Abstract
Context:
Studies on physical function trajectories in older adults during chemotherapy remain limited.
Objectives:
Determine demographic, clinical, and symptom characteristics associated with initial levels as well as trajectories of physical function over two cycles of chemotherapy in adults age ≥65 with breast, gastrointestinal, gynecological, or lung cancer.
Methods:
Older adults with cancer (n=363) who had received chemotherapy within the preceding four weeks were assessed six times over two cycles of chemotherapy using the Short Form-12 Physical Component Summary (PCS) score. Hierarchical linear modeling was used to evaluate for inter-individual variability in initial levels and trajectories of PCS scores.
Results:
Mean age was 71.4 years (SD 5.5). Mean PCS score at enrollment was 40.5 (SD 0.45). On average, PCS scores decreased slightly (i.e., 0.21 points) at each subsequent assessment. Lower PCS scores at enrollment were associated with older age, greater comorbidity, being unemployed, lack of regular exercise, higher morning fatigue, lower evening energy, occurrence of pain, lower trait anxiety, and lower attentional function. Only higher morning fatigue and lower enrollment PCS scores were associated with decrements in physical function over time.
Conclusion:
While several symptoms were associated with decrements in PCS scores at enrollment in older adults with cancer receiving chemotherapy, morning fatigue was the only symptom associated with decreases in physical function over time. Regular assessments of symptoms and implementation of evidence-based interventions should be considered to maintain physical function in older adults during chemotherapy.
Keywords: physical function, older adults, chemotherapy, fatigue, hierarchical linear modeling
Introduction
As the incidence of cancer among older adults in the U.S. increases to 2.3 million by 2030 (1), the impact of cancer treatment on physical function will become increasingly important. Pretreatment functional impairment and decline during treatment are associated with worse quality of life (2–4) and overall survival (5, 6). In addition, the impact of cancer treatment on physical function is critically important to patients. In a study of adults age ≥60 with limited life expectancy, more than 70% of those with cancer reported that they would not choose a treatment that results in functional impairment, even if it improved survival (7).
Despite the importance of functional outcomes to older cancer patients, studies on the effect of treatment on physical function remain limited (6, 8–13), with only two studies focused specifically on physical function during chemotherapy (6, 8). In these European studies, pretreatment depression, abnormal nutritional status, and dependency in instrumental activities of daily living (IADL) were associated with decrements in activities of daily living (ADL) during chemotherapy (6, 8). Chemotherapy for a new diagnosis of cancer was associated with decrements in IADL (6). However, both of these studies examined changes in physical function between only two time points, which may miss acute within cycle, potentially non-linear changes in physical function during treatment. Acute changes in physical function after each chemotherapy infusion may be especially important in older adults with cancer since their limited physiologic reserve may make recovering from any functional decline more challenging (14). Furthermore, while both studies examined the association between depression and functional decline during chemotherapy, the impact of other symptoms such as morning and evening fatigue, morning and evening energy, sleep disturbance, and state and trait anxiety on physical function remains unknown.
Given the limited research on changes in and predictors of decrements in physical function in older adults during chemotherapy, the purposes of our study, in a sample of older adults with breast, gastrointestinal (GI), gynecological (GYN), and lung cancer who received chemotherapy (n=363), were to evaluate for inter-individual differences in physical function and to determine which demographic, clinical, and symptom characteristics were associated with initial levels as well as with trajectories of physical function over six time points during two cycles of chemotherapy.
Patients and Methods
Patients and Settings
The procedures for the parent cohort study are described in detail elsewhere (15, 16). The objective of the parent study was to characterize symptom clusters in cancer patients receiving chemotherapy (16–19). Eligible patients in the parent study were ≥18 years of age; had a diagnosis of breast, GI, GYN, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles; and were able to read, write, and understand English. We chose to enroll patients who had received at least one prior cycle of chemotherapy to better understand their ongoing risk of decrements in physical function during subsequent cycles of chemotherapy. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs. A total of 2,234 patients were approached and 1,343 consented to participate (60.1% response rate). For this analysis, patients who were ≥65 years of age (n=363) were included.
Instruments
Demographic and clinical characteristics
Patients completed a demographics questionnaire, the Karnofsky Performance Status (KPS) scale (20–22), and Self-Administered Comorbidity Questionnaire (23). Medical records were reviewed for disease and treatment characteristics. The MAX2 index (24, 25) estimated the average risk for grade 3 to 4 toxicity for each chemotherapy regimen.
Assessment of physical function
Changes in physical function over two cycles of chemotherapy were assessed using the Physical Component Summary (PCS) score from the Medical Outcomes Study-Short Form-12 (SF-12) (26–40), which assesses various aspects of physical and mental health. The PCS score consists of 6 items: health limitations with moderate activities, climbing several flights of stairs, accomplishing less than you would like, limitations in work or other activities; pain interference with normal work; and overall health rating. PCS scores can range from 0 to 100, with higher scores indicating better physical functioning. PCS results are scored using a norm-based algorithm with a standardized mean of 50 and standard deviation (SD) of 10 in the general U.S. adult population (41). The SF-12 and PCS score have well established validity and reliability (26) and have been used in other studies of patients with cancer (42, 43).
