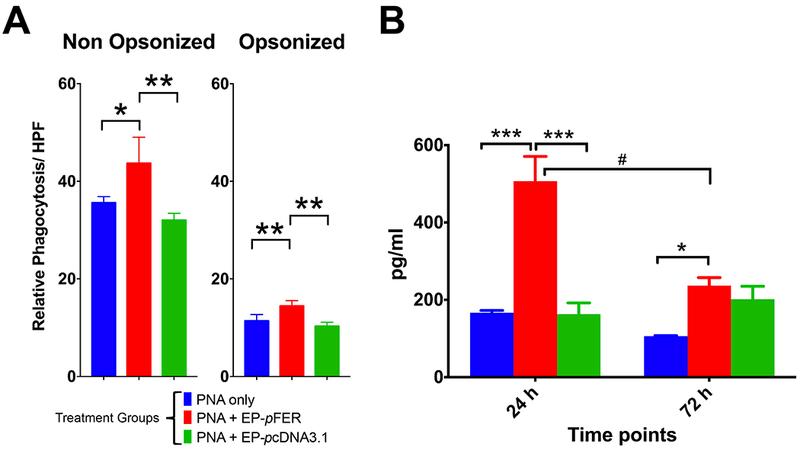
A. In vitro opsonized and non-opsonized bacterial phagocytosis of FITC-labeled heat killed Klebsiella pneumoniae was superior in the FER treated group. B. Higher activity of MPO was detected in the FER group only at early time point, suggesting an intense but short lived oxidative burst in which bacterial removal is occurring. N=6. Two-way ANOVA with Tukey’s multiple comparison tests (comparison among treatment groups: * p < 0.05; ** p < 0.01; *** p < 0.001 -- comparison among time points: # p < 0.05; ## p < 0.01; ### p < 0.001).
Electroporation-mediated delivery of FER gene increases phosphorylation of STAT proteins, with active translocation of STAT-3 into the nucleus in Klebsiella pneumonia.
STAT proteins are important transcription factors that regulate the inflammatory response and are essential to fight bacterial infection(23). In total lung lysates, at the 24 h time point, we found that the electroporation of FER gene had induced higher phosphorylation of STAT-3 levels than the PNA-only control (Figure 7A). Additionally, we found a higher proportion of activated protein transported into the nucleus (its primary site of action) in the FER-electroporation treated group (Figure 7B). From these groups, we asked the question if newly recruited BAL cells had STAT protein activation. Using flow cytometry and cell gating for Ly6C expressing monocytes and macrophages recovered from BAL at 24 h we found significant increases of phosphorylated isoforms of STAT-1 and STAT-6 proteins only within the FER-electroporated group after pneumonia (Figure 7C and 7D, dark line-group).
Figure 7. FER electroporation accelerates STAT transcription factor phosphorylation and translocation into the nucleus during pneumonia.

A. FER induces amore robust phosphorylation of STAT-3 at earlier stages (24 h). B. FER treated animals had a higher rate of translocation of phosphor-STAT-3 into the nucleus, its site of action compared to PNA-only group. C-D. Flow cytometry, showing cell gating strategy and D levels anti-phospho-STAT-1 and anti-phospho-STAT-6 stained of cell sorted Ly6C+ cells from BAL after pneumonia insult and treated with FER electroporation.
Electroporation-mediated delivery of FER gene induces both pro and anti-inflammatory cytokines during Klebsiella pneumonia
We analyzed the production of several inflammatory mediators in BAL by ELISA. As anticipated several important pro-inflammatory mediators had increased levels (TNF-α, KC, CCL-2, CXCL-10) in the FER-treated animals over control mostly at the 24 h time point (Figure 8A). More importantly, we found that INF-γ, critical for bacterial clearance, was indeed significantly elevated (~ 4 fold) in FER-electroporated animals over the PNA-only group (Figure 8B). Surprisingly, EP mediated gene transfer of FER treatment also showed significant elevations of anti-inflammatory cytokines IL-1 receptor antagonist (IL-1RA) and RAGE (Figure 8C) at 24 h counterbalancing the effects of inflammatory mediators.
Figure 8. ELISA of pro-inflammatory and anti-inflammatory cytokines in BAL.