Assessment of symptoms
To evaluate common symptoms, patients completed separate Lee Fatigue Scale questionnaires (44) that evaluated diurnal variations (i.e., morning and evening) in fatigue severity and decrements in energy. Morning and evening fatigue and morning and evening energy are distinct symptoms both from phenotypic and genotypic perspectives (45–50). In addition, patients completed the General Sleep Disturbance Scale (51), Center for Epidemiological Studies-Depression scale (52), Brief Pain Inventory (53), Attentional Function Index (54), and Spielberger State-Trait Anxiety Inventories (55). State anxiety measures a person’s temporary anxiety response to a specific situation while trait anxiety measures a person’s predisposition to anxiety as part of one’s personality.
Study Procedures
The study was approved by the Institutional Review Board at each study site. Written informed consent was obtained from all participants. Depending on the length of their chemotherapy cycles (i.e., 14 days, 21 days, 28 days), patients completed study questionnaires in their homes a total of six times over two cycles of chemotherapy: prior to chemotherapy administration (i.e., recovery from previous chemotherapy cycle; assessments 1 and 4), approximately 1 week after chemotherapy administration (i.e., acute symptoms in the week after infusion; assessments 2 and 5), and approximately 2 weeks after chemotherapy administration (i.e., potential nadir; assessments 3 and 6).
Statistical Analyses
Descriptive statistics and frequency distributions were generated on the sample characteristics and symptom severity scores at enrollment using the Statistical Package for the Social Sciences (SPSS, version 24, IBM Corporation, Armonk, NY).
Hierarchical linear modeling (HLM) based on full maximum likelihood estimation was performed in two stages using software developed by Raudenbush and Bryk (56). The HLM methods are described in detail elsewhere (15). In brief, the HLM analysis evaluated for changes over time in PCS scores. During stage 1, intra-individual variability in PCS scores over time was examined. Three level 1 models were compared to determine whether the patients’ level of physical function did not change over time (i.e., no time effect), changed at a constant rate (i.e., linear time effect), or changed at a rate that accelerated or decelerated over time (i.e., quadratic effect). Then, the level 2 model was constrained to be unconditional (i.e., no predictors) and likelihood ratio tests were used to determine the best model.
The second stage of the HLM analysis examined inter-individual differences in the trajectories of PCS scores by modeling individual change parameters (i.e., intercept, linear slope) as a function of proposed predictors at level 2. Supplementary Table 1 presents a list of demographic, clinical, and symptom characteristics that were evaluated as potential predictors based on a literature review of physical function in cancer patients (6, 8–10, 12, 13). To improve estimation efficiency and construct a parsimonious model, bivariable exploratory level 2 analyses were performed in which each characteristic was added as a predictor to determine whether it improved the model. Characteristics with an absolute t-value <2.0 were dropped from subsequent models. All potential significant predictors from the exploratory analyses were entered into the HLM models to predict each change parameter. Only those characteristics that maintained a statistically significant contribution in conjunction with other characteristics were retained in the final HLM model. A p-value of <0.05 indicates statistical significance.
One advantage of HLM is that patients with some missing data on the dependent variable (i.e., PCS score) are not eliminated from the analysis. They contribute as many assessments as were possible for them to provide. In contrast, missing data are not allowed for predictor variables so patients with any missing predictor variables were not included in the HLM analyses
Results
Sample Characteristics
Demographic, clinical, and symptom characteristics of the sample (N=363) are presented in Table 1. The sample was predominately female (68.3%) with a mean age of 71.4 (SD 5.5) years. Patients had an average of 16.5 (SD 3.1) years of education, BMI of 26.1 (SD 5.3), and KPS score of 82.6 (SD12.6). Patients were 2.9 (SD 5.2) years from their cancer diagnosis (median 0.49 years) and primarily being treated with 21-day chemotherapy cycles (55.1%) for metastatic disease (73.6%). At enrollment, the mean morning energy score on the Lee Fatigue Scale was below the clinically meaningful cutoff. Over 67% of patients reported experiencing pain and 25.1% had a depression score of ≥16 suggesting depressive symptoms that warrant a clinical evaluation. In addition, the mean sleep disturbance and trait anxiety scores were above the cut-off scores for clinically meaningful levels of sleep disturbance and trait anxiety, respectively.
Table 1.
Demographic, clinical, and symptom characteristics of older adults with cancer receiving chemotherapy (N=363).
| Characteristics | n (%) or Mean (SD) | ||
|---|---|---|---|
| Demographic Characteristics | |||
| Age, years; mean (SD) | 71.4 (5.5) | ||
| Age, years; median (range) | 69 (65–90) | ||
| Female gender | 248 (68.3) | ||
| Ethnicity | |||
| White | 289 (80.1) | ||
| Black | 24 (6.6) | ||
| Asian/Pacific Islander | 23 (6.4) | ||
| Hispanic/Mixed/Other | 25 (6.9) | ||
| Education, years; mean (SD) | 16.5 (3.1) | ||
| Married or partnered | 211 (59.1) | ||
| Lives alone | 106 (29.8) | ||
| Currently employed | 78 (21.7) | ||
| Child care responsibilities | 17 (4.8) | ||
| Income | |||
| Less than $30,000 | 75 (23.9) | ||
| $30,000 to <$70,000 | 79 (25.2) | ||
| $70,000 to <$100,000 | 55 (17.5) | ||
| More than $100,000 | 105 (33.4) | ||
| Clinical Characteristics | |||
| Number of comorbidities, mean (SD) | 2.8 (1.5) | ||
| Self-administered Comorbidity Questionnaire score, mean (SD) | 6.2 (3.4) | ||
| Specific comorbidities reported | |||
| Hypertension | 167 (46.0) | ||
| Back pain | 95 (26.2) | ||
| Osteoarthritis | 85 (23.4) | ||
| Lung disease | 73 (20.1) | ||
| Depression | 64 (17.6) | ||
| Diabetes | 52 (14.3) | ||
| Heart disease | 42 (11.6) | ||
| Anemia | 33 (9.1) | ||
| Liver disease | 26 (7.2) | ||
| Ulcer or stomach disease | 16 (4.4) | ||
| Rheumatoid arthritis | 13 (3.6) | ||
| Kidney disease | 7 (1.9) | ||
| Body mass index, kg/m2; mean (SD) | 26.1 (5.3) | ||
| Hemoglobin, gm/dL; mean (SD) | 11.5 (1.4) | ||
| Karnofsky Performance Status score, mean (SD) | 82.6 (12.6) | ||
| Karnofsky Performance Status score, median (range) | 90 (40–100) | ||
| Current or former smoker | 169 (47.5) | ||
| Exercise on a regular basis | 235 (66.2) | ||
| Cancer diagnosis | |||
| Breast | 84 (23.1) | ||
| Gastrointestinal | 119 (32.8) | ||
| Gynecological | 79 (21.8) | ||
| Lung | 81 (22.3) | ||
| Time since cancer diagnosis, years; mean (SD) | 2.9 (5.2) | ||
| Time since cancer diagnosis, years; median (range) | 0.49 (0.06–38.3) | ||
| Any prior cancer treatments | 269 (76.2) | ||
| Number prior cancer treatments, mean (SD) | 1.7 (1.5) | ||
| Chemotherapy MAX2 index, mean (SD) | 0.152 (0.1) | ||
| Chemotherapy cycle length | |||
| 14 days | 124 (34.2) | ||
| 21 days | 200 (55.1) | ||
| 28 days | 39 (10.7) | ||
| Metastatic disease at time of study | 265 (73.6) | ||
| Number of metastatic sites including lymph node involvement, mean (SD) | 1.4 (1.2) | ||
| Number of metastatic sites excluding lymph node involvement, mean (SD) | 0.9 (1.1) | ||
| Symptom Characteristics at Enrollmenta | |||
| Lee Fatigue Scale: morning fatigue score, mean (SD) | 2.6 (2.1) | ||
| Lee Fatigue Scale: evening fatigue score, mean (SD) | 4.8 (2.2) | ||
| Lee Fatigue Scale: morning energy score, mean (SD) | 4.3 (2.5) | ||
| Lee Fatigue Scale: evening energy score, mean (SD) | 3.8 (2.1) | ||
| General Sleep Disturbance Scale score, mean (SD) | 48.7 (18.5) | ||
| Center for Epidemiological Studies-Depression Scale score, mean (SD) | 10.9 (9.1) | ||
| Pain present | 242 (67.2) | ||
| State Anxiety score, mean (SD) | 32.0 (12.0) | ||
| Trait Anxiety score, mean (SD) | 33.8 (10.5) | ||
| Attentional Function Index score, mean (SD) | 6.5 (1.8) | ||
Abbreviations: gm/dL, grams per deciliter; kg/m2, kilograms per meters squared; SD, standard deviation.
Clinically meaningful symptom cut-point scores: Lee Fatigue Scale score ≥3.2 for morning fatigue, ≥5.6 for evening fatigue, ≤6.2 for morning energy, ≤3.5 for evening energy;25 General Sleep Disturbance Scale score ≥43;26 Center for Epidemiological Studies-Depression Scale score ≥16;27 State Anxiety score ≥32.2; Trait Anxiety score ≥31.8;30 Attentional Function Index score ≤5.29 Higher scores for Lee Fatigue Scale, General Sleep Disturbance Scale, Center for Epidemiological Studies-Depression Scale, State Anxiety Scale, and Trait Anxiety Scale indicate higher levels of symptoms. Lower scores on the Attentional Function Index and Lee Energy Scale indicate worse attentional function and lower levels of energy, respectively.
Changes in Physical Function Over Time
The first HLM analysis examined how physical function (i.e., PCS scores) changed over two cycles of chemotherapy. As shown in Figure 1A, a linear model fit the data best. As shown in Table 2, the intercept in the unconditional model represents the estimated level of physical function (i.e., PCS score of 40.719 on a 0 to 100 scale) prior to the initiation of the next cycle of chemotherapy (i.e., Assessment 1). The estimated linear rate of change in physical function for each additional assessment was -0.212 (p <.01). As illustrated in Figure 1A, physical function decreased slightly over the two cycles of chemotherapy.
Figure 1.
A – Model of mean Physical Component Summary scores for six assessment points over two cycles of chemotherapy: prior to chemotherapy administration (i.e., recovery from previous chemotherapy cycle; assessments 1 and 4), approximately 1 week after chemotherapy administration (i.e., acute symptoms in the week after infusion; assessments 2 and 5), and approximately 2 weeks after chemotherapy administration (i.e., potential nadir; assessments 3 and 6). B – Spaghetti plot of individual Physical Component Summary score trajectories for a 10% random sample of patients over two cycles of chemotherapy.
Table 2.