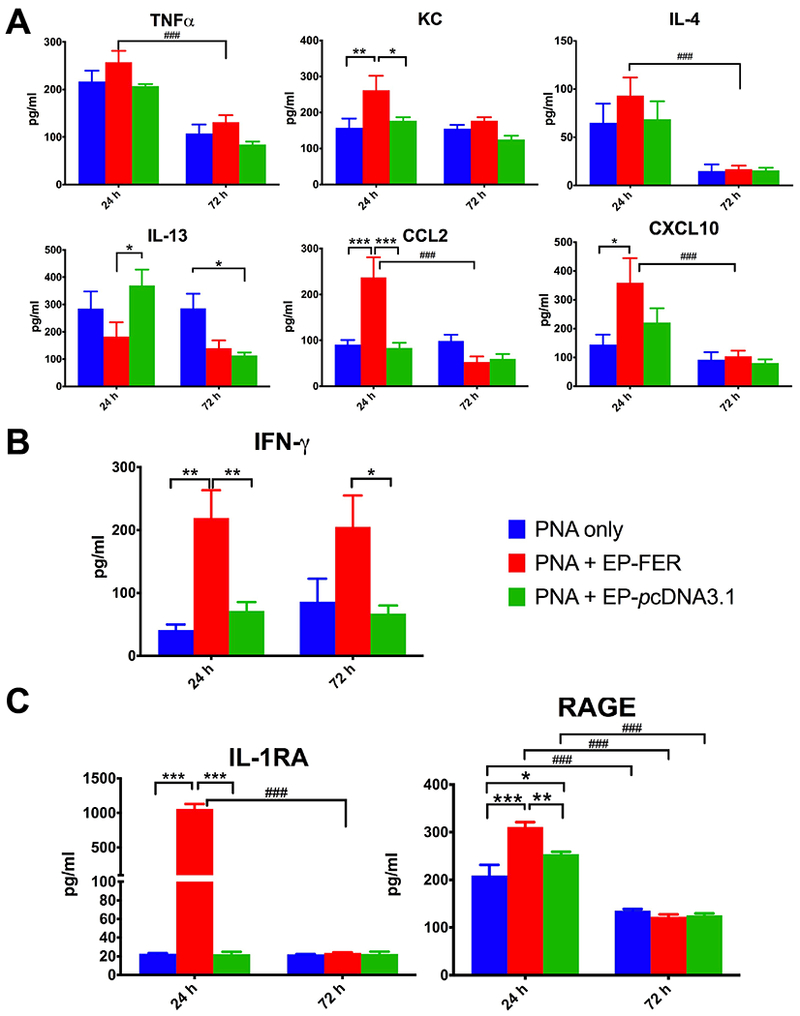
A. Increases of important pro-inflammatory cytokines were detected mostly at 24 h. B. IFN-γ a cytokine critical in bacterial removal remained at higher levels at both assessed time points (24 h, 72 h). C. IL-1RA and RAGE anti-inflammatory cytokines were also increased suggesting a counter-regulatory mechanism of control of FER induced inflammation. N=8. Two-way ANOVA with Tukey’s multiple comparison tests (comparison among treatment groups: * p < 0.05; ** p < 0.01; *** p < 0.001 -- comparison among time points: # p < 0.05; ## p < 0.01; ### p < 0.001)
Electroporation-mediated delivery of FER gene induces changes in gene programming in lung tissue and BAL cells during Klebsiella pneumonia
We next asked if effects of FER were mediated at transcriptional level. Previously recognized genes for mediators of bacterial clearance and the lung’s inflammatory response were selected and assessed by real time PCR (TaqMan) from total lung and BAL cells RNA extracts. Each compartment (cells from alveolar space vs. cells from lung parenchyma) exhibited a unique gene program after FER stimulation. As for TaqMan transcripts, the use of an empty vector or saline only electroporation in the presence of pneumonia was not much different from the untreated control. In the lung parenchyma (Figure 9A), we found very significant elevations of transcription for several pro-inflammatory signaling transcription factors mediators and end-product cytokines (IL-1β, Tnf-α, Nrf2 and Nrlp3), over naïve animals and much higher than their pneumonia-only counter parts. Interestingly, in the earlier time point Hsp90 a STAT-3 chaperone protein, showed subdued expression in lung extracts, yet its expression was significantly higher in FER-stimulated BAL cells (Figure 9B) within this same time frame, thus indicating a possible genetic counter regulation control, specific for these cells. In the lung, all major effects in transcription appear to taper off at 72 h, same time in which EP-mediated human FER overexpression disappears. However that of Hsp90 continues to increase in FER treated animals while opposite effect is seen in PNA only untreated animals. As for BAL cells, the effects on transcription were modest as compared to lung parenchymal lysates. However we did observe that pro-inflammatory Cxcl2, Ifn-γ, Nrlp3 and Nrf2 transcription had increases at several order of magnitude superior to their pneumonia only counter parts in the assessed time point (24 h) (Figure 9B).