Hierarchical linear model of physical function.
| Physical Function Model Characteristics | Coefficient (SE) |
|
|---|---|---|
| Unconditional Model | Final Model | |
| Fixed Effects | ||
| Intercept | 40.719 (.544)+ | 40.536 (0.450)+ |
| Linear rate of change per assessment | −0.212 (.079)** | −0.212 (0.077)** |
| Time invariant covariates | ||
| Intercept | ||
| Age | −0.176 (0.082)* | |
| Employed | 2.479 (1.107)* | |
| SCQ score | −0.577 (0.147)+ | |
| Exercise on a regular basis | 4.141 (0.980)+ | |
| Morning fatigue score at enrollment | −0.871 (0.278)** | |
| Evening energy score at enrollment | 0.576 (0.223)* | |
| Pain present at enrollment | −3.821 (1.029)+ | |
| Trait Anxiety score at enrollment | 0.120 (0.057)* | |
| Attentional Function Index score at enrollment | 0.754 (0.341)* | |
| Linear slope | ||
| Morning fatigue score at enrollment | −0.080 (0.038)* | |
| PCS score at enrollment | −0.020 (0.007)** | |
| Variance components | ||
| In intercept | 96.256+ | 62.198+ |
| In linear slope | 0.753+ | 0.625+ |
| Goodness-of-fit deviance (parameters estimated) | 11768.510 (6) | 11613.847 (17) |
| Model comparison (x2) | 154.663 (11)+ | |
p <0.05;
p <0.01;
p <0.001
Abbreviations: PCS, Physical Component Summary; SCQ, Self-Administered Comorbidity Questionnaire; SE, standard error.
While a small sample-wide decline in PCS scores was found over time, there was considerable inter-individual variability in the intercept for physical function and moderate inter-individual variability in the slope (Table 2). A spaghetti plot of a random 10% of the sample demonstrates the inter-individual variability in PCS scores over the two cycles of chemotherapy (Figure 1B). These results supported additional analyses of predictors of inter-individual differences in initial levels as well as in the trajectories of PCS scores.
Characteristics Associated with Inter-Individual Differences in Functional Status
The second stage of the HLM analysis evaluated how the pattern of change over time in physical function varied based on demographic, clinical, and symptom characteristics. While 18 characteristics were associated with PCS score at enrollment in exploratory analyses (Supplementary Table 1), only nine characteristics were associated with PCS score at enrollment in the final HLM model (Table 2). Lower PCS scores at enrollment were associated with older age, greater comorbidity, being unemployed, lack of regular exercise, higher morning fatigue, lower evening energy, occurrence of pain, lower trait anxiety, and lower attentional function. Only higher morning fatigue (p=0.04) and lower enrollment PCS score (p=0.01) were associated with decrements in PCS score over time. Of note, neither the MAX2 index nor chemotherapy cycle length were associated with PCS scores at enrollment or with decrements in PCS scores over time.
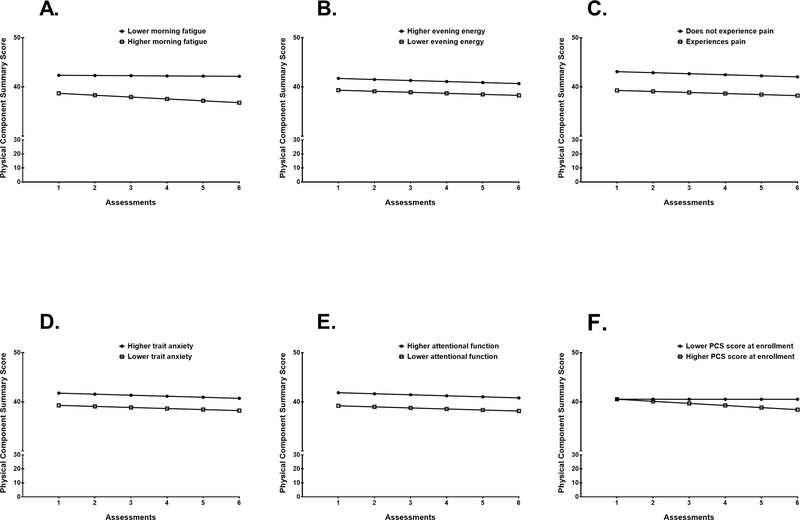
To illustrate the effects of each of these characteristics on patients’ enrollment levels and trajectories of physical function, Figures 2A through 2D display the adjusted change curves for PCS scores estimated based on differences in age (i.e., younger/older calculated based on one SD below and above the mean age), employment status, comorbidity score (i.e., lower/higher calculated based on one SD below and above the mean comorbidity score), and exercise status, respectively. Figures 3A through 3E display the adjusted change curves for PCS scores based on differences in symptoms at enrollment: morning fatigue, evening energy, occurrence of pain, trait anxiety, and attentional function (i.e., lower/higher calculated based on one SD below and above the mean score for each symptom). Figure 3F displays the adjusted change curve for physical function based on differences in PCS score at enrollment. All mean PCS scores for the various characteristics depicted in the figures are estimated or predicted means based on the HLM analyses.
Figure 2A-D –
Influence of (A) age, (B) employment status, (C) Self-Administered Comorbidity Questionnaire score, and (D) exercise on a regular basis at enrollment on inter-individual differences in the intercept for physical function. In (A) and (C), values for one SD below and above the mean are plotted as examples. Patients were assessed six times over two cycles of chemotherapy: prior to chemotherapy administration (i.e., recovery from previous chemotherapy cycle; assessments 1 and 4), approximately 1 week after chemotherapy administration (i.e., acute symptoms in the week after infusion; assessments 2 and 5), and approximately 2 weeks after chemotherapy administration (i.e., potential nadir; assessments 3 and 6).