Figure 9. Real-time PCR of pro and anti-inflammatory genes after EP-mediated delivery of FER gene post Klebsiella pneumonia challenge.

A. Total lung lysate expression. Significant effects in genetic expression for several pro-inflammatory and chemotactic genes were seen at 24 h, tapering off at 72 h. B. RT-PCR in BAL cells at 24 h, showing a pro-inflammatory genetic program for some transcripts after FER electroporation. Heat Shock Protein 90 (Hsp90) gene, an important chaperone for STAT proteins, was also increased suggesting a regulatory feedback mechanism after FER stimulation. N=8. For lung parenchyma two-way ANOVA with Tukey’s multiple comparison tests (comparison among treatment groups: * p < 0.05; ** p < 0.01; p < 0.001 -- comparison among time points: # p < 0.05; ## p < 0.01; ### p < 0.001) was performed. For BAL cells an unpaired T-test (* p < 0.05; ** p < 0.01; *** p < 0.001) was performed.
EP of FER reduces bacterial counts in the lung and increases recruitment of inflammatory cells after Methicillin-Resistant Staphylococcus aureus (MRSA) inoculation
In order to ascertain if the effects of EP of FER gene were exclusive to Klebsiella pneumoniae or could be other clinically relevant organisms, we performed a parallel experiment with an inoculation of 107 CFU of MRSA in AJ strain mice with known susceptibility to Gram-positive infections (Figure 10). We found a significant 3-order magnitude decrease in the number of organisms at 24 h in lung homogenates. There was an increased recruitment of inflammatory cells both in BAL as well as in total lung, thus suggesting that, as in Klebsiella, reductions in MRSA counts are related to an enhanced innate immune response. Taqman transcripts at 24 h resulted in a very similar pattern to those obtained during FER treatment in Klebsiella pneumonia (Figure 11) indicating that responsive effects on FER inducible transcriptional program are not unique to a single organism.
Figure 10. FER lung gene delivery can inhibit Methicillin Resistant Staphylococcus aureus growth in the lung and is also associated with fast recruitment of innate immune cells in the lung.
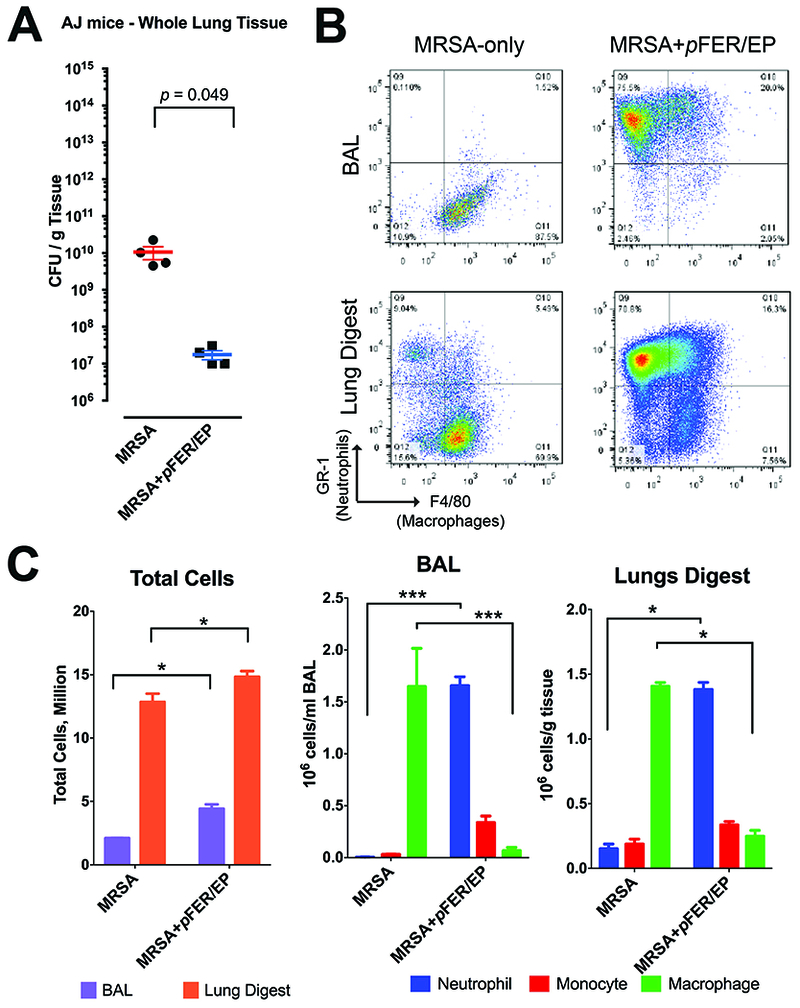
A. Lungs harvested at 24 h. FER mediated gene delivery reduced bacterial load by several orders of magnitude compared to pneumonia control. N=4. Unpaired T-test was performed. B. Flow cytometry at 24 h, stained for Gr-1, F4/80, CD11b and CD206, examples of gates utilized for Neutrophils and Macrophages. C. Flow cytometry results showing enhanced recruitment of innate immune cells after MRSA challenge. N=4. Two-way ANOVA with Tukey’s multiple comparison tests (comparison among treatment groups: * p < 0.05; ** p < 0.01; *** p < 0.001).
Figure 11. Real-time PCR after EP-mediate delivery of FER gene post Methicillin-resistant Staphylococcus aureus challenge.

Animals received an inoculum of 107 MRSA followed by electrogene transfer of FER plasmid. Lungs were harvest at 24 h and RNA extraction from total lung lysates was performed after bronchial alveolar lavage. Similar transcriptional effects to those obtain in Klebsiella experiments, suggesting a universal pathway, non-dependent on type bacteria. N=4 Unpaired T-test (* p < 0.05)
High expression of FER in grossly inflamed human lung.
Samples of human lung tissue obtained from discarded lungs deemed as unsuitable for transplantion were obtained during multi-organ procurements. Grossly abnormal lung (atelectatic and inflamed) as well as “normal-looking” areas of lungs was excised for comparison. These lungs had been rejected to proceed for transplant due to pneumonia per procurement team, but no further clinical information was provided (specific admission diagnoses, clinical course, unknown time frame from moment of diagnosis, specific radiological/bronchoscopic findings, microbiology and cause of death). Frozen sections were cut in OCT-media and stained with antibody against human FER. We found that the more severe grossly abnormal looking areas of lung parenchyma had a higher expression of FER compared to better-inflated lung (Figure 12 A-C). Furthermore, TaqMan for several transcripts was performed showing spatial correlation of FER with Cxcl2, IL-1β, IL-6, Nos2 and Tnf-α. Taken together these findings suggest a possible role of FER in the human lung inflammatory response (Figure 12D).
Figure 12. FER is present in inflamed areas of human lung.

A-C. Immunohistochemistry staining with antibodies against human FER (TRITC-red stain) from better appearing to grossly inflamed looking areas of lung discarded for transplant due to reported pneumonia (DAPI nuclear blue counter stain). D. RT-PCR analysis of transcripts obtained from these same contrasting areas. FER was highly present inflamed areas of lung, confirmed with RT-PCR. Additionally, several transcripts showed similar levels of up-regulation as in the murine model after FER overexpression. (N=3)
DISCUSSION
FER is a cytosolic non-receptor, non-transmembrane member of the fps/fes (feline sarcoma) family(24, 25). It is ubiquitously distributed among diverse cells in the body and evolutionary conserved. It plays a significant role as downstream up regulator of cell surface receptors for growth factors (EGFR(26, 27), KIT(28, 29), PDGFR(30)), thus presumed by some as a proto-oncogene and associated with cell transformation, oncogenesis and metastatic potential for Acute Myleoid Leukemia(31–33), Breast(27), Lung(26, 34) and Prostate(23) cancers. However and in contrast to these negative attributes, FER activation has been considered essential in chemotaxis(35–37), myeloid differentiation(38, 39), cytoskeleton (actin and microtubule) assembly(40–42), cell adhesion(41, 43) and cell migration(35, 36, 44, 45), all which are important aspects of adequate immune cell function. In transgenic mice, expressing a kinase defective variant of FER, significant inflammation after lipopolysaccharide challenge (LPS) in gut epithelia can be seen producing significant barrier function disruption. Considering the similarities of gut and lung epithelia, dysfunction of FER may have implications in the physiopathology of acute lung injury. Thus it is possible that its biological significance is time, space, dose and stimulus-type, dependent, rather than its sole presence or absence within a cell(46).