Figure 3A-F –
Influence of symptoms including (A) morning fatigue, (B) evening energy, (C) pain, (D) trait anxiety, (E) attentional function, and (F) Physical Component Summary (PCS) score at enrollment on inter-individual differences in the intercept for physical function. For (A) morning fatigue and (F) PCS score at enrollment, inter-individual differences in the slope parameters for physical function are shown. In (F), values for one SD below and above the mean PCS score at enrollment are plotted as examples. Patients were assessed six times over two cycles of chemotherapy: prior to chemotherapy administration (i.e., recovery from previous chemotherapy cycle; assessments 1 and 4), approximately 1 week after chemotherapy administration (i.e., acute symptoms in the week after infusion; assessments 2 and 5), and approximately 2 weeks after chemotherapy administration (i.e., potential nadir; assessments 3 and 6).
Discussion
In a large sample of older adults with cancer who were entering a second or subsequent cycle of chemotherapy, we identified numerous demographic (i.e., older age, not working), clinical (i.e., higher comorbidity, lack of regular exercise), and symptom characteristics (i.e., higher morning fatigue, lower evening energy, occurrence of pain, higher trait anxiety, lower attentional function) associated with lower levels of physical function at enrollment. In contrast, only morning fatigue and PCS scores at enrollment were associated with modest decrements in physical function over time. This study is the first to identify that higher levels of morning fatigue was the only symptom associated with functional decline during chemotherapy. In addition, this study is the first to assess physical function at multiple time points over two cycles of chemotherapy in older adults and to analyze changes in physical function as a trajectory, rather than as a dichotomous outcome of functional decline between two time points. It should be noted that at enrollment, the mean PCS score for our sample was 4.2 points lower than the age-based normative score of 44.9 (adults age 65 to 74 years) based on the 2001 Utah Health Status Survey (57).
Fatigue is one of the most common and distressing symptoms reported by patients with cancer and can result in decreased quality of life (58–60). In this study, we identified that higher levels of morning fatigue was associated with modest decreases in physical function during chemotherapy. Specifically, as illustrated in Figure 3A, the predicted mean PCS score for Assessment 1 (intercept) was 42.4 for patients with higher morning fatigue (one SD above the mean morning fatigue score) and 38.7 for patients with lower morning fatigue (one SD below the mean evening fatigue score). At Assessment 6, the predicted mean PCS score was 42.2 for patients with higher morning fatigue and 36.8 for patients with lower morning fatigue.
In comparison, evening fatigue, morning energy, and evening energy were not associated with changes in physical function over time. Of note, both higher morning fatigue and lower evening energy were associated with lower levels of physical function at enrollment. This finding highlights the importance of assessing for diurnal variations in fatigue severity (61) as well as decrements in energy (62, 63). In addition, research from our group demonstrated distinct characteristics associated with the trajectories of morning and evening fatigue and energy in patients with cancer (15, 45, 64, 65), supporting the need for comprehensive assessment of fatigue both in research and clinical care. Furthermore, management strategies for cancer-related fatigue, such as energy conservation and exercise (66, 67), may impact morning and evening fatigue differently. Therefore, morning and evening fatigue should be evaluated as distinct outcomes in addition to overall fatigue to determine how interventions modify each symptom.
In a prior study of physical function among older adults with cancer receiving chemotherapy (6), no association was found between fatigue and functional decline. The lack of association between fatigue and functional decline in the prior study may be related to differences in the measurement of fatigue (single global measure using the Mobility-Tiredness Test versus morning and evening fatigue and energy levels using the Lee Fatigue Scale in our study), measurement of physical function (change in ADL and IADL versus PCS score in our study), and the timing of the assessments (two time points prior to treatment and at two to three months versus six time points over two cycles of chemotherapy in our study). In another study of functional decline during first-line chemotherapy in older adults (8), its association with fatigue was not evaluated. Therefore, our result warrants confirmation in future research to determine if improvements in morning fatigue may mitigate decrements in physical function.
In addition, we found that lower physical function at enrollment (i.e., prior to next dose of chemotherapy) was associated with older age, greater comorbidity, being unemployed, lack of regular exercise, presence of pain, lower trait anxiety, and lower attentional function. While older age, greater comorbidity, and pain are risk factors for decrements in physical function (9, 10, 68), unemployment and lack of regular exercise may reflect risk factors for and/or outcomes of poorer physical function. Interestingly in our sample, higher trait anxiety, a disposition toward experiencing anxiety (69), was associated with higher physical function scores at enrollment. Higher trait anxiety was associated with moderate intensity physical activity in a study of community dwelling older adults (70), which supports our finding. While the association between lower attentional function and lower physical function suggests that both processes are interrelated (71), further research is needed to understand how the two processes interact in older adults during cancer treatment.
Consistent with a previous report (6), we did not identify an association between depression at enrollment and initial levels or trajectories of physical function in our sample of older adults with cancer. The proportion of patients reporting depressive symptoms in the previous report (20.6%) (6) was similar to the 25.1% in our study. These findings contrast with another study, where 44.5% of patients reported depressive symptoms and a positive association was found between depression and increased risk of functional decline after one cycle of first-line chemotherapy (8). Additional research is needed to better characterize the association between depression and functional decline in older adults with cancer.