FER is a challenging molecule to study, as it does not have any known ligand or specific inhibitor. We have adopted the use electroporation gene delivery as a method to induce its overexpression. Our laboratory and others has been able to reproducibly achieve high levels of transgene expression using electroporation in the lung that are comparable to best of viruses, regardless of the underlying insult (lipopolysaccharide-LPS, lung contusion, pneumonia or its combination), targeting multiple cells within the electrical field and with minimal side effects(17, 47–50). In sterile models of LPS and LC injury, while electroporation was able to induce transgene reporter expression in damaged targeted areas, it was significantly dampened as compared to un-injured control(17, 48, 50). In the present study, however, we found that in the live organism Klebsiella pneumonia model was associated with increased electroporation expression of luciferase in infected animals. Given that the inflammatory cell infiltration (monocytes and macrophages) is provoked by live bacteria a plausible explanation is provision of increased number of targets for plasmids to express. An alternative mechanism could be the increased lung permeability and therefore movement of DNA or induction of the transcriptional machinery in epithelial cells and other targets within the lung(51). While these observations are encouraging for therapeutic use of electroporation, the specific mechanisms require further investigation to avoid potential side effects and should be subject of future studies.
In our previous publication(13), we demonstrated that the transient overexpression of FER using electroporation (EP) mediated gene delivery was beneficial in improving survival in a murine model of combined lung contusion (LC) and pneumonia (PNA) thus confirming observational findings from a GWAS trial performed on public bio repositories obtained from diverse sepsis trials (GenOSept; VASST, PROWESS, GAins)(12). To gain a better understanding of FER’s participation in lung inflammation, we decided to perform this present complementary study in a more simplified model of primary bacterial (Gram negative) pneumonia. LC introduces several physiologic (permeability injury, alteration of surfactant, loss of compliance, hypoxia) and immunologic derangements (neutrophilia and macrophage depletion) in the lung(18, 52–55). It was unclear from our prior pilot study whether mechanisms of benefit seen from EP-induced FER overexpression are related to infection control or lung physiologic restoration. Based on our current findings it appears to be the former. As in our previous model of trauma-related secondary pneumonia, our results show that FER gene delivery improved survival. A single application of FER was able to eradicate Klebsiella sp infection in the lung and avoids bacteremia. Additionally EP-mediated delivery of human FER gene in mouse lungs resulted in early recruitment and mobilization of inflammatory monocytes in bronchial alveolar lavage fluid. Bacterial clearance is correlated to the presence of these newly recruited inflammatory monocytes in the lung. These cells had high levels of expression of Toll-like receptor proteins 2 and 4 (TLR-2/TLR-4), part of pattern recognition molecular inflammatory pathways important for bacterial clearance. Notably, we found that these cells had higher phagocytic capacity and higher levels of bactericidal myeloperoxidase (MPO). FER induces an early inflammatory milieu that favors bacterial killing shown by increases in pro-inflammatory cytokines (IFN-γ, TNF-α, KC) and several chemotactic chemokines (KC, CCL2, CXCL10) resulting in improved recruitment. At the same time we found that FER stimulated IL-1RA and RAGE suggesting that this particular inflammatory response is tightly regulated.
In our model, FER appears to be working via activation of Signal Transducer and Activator of Transcription (STAT) transcription factors (1,3 and 6). The translocation of these transcription factors into the nucleus is extremely important as it induces a transcription program that favors the production and maintenance of a bactericidal inflammatory milieu. In the FER treated group, the transcription and expression of some participants of the inflammatory response was tremendously stimulated (up to 4 orders of magnitude). These changes in gene transcription are transient and only persist while FER is being overexpressed (~24-48 h as it is being driven by short live SV40 promoter)(13). FER-induced STAT proteins seem to operate in tandem with other transcription factors such NRF2. The inflammasome molecule NRLP3, which participates in the maturation and release of pre-formed cytokines, was also stimulated by FER and could be an additional benefit in improving responsiveness of cells to bacterial pneumonia. Interestingly, Heat Shock Protein 90 (Hsp90) the chaperone molecule for STAT proteins had dual expression. While its expression was being inhibited in the lung, it had higher expression in BAL cells thus possibly avoiding the expansion of exaggerated inflammatory response, and suggesting a controlling feedback mechanism at cytosolic level. Future experiments will assess if targeting Hsp90 with direct pharmacologic inhibition in combination with FER overexpression will be summative and improve the inflammatory response; or on the contrary, will be deleterious by annulling the controlling effects of FER exerted on inflammatory cells, especially at later stages when Hsp90 levels are noticeably high(56–58).
The resultant reduction of bacterial counts, coupled with early inflammatory cell recruitment and repeated transcriptional program obtained in our preliminary experiments with MRSA pneumonia indicate that FER related effects are not unique to Klebsiella, but are could be generalized to all bacterial infections within the lung and provides a very promising avenue of inquiry.
This study is not without limitations. First and foremost, the lack of a known ligand for FER impedes its direct pharmacological activation or blockade for a cleaner experimental approach. Second, although the majority of beneficial effects seem to be related to FER’s modulation on BAL leukocytes, our previous publication showed that EP delivery of FER also targeted alveolar epithelial cells, whose function could also be influenced by FER overexpression and are important for lung homeostasis. Specifically, FER is known to interact with signaling pathways that regulate cytoskeleton assembly and cell adhesion, which are critical components of epithelial barrier function integrity. Indeed, further experimental analysis and labeling approaches are required to identify FER’s true cellular target and cascading pathways, and for such purpose, conditional knockouts are being developed to answer mechanistically some of these questions. Finally, Nrf2, Nrlp3, Tnf-α, and Hsp90 may not be the only transcripts that are regulated by FER, indicating the need of a wider throughput analysis (using DNAseq or RNAseq platforms) of individual lung cells. We also recognize the caveats of extrapolating our murine model of pneumonia to serve as a recapitulation of human clinical disease(59), nevertheless observing FER in inflamed areas of human lungs with a similar transcriptional expression as seen in our murine model suggests evolutionary conserved function across species in lung inflammation and are worth studying. Indeed, observations from previously mentioned GWAS study have been recently complemented by a report from Hinz et al(60) in which the low-expressor form FER rs4957796 T/T small nucleotide polymorphism (SNP) allele was associated with unfavorable survival in patients with severe Adult Respiratory Distress Syndrome (ARDS) due to pneumonia.
In summary, EP-mediated FER gene delivery improves survival after bacterial pneumonia, through activation of STAT pathways leading to early transcriptional up-regulation of Nrf2, Nrlp3, Tnf-α. Consequently, elevated levels of CCL-2, CXCL2, KC and IFNγ found in the BAL result in robust recruitment of inflammatory monocytes contribution to the containment and elimination of bacterial organisms from the lung, thus avoiding bacteremia. This inflammatory response was counter-regulated by Hsp90 at the cellular level and IL-1RA and RAGE at the tissue level. Our observations of the effects of FER’s overexpression potentiating competent inflammatory responses during infection are novel to the lung and could constitute a promising therapeutic strategy against severe pneumonia.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support from the National Institutes of Health-R01GM111305 (KR) and K12HL133304 (DMA) as well as the Michigan Center for Integrative Research in Critical Care (DMA). We also thank Dr. Eric White (Gift of Life of Michigan) and John Erby Wilkinson (Department of Pathology/ULAM, University of Michigan Medical School) for their help in obtaining, processing and staining human lung tissue.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 2.Heron M Deaths: Leading Causes for 2011. Natl Vital Stat Rep. 2015;64(7):1–96. [PubMed] [Google Scholar]
- 3.Epstein L, Dantes R, Magill S, Fiore A. Varying Estimates of Sepsis Mortality Using Death Certificates and Administrative Codes--United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2016;65(13):342–5. [DOI] [PubMed] [Google Scholar]
- 4.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. [DOI] [PubMed] [Google Scholar]
- 5.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med. 2017;377(5):414–7. [DOI] [PubMed] [Google Scholar]
- 6.Patel G, Perez F, Bonomo RA. Carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii: assessing their impact on organ transplantation. Curr Opin Organ Transplant. 2010. [DOI] [PubMed] [Google Scholar]
- 7.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51 Suppl 1:S81–7. [DOI] [PubMed] [Google Scholar]
- 8.Keen EF, 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns. 2010;36(6):819–25. [DOI] [PubMed] [Google Scholar]
- 9.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. [DOI] [PubMed] [Google Scholar]