While a previous study found that older adults receiving first-line chemotherapy for a new diagnosis of cancer were more likely to experience decline in IADL than those treated for progression/relapse (6), we did not detect any associations between time since cancer diagnosis or the number of prior cancer treatments and decrements in physical function in our sample. Because all of our patients had received at least one cycle of chemotherapy within the four weeks prior to enrollment, patients treated for a new diagnosis may have already experienced some decrements in physical function before our assessments.
Strengths of our study include the focused appraisal of physical function at multiple points over two cycles of chemotherapy, which allows for the examination of acute within cycle changes in function that may occur as a result of toxicities immediately after chemotherapy infusion into nadir and recovery. While prior studies of physical function in older adults during chemotherapy have focused on changes after one cycle (8) or several months after the initiation of chemotherapy (6), our study design allowed us to evaluate for acute changes in physical function within a chemotherapy cycle. In addition, we analyzed physical function over time as a trajectory, rather than a dichotomous outcome of functional decline between two time points. This approach allowed us to examine more subtle changes in physical function and evaluate for different possible trajectories (e.g., linear, quadratic).
Our study has several limitations. First, while patients were uniformly assessed at three specific points (i.e., prior to chemotherapy administration and at approximately 1 and 2 weeks after administration) across two cycles of chemotherapy, they were recruited at various cycles in their chemotherapy treatment. As a result, changes in physical function from the initiation of chemotherapy cannot be evaluated in this study. In addition, because our study design may have excluded older adults who discontinued chemotherapy after their initial cycle(s) due to functional decline, our results may underestimate the true degree of functional decline in this population. Furthermore, additional common geriatric assessment domains such as nutrition (72, 73) and frailty (74, 75) that could contribute to decrements in physical function were not assessed. Lastly, physical function was not assessed using objective measures (e.g., gait speed) or assessments of ADL or IADL. However, the SF-12 PCS score is a valid and reliable measure of physical function that includes assessments of ability to perform moderate activities, climb stairs, and accomplish work and other activities (27–40). Although the number of studies of serial comprehensive geriatric assessment during cancer treatment is increasing, the large number of studies that measure quality of life using instruments that include physical function subscales such as the SF-12, SF-36, and European Organization for Research and Treatment of Cancer QLQ-C30 (76) provides additional opportunities to understand changes in physical function during cancer treatment.
It is important to note that within the geriatric oncology literature, it is crucial to highlight analyses that include function as an outcome since functional outcomes are extremely important to older adults with cancer and are not assessed in many studies. Given the numerous studies that have used the SF-12 or SF-36, which closely overlaps other measures of physical function such as the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function item bank (77, 78), secondary analyses of cancer studies with SF-12 or SF-36 data are important to suggest future directions for research.
Conclusions
This study is the first to identify morning fatigue as a potentially modifiable characteristic associated with decrements in physical function in older adults with cancer who were entering a second or subsequent cycle of chemotherapy. Interventions focused on improving morning fatigue may prevent functional decline in older adults receiving chemotherapy and should be studied. Since our study enrolled older adults who had already started their chemotherapy treatment, the current findings should be confirmed in patients who are assessed at the initiation of chemotherapy and followed through to treatment completion or discontinuation. Future studies need to investigate the impact of multiple co-occurring symptoms and symptom clusters on the trajectories of physical function during chemotherapy.
Supplementary Material
Supplementary Table 1. Characteristics evaluated as potential predictors of intercept and linear slope for physical function.
ACKNOWLEDGEMENTS
This study was funded by the National Cancer Institute (NCI; R01CA134900). Dr. Wong is supported by the National Institute on Aging (NIA; T32AG000212, R03AG056439, P30AG044281) and National Center for Advancing Translational Sciences (KL2TR001870). Dr. Ritchie is supported by the NIA (P30AG044281). Dr. Steinman is supported by the NIA (K24AG049057, P30AG044281). Dr. Walter is supported by the NIA (K24AG041180, P30AG044281). Dr. Miaskowski is supported by a grant from the American Cancer Society and NCI (K05CA168960). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the American Cancer Society.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65. [DOI] [PubMed] [Google Scholar]
- 2.Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: A short review. Eur J Cancer 2007;43:2203–2210. [DOI] [PubMed] [Google Scholar]
- 3.Esbensen BA, Osterlind K, Roer O, Hallberg IR. Quality of life of elderly persons with newly diagnosed cancer. Eur J Cancer Care (Engl) 2004;13:443–53. [DOI] [PubMed] [Google Scholar]
- 4.Wedding U, Rohrig B, Klippstein A, et al. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients. Support Care Cancer 2007;15:1097–104. [DOI] [PubMed] [Google Scholar]
- 5.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the Multicenter Italian Lung Cancer in the Elderly study. J Clin Oncol 2005;23:6865–72. [DOI] [PubMed] [Google Scholar]
- 6.Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol 2017;8:196–205. [DOI] [PubMed] [Google Scholar]
- 7.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346:1061–6. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 2013;31:3877–82. [DOI] [PubMed] [Google Scholar]