- 10.Dobias J, Poirel L, Nordmann P. Cross-resistance to human cationic antimicrobial peptides and to polymyxins mediated by the plasmid-encoded MCR-1? Clin Microbiol Infect. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Dean DA. Gene therapy for ALI/ARDS. Crit Care Clin. 2011;27(3):705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rautanen A, Mills TC, Gordon AC, Hutton P, Steffens M, Nuamah R, et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med. 2015;3(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgachev VA, Goldberg R, Suresh MV, Thomas B, Talarico N, Hemmila MR, et al. Electroporation-mediated delivery of the FER gene in the resolution of trauma-related fatal pneumonia. Gene Ther. 2016;23(11):785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolgachev VA, Yu B, Sun L, Shanley TP, Raghavendran K, Hemmila MR. Interleukin 10 overexpression alters survival in the setting of gram-negative pneumonia following lung contusion. Shock. 2014;41(4):301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolgachev VA, Yu B, Reinke JM, Raghavendran K, Hemmila MR. Host susceptibility to gram-negative pneumonia after lung contusion. The journal of trauma and acute care surgery. 2012;72(3):614–22; discussion 22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolgachev V; Panicker S; Balijepalli S; Suresh MV.; Raghavendran K.; Machado-Aranda D. Overexpression of FER gene by Electroporation Enhances Survival In Trauma Complicated Fatal Pneumonia Via Fast Recruitment and Activation of Monocytes. Shock 2017;47(Suppl 1):Abstract P173. [Google Scholar]
- 17.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003;10(18):1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh MV, Thomas B, Machado-Aranda D, Dolgachev VA, Kumar Ramakrishnan S, Talarico N, et al. Double-Stranded RNA Interacts With Toll-Like Receptor 3 in Driving the Acute Inflammatory Response Following Lung Contusion. Crit Care Med. 2016;44(11):e1054–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suresh MV, Ramakrishnan SK, Thomas B, Machado-Aranda D, Bi Y, Talarico N, et al. Activation of hypoxia-inducible factor-1alpha in type 2 alveolar epithelial cell is a major driver of acute inflammation following lung contusion. Critical care medicine. 2014;42(10):e642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taddonio MA, Dolgachev V, Bosmann M, Ward PA, Su G, Wang SC, et al. Influence of lipopolysaccharide-binding protein on pulmonary inflammation in gram-negative pneumonia. Shock. 2015;43(6):612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou R, Norton JE, Zhang N, Dean DA. Electroporation-mediated transfer of plasmids to the lung results in reduced TLR9 signaling and inflammation. Gene Ther. 2007;14(9):775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhan U, Trujillo G, Lyn-Kew K, Newstead MW, Zeng X, Hogaboam CM, et al. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immun. 2008;76(7):2895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha J, Zouanat FZ, Zoubeidi A, Hamel L, Benidir T, Scarlata E, et al. The Fer tyrosine kinase acts as a downstream interleukin-6 effector of androgen receptor activation in prostate cancer. Mol Cell Endocrinol. 2013;381(1–2):140–9. [DOI] [PubMed] [Google Scholar]
- 24.Hao QL, Heisterkamp N, Groffen J. Isolation and sequence analysis of a novel human tyrosine kinase gene. Mol Cell Biol. 1989;9(4):1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao QL, Ferris DK, White G, Heisterkamp N, Groffen J. Nuclear and cytoplasmic location of the FER tyrosine kinase. Mol Cell Biol. 1991;11(2):1180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn J, Truesdell P, Meens J, Kadish C, Yang X, Boag AH, et al. Fer protein-tyrosine kinase promotes lung adenocarcinoma cell invasion and tumor metastasis. Mol Cancer Res. 2013;11(8):952–63. [DOI] [PubMed] [Google Scholar]
- 27.Sangrar W, Shi C, Mullins G, LeBrun D, Ingalls B, Greer PA. Amplified Ras-MAPK signal states correlate with accelerated EGFR internalization, cytostasis and delayed HER2 tumor onset in Fer-deficient model systems. Oncogene. 2015;34(31):4109–17. [DOI] [PubMed] [Google Scholar]
- 28.Kwok E, Everingham S, Zhang S, Greer PA, Allingham JS, Craig AW. FES kinase promotes mast cell recruitment to mammary tumors via the stem cell factor/KIT receptor signaling axis. Mol Cancer Res. 2012;10(7):881–91. [DOI] [PubMed] [Google Scholar]
- 29.Ding L, Dolgachev V, Wu Z, Liu T, Nakashima T, Wu Z, et al. Essential role of stem cell factor-c-Kit signalling pathway in bleomycin-induced pulmonary fibrosis. The Journal of pathology. 2013;230(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40(5):1700–5. [DOI] [PubMed] [Google Scholar]
- 31.Nagamura-Inoue T, Tamura T, Ozato K. Transcription factors that regulate growth and differentiation of myeloid cells. Int Rev Immunol. 2001;20(1):83–105. [DOI] [PubMed] [Google Scholar]
- 32.Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321(1):35–44. [DOI] [PubMed] [Google Scholar]