- 9.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res 2001;50:222–32. [DOI] [PubMed] [Google Scholar]
- 10.Given CW, Given B, Azzouz F, Stommel M, Kozachik S. Comparison of changes in physical functioning of elderly patients with new diagnoses of cancer. Med Care 2000;38:482–93. [DOI] [PubMed] [Google Scholar]
- 11.van Abbema D, van Vuuren A, van den Berkmortel F, et al. Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. Journal of Geriatric Oncology 2017;8:176–184. [DOI] [PubMed] [Google Scholar]
- 12.Owusu C, Margevicius S, Schluchter M, et al. Vulnerable elders survey and socioeconomic status predict functional decline and death among older women with newly diagnosed nonmetastatic breast cancer. Cancer 2016;122:2579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puts MTE, Monette J, Girre V, et al. Changes in functional status in older newly-diagnosed cancer patients during cancer treatment: A six-month follow-up period. Results of a prospective pilot study. Journal of Geriatric Oncology 2011;2:112–120. [Google Scholar]
- 14.Repetto L Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 2003;1:18–24. [PubMed] [Google Scholar]
- 15.Wright F, D'Eramo Melkus G, Hammer M, et al. Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage 2015;50:176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer 2014;120:2371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward Sullivan C, Leutwyler H, Dunn LB, et al. Differences in symptom clusters identified using symptom occurrence rates versus severity ratings in patients with breast cancer undergoing chemotherapy. Eur J Oncol Nurs 2017;28:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan CW, Leutwyler H, Dunn LB, et al. Stability of symptom clusters in patients with breast cancer receiving chemotherapy. J Pain Symptom Manage 2018;55:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miaskowski C, Cooper BA, Aouizerat B, et al. The symptom phenotype of oncology outpatients remains relatively stable from prior to through 1 week following chemotherapy. Eur J Cancer Care (Engl) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnofsky D Performance scale, New York: Plenum Press, 1977. [Google Scholar]
- 21.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer 2008;113:2205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer 2001;85:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. [DOI] [PubMed] [Google Scholar]
- 24.Extermann M, Chen H, Cantor AB, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer 2002;38:1466–73. [DOI] [PubMed] [Google Scholar]
- 25.Extermann M, Bonetti M, Sledge GW, et al. MAX2--a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer 2004;40:1193–8. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, Jr., Kosinski M, Keller SD A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 27.Annunziata MA, Muzzatti B, Giovannini L, et al. Is long-term cancer survivors' quality of life comparable to that of the general population? An italian study. Support Care Cancer 2015;23:2663–8. [DOI] [PubMed] [Google Scholar]
- 28.Briggs KK, Soares E, Bhatia S, Philippon MJ. Postoperative alpha angle not associated with patient-centered midterm outcomes following hip arthroscopy for FAI. Knee Surg Sports Traumatol Arthrosc 2018. [DOI] [PubMed] [Google Scholar]
- 29.Gallo RA, Plakke M, Mosher T, Black KP. Outcomes following impaction bone grafting for treatment of unstable osteochondritis dissecans. Knee 2016;23:495–500. [DOI] [PubMed] [Google Scholar]
- 30.Gautschi OP, Corniola MV, Smoll NR, et al. Sex differences in subjective and objective measures of pain, functional impairment, and health-related quality of life in patients with lumbar degenerative disc disease. Pain 2016;157:1065–71. [DOI] [PubMed] [Google Scholar]
- 31.Gautschi OP, Smoll NR, Corniola MV, et al. Sex differences in lumbar degenerative disc disease. Clin Neurol Neurosurg 2016;145:52–7. [DOI] [PubMed] [Google Scholar]
- 32.Gerhardt D, De Visser E, Hendrickx BW, Schreurs BW, Van Susante JLC. Bone mineral density changes in the graft after acetabular impaction bone grafting in primary and revision hip surgery. Acta Orthop 2018;89:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard LM, Flach C, Mehay A, Sharp D, Tylee A. The prevalence of suicidal ideation identified by the Edinburgh Postnatal Depression Scale in postpartum women in primary care: findings from the RESPOND trial. BMC Pregnancy Childbirth 2011;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendes de Leon CF, Czajkowski SM, Freedland KE, et al. The effect of a psychosocial intervention and quality of life after acute myocardial infarction: the Enhancing Recovery in Coronary Heart Disease (ENRICHD) clinical trial. J Cardiopulm Rehabil 2006;26:9–13; quiz 14–5. [DOI] [PubMed] [Google Scholar]
- 35.Newman JT, Briggs KK, McNamara SC, Philippon MJ. Revision Hip Arthroscopy: A Matched-Cohort Study Comparing Revision to Primary Arthroscopy Patients. Am J Sports Med 2016;44:2499–2504. [DOI] [PubMed] [Google Scholar]
- 36.Preede L, Saebu M, Perrin PB, et al. One-year trajectories of mental and physical functioning during and after rehabilitation among individuals with disabilities. Health Qual Life Outcomes 2015;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roelen C, van Rhenen W, Schaufeli W, et al. Mental and physical health-related functioning mediates between psychological job demands and sickness absence among nurses. J Adv Nurs 2014;70:1780–92. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y, Green P, Maurer M, et al. Relationship Between Accelerometer-Measured Activity and Self-Reported or Performance-Based Function in Older Adults with Severe Aortic Stenosis. Curr Geriatr Rep 2015;4:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Oldenrijk J, Scholtes VAB, van Beers L, et al. Better early functional outcome after short stem total hip arthroplasty? A prospective blinded randomised controlled multicentre trial comparing the Collum Femoris Preserving stem with a Zweymuller straight cementless stem total hip replacement for the treatment of primary osteoarthritis of the hip. BMJ Open 2017;7:e014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Brown JC, Schmitz KH. Association between Body Mass Index and Physical Function among Endometrial Cancer Survivors. PLoS One 2016;11:e0160954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware J, Kosinski M, Keller S. SF-12: How to score the SF-12 physical and mental health summary scales, Second ed. Boston, MA: The Health Institute, New England Medical Center, 1995. [Google Scholar]