- 33.Metz S, Naeth G, Heinrich PC, Muller-Newen G. Novel inhibitors for murine and human leukemia inhibitory factor based on fused soluble receptors. J Biol Chem. 2008;283(10):5985–95. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami M, Morita S, Sunohara M, Amano Y, Ishikawa R, Watanabe K, et al. FER overexpression is associated with poor postoperative prognosis and cancer-cell survival in non-small cell lung cancer. International journal of clinical and experimental pathology. 2013;6(4):598–612. [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons SA, Mewburn JD, Truesdell P, Greer PA. The Fps/Fes kinase regulates leucocyte recruitment and extravasation during inflammation. Immunology. 2007;122(4):542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khajah M, Andonegui G, Chan R, Craig AW, Greer PA, McCafferty DM. Fer kinase limits neutrophil chemotaxis toward end target chemoattractants. J Immunol. 2013;190(5):2208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SJ, Lo M, Kuo RL, Shih SR, Ojcius DM, Lu J, et al. The pathological effects of CCR2+ inflammatory monocytes are amplified by an IFNAR1-triggered chemokine feedback loop in highly pathogenic influenza infection. J Biomed Sci. 2014;21:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zirngibl RA, Senis Y, Greer PA. Enhanced endotoxin sensitivity in fps/fes-null mice with minimal defects in hematopoietic homeostasis. Mol Cell Biol. 2002;22(8):2472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Ogata Y, Feldman RA. Fes tyrosine kinase promotes survival and terminal granulocyte differentiation of factor-dependent myeloid progenitors (32D) and activates lineage-specific transcription factors. J Biol Chem. 2003;278(17):14978–84. [DOI] [PubMed] [Google Scholar]
- 40.Kim L, Wong TW. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J Biol Chem. 1998;273(36):23542–8. [DOI] [PubMed] [Google Scholar]
- 41.Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, et al. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci. 2004;117(Pt 15):3207–19. [DOI] [PubMed] [Google Scholar]
- 42.McPherson VA, Everingham S, Karisch R, Smith JA, Udell CM, Zheng J, et al. Contributions of F-BAR and SH2 domains of Fes protein tyrosine kinase for coupling to the FcepsilonRI pathway in mast cells. Mol Cell Biol. 2009;29(2):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. J Cell Biol. 1998;141(6):1449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig AW, Greer PA. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol Cell Biol. 2002;22(18):6363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27(17):6140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greer P Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol. 2002;3(4):278–89. [DOI] [PubMed] [Google Scholar]
- 47.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GR, Yeldandi AV, et al. Gene transfer of the Na+,K+-ATPase beta1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005;171(3):204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, et al. Electroporation-mediated gene transfer of the Na+,K+ -ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176(6):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman CD, Geiger RC, Dean DA. Electroporation- and mechanical ventilation-mediated gene transfer to the lung. Gene Ther. 2010;17(9):1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na(+)/K(+)-ATPase pump reduced lung injury in a mouse model of lung contusion. The journal of trauma and acute care surgery. 2012;72(1):32–9; discussion 9–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holden VI, Breen P, Houle S, Dozois CM, Bachman MA. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1alpha Stabilization during Pneumonia. MBio. 2016;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoth JJ, Wells JD, Hiltbold EM, McCall CE, Yoza BK. Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock. 2011;35(6):604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, et al. Role of Macrophage Chemoattractant Protein 1 in Acute Inflammation Following Lung Contusion. Am J Respir Cell Mol Biol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado-Aranda D, Wang Z, Yu B, Suresh MV, Notter RH, Raghavendran K. Increased phospholipase A2 and lyso-phosphatidylcholine levels are associated with surfactant dysfunction in lung contusion injury in mice. Surgery. 2013;153(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado-Aranda D M VS Yu B, Dolgachev V, Hemmila MR, Raghavendran K. Alveolar macrophage depletion increases the severity of acute inflammation following nonlethal unilateral lung contusion in mice. J Trauma Acute Care Surg. 2014;76(4):982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, et al. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med. 2007;176(7):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77(12):1763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Munoz-Garcia B, Ramos-Mozo P, Ortega L, et al. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res. 2010;86(2):330–7. [DOI] [PubMed] [Google Scholar]
- 59.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinz J, Buttner B, Kriesel F, Steinau M, Frederik Popov A, Ghadimi M, et al. The FER rs4957796 TT genotype is associated with unfavorable 90-day survival in Caucasian patients with severe ARDS due to pneumonia. Sci Rep. 2017;7(1):9887. [DOI] [PMC free article] [PubMed] [Google Scholar]