- 42.Jacobs BL, Lopa SH, Yabes JG, et al. Association of functional status and treatment choice among older men with prostate cancer in the Medicare Advantage population. Cancer 2016;122:3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroenke K, Johns SA, Theobald D, Wu J, Tu W . Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Support Care Cancer 2013;21:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res 1991;36:291–8. [DOI] [PubMed] [Google Scholar]
- 45.Aouizerat BE, Dhruva A, Paul SM, et al. Phenotypic and molecular evidence suggests that decrements in morning and evening energy are distinct but related symptoms. J Pain Symptom Manage 2015;50:599–614.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eshragh J, Dhruva A, Paul SM, et al. Associations Between Neurotransmitter Genes and Fatigue and Energy Levels in Women After Breast Cancer Surgery. J Pain Symptom Manage 2017;53:67–84.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kober KM, Smoot B, Paul SM, et al. Polymorphisms in Cytokine Genes Are Associated With Higher Levels of Fatigue and Lower Levels of Energy in Women After Breast Cancer Surgery. J Pain Symptom Manage 2016;52:695–708.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abid H, Kober KM, Smoot B, et al. Common and Distinct Characteristics Associated With Trajectories of Morning and Evening Energy in Oncology Patients Receiving Chemotherapy. J Pain Symptom Manage 2017;53:887–900.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright F, Hammer M, Paul SM, et al. Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine 2017;91:187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kober KM, Cooper BA, Paul SM, et al. Subgroups of Chemotherapy Patients With Distinct Morning and Evening Fatigue Trajectories. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2016;24:1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KA. Self-reported sleep disturbances in employed women. Sleep 1992;15:493–8. [DOI] [PubMed] [Google Scholar]
- 52.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 53.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17:197–210. [DOI] [PubMed] [Google Scholar]
- 54.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology 2011;20:194–202. [DOI] [PubMed] [Google Scholar]
- 55.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire, Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
- 56.Raudenbush SW, Bryk A. Hierarchical linear models: Applications and data analysis methods, 2nd ed. Thousand Oaks, CA: Sage Publications, 2002. [Google Scholar]
- 57.Office of Public Health Assessment. Health Status in Utah: The Medical Outcomes Study SF-12 (2001 Utah Health Status Survey Report). In: Utah Department of Health, ed. Salt Lake City, UT: 2004. [Google Scholar]
- 58.Storey DJ, Waters RA, Hibberd CJ, et al. Clinically relevant fatigue in cancer outpatients: The Edinburgh Cancer Centre symptom study. Ann Oncol 2007;18:1861–9. [DOI] [PubMed] [Google Scholar]
- 59.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: The scale of the problem. Oncologist 2007;12 Suppl 1:4–10. [DOI] [PubMed] [Google Scholar]
- 60.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist 2000;5:353–360. [DOI] [PubMed] [Google Scholar]
- 61.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: Variability in fatigue levels in cancer patients. Psychosomatics 2007;48:247–252. [DOI] [PubMed] [Google Scholar]
- 62.Lerdal A A theoretical extension of the concept of energy through an empirical study. Scandinavian Journal of Caring Sciences 2002;16:197–206. [DOI] [PubMed] [Google Scholar]
- 63.Lerdal A, Kottorp A, Gay C, et al. A Rasch analysis of assessments of morning and evening fatigue in oncology patients using the Lee Fatigue Scale. J Pain Symptom Manage 2016;51:1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kober KM, Cooper BA, Paul SM, et al. Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer 2016;24:1473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abid H, Kober KM, Smoot B, et al. Common and distinct characteristics associated with trajectories of morning and evening energy in oncology patients receiving chemotherapy. J Pain Symptom Manage 2017;53:887–900 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol 2017;3:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cancer-related fatigue (Version 2.2017). Available from: http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed Sep 4 2017, 2017. [DOI] [PMC free article] [PubMed]
- 68.Vallerand AH, Templin T, Hasenau SM, Riley-Doucet C. Factors that affect functional status in patients with cancer-related pain. Pain 2007;132:82–90. [DOI] [PubMed] [Google Scholar]
- 69.Elwood LS, Wolitzky-Taylor K, Olatunji BO. Measurement of anxious traits: A contemporary review and synthesis. Anxiety Stress Coping 2012;25:647–66. [DOI] [PubMed] [Google Scholar]
- 70.Smith JC, Zalewski KR, Motl RW, Van Hart M, Malzahn J. The contributions of self-efficacy, trait anxiety, and fear of falling to physical activity behavior among residents of continuing care retirement communities. 2010 2010;1. [Google Scholar]
- 71.Montero-Odasso M, Bherer L, Studenski S, et al. Mobility and cognition in seniors. Report from the 2008 Institute of Aging (CIHR) Mobility and Cognition Workshop. Can Geriatr J 2015;18:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol 2012;30:1829–34. [DOI] [PubMed] [Google Scholar]
- 74.Huisingh-Scheetz M, Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol 2017;8:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. J Natl Compr Canc Netw 2017;15:894–902. [DOI] [PubMed] [Google Scholar]
- 76.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 77.Rose M, Bjorner JB, Gandek B, et al. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol 2014;67:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fries JF, Witter J, Rose M, et al. Item Response Theory, Computerized Adaptive Testing, and PROMIS: Assessment of Physical Function. J Rheumatol 2014;41:153–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Characteristics evaluated as potential predictors of intercept and linear slope for physical function.